Published online Apr 27, 2018. doi: 10.4254/wjh.v10.i4.402
Peer-review started: January 20, 2018
First decision: February 28, 2018
Revised: March 10, 2018
Accepted: April 2, 2018
Article in press: April 3, 2018
Published online: April 27, 2018
Metastatic hepatic leiomyosarcoma is a rare malignant smooth muscle tumor. We report a case of metastatic hepatic leiomyosarcoma associated with smooth muscle tumor of uncertain malignant potential (STUMP). A 68-year-old female presented with a liver mass (60 mm × 40 mm, Segment 4). She underwent left salpingo-oophorectomy for an ovary tumor with STUMP in a broad ligament 6 years ago. Though FDG-PET showed obvious metabolically active foci, abnormal metabolically active foci other than the lesion were not detected. A malignant liver tumor was strongly suspected and laparoscopic partial liver resection was performed with vessel-sealing devices using the crush clamping method and Pringle maneuver. Immunohistochemical findings revealed metastatic liver leiomyosarcoma associated with STUMP in a broad ligament. This case is an extremely rare case of malignant transformation from primary STUMP to metastatic hepatic leiomyosarcoma. It provides important evidence regarding the treatment for metastatic hepatic leiomyosarcoma associated with STUMP.
Core tip: Metastatic hepatic leiomyosarcoma is a rare malignant smooth muscle tumor. This case report presents a case of smooth muscle tumor of uncertain malignant potential (STUMP) occurring in a broad ligament that developed into metastatic hepatic leiomyosarcoma over a period of 6 years, and an ultrasound-guided pure laparoscopic partial liver resection with vessel-sealing devices using the crush clamping method and Pringle maneuver was performed safely. This case is an extremely rare case of malignant transformation from primary STUMP to metastatic hepatic leiomyosarcoma. We share this case to provide important evidence regarding the treatment for metastatic hepatic leiomyosarcoma associated with STUMP.
- Citation: Fukui K, Takase N, Miyake T, Hisano K, Maeda E, Nishimura T, Abe K, Kozuki A, Tanaka T, Harada N, Takamatsu M, Kaneda K. Review of the literature laparoscopic surgery for metastatic hepatic leiomyosarcoma associated with smooth muscle tumor of uncertain malignant potential: Case report. World J Hepatol 2018; 10(4): 402-408
- URL: https://www.wjgnet.com/1948-5182/full/v10/i4/402.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i4.402
Metastatic hepatic malignancies are encountered more frequently (approximately 18-40 times) than primary liver cancers[1]. Among such malignancies, metastatic hepatic leiomyosarcoma is a rare malignant smooth muscle tumor (SMT). The hepatic metastasis associated with primary female genital system arises not only in leiomyosarcoma but also in other SMTs including leiomyoma and smooth uterine muscle of uncertain malignant potential (STUMP)[2,3]. However, STUMP usually does not metastasize because it is a mesenchymal uterine tumor lying between benign leiomyomas and leiomyosarcomas[4]. We herein report a case of metastatic hepatic leiomyosarcoma associated with STUMP in a broad ligament. A pure laparoscopic partial liver resection was performed successfully.
A 68-year-old female who had been admitted to another facility presented with a liver mass, which was palpable from the body surface. She had non-alcoholic steatohepatitis (NASH) and cholelithiasis in her past medical history. She underwent hysterectomy for uterine leiomyoma 21 years earlier and left salpingo-oophorectomy for an ovary tumor with STUMP in a broad ligament 6 years earlier.
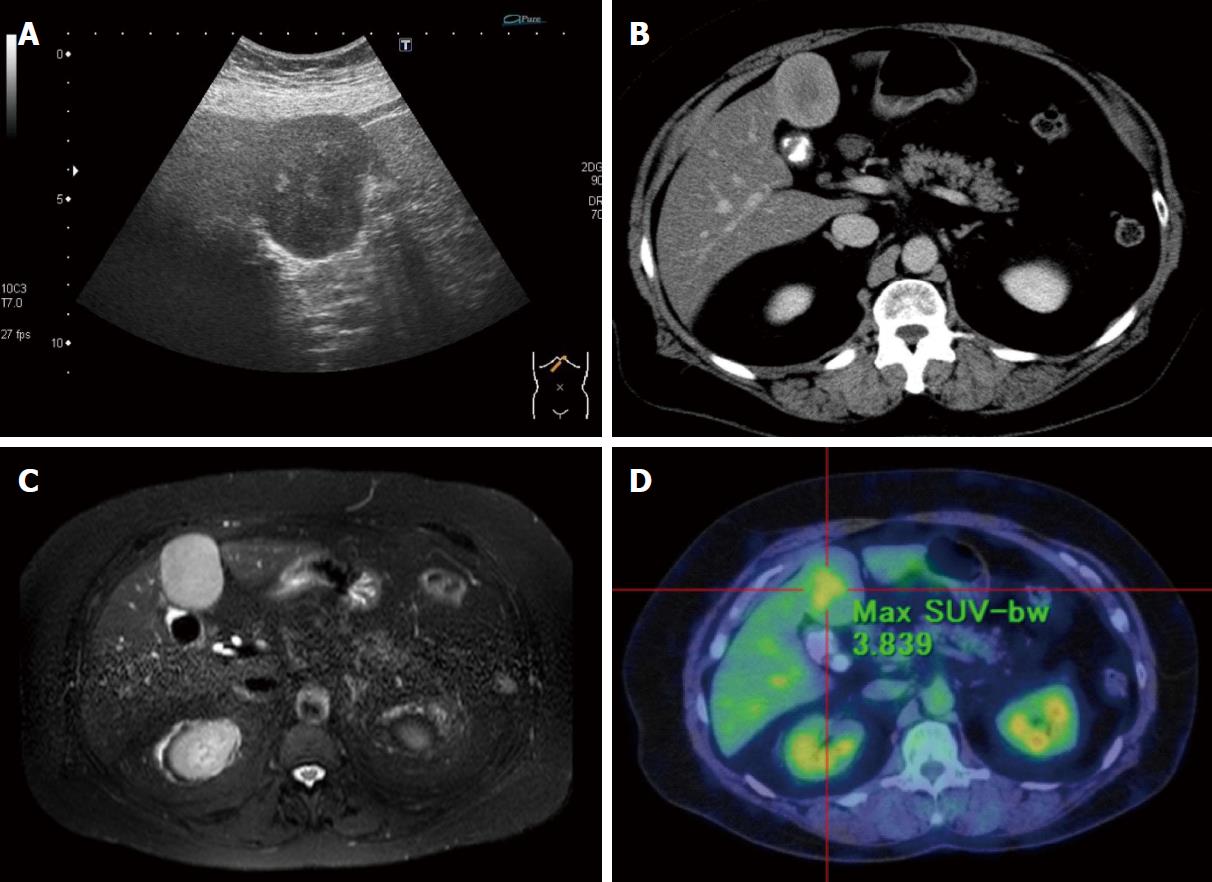
Her laboratory tests showed a mild dysfunction of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) which would be consistent with suspected NASH. She had no hepatitis virus infection, and tumor markers including alpha-fetoprotein and protein induced by vitamin K absence-2 (PIVKA-2) were negative. Ultrasonography of the liver showed a hypoechoic smooth mass (60 mm × 40 mm) with a heterogeneous hyperechoic region in the median section of the left lobe of the liver (Segment 4) (Figure 1A). Contrast-enhanced computed tomography (CE-CT) and ethoxybenzyl-magnetic resonance imaging (EOB-MRI) showed a heterogeneous mass enhancement (Figure 1B and C). Though fluorodeoxyglucose-positron emission tomography (FDG-PET) also showed obvious metabolically active foci (Figure 1D), no other abnormal metabolically active foci were detected in addition to the lesion.
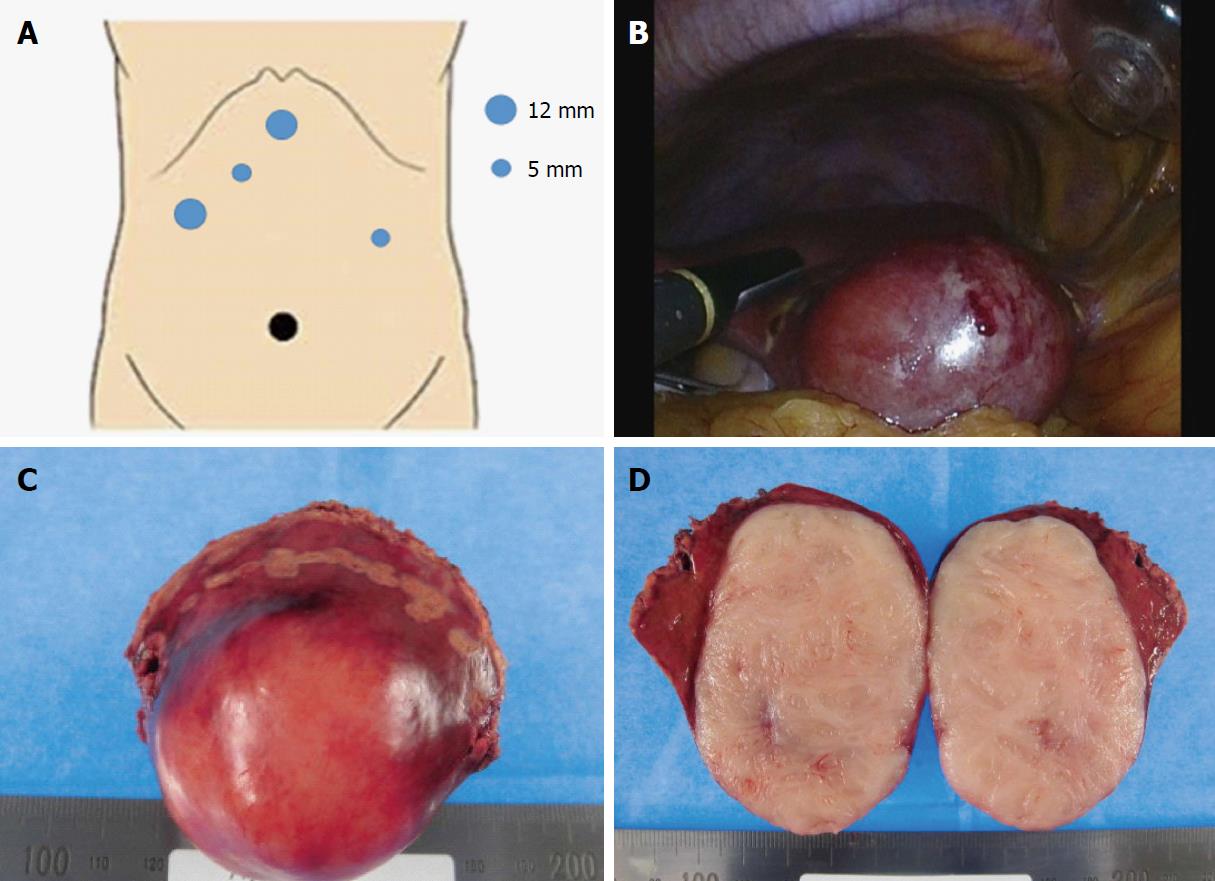
Though the clinical findings did not lead to a preoperative diagnosis because of the non-specific characteristics, malignant liver tumor was strongly suspected and a pure laparoscopic partial liver resection was performed. Briefly, 5 ports were placed with the patient in the supine position. Three ports were placed in the epigastric region. The other 2 ports were placed in the umbilicus and left lateral abdominal region (Figure 2A). As for the liver resection, we performed ultrasound-guided pure laparoscopic partial liver resection with vessel-sealing devices using the crush clamping method and Pringle maneuver (Figure 2B). The resected partial liver was retrieved from the umbilical port with auxiliary incision. The surgical specimen showed a white colored solid mass with smooth surface (Figure 2C and D).
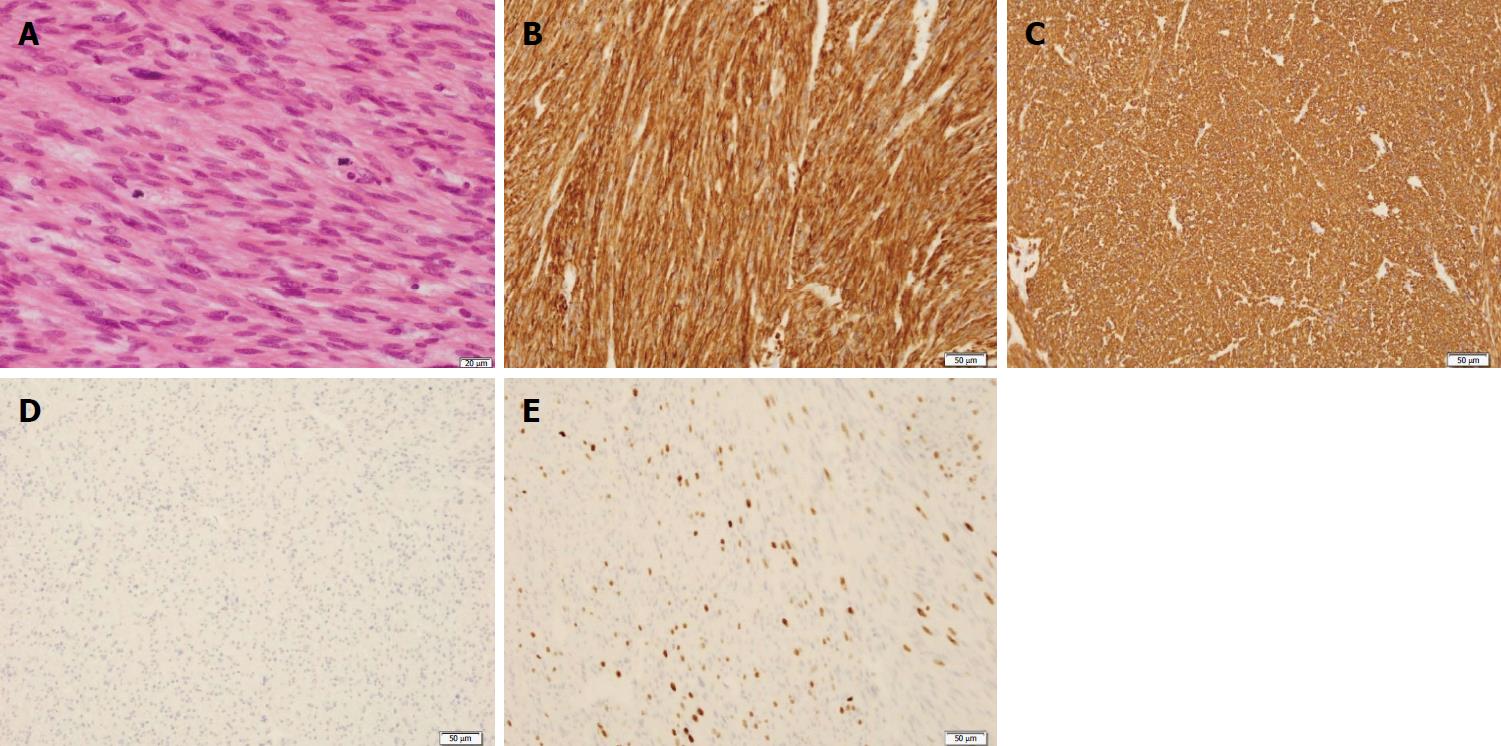
Hematoxylin and eosin (H-E) stain showed the proliferation of spindle-shaped cells with enlarged nuclei and eosinophilic cytoplasm, and the cells exhibited diffuse moderate-to-severe atypia and multiple mitoses. The mitotic count activity showed the presence of more than 10 mitotic figures (MFs)/10 high power fields (HPFs) (Figure 3A). Immunohistochemical findings were strongly positive for smooth muscle actin (SMA) and desmin, and negative for c-kit (Figure 3B-D). In addition, the expressions of cluster of differentiation 34 (CD34) and s-100 were negative (data not shown). Moreover, more than 10% ki-67 positive cells were seen in the lesion (Figure 3E).
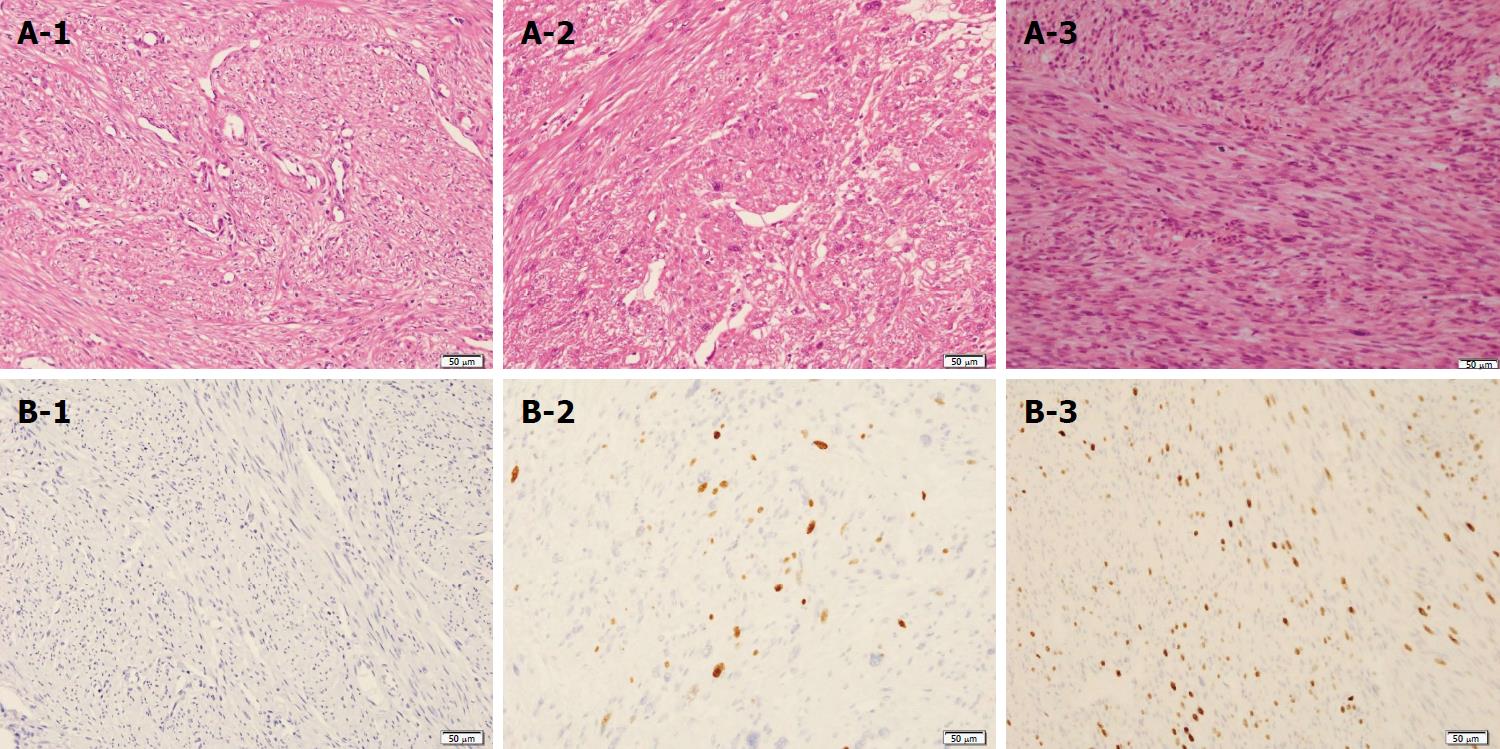
Next, we histologically reconfirmed the past resected specimens including uterus and broad ligament specimens to explore the primary lesion. In the uterus, MFs were absent, and the ki-67 proliferation-index (MIB-1) was zero (Figure 4A-1 and 4B-1). The pathological findings of MFs and the MIB-1 index showed malignant transformation from STUMP to leiomyosarcoma (Figure 4A-2 and A-3) (Figure 4B-2 and B-3). The final diagnosis was STUMP in a broad ligament that developed into metastatic hepatic leiomyosarcoma over a period of 6 years.
She was discharged 8 d after the operation without any complications, and she was not received adjuvant systemic treatment. However, follow-up CE-CT and EOB-MRI studies showed recurrent metastatic hepatic leiomyosarcoma in the edge of Segment 5 (12 mm × 9 mm) 7 mo after the initial surgery. We performed pure laparoscopic partial resection with these procedures safely in both primary and secondary resections because of the minimum postoperative abdominal adhesions. No evident disease recurrence has been seen in the 4 mo since the secondary surgery.
SMTs are broadly categorized into 6 major histological types: leiomyoma, mitotically active leiomyoma, cellular leiomyoma, atypical leiomyoma, STUMP and leiomyosarcoma. SMTs are diagnosed based on the assessment of three histopathological characteristics: Mitotic count activity (MFs/10 HPFs), the presence or absence of coagulative tumor cell necrosis, and the degree of cytological atypia, and the histopathological features determine the difference between benign leiomyomas and malignant leiomyosarcomas[5]. In addition, Mayerhofer et al[6] indicated that the expression of ki-67 in SMTs may be a useful immunohistochemical parameter for distinguishing between malignant histology and borderline histology. However, it is difficult to categorize them clearly because there are various opinions regarding the pathological diagnostic criteria of SMTs.
There is general acceptance of the notion that STUMP has characteristics lying between benign leiomyomas and leiomyosarcomas[5,7]. In fact, the WHO classification also indicates that STUMP is difficult to unequivocally diagnose as benign or malignant[2]. The criteria used for the diagnosis of STUMP were the presence of coagulative necrosis, low mitotic count (less than or equal to 10 per 10 HPFs) and the absence of atypia, or a mitotic count greater than 10 per 10 HPFs, focal mild to moderate atypia in the absence of coagulative necrosis, or a mitotic count equal to or less than 10 per 10 HPFs, and focal moderate-to-severe atypia in the absence of coagulative necrosis[8]. It is known that STUMP occurs in broad ligaments as well as the uterus. STUMP in broad ligaments is very rare, with only a few cases reported in the medical literature[8]. A previous study reported based on molecular analysis that most atypical leiomyoma including STUMP and leiomyosarcoma had a similar origin and may represent different stages of tumorigenesis[9]. Leiomyosarcoma is considered to arise de novo or it may develop from preexisting atypical leiomyoma, while the mechanism of malignant transformation has not been determined conclusively. Zhang et al[9] demonstrated that p53 mutation and PTEN deletions were significantly higher in leiomyosarcoma, atypical leiomyoma and STUMP compared with other uterine SMTs.
The common sites of metastatic hepatic leiomyosarcoma are the stomach (31%), retroperitoneum (19%), small bowel (15%) and vena cava (4%)[10]. Also, few hepatic leiomyosarcomas are known to have occurred in the female genital system. Among the soft tissue sarcomas, the most common site of leiomyosarcoma was the retroperitoneum[11]. However, these are generally distant metastases of leiomyosarcoma rather than distant metastases of other SMTs without leiomyosarcoma. Few studies have reported that the lung and liver are two common sites of metastases of STUMP[2,8,12,13]. We searched all common literature search engines (PubMed, Medline, Google Scholar and Embase). To our knowledge, only 2 cases involving malignant transformation of metastatic hepatic leiomyosarcoma associated with STUMP have been reported[12].
It is very difficult to make a definitive diagnosis of leiomyosarcoma preoperatively. Also, primary or metastatic hepatic leiomyosarcoma falls into the preoperative diagnostic dilemma because of the non-specific diagnostic imaging and poor clinical presentation[14]. In addition, although several studies have shown the outcome of metastatic hepatic leiomyosarcoma, definitive evidence is lacking. Because metastases from leiomyosarcoma are usually not sensitive to chemotherapy or chemoembolization, the outcome is often poor, with only short survival. Without treatment, the median survival of patients with liver metastases is no more than 14 mo[10]. Currently, the best proven approach to metastatic hepatic leiomyosarcoma is a radical cure excision involving the complete removal of all tumors. Lang et al[10] reported that the 5-year survival rate was 13% for all patients and 20% after R0 resection for metastatic hepatic leiomyosarcoma. Recent studies have indicated that liver resection is superior to chemoradiotherapy as a treatment for metastatic hepatic leiomyosarcoma[15-17]. However, standard treatments for metastatic hepatic leiomyosarcoma have not been established. Two agents have been considered to be active in soft tissue sarcomas in general, doxorubicin and ifosfamide[18,19]. In recent years, trabectedin, a marine-derived drug, exhibited activity in patients with metastatic soft tissue sarcoma after the failure of conventional chemotherapy[20]. Radiotherapy has been shown to improve local control and local recurrence but without any improvement in overall survival[21]. A molecular targeted study also demonstrated that the expression of LMP2 significantly blocked the tumorigenesis of leiomyosarcoma in vitro[22].
Laparoscopic hepatic surgery produces relatively less peritoneal trauma and blood loss than conventional laparotomy and may result in decreased perioperative complications[23,24]. Therefore, it has recently been considered a better operative approach and has increasingly been performed worldwide. Moreover, ultrasound-guided laparoscopic liver resection with vessel-sealing devices using the crush clamping method and Pringle maneuver is a minimally invasive, safe and effective procedure for patients with primary and metastatic hepatic tumor[24-26]. A recent study reported that laparoscopic intraoperative ultrasound examination had a higher detection rate regarding liver metastases, when compared to CE-CT and MRI[26]. Therefore, it enables a more accurate R0 resection. The Pringle maneuver is the simplest method of vascular occlusion by clamping of the hepatoduodenal ligament to occlude total inflow to the liver. This method ensures safety and prevent major accidental blood loss during laparoscopic liver resection[25]. The crush clamping method for hepatic parenchymal transection is well-known. Moreover, a combination technique with vessel-sealing devices in laparoscopic liver resection have been applied. This technique shows lower blood loss, shorter transection time, and reduces rates of post-hepatectomy complications[24]. We also performed laparoscopic partial resection with these procedures safely in both primary and secondary resections. However, excessive intraoperative blood loss can occur during laparoscopic liver resection because active bleeding is difficult to control, as the conversion to laparotomy takes time[25]. In addition, laparoscopic partial resections of segment 7 and segment 8 on the dorsal liver head side are considered relatively difficult because of the anatomical features[27]. Therefore, it is necessary to carefully identify the surgical indications for laparoscopic liver resection.
In conclusion, we documented a STUMP in a broad ligament that developed into metastatic hepatic leiomyosarcoma over a period of 6 years. An ultrasound-guided pure laparoscopic partial liver resection was safely performed. There are few case reports and case series regarding metastatic liver leiomyosarcoma, however, this is an extremely rare case that described malignant transformation from primary STUMP to metastatic hepatic leiomyosarcoma. This case provides important evidence regarding the treatment for metastatic hepatic leiomyosarcoma associated with STUMP.
Metastatic hepatic leiomyosarcoma associated with STUMP in a broad ligament was treated by laparoscopic partial liver resection.
Preoperative imaging tests and past medical history strongly suggested that the lesion was an atypical malignant tumor.
Hepatocellular carcinoma, Hepatic hemangioma, Metastatic hepatic tumor.
Mild dysfunction of aspartate aminotransferase and alanine aminotransferase consistent with suspected NASH.
CE-CT and EOB-MRI showed a heterogeneous mass enhancement, and FDG-PET strongly suggested malignant liver tumor.
We diagnosed the condition as metastatic hepatic leiomyosarcoma.
The patients were treated with ultrasound-guided pure laparoscopic partial liver resection with vessel-sealing devices using the crush clamping method and Pringle maneuver.
We share this case to provide important knowledge regarding the appropriate treatment method for metastatic hepatic leiomyosarcoma.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Coelho FF, Mentes O, Wu SD S- Editor: Cui LJ L- Editor: A E- Editor: Wang CH
| 1. | Namasivayam S, Martin DR, Saini S. Imaging of liver metastases: MRI. Cancer Imaging. 2007;7:2-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Dall’Asta A, Gizzo S, Musarò A, Quaranta M, Noventa M, Migliavacca C, Sozzi G, Monica M, Mautone D, Berretta R. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): pathology, follow-up and recurrence. Int J Clin Exp Pathol. 2014;7:8136-8142. [PubMed] [Cited in This Article: ] |
| 3. | Nucci MR, Drapkin R, Dal Cin P, Fletcher CD, Fletcher JA. Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenetic implications. Am J Surg Pathol. 2007;31:737-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kotsopoulos IC, Barbetakis N, Asteriou C, Voutsas MG. Uterine smooth muscle tumor of uncertain malignant potential: A rare cause of multiple pulmonary nodules. Indian J Med Paediatr Oncol. 2012;33:176-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Kalogiannidis I, Stavrakis T, Dagklis T, Petousis S, Nikolaidou C, Venizelos I, Rousso D. A clinicopathological study of atypical leiomyomas: Benign variant leiomyoma or smooth-muscle tumor of uncertain malignant potential. Oncol Lett. 2016;11:1425-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Mayerhofer K, Lozanov P, Bodner K, Bodner-Adler B, Kimberger O, Czerwenka K. Ki-67 expression in patients with uterine leiomyomas, uterine smooth muscle tumors of uncertain malignant potential (STUMP) and uterine leiomyosarcomas (LMS). Acta Obstet Gynecol Scand. 2004;83:1085-1088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Ng JS, Han A, Chew SH, Low J. A clinicopathologic study of uterine smooth muscle tumours of uncertain malignant potential (STUMP). Ann Acad Med Singapore. 2010;39:625-628. [PubMed] [Cited in This Article: ] |
| 8. | Wahal SP, Mardi K, Sharma S. “Stump” of broad ligament: A rare entity with review of literature. South Asian J Cancer. 2013;2:118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Zhang Q, Ubago J, Li L, Guo H, Liu Y, Qiang W, Kim JJ, Kong B, Wei JJ. Molecular analyses of 6 different types of uterine smooth muscle tumors: Emphasis in atypical leiomyoma. Cancer. 2014;120:3165-3177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Lang H, Nussbaum KT, Kaudel P, Frühauf N, Flemming P, Raab R. Hepatic metastases from leiomyosarcoma: A single-center experience with 34 liver resections during a 15-year period. Ann Surg. 2000;231:500-505. [PubMed] [Cited in This Article: ] |
| 11. | Jaques DP, Coit DG, Casper ES, Brennan MF. Hepatic metastases from soft-tissue sarcoma. Ann Surg. 1995;221:392-397. [PubMed] [Cited in This Article: ] |
| 12. | Atkins KA, Arronte N, Darus CJ, Rice LW. The Use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008;32:98-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009;33:992-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Lv WF, Han JK, Cheng DL, Tang WJ, Lu D. Imaging features of primary hepatic leiomyosarcoma: A case report and review of literature. Oncol Lett. 2015;9:2256-2260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Faraj W, El-Kehdy J, Nounou GE, Deeba S, Fakih H, Jabbour M, Haydar A, El Naaj AA, Abou-Alfa GK, O’Reilly EM. Liver resection for metastatic colorectal leiomyosarcoma: a single center experience. J Gastrointest Oncol. 2015;6:E70-E76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 16. | Hamed MO, Roberts KJ, Merchant W, Lodge JP. Contemporary management and classification of hepatic leiomyosarcoma. HPB (Oxford). 2015;17:362-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Lin YH, Lin CC, Concejero AM, Yong CC, Kuo FY, Wang CC. Surgical experience of adult primary hepatic sarcomas. World J Surg Oncol. 2015;13:87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, Verweij J, Santoro A, Buesa J, Tursz T. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 442] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Duffaud F, Ray-Coquard I, Salas S, Pautier P. Recent advances in understanding and managing leiomyosarcomas. F1000Prime Rep. 2015;7:55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K, Tawbi H. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol. 2016;34:786-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 552] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 21. | Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1160] [Cited by in F6Publishing: 1058] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 22. | Hayashi T, Horiuchi A, Sano K, Hiraoka N, Kasai M, Ichimura T, Sudo T, Tagawa Y, Nishimura R, Ishiko O. Potential role of LMP2 as tumor-suppressor defines new targets for uterine leiomyosarcoma therapy. Sci Rep. 2011;1:180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Kavic SM, Kavic SM. Adhesions and adhesiolysis: the role of laparoscopy. JSLS. 2002;6:99-109. [PubMed] [Cited in This Article: ] |
| 24. | Nanashima A, Abo T, Arai J, Takagi K, Matsumoto H, Takeshita H, Tsuchiya T, Nagayasu T. Usefulness of vessel-sealing devices combined with crush clamping method for hepatectomy: a retrospective cohort study. Int J Surg. 2013;11:891-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Piardi T, Lhuaire M, Memeo R, Pessaux P, Kianmanesh R, Sommacale D. Laparoscopic Pringle maneuver: how we do it? Hepatobiliary Surg Nutr. 2016;5:345-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Ellebæk SB, Fristrup CW, Mortensen MB. Intraoperative Ultrasound as a Screening Modality for the Detection of Liver Metastases during Resection of Primary Colorectal Cancer - A Systematic Review. Ultrasound Int Open. 2017;3:E60-E68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Takahashi Y, Katagiri S, Ariizumi SI, Kotera Y, Egawa H, Wakabayashi G, Kaneko H, Yamamoto M. Laparoscopic Hepatectomy: Current State in Japan Based on the 4th Nationwide Questionnaire. Gastroenterol Res Pract. 2017;2017:6868745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |