Published online Apr 26, 2022. doi: 10.4252/wjsc.v14.i4.267
Peer-review started: January 11, 2022
First decision: March 11, 2022
Revised: March 19, 2022
Accepted: April 25, 2022
Article in press: April 25, 2022
Published online: April 26, 2022
Cancer stem cells (CSCs) possess self-renewal and differentiation potential, which may be related to recurrence, metastasis, and radiochemotherapy resistance during tumor treatment. Understanding the mechanisms via which CSCs maintain self-renewal may reveal new therapeutic targets for attenuating CSC resistance and extending patient life-span. Recent studies have shown that amino acid metabolism plays an important role in maintaining the self-renewal of CSCs and is involved in regulating their tumorigenicity characteristics. This review summarizes the relationship between CSCs and amino acid metabolism, and discusses the possible mechanisms by which amino acid metabolism regulates CSC characteristics particularly self-renewal, survival and stemness. The ultimate goal is to identify new targets and research directions for elimination of CSCs.
Core Tip: Amino acid metabolism plays an important role in maintaining the stemness of cancer stem cells (CSCs) and is involved in regulating their self-renewal and differentiation potential. This review summarizes the relationship between CSCs and amino acid metabolism and discusses possible mechanisms via which amino acid metabolism regulates the self-renewal and differentiation potential of CSCs. The ultimate goal is to identify new targets and research directions for elimination of CSCs.
- Citation: Zhang Q, Li W. Correlation between amino acid metabolism and self-renewal of cancer stem cells: Perspectives in cancer therapy. World J Stem Cells 2022; 14(4): 267-286
- URL: https://www.wjgnet.com/1948-0210/full/v14/i4/267.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i4.267
The concept of cancer stem cells (CSCs) has emerged in recent years. CSCs are a population of self-renewing cell types identified in many types of liquid and solid tumors, and persist predominantly in a low pH, low O2, and nutrient-deficient tumor microenvironment (TME)[1,2]. CSCs possess the capability to initiate cancer development, recurrence and metastasis[1-4], and play important roles in radio-, chemo- and immunotherapy resistance[5,6]. The TME is a dynamic milieu comprising of cancer cells and stromal cells[7-9] and provides specific conditions favorable to tumor growth such as low pH, hypoxia, ischemia, and limited nutrients[7]. TME regulates the morphology of cancer cells, induces tumor cell activation and CSC production, mediates immunosuppression, and determines tumor response to treatment[2,7,10-12]. The CSC niche is a part of the TME, in which perivascular, invasive, and hypoxic niches are involved in the generation and maintenance of CSCs[13,14]. CSCs also rebuild the microenvironment by transdifferentiation into vascular endothelial cells, fibroblasts, and pericytes[7,14]. CSCs obtain nutrients from TME to support their proliferation[15]. Owing to the increased interest in CSCs, the role of metabolism in the regulation of CSC biology is now being extensively investigated.
Amino acids are indispensable nutrients for the body and play important roles in TME[16]. The human body contains twenty amino acids, which are divided as essential and nonessential. Among these, eight essential amino acids are obtained from food, as they cannot be synthesized by the body or their rate of synthesis cannot meet the body requirements[17]. Although nonessential amino acids can be synthesized by the body, they are equally or more important than essential amino acids for cancer progression[18]. Arginine and histidine are semi-essential amino acids as their organic synthesis is not sufficient for metabolic requirements, and hence they have to be obtained from the environment[19]. However, some researchers consider cysteine and tyrosine as semi-essential amino acids as well because they can be converted from methionine and phenylalanine in vivo; thus, conversion and food intake can complement each other[20,21]. In this review we have described these four amino acids as nonessential amino acids.
In addition to being the building blocks of proteins, amino acids participate in many biosynthetic pathways as intermediate metabolites[22]. Previously, researchers have studied the relationship between tumors and amino acid metabolism. In tumor cells, nonessential amino acids may act as essential amino acids to meet the requirement of abnormal proliferation[23]. For example, glutamine is considered to be a “conditional” essential amino acid[24], and therefore, it has been proposed that amino acid metabolism-related enzymes may be used to disrupt amino acid metabolism in targeted therapy[25]. Whether CSCs also harbor similar therapeutic targets warrants detailed investigation, but the exact relationship between CSCs and amino acid metabolism is not completely elucidated. This review tries to summarize the relationship between CSC self-renewal and other characteristics and amino acid metabolism to provide new targets for cancer therapy.
In recent years, researchers have focused on the differences in amino acid metabolism between CSCs and tumor cells, in which essential amino acids play a major role. Several studies have investigated the relationship between methionine and tryptophan metabolism and CSCs; however, studies on phenylalanine metabolism are lacking, and those on the metabolism of the other five essential amino acids are limited. In this review, we have attempted to summarize the role of metabolism of these amino acids in CSC self-renewal.
The methionine cycle maintains the balance of methionine levels in vivo. Homocysteine, an intermediate of the methionine cycle, regenerates methionine and tetrahydrofolate (THF) with one-carbon THF (1C-THF) catalyzed by methyltransferase (MTase), while methionine reenters the methionine cycle. THF acts as a carrier in the transfer and utilization of 1C unit, which is crucial for biosynthesis of nucleic acids, DNA stability, and gene expression[26,27]. As conversion of homocysteine to methionine is folic acid-dependent, content of folic acid affects the tumorsphere-forming ability, nucleotide biosynthesis, and DNA methylation in colon cancer cells and glioblastoma cells[27-29]. Cancer cells consume higher amount of methionine than normal cells in some malignant tumors[30,31]; hence, methionine and its derivatives may be labeled with radionuclides in clinics for identification of malignant recurrent glioma, meningioma, as well as prostate cancer and multiple myeloma[28,32-34]. The methionine cycle is enhanced in CSCs of various cancers, such as lung, breast cancer, osteosarcoma, and brain tumor, owing to their disordered proliferation and higher rate of DNA biosynthesis[28,35,36]. As the concentration of methionine increases, the glioblastoma tumorsphere formation ability that supports the self-renewal capacity of CSCs increases; while methionine deprivation not only promotes embryonic stem cell (ESC) differentiation but also weakens clonal formation and tumorigenicity of lung and breast cancer tumorsphere cells, which can be rescued by the re-addition of methionine[28,36]. It was further found that betaine, synthesized from choline, provides methyl group to homocysteine under the action of betaine homocysteine MTas; this in turn leads to the recycling of methionine[29,37,38]. Stem cell reprogramming requires methionine metabolism and the choline/betaine axis to jointly regulate intracellular homocysteine, abnormality in which causes oxidative stress, mitochondrial toxicity, and inflammation[29].
S-adenosine methionine (SAM) is a crucial intermediate of the methionine cycle, which acts as a direct donor of the methyl group and is involved in genome methylation in vivo[28,39,40]. In gastric CSCs, higher methylation of miR-7-5p promoter region reduces its intracellular expression, while in methionine-deficient medium, miR-7-5p expression is up-regulated and inhibits the formation of gastric CSCs by targeting Notch and Hedgehog signaling pathways[41]. Nuclear reprogramming is usually accompanied by an increase in DNA methylation level in ESCs[29]. Methionine adenosyltransferase (MAT) catalyzes the production of SAM and has two isozymes, MATα1 and MATα2[42]. MATα1 is mainly expressed in hepatocytes, while MATα2 is present in extrahepatic tissue[42,43]. MATα2, which maintains the epigenome of CSCs, is a key enzyme involved in the SAM generation in lung, breast, and liver CSCs[36,39,44]. The inhibition of tumorsphere formation and genome methylation by MATα2 inhibitors FIDAS-5 and cycloleucine demonstrates that methionine circulation is necessary to maintain CSC self-renewal and tumorigenicity[36,39]. Another study found that sirtuin 1 (SIRT1), a NAD+-dependent protein deacetylase, regulated methionine metabolism and histone methylation by regulating MYC-mediated MAT expression in mouse ESCs (mESC)[45,46]. Nicotinamide N-methyltransferase (NNMT) catalyzes the transfer of methyl groups from SAM to nicotinamide and is overexpressed in a variety of cancer cells. NNMT promotes cancer cell invasion, migration, and proliferation by inhibiting the methylation potential of cancer cells[47,48]. Overexpression of NNMT in glioblastoma mesenchymal stem-like cells promotes hypomethylation of mesenchymal subtype genes by down-regulating DNA methyltransferase 1 (DNMT1) and DNMT3A[49]. Ras, Stat3, and nuclear factor-kappaB (NF-κB) signaling pathways upregulate NNMT in cancer cells, which may be related to the epithelial-to-mesenchymal transition (EMT)[47]. Several other enzymes that catalyze SAM, such as DNMT1/3L, AMD1, SRM, and MTAP, are downregulated in colon CSCs. The reduction of DNMT1/3L, which catalyzes the transfer of methyl groups from SAM to DNA, leads to the accumulation of SAM in CSCs and thus affects DNA methylation[50].
Overall, maintenance of CSC phenotype mainly requires methionine cycle and folic acid cycle, as they either directly supply CSCs with nutrients or participate in genome methylation as methyl donors. Therefore, reducing the exogenous intake of methionine and folic acid or blocking the methionine cycle may be new therapeutic directions, which are worth investigating[26,51,52].
Tryptophan is a source of the 1C unit and high consumption causes changes in TME. Tryptophan 2,3-dioxygenase (TDO2), a rate-limiting enzyme in tryptophan metabolism, was overexpressed in esophageal CSCs and may promote their production by inducing Oct4 and CD44 expression and activating EGFR pathway, which stimulates EMT and invasion of esophageal CSCs[53,54]. TDO2 is involved in the formation of tumorspheres of esophageal CSCs and TDO2 suppression reduces the size and number of spheres[53]. Indoleamine-2,3-dioxygenase-1 (IDO1), one of two IDO isozymes, is another rate-limiting enzyme that catalyzes the production of kynurenine in tryptophan metabolism. Similar to TDO2, the expression of IDO1 is increased in breast and prostate CSCs as well as mesothelioma stem cells[55]. The increased IDO1 promotes immune escape by depleting tryptophan in TME and inducing the binding of tryptophan catabolites to aryl hydrocarbon receptor (AhR) resulting in regulatory T cell activation; this can be reversed by IDO1 inhibitors such as LW106[55-57]. Additionally, IDO also regulates tumor-related immune responses through molecular stress response pathways, mTOR kinase, and NF-κB pathway[56,58,59]. IDO1 and kynurenine pathway metabolites may promote colon cancer cell proliferation and cancer-therapy resistance by altering the PI3K/Akt and β-catenin pathways, which are known to be beneficial for self-renewal of colon CSCs[60-63]. 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) is a tryptophan metabolite. ITE reduces the expression of Oct4 in CSCs by activating the AhR transcriptional pathway, thereby inducing CSC differentiation and ultimately reducing CSC tumorigenicity[53]. Tryptophan deprivation in TME decreases endogenous ITE level and increases Oct4 expression in CSCs, which subsequently maintains the stemness of CSCs[53,64]. Recent findings on ITE synthesis and stimulation of the AhR transcriptional pathway have provided crucial targets for the treatment of CSCs[64,65]. Tryptophan derivative, melatonin, may inhibit the proliferation and tumorigenicity of glioma stem cells by inhibiting the zeste homologue 2 and Notch pathways that are important for the survival of glioma stem-like cells[66,67]. In conclusion, tryptophan metabolic enzymes or metabolites, rather than tryptophan itself, are more essential for CSC self-renewal and survival[53,56] and provide new directions for eliminating CSCs.
Threonine is involved in the synthesis of nucleotides and is an important nutrient for mESCs[68]. Threonine content was significantly increased during tumorsphere formation of the colon cancer HCT116 cell line, but it was not explored in-depth[69]. Glycine and acetyl-CoA, produced by threonine dehydrogenase (TDH)-mediated threonine metabolism, are involved in various biosynthetic pathways. Additionally, glycine produces 1C-THF, which results in SAM synthesis via the methionine cycle. SAM ultimately regulates epigenetic modifications in ESCs, which play a significant role in their stemness maintenance and self-renewal[68,70]. TDH is highly expressed in mESCs, and inhibition of TDH or depletion of threonine in the growth medium reduces trimethylation of histone H3 Lysine 4 (H3K4me3) and ESC growth[68,71].
Studies on the relationship between lysine metabolism and CSCs are limited. Only a few studies have suggested that lysine metabolism in CD110+ colorectal CSCs not only reduces the production of reactive oxygen species (ROS), which suppress the proliferation of cancer cells, but also maintains the self-renewal of CSCs by activating the Wnt signaling pathway[72,73]. Instead, researchers have extensively investigated the role of epigenetic modification of histone lysine residues in CSCs[74,75], which may be of greater relevance.
Studies on the relationship between leucine or isoleucine metabolism and CSCs are also limited. Several studies have focused on the leucine-rich repeat of G-protein coupled receptor 5, a gastrointestinal CSC biomarker[71,76-78]. A few studies have found that leucine and isoleucine inhibit the stemness and self-renewal of EpCAM+ hepatocellular carcinoma stem cells by activating the mammalian target of rapamycin pathway complex 1 (mTORC1), in addition to enhanced chemotherapy sensitivity[72,79]. But a recent study suggested that the reduction of leucine caused apoptosis of CD13+ CSCs in hepatocellular carcinoma, but the specific mechanism is not yet clear[80].
Valine is reported to be elevated in canine mammary CSCs[72] and a decrease in valine can cause apoptosis of CD13+ CSCs in hepatocellular carcinoma by unknown mechanisms[80]. 3-Hydroxyisobutyryl-CoA hydrolase (HIBCH), which catalyzes 3-hydroxyisobutyryl-CoA to 3-hydroxyisobutyrate, is a key enzyme in valine metabolism and is highly expressed in a colorectal cancer, prostate cancer, and brain tumor. Elevated HIBCH promotes the initiation and progression of colorectal cancer by increasing the proliferation of tumor cells and the resistance to bevacizumab, while reducing cancer cell autophagy[81,82]. In brain tissue with breast cancer metastasis, HIBCH expression was significantly increased in the areas of reactive gliosis associated with metastatic cells, tumor margins, and hemorrhagic areas, which may provide metabolic substrates[82]. Although studies on metabolism of these five amino acids in CSCs is limited, we confirm their involvement in maintaining self-renewal, survival, and drug resistance of CSCs.
Humans do not have a dietary requirement for nonessential amino acids; however, they have crucial roles to play in CSC survival. To date, only one study illustrates the role of histidine metabolism in the central nervous system of Drosophila[83]. This section will further enumerate the roles of other nonessential amino acid metabolism in CSC biology.
Serine and glycine are commonly obtained via a branch of glycolysis and subsequent biosynthetic pathways. They can be interconverted by serine hydroxymethyl transferase (SHMT1/2), and participate in the folic acid cycle by providing a carbon unit[84]. Hence, in this review, we have jointly discussed the relationship between serine and glycine metabolism and CSCs. In colon CSCs, canine mammary CSCs, and neuroblastoma stem-like cells, the level of glycine is significantly higher than that in normal cancer cells[69,72,85-87]. If levels of glycine in colon CSC spheres are reduced, EMT suppression and induction of CSC apoptosis will occur[69]. Glycine decarboxylase (GLDC) is highly expressed in several cancers, including lung, ovarian, cervical, prostate, lymphoma, and breast except gastric cancer, catalyzes the conversion of glycine to 1C-THF, and participates in the methionine cycle[36,84,88-90]. The silent GLDC in gastric cancer may be due to hypermethylation of CpG islands in the promoter region of GLDC, which causes invasion and migration of gastric cancer cells[90,91]. GLDC is also related to bone metastases from breast cancer and may increase the aggressiveness of malignant tumors by aiding their metabolic adaptation to hypoxia[89,92]. Overexpressed GLDC in non-small cell lung CSCs alters glycolysis, promotes cellular transformation and synthesis of pyrimidines for cell proliferation that eventually promotes tumorigenesis[88,90]. GLDC knockout suppresses colony formation and CD166 on the surface markers of lung CSCs and reduces tumorigenicity[36,88]. Glycine metabolism via glycine and GLDC is a requirement to drive CSCs and promote tumorigenesis[72,88]. Recently, it was found that a new splice variant of GLDC is overexpressed in non-small cell lung CSCs; its tumorigenic ability is similar to that of GLDC and can be exerted by activating MAPK/ERK signaling pathway and regulating cyclin[93]. The binding of c-Myc to GLDC promoter also results in GLDC overexpression in ESCs, which is critical for maintaining their stemness by adjusting H3K4me3 levels; however, whether this is related to c-Myc in CSCs is not yet clear[94-96]. In glioblastoma multiforme, GLDC knockdown results in conversion of excess glycine into toxic aminoacetone and methylglyoxal by glycine C-acetyltransferase (GCAT), leading to highly expressing SHMT2 cell growth arrest[97]. Importantly, GCAT silencing and preemptive knockdown of SHMT2 can suppress the toxicity due to GLDC knockdown[97]. Thus, excess glycine is probably toxic to CSCs, and inducing excessive accumulation of glycine in CSC cytoplasm may be a new treatment strategy for glioblastoma[98].
Serine is involved in nucleotide and one-carbon unit biosynthesis. It plays an important role in tumor cell proliferation and is found at a high level in colorectal CSCs, ovarian clear cell adenocarcinoma, cervical squamous cell carcinoma, and neuroblastoma stem-like cells[69,87,99]. In ovarian clear cell adenocarcinoma and cervical squamous cell carcinoma stem-like cells, the high levels of serine are accompanied by elevated levels of aspartate, glutamate, and glutamine, all of which are involved in the tricarboxylic acid cycle (TCA cycle)[99]. In neuroblastoma stem-like cells, upregulated activating transcription factor 4 can activate genes of the glycine/serine pathway to promote formation of tumorspheres[87,100]. Moreover, in melanoma stem-like cells, up-regulated phosphoenolpyruvate carboxykinase, an enzyme in gluconeogenesis, promotes tumorigenesis by promoting glycolysis and serine/glycine pathway[101]. Phosphoglycerate dehydrogenase (PHGDH) is the first key enzyme in the glycolytic serine biosynthetic pathway and is overexpressed in breast cancer[102-104]. The serine synthesized by PHGDH is converted to glycine by SHMT and then forms glutathione (GSH) to maintain intracellular redox balance[105,106]. PHGDH was found to be preferentially expressed in hypoxia-induced breast CSCs and preserved the breast CSC stemness by maintaining the balance of redox reactions and shunting a portion of glucose-derived 3-phosphoglycerate[103,104]. The shunt from glucose metabolism to serine metabolism produces NADPH, which can maintain the reduced state of GSH and forms an antioxidant barrier in breast CSCs[104,107]. Additionally, high intra-tumoral co-expression of PHGDH and Oct4 in NT2/D1 (embryonal carcinoma stem-like cells) is beneficial for the survival of CSCs[103]. PHGDH can interact with kinesin family member 15, which is overexpressed in liver cancer cells and liver CSCs, and increase its stability to promote the liver CSC phenotype[108]. Because CSCs are more dependent on mitochondrial metabolic pathways than glycolytic pathways, mitochondrial inhibitors can limit their growth[107,109,110]. However, increased intracellular PHGDH expression was observed after the use of mitochondrial inhibitors, indicating that PHGDH may play a protective role against mitochondrial inhibitors in CSCs. Additionally, increasing the intake of exogenous serine or synthesis of intracellular serine also countervails the damage to CSCs caused by mitochondrial inhibitors[107]. PHGDH deficiency suppresses tumorsphere formation and reduces expression of stem factors (Oct4, Sox2, Nanog, Bmi-1) in breast CSCs, embryonal carcinoma, and brain tumor stem-like cells, and also impairs metastasis from breast to lung and increases chemotherapy sensitivity[103,104,106]. Mechanistically, the inhibition of PHGDH not only results in redox imbalance but also promotes the differentiation of CSCs through the degradation of Oct4 and the differential ubiquitination of β3-tubulin; it also promotes p-AMPK mediated-Beclin-1 dependent autophagy in a p-mTOR-independent manner. These findings suggest that PHGDH is necessary for maintaining CSC stemness and self-renewal and may be a new metabolic target for eradication of CSCs[103,104,108]. Other enzymes, including SHMT1/2, phosphoserine phosphatase, phosphoserine aminotransferase, and GCAT, required for glycine/serine metabolism, are up-regulated in non-small cell lung CSCs with different amplitudes and promote tumorigenesis by up-regulating glycine/serine metabolism[88].
Glutamate and glutamine, often upregulated in CSCs, have an amine group (-NH2) difference and glutamine is converted to glutamate via deamination by glutaminase (GLS), which constitutes the first step of glutaminolysis[111]. Glutaminolysis, a series of reactions in which glutamine is degraded to produce metabolic components and energy, may either replace or complement glucose dependence of cancer cells and CSCs[111-113]. Glutamine and glutamate are structurally similar and their roles in vivo are interrelated[114]. Glutamine is used in the biosynthesis of nucleotides, lipids, and amino acids; glutamate forms α-ketoglutarate (α-KG) catalyzed by glutamate dehydrogenase (GDH or GLUD), thereby producing ATP for cellular activities. Interestingly, the biological functions of both these amino acids may be specific to the cancer types[115-118].
Glutamine acts as a “conditionally” essential amino acid in multiple CSCs because the biosynthesis of three major nutrients and nucleic acids requires glutamine to provide the source of carbon and amino nitrogen[99,111,112,117,119]. The glutamine transporter ASCT2 (also known as SLC1A5), encoded by SLC1A5, is highly expressed in various CSCs and is associated with tumor progression and poor prognosis[120,121]. CD9-mediated ASCT2 plasma membrane localization increases glutamine uptake and provides energy for CSC growth in pancreatic ductal adenocarcinoma[122]. The up-regulation of MYC-regulated ASCT2 and GLS1 in colorectal CSCs increases glutamine metabolism and metformin resistance[118,123-125]. MYC in CSCs is regulated by the tumor suppressor TP73/p73, and loss of TP73/p73 reduces the expression of MYC and GLS, thereby inhibiting ASCT2 and reducing glutamine-uptake and glutamine metabolism[126,127]. ASCT2 also activates the downstream mTORC1 signaling pathway to promote the growth of prostate cancer cells or melanoma cells by increasing glutamine uptake[128,129]. Of the other SLC1A family members, the upregulation of SLC1A3 (also called glutamate aspartate transporter, GLAST) in CD133+ thyroid CSCs depends on the activation of the NF-κB pathway; SLC1A3 expression in glioblastoma stem-like cells depends on the activation of the STAT3 pathway triggered by glutamate, whereas SLC1A6 that acts as a glutamate exporter is down-regulated in EMT[130-132]. The higher glutamine and glutamate levels in ovarian clear cell adenocarcinoma and cervical squamous cell carcinoma stem-like cells are related to TCA cycle; in glioblastoma stem-like cells with high GLS expression, GLS inhibition attenuates the influx of glutamine metabolites into the TCA cycle[99,111]. Exogenous glutamine via GLS induces tumorsphere formation and expression of ALDH, a stem cell marker of head and neck squamous cell carcinoma, which can be prevented by glutamine deprivation and GLS inhibitors[112]. Glutamine not only promotes the expression of CSC markers and self-renewal potential of pancreatic CSCs, but also increases radiotherapy resistance by maintaining ROS stability[118,133]. Glutamine also promotes clonogenic formation and stemness marker expression in non-small cell lung CSCs and hepatocellular carcinoma CSCs via the maintenance of redox balance and activation of the Wnt/β-catenin signaling pathway[119,134]. Additionally, the AMPK-mTOR pathway is involved in the regulation of glutamine metabolism on the metformin sensitivity of colorectal CSCs; in absence of glutamine, the activation of AMPK and inhibition of mTOR will increase the sensitivity of metformin-resistant SW620 colorectal CSCs to metformin; however, as metformin-sensitive HT29 CSCs have an activated AMPK pathway, inhibition of glutamine metabolism will enhance the inhibitory effect of metformin[118,125]. Moreover, mTOR inhibition in ovarian clear cell adenocarcinoma stem-like cells in absence of glutamine confirms that glutamine regulates CSCs through the mTOR pathway[135]. The ammonia molecule released by glutaminolysis also neutralizes the excess acid produced by the Warburg effect in epithelial CSCs, in which stemness and EMT are uncoupled[136]. α-KG, another metabolite produced during glutaminolysis in ESCs, regulates the demethylation of DNA/histone to maintain pluripotency[137]. If the overexpressed GDH1 is suppressed in CSCs, the level of α-KG will be reduced, which not only reduces the production of ATP but also produces a large amount of ROS to damage CSCs[132].
GLS has two isoenzyme forms, GLS1 and GLS2; GLS1 is a tumor promoter in many cancers, while GLS2 appears to be a tumor suppressor[123,134,138]. Recently, studies have found that GLS1 induced by distal-less homeobox-2 promotes the progression of transcription factor Snail-mediated EMT by negatively regulating p53 in colon and breast cancer. However, GLS2 inhibits Snail to prevent EMT in hepatocellular carcinoma independent of glutaminase activity; during breast cancer EMT, GLS2 and glutamine utilization are reduced, which can be rescued by the suppression of transcription factor, FOXC2[117,123,139,140]. In intrahepatic cholangiocarcinoma and lung cancer, the expression of GLS1 is negatively correlated with the expression of E-cadherin but positively correlated with that of vimentin, and cells with low E-cadherin/high vimentin are more sensitive to GLS1 inhibitors[138,141]. Additionally, aspartate aminotransferase (GOT1) is upregulated and system L-type amino acid transporter 1 is down-regulated in ovarian clear cell adenocarcinoma stem-like cells[135,142]. Glutamine depletion and ASCT2/SLC1A3/GLS/GDH/GOT1 inhibition increase CSC apoptosis and sensitivity to therapy, all of which are new ways for CSC therapy[111,119,128,133,134]. For instance, GLS inhibitors CB839 and compound 968 suppress cloning ability of high GLS-expressing glioblastoma stem-like cells and reduce expression of stemness marker CD133. CB839 also selectively leads to cell cycle arrest without inducing apoptosis[111]. Other GLS inhibitors, such as BPTES and Zaprinast, effectively sensitizes pancreatic CSCs to radiotherapy and induces apoptosis through intracellular ROS accumulation[133]. SLC1A3 knockdown reduces intracellular glutamate levels and inhibits the self-renewal activity and tumorigenicity of CD133 + thyroid CSCs; SLC1A3 inhibitor UCPH-101 induces apoptosis of glioblastoma stem-like cells[130,131].
Cysteine is a special amino acid that can be obtained not only from cystine conversion but also via homocysteine transsulfuration[31,143]. The cystathionine produced from homocysteine by cystathionine β-synthase (CBS) is further converted to cysteine by cystathionine γ-lyase (CGL). Cysteine then produces GSH so as to maintain the redox balance[143]. Increased homocysteine to cysteine metabolism is observed in tamoxifen-resistant breast cancer, and both CBS and CGL are significantly upregulated in CD133+ colon CSCs[50,144]. The cystine–glutamate antiporter xCT (SLC7A11) on the cell membrane, which is stabilized by CD44/CD44 variant (CD44v) and overexpressed in breast CSCs, is associated with cystine intake as well as cysteine and GSH production[145-149]. CD133 in liver CSCs, CD44v in lung CSCs, and CD44v8-10 in esophageal squamous cell carcinoma and urothelial cancer stem-like cells upregulate or stabilize xCT against intercellular ROS, and overexpressed CD44v in lung CSCs is not related to stem-like properties[150-153]. Inhibition of xCT leads to changes in redox levels of breast CSCs, decreased survival rate, and reduced self-renewal[146,147]. Sulfasalazine (SSZ), an inhibitor of xCT, selectively inhibits CD44+/CD44v+ CSCs, such as those in gastrointestinal tumors, metastatic bladder cancer, esophageal squamous cell carcinoma, and glioma, decreases intracellular GSH levels, and increases ROS levels[145,152,154-156]. The same SSZ effect also occurs in CD133+ liver CSCs[150,157]. In CD44vhigh head and neck squamous cell carcinoma cells, the cytotoxicity of SSZ depends on ASCT2-dependent glutamine uptake and GDH-mediated production of α-KG; GDH depletion and ASCT2 inhibition not only significantly attenuate SSZ-induced intracellular ROS accumulation but also weaken the inhibitory effect of SSZ on cell survival[120]. A phase I study on combined drug therapy in advanced non-small cell lung cancer (UMIN000017854) proposed that SSZ 1.5 g/day can be safely used in combination with standard-dose cisplatin and pemetrexed, but its side effects include intestinal toxicity and limited absorption[158]. Another phase 1 study in patients with refractory cisplatin CD44v+ gastric cancer (UMIN000015595) showed that a combination of 6 g dose of SSZ and cisplatin is feasible, but side effects and disappearance of the inhibitory effect of SSZ on xCT after oral administration were reported[159]. In addition, vaccines against xCT antigens induce xCT antibody production that mediates antibody-dependent cell cytotoxicity, resulting in redox imbalance, inhibition of breast CSC phenotype and self-renewal, increased chemosensitivity, delay in primary tumor growth, and impaired pulmonary metastasis[146,147,160,161]. Therefore, targeted inhibition and immunotargeting therapy of xCT promotes CSC apoptosis, which may provide new methods for adjuvant anti-cancer therapy[147,150,154,155,160]. Glutamate cysteine ligase (GCL) catalyzes condensation of cysteine, produced by above mentioned pathways, and glutamate to form γ-glutamyl-cysteine, which reacts with glycine to form GSH by the action of glutathione synthetase[162]. GCL is composed of a catalytic subunit (GCLC) and a modifier subunit (GCLM); upregulated GCLC, regulated by nuclear factor erythroid-derived 2-like 2, in breast CSCs can mediate the production of GSH to upregulate the expression of FoxO3a and Bmi-1, which are essential for maintaining stemness, whereas GCLM is induced in a HIF-1 dependent manner during chemotherapy or hypoxia[162,163]. In short, GSH is the key to elucidating the role of cysteine metabolism in CSCs.
Aspartate and asparagine differ in cellular functions owing to their structural differences. In tumor cells, asparagine is involved in the synthesis of proteins and is a nitrogen source for the synthesis of purines and pyrimidines[164]. Recently, studies have focused on the regulatory role of asparagine in cancers. Asparagine regulates the cellular adaptation to glutamine depletion and inhibits glutamine depletion-mediated apoptosis; in case of sufficient availability of other amino acids, asparagine depletion also causes apoptosis[165]. Asparagine synthetase, which synthesizes asparagine from glutamine, is associated with tumorigenesis in lung cancer and poor prognosis in glioma and neuroblastoma as well as plays a crucial role in glutamine-dependent survival[165,166]. Asparagine can also be used as an exchange factor for cellular uptake of amino acids, such as serine, arginine, and histidine, thereby activating mTORC1 and regulating amino acid metabolism[167]. Aspartate is also involved in the synthesis of nucleotides[168]. Aspartate and asparagine are upregulated in osteosarcoma stem-like cells, and GOT1, an enzyme that converts aspartate to oxaloacetate, is upregulated in ovarian clear cell adenocarcinoma stem-like cells[35,135,169]. The upregulated aspartate in ovarian clear cell adenocarcinoma and cervical squamous cell carcinoma stem-like cells may be involved in TCA cycle reactions[99]. Although research on aspartate and asparagine in CSCs is limited, their established functions in cancer cells provides a basis for further research on CSCs.
A recent study revealed that alanine and proline levels are increased in canine mammary CSCs, which may be related to maintenance of stemness[72]. Over-expression of glutamic pyruvate transaminase 2, which catalyzes the reaction between alanine and α-KG to form pyruvate and glutamate, reduces the level of α-KG in cells, thereby leading to proline hydroxylase 2 activity inhibition and HIF-1α stabilization. HIF-1α in turn activates the sonic hedgehog signaling pathway and promotes breast cancer tumorigenesis and CSC growth[170,171]. Arginine and proline metabolism are upregulated in osteosarcoma stem-like cells. The level of arginine and ornithine, converted from arginine via arginase, increases significantly and participates in cell proliferation and urea cycle[35]. Addition of proline to Dulbecco's minimum essential media allows ESC to maintain pluripotency. Moreover, proline also induces ESC transformation to mesenchymal-like state and genome-wide reprogramming involving H3K9 and H3K36 methylation[172,173]. TP73/p73 regulates proline metabolism in CSCs; loss of TP73/p73 reduces proline synthesis by inhibiting pyrroline-5-carboxylate reductase 1, which catalyzes proline formation from pyrroline-5-carboxylate[126]. In addition, proline metabolism also plays an important role in the self-renewal of human breast CSCs via proline dehydrogenase (PRODH), and inhibiting PRODH damages spheroidal growth and metastasis[72]. CD13+ CSCs are habituated to tyrosine metabolism in hepatocellular carcinoma; acetyl-CoA, produced by tyrosine metabolism, not only enters the TCA cycle to provide energy to CD13+ CSCs, but also promotes the transcription factor Foxd3 acetylation to maintain the CD13+ CSC self-renewal[80]. Due to the lack of phenylalanine hydroxylase in CD13+ CSCs, deprivation of phenylalanine has no effect on cell survival[80]. Thus, the metabolism of arginine, alanine, proline, and tyrosine seem to be necessary in CSCs of specific cancer species.
In addition to regulating CSCs, amino acid metabolism is also interconnected with TME or CSC niches as TME plays an important role in maintaining the self-renewal of CSCs[119,174]. As microenvironments of different cancer types exhibit variable conditions (glucose concentration and oxygen tension), CSCs display diverse metabolic phenotypes to adapt to these microenvironments[113,175-177]. For instance, in the absence of glucose, glutamine compensates for the shortage of glucose[12,113]. However, in the absence of glutamine, extracellular asparagine becomes critical because intracellular asparagine is redirected to glutamine synthesis to avoid apoptosis[165,178,179]. Glutamine metabolism is also affected in TME as interleukin-4 (IL-4), secreted by immune cells, increases ASCT2 expression in breast cancer cells[180,181]. Growth factor IL-3 in TME, through binding to IL-3Rα, up-regulates ASCT2 expression and promotes glutamine uptake via the JAK/STAT pathway[180,182]. Hypoxic microenvironment causes the accumulation of lactate, which affects ASCT2 and GLS1 expression by activating c-Myc[180,183]. Glutamine-dependent ovarian cancer cells form a glutamine loop with cancer-associated fibroblasts (CAFs) within TME; tumor cells convert glutamine to glutamate, which is regenerated into glutamine by CAFs to supply to tumor cells[12,184]. CAFs also secrete cysteine and GSH, which are absorbed by ovarian cancer cells to induce resistance to platinum-based chemotherapy. However, drug resistance induced by TME is destroyed by effector T cells, which suppress xCT expression of CAFs through the JAK/STAT1 pathway[180,185].
Cancer cell metabolism produces an acidic, hypoxic, and malnourished TME, which is detrimental to the antitumor immune response[186]. The main amino acids in the tumor immune microenvironment are tryptophan and arginine, whose increased catabolism is a common marker of TME[187]. Cells that decompose tryptophan and arginine, such as myeloid-derived suppressor cells and tumor-associated dendritic cells, induce regulatory T cells and suppress effector T cells to suppress antitumor immunity and promote tumorigenesis[187,188]. Interestingly, tryptophan has a significant effect on T cell survival and function[189]. Tumor cells that overexpress IDO show reduced extracellular tryptophan, which affects the effector function of T cells[189]. Whereas kynurenine, an immunosuppressive product of tryptophan metabolism, induces CD4+ T cells to differentiate into regulatory T cells by activating AhR, which weakens the ability of the immune system to recognize and kill cancer cells[178,190]. However, it does not seem to be a contradiction, as the expression of IDO, extracellularly consumed tryptophan, and synthesized kynurenine all synergistically inhibit T cell proliferation and activation[188]. The increased IDO1 in breast and prostate CSCs also activates regulatory T cells through the kynurenine pathway to promote immune escape[55]. The presence of arginine promotes the effector function and survival of T cells, which indicates that the lack of arginine in TME leads to T cell dysfunction[12,184,188]. Citrulline and ornithine, downstream metabolites of arginine, also affect T cell activation[178]. Glutamine is not only used for cancer cell metabolism, but also provides nitrogen and carbon sources for active T cells in TME[178,191]. Glutamine metabolism is essential to B cell proliferation and differentiation into plasma cells, and macrophage antigen presentation and phagocytosis[188,189]. Amino acids such as serine and alanine are also critical to the tumor immune microenvironment. Serine provides purines for T cell proliferation, but has no effect on T cell function. Conversely, alanine affects T cell effector function and proinflammatory cytokine secretion by promoting T cell protein synthesis and initial activation[189,192]. In addition to tumor metabolism and immune microenvironment, amino acid metabolism also has an influence on the structural microenvironment. Collagen, the main component of the extracellular matrix in TME, is degraded to proline by the action of metalloproteinases and collagenases. The extracellular proline is an energy source for tumor cells and may be resynthesized into collagen to promote the extracellular matrix remodeling, which is responsible for cancer cell reprogramming[174,193,194]. High-density extracellular collagen matrix also shifts the metabolism of metastatic breast cancer 4T1 cell line from glucose to glutamine[195]. All the above evidence shows that amino acid metabolism, cancer cells or CSCs, and TME form a complex regulatory network that can be efficiently applied in clinical research.
The concept of CSCs, a class of cells with potential for self-renewal and differentiation, was proposed owing to the emergence of recurrence, metastasis, and drug or radiotherapy resistance in tumors, and renders tumor treatment challenging[196]. Currently, researchers are focusing on issues related to the metabolism within CSCs and attempting to identify new research directions and therapeutic targets to eliminate CSC population. In TME, researchers have indicated the involvement of amino acids as a nutrition and energy source, apart from glycolysis, by demonstrating abnormal mitochondrial function in tumor cells[197,198]. Amino acids are not only involved in protein synthesis, but also participate in important biosynthetic pathways as intermediate metabolites. As amino acids are important nutritional components in TME, an increasing number of studies are focusing on the role of amino acids in self-renewal and other biological characteristics of CSCs. This review focuses on the role of amino acid metabolism in CSC biology, particularly self-renewal and their mechanism of action.
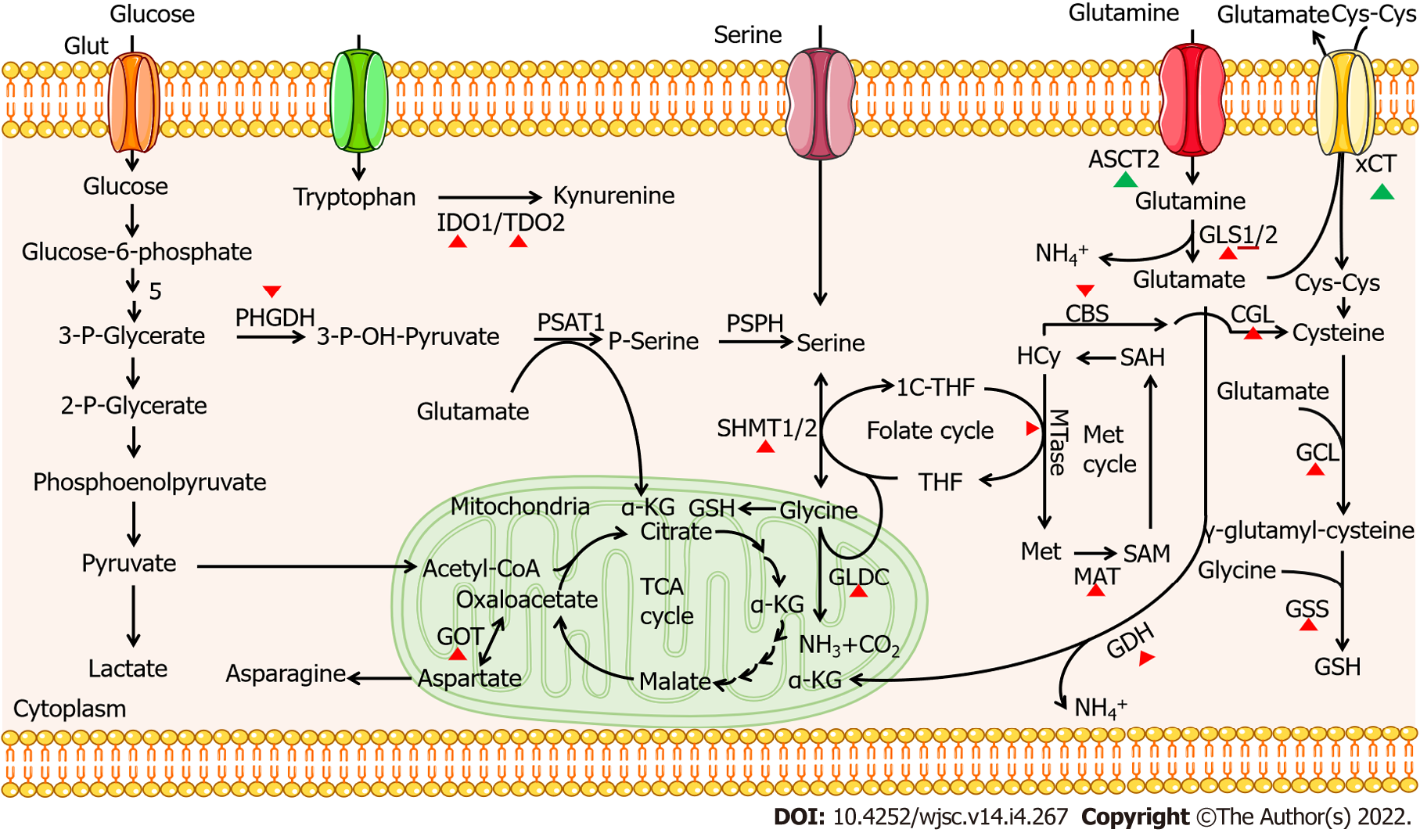
The role of 20 amino acids in CSCs is summarized in Table 1. The metabolism of certain amino acid plays an important role in the self-renewal of CSCs, such as methionine, tryptophan, glycine, serine, and glutamine. The effects of amino acids depletion in TME or inhibition of key enzymes on the self-renewal and survival of CSCs further illustrate the influence of amino acid metabolism on the characteristics of CSCs and provide potential targets for cancer therapy. An increasing number of clinical trials focus on targeting key proteins in amino acid metabolism pathways in CSCs. For example, xCT, a cystine-glutamate antiporter, plays an important role in CSC self-renewal; clinical studies involving its inhibitor, SSZ, in advanced non-small cell lung cancer (UMIN000017854) and refractory cisplatin CD44v+ gastric cancer (UMIN000015595) suggest the potential feasibility of targeting amino acid metabolism transporters for tumor therapy[158,159]. In two clinical cases of CD44v9-positive urogenital cancer, SSZ was also used as a new adjuvant treatment approach[199]. Further, parthenolide and piperlongumine targeting aberrant glutathione metabolism in leukemia stem cells[200]; pegcrisantaspase depleting plasma glutamine and asparagine in relapsed/refractory acute myeloid leukemia[201]; and L-asparaginase exhausting asparagine in acute lymphoblastic leukaemia[202] highlight the extensive prospect of targeting amino acid metabolism in cancer therapy. Additionally, key metabolic enzymes also act as potential targets for CSC-based cancer therapy; they are listed in Table 2 and Figure 1.
| Amino acid metabolism | Possible mechanisms in CSCs | Role in CSC properties |
| Methionine metabolism | Direct nutrients; Genetic modification; DNA biosynthesis | Self-renewal; tumorigenicity |
| Tryptophan metabolism | Immune escape and resistance; regulates stem genes and signal pathway | Self-renewal; survival |
| Threonine metabolism | Upregulated in colon CSCs (HCT116), but mechanism is unknown | Self-renewal |
| Lysine metabolism | Reduces ROS and activates Wnt pathway | Self-renewal |
| Leucine metabolism | Regulates CD13+ CSCs survival in hepatocellular carcinoma, but mechanism is unknown; Inhibits stemness and growth of EpCAM+ hepatocellular carcinoma stem cells by activating MTORC1 | Stemness; survival |
| Valine metabolism | Regulates CD13+ CSCs survival in hepatocellular carcinoma, but mechanism is unknown | survival |
| Phenylalanine metabolism | Unknown | Unknown |
| Isoleucine metabolism | Inhibits stemness and growth of EpCAM+ hepatocellular carcinoma stem cells by activating MTORC1 | Stemness |
| Histidine metabolism | Unknown | Unknown |
| Glycine metabolism | Direct nutrients within a certain range; epigenetic modification; DNA synthesis; regulates redox homeostasis; carries out TCA cycle | Self-renewal; survival; tumorigenicity; metastasis |
| Serine metabolism | Regulates redox homeostasis; shunts glucose metabolism; carries out TCA cycle; influences T cell proliferation | Self-renewal; survival; tumorigenicity; stemness; metastasis; resistance |
| Glutamine metabolism | Direct nutrients; carries out TCA cycle; synthesis of nucleic acids; maintains redox balance; regulates tumor immunity | Self-renewal; survival; tumorigenicity; stemness; resistance |
| Glutamate metabolism | Carries out TCA cycle; participates serine metabolism; maintains redox balance | Self-renewal; survival; tumorigenicity; stemness |
| Cysteine metabolism | Mainly maintains redox balance | Self-renewal; survival; tumorigenicity; resistance; metastasis |
| Aspartate metabolism | Replenishes TCA cycle; synthesis of nucleic acids | Survival |
| Asparagine metabolism | Replenishes TCA cycle; synthesis of nucleic acids; exchanges amino acids | Survival |
| Alanine metabolism | Upregulated in breast CSCs, but mechanism is unknown; regulates T cell function | Self-renewal;stemness; tumorigenicity |
| Arginine metabolism | Participates in cell proliferation and urea cycle; regulates tumor immunity | Self-renewal |
| Proline metabolism | Maybe epigenetic modification and transform steadily; synthesize collagen | Self-renewal; stemness; metastasis |
| Tyrosine metabolism | Provides energy; Foxd3 acetylation | Self-renewal |
| Enzyme | Role in amino acid metabolism | CSC therapy |
| MTase | Translates homocysteine to methionine | Inhibition |
| MATα2 | Induces the production of SAM | Inhibition |
| IDO1 | Catalyzes tryptophan into kynurenine | Inhibition |
| TDO2 | Catalyzes tryptophan into kynurenine | Inhibition |
| GLDC | Catalyzes glycine into NH3, CO2 and CH2-THF | Inhibition (except gastric cancer, better inhibit SHMT and GCAT simultaneously) |
| PHGDH | Catalyzes 3P-glycerate into 3-P-OH-pyruvate | Inhibition |
| SHMT1/2 | Completes the conversion between serine and glycine | Inhibition |
| GLS1 | Catalyzes glutamine into glutamate | Inhibition |
| GDH | Catalyzes glutamate into α-KG | Inhibition |
| CBS | Translates homocysteine to cystathionine | Inhibition |
| CGL | Catalyzes cystathionine to cysteine | Inhibition |
| GCL | Catalyzes the production of γ-glutamyl-cysteine | Inhibition |
| GSS | Catalyzes GSH production | Inhibition |
| GOT1 | Catalyzes the production of oxaloacetate from aspartate | Inhibition |
| GPT2 | Catalyzes transamination between alanine and α-KG to pyruvate and glutamate | Inhibition |
| PRODH | Oxidize proline to glutamate | Inhibition |
In conclusion, the role of amino acid metabolism is varied in different cancer types and metabolism of amino acids are interlinked, which adds to the complexity of TME. Based on these reports, we expect the future research on amino acid metabolism to be based on cancer types, amino acid interrelations, and TME. Only through this research path, can we propose better solutions for CSC clinical therapy and ultimately prolong patient life-expectancy.
We thank all the reviewers for their valuable suggestions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bragança J, Portugal; Saleem S, Pakistan S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 2. | Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234:8381-8395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 3. | Akbulut H, Babahan C, Abgarmi SA, Ocal M, Besler M. Recent Advances in Cancer Stem Cell Targeted Therapy. Crit Rev Oncog. 2019;24:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Najafi M, Mortezaee K, Ahadi R. Cancer stem cell (a)symmetry & plasticity: Tumorigenesis and therapy relevance. Life Sci. 2019;231:116520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Tang T, Guo C, Xia T, Zhang R, Zen K, Pan Y, Jin L. LncCCAT1 Promotes Breast Cancer Stem Cell Function through Activating WNT/β-catenin Signaling. Theranostics. 2019;9:7384-7402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Najafi M, Farhood B, Mortezaee K, Kharazinejad E, Majidpoor J, Ahadi R. Hypoxia in solid tumors: a key promoter of cancer stem cell (CSC) resistance. J Cancer Res Clin Oncol. 2020;146:19-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Najafi M, Goradel NH, Farhood B, Salehi E, Solhjoo S, Toolee H, Kharazinejad E, Mortezaee K. Tumor microenvironment: Interactions and therapy. J Cell Physiol. 2019;234:5700-5721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 8. | Jiang E, Yan T, Xu Z, Shang Z. Tumor Microenvironment and Cell Fusion. Biomed Res Int. 2019;2019:5013592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int J Mol Med. 2016;38:3-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 10. | Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2935] [Cited by in F6Publishing: 3233] [Article Influence: 359.2] [Reference Citation Analysis (0)] |
| 11. | Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, Tuccitto A, Tagliabue E, Castelli C, Rivoltini L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017;43:74-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 12. | Anderson KG, Stromnes IM, Greenberg PD. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer Cell. 2017;31:311-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 434] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 13. | Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 883] [Cited by in F6Publishing: 1021] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 14. | Eun K, Ham SW, Kim H. Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep. 2017;50:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 15. | Natarajan SK, Venneti S. Glutamine Metabolism in Brain Tumors. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Korshunov DA, Kondakova IV, Shashova EE. Modern Perspective on Metabolic Reprogramming in Malignant Neoplasms. Biochemistry (Mosc). 2019;84:1129-1142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Yue M, Jiang J, Gao P, Liu H, Qing G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis. Cell Rep. 2017;21:3819-3832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 18. | Phang JM, Liu W, Hancock C. Bridging epigenetics and metabolism: role of non-essential amino acids. Epigenetics. 2013;8:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Wijnands KA, Castermans TM, Hommen MP, Meesters DM, Poeze M. Arginine and citrulline and the immune response in sepsis. Nutrients. 2015;7:1426-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Ding S, Fang J, Liu G, Veeramuthu D, Naif Abdullah AD, Yin Y. The impact of different levels of cysteine on the plasma metabolomics and intestinal microflora of sows from late pregnancy to lactation. Food Funct. 2019;10:691-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Friedman M, Levin CE. Nutritional and medicinal aspects of D-amino acids. Amino Acids. 2012;42:1553-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Tabe Y, Lorenzi PL, Konopleva M. Amino acid metabolism in hematologic malignancies and the era of targeted therapy. Blood. 2019;134:1014-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 23. | Combs JA, DeNicola GM. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 24. | Bernfeld E, Foster DA. Glutamine as an Essential Amino Acid for KRas-Driven Cancer Cells. Trends Endocrinol Metab. 2019;30:357-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Zou S, Wang X, Liu P, Ke C, Xu S. Arginine metabolism and deprivation in cancer therapy. Biomed Pharmacother. 2019;118:109210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 26. | Hanley MP, Kadaveru K, Perret C, Giardina C, Rosenberg DW. Dietary Methyl Donor Depletion Suppresses Intestinal Adenoma Development. Cancer Prev Res (Phila). 2016;9:812-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Farias N, Ho N, Butler S, Delaney L, Morrison J, Shahrzad S, Coomber BL. The effects of folic acid on global DNA methylation and colonosphere formation in colon cancer cell lines. J Nutr Biochem. 2015;26:818-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Zgheib R, Battaglia-Hsu SF, Hergalant S, Quéré M, Alberto JM, Chéry C, Rouyer P, Gauchotte G, Guéant JL, Namour F. Folate can promote the methionine-dependent reprogramming of glioblastoma cells towards pluripotency. Cell Death Dis. 2019;10:596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Fernández-Arroyo S, Cuyàs E, Bosch-Barrera J, Alarcón T, Joven J, Menendez JA. Activation of the methylation cycle in cells reprogrammed into a stem cell-like state. Oncoscience. 2015;2:958-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Lopci E, Novellis P, Testori A, Rahal D, Voulaz E, Bottoni E, Ferraroli GM, Crepaldi A, Ceresoli GL, Perrino M, Castello A, Alloisio M, Veronesi G, Zucali PA. In-vivo imaging of methionine metabolism in patients with suspected malignant pleural mesothelioma. Nucl Med Commun. 2019;40:1179-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Schrier MS, Trivedi MS, Deth RC. Redox-Related Epigenetic Mechanisms in Glioblastoma: Nuclear Factor (Erythroid-Derived 2)-Like 2, Cobalamin, and Dopamine Receptor Subtype 4. Front Oncol. 2017;7:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Arora G, Sharma P, Sharma A, Mishra AK, Hazari PP, Biswas A, Garg A, Aheer D, Kumar R. 99mTc-Methionine Hybrid SPECT/CT for Detection of Recurrent Glioma: Comparison With 18F-FDG PET/CT and Contrast-Enhanced MRI. Clin Nucl Med. 2018;43:e132-e138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Mesguich C, Zanotti-Fregonara P, Hindié E. New Perspectives Offered by Nuclear Medicine for the Imaging and Therapy of Multiple Myeloma. Theranostics. 2016;6:287-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Hong H, Zhang Y, Sun J, Cai W. Positron emission tomography imaging of prostate cancer. Amino Acids. 2010;39:11-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Zhong Z, Mao S, Lin H, Li H, Lin J, Lin JM. Alteration of intracellular metabolome in osteosarcoma stem cells revealed by liquid chromatography-tandem mass spectrometry. Talanta. 2019;204:6-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Wang Z, Yip LY, Lee JHJ, Wu Z, Chew HY, Chong PKW, Teo CC, Ang HY, Peh KLE, Yuan J, Ma S, Choo LSK, Basri N, Jiang X, Yu Q, Hillmer AM, Lim WT, Lim TKH, Takano A, Tan EH, Tan DSW, Ho YS, Lim B, Tam WL. Methionine is a metabolic dependency of tumor-initiating cells. Nat Med. 2019;25:825-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 37. | Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients. 2013;5:3481-3495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 38. | Robinson JL, McBreairty LE, Randell EW, Brunton JA, Bertolo RF. Restriction of dietary methyl donors limits methionine availability and affects the partitioning of dietary methionine for creatine and phosphatidylcholine synthesis in the neonatal piglet. J Nutr Biochem. 2016;35:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Strekalova E, Malin D, Weisenhorn EMM, Russell JD, Hoelper D, Jain A, Coon JJ, Lewis PW, Cryns VL. S-adenosylmethionine biosynthesis is a targetable metabolic vulnerability of cancer stem cells. Breast Cancer Res Treat. 2019;175:39-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Quinlan CL, Kaiser SE, Bolaños B, Nowlin D, Grantner R, Karlicek-Bryant S, Feng JL, Jenkinson S, Freeman-Cook K, Dann SG, Wang X, Wells PA, Fantin VR, Stewart AE, Grant SK. Targeting S-adenosylmethionine biosynthesis with a novel allosteric inhibitor of Mat2A. Nat Chem Biol. 2017;13:785-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Xin L, Liu L, Liu C, Zhou LQ, Zhou Q, Yuan YW, Li SH, Zhang HT. DNA-methylation-mediated silencing of miR-7-5p promotes gastric cancer stem cell invasion via increasing Smo and Hes1. J Cell Physiol. 2020;235:2643-2654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Murray B, Barbier-Torres L, Fan W, Mato JM, Lu SC. Methionine adenosyltransferases in liver cancer. World J Gastroenterol. 2019;25:4300-4319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Lozano-Rosas MG, Chávez E, Velasco-Loyden G, Domínguez-López M, Martínez-Pérez L, Chagoya De Sánchez V. Diminished S-adenosylmethionine biosynthesis and its metabolism in a model of hepatocellular carcinoma is recuperated by an adenosine derivative. Cancer Biol Ther. 2020;21:81-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Pascale RM, Peitta G, Simile MM, Feo F. Alterations of Methionine Metabolism as Potential Targets for the Prevention and Therapy of Hepatocellular Carcinoma. Medicina (Kaunas). 2019;55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Tang S, Fang Y, Huang G, Xu X, Padilla-Banks E, Fan W, Xu Q, Sanderson SM, Foley JF, Dowdy S, McBurney MW, Fargo DC, Williams CJ, Locasale JW, Guan Z, Li X. Methionine metabolism is essential for SIRT1-regulated mouse embryonic stem cell maintenance and embryonic development. EMBO J. 2017;36:3175-3193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Qiao PF, Yao L, Zeng ZL. Catalpolmediated microRNA34a suppresses autophagy and malignancy by regulating SIRT1 in colorectal cancer. Oncol Rep. 2020;43:1053-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Ulanovskaya OA, Zuhl AM, Cravatt BF. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat Chem Biol. 2013;9:300-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 48. | Sperber H, Mathieu J, Wang Y, Ferreccio A, Hesson J, Xu Z, Fischer KA, Devi A, Detraux D, Gu H, Battle SL, Showalter M, Valensisi C, Bielas JH, Ericson NG, Margaretha L, Robitaille AM, Margineantu D, Fiehn O, Hockenbery D, Blau CA, Raftery D, Margolin AA, Hawkins RD, Moon RT, Ware CB, Ruohola-Baker H. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol. 2015;17:1523-1535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 49. | Jung J, Kim LJ, Wang X, Wu Q, Sanvoranart T, Hubert CG, Prager BC, Wallace LC, Jin X, Mack SC, Rich JN. Nicotinamide metabolism regulates glioblastoma stem cell maintenance. JCI Insight. 2017;2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 50. | Chen KY, Liu X, Bu P, Lin CS, Rakhilin N, Locasale JW, Shen X. A metabolic signature of colon cancer initiating cells. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:4759-4762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Guéant JL, Oussalah A, Zgheib R, Siblini Y, Hsu SB, Namour F. Genetic, epigenetic and genomic mechanisms of methionine dependency of cancer and tumor-initiating cells: What could we learn from folate and methionine cycles. Biochimie. 2020;173:123-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Momparler RL, Côté S. Targeting of cancer stem cells by inhibitors of DNA and histone methylation. Expert Opin Investig Drugs. 2015;24:1031-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Pham QT, Oue N, Sekino Y, Yamamoto Y, Shigematsu Y, Sakamoto N, Sentani K, Uraoka N, Yasui W. TDO2 Overexpression Is Associated with Cancer Stem Cells and Poor Prognosis in Esophageal Squamous Cell Carcinoma. Oncology. 2018;95:297-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Sato F, Kubota Y, Natsuizaka M, Maehara O, Hatanaka Y, Marukawa K, Terashita K, Suda G, Ohnishi S, Shimizu Y, Komatsu Y, Ohashi S, Kagawa S, Kinugasa H, Whelan KA, Nakagawa H, Sakamoto N. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biol Ther. 2015;16:933-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Stapelberg M, Zobalova R, Nguyen MN, Walker T, Stantic M, Goodwin J, Pasdar EA, Thai T, Prokopova K, Yan B, Hall S, de Pennington N, Thomas SR, Grant G, Stursa J, Bajzikova M, Meedeniya AC, Truksa J, Ralph SJ, Ansorge O, Dong LF, Neuzil J. Indoleamine-2,3-dioxygenase elevated in tumor-initiating cells is suppressed by mitocans. Free Radic Biol Med. 2014;67:41-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Wu H, Gong J, Liu Y. Indoleamine 2, 3-dioxygenase regulation of immune response (Review). Mol Med Rep. 2018;17:4867-4873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 57. | Fu R, Zhang YW, Li HM, Lv WC, Zhao L, Guo QL, Lu T, Weiss SJ, Li ZY, Wu ZQ. LW106, a novel indoleamine 2,3-dioxygenase 1 inhibitor, suppresses tumour progression by limiting stroma-immune crosstalk and cancer stem cell enrichment in tumour micro-environment. Br J Pharmacol. 2018;175:3034-3049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 59. | Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 362] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 60. | Bishnupuri KS, Alvarado DM, Khouri AN, Shabsovich M, Chen B, Dieckgraefe BK, Ciorba MA. IDO1 and Kynurenine Pathway Metabolites Activate PI3K-Akt Signaling in the Neoplastic Colon Epithelium to Promote Cancer Cell Proliferation and Inhibit Apoptosis. Cancer Res. 2019;79:1138-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 61. | Thaker AI, Rao MS, Bishnupuri KS, Kerr TA, Foster L, Marinshaw JM, Newberry RD, Stenson WF, Ciorba MA. IDO1 metabolites activate β-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145:416-25.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 62. | Chen J, Shao R, Li F, Monteiro M, Liu JP, Xu ZP, Gu W. PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clin Exp Pharmacol Physiol. 2015;42:1317-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1301] [Cited by in F6Publishing: 1412] [Article Influence: 100.9] [Reference Citation Analysis (1)] |
| 64. | Cheng J, Li W, Kang B, Zhou Y, Song J, Dan S, Yang Y, Zhang X, Li J, Yin S, Cao H, Yao H, Zhu C, Yi W, Zhao Q, Xu X, Zheng M, Zheng S, Li L, Shen B, Wang YJ. Tryptophan derivatives regulate the transcription of Oct4 in stem-like cancer cells. Nat Commun. 2015;6:7209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Kolluri SK, Jin UH, Safe S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch Toxicol. 2017;91:2497-2513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 66. | Zheng X, Pang B, Gu G, Gao T, Zhang R, Pang Q, Liu Q. Melatonin Inhibits Glioblastoma Stem-like cells through Suppression of EZH2-NOTCH1 Signaling Axis. Int J Biol Sci. 2017;13:245-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 67. | Gürsel DB, Berry N, Boockvar JA. The contribution of Notch signaling to glioblastoma via activation of cancer stem cell self-renewal: the role of the endothelial network. Neurosurgery. 2012;70:N19-N21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Chen G, Wang J. A regulatory circuitry locking pluripotent stemness to embryonic stem cell: Interaction between threonine catabolism and histone methylation. Semin Cancer Biol. 2019;57:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Terasaki M, Mima M, Kudoh S, Endo T, Maeda H, Hamada J, Osada K, Miyashita K, Mutoh M. Glycine and succinic acid are effective indicators of the suppression of epithelial-mesenchymal transition by fucoxanthinol in colorectal cancer stem-like cells. Oncol Rep. 2018;40:414-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Chen G, Wang J. Threonine metabolism and embryonic stem cell self-renewal. Curr Opin Clin Nutr Metab Care. 2014;17:80-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T, Unternaehrer JJ, Zhu H, Asara JM, Daley GQ, Cantley LC. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339:222-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 470] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 72. | Michishita M, Saito N, Nozawa S, Furumoto R, Nakagawa T, Sato T, Ochiai K, Azakami D, Katayama K, Nakahira R, Tazaki H, Machida Y, Ishiwata T. Metabolite profiling in sphere-forming cells from canine mammary adenocarcinoma cell lines using gas chromatography-mass spectrometry. J Vet Med Sci. 2019;81:1238-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Wu Z, Wei D, Gao W, Xu Y, Hu Z, Ma Z, Gao C, Zhu X, Li Q. TPO-Induced Metabolic Reprogramming Drives Liver Metastasis of Colorectal Cancer CD110+ Tumor-Initiating Cells. Cell Stem Cell. 2015;17:47-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 74. | Gu B, Lee MG. Histone H3 Lysine 4 methyltransferases and demethylases in self-renewal and differentiation of stem cells. Cell Biosci. 2013;3:39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Völkel P, Dupret B, Le Bourhis X, Angrand PO. Diverse involvement of EZH2 in cancer epigenetics. Am J Transl Res. 2015;7:175-193. [PubMed] [Cited in This Article: ] |
| 76. | Zavros Y. Initiation and Maintenance of Gastric Cancer: A Focus on CD44 Variant Isoforms and Cancer Stem Cells. Cell Mol Gastroenterol Hepatol. 2017;4:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | Nakajima T, Uehara T, Iwaya M, Kobayashi Y, Maruyama Y, Ota H. Characterization of LGR5 expression in poorly differentiated colorectal carcinoma with mismatch repair protein deficiency. BMC Cancer. 2020;20:319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Shekarriz R, Montazer F, Alizadeh-Navaei R. Overexpression of cancer stem cell marker Lgr5 in colorectal cancer patients and association with clinicopathological findings. Caspian J Intern Med. 2019;10:412-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 79. | Nishitani S, Horie M, Ishizaki S, Yano H. Branched chain amino acid suppresses hepatocellular cancer stem cells through the activation of mammalian target of rapamycin. PLoS One. 2013;8:e82346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Sun L, Zhang L, Chen J, Li C, Sun H, Wang J, Xiao H. Activation of Tyrosine Metabolism in CD13+ Cancer Stem Cells Drives Relapse in Hepatocellular Carcinoma. Cancer Res Treat. 2020;52:604-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Shan Y, Gao Y, Jin W, Fan M, Wang Y, Gu Y, Shan C, Sun L, Li X, Yu B, Luo Q, Xu Q. Targeting HIBCH to reprogram valine metabolism for the treatment of colorectal cancer. Cell Death Dis. 2019;10:618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Kalita-de Croft P, Straube J, Lim M, Al-Ejeh F, Lakhani SR, Saunus JM. Proteomic Analysis of the Breast Cancer Brain Metastasis Microenvironment. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Froldi F, Pachnis P, Szuperák M, Costas O, Fernando T, Gould AP, Cheng LY. Histidine is selectively required for the growth of Myc-dependent dedifferentiation tumours in the Drosophila CNS. EMBO J. 2019;38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Kim SK, Jung WH, Koo JS. Differential expression of enzymes associated with serine/glycine metabolism in different breast cancer subtypes. PLoS One. 2014;9:e101004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 85. | Terasaki M, Matsumoto N, Hashimoto R, Endo T, Maeda H, Hamada J, Osada K, Miyashita K, Mutoh M. Fucoxanthin administration delays occurrence of tumors in xenograft mice by colonospheres, with an anti-tumor predictor of glycine. J Clin Biochem Nutr. 2019;64:52-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Terasaki M, Ogawa Y, Endo T, Maeda H, Hamada J, Osada K, Miyashita K, Mutoh M. Glycine Is a Predictor for a Suppressive Effect of Fucoxanthinol on Colonosphere Formation Under Hypoxia. Anticancer Res. 2018;38:2169-2179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Liu M, Xia Y, Ding J, Ye B, Zhao E, Choi JH, Alptekin A, Yan C, Dong Z, Huang S, Yang L, Cui H, Zha Y, Ding HF. Transcriptional Profiling Reveals a Common Metabolic Program in High-Risk Human Neuroblastoma and Mouse Neuroblastoma Sphere-Forming Cells. Cell Rep. 2016;17:609-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 497] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 89. | Zhao C, Lou Y, Wang Y, Wang D, Tang L, Gao X, Zhang K, Xu W, Liu T, Xiao J. A gene expression signature-based nomogram model in prediction of breast cancer bone metastases. Cancer Med. 2019;8:200-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Min HL, Kim J, Kim WH, Jang BG, Kim MA. Epigenetic Silencing of the Putative Tumor Suppressor Gene GLDC (Glycine Dehydrogenase) in Gastric Carcinoma. Anticancer Res. 2016;36:179-187. [PubMed] [Cited in This Article: ] |
| 91. | Zhuang H, Li Q, Zhang X, Ma X, Wang Z, Liu Y, Yi X, Chen R, Han F, Zhang N, Li Y. Downregulation of glycine decarboxylase enhanced cofilin-mediated migration in hepatocellular carcinoma cells. Free Radic Biol Med. 2018;120:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 92. | Berezowska S, Galván JA, Langer R, Bubendorf L, Savic S, Gugger M, Schmid RA, Marti TM. Glycine decarboxylase and HIF-1α expression are negative prognostic factors in primary resected early-stage non-small cell lung cancer. Virchows Arch. 2017;470:323-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Yuan Y, Sun L, Wang X, Chen J, Jia M, Zou Y, Sa H, Cai Y, Xu Y, Sun C, Guo Y, Li H, Ma K. Identification of a new GLDC gene alternative splicing variant and its protumorigenic roles in lung cancer. Future Oncol. 2019;15:4127-4139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Kang PJ, Zheng J, Lee G, Son D, Kim IY, Song G, Park G, You S. Glycine decarboxylase regulates the maintenance and induction of pluripotency via metabolic control. Metab Eng. 2019;53:35-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Arribas-Carreira L, Bravo-Alonso I, López-Márquez A, Alonso-Barroso E, Briso-Montiano Á, Arroyo I, Ugarte M, Pérez B, Pérez-Cerdá C, Rodríguez-Pombo P, Richard E. Generation and characterization of a human iPSC line (UAMi005-A) from a patient with nonketotic hyperglycinemia due to mutations in the GLDC gene. Stem Cell Res. 2019;39:101503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Tian S, Feng J, Cao Y, Shen S, Cai Y, Yang D, Yan R, Wang L, Zhang H, Zhong X, Gao P. Glycine cleavage system determines the fate of pluripotent stem cells via the regulation of senescence and epigenetic modifications. Life Sci Alliance. 2019;2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, Shelton LM, Gui DY, Kwon M, Ramkissoon SH, Ligon KL, Kang SW, Snuderl M, Vander Heiden MG, Sabatini DM. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520:363-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 98. | Kahlert UD, Mooney SM, Natsumeda M, Steiger HJ, Maciaczyk J. Targeting cancer stem-like cells in glioblastoma and colorectal cancer through metabolic pathways. Int J Cancer. 2017;140:10-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 99. | Sato M, Kawana K, Adachi K, Fujimoto A, Yoshida M, Nakamura H, Nishida H, Inoue T, Taguchi A, Takahashi J, Eguchi S, Yamashita A, Tomio K, Wada-Hiraike O, Oda K, Nagamatsu T, Osuga Y, Fujii T. Spheroid cancer stem cells display reprogrammed metabolism and obtain energy by actively running the tricarboxylic acid (TCA) cycle. Oncotarget. 2016;7:33297-33305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 100. | Zhao E, Ding J, Xia Y, Liu M, Ye B, Choi JH, Yan C, Dong Z, Huang S, Zha Y, Yang L, Cui H, Ding HF. KDM4C and ATF4 Cooperate in Transcriptional Control of Amino Acid Metabolism. Cell Rep. 2016;14:506-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 101. | Li Y, Luo S, Ma R, Liu J, Xu P, Zhang H, Tang K, Ma J, Zhang Y, Liang X, Sun Y, Ji T, Wang N, Huang B. Upregulation of cytosolic phosphoenolpyruvate carboxykinase is a critical metabolic event in melanoma cells that repopulate tumors. Cancer Res. 2015;75:1191-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | Pacold ME, Brimacombe KR, Chan SH, Rohde JM, Lewis CA, Swier LJ, Possemato R, Chen WW, Sullivan LB, Fiske BP, Cho S, Freinkman E, Birsoy K, Abu-Remaileh M, Shaul YD, Liu CM, Zhou M, Koh MJ, Chung H, Davidson SM, Luengo A, Wang AQ, Xu X, Yasgar A, Liu L, Rai G, Westover KD, Vander Heiden MG, Shen M, Gray NS, Boxer MB, Sabatini DM. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat Chem Biol. 2016;12:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 103. | Sharif T, Martell E, Dai C, Ghassemi-Rad MS, Lee K, Singh SK, Weaver ICG, Gujar S. Phosphoglycerate dehydrogenase inhibition induces p-mTOR-independent autophagy and promotes multilineage differentiation in embryonal carcinoma stem-like cells. Cell Death Dis. 2018;9:990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Samanta D, Semenza GL. Serine Synthesis Helps Hypoxic Cancer Stem Cells Regulate Redox. Cancer Res. 2016;76:6458-6462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 105. | Zhang T, Gillies MC, Madigan MC, Shen W, Du J, Grünert U, Zhou F, Yam M, Zhu L. Disruption of De Novo Serine Synthesis in Müller Cells Induced Mitochondrial Dysfunction and Aggravated Oxidative Damage. Mol Neurobiol. 2018;55:7025-7037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 106. | Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36:252-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 107. | Subedi A, Muroi M, Futamura Y, Kawamura T, Aono H, Nishi M, Ryo A, Watanabe N, Osada H. A novel inhibitor of tumorspheres reveals the activation of the serine biosynthetic pathway upon mitochondrial inhibition. FEBS Lett. 2019;593:763-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Li Q, Qiu J, Yang H, Sun G, Hu Y, Zhu D, Deng Z, Wang X, Tang J, Jiang R. Kinesin family member 15 promotes cancer stem cell phenotype and malignancy via reactive oxygen species imbalance in hepatocellular carcinoma. Cancer Lett. 2020;482:112-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 109. | Subedi A, Futamura Y, Nishi M, Ryo A, Watanabe N, Osada H. High-throughput screening identifies artesunate as selective inhibitor of cancer stemness: Involvement of mitochondrial metabolism. Biochem Biophys Res Commun. 2016;477:737-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Loureiro R, Mesquita KA, Magalhães-Novais S, Oliveira PJ, Vega-Naredo I. Mitochondrial biology in cancer stem cells. Semin Cancer Biol. 2017;47:18-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 111. | Koch K, Hartmann R, Tsiampali J, Uhlmann C, Nickel AC, He X, Kamp MA, Sabel M, Barker RA, Steiger HJ, Hänggi D, Willbold D, Maciaczyk J, Kahlert UD. A comparative pharmaco-metabolomic study of glutaminase inhibitors in glioma stem-like cells confirms biological effectiveness but reveals differences in target-specificity. Cell Death Discov. 2020;6:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 112. | Kamarajan P, Rajendiran TM, Kinchen J, Bermúdez M, Danciu T, Kapila YL. Head and Neck Squamous Cell Carcinoma Metabolism Draws on Glutaminolysis, and Stemness Is Specifically Regulated by Glutaminolysis via Aldehyde Dehydrogenase. J Proteome Res. 2017;16:1315-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 113. | Chae YC, Kim JH. Cancer stem cell metabolism: target for cancer therapy. BMB Rep. 2018;51:319-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 100] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Tapiero H, Mathé G, Couvreur P, Tew KD. II. Glutamine and glutamate. Biomed Pharmacother. 2002;56:446-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 115. | Tian Y, Du W, Cao S, Wu Y, Dong N, Wang Y, Xu Y. Systematic analyses of glutamine and glutamate metabolisms across different cancer types. Chin J Cancer. 2017;36:88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 116. | Plaitakis A, Kalef-Ezra E, Kotzamani D, Zaganas I, Spanaki C. The Glutamate Dehydrogenase Pathway and Its Roles in Cell and Tissue Biology in Health and Disease. Biology (Basel). 2017;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 117. | Lee SY, Ju MK, Jeon HM, Lee YJ, Kim CH, Park HG, Han SI, Kang HS. Oncogenic Metabolism Acts as a Prerequisite Step for Induction of Cancer Metastasis and Cancer Stem Cell Phenotype. Oxid Med Cell Longev. 2018;2018:1027453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 118. | Peixoto J, Lima J. Metabolic traits of cancer stem cells. Dis Model Mech. 2018;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 119. | Liao J, Liu PP, Hou G, Shao J, Yang J, Liu K, Lu W, Wen S, Hu Y, Huang P. Regulation of stem-like cancer cells by glutamine through β-catenin pathway mediated by redox signaling. Mol Cancer. 2017;16:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 120. | Okazaki S, Umene K, Yamasaki J, Suina K, Otsuki Y, Yoshikawa M, Minami Y, Masuko T, Kawaguchi S, Nakayama H, Banno K, Aoki D, Saya H, Nagano O. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019;110:3453-3463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 121. | Lu H, Li X, Lu Y, Qiu S, Fan Z. ASCT2 (SLC1A5) is an EGFR-associated protein that can be co-targeted by cetuximab to sensitize cancer cells to ROS-induced apoptosis. Cancer Lett. 2016;381:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 122. | Wang VM, Ferreira RMM, Almagro J, Evan T, Legrave N, Zaw Thin M, Frith D, Carvalho J, Barry DJ, Snijders AP, Herbert E, Nye EL, MacRae JI, Behrens A. CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat Cell Biol. 2019;21:1425-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 123. | Ramirez-Peña E, Arnold J, Shivakumar V, Joseph R, Vidhya Vijay G, den Hollander P, Bhangre N, Allegakoen P, Prasad R, Conley Z, Matés JM, Márquez J, Chang JT, Vasaikar S, Soundararajan R, Sreekumar A, Mani SA. The Epithelial to Mesenchymal Transition Promotes Glutamine Independence by Suppressing GLS2 Expression. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 124. | Hanaford AR, Alt J, Rais R, Wang SZ, Kaur H, Thorek DLJ, Eberhart CG, Slusher BS, Martin AM, Raabe EH. Orally bioavailable glutamine antagonist prodrug JHU-083 penetrates mouse brain and suppresses the growth of MYC-driven medulloblastoma. Transl Oncol. 2019;12:1314-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 125. | Kim JH, Lee KJ, Seo Y, Kwon JH, Yoon JP, Kang JY, Lee HJ, Park SJ, Hong SP, Cheon JH, Kim WH, Il Kim T. Effects of metformin on colorectal cancer stem cells depend on alterations in glutamine metabolism. Sci Rep. 2018;8:409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 126. | Sharif T, Martell E, Dai C, Singh SK, Gujar S. Regulation of the proline regulatory axis and autophagy modulates stemness in TP73/p73 deficient cancer stem-like cells. Autophagy. 2019;15:934-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Sharif T, Dai C, Martell E, Ghassemi-Rad MS, Hanes MR, Murphy PJ, Kennedy BE, Venugopal C, Subapanditha M, Giacomantonio CA, Marcato P, Singh SK, Gujar S. TAp73 Modifies Metabolism and Positively Regulates Growth of Cancer Stem-Like Cells in a Redox-Sensitive Manner. Clin Cancer Res. 2019;25:2001-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Wang Q, Hardie RA, Hoy AJ, van Geldermalsen M, Gao D, Fazli L, Sadowski MC, Balaban S, Schreuder M, Nagarajah R, Wong JJ, Metierre C, Pinello N, Otte NJ, Lehman ML, Gleave M, Nelson CC, Bailey CG, Ritchie W, Rasko JE, Holst J. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol. 2015;236:278-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 129. | Wang Q, Beaumont KA, Otte NJ, Font J, Bailey CG, van Geldermalsen M, Sharp DM, Tiffen JC, Ryan RM, Jormakka M, Haass NK, Rasko JE, Holst J. Targeting glutamine transport to suppress melanoma cell growth. Int J Cancer. 2014;135:1060-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 130. | Wang C, Wang Z, Liu W, Ai Z. CD133 promotes the self-renewal capacity of thyroid cancer stem cells through activation of glutamate aspartate transporter SLC1A3 expression. Biochem Biophys Res Commun. 2019;511:87-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Corbetta C, Di Ianni N, Bruzzone MG, Patanè M, Pollo B, Cantini G, Cominelli M, Zucca I, Pisati F, Poliani PL, Finocchiaro G, Pellegatta S. Altered function of the glutamate-aspartate transporter GLAST, a potential therapeutic target in glioblastoma. Int J Cancer. 2019;144:2539-2554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Farris JC, Pifer PM, Zheng L, Gottlieb E, Denvir J, Frisch SM. Grainyhead-like 2 Reverses the Metabolic Changes Induced by the Oncogenic Epithelial-Mesenchymal Transition: Effects on Anoikis. Mol Cancer Res. 2016;14:528-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 133. | Li D, Fu Z, Chen R, Zhao X, Zhou Y, Zeng B, Yu M, Zhou Q, Lin Q, Gao W, Ye H, Zhou J, Li Z, Liu Y. Inhibition of glutamine metabolism counteracts pancreatic cancer stem cell features and sensitizes cells to radiotherapy. Oncotarget. 2015;6:31151-31163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 134. | Li B, Cao Y, Meng G, Qian L, Xu T, Yan C, Luo O, Wang S, Wei J, Ding Y, Yu D. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine. 2019;39:239-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 135. | Sato M, Kawana K, Adachi K, Fujimoto A, Yoshida M, Nakamura H, Nishida H, Inoue T, Taguchi A, Ogishima J, Eguchi S, Yamashita A, Tomio K, Wada-Hiraike O, Oda K, Nagamatsu T, Osuga Y, Fujii T. Targeting glutamine metabolism and the focal adhesion kinase additively inhibits the mammalian target of the rapamycin pathway in spheroid cancer stem-like properties of ovarian clear cell carcinoma in vitro. Int J Oncol. 2017;50:1431-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 136. | Aguilar E, Marin de Mas I, Zodda E, Marin S, Morrish F, Selivanov V, Meca-Cortés Ó, Delowar H, Pons M, Izquierdo I, Celià-Terrassa T, de Atauri P, Centelles JJ, Hockenbery D, Thomson TM, Cascante M. Metabolic Reprogramming and Dependencies Associated with Epithelial Cancer Stem Cells Independent of the Epithelial-Mesenchymal Transition Program. Stem Cells. 2016;34:1163-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 137. | Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 674] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 138. | Cao J, Zhang C, Jiang GQ, Jin SJ, Gao ZH, Wang Q, Yu DC, Ke AW, Fan YQ, Li DW, Wang AQ, Bai DS. Expression of GLS1 in intrahepatic cholangiocarcinoma and its clinical significance. Mol Med Rep. 2019;20:1915-1924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | Bhattacharya D, Scimè A. Metabolic Regulation of Epithelial to Mesenchymal Transition: Implications for Endocrine Cancer. Front Endocrinol (Lausanne). 2019;10:773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 140. | Kuo TC, Chen CK, Hua KT, Yu P, Lee WJ, Chen MW, Jeng YM, Chien MH, Kuo KT, Hsiao M, Kuo ML. Glutaminase 2 stabilizes Dicer to repress Snail and metastasis in hepatocellular carcinoma cells. Cancer Lett. 2016;383:282-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 141. | Ulanet DB, Couto K, Jha A, Choe S, Wang A, Woo HK, Steadman M, DeLaBarre B, Gross S, Driggers E, Dorsch M, Hurov JB. Mesenchymal phenotype predisposes lung cancer cells to impaired proliferation and redox stress in response to glutaminase inhibition. PLoS One. 2014;9:e115144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 142. | Sato M, Kawana K, Adachi K, Fujimoto A, Taguchi A, Fujikawa T, Yoshida M, Nakamura H, Nishida H, Inoue T, Ogishima J, Eguchi S, Yamashita A, Tomio K, Arimoto T, Wada-Hiraike O, Oda K, Nagamatsu T, Osuga Y, Fujii T. Low uptake of fluorodeoxyglucose in positron emission tomography/computed tomography in ovarian clear cell carcinoma may reflect glutaminolysis of its cancer stem cell-like properties. Oncol Rep. 2017;37:1883-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 143. | Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 660] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 144. | Ryu CS, Kwak HC, Lee JY, Oh SJ, Phuong NT, Kang KW, Kim SK. Elevation of cysteine consumption in tamoxifen-resistant MCF-7 cells. Biochem Pharmacol. 2013;85:197-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 145. | Haryu S, Saito R, Jia W, Shoji T, Mano Y, Sato A, Kanamori M, Sonoda Y, Sampetrean O, Saya H, Tominaga T. Convection-enhanced delivery of sulfasalazine prolongs survival in a glioma stem cell brain tumor model. J Neurooncol. 2018;136:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 146. | Bolli E, O'Rourke JP, Conti L, Lanzardo S, Rolih V, Christen JM, Barutello G, Forni M, Pericle F, Cavallo F. A Virus-Like-Particle immunotherapy targeting Epitope-Specific anti-xCT expressed on cancer stem cell inhibits the progression of metastatic cancer in vivo. Oncoimmunology. 2018;7:e1408746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 147. | Quaglino E, Conti L, Cavallo F. Breast cancer stem cell antigens as targets for immunotherapy. Semin Immunol. 2020;47:101386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 148. | Digomann D, Linge A, Dubrovska A. SLC3A2/CD98hc, autophagy and tumor radioresistance: a link confirmed. Autophagy. 2019;15:1850-1851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 149. | Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 865] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 150. | Song Y, Park IS, Kim J, Seo HR. Actinomycin D inhibits the expression of the cystine/glutamate transporter xCT via attenuation of CD133 synthesis in CD133+ HCC. Chem Biol Interact. 2019;309:108713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 151. | Horibe S, Kawauchi S, Tanahashi T, Sasaki N, Mizuno S, Rikitake Y. CD44v-dependent upregulation of xCT is involved in the acquisition of cisplatin-resistance in human lung cancer A549 cells. Biochem Biophys Res Commun. 2018;507:426-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 152. | Kagami T, Yamade M, Suzuki T, Uotani T, Tani S, Hamaya Y, Iwaizumi M, Osawa S, Sugimoto K, Baba S, Sugimura H, Miyajima H, Furuta T. High expression level of CD44v8-10 in cancer stem-like cells is associated with poor prognosis in esophageal squamous cell carcinoma patients treated with chemoradiotherapy. Oncotarget. 2018;9:34876-34888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 153. | Hagiwara M, Kikuchi E, Tanaka N, Kosaka T, Mikami S, Saya H, Oya M. Variant isoforms of CD44 involves acquisition of chemoresistance to cisplatin and has potential as a novel indicator for identifying a cisplatin-resistant population in urothelial cancer. BMC Cancer. 2018;18:113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 154. | Shitara K, Doi T, Nagano O, Imamura CK, Ozeki T, Ishii Y, Tsuchihashi K, Takahashi S, Nakajima TE, Hironaka S, Fukutani M, Hasegawa H, Nomura S, Sato A, Einaga Y, Kuwata T, Saya H, Ohtsu A. Dose-escalation study for the targeting of CD44v+ cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer. 2017;20:341-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 155. | Ogihara K, Kikuchi E, Okazaki S, Hagiwara M, Takeda T, Matsumoto K, Kosaka T, Mikami S, Saya H, Oya M. Sulfasalazine could modulate the CD44v9-xCT system and enhance cisplatin-induced cytotoxic effects in metastatic bladder cancer. Cancer Sci. 2019;110:1431-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 156. | Miyoshi S, Tsugawa H, Matsuzaki J, Hirata K, Mori H, Saya H, Kanai T, Suzuki H. Inhibiting xCT Improves 5-Fluorouracil Resistance of Gastric Cancer Induced by CD44 Variant 9 Expression. Anticancer Res. 2018;38:6163-6170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 157. | Song Y, Jang J, Shin TH, Bae SM, Kim JS, Kim KM, Myung SJ, Choi EK, Seo HR. Sulfasalazine attenuates evading anticancer response of CD133-positive hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2017;36:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 158. | Otsubo K, Nosaki K, Imamura CK, Ogata H, Fujita A, Sakata S, Hirai F, Toyokawa G, Iwama E, Harada T, Seto T, Takenoyama M, Ozeki T, Mushiroda T, Inada M, Kishimoto J, Tsuchihashi K, Suina K, Nagano O, Saya H, Nakanishi Y, Okamoto I. Phase I study of salazosulfapyridine in combination with cisplatin and pemetrexed for advanced non-small-cell lung cancer. Cancer Sci. 2017;108:1843-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 159. | Shitara K, Doi T, Nagano O, Fukutani M, Hasegawa H, Nomura S, Sato A, Kuwata T, Asai K, Einaga Y, Tsuchihashi K, Suina K, Maeda Y, Saya H, Ohtsu A. Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407). Gastric Cancer. 2017;20:1004-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 160. | Donofrio G, Tebaldi G, Lanzardo S, Ruiu R, Bolli E, Ballatore A, Rolih V, Macchi F, Conti L, Cavallo F. Bovine herpesvirus 4-based vector delivering the full length xCT DNA efficiently protects mice from mammary cancer metastases by targeting cancer stem cells. Oncoimmunology. 2018;7:e1494108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 161. | Ruiu R, Rolih V, Bolli E, Barutello G, Riccardo F, Quaglino E, Merighi IF, Pericle F, Donofrio G, Cavallo F, Conti L. Fighting breast cancer stem cells through the immune-targeting of the xCT cystine-glutamate antiporter. Cancer Immunol Immunother. 2019;68:131-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 162. | Lu H, Samanta D, Xiang L, Zhang H, Hu H, Chen I, Bullen JW, Semenza GL. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc Natl Acad Sci U S A. 2015;112:E4600-E4609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 163. | Kim DH, Jang JH, Kwon OS, Cha HJ, Youn HJ, Chun KS, Surh YJ. Nuclear Factor Erythroid-Derived 2-Like 2-Induced Reductive Stress Favors Self-Renewal of Breast Cancer Stem-Like Cells via the FoxO3a-Bmi-1 Axis. Antioxid Redox Signal. 2020;32:1313-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 164. | Zhu Y, Li T, Ramos da Silva S, Lee JJ, Lu C, Eoh H, Jung JU, Gao SJ. A Critical Role of Glutamine and Asparagine γ-Nitrogen in Nucleotide Biosynthesis in Cancer Cells Hijacked by an Oncogenic Virus. mBio. 2017;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 165. | Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR, Pawel B, Baggs J, Cherry S, Rabinowitz JD, Thompson CB. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell. 2014;56:205-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 292] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 166. | Xu Y, Lv F, Zhu X, Wu Y, Shen X. Loss of asparagine synthetase suppresses the growth of human lung cancer cells by arresting cell cycle at G0/G1 phase. Cancer Gene Ther. 2016;23:287-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 167. | Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016;7:11457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 317] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 168. | Patel D, Menon D, Bernfeld E, Mroz V, Kalan S, Loayza D, Foster DA. Aspartate Rescues S-phase Arrest Caused by Suppression of Glutamine Utilization in KRas-driven Cancer Cells. J Biol Chem. 2016;291:9322-9329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 169. | Anglin J, Zavareh RB, Sander PN, Haldar D, Mullarky E, Cantley LC, Kimmelman AC, Lyssiotis CA, Lairson LL. Discovery and optimization of aspartate aminotransferase 1 inhibitors to target redox balance in pancreatic ductal adenocarcinoma. Bioorg Med Chem Lett. 2018;28:2675-2678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 170. | Cao Y, Lin SH, Wang Y, Chin YE, Kang L, Mi J. Glutamic Pyruvate Transaminase GPT2 Promotes Tumorigenesis of Breast Cancer Cells by Activating Sonic Hedgehog Signaling. Theranostics. 2017;7:3021-3033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 171. | Ouyang Q, Nakayama T, Baytas O, Davidson SM, Yang C, Schmidt M, Lizarraga SB, Mishra S, Ei-Quessny M, Niaz S, Gul Butt M, Imran Murtaza S, Javed A, Chaudhry HR, Vaughan DJ, Hill RS, Partlow JN, Yoo SY, Lam AT, Nasir R, Al-Saffar M, Barkovich AJ, Schwede M, Nagpal S, Rajab A, DeBerardinis RJ, Housman DE, Mochida GH, Morrow EM. Mutations in mitochondrial enzyme GPT2 cause metabolic dysfunction and neurological disease with developmental and progressive features. Proc Natl Acad Sci U S A. 2016;113:E5598-E5607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 172. | Phang JM, Liu W, Hancock CN, Fischer JW. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care. 2015;18:71-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 173. | Comes S, Gagliardi M, Laprano N, Fico A, Cimmino A, Palamidessi A, De Cesare D, De Falco S, Angelini C, Scita G, Patriarca EJ, Matarazzo MR, Minchiotti G. L-Proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling H3K9 and H3K36 methylation. Stem Cell Reports. 2013;1:307-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 174. | D'Aniello C, Patriarca EJ, Phang JM, Minchiotti G. Proline Metabolism in Tumor Growth and Metastatic Progression. Front Oncol. 2020;10:776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 175. | Deshmukh A, Deshpande K, Arfuso F, Newsholme P, Dharmarajan A. Cancer stem cell metabolism: a potential target for cancer therapy. Mol Cancer. 2016;15:69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 176. | Bezuidenhout N, Shoshan M. A Shifty Target: Tumor-Initiating Cells and Their Metabolism. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 177. | Libby CJ, Tran AN, Scott SE, Griguer C, Hjelmeland AB. The pro-tumorigenic effects of metabolic alterations in glioblastoma including brain tumor initiating cells. Biochim Biophys Acta Rev Cancer. 2018;1869:175-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 178. | Fahrmann JF, Vykoukal JV, Ostrin EJ. Amino Acid Oncometabolism and Immunomodulation of the Tumor Microenvironment in Lung Cancer. Front Oncol. 2020;10:276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 179. | Pavlova NN, Hui S, Ghergurovich JM, Fan J, Intlekofer AM, White RM, Rabinowitz JD, Thompson CB, Zhang J. As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid. Cell Metab. 2018;27:428-438.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 180. | Yang L, Venneti S, Nagrath D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu Rev Biomed Eng. 2017;19:163-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 327] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 181. | Venmar KT, Kimmel DW, Cliffel DE, Fingleton B. IL4 receptor α mediates enhanced glucose and glutamine metabolism to support breast cancer growth. Biochim Biophys Acta. 2015;1853:1219-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 182. | Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784-2799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 183. | Pérez-Escuredo J, Dadhich RK, Dhup S, Cacace A, Van Hée VF, De Saedeleer CJ, Sboarina M, Rodriguez F, Fontenille MJ, Brisson L, Porporato PE, Sonveaux P. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle. 2016;15:72-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 184. | Sun L, Suo C, Li ST, Zhang H, Gao P. Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. Biochim Biophys Acta Rev Cancer. 2018;1870:51-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 185. | Wang W, Kryczek I, Dostál L, Lin H, Tan L, Zhao L, Lu F, Wei S, Maj T, Peng D, He G, Vatan L, Szeliga W, Kuick R, Kotarski J, Tarkowski R, Dou Y, Rattan R, Munkarah A, Liu JR, Zou W. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell. 2016;165:1092-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 186. | Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, Tam A, Blosser RL, Prchalova E, Alt J, Rais R, Slusher BS, Powell JD. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 582] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 187. | Lemos H, Huang L, Prendergast GC, Mellor AL. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat Rev Cancer. 2019;19:162-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 188. | Li Y, Wan YY, Zhu B. Immune Cell Metabolism in Tumor Microenvironment. Adv Exp Med Biol. 2017;1011:163-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 189. | Cassim S, Pouyssegur J. Tumor Microenvironment: A Metabolic Player that Shapes the Immune Response. Int J Mol Sci. 2019;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 190. | Lyssiotis CA, Kimmelman AC. Metabolic Interactions in the Tumor Microenvironment. Trends Cell Biol. 2017;27:863-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 516] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 191. | Klysz D, Tai X, Robert PA, Craveiro M, Cretenet G, Oburoglu L, Mongellaz C, Floess S, Fritz V, Matias MI, Yong C, Surh N, Marie JC, Huehn J, Zimmermann V, Kinet S, Dardalhon V, Taylor N. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci Signal. 2015;8:ra97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 334] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 192. | Ghosh-Choudhary S, Liu J, Finkel T. Metabolic Regulation of Cell Fate and Function. Trends Cell Biol. 2020;30:201-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 193. | Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, Lac S, Roques J, Lavaut MN, Berthezène P, Rubis M, Secq V, Garcia S, Moutardier V, Lombardo D, Iovanna JL, Tomasini R, Guillaumond F, Vander Heiden MG, Vasseur S. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun. 2017;8:16031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 194. | Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32:795-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 383] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 195. | Morris BA, Burkel B, Ponik SM, Fan J, Condeelis JS, Aguirre-Ghiso JA, Castracane J, Denu JM, Keely PJ. Collagen Matrix Density Drives the Metabolic Shift in Breast Cancer Cells. EBioMedicine. 2016;13:146-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 196. | Bai X, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 197. | Ananieva E. Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J Biol Chem. 2015;6:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 111] [Cited by in F6Publishing: 109] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 198. | Mathupala SP. Metabolic targeting of malignant tumors: small-molecule inhibitors of bioenergetic flux. Recent Pat Anticancer Drug Discov. 2011;6:6-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 199. | Takayama T, Kubo T, Morikawa A, Morita T, Nagano O, Saya H. Potential of sulfasalazine as a therapeutic sensitizer for CD44 splice variant 9-positive urogenital cancer. Med Oncol. 2016;33:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 200. | Pei S, Minhajuddin M, Callahan KP, Balys M, Ashton JM, Neering SJ, Lagadinou ED, Corbett C, Ye H, Liesveld JL, O'Dwyer KM, Li Z, Shi L, Greninger P, Settleman J, Benes C, Hagen FK, Munger J, Crooks PA, Becker MW, Jordan CT. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J Biol Chem. 2013;288:33542-33558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 201. | Emadi A, Kapadia B, Bollino D, Bhandary B, Baer MR, Niyongere S, Strovel ET, Kaizer H, Chang E, Choi EY, Ma X, Tighe KM, Carter-Cooper B, Moses BS, Civin CI, Mahurkar A, Shetty AC, Gartenhaus RB, Kamangar F, Lapidus RG. Venetoclax and pegcrisantaspase for complex karyotype acute myeloid leukemia. Leukemia. 2021;35:1907-1924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 202. | Patil S, Coutsouvelis J, Spencer A. Asparaginase in the management of adult acute lymphoblastic leukaemia: is it used appropriately? Cancer Treat Rev. 2011;37:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |