Published online Dec 27, 2020. doi: 10.4254/wjh.v12.i12.1276
Peer-review started: July 28, 2020
First decision: August 22, 2020
Revised: September 7, 2020
Accepted: November 5, 2020
Article in press: November 5, 2020
Published online: December 27, 2020
Malnutrition in cirrhotic patients is correlated with mortality and a better response to liver transplantation. However, recovery of the nutritional status in these patients is a challenge due to the difficulty in establishing a reliable nutritional diagnosis. The bioelectrical impedance vector analysis (BIVA) method appears as a feasible tool in clinical practice to define the physiological state of cirrhotic patients by assessing hydration and body cellularity.
To evaluate body composition in cirrhotic patients using BIVA.
This retrospective cross-sectional study was carried out by following cirrhotic outpatients at a hospital in Porto Alegre, Brazil. A tetrapolar bioelectrical impedance analysis device was used to evaluate cellularity and hydration and to perform the BIVA. The BIVA graphic was elaborated by software and for statistical analysis a significance level of 5% (P ≤ 0.05) was considered.
One hundred and ninety patients, 61.1% males, with a mean age of 56.6 ± 11.0 years, were evaluated. Of these, 56.3% had Child-Turcotte-Pugh (CTP) A score, and the prevalent etiology was hepatitis C virus (47.4%). The patients were classified according to cellularity and hydration by the quadrants and ellipses of the BIVA method, quadrant 1 (47.9%); quadrant 2 (18.9%); quadrant 3 (14.2%); and quadrant 4 (18.9%). Those classified in quadrant 1 and 2 had a higher phase angle compared to those in quadrants 3 and 4 (P < 0.001). Quadrant 2 patients had a lower average age than the other groups. The association with CTP score showed that patients in quadrant 2 had a higher proportion of CTP A, and those in quadrant 4 had a higher proportion of CTP C (P < 0.052).
The BIVA method allows identification of the cellularity and hydration status of cirrhotic patients, and its association with clinical factors determines the disease severity, age and prognostic index.
Core Tip: Using the bioelectrical impedance vector analysis method, it is feasible in clinical practice to identify hydration and cellularity status in patients with liver cirrhosis, regardless of their etiology. This tool allows health professionals to establish an effective treatment for these patients with the objectives of clinical improvement, a better quality of life and better response to orthotopic liver transplantation.
- Citation: Fernandes SA, Leonhardt LR, da Silva DM, Alves FD, Marroni CA. Bioelectrical impedance vector analysis evaluates cellularity and hydration in cirrhotic patients. World J Hepatol 2020; 12(12): 1276-1288
- URL: https://www.wjgnet.com/1948-5182/full/v12/i12/1276.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i12.1276
One of the main clinical complications of liver cirrhosis is protein-calorie malnutrition, which has a prevalence ranging from 10% to 100%, regardless of the stage and etiology of the disease. It has been observed in different studies that the general prognosis of the disease worsens in the presence of malnutrition, contributing negatively to the quality of life of patients[1-7].
The evaluation of body composition in cirrhotic patients is a challenge, as there is no recognized gold standard. Anthropometric measurements are non-invasive and low-cost methods, but they can be impaired by changes in hydration status (ascites and edema) and have moderate reliability due to interobserver variation[6,8]. Among the most frequently used methods for assessing body composition, electrical bioimpedance (BIA) is capable of providing information on lean mass and fat mass through the parameters of electrical current of tissues, resistance (R) and reactance (Xc), which help to identify the functionality and integrity of cell membranes[9].
Using R and Xc, the phase angle (PA), a marker of the nutritional status independent of the device’s pre-established formulas can be calculated, where the patient’s hydration, for example in cirrhotic patients, could generate estimation errors. In addition, PA values have been shown to be an excellent prognostic index in several clinical conditions[6,10-15]. The BIA can further provide angular vectors of alterations in body fluid levels and cellularity of the patient, and this method is known as bioelectrical impedance vector analysis (BIVA)[16-18].
The PA provides us with a large amount of data that, analyzed in a specific way, with specific statistical programs, allows new analyses of body composition to deepen our knowledge. BIVA uses graphic vectors for the analysis of BIA data, where impedance is plotted as a vector by its components R (X axis) and Xc (Y axis) after standardization by weight[11,12,18].
The electrical properties of the tissues (R and Xc) must be standardized by sex and race, with their tolerance intervals, in relation to a given population. The resulting graph provides ellipses of tolerance, i.e., 50%, 75% and 95% percentiles (confidence intervals) that are divided into quadrants that represent groups of patients with more or less hydration, more or less cellularity. The advantage of this method is that it allows simultaneous information on changes in body hydration or soft tissue mass, regardless of body weight. Thus, BIVA is able to correctly interpret, even if the patient is extremely heavy, the distribution of water volume in different diseases, and assess the general composition of the body[19-21].
To date, no studies have analyzed body composition regarding hydration and cellularity in cirrhotic patients using the BIVA method. Therefore, the main objective of this study was to evaluate the results of BIVA regarding hydration and cellularity, and compare them with the PA and other clinical parameters in cirrhotic patients.
This was a retrospective cross-sectional study with data collected between May 2007 and December 2015, at the Santa Casa de Misericórdia Hospital Complex in Porto Alegre, RS, Brazil.
A total of 224 patients with cirrhosis undergoing outpatient follow-up were included in the data collection. Of these, 34 were excluded due to incomplete data and 190 patients were included in the final analysis. The etiology of cirrhosis was as follows: Hepatitis C virus (HCV), hepatitis B virus, alcohol, autoimmune, non-alcoholic fatty liver disease, cryptogenic or cholestatic disease, and some patients had two concomitant etiologies.
Data on age, gender, socioeconomic status, social history (smoking and alcohol consumption), and chronic diseases were obtained. In addition to the anamnesis, data on the etiology of cirrhosis, staging of the disease, medications used, complementary examinations, laboratory, imaging or anatomopathological data were obtained.
Body mass measurement was verified by the Filizola® scale, with a scale of 100 g, previously calibrated. The patients were measured wearing light clothing and barefoot. Height was determined with a fixed stadiometer on the wall, with the patient standing erect and barefoot.
The BIA evaluation was performed in the outpatient department, without previous specific preparation for fasting. The patients were evaluated in a comfortable dorsal decubitus position and relaxed, without shoes, socks and metallic fittings. According to the procedure, the legs were spread apart, hands open and supported on the stretcher. Skintak® electrodes were used as follows: One electrode was placed at the base of the middle toe on the right foot and another electrode slightly above the line of the ankle joint between the medial and lateral malleoli. Another pair of electrodes was distributed at the base of the middle finger of the right hand, and slightly above the line of the right wrist joint, coinciding with the styloid process.
The device used was Biodynamics®, model 450, with an electric current intensity of 800 µA and frequency of 50 kHz. Nominal voltage was 8.4 V, with a rated capacity of 600 mA/h. The amplitude of R was 200-1500 Ω, with a resolution of 0.1 Ω and accuracy of 0.1%. The amplitude of Xc was 0-300 Ω, the resolution was 0.1 Ω and the precision was 0.2%. The unit also had a 0°-20° PA amplitude, 0.1° resolution and 0°-2° accuracy.
The PA was automatically provided by the equipment from the values of R and Xc. PA was classified according to the cut-off point of 5.4°, based on the reference parameters of the study by Fernandes et al[6] and Selberg et al[22], in which values below this point are considered predictive of a bad prognosis, and the values above are predictive of a good prognosis.
In this method, the raw measures of the BIA (R and Xc) are used graphically, standardized by height in meters, and plotted as vector bivariate points, with their confidence and tolerance intervals, which are ellipses in the graphical plane RXc. The method is based on the analysis of the bivariate distribution of vector impedance in a healthy population. Graph RXc can be observed with the tolerance intervals of 50%, 75% and 95% of the impedance value (i.e., the ellipses containing the vector values and the probabilities of 50%, 75% and 95%)[19-21].
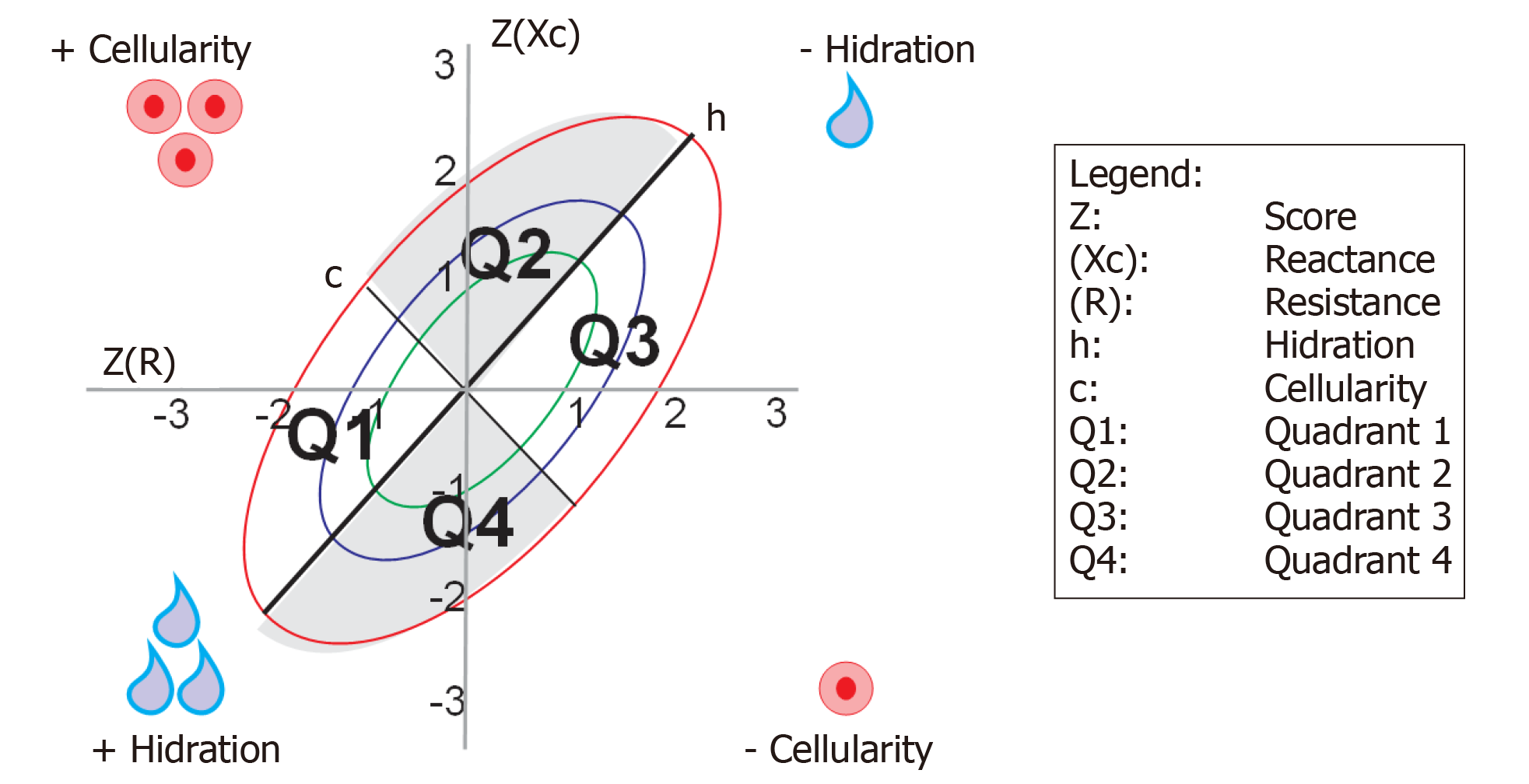
The upward or downward displacement of vectors in the direction of the largest axis (h) of the ellipse indicates progressive change in tissue hydration (dehydration towards the upper pole, hyperhydration with apparent edema toward the lower pole). Vectors migrating towards the lower axis (c) above, to the left, indicate more body cell mass and below, to the right, less body cell mass[19].
BIVA measurement points were determined for each quadrant, considering areas between the h and c axes, according to body conditions (hydration and cellularity), being classified as: Quadrant 1 (Q1): More cellularity, more hydration; quadrant 2 (Q2): More cellularity, less hydration; quadrant 3 (Q3): Less cellularity, less hydration; and quadrant 4 (Q4): Less cellularity, more hydration (Figure 1).
Quantitative variables were described by the mean ± SD and categorical variables by absolute and relative frequencies. One-way ANOVA was used to compare the means, complemented by the Tukey’s test. In the comparison of proportions, the Chi-square test was applied along with the analysis of the adjusted residuals. For control of confounding factors, the Poisson regression analysis was applied to the factors that presented a P < 0.10 in the bivariate analysis.
The significance level adopted was 5% (P ≤ 0.05) and the analyses were performed using the SPSS program version 21.0.
A total of 190 patients with a mean age of 56.6 ± 11.0 years were evaluated. Sixty-one percent of the patients were male. Of these, 56.3% had Child-Turcotte-Pugh (CTP) A score, and the most prevalent etiology was HCV (47.4%). The characteristics of the studied population are presented in Table 1.
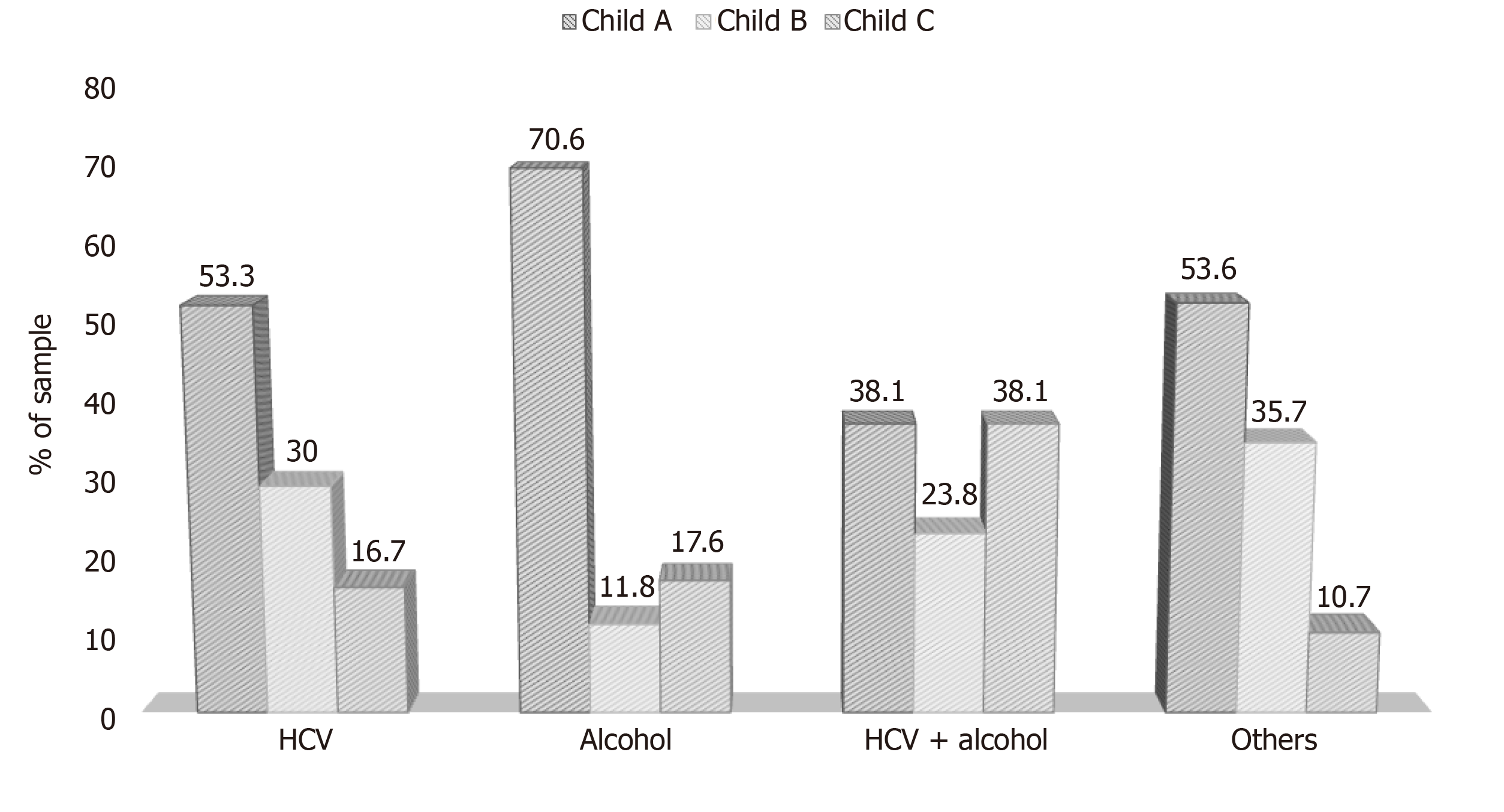
A significant association (P = 0.025) between etiology and CTP score was observed, with CTP A more prevalent in patients with an etiology related to alcohol. Also, patients with HCV + alcohol etiology had a higher prevalence of CTP C (Figure 2).
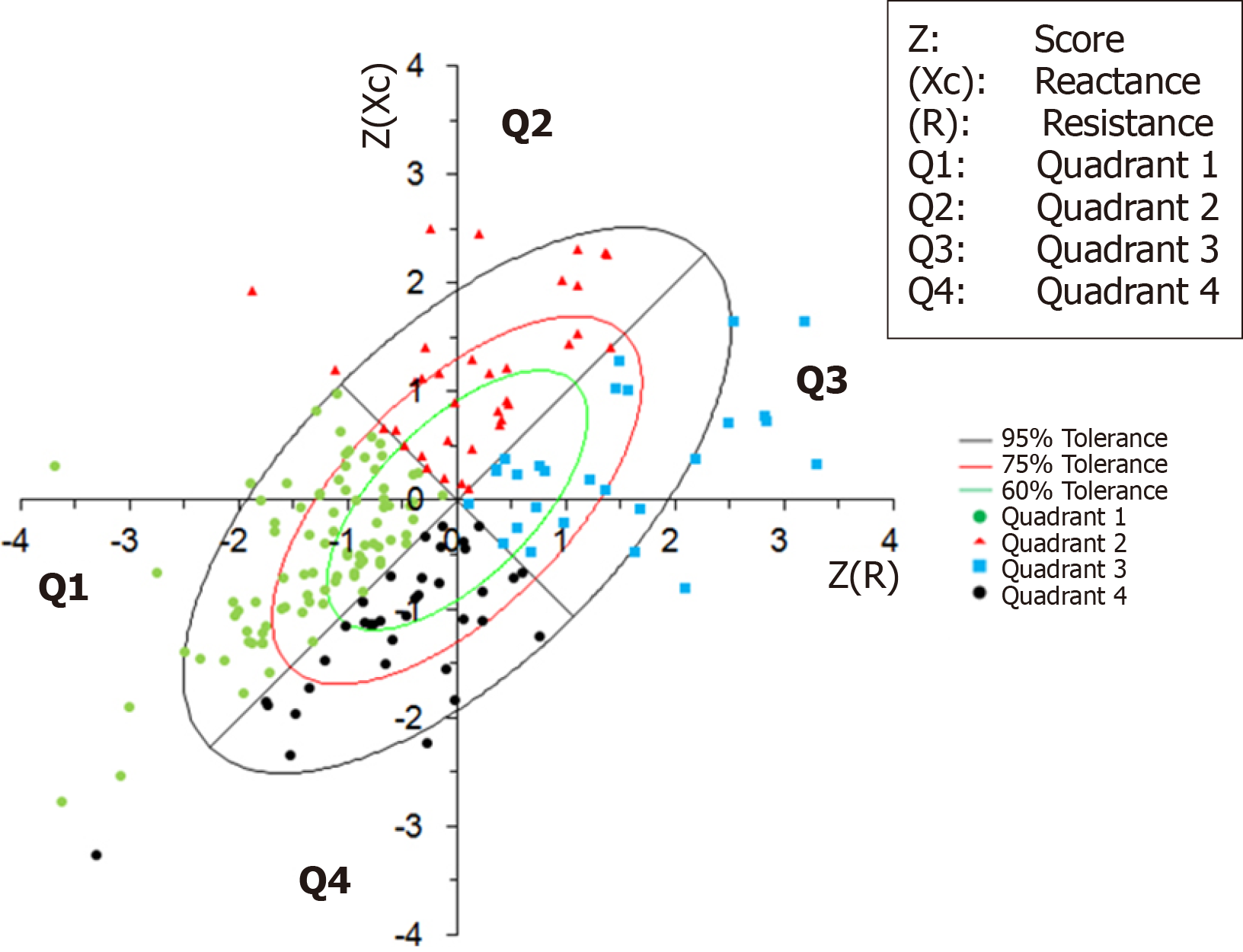
The patient sample was plotted on the RXc chart and classified by BIVA quadrants, according to hydration and cellularity (Figure 3).
The patients were evaluated by the BIVA method in relation to age, sex, disease staging (CTP score), etiology and PA (Table 2). The Q2 patients had a lower mean age than those in the other quadrants (P < 0.001). Patients classified in Q1 and Q2 had higher PA than those in Q3 and Q4 (P < 0.001).
| Variables1 | Total sample (n = 190) | Classification quadrants - BIVA | ||||
| Q1 (n = 91; 47.9%) | Q2 (n = 36; 18.9%) | Q3 (n = 27; 14.2%) | Q4 (n = 36; 18.9%) | P value | ||
| Age (yr) | 56.6 ± 11.0 | 56.8 ± 9.64 | 50.3 ± 14.33 | 57.7 ± 9.24 | 61.6 ± 8.94 | < 0.001 |
| Gender | 0.642 | |||||
| Male | 116 (61.1) | 54 (59.3) | 20 (55.6) | 17 (63.0) | 25 (69.4) | |
| Female | 74 (38.9) | 37 (40.7) | 16 (44.4) | 10 (37.0) | 11 (30.6) | |
| Child-Turcotte-Pugh | 0.052 | |||||
| A | 107 (56.3) | 52 (57.1) | 27 (75.0) | 13 (48.1) | 15 (41.7) | |
| B | 48 (25.3) | 25 (27.5) | 5 (13.9) | 9 (33.3) | 9 (25.0) | |
| C | 35 (18.4) | 14 (15.4) | 4 (11.1) | 5 (18.5) | 12 (33.3) | |
| Etiology | 0.380 | |||||
| HCV | 90 (47.4) | 45 (49.5) | 16 (44.4) | 13 (48.1) | 16 (44.4) | |
| Alcohol | 51 (26.8) | 29 (31.9) | 7 (19.4) | 7 (25.9) | 8 (22.2) | |
| HCV + alcohol | 21 (11.1) | 6 (6.6) | 4 (11.1) | 4 (14.8) | 7 (19.4) | |
| Other2 | 28 (14.7) | 11 (12.1) | 9 (25.0) | 3 (11.1) | 5 (13.9) | |
| Phase angle | 6.06 ± 2.20 | 6.49 ± 2.444 | 7.30 ± 2.124 | 5.07 ± 0.673 | 4.46 ± 0.703 | < 0.001 |
There was an association between the BIVA quadrants and CTP classification. The patients classified in Q2 had a significantly higher proportion of CTP A than the other quadrants. In addition, Q4 patients had a significantly higher CTP ratio than those in the other quadrants.
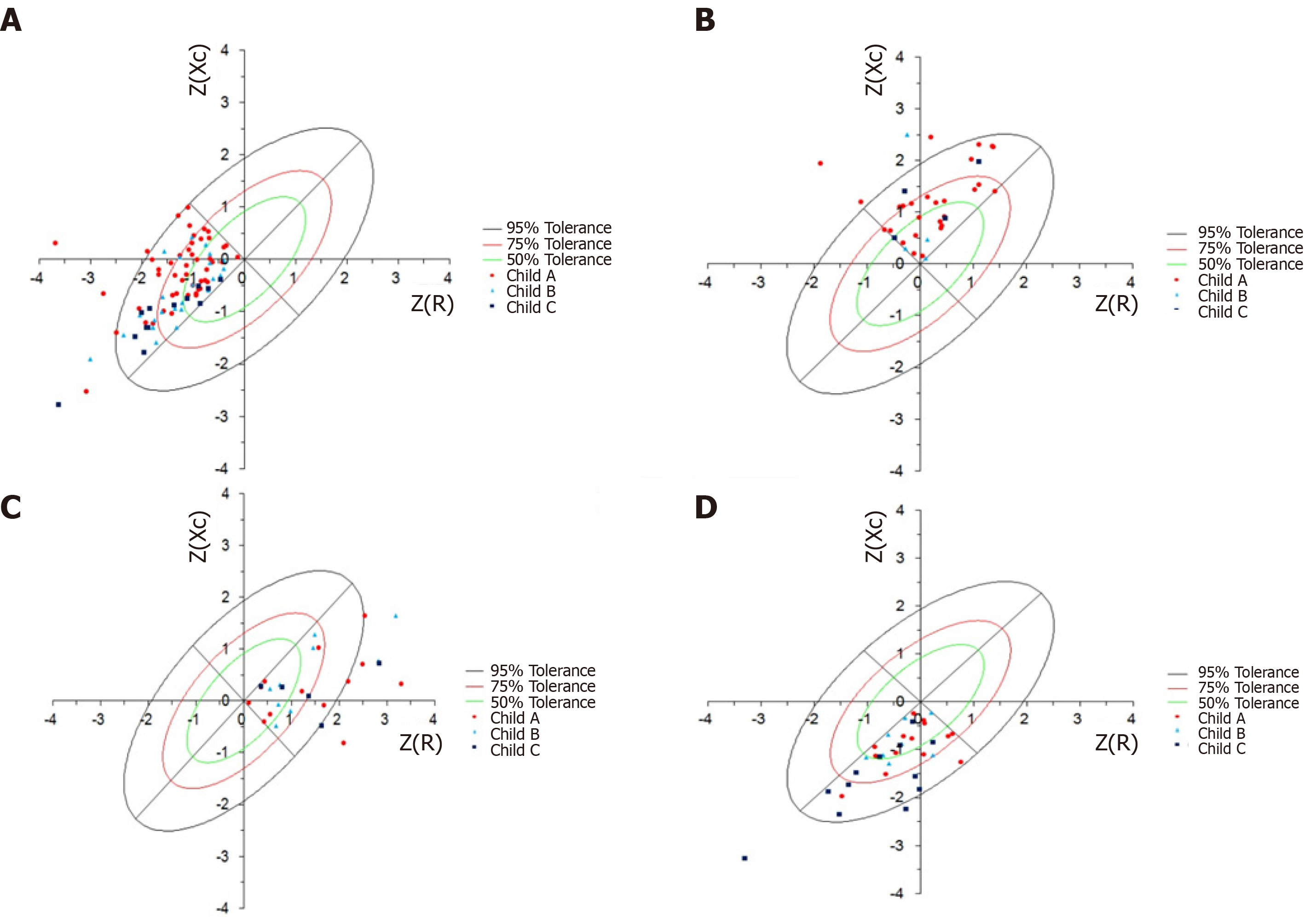
The association between staging and the CTP score according to the different BIVA quadrants is plotted on the BIVA graph (Figure 4).
Graphical representation of the BIVA shows the distribution of the patients evaluated according to staging of the disease. According to the CTP score, more severe disease was observed in Q4, using the prognosis by the PA values; the opposite was observed in Q2 (P < 0.002).
The sample showed an association between the BIVA quadrants and prognosis, using the PA values. There was a statistically significant association between the BIVA and PA classifications (P < 0.002), with the values of PA corresponding to a better prognosis in Q1 and Q2 (P < 0.007).
The population was evaluated according to age group in relation to PA, BIVA quadrants, gender, disease staging (CTP score) and etiology (Table 3). It was observed that PA was significantly lower in patients aged 50 years or older when compared to those younger than 40 years. Patients younger than 40 years also showed an association with BIVA Q2, and these same patients had a higher prevalence of other etiologies (Table 3).
| Variables1 | Age (yr) | ||||
| 20-39 (n = 11; 5.8%) | 40-49 (n = 32; 16.8%) | 50-59 (n = 66; 34.7%) | ≥ 60 (n = 81; 42.6%) | P value | |
| PA | 7.67 ± 2.504 | 6.68 ± 2.633, 4 | 5.79 ± 1.613 | 5.83 ± 2.293 | 0.014 |
| PA classification | 0.007 | ||||
| Good prognosis | 11 (100)5 | 24 (75.0) | 43 (65.2) | 43 (53.1) | |
| Bad prognosis | 0 (0.0) | 8 (25.0) | 23 (34.8) | 38 (46.9)5 | |
| BIVA Quadrant classification | 0.002 | ||||
| Q1 | 4 (36.4) | 15 (46.9) | 36 (54.5) | 36 (44.4) | |
| Q2 | 7 (63.6)5 | 8 (25.0) | 10 (15.2) | 11 (13.6) | |
| Q3 | 0 (0.0) | 6 (18.8) | 5 (7.6) | 16 (19.8) | |
| Q4 | 0 (0.0) | 3 (9.4) | 15 (22.7) | 18 (22.2) | |
| Gender | 0.162 | ||||
| Male | 9 (81.8) | 22 (68.8) | 42 (63.6) | 43 (53.1) | |
| Female | 2 (18.2) | 10 (31.3) | 24 (36.4) | 38 (46.9) | |
| Child-Turcotte-Pugh | 0.194 | ||||
| A | 9 (81.8) | 18 (56.3) | 31 (47.0) | 49 (60.5) | |
| B | 2 (18.2) | 8 (25.0) | 17 (25.8) | 21 (25.9) | |
| C | 0 (0.0) | 6 (18.8) | 18 (27.3) | 11 (13.6) | |
| Etiology | 0.030 | ||||
| HCV | 1 (9.1) | 14 (43.8) | 34 (51.5) | 41 (50.6) | |
| Alcohol | 3 (27.3) | 9 (28.1) | 16 (24.2) | 23 (28.4) | |
| HCV + alcohol | 1 (9.1) | 4 (12.5) | 6 (9.1) | 10 (12.3) | |
| Other2 | 6 (54.5)5 | 5 (15.6) | 10 (15.2) | 7 (8.6) | |
When adjusted for age, patients with the etiology related to HCV + alcohol had a significantly higher prevalence of being classified as CTP C [hazard rate (HR) = 2.28, 95%CI: 1.12-4.67, P = 0.024] when compared to patients with HCV only. Also, patients with alcohol-related etiology had a 31% higher prevalence of CTP A when compared to those with HCV (HR = 1.31, 95%CI: 1.01-1.71, P = 0.044). When adjusted for age, CTP C patients had a 17% higher prevalence of being in Q4 by BIVA (HR = 1.17, 95%CI: 1.04-1.33, P = 0.012) when compared to Child A patients. The prevalence of bad prognosis by PA was approximately 5 times higher in patients classified in quadrants 3 (HR = 4.47, 95%CI: 2.70-7.40, P < 0.001) and 4 (HR = 5.64, 95%CI: 3.54-8.97, P < 0.001) when compared to patients in quadrants 1 and 2. When adjusted for age and CTP, the effect measures did not change in quadrants 3 (HR = 4.18, 95%CI: 2.51-6.97, P < 0.001) and 4 (HR = 5.01, 95%CI: 3.10-8.10, P < 0.001).
There was a statistically significant association between the classification of PA and CTP score (P < 0.001). Patients with CTP A were associated with a good prognosis and were classified in quadrants 1 and 2, and patients with CTP C had a bad prognosis and were classified in quadrants 3 and 4 (Table 4).
| Variables1 | Classification PA – 5.4° | P value | |
| Good prognosis (> 5.4°; n = 121) | Bad prognosis (< 5.4°; n = 69) | ||
| Age (yr) | 54.2 ± 11.3 | 60.9 ± 9.0 | < 0.001 |
| Gender | 0.040 | ||
| Male | 81 (66.9) | 35 (50.7) | |
| Female | 40 (33.1) | 34 (49.3) | |
| Child-Turcotte-Pugh | < 0.001 | ||
| A | 81 (66.9)3 | 26 (37.7) | |
| B | 26 (21.5) | 22 (31.9) | |
| C | 14 (11.6) | 21 (30.4)3 | |
| Etiology | 0.060 | ||
| HCV | 51 (42.1) | 39 (56.5) | |
| Alcohol | 39 (32.2) | 12 (17.4) | |
| HCV + alcohol | 11 (9.1) | 10 (14.5) | |
| Other2 | 20 (16.5) | 8 (11.6) | |
The evaluation of body composition in cirrhotic patients presents some difficulties in measurement due to its peculiarities, and relevant studies suggest that there is no gold standard for diagnosing clinical conditions, such as malnutrition, in these patients.
In the present study of adult cirrhotic patients, a higher proportion of males was observed, which was in accordance with previous studies[23]. With regard to the classification of CTP, there is a stepwise progression of CTP A, B and C, and because these were outpatients, there was a greater number of CTP A and B than CTP C patients. The etiology of cirrhosis in this study was predominantly due to HCV, alcohol, and HCV associated with alcohol, and was a regional peculiarity, and may differ from other geographical locations[6].
According to the BIVA, it was possible to differentiate patients according to the disease stage. Younger patients in the sample (50.3 ± 14.3 years) were less hydrated and had more cellularity (Q2), according to the BIVA, and with a greater number of patients classified as CTP A presenting higher values of PA, reflecting a better prognosis. On the other hand, patients classified as more hydrated (water retention) and with lower cellularity (Q4) were older (61.6 ± 8.9 years), mostly with CTP C and with lower PA, and a possible association between greater severity and bad prognosis.
The BIVA has been studied in several clinical situations in an attempt to understand the human body composition in relation to hydration and cellularity alterations, such as heart failure, compensated cirrhosis, hemodialysis, chronic obstructive pulmonary disease and cancer[12,17,24-26]. Norman et al[9] established that the BIVA method reflects the actual state of hydration and composition of the cell mass, recognizing its importance in the evaluation and monitoring of possible modifications of body composition. This method has become an important tool in the management of cirrhotic patients.
The use of the BIVA method to determine the status of body fluids is well established in the literature and has been gaining prominence in the management of several diseases[26]. A classic example where the BIVA method can be used systematically is in the assessment of body fluids of patients the in intensive care unit (ICU), mainly with the diagnosis of acute kidney injury (AKI), which is associated with increased mortality due to the disturbance in water balance[27]. In the study by Hise et al[28] which evaluated critically ill patients with AKI using the BIVA method, it was observed that the survivors presented vectors of longer and steeper groups, characterized by higher values of R and Xc (P < 0.05).
When the patients were plotted according to the clinical classification of CTP, it was possible to conclude that CTP A patients had higher cellularity and lower hydration, with PA values indicating a good prognosis. It was also possible to identify that patients with CTP C were more prevalent in Q4 and had a bad prognostic value assessed by PA (P < 0.001). These data corroborate with the findings of Fernandes et al[6], in which the PA was associated with staging of the disease via the CTP score.
Guglielmi et al[29] evaluated 810 cirrhotic patients with different etiologies using the BIVA method and compared them with a control group of 208 healthy individuals, and showed differences in hydration between the two groups. Similar findings were observed in the present study, where higher hydration status was associated with patients who had decompensated cirrhosis. On the other hand, the above controlled study did not evaluate the participants’ cellularity.
The mean PA in this sample was 6.06 ± 2.20, similar to the findings of Fernandes et al[6] and Selberg et al[22], where values below 5.4° were characterized as having a poor prognosis. When we analyzed the patients divided into two groups according to the PA cut-off point of 5.4°, differences were observed between the two groups, with younger patients, males and those with CTP A having a good prognosis, which may be indicative of the absence of sarcopenia in this population. Sarcopenia is characterized by progressive loss of skeletal muscle mass and strength, negatively influencing body homeostasis associated with functional limitations and morbidity and mortality[30,31].
Studies have shown that PA is a good prognostic indicator in severe clinical situations[6,10-14]. Gupta et al[10] demonstrated that PA was a more potent indicator of survival than traditional nutritional assessment parameters, such as albumin, prealbumin and transferrin in patients with advanced pancreatic cancer, and showed that the cut-off point for PA was 5.0°. In a similar study of patients with advanced lung cancer, the patients were stratified using a cut-off point for PA of 4.5°[11]. Alves et al[12], in a study of chronic heart failure, identified that the BIVA method associated with PA was capable of identifying significant changes in the hydration state during the acute decompensation phase of the disease.
Stapel et al[32] when assessing 196 patients in the ICU showed that patients with higher PA had a lower 90-day mortality rate than those patients with a low PA (5.0° ± 1.3° vs 4.1° ± 1.2°, P < 0.001). It is important to highlight that BIA was performed within 24 h of the patient’s admission to the ICU, clearly showing that PA reflects the patient’s physiological status (catabolism) and can be classified as a biological marker, as described by Marroni et al[15].
Ruiz-Margáin et al[17], in a pilot study of patients with compensated cirrhosis, the cut-off point for PA of 4.9° was established, indicating this bad prognostic factor is an independent risk factor of mortality. Belarmino et al[33] obtained similar findings in a study of cirrhotic patients using the PA cut-off of ≤ 4.9° established by Ruiz-Margáin et al[17], and observed that PA is an independent prognostic factor associated with mortality, and identified associations with poorer metabolic profiles, nutrition and disease progression. However, these two studies did not evaluate cellularity and body fluid in cirrhotic patients; thus, clinical and nutritional behaviors were not assessed early.
There are some limitations in the present study, such as the use of an Italian population as a reference, and the Piccoli Software[19] to calculate the BIVA, as there are no data available for the Brazilian population. The population in the study region suffered great miscegenation, having a high Italian genetic component and therefore we believe that it does not significantly compromise the results.
BIVA offers advantages over traditional methods in evaluating body composition, due to its non-invasive nature and simplicity. BIVA has a methodological advantage over traditional BIA calculations due to its independence from regression equations. In addition, BIVA can facilitate longitudinal assessment of changes in body composition over time. These properties are useful for assessing nutrition and hydration in cirrhotic patients, who are unable to tolerate more invasive assessment methods. This research demonstrates the potential of using published BIVA data for further analysis, especially in decompensated cirrhotic patients.
The evaluation at different points in the disease trajectory can demonstrate changes in body composition over time. Our data demonstrate that body composition appears to be related to the clinical status of cirrhotic patients.
The main limitation of this study is that nutritional screening tools were not used, which makes it difficult to compare the nutritional basis. Therefore, our ability to assess how BIVA relates to nutritional status is limited.
A small number of studies were evaluated in this analysis, which only included English language studies, and it is possible that studies using BIVA in different cultural contexts have been excluded. There are challenges in using the BIVA method correctly when there is variability in how reference populations are chosen. The BIVA method does not provide quantitative data on body composition variables; therefore, stratification is required, according to clinical variables of BIVA data to determine clinically significant outcomes.
As already mentioned, evaluations of BIA were performed in clinical medical consultations, and not performed with the recommended preparation for the use of BIA. However, as the results of this study do not depend on pre-established formulas of the apparatus, where hydration is a limiting factor, we believe that this did not influence the results.
This study demonstrated the potential of using the BIVA method to perform comparative, multigroup analyses of body composition, to compare differences in cirrhotic patients according to the stage and type of disease. This has the potential to personalize therapeutic, nutritional and hydration interventions according to an individual’s physiology.
More studies are needed to recommend the BIVA method for routine clinical use, due to the limited number of studies using this method.
In conclusion, the BIVA method allows identification of the cellularity and hydration status of cirrhotic patients associated with clinical factors to determine the severity of the disease, such as age, staging and PA. The BIVA method is a new tool for evaluating the body composition of cirrhotic patients, especially in patients with asymmetry, allowing an early and specific nutritional assessment in each case, which will help to improve the clinical condition of these patients.
One of the main clinical complications of liver cirrhosis is protein-calorie malnutrition, the prevalence of which can vary from 10% to 100%, regardless of the stage and etiology of the disease, but which negatively interferes with the general prognosis of the disease. Therefore, determining the behavior of body composition (cellularity and hydration) using the bioelectrical impedance vector analysis (BIVA) method, seems to be a promising method for improving the health of patients with liver cirhosis, expanding their life expectancy and quality of life.
There are few studies on the assessment of body composition and functioning in cirrhotic patients, which directly impacts the overall clinical management of these patients. We believe that with the BIVA method we can gain a new tool for analyzing body homeostasis in this population.
The aim of this study was to evaluate the results of the BIVA regarding hydration and cellularity, and compare them with the phase angle and other clinical parameters in cirrhotic patients.
This was a retrospective cross-sectional study with data collected between May 2007 and December 2015, at the Santa Casa de Misericórdia Hospital Complex in Porto Alegre, RS, Brazil. The data obtained were related to the protocol for routine pre- and postoperative care at the service’s outpatient clinic. Quantitative variables were described by the mean and standard deviation and the categorical variables by absolute and relative frequencies. One-way ANOVA was used to compare the means, complemented by Tukey’s test. In the comparison of proportions, the Chi-square test was applied along with the analysis of adjusted residuals. For control of confounding factors, the Poisson regression analysis was applied to the factors that presented a P < 0.10 in the bivariate analysis.
A total of 190 patients with cirrhosis undergoing outpatient follow-up were included for data collection. The BIVA method showed an association with the staging of cirrhosis, showing worsening of cellularity (integrity and functionality) and worsening of the hydroelectrolytic distribution in patients with greater disease severity.
The BIVA method makes it possible to identify the cellularity and hydration status of cirrhotic patients, being associated with clinical factors that determine the severity of the disease, such as age, staging and PA.
The BIVA method is a new tool for evaluating body composition in cirrhotic patients, especially in those with asymmetry, allowing an early and specific nutritional assessment in each case, and helps to improve the clinical condition of these patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Skrypnik D S-Editor: Chen XF L-Editor: Webster JR P-Editor: Xing YX
| 1. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1199] [Cited by in F6Publishing: 1220] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25:281-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Muir AJ. Understanding the Complexities of Cirrhosis. Clin Ther. 2015;37:1822-1836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17:761-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Castellanos Fernández M, Santana Porbén S, García Jordá E, Rodríguez de Miranda A, Barreto Penié J, López Díaz Y, Martínez González C. [Influence of hyponutrition on occurrence of complications and mortality among cirrhosis patients]. Nutr Hosp. 2008;23:68-74. [PubMed] [Cited in This Article: ] |
| 6. | Fernandes SA, Bassani L, Nunes FF, Aydos ME, Alves AV, Marroni CA. Nutritional assessment in patients with cirrhosis. Arq Gastroenterol. 2012;49:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Fernandes SA, Gonzalez MC, Bassani L, Miranda D, Pivatto B, Harter DL, Marroni CA. Is the Phase Angle, a Prognostic Indicator for Nutritional Status in Cirrhotic Patients? J Antivir Antiretrovir. 2013;S3:1-4. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Avesani CM, Draibe SA, Kamimura MA, Cendoroglo M, Pedrosa A, Castro ML, Cuppari L. Assessment of body composition by dual energy X-ray absorptiometry, skinfold thickness and creatinine kinetics in chronic kidney disease patients. Nephrol Dial Transplant. 2004;19:2289-2295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31:854-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 432] [Cited by in F6Publishing: 551] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 10. | Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, Lammersfeld CA. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr. 2004;92:957-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Toso S, Piccoli A, Gusella M, Menon D, Bononi A, Crepaldi G, Ferrazzi E. Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition. 2000;16:120-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Alves FD, Souza GC, Aliti GB, Rabelo-Silva ER, Clausell N, Biolo A. Dynamic changes in bioelectrical impedance vector analysis and phase angle in acute decompensated heart failure. Nutrition. 2015;31:84-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Pupim LB, Kent P, Ikizler TA. Bioelectrical impedance analysis in dialysis patients. Miner Electrolyte Metab. 1999;25:400-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Ott M, Fischer H, Polat H, Helm EB, Frenz M, Caspary WF, Lembcke B. Bioelectrical impedance analysis as a predictor of survival in patients with human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:20-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Marroni CA, Miranda D, Boemeke L, Fernandes SA. Phase Angle Bioelectrical Impedance Analysis (BIA) as a Biomarker Tool for Liver Disease. In: Patel VB, Preedy VR. Biomarkers in Liver Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Dordrecht: Springer, 2017: 735-751. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 16. | Máttar JA. Application of total body bioimpedance to the critically ill patient. Brazilian Group for Bioimpedance Study. New Horiz. 1996;4:493-503. [PubMed] [Cited in This Article: ] |
| 17. | Ruiz-Margáin A, Macías-Rodríguez RU, Duarte-Rojo A, Ríos-Torres SL, Espinosa-Cuevas Á, Torre A. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: a prospective cohort study. Dig Liver Dis. 2015;47:309-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Piccoli A. Bioelectric impedance measurement for fluid status assessment. Contrib Nephrol. 2010;164:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Piccoli A, Pastori G. BIVA Software 2002. 2002. Available from: http://www.renalgate.it/formule_calcolatori/BIVAguide.pdf. [Cited in This Article: ] |
| 20. | Piccoli A, Rossi B, Pillon L, Bucciante G. A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney Int. 1994;46:534-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 438] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Piccoli A, Pillon L, Dumler F. Impedance vector distribution by sex, race, body mass index, and age in the United States: standard reference intervals as bivariate Z scores. Nutrition. 2002;18:153-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86:509-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 23. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2042] [Cited by in F6Publishing: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 24. | Nescolarde L, Piccoli A, Román A, Núñez A, Morales R, Tamayo J, Doñate T, Rosell J. Bioelectrical impedance vector analysis in haemodialysis patients: relation between oedema and mortality. Physiol Meas. 2004;25:1271-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Walter-Kroker A, Kroker A, Mattiucci-Guehlke M, Glaab T. A practical guide to bioelectrical impedance analysis using the example of chronic obstructive pulmonary disease. Nutr J. 2011;10:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Lukaski HC, Vega Diaz N, Talluri A, Nescolarde L. Classification of Hydration in Clinical Conditions: Indirect and Direct Approaches Using Bioimpedance. Nutrients. 2019;11:809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL; Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 702] [Cited by in F6Publishing: 668] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 28. | Hise ACDR, Gonzalez MC. Assessment of hydration status using bioelectrical impedance vector analysis in critical patients with acute kidney injury. Clin Nutr. 2018;37:695-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Guglielmi FW, Mastronuzzi T, Pietrini L, Panarese A, Panella C, Francavilla A. The RXc graph in evaluating and monitoring fluid balance in patients with liver cirrhosis. Ann N Y Acad Sci. 1999;873:105-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Kim JH, Lim S, Choi SH, Kim KM, Yoon JW, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J Gerontol A Biol Sci Med Sci. 2014;69:1244-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 306] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 32. | Stapel SN, Looijaard WGPM, Dekker IM, Girbes ARJ, Weijs PJM, Oudemans-van Straaten HM. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur J Clin Nutr. 2018;72:1019-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 33. | Belarmino G, Gonzalez MC, Torrinhas RS, Sala P, Andraus W, D'Albuquerque LA, Pereira RM, Caparbo VF, Ravacci GR, Damiani L, Heymsfield SB, Waitzberg DL. Phase angle obtained by bioelectrical impedance analysis independently predicts mortality in patients with cirrhosis. World J Hepatol. 2017;9:401-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |