Published online Oct 26, 2013. doi: 10.4252/wjsc.v5.i4.163
Revised: September 1, 2013
Accepted: October 16, 2013
Published online: October 26, 2013
Pluripotent stem cells, which are capable of differentiating in various species of cells, are hoped to be donor cells in transplantation in regenerative medicine. Embryonic stem (ES) cells and induced pluripotent stem cells have the potential to differentiate in approximately all species of cells. However, the proliferating ability of these cells is high and the cancer formation ability is also recognized. In addition, ethical problems exist in using ES cells. Somatic stem cells with the ability to differentiate in various species of cells have been used as donor cells for neuronal diseases, such as amyotrophic lateral sclerosis, spinal cord injury, Alzheimer disease, cerebral infarction and congenital neuronal diseases. Human mesenchymal stem cells derived from bone marrow, adipose tissue, dermal tissue, umbilical cord blood and placenta are usually used for intractable neuronal diseases as somatic stem cells, while neural progenitor/stem cells and retinal progenitor/stem cells are used for a few congenital neuronal diseases and retinal degenerative disease, respectively. However, non-treated somatic stem cells seldom differentiate to neural cells in recipient neural tissue. Therefore, the contribution to neuronal regeneration using non-treated somatic stem cells has been poor and various differential trials, such as the addition of neurotrophic factors, gene transfer, peptide transfer for neuronal differentiation of somatic stem cells, have been performed. Here, the recent progress of regenerative therapies using various somatic stem cells is described.
Core tip: Pluripotent stem cells, which are capable of differentiating in various species of cells, are hoped to be donor cells in transplantation in regenerative medicine. Somatic stem cells with the ability to differentiate in various species of cells have been used as donor cells for neuronal diseases, such as spinal cord injury, cerebral infarction, amyotrophic lateral sclerosis, Parkinson’s disease and multiple sclerosis. Here, the recent progress of regenerative therapies using various somatic stem cells is described.
- Citation: Kanno H. Regenerative therapy for neuronal diseases with transplantation of somatic stem cells. World J Stem Cells 2013; 5(4): 163-171
- URL: https://www.wjgnet.com/1948-0210/full/v5/i4/163.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i4.163
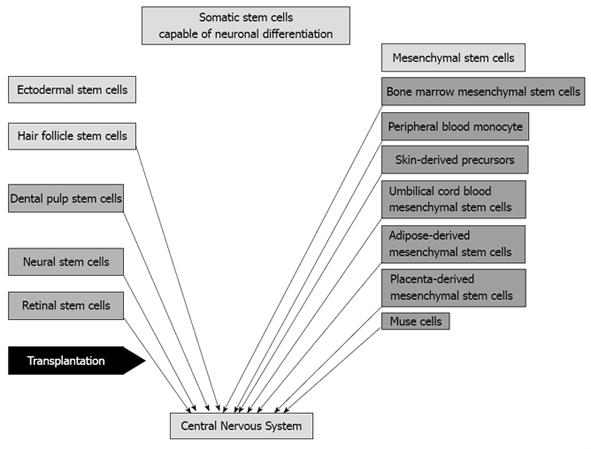
Pluripotent stem cells, which are capable of differentiating in various species of cells, are hoped to be donor cells in transplantation in regenerative medicine. Human embryonic stem (ES) cells[1] and induced pluripotent (iPS) cells[2] have the potential to differentiate in approximately all species of cells. However, the proliferating ability of these cells is high and the cancer formation ability is also recognized[2,3]. Ethical problems exist in using ES cells[4], while iPS cells produced from the patients themselves have little ethical problems. Gene transfer, particularly oncogene transfer, is associated with DNA change and cancer formation[2]. Omission of oncogene c-Myc from the defined four factors was tried and the cancer formation rate decreased[5]. In addition, no integration of defined factors into the genome was tried and brought good results[6]. However, cancer formation problems remain completely unsolved. It is probable that somatic stem cells reside in all organ tissues. In addition, truly pluripotent somatic stem cells, such as multilineage-differentiating stress enduring (MUSE) cells, are also probably harbored in all organ tissues[7,8]. However, it has been reported that the capability of neuronal differentiation is recognized in only mesenchymal or ectodermal stem cells[9,10]. Mesenchymal stem cells include bone marrow mesenchymal stem cells[11], adipose-derived mesenchymal stem cells[12], skin-derived precursors[13], umbilical cord blood-derived mesenchymal stem cells[14], placenta-derived mesenchymal stem cells, peripheral blood monocytes and MUSE cells[7], while ectodermal stem cells include hair follicle stem cells[15], dental pulp-derived stem cells[16], retinal progenitor/stem cells and neural progenitor/stem cells[17] (Figure 1). Although recent clinical trials of regenerative therapy for neuronal disease with transplantation of somatic stem cells has been performed with neural stem cells[18,19], bone marrow mesenchymal stem cells[20-25] and adipose mesenchymal stem cells[26], most of them stay at the level of confirmation of safety, but the efficacy of the therapies has not been shown (Table 1). On the other hand, numerous studies of transplantation of somatic stem cells using neuronal disease models have been reported and most studies have confirmed it to be efficient for the repair of neuronal diseases[27-34]. Ectodermal stem cells and mesodermal (mesenchymal) stem cells potentially differentiate to neurons, while it seems that endodermal stem cells do not differentiate to neurons without dedifferentiation or induction to iPS cells. Being different from iPS cells, these stem cells do not basically transform or dedifferentiate to cancer cells. The clinical application of somatic stem cells has a greater advantage than iPS cells. The regenerative effect of transplantation of somatic stem cells is considered to be mostly derived from trophic factors secreted from somatic stem cells. It is reported that the transplantation effect of adipose-derived stem cells is greater than bone marrow mesenchymal stem cells because the former cells secrete more vascular endothelial growth factor (VEGF) or hepatocyte growth factor (HGF) than the latter[35]. To survive as functional cells appropriate in the niche, it is necessary that transplanted cells differentiate to appropriate cells or somatic stem cells differentiate to appropriately functional cells before transplantation[36]. Naïve somatic stem cells scarcely differentiate to appropriate cells in the niche. Therefore, for example, it is necessary that transplanted somatic neuronal cells in the nervous system are differentiated to neuronal cells. Here, I describe regenerative therapy for neuronal diseases with transplantation of somatic stem cells.
| Kind of cell | Disease | Ref. |
| Neural stem cell | Pelizaeus-Merzbacher disease | [19] |
| Neuronal ceroid lipofuscinosis | [18] | |
| Bone marrow mesenchymal stem cell | Alzheimer’s disease | [58] |
| Parkinson’s disease | [20,59,60] | |
| Amyotrophic lateral sclerosis | [61-71] | |
| Multiple sclerosis | [21,22] | |
| Cerebral infarction | [57,73,74] | |
| Spinal cord injury | [23-25,77,78] | |
| Adipose mesenchymal stem cell | Parry-Romberg syndrome | [26] |
It is difficult to obtain human neural stem/progenitor cells but they are easily obtained from human fetal brains without ethical problems. The use of these human cells is accompanied with a great ethical problem[37,38]. Previously, tissues of striatum and substantia nigra richly containing dopaminergic neurons were obtained from human fetal brain and were implanted into the striatum of Parkinson’s disease patients. As a result, symptoms of a part of Parkinson’s patients dramatically improved[39,40]. However, these clinical trials were stopped due to the difficulty of obtaining fetal brain tissue and a great ethical problem in using an abortion fetus. Neural stem cells reside in the subventricular zone and hippocampus. It is more difficult to obtain autologous cells from the brain. Therefore, transplantation of autologous neural stem cells has not been tried for neuronal regeneration. In addition, few clinical applications of allogenic transplantation of human neural stem cells have been performed[41,42].
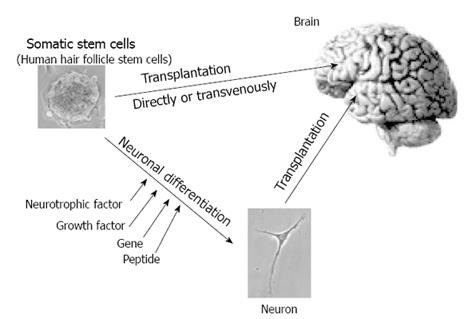
In place of transplantation of human neural stem cells, activation of endogenous neural stem cells using fibroblast growth factor 2 (FGF-2), epidermal growth factor (EGF), erythropoietin and brain derived neurotrophic factor have been investigated[41,42]. Murine or rodent neural stem/progenitor cells are frequently used for regenerative research. Transplantations of neural stem/progenitor cells have been used for a Parkinson’s disease model[36], cerebral infarction model[43], spinal cord injury model[27], retinal disease model[44] and so on. However, transplantation of neural stem/progenitor cells without treatment is not useful for regeneration of neural tissue because non-treated neural stem/progenitor cells cannot survive in the recipient’s neural tissue and in addition cannot differentiate to a neuron[36]. Before transplantation, treatment of neuronal differentiation is effective for survival as a neuron. The addition of neurotrophic factors such as FGF8, sonic hedgehog and glial cell line-derived neurotrophic factor leads to neuronal differentiation[45]. In addition, gene transfer to cells is useful for neuronal differentiation in neural stem/progenitor cells. Gene transfers of Math-1[46], Ascl-1[47], Nurr-1[48] and von Hippel-Lindau (VHL)[49] show neuronal differentiation in neural stem/progenitor cells. Neuronal differentiation of intracellular transfer of protein or peptide is also reported. Intracellular transfer of VHL peptide, consisting of an amino-acid sequence of binding sites to elongin C, is useful for neuronal differentiation in neural progenitor cells (Figure 2). VHL peptide linked with protein transduction domain peptide shows high efficacy and rapid intracellular transduction. Transplantation of VHL peptide-treated neural stem cells promoted recovery in injured rat spinal cord[27]. Clinical applications using human allogenic neural stem cells have been tried for neuronal ceroid lipofuscinosis[41] and Pelizaeus-Merzbacher disease, both of which are hereditary intractable neuronal diseases[42] (Table 1)..
Recently, retinal progenitor/stem cells (RSCs) have been identified in not only embryonic and newborn retina but also in adult ciliary epithelium (CE) of rodents and humans[50-54]. Their niche has been suggested to be in the pigmented or nonpigmented epithelial layer of the ciliary margin at the peripheral edge of the retina. Since the majority of the differentiated cells were photoreceptor cells[54], transplantation of RSCs has shown their potential as tools for cell replacement in retinal degenerative diseases.
Bone marrow mesenchymal stem cells are also called bone marrow stromal cells and have been reported to be able to differentiate cells of bone, cartilage, adipose tissue, liver and neural tissue[55]. Transplantation of the bone marrow mesenchymal cells has been applied for cerebral infarction[56,57]. These cells are transplanted via intravenous transfusion and a part of them have been demonstrated to penetrate the blood brain barrier (BBB), but these penetrated cells scarcely survive and function as neurons in the brain[56,58]. Even if these cells do not differentiate to neural cells in the brain, these cells secrete neurotrophic factors which may have effects on neural tissue repair[58]. When the bone marrow stromal cells are transferred with the gene of Notch intracellular domain and neurotrophic factors were added, these cells mostly differentiated to neurons[55]. Clinical application with transplantation of bone marrow mesenchymal stem cells to neuronal degenerative disease patients of Alzheimer disease[57], Parkinson’s disease[20,59,60], amyotrophic lateral sclerosis[61-71] and multiple sclerosis[21,22] have been tried, but those effects have not been fully established. Those induced neurons are transplanted to cerebral infarction model rats into the brain and the major part of the transplanted cells differentiated to neurons and the symptoms of the model rats improved[72]. VHL peptide-transferred bone marrow stromal cells partially differentiate to neurons and transplantation of those induced neurons improved the behavior of the spinal cord injury rats[28]. Human autologous bone marrow-derived mesenchymal stem cells have been transfused to brain ischemic disease patients[73,74]. The results of the clinical trials appear to be feasible and safe and occasionally an improving effect is observed. Several human clinical applications for spinal cord injury with transplantation of bone marrow mesenchymal stem cells have been reported[23-25,72-78] (Table 1). Among them, improvement of motor function and electrophysiological findings have been recognized[25].
Skin-derived precursors (SKPs), which are also called dermal papilla stem cells, are reported to differentiate into various types of cells, including neuronal cells[79-81]. Although these cells are considered to originate from mesenchymal tissue in dermis, they differentiate to not only mesenchymal-derived cells, such as smooth muscle cells and adipose cells, but also epithelial lineage cells, such as neurons, glia and keratinocytes. In addition, nestin-expressing hair follicle stem cells residing at the hair follicle bulge region in mice and at the outer root sheath of hair follicle beneath sebaceous glands in humans are reported to differentiate to epithelial lineage cells, including neuronal cells[82,83], and they are also called neural crest stem cells [84]. These cells might contribute to neuronal regenerative therapy, repairing not only peripheral nerves but also the central nervous system, including brain and spinal cord[85,86] (Figure 2). It is reported that VHL peptide-transferred rodent SKPs were transplanted into cerebrum in Parkinson’s disease rat models and they differentiated to dopaminergic neurons in the cerebrum with improvement of their symptoms[29,30]. This report suggested that SKPs are hopeful sources of donor cells to transplant into the nervous system for neuronal regenerative therapy.
Adipose tissue-derived mesenchymal stem cells are similar to bone marrow-derived mesenchymal stem cells. These cells differentiate to various types of cells, derived from not only mesenchymal organs but also epithelial and endogenous organs. Recently, directed differentiation of motor neuron cell-like cells from human adipose-derived stem cells was induced with retinoic acid and sonic hedgehog[87,88], and the potential application for Huntington’s disease or intracerebral hemorrhage is promising[89,90]. The application to animal models and also the human clinical application have been tried using those cells, but their human clinical application is still limited[91]. It is reported that adipose-tissue derived mesenchymal stem cells secret trophic factors, such as VEGF and HGF, which contribute to repair for ischemic brain tissue[35].
Human umbilical cord blood contains hematopoietic stem cells and mesenchymal stem cells. Umbilical cord blood-derived mesenchymal stem cells (UCBSCs) differentiate to neuronal cells and are clinically promising as a regenerative cell therapy for neuronal disease. It reported that neuronal differentiation of UCBSCs is mediated by protein kinase and that estrogen stimulates the neuronal differentiation of human UCBSCs[92,93]. UCBSCs differentiated to dopaminergic neurons in vitro. Transplantation of those cells is applied to neuronal disease models[94,95].
Dental pulp stem cells (DPSCs) are putatively neural crest cell-derived[96] and thus differentiate to neurons[97]. Therefore, these cells are promising as donor cells of neuronal regenerative cell therapy. Transplantation of DPSCs is applied to neuronal disease models such as spinal cord injury[98]. It is suggested that implanted adult human dental pulp stem cells induce endogenous axon guidance[99]. In addition, it is suggested that human DPSCs differentiate towards functionally active neurons in an appropriate environment[16].
Placenta-derived mesenchymal stem cells are from mesenchymal somatic stem cells and differentiate to cells of neuronal phenotype in the appropriate niche conditions[100]. These cells differentiate to dopaminergic neuron-like cells in vitro[101]. In addition, intracerebral transplantation of these cells has been reported[102]. The transplantation of placenta-derived mesenchymal stem cells is promising for regenerative therapy for intractable neuronal diseases.
Peripheral blood monocytes include mesenchymal stem cells that are multipotential and capable of differentiating to neuronal lineage cells. These cells have the advantage of being obtained from an easily accessible minimally invasive procedure. With treatments of macrophage colony-stimulating growth factor and thereafter NGF, these cells express neuron specific enolase, neurofilament and microtubule associated protein 1-B that are neuronal markers[103]. These cells differentiate to microglia that is supportive for neuronal tissue[104] and are promising candidates as donor cells of autologous transplantation for neuronal regeneration.
Multilineage-differentiating stress enduring (MUSE) cells are pluripotent stem cells resembling ES or iPS cells[105]. These cells are derived from skin fibroblast or mesenchymal stromal cells[7,106]. Among stress (long-time heparin treatment) enduring fibroblasts, multilineage-differentiating stem cells were found. It is reported that these cells can differentiate to tri-dermal cells[7]. They are promising as donor cells for regenerative cell therapy[8]. Since they differentiated to neural lineage cells such as neuron and glia, they are hoped to be donor cells for neuronal regenerative cell therapy[107]. MUSE cells are the most promising somatic stem cells and the obtaining method is established. The autologous transplantation of MUSE cells obtained from autologous fibroblast or mesenchymal stem cells is useful for neuronal regenerative cell therapy. The necessity of cell sorting using anti-SSEA-3 antibody is a limiting factor in generating MUSE cells[7]. However, since the generative rate of MUSE cells is small but stable, use of MUSE cells is very promising as donor cells of transplantation of cell therapy for regeneration of neuronal disease.
Endoderm-derived somatic stem cells capable of neuronal differentiation are rare. When normal thyrocytes are cultured in non-serum small airway growth medium (SAGM) and their neuronal differentiation is induced, they express neuronal marker beta-III-tubulin[108]. This result suggests that thyroid cells derived from endoderm are capable of differentiating to neurons. Although direct conversion of hepatocytes derived from endoderm to neurons using defined factors has been recently reported[109], it is a report using a reprogramming method like iPS cells. Principally, it is likely that endoderm-derived stem cells are difficult to differentiate to neurons.
Cell transplantation therapy using somatic stem cells is very promising. At present, the kinds of clinically used somatic stem cells are mostly limited to neural stem cells and bone marrow mesenchymal stem cells. Other somatic stem cells are scarcely used for clinical applications. However, therapeutic levels of somatic stem cell therapy still mostly stay at the confirmation of safety and feasibility. Undoubtedly, neuronal regenerative therapy with transplantation of somatic stem cells will be applied to intractable neuronal diseases and spread throughout the world in the future.
P- Reviewer Wen TQ S- Editor Zhai HH L- Editor Roemmele A E- Editor Lu YJ
| 1. | Ben-Shushan E, Thompson JR, Gudas LJ, Bergman Y. Rex-1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to an octamer site and a novel protein, Rox-1, binding to an adjacent site. Mol Cell Biol. 1998;18:1866-1878. [PubMed] [Cited in This Article: ] |
| 2. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14327] [Cited by in F6Publishing: 13579] [Article Influence: 848.7] [Reference Citation Analysis (0)] |
| 3. | Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Denker HW. Potentiality of embryonic stem cells: an ethical problem even with alternative stem cell sources. J Med Ethics. 2006;32:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2072] [Cited by in F6Publishing: 1907] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 6. | Jin ZB, Okamoto S, Xiang P, Takahashi M. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling. Stem Cells Transl Med. 2012;1:503-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, Tanimura Y, Tsuchiyama K, Kikuchi T, Goda M. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci USA. 2011;108:9875-9880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Wakao S, Kitada M, Kuroda Y, Dezawa M. Isolation of adult human pluripotent stem cells from mesenchymal cell populations and their application to liver damages. Methods Mol Biol. 2012;826:89-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Tsai CC, Hung SC. Functional roles of pluripotency transcription factors in mesenchymal stem cells. Cell Cycle. 2012;11:3711-3712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Zhao H, Sun N, Young SR, Nolley R, Santos J, Wu JC, Peehl DM. Induced pluripotency of human prostatic epithelial cells. PLoS One. 2013;8:e64503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 12. | Dhanasekaran M, Indumathi S, Poojitha R, Kanmani A, Rajkumar JS, Sudarsanam D. Plasticity and banking potential of cultured adipose tissue derived mesenchymal stem cells. Cell Tissue Bank. 2013;14:303-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Yang LY, Zheng JK, Liu XM, Hui GZ, Guo LH. Culture of skin-derived precursors and their differentiation into neurons. Chin J Traumatol. 2004;7:91-95. [PubMed] [Cited in This Article: ] |
| 14. | Qiu P, Song W, Niu Z, Bai Y, Li W, Pan S, Peng S, Hua J. Platelet-derived growth factor promotes the proliferation of human umbilical cord-derived mesenchymal stem cells. Cell Biochem Funct. 2013;31:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102:5530-5534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787-1795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 17. | Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Selden NR, Al-Uzri A, Huhn SL, Koch TK, Sikora DM, Nguyen-Driver MD, Guillaume DJ, Koh JL, Gultekin SH, Anderson JC. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J Neurosurg Pediatr. 2013;11:643-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4:155ra137. [PubMed] [Cited in This Article: ] |
| 20. | Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, Rao DK, Das M, Jan M, Gupta PK. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155:62-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 21. | Liang J, Zhang H, Hua B, Wang H, Wang J, Han Z, Sun L. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler. 2009;15:644-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, Scolding N, Slavin S, Le Blanc K, Uccelli A. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 2010;16:503-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, Park HS. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11:913-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25:2066-2073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Park JH, Kim DY, Sung IY, Choi GH, Jeon MH, Kim KK, Jeon SR. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70:1238-147; discussion 1247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Koh KS, Oh TS, Kim H, Chung IW, Lee KW, Lee HB, Park EJ, Jung JS, Shin IS, Ra JC. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg. 2012;69:331-337. [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Maeda K, Kanno H, Yamazaki Y, Kubo A, Sato F, Yamaguchi Y, Saito T. Transplantation of Von Hippel-Lindau peptide delivered neural stem cells promotes recovery in the injured rat spinal cord. Neuroreport. 2009;20:1559-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Yamazaki Y, Kanno H, Maeda K, Yoshida T, Kobayashi N, Kubo A, Yamaguchi Y, Saito T. Engrafted VHL peptide-delivered bone marrow stromal cells promote spinal cord repair in rats. Neuroreport. 2010;21:287-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Kubo A, Yoshida T, Kobayashi N, Yokoyama T, Mimura T, Nishiguchi T, Higashida T, Yamamoto I, Kanno H. Efficient generation of dopamine neuron-like cells from skin-derived precursors with a synthetic peptide derived from von Hippel-Lindau protein. Stem Cells Dev. 2009;18:1523-1532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Higashida T, Jitsuki S, Kubo A, Mitsushima D, Kamiya Y, Kanno H. Skin-derived precursors differentiating into dopaminergic neuronal cells in the brains of Parkinson disease model rats. J Neurosurg. 2010;113:648-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Heo JS, Choi SM, Kim HO, Kim EH, You J, Park T, Kim E, Kim HS. Neural transdifferentiation of human bone marrow mesenchymal stem cells on hydrophobic polymer-modified surface and therapeutic effects in an animal model of ischemic stroke. Neuroscience. 2013;238:305-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Koh SH, Kim KS, Choi MR, Jung KH, Park KS, Chai YG, Roh W, Hwang SJ, Ko HJ, Huh YM. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, Wagner J, Shumsky JS, Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Kanno H, Kubo A, Yoshizumi T, Mikami T, Maegawa J. Isolation of Multipotent Nestin-Expressing Stem Cells Derived from the Epidermis of Elderly Humans and TAT-VHL Peptide-Mediated Neuronal Differentiation of These Cells. Int J Mol Sci. 2013;14:9604-9617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13:675-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 36. | Yamada H, Dezawa M, Shimazu S, Baba M, Sawada H, Kuroiwa Y, Yamamoto I, Kanno H. Transfer of the von Hippel-Lindau gene to neuronal progenitor cells in treatment for Parkinson’s disease. Ann Neurol. 2003;54:352-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Turner DA, Kearney W. Scientific and ethical concerns in neural fetal tissue transplantation. Neurosurgery. 1993;33:1031-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Boer GJ. Ethical guidelines for the use of human embryonic or fetal tissue for experimental and clinical neurotransplantation and research. Network of European CNS Transplantation and Restoration (NECTAR). J Neurol. 1994;242:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | López-Lozano JJ, Bravo G, Brera B, Dargallo J, Salmeán J, Uría J, Insausti J, Millán I. Long-term follow-up in 10 Parkinson’s disease patients subjected to fetal brain grafting into a cavity in the caudate nucleus: the Clinica Puerta de Hierro experience. CPH Neural Transplantation Group. Transplant Proc. 1995;27:1395-1400. [PubMed] [Cited in This Article: ] |
| 40. | Bhattacharya N, Chhetri MK, Mukherjee KL, Ghosh AB, Samanta BK, Mitra R, Bhattacharya M, Bhattacharya S, Bandopadhyaya T. Can human fetal cortical brain tissue transplant (up to 20 weeks) sustain its metabolic and oxygen requirements in a heterotopic site outside the brain? A study of 12 volunteers with Parkinson’s disease. Clin Exp Obstet Gynecol. 2002;29:259-266. [PubMed] [Cited in This Article: ] |
| 41. | Uzun G, Subhani D, Amor S. Trophic factors and stem cells for promoting recovery in stroke. J Vasc Interv Neurol. 2010;3:3-12. [PubMed] [Cited in This Article: ] |
| 42. | Leker RR, Lasri V, Chernoguz D. Growth factors improve neurogenesis and outcome after focal cerebral ischemia. J Neural Transm. 2009;116:1397-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol Dis. 2013;52:191-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 44. | Mellough CB, Cui Q, Harvey AR. Treatment of adult neural progenitor cells prior to transplantation affects graft survival and integration in a neonatal and adult rat model of selective retinal ganglion cell depletion. Restor Neurol Neurosci. 2007;25:177-190. [PubMed] [Cited in This Article: ] |
| 45. | Kim TE, Lee HS, Lee YB, Hong SH, Lee YS, Ichinose H, Kim SU, Lee MA. Sonic hedgehog and FGF8 collaborate to induce dopaminergic phenotypes in the Nurr1-overexpressing neural stem cell. Biochem Biophys Res Commun. 2003;305:1040-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Nakamichi N, Ishioka Y, Hirai T, Ozawa S, Tachibana M, Nakamura N, Takarada T, Yoneda Y. Possible promotion of neuronal differentiation in fetal rat brain neural progenitor cells after sustained exposure to static magnetism. J Neurosci Res. 2009;87:2406-2417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Jessberger S, Toni N, Clemenson GD, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 48. | Sakurada K, Ohshima-Sakurada M, Palmer TD, Gage FH. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development. 1999;126:4017-4026. [PubMed] [Cited in This Article: ] |
| 49. | Kanno H, Saljooque F, Yamamoto I, Hattori S, Yao M, Shuin T, U HS. Role of the von Hippel-Lindau tumor suppressor protein during neuronal differentiation. Cancer Res. 2000;60:2820-2824. [PubMed] [Cited in This Article: ] |
| 50. | Ahmad I, Dooley CM, Thoreson WB, Rogers JA, Afiat S. In vitro analysis of a mammalian retinal progenitor that gives rise to neurons and glia. Brain Res. 1999;831:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Yang P, Seiler MJ, Aramant RB, Whittemore SR. In vitro isolation and expansion of human retinal progenitor cells. Exp Neurol. 2002;177:326-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Klassen H, Ziaeian B, Kirov II, Young MJ, Schwartz PH. Isolation of retinal progenitor cells from post-mortem human tissue and comparison with autologous brain progenitors. J Neurosci Res. 2004;77:334-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 334] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 54. | Coles BL, Angénieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci USA. 2004;101:15772-15777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 55. | Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701-1710. [PubMed] [Cited in This Article: ] |
| 56. | Rosado-de-Castro PH, Schmidt Fda R, Battistella V, Lopes de Souza SA, Gutfilen B, Goldenberg RC, Kasai-Brunswick TH, Vairo L, Silva RM, Wajnberg E. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen Med. 2013;8:145-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28:329-343. [PubMed] [Cited in This Article: ] |
| 58. | Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 59. | Bahat-Stroomza M, Barhum Y, Levy YS, Karpov O, Bulvik S, Melamed E, Offen D. Induction of adult human bone marrow mesenchymal stromal cells into functional astrocyte-like cells: potential for restorative treatment in Parkinson’s disease. J Mol Neurosci. 2009;39:199-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Levy YS, Bahat-Stroomza M, Barzilay R, Burshtein A, Bulvik S, Barhum Y, Panet H, Melamed E, Offen D. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson’s disease. Cytotherapy. 2008;10:340-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Prabhakar S, Marwaha N, Lal V, Sharma RR, Rajan R, Khandelwal N. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: a pilot study. Neurol India. 2012;60:465-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Baek W, Kim YS, Koh SH, Lim SW, Kim HY, Yi HJ, Kim H. Stem cell transplantation into the intraventricular space via an Ommaya reservoir in a patient with amyotrophic lateral sclerosis. J Neurosurg Sci. 2012;56:261-263. [PubMed] [Cited in This Article: ] |
| 63. | Blanquer M, Moraleda JM, Iniesta F, Gómez-Espuch J, Meca-Lallana J, Villaverde R, Pérez-Espejo MÁ, Ruíz-López FJ, García Santos JM, Bleda P. Neurotrophic bone marrow cellular nests prevent spinal motoneuron degeneration in amyotrophic lateral sclerosis patients: a pilot safety study. Stem Cells. 2012;30:1277-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, Servo S, Carriero A, Monaco F, Fagioli F. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14:56-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 65. | Gamez J, Carmona F, Raguer N, Ferrer-Sancho J, Martín-Henao GA, Martí-Beltrán S, Badia M, Gratacós M, Rodriguez-Gónzalez E, Seoane JL. Cellular transplants in amyotrophic lateral sclerosis patients: an observational study. Cytotherapy. 2010;12:669-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Choi MR, Kim HY, Park JY, Lee TY, Baik CS, Chai YG, Jung KH, Park KS, Roh W, Kim KS. Selection of optimal passage of bone marrow-derived mesenchymal stem cells for stem cell therapy in patients with amyotrophic lateral sclerosis. Neurosci Lett. 2010;472:94-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 67. | Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K, Testa L, Stecco A, Tarletti R, Miglioretti M. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol. 2010;223:229-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 68. | Martinez HR, Gonzalez-Garza MT, Moreno-Cuevas JE, Caro E, Gutierrez-Jimenez E, Segura JJ. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy. 2009;11:26-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Deda H, Inci MC, Kürekçi AE, Sav A, Kayihan K, Ozgün E, Ustünsoy GE, Kocabay S. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy. 2009;11:18-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, Boccaletti R, Testa L, Livigni S, Fagioli F. Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res. 2006;28:523-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 71. | Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Oliveri G, Olivieri C, Pastore I, Marasso R, Madon E. Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:158-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 72. | Mimura T, Dezawa M, Kanno H, Yamamoto I. Behavioral and histological evaluation of a focal cerebral infarction rat model transplanted with neurons induced from bone marrow stromal cells. J Neuropathol Exp Neurol. 2005;64:1108-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 834] [Cited by in F6Publishing: 827] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 74. | Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790-1807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 75. | Yazdani SO, Hafizi M, Zali AR, Atashi A, Ashrafi F, Seddighi AS, Soleimani M. Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy. 2013;15:782-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 76. | Sharma A, Gokulchandran N, Chopra G, Kulkarni P, Lohia M, Badhe P, Jacob VC. Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplant. 2012;21 Suppl 1:S79-S90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114:935-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 78. | Saito F, Nakatani T, Iwase M, Maeda Y, Murao Y, Suzuki Y, Fukushima M, Ide C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor Neurol Neurosci. 2012;30:127-136. [PubMed] [Cited in This Article: ] |
| 79. | Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, Xie Y, Miller FD, Tetzlaff W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545-9559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 80. | Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 461] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 81. | Hunt DP, Morris PN, Sterling J, Anderson JA, Joannides A, Jahoda C, Compston A, Chandran S. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2008;26:163-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 82. | Amoh Y, Mii S, Aki R, Hamada Y, Kawahara K, Hoffman RM, Katsuoka K. Multipotent nestin-expressing stem cells capable of forming neurons are located in the upper, middle and lower part of the vibrissa hair follicle. Cell Cycle. 2012;11:3513-3517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10:830-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 84. | Krejčí E, Grim M. Isolation and characterization of neural crest stem cells from adult human hair follicles. Folia Biol (Praha). 2010;56:149-157. [PubMed] [Cited in This Article: ] |
| 85. | Petit I, Kesner NS, Karry R, Robicsek O, Aberdam E, Müller FJ, Aberdam D, Ben-Shachar D. Induced pluripotent stem cells from hair follicles as a cellular model for neurodevelopmental disorders. Stem Cell Res. 2012;8:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Esmaeilzade B, Nobakht M, Joghataei MT, Rahbar Roshandel N, Rasouli H, Samadi Kuchaksaraei A, Hosseini SM, Najafzade N, Asalgoo S, Hejazian LB. Delivery of epidermal neural crest stem cells (EPI-NCSC) to hippocamp in Alzheimer’s disease rat model. Iran Biomed J. 2012;16:1-9. [PubMed] [Cited in This Article: ] |
| 87. | Cardozo A, Ielpi M, Gómez D, Argibay P. Differential expression of Shh and BMP signaling in the potential conversion of human adipose tissue stem cells into neuron-like cells in vitro. Gene Expr. 2010;14:307-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Abdanipour A, Tiraihi T. Induction of adipose-derived stem cell into motoneuron-like cells using selegiline as preinducer. Brain Res. 2012;1440:23-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Lee ST, Chu K, Jung KH, Im WS, Park JE, Lim HC, Won CH, Shin SH, Lee SK, Kim M. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. 2009;66:671-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 90. | Chen J, Tang YX, Liu YM, Chen J, Hu XQ, Liu N, Wang SX, Zhang Y, Zeng WG, Ni HJ. Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther. 2012;18:847-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Safford KM, Rice HE. Stem cell therapy for neurologic disorders: therapeutic potential of adipose-derived stem cells. Curr Drug Targets. 2005;6:57-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Wang TT, Tio M, Lee W, Beerheide W, Udolph G. Neural differentiation of mesenchymal-like stem cells from cord blood is mediated by PKA. Biochem Biophys Res Commun. 2007;357:1021-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Kang JH, Lee CK, Kim JR, Yu SJ, Jo JH, Do BR, Kim HK, Kang SG. Estrogen stimulates the neuronal differentiation of human umbilical cord blood mesenchymal stem cells (CD34-). Neuroreport. 2007;18:35-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Low CB, Liou YC, Tang BL. Neural differentiation and potential use of stem cells from the human umbilical cord for central nervous system transplantation therapy. J Neurosci Res. 2008;86:1670-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Schira J, Gasis M, Estrada V, Hendricks M, Schmitz C, Trapp T, Kruse F, Kögler G, Wernet P, Hartung HP. Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain. 2012;135:431-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671-1679. [PubMed] [Cited in This Article: ] |
| 97. | Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, Field J, Hamilton-Bruce MA, Warming S, Manavis J. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med. 2012;1:177-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 98. | Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80-90. [PubMed] [Cited in This Article: ] |
| 99. | Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009;27:2229-2237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 100. | Martini MM, Jeremias Tda S, Kohler MC, Marostica LL, Trentin AG, Alvarez-Silva M. Human placenta-derived mesenchymal stem cells acquire neural phenotype under the appropriate niche conditions. DNA Cell Biol. 2013;32:58-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Chen L, He DM, Zhang Y. The differentiation of human placenta-derived mesenchymal stem cells into dopaminergic cells in vitro. Cell Mol Biol Lett. 2009;14:528-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Nazarov I, Lee JW, Soupene E, Etemad S, Knapik D, Green W, Bashkirova E, Fang X, Matthay MA, Kuypers FA. Multipotent stromal stem cells from human placenta demonstrate high therapeutic potential. Stem Cells Transl Med. 2012;1:359-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 103. | Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci USA. 2003;100:2426-2431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 370] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 104. | Schmitz G, Leuthäuser-Jaschinski K, Orsó E. Are circulating monocytes as microglia orthologues appropriate biomarker targets for neuronal diseases? Cent Nerv Syst Agents Med Chem. 2009;9:307-330. [PubMed] [Cited in This Article: ] |
| 105. | Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc. 2013;8:1391-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 106. | Heneidi S, Simerman AA, Keller E, Singh P, Li X, Dumesic DA, Chazenbalk G. Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS One. 2013;8:e64752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 107. | Dezawa M. [Tissue repairing cells that exist among mesenchymal stem cells; their potential for cell-based therapy]. Nihon Rinsho. 2011;69:2128-2135. [PubMed] [Cited in This Article: ] |
| 108. | Suzuki K, Mitsutake N, Saenko V, Suzuki M, Matsuse M, Ohtsuru A, Kumagai A, Uga T, Yano H, Nagayama Y. Dedifferentiation of human primary thyrocytes into multilineage progenitor cells without gene introduction. PLoS One. 2011;6:e19354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Südhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |