Published online Jan 27, 2018. doi: 10.4254/wjh.v10.i1.116
Peer-review started: November 7, 2017
First decision: December 4, 2017
Revised: December 15, 2017
Accepted: January 15, 2018
Article in press: January 15, 2018
Published online: January 27, 2018
To examine the largest tertiary referral center in southern and central Vietnam from 2010 to 2016, evaluating epidemiological trends of hepatocellular carcinoma (HCC) and viral hepatitis B-C in this resource-limited setting.
We extracted data of patients receiving care from Cho Ray Hospital (Ho Chi Minh City), the largest oncology referral center in southern and central Vietnam, from 2010 to 2016. We collected information on patient age, gender, geographic distribution, and disease characteristics including disease stage, tumor biomarker levels [serum alpha-fetoprotein (AFP), AFP-L3 isoform percentage, and prothrombin induced by induced by vitamin K absence-II], and serological testing for hepatitis B virus (HBV) and hepatitis C virus (HCV) infections.
Data from 24091 HCC patients were extracted, with sample demographics comprising mostly male (81.8%) and older age (however with 8.5% younger than 40 years old). This patient sample included a geographic catchment population of 56 million people (60% of the country’s total population of 92.7 million), derived from 38 provinces and municipalities in Vietnam. Chronic HBV infection was found in 62.3% of cases, and chronic HCV infection in 26.0%. HBV and HCV co-infection was seen in 2.7%. Cirrhosis was found in an estimated 30% to 40% of cases. Nine percent of patients were not found to have chronic viral hepatitis. Twenty three point two percent of the patients had a normal AFP level. A total of 2199 patients were tested with AFP-L3 and PIVKA II over two years, with 57.7% having elevated AFP-L3%, and 88.5% with elevated PIVKA II levels. Over this 7-year period, the incidence of HCC increased, with a large proportion of cases (overall 40.8%) presenting initially an advanced stage, not amendable to surgical or locoregional therapy.
HCC contributes significant health care burden in southern and central Vietnam, with increasing case volume over this seven-year period. Viral hepatitis likely explains this high HCC prevalence.
Core tip: Hepatocellular carcinoma remains a serious public health issue in Vietnam, and is closely associated with chronic hepatitis B and C virus (HBV and HCV) infections. In one of the largest tertiary referral hospitals in southern and central Vietnam, the clinical volume has been increasing from 2010 to 2016, with most patients having chronic HBV or HCV infections, and most patients initially at an advanced stage, precluding curative treatment. Public health, policy, and institutional efforts are needed to reduce the burden that this disease places on the Vietnamese people in Vietnam.
- Citation: Nguyen-Dinh SH, Do A, Pham TND, Dao DY, Nguy TN, Chen Jr MS. High burden of hepatocellular carcinoma and viral hepatitis in Southern and Central Vietnam: Experience of a large tertiary referral center, 2010 to 2016. World J Hepatol 2018; 10(1): 116-123
- URL: https://www.wjgnet.com/1948-5182/full/v10/i1/116.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i1.116
Hepatocellular carcinoma (HCC) results in significant morbidity and mortality, and has now become the world’s second deadliest cancer (after lung cancer)[1] and one of the most common especially in the developing world[2]. HCC is etiologically linked to viral hepatitis, particularly chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. HBV and HCV account for approximately 80% to 90% of HCC cases worldwide, with both infections demonstrating wide regional variability but is known to be highly endemic in Vietnam[3-5]. Although a variety of modalities exist to treat HCC including surgical resection, locoregional ablative therapies, systemic chemotherapy, and liver transplantation, most individuals present later in their disease course and thus are limited in their ability to receive curative treatment[6,7]. HCC is a serious public health issue in Vietnam, with varied reported age adjusted incidence rates but generally thought to be greater than 20 per 100000 people, compared to that experienced in industrialized countries which are estimated to be less than 5 per 100000 people[6,8].
Viral hepatitis is thought to account for many cases of HCC, and epidemiological studies report high HBV and HCV infection rates among Vietnamese people, but have been limited to regional data (provincial or city-wide data) primarily reporting data obtained in northern regions[9-13]. Compounding the issue of high disease prevalence, the burden of disease is suggested to continue to rise in the coming decade, despite a country-wide universal infant immunization program established in 2003, though it has also been reported to have reduced chronic HBV rates[14,15]. It is difficult to ascertain whether efforts in Vietnam have been effective thus far, and challenges associated with reliance of survey-based data, incomplete death reporting, wide geographic dispersal of deaths, and travel between households, and limited testing without systematic screening, are potential limitations to obtaining accurate epidemiological estimates of disease burden in this resource-limited setting[16].
There is no comprehensive national nor regional cancer registry in Vietnam, and limited information in southern and central Vietnam is available compared to the northern regions. Thus, the purpose of this study is to describe and report the experience of Cho Ray Hospital, the largest tertiary referral center in southern and central Vietnam, to better elucidate the HCC disease burden placed on this resource-limited medical system, as well as geographic and demographic epidemiological trends from 2010 to 2016. We hypothesized that this large referral center, despite being one of the largest in Vietnam, is experiencing an increasing volume of patients with HCC during the study time period. Additionally, as chronic HBV is estimated to account for approximately 50% of all HCC cases, we also hypothesize that the majority of patients with HCC will have comorbid HBV infection, and will comprise a wide range of ages owing to high prevalence of vertical HBV transmission.
We conducted a prevalence study across a seven-year time period to examine the public health burden of HCC in southern and central Vietnam, using a large database of patients with liver cancer receiving care at Cho Ray Hospital (CRH), the largest tertiary referral center in Southern and Central Vietnam. CRH is located in Ho Chi Minh City and has a catchment area for HCC referral thought to be 56 million people (approximately 60% of the country’s total population of 92.7 million people), including 90% of southern and central Vietnam. This hospital has 1200 inpatient beds and serves approximately 67000 inpatients and 457000 outpatients per year[17]. Patients are cared for by providers of the Liver Cancer Center, composed of 12 medical hepatologists and surgeons. All patients with newly-diagnosed HCC or referred for further management of HCC after diagnosis, were included in this study. Referral for HCC management is generally the reason these patients receive care for HCC at CRH. We collected and analyzed data from January 1st, 2010 through December 31st, 2016. All patients of Vietnamese origin were included for analysis. HCC diagnosis, surveillance, and management was made according to Japan Society of Hepatology clinical guideline recommendations, including imaging characteristics (ultrasonography, computed tomography, and magnetic resonance imaging) with tumor characteristics, in conjunction with Child-Pugh classification stage and tumor marker levels[18].
Patient demographic information was obtained, including age in years, gender (male or female), region of residence (province or municipality), serological testing including serum HBV surface antigen (HBsAg) and anti-HCV antibody, and tumor markers [serum alpha-fetoprotein (AFP), AFP-L3 isoform percentage, and prothrombin induced by induced by vitamin K absence-II (PIVKA-II, also known as des-gamma-carboxy prothrombin, or DCP)]. Etiology of liver disease was classified as due to viral hepatitis (HBV, HCV, or HBV+HCV infections), or other. Chronic HCV infection was defined as having a positive anti-HCV antibody result. Chronic HBV infection was defined as having a positive HBsAg level. Patients were determined to have cirrhosis based on suggestive imaging findings on ultrasound or computed tomography (CT) imaging with or without evidence of liver chemistry abnormalities [aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, bilirubin]. Staging and management of HCC was based on Vietnamese country-wide guidelines, adapted from the Asia-Pacific Association for the Study of the Liver HCC Guidelines, which has been most recently updated in 2017[18-21]. Local institutional review board approval was obtained for this study. Disease severity was classified as advanced (no treatment possible other than palliative care) vs other.
Descriptive statistics were generated for this patient dataset, with association testing performed with chi-square for proportions of categorical variables, respectively. Trends testing was performed with Manel-Haenzel tests for categorical variables. All analyses were performed using SAS 9.4 for Windows (Cary, NC, United States).
After applying exclusion criteria, we extracted data from 24091 Vietnamese patients with HCC from 2010 to 2016. Patient demographic and disease characteristic distributions are summarized in Table 1. An increasing case volume in Cho Ray Hospital was observed during this period (2793 in 2010 with annual increase to 4069 in 2016). The majority of the sample were males (81.8%) and comprised a wide range of ages: A plurality were 50 to 60 years old (29.9%), but a majority of patients were between 40 to 70 years old (72.4%). A notable proportion of patients (8.5%) in the sample were younger than 40 years old.
| Characteristic | 2010 (n = 2793) | 2011 (n = 3111) | 2012 (n = 3349) | 2013 (n = 3471) | 2014 (n = 3494) | 2015 (n = 3804) | 2016 (n = 4069) | Total (n = 24091) | P value |
| Male gender | 82.6 | 82.77 | 82.23 | 80.44 | 81.2 | 81.2 | 82.13 | 81.76 | 0.17 |
| Age (yr) | |||||||||
| ≤ 40 | 10.31 | 8.94 | 9.14 | 8.58 | 7.76 | 7.6 | 7.77 | 8.49 | < 0.0001 |
| 41-50 | 19.3 | 18.58 | 17.44 | 17.26 | 17.32 | 17.43 | 16.76 | 17.64 | |
| 51-60 | 30.68 | 30.57 | 29.23 | 30.83 | 29.28 | 29.86 | 29.29 | 29.92 | |
| 61-70 | 21.48 | 22.6 | 24.37 | 23.22 | 26.24 | 26.63 | 27.89 | 24.86 | |
| > 70 | 18.22 | 19.32 | 19.83 | 20.11 | 19.4 | 18.48 | 18.28 | 19.08 | |
| Advanced disease stage | 40.57 | 43.68 | 40.52 | 37.65 | 40.78 | 40.75 | 41.68 | 40.79 | 0.76 |
| HBsAg and anti-HCV testing available (n)1 | n = 2505 | n = 2405 | n = 2773 | n = 3153 | n = 3225 | n = 3616 | n = 3907 | n = 21584 | < 0.0001 |
| HBV infection | 61.72 | 69.94 | 66.35 | 60.1 | 59.69 | 60.87 | 60.23 | 62.28 | |
| HCV infection | 24.79 | 27.32 | 28.96 | 25.06 | 27.13 | 25.06 | 24.98 | 26 | |
| HBV and HCV coinfection | 2.71 | 2.74 | 2.52 | 3.04 | 2.95 | 2.41 | 2.46 | 2.68 | |
| Non-HBV or HCV liver disease | 10.78 | 0 | 2.16 | 11.8 | 10.23 | 11.67 | 12.34 | 8.97 |
A large proportion of individuals (9827 patients, 40.8% of total sample), comprising of 8491 (86.4%) males and 1336 (13.6%) females, presented with advanced HCC, only amendable to palliative care (Table 2). In this sample and across years, only 47.0% to 50.5% of patients with HCC had serum AFP levels > 400 ng/mL, otherwise with the next-largest group (ranging 21.6% to 24.0%) had normal levels ≤ 20 ng/mL. AFP level subgroups did not demonstrate a trend during the study time period (P = 0.33).
| AFP level (ng/mL) | 2010 (n = 2793) | 2011 (n = 3111) | 2012 (n = 3349) | 2013 (n = 3471) | 2014 (n = 3494) | 2015 (n = 3804) | 2016 (n = 4069) | Total (n = 24091) |
| < 20 | 23.24 | 22.53 | 21.59 | 26.22 | 22.41 | 22.32 | 23.99 | 23.21 |
| > 20-100 | 15.43 | 14.14 | 15.17 | 13.71 | 15.00 | 14.54 | 14.48 | 14.62 |
| > 100-200 | 6.30 | 5.37 | 5.11 | 5.36 | 6.70 | 6.34 | 6.41 | 5.96 |
| > 200-400 | 5.87 | 5.63 | 5.32 | 5.30 | 5.38 | 5.81 | 5.97 | 5.62 |
| > 400 | 47.65 | 50.50 | 50.82 | 46.99 | 48.20 | 49.50 | 47.53 | 48.72 |
| Not recorded | 1.50 | 1.83 | 2.00 | 2.42 | 2.32 | 1.5 | 1.62 | 1.88 |
New serum tumor biomarker tests AFP-L3 percentage and PIVKA II levels were obtained starting in 2015. In 2015, 23.3% of patients received testing with these biomarkers, increasing to 32.2% in 2016. In these patients, 56.4% to 58.6% of patients had an elevated AFP-L3% (greater than 10%, the upper limit of normal), while 88.3% to 88.7% of patients had elevated PIVKA II levels (> 40 mAU/mL, the upper limit of normal).
Most patients with HCC had available viral hepatitis serologies (89.6%) with HBsAg and anti-HCV antibody testing. Of those tested, 59.7% to 69.9% (overall 62.3%) were found to have chronic HBV infection, while 24.8% to 28.9% (overall 26.0%) had chronic HCV infection. 2.4 to 3.0% (overall 2.7%) of patients had HBV-HCV co-infection. Mono-infection subgroups demonstrated an upward trend during the study period (P < 0.001). 9.0% of the tested sample had negative viral hepatitis makers (negative for both HBsAg and anti-HCV antibody). Cirrhosis was estimated to be present in 30% to 40% of individuals presenting for care, based on presence of hepatic decompensation or suggestive imaging (ultrasound, computed tomography, or magnetic resonance imaging) with hepatic macronodular changes and portal hypertension when available.
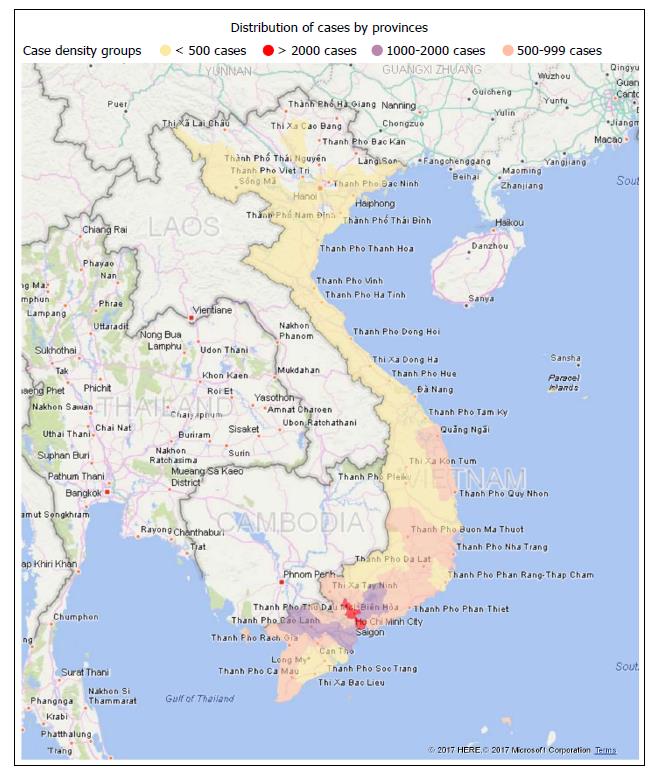
Figure 1 illustrates the geographical distribution from which the sample of HCC patients were derived, which included a total of 38 provinces. Most patients came from Southern Vietnam, with the provinces with most patients including Ho Chi Minh City (3830 patients, 15.89% of total), Tien Giang (1618 patients, 6.72% of total), Dong Nai (1567 patients, 6.50% of total), An Giang (1349 patients, 5.60% of total).
We report over 24000 cases of HCC seen over a 7-year period at a tertiary referral hospital, showing an increasing burden of HCC based on the experience of Cho Ray Hospital, with a high frequency of individuals initially presenting with untreatable, advanced disease. Most cases likely resulted from chronic HBV and/or HCV infection. Additionally, we find that men are disproportionately affected compared to women, and that a wide range of ages are affected, including a non-negligible proportion of patients younger than 40 years old (8.5%), which is generally thought to be an uncommon occurrence[2]. This sample entails a broad expanse of southern and central Vietnam, and covers seven recent years. Additionally, AFP tumor markers were elevated in only approximately half of these patients, demonstrating insufficiency of this sole biomarker in appropriate screening for these patients.
The strengths of this observational study are considerable. Data on viral hepatitis and HCC from Vietnam are rare. To the best of our knowledge, this study represents the largest number of HCC cases reported and analyzed from Vietnam since its reunification in 1975. Cho Ray Hospital is the largest oncology center in southern and central Vietnam. These patients comprise a set of varied demographic and clinical characteristics including viral hepatitis status, disease stage, tumor biomarkers levels, and other indicators were meticulously compiled and analyzed. This data illustrates a clearer image of the markedly severe epidemic that viral hepatitis imposes upon the Vietnamese people, and the marked geographical differences seen between industrialized and rural countries highlights the potential for improved public health with appropriate and sufficient resources.
Our data suggest a potential role for additional tumor biomarker testing with a relatively large proportion of people demonstrating elevated AFP-L3 and PIVKA II levels. The Japan Society of Hepatology has recommended HCC surveillance using these biomarkers in supplement to AFP and imaging, and data supports use of these additional biomarkers as a surveillance tool to determine risk of HCC development, in supplement to AFP levels[18,22-24].
Despite these considerable strengths, we are aware of limitations of our findings. Our data were derived from a cross sectional rather than longitudinal database thus limiting data on patient outcomes. As Cho Ray Hospital is a tertiary referral center, many patients had been recently diagnosed with HCC or the clinical suspicion exists before referral. In addition, inadequate knowledge of formal screening and surveillance best practices for HCC among health care providers resulted in late entry to care. Resource limitations in the setting of large patient population-to-provider ratios, access to timely laboratory and imaging testing, and challenges to universal direct-acting antiviral therapies may all play a role in contributing to the high observed disease burden. Additionally, further studies with additional detailed assessment of clinical staging and tumor characteristics with a standardized system (e.g., Child-Pugh or Barcelona Clinic Liver Cancer classification), prior vaccination/treatment and viral hepatitis details (genotype, viral load), in conjunction with patient outcomes and evaluation for additional etiologies of liver disease such as alcohol use or non-alcoholic steatohepatitis, would enhance our knowledge of anticipated disease burden in Vietnam. Although the catchment area of this medical center is expansive, it does not cover northern Vietnam and thus does not allow for national-level estimates.
Nevertheless, there is much untapped potential and much to be done. Vietnam is a high endemic area for HBV and HCV, with consequent HCC. While lung cancer leads all cancer deaths in Vietnam accounting for 5.95% of all mortality, liver cancer is second of all cancer deaths, accounting for 2.42% but has been increasing at 2.34% annually[25]. To characterize the situation in Vietnam as “epidemic” for HCC compared to the rest of the world would not be an understatement. Additionally, as the window for early-stage HCC diagnosis is thought to be small, measures to quickly identify and provide care linkage, even if on small scales, will likely result in a large magnitude effect given the endemic and epidemic nature of viral hepatitis in Vietnam.
The burden of HCC at the Cho Ray Hospital tertiary referral center is substantial and does not appear to subside in the foreseeable future. Certainly, from a public health perspective, screening for viral hepatitis, both HBV and HCV, with linkage to care is highly warranted. For those not already infected and without natural immunity, HBV vaccinations should be administered and education regarding protecting against HCV transmission should be offered through culturally and linguistically competent messaging. For those who are chronically infected, clinical management and follow up are necessary, but how this occurs should be determined based on future needs assessments and can potentially take the form of augmentation of specialist hepatologists, more education to general providers, or increasing facility access for liver-related diagnostics and care (e.g., elastography, advanced laboratory testing such as tumor markers).
Systematic, institutional, and programmatic efforts all have a role to play in combating HCC, and adoption of birth dose provision through a large-scale childhood HBV vaccination program has been reported to reduce chronic HBV infection rates[15]. Consistent linkage and maintenance of care is paramount, especially as HCC often occurs on the backdrop of chronic diseases (viral hepatitis and cirrhosis) which warrant consistent, regular medical care. Additionally, the need for ongoing and updated information will be important in the coming years to assess progress. A formal, national registry to capture all cases, associated risk factors, and treatment histories collected and analyzed in a standardized manner should allow medical providers and governmental policymakers to better understand high-yield interventional points as well as high-risk populations or sub-populations, to affect the highest impact while minimizing cost.
Additionally, clinical practice standardization is important as data quality in concert with collection will allow for the most accurate public health response. For example, at the institutional level, standardization of disease staging and management will allow for better disease characterization across medical institutions, although it is known that the Barcelona Clinic Liver Cancer (BCLC) staging system is not necessarily utilized by many providers in the Asian-Pacific region, formal staging using the Asia-Pacific Clinical Practice Guidelines or other system will allow for standardization and thus comparability[21,26].
However, specialists in viral hepatitis care and HCC in Vietnam are quite limited. A huge demand for training by the primary care providers and their support personnel exists for the kind of training offered by the Vietnam Viral Hepatitis Alliance where its two annual conferences attracted 350 in its inaugural year, increasing to 500 in its second year. Training both primary care providers along with their support staff so that their knowledge of viral hepatitis and HCC and their practical roles in care are needed. Additionally, health education for the public at large is essential. From research conducted among overseas Vietnamese in the United States, the odds of Vietnamese patients being screened for HBV is 4-fold if the provider recommends it and increases to nearly 9-fold if both the provider and the patient ask for it[27]. We anticipate that the dual targeting of providers and patients will bring about synergy in promoting serological testing for viral hepatitis as the first community-based intervention to stem the tide of HCC in Vietnam.
In the future, trends in chronic liver disease seen in industrialized countries could be expected to be mirrored in Vietnam as well. With globalization of convenience foods that favor taste over nutrition as well as other geopolitical and economy-based factors, concern for obesity and metabolic-associated diseases will likely become a larger issue in Vietnam in the future. From a hepatology perspective, there is known concern about increasing diabetes rates, and given worldwide trends of non-alcoholic fatty liver disease increasing in industrialized countries this may contribute to the growing burden of HCC in Vietnam as well[28-30].
In conclusion, from 2010 to 2016, we find an increasing number of patients receiving HCC care at Cho Ray Hospital, a large tertiary referral center serving southern and central Vietnam, with most patients having an advanced disease stage not amendable to treatment, and additionally these cases are reasonably attributed to chronic HBV or HCV infection. Future efforts in public health efforts to screen, provide linkage to care, to provide surveillance, and to educate health care providers, will all play an important role in curtailing the burgeoning of this disease throughout Vietnam in the coming years.
Hepatocellular carcinoma (HCC) is currently the world’s second deadliest cancer. HCC is closely linked to viral hepatitis, which in turns has been reported to have high epidemiologic heterogeneity especially in the developing world. There is limited epidemiological data on HCC reported in Vietnam, particularly the southern and central regions.
This study primarily seeks to elucidate the epidemiological characteristics of HCC in southern and central Vietnam. These results have significant policy and research implications in establishing priorities for public health interventions, financial allocations, and driving knowledge acquisition in further large-scale observational studies and potential biomarker testing expansion.
The authors sought to evaluate the burden of HCC and characteristics of patients presenting with HCC, as well as potential disease etiology.
The authors conducted an epidemiological observational study from 2000 to 2016, using a large database of patients with liver cancer who receive care at Cho Ray Hospital, the largest tertiary referral center in southern and central Vietnam. Information on patient demographic information, disease staging, and tumor marker results were extracted.
Analysis was performed on 24091 patients from 2010 to 2016, with increasing disease frequency noted (2793 patients in 2010 to 4069 in 2016). Most patients were male (86.4%), most patients presented with advanced disease (40.8%). Most patients were found to have viral hepatitis (89.6%), with 62.3% with HBV, 26.0% with HCV, and 2.7% with HBV-HCV coinfection. Eight point five percent of patients were younger than 40 years old.
In the largest epidemiological study conducted for liver cancer in Vietnam to date, we find high and increasing disease burden from 2010 to 2016, which manifests as advanced disease and co-prevalent with viral hepatitis. Demographic patterns suggest higher disease burden on males and disproportionate burden on younger patients.
These findings emphasize the importance of developing systems and methods to better understand epidemiology of liver cancer, as well as for linkage to care, evaluation, and treatment of both liver cancer and viral hepatitis. Future research should focus on health care services and policy implications for disease screening and treatment outcomes for this population.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Vietnam
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Berkane S, Christodoulidis G, Namisaki T, Sazci A, Tajiri K, Zhu X S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19774] [Article Influence: 2197.1] [Reference Citation Analysis (17)] |
| 2. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2183] [Cited by in F6Publishing: 2337] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 3. | Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 487] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206-S214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 394] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 5. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 6. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2881] [Cited by in F6Publishing: 2977] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 7. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 908] [Cited by in F6Publishing: 933] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 8. | Sereno L, Mesquita F, Kato M, Jacka D, Nguyen TT, Nguyen TN. Epidemiology, responses, and way forward: the silent epidemic of viral hepatitis and HIV coinfection in Vietnam. J Int Assoc Physicians AIDS Care (Chic). 2012;11:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Anh PT, Parkin DM, Hanh NT, Duc NB. Cancer in the population of Hanoi, Vietnam, 1988-1990. Br J Cancer. 1993;68:1236-1242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Do SH, Yamada H, Fujimoto M, Ohisa M, Matsuo J, Akita T, Katayama K, Van Nguyen N, Miyakawa Y, Tanaka J. High prevalences of hepatitis B and C virus infections among adults living in Binh Thuan province, Vietnam. Hepatol Res. 2015;45:259-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Cordier S, Le TB, Verger P, Bard D, Le CD, Larouze B, Dazza MC, Hoang TQ, Abenhaim L. Viral infections and chemical exposures as risk factors for hepatocellular carcinoma in Vietnam. Int J Cancer. 1993;55:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Nguyen VT, McLaws ML, Dore GJ. Highly endemic hepatitis B infection in rural Vietnam. J Gastroenterol Hepatol. 2007;22:2093-2100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ishizaki A, Tran VT, Nguyen CH, Tanimoto T, Hoang HTT, Pham HV, Phan CTT, Bi X, Pham TV, Ichimura H. Discrepancies in prevalence trends for HIV, hepatitis B virus, and hepatitis C virus in Haiphong, Vietnam from 2007 to 2012. PLoS One. 2017;12:e0179616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Nguyen VT, Law MG, Dore GJ. An enormous hepatitis B virus-related liver disease burden projected in Vietnam by 2025. Liver Int. 2008;28:525-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Nguyen TH, Vu MH, Nguyen VC, Nguyen LH, Toda K, Nguyen TN, Dao S, Wannemuehler KA, Hennessey KA. A reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine. 2014;32:217-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Ngo AD, Rao C, Hoa NP, Adair T, Chuc NT. Mortality patterns in Vietnam, 2006: Findings from a national verbal autopsy survey. BMC Res Notes. 2010;3:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Cho Ray Hospital: main page [cited 2017 Oct 15]. Available from: http://www.choray.vn/. [Cited in This Article: ] |
| 18. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45:123-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 19. | Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, Yamashita T, Kokudo N, Tanaka M, Takayama T. Management of hepatocellular carcinoma: Report of Consensus Meeting in the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:667-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 439] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 21. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 986] [Cited by in F6Publishing: 1344] [Article Influence: 192.0] [Reference Citation Analysis (0)] |
| 22. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Yu R, Tan Z, Xiang X, Dan Y, Deng G. Effectiveness of PIVKA-II in the detection of hepatocellular carcinoma based on real-world clinical data. BMC Cancer. 2017;17:608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, Jang MK, Lee JH, Kim JS, Kim HY. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2015;21:3928-3935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 44] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | 2016 Global Burden of Disease 2017 [cited 2017 Oct 15]. Available from: https://vizhub.healthdata.org/gbd-compare/. [Cited in This Article: ] |
| 26. | Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:7-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 27. | Nguyen TT, McPhee SJ, Stewart S, Gildengorin G, Zhang L, Wong C, Maxwell AE, Bastani R, Taylor VM, Chen MS Jr. Factors associated with hepatitis B testing among Vietnamese Americans. J Gen Intern Med. 2010;25:694-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Khue NT. Diabetes in Vietnam. Ann Glob Health. 2015;81:870-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Tabibian JH, Lazo M, Durazo FA, Yeh HC, Tong MJ, Clark JM. Nonalcoholic fatty liver disease across ethno-racial groups: do Asian-American adults represent a new at-risk population? J Gastroenterol Hepatol. 2011;26:501-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Wong RJ, Ahmed A. Obesity and non-alcoholic fatty liver disease: Disparate associations among Asian populations. World J Hepatol. 2014;6:263-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (1)] |