Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.879
Peer-review started: March 4, 2020
First decision: April 25, 2020
Revised: July 2, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 26, 2020
Mesenchymal stem cells (MSCs) have been reported to possess immune regulatory effects in innate and adaptive immune reactions. MSCs can mediate intercellular communications by releasing extracellular vesicles (EVs), which deliver functional molecules to targeted cells. MSC derived EVs (MSC-EVs) confer altering effects on many immune cells, including T lymphocytes, B lymphocytes, natural killer cells, dendritic cells, and macrophages. A large number of studies have suggested that MSC-EVs participate in regulating autoimmunity related diseases. This characteristic of MSC-EVs makes them be potential biomarkers for the diagnosis and treatment of autoimmunity related diseases.
To verify the potential of MSC-EVs for molecular targeted therapy of autoimmunity related diseases.
Literature search was conducted in PubMed to retrieve the articles published between 2010 and 2020 in the English language. The keywords, such as “MSCs,” “EVs,” “exosome,” “autoimmunity,” “tumor immunity,” and “transplantation immunity,” and Boolean operator “AND” and “NOT” coalesced admirably to be used for searching studies on the specific molecular mechanisms of MSC-EVs in many immune cell types and many autoimmunity related diseases. Studies that did not investigate the molecular mechanisms of MSC-EVs in the occurrence and development of autoimmune diseases were excluded.
A total of 96 articles were chosen for final reference lists. After analyzing those publications, we found that it had been well documented that MSC-EVs have the ability to induce multiple immune cells, like T lymphocytes, B lymphocytes, natural killer cells, dendritic cells, and macrophages, to regulate immune responses in innate immunity and adaptive immunity. Many validated EVs-delivered molecules have been identified as key biomarkers, such as proteins, lipids, and nucleotides. Some EVs-encapsulated functional molecules can serve as promising therapeutic targets particularly for autoimmune disease.
MSC-EVs play an equally important part in the differentiation, activation, and proliferation of immune cells, and they may become potential biomarkers for diagnosis and treatment of autoimmunity related diseases.
Core tip: Mesenchymal stem cells (MSCs) have been reported to possess immunomodulatory effects on autoimmune responses. MSCs can mediate intercellular communications by releasing extracellular vesicles (EVs), which deliver functional molecules to targeted cells. MSC derived EVs (MSC-EVs) exert immunomodulatory effects on many immune cells. A large number of studies have suggested that MSC-EVs and the encapsulated bioactive molecules are potential targets for autoimmune disease, cancer, and other diseases. However, there is still a long way for investigating the molecular mechanism of MSC-EVs in autoimmunity. This review will focus on the immunomodulatory function and underlying mechanism of MSC-EVs in autoimmunity related diseases.
- Citation: Wang JH, Liu XL, Sun JM, Yang JH, Xu DH, Yan SS. Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity: A systematic review. World J Stem Cells 2020; 12(8): 879-896
- URL: https://www.wjgnet.com/1948-0210/full/v12/i8/879.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i8.879
Mesenchymal stem cells (MSCs) are a group of common multipotent progenitor cells, which can be found in bone marrow[1,2], synovium[3,4], umbilical cord[5], and adipose tissue[1,6]. They are characterized by a multilineage differentiation potential and paracrine function[7]. There is growing evidence that MSCs exert immunomodulatory effects through their paracrine function[8], in which multiple small molecules, including extracellular vesicles (EVs), cytokines, chemokines, growth factors, and interleukin(IL), are secreted to the extracellular microenvironment in animal models[9]. Recently, numerous studies demonstrated that MSCs can be used in clinical therapy for immunomodulation and regenerative medicine in vivo and in vitro[10-12]. Despite great improvements in the MSC therapeutic strategies for autoimmune diseases, treatment failures are still common and there is no doubt that it is imperative to carry out more studies to investigate the specific molecular mechanisms. EVs are key components of the paracrine process that play a vital role in intercellular communication by transmitting biological molecules in pathological and physiological conditions.
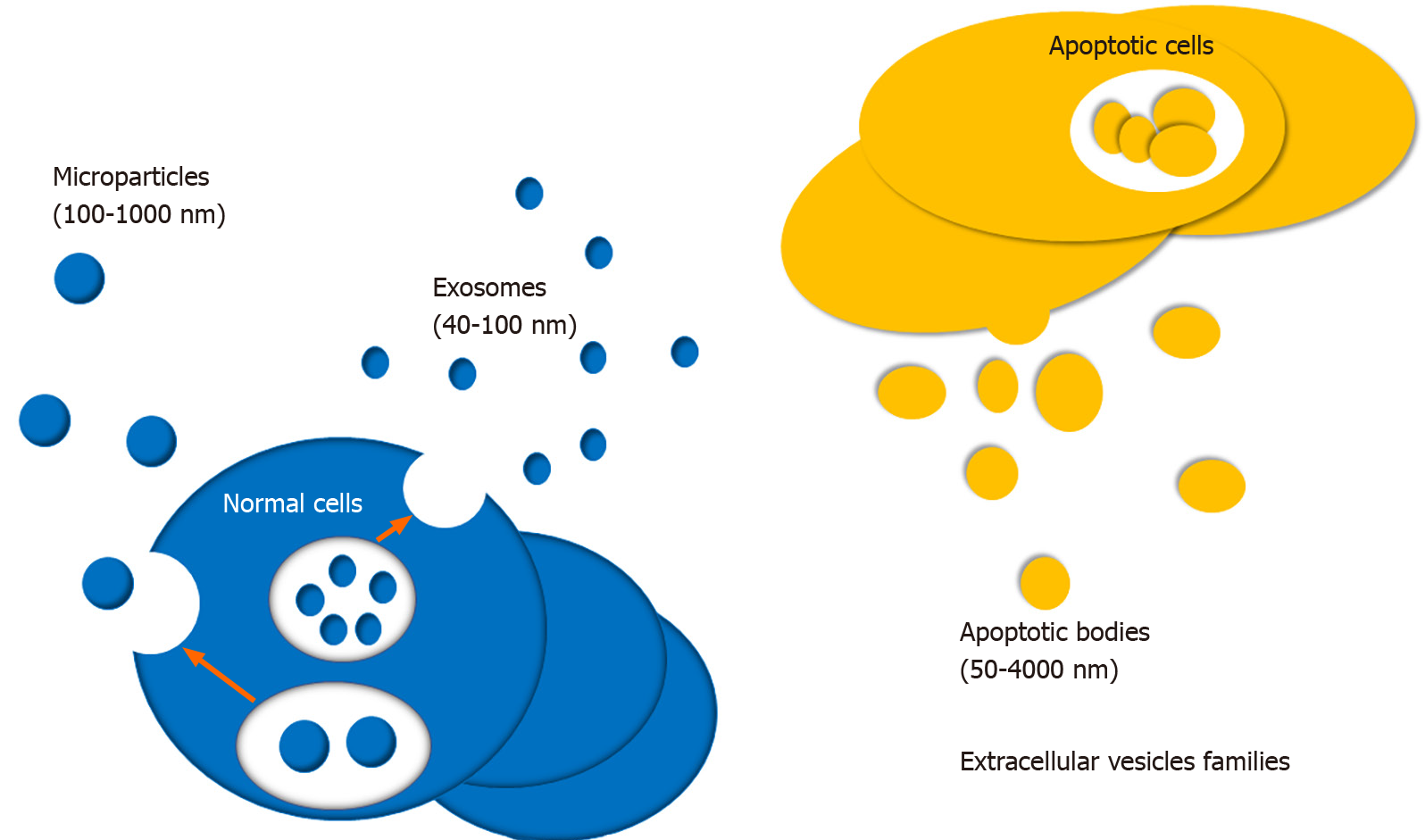
EV is newly identified small vesicle wrapped in lipid membranes, which is widely produced by many cells and secreted into the extracellular microenvironment. In 1967, Wolf first discovered EVs and described them as function-free platelet wastes[13]. EVs can be isolated from various extracellular fluids, like blood, urine, saliva, tear, cerebrospinal fluid, milk, and so on, and various cells, including stem cells[14-18], primary cells of the immune and nervous system[19-22], and multiple cancer cell types[23-25]. Their encapsulated functional molecules can be novel biomarkers and therapeutic targets for many kinds of diseases, for instance, cancer, autoimmune diseases, and neurodegenerative disorders. The role of EVs in immunity and inflammation regulations has been attracting attention during the past few decades. According to diameter, EVs can be divided into three types, including apoptotic bodies, microparticles, and exosomes (Figure 1)[26,27]. Exosomes are the most common EVs with a diameter of 50-100 nm[28]. Exosomes were first discovered in sheep reticulocytes, by electron microscopy[29]. Microparticles, also called microvesicles, are submicronic vesicles with a diameter of 100-1000 nm, which are formed by budding of the cellular membrane after cell stimulation or stress, such as cell activation, apoptosis, and hypoxia. Apoptotic bodies also belong to EVs with a diameter of 50-4000 nm. They are usually released during the stage of cell apoptosis. EVs participate in the intercellular communication by delivering numerous proteins and nucleotides with biological activity, and nucleotides include microRNAs (miRNAs), long non-coding RNAs (lncRNAs), mRNA, and even extra-chromosomal DNA[30,31]. They play vital roles in regulating inflammation, immune response, vascular reactivity, and tissue repair[32,33]. During the past few decades, MSC derived EVs (MSC-EVs) have been implicated in regulating inflammation and autoimmunity[7]. It has been well established that EVs are involved in regulating autoimmune disorders by delivering a large number of bioactive molecules, including cytokines, enzymes, transcription factors, cytokines receptor antagonists, miRNAs, lncRNAs, and circRNAs[34]. MSCs, as a specific group of cells, are multipotent stem cells characterized by immunomodulatory and self-renew properties[35,36]. Cosenza et al[37] have reported the important pathogenic or therapeutic role of MSC-EVs in rheumatic diseases. Therefore, we proposed that MSC-EVs can become potential biotargets for the development of novel molecular targeted drugs in autoimmune related diseases based on the above conclusions. This systematic review will provide in-depth knowledge of biogenesis and functional roles of MSC-EVs, especially exosomes, in autoimmunity.
The key words “MSCs,” “EVs,” “exosome,” “autoimmunity,” “tumor immunity,” and “transplantation immunity” were used to retrieve relevant articles published in English from 2010 to 2020 in PubMed database. Besides, Boolean operator “AND” and “NOT” were combined admirably with those keywords to search the related articles. Reference lists from those articles were reviewed to exclude irrelevant articles. Manuscripts available were reviewed and recognized by using document management tool. All available information was obtained by skimming the abstracts of searched articles. Data were analyzed using descriptive statistics.
All repetitive documents were excluded, and the remainder needed to be restored for reading. Nevertheless, full text retrieval was performed due to many documents with unavailable abstract.
This article was a systematic review and no statistical method was used in this article.
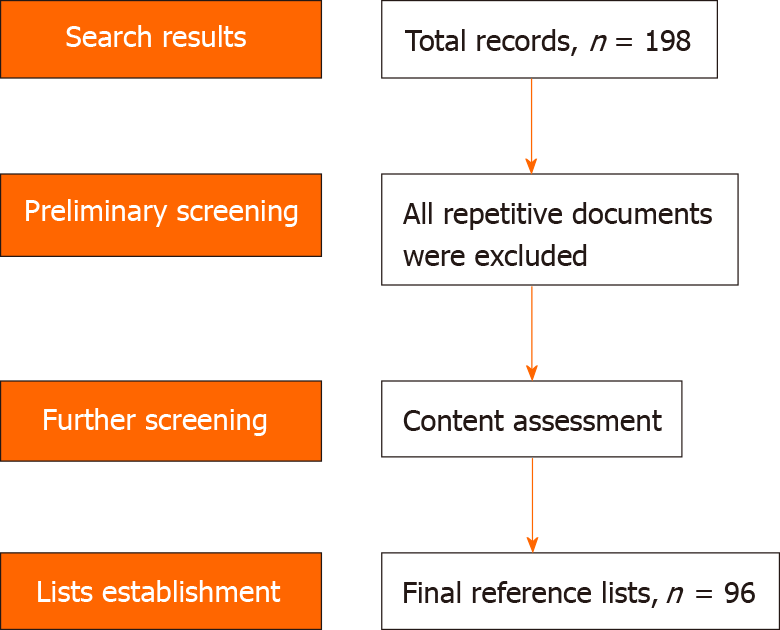
Initially, we retrieved 198 records for this review. Then, repetitive and irrelevant documents were excluded, and we retained ultimately 96 high-quality papers with innovative viewpoints for reference lists. The screening process of those documents is showed in Figure 2.
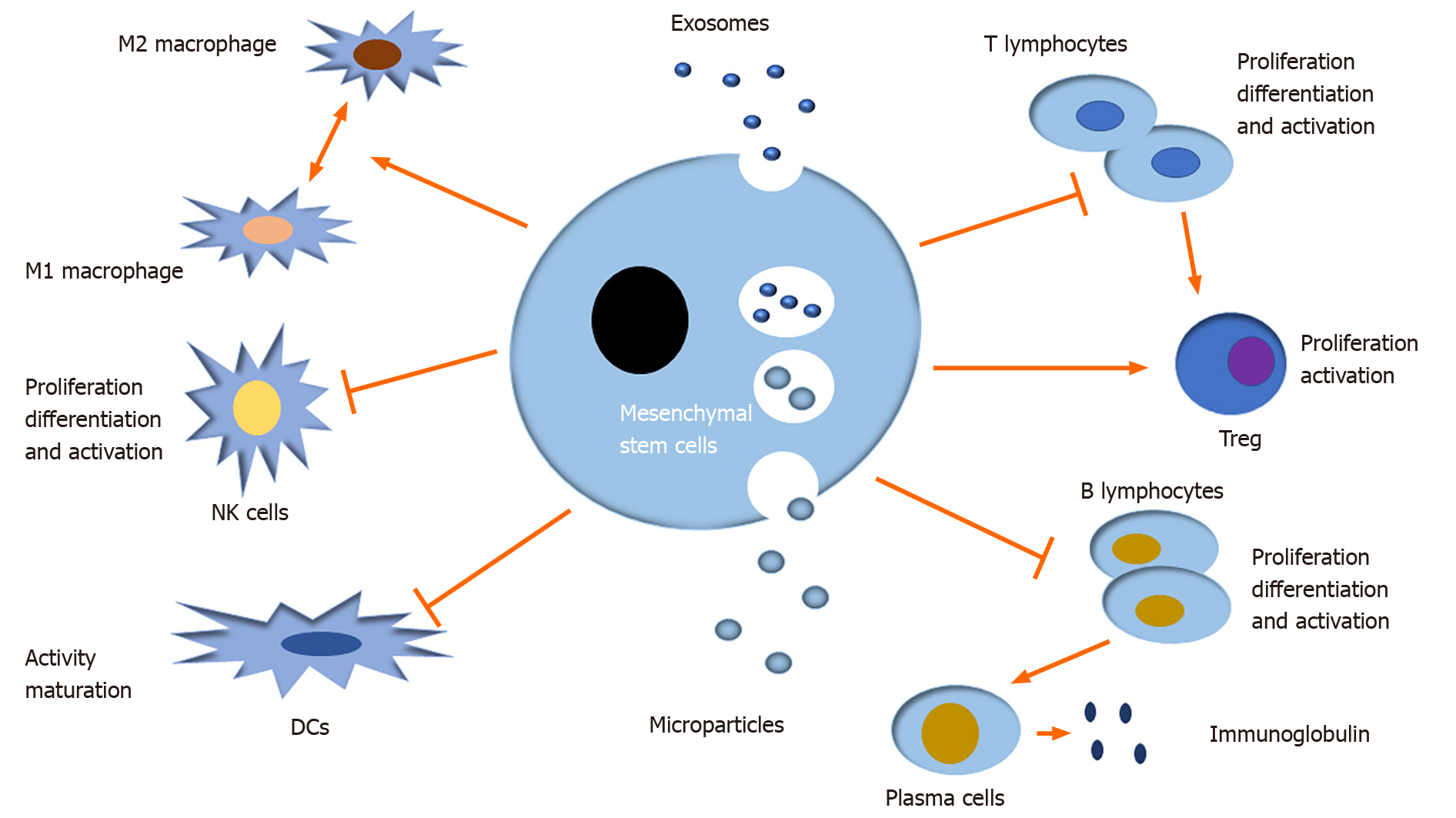
EVs can regulate many immune and inflammatory responses by mediating intercellular communication. Moreover, MSC-EVs have been well documented to induce multiple immune cells to mediate immune responses in innate immunity and adaptive immunity, namely, they modulate the differentiation, activation, and proliferation of immune cells, like T lymphocytes, B lymphocytes, natural killer cells (NKs), dendritic cells (DCs), and macrophages in the autoimmune system (Figure 3)[38-41].
There is growing evidence that MSC-EVs serving as a type of signal molecules play major biological roles in the initiation, maintenance, and progression of multiple autoimmune related diseases, such as autoimmune diseases, cancer, and graft-versus-host disease. The features of MSC-EVs immunomodulation and their therapeutic potential in autoimmune related diseases are summarized in Tables 1 and 2.
| Disease | EVs | Expression | MSC-EVs source/ Target molecules | Target immune cells | Pathway(s) involved | Ref. |
| RA | MSC derived exosomal miR-150-5p | Down | Bone marrow derived MSC-EVs/MMP 14 and VEGF | Macrophages | TGF-β pathway | Chen et al[80] |
| Exosome-encapsulated miR-548a-3p | Down | TLR4 | Macrophages | MiR-548a-3p/TLR4/NF-κB axis | Wang et al[81] | |
| Exosome-encapsulated miR-6089 | Down | TLR4 | Macrophages | TLR4/NF-κB signaling pathway | Xu et al[82] | |
| Exosome-derived lncRNA Hotair | Up | MMP-2 and MMP-13 | Macrophages | - | Song et al[83] | |
| Exosomal miR-17 | Up | TGFBR II | T cells | - | Wang et al[84] | |
| MicroRNA-155 | Up | SHIP-1 | Macrophages | - | Kurowska-Stolarska et al[85] | |
| MicroRNA-146 | Up | - | Macrophages, T cells, B cells | - | Nakasa et al[86] | |
| SLE | Exosomal miR-26a | Up | Podocyte proteins, actin family members, and intermediate filaments | Podocytes | - | Ichii et al[99] |
| Exosomal miRNA-146a | Up | - | - | Interferon-γ pathway | Perez-Hernandez et al[100] | |
| pSS | EV derived LCN2 | Up | TNF-α | B cells | TNF-α signaling | Aqrawi et al[107] |
| EV derived APMAP | Up | TNF-α | B cells | TNF-α signaling | Aqrawi et al[107] | |
| EV derived CPNE1 | Up | TNF-α | B cells | TNF-α signaling | Aqrawi et al[107] | |
| IBD | MSC-EVs | Up | Bone marrow derived MSC-EVs | Macrophages | JAK1/STAT1/STAT6 signaling pathway | Cao et al[113] |
| Breast cancer | Exosomal PD-L1 | Down | PD-1 | T cells | PD-L1/ PD-1 pathway | Yang et al[120] |
| Lung cancer | EV derived miR-103a | Up | Lung cancer cell derived EVs/PTEN | Macrophages | PI3K/ AKT and STAT3 axis | Hsu et al[121] |
| Pancreatic cancer | Exosomal miR-301a-3p | Up | PTEN | Macrophages | PI3Kγ signaling pathway | Wang et al[122] |
| GVHD | MSC-EVs | Up | T cell derived EVs | - | - | Park et al[126] |
| Disease | EVs | Experimental sample | Therapeutic potential | Ref. |
| RA | MSC derived exosomal miR-150-5p | Collagen induced arthritis mouse model | MiR-150-5p could reduce joint destruction by inhibiting synoviocyte hyperplasia and angiogenesis | Chen et al[80] |
| Exosome-encapsulated miR-548a-3p | Macrophage-like cells | MiR-548a-3p could inhibit the proliferation and activation of pTHP-1 cells via the TLR4/NF-κB signaling pathway | Wang et al[81] | |
| Exosome-encapsulated miR-6089 | Macrophage-like cells | MiR-6089 could regulate LPS/TLR4-mediated inflammatory response | Xu et al[82] | |
| Exosome-derived lncRNA Hotair | Blood mononuclear cells | Hotair may contribute to the dissolution of bone and cartilage matrix through activation of MMP-2 and MMP-13 in osteoclasts and RA synoviocytes. Hotair is more stable and easily detected in body fluid | Song et al[83] | |
| Exosomal miR-17 | Blood mononuclear cells | MiR-17 can suppress regulatory T cell differentiation by inhibiting the expression of TGFBR II | Wang et al[84] | |
| MicroRNA-155 | MiR-155–deficient mice | MiR-155–deficient mice are resistant to collagen-induced arthritis, and antigen-specific Th17 cell and autoantibody responses are suppressed markedly to reduce articular inflammation | Kurowska-Stolarska et al[85] | |
| MicroRNA-146 | Human RA synovial fibroblasts | MiR-146a is expressed in the superficial and sublining layers of synovial tissue, like synovial fibroblasts, macrophages, T cells, and B cells | Nakasa et al[86] | |
| SLE | Exosomal miR-26a | Female B6.MRLc1 and C57BL/6 mice; C57BL/6 (9 mo of age) | Podocytes mainly expresse miR-26a in mouse kidneys. Glomerular miR-26a expression in B6.MRLc1 mice correlates negatively with the urinary albumin levels and podocyte specific gene expression | Ichii et al[99] |
| Exosomal miRNA-146a | Urine sample of SLE patients | Up-regulated exosomal miRNA-146a is found in the presence of active lupus nephritis | Perez-Hernandez et al[100] | |
| pSS | EV derived LCN2 | Saliva and tear samples from pSS patients and healthy controls | EV derived LCN2 is over-expressed in pSS patients | Aqrawi et al[107] |
| EV derived APMAP | Saliva and tear samples from pSS patients and healthy controls | EV derived APMAP is over-expressed in pSS patients | Aqrawi et al[107] | |
| EV derived CPNE1 | Saliva and tear samples from pSS patients and healthy controls | EV derived CPNE1 is over-expressed in pSS patients | Aqrawi et al[107] | |
| IBD | MSC-EVs | LPS treated macrophages and an in vivo DSS induced mouse model | EVs promote the up-regulation of pro-inflammatory factors (TNF-α, IL-6, and IL-12) and down-regulation of the anti-inflammatory factor IL-10 in LPS-induced macrophages. EVs promote polarization of M1-like macrophages to an M2-like state | Cao et al[113] |
| Breast cancer | Exosomal PD-L1 | MDA-MB-231 (231) human breast cancer cells and 4T1 mouse mammary tumor cells with PD-L1 expression or PD-L1KO | Exosomal PD-L1 bind to PD-1 on T cells to inhibit T cell activation and killing activities | Yang et al[120] |
| Lung cancer | EV derived miR-103a | Human adenocarcinoma cell lines NCI-H1437, NCI-H1792, and NCI-H2087 and human embryonic kidney HEK293 cells | miRNA inhibitor could inhibit effectively miR-103a mediated M2-type polarization, improving the cytokine prolife of tumor infiltration macrophages | Hsu et al[121] |
| Pancreatic cancer | Exosomal miR-301a-3p | Pancreatic cancer blood samples, Pancreatic cancer cell lines PANC-1, BxPC-3 and monocytic cell line THP-1 | Pancreatic cells generate miR-301a-3p-rich exosomes in a hypoxic microenvironment, which polarize macrophages to promote malignant behaviors of cancer cells | Wang et al[122] |
| GVHD | MSC-EVs | Kidney samples from acute cellular rejection | iKEA (integrated kidney exosome analysis) shows a high level of CD3-positive EVs in kidney rejection patients and achieved high detection accuracy (91.1%) | Park et al[126] |
T lymphocytes are important immune cells in adaptive immunity and play a significant role in the occurrence and development of many autoimmune and inflammatory diseases. MSC derived exosomes and microparticles down-regulate T cell proliferation, and CD4+ and CD8+ T cell subsets decrease significantly in quantity[7]. Adipose mesenchymal stem cell (AMSC) derived exosomes depress the activity of T cells, and up-regulate IL-4, IL-10, and transforming growth factor-β and down-regulate IL-17 and interferon-γ in streptozotocin induced type-1 diabetes mellitus mice, thus deadening the progression of diseases[42]. MSCs have been extensively reported to decorate the activation of CD4+ T cells by some specific T cell effector cytokines or direct contact, down-regulating their immune activity and converting them to a regulatory phenotype (Treg)[43,44]. Programmed death-1 (PD-1) is a valuable cytokine inducing T cell activity. Research shows that MSCs express and secrete PD-1 ligands (PD-L1 and PD-L2) to regulate T cell dependent immune responses by binding with PD-1[45], suggesting that MSCs possess immunosuppressive properties via the modulation of T cells. AMSCs under stimulation with IFN-γ can secret a big body of exosomes to the conditioned medium, and importantly, T cells isolated from that medium are significantly inhibited in activity and proliferation[46]. In a word, MSC-EVs down-regulate the activity and proliferation of T cells to inhibit T dependent autoimmune responses.
B lymphocytes are also vital immune cells in adaptive immunity. A growing number of studies suggest that MSCs possess an immunomodulatory effect on B cells, but the molecular mechanisms involved are still mysterious[47]. Nevertheless, there is little research on the role of MSC-EVs in mediating the regulatory effect of B cells on inflammatory and immune responses. Membrane vesicles derived from MSCs inhibit both B cell proliferation and differentiation in a dose-dependent fashion[48]. Traggiai E and his colleagues found that MSCs positively influence the proliferation and differentiation of B cells into plasma cells secreting more immunoglobulins[47]. Thus, MSCs promote downstream immune responses by mediating the conversion of B cells. Systemic lupus erythematosus (SLE) is a typical autoimmune disease characterized by constantly producing various antibodies to counter autologous cells. It is well established that B cells play a critical role in autoimmune responses via autoantibodies dependent mechanisms. Therefore, we infer that MSCs mediated cell conversion can boost the inflammatory progression. Therefore, MSCs can serve as a potential therapeutic tool in autoimmune diseases.
Monocytes are secreted from bone marrow into the circulatory system and transported to target tissue, where they differentiate into mature macrophages[49]. Macrophages are critical effectors and regulators of the immune system and play a central role in inflammation[50]. It has been well documented that macrophages can be divided into two subpopulations: The classic M1 and the alternative M2 macrophages under microenvironmental factors. The classical M1 macrophages are induced by TLR ligands and IFN-γ and alternative M2 macrophages are induced by the immune complex IL-4/IL-13[51,52]. M1 macrophages are characterized by strong microbicidal and tumoricidal activity, which can promote Th1 related inflammatory responses by releasing a range of proinflammatory cytokines, such as IL-6, IL-12, and TNF-α[53], whereas M2 macrophages with anti-inflammatory function produce less proinflammatory cytokines and more IL-10 and other anti-inflammatory factors[54]. In short, both M1 and M2 macrophages contribute to the balance between destruction and repair of tissue in pathological conditions. A study suggested that after coculture of AMSCs with inflammatory cytokines IFN-γ and TNF-α, a higher level of exososmes can be detected in the medium supernatant, which induce M1 differentiate to anti-inflammatory M2 phenotype[55]. Adipose tissue accumulating constantly in the body leads to obesity and inflammatory responses, which increase the risk of incidence of many chronic diseases, including type 2 diabetes, cardiovascular events, and part of cancers[56-58]. Previous studies have revealed that the invasion of macrophages and T cells promote the formation of chronic inflammation in white adipose tissues[59,60]. In high fat diet fed mice, AMSC derived exosomes promote white adipose tissue hypertrophy by inducing M2 macrophage polarization[61]. A study by Németh et al[62] showed that endotoxin stimulated MSCs induce M2 macrophage polarization to release IL-10 and attenuate sepsis via the NF-κB signal pathway in a mouse model[62]. MSC-EVs induce the production of M2 macrophages with anti-inflammatory properties to restrain many relevant immune responses.
NK cells, a vital cell type in the innate immune system, mediate cytotoxic activity and produce certain cytokines and chemokines to mediate antigen presentation, antiviral responses, autoimmune responses, and the occurrence of various autoimmune diseases[63]. A previous result showed that MSC-EVs injected into periocular tissue depress the transfer of CD161+ NK cells, delay the progression of disease, and restore damaged tissue in autoimmune uveitis rat models[64,65]. Decidua parietalis MSCs release IL-2 to CD69 (NK cell receptor) to stimulate IL-2 dependent NK cells and thus promote the proliferation of activated NK cells[66]. Thus, decidua parietalis MSCs induce directly the activity of NK cells through IL-2 and CD69. Recent research suggested that fetal liver MSC derived exosomes carrying LAP, TGFβ, and TSP1 restrain the proliferation and activation of NK cells via TGFβ/Smad2/3 signaling[67]. Although available data show that MSC-EVs depress the activation and proliferation of NK cells, the research on that is limited in quantity and more studies need to be carried out in the future.
DCs, important bone marrow derived APCs, present multiple antigenic peptides (major histocompatibility complex - peptide complexes) to other immune cells, like T cells, and play a key role in bridging innate to adaptive immune systems. Coculture of DCs with MSC-EVs led to down-regulated cellular surfactants and IL-10, IL-6, and IL-17 and up-regulated the number of regulatory T cells[68], and the activity and maturation of DCs are apparently restrained. Many studies suggest that these MSC-EVs stimulate immature DCs to release TGF-β and PGE2, and regulate the immunocompetence of T cells in DC and T cell culture medium. Those small molecules mediate autoimmune responses with unclear mechanism. MSCs induce mature DCs to immature status with low immunogenicity and immunoregulatory property. The immature DCs express less immunomodulatory factors Ia, CD11c, CD80, CD86, and CD40, except for increased CD11[39]. Overall, MSC-EVs down-regulate the immune activity of DCs and T cell dependent adaptive immune responses indirectly. Nevertheless, the research on the interaction between DCs and MSC-EVs is limited, and the exact molecular mechanisms warrant further studies.
MSC-EVs have been suggested in many kinds of diseases, which can serve as promising strategies for autoimmune disease diagnosis and treatment, such as rheumatoid arthritis (RA), SLE, primary Sjgren's syndrome (pSS), systemic sclerosis, and inflammatory bowel diseases (IBD) due to their vital role in intercellular communications. Nevertheless, the precise molecular mechanism underlying EV regulation in autoimmunity warrants in-depth investigation.
Epidemiological survey and analysis suggest that the incidence of autoimmune diseases has been increasing year by year over the past several decades[69]. Autoimmune diseases usually influence multiple organs and systems, such as the motor system, respiratory system, digestive system, and circulatory system[70]. They lead to a heavy burden to public health. It is well known that some autoimmune diseases are genetically susceptible[71]. Women tend to be affected by some autoimmune diseases, and approximately 90% of patients with autoimmune disease are female[72]. Currently, glucocorticoids and immunosuppressive drugs are still the most frequently used non-specific therapeutic agents. That traditional therapeutic strategy causes many adverse reactions, such as opportunistic infections and metabolic abnormalities, and the development of biological molecular targeted drugs to cause slower disease progression is a priority. Accumulating data reveal the biological features of MSCs in relieving immune cell-driven systemic inflammatory responses to down-regulate immune responses, such as autoimmune diseases[73], and MSC-EVs are a significant regulator[74]. The current knowledge of EVs in autoimmune diseases will be discussed in detail in the following text.
RA is one of the most common chronic and systemic autoimmune diseases involving multiple systems, which is characterized by the destruction of synovial joints. The representative clinical manifestations are redness, swelling, and pain of distal joints, especially small joints of hands and feet[75]. Many researchers have suggested that the occurrence of RA is caused by many complex factors, such as genetic factors and environmental factors[76,77]. Dysregulation of immune responses occupies a necessary position in RA.
Increasing data have revealed EVs as critical regulators in the pathogenesis of RA by delivering specific functional molecules to targeted cells. Previously, the effectiveness of MSC therapies has been elucidated in cartilage repair in both animal studies[78] and human clinical trials[79]. Previous studies have revealed that EVs generated by MSCs play a critical role in protecting against cartilage destruction and enhancing cartilage regeneration. Particularly, exosomal noncoding RNAs (ncRNAs), including miRNAs and lncRNAs, have been implicated in regulating inflammation and immune response. MSC derived exosomal miR-150-5p down-regulated inflammatory responses and reduced joint destruction and vasculitis by targeting matrix metalloproteinase 14 (MMP14) and vascular endothelial growth factor in a collagen-induced arthritis mouse model, which is considered as a potential therapeutic biomarker for RA[80]. We have previously demonstrated the important role of exosome-encapsulated miR-6089 and miR-548a-3p in affecting macrophage-mediated inflammatory response in RA[81,82]. Exosome-derived lncRNA Hotair affected the migration of activated macrophages and significantly decreased the levels of MMP-2 and MMP-13, suggesting that it is a potential biomarker for RA[83]. A study by Wang et al[84] has shown that exosomal miR-17 inhibits regulatory T cells by targeting TGFBR II in RA[84]. Besides, exosomes-encapsulated miR-155 and miR-146a produced by DCs can serve as important regulators in immune response and inflammatory response in RA[85-87]. It has been shown that the expression of exosomal amyloid A is positively correlated with anti-CCP antibody and CRP, suggesting a vital role of exosomal protein in predicating the disease activity of RA patients[88]. Taken together, exosomal ncRNAs play critical roles in regulating immune and inflammatory cells and thus participate in the occurrence and development of RA. Nevertheless, more studies are warranted to explore the molecular mechanisms of those exosomes harboring ncRNAs in the pathogenesis of RA.
SLE is a systemic autoimmune disease with various autoantibodies, which usually affects multi-organ systems due to enhanced inflammation and complex autoimmune disorders[89,90]. It has been well established that SLE is caused by the abundant activation of T and B lymphocytes, elevated pro-inflammatory cytokines, sedimentation of immune complex substance, and finally multiple organ damage, while the kidney is the most commonly involved organ in SLE and lupus nephritis (LN) is often caused[91]. EVs are significant regulators in mediating cell-to-cell communications involved in inflammation and immune regulations. Mounting evidence has suggested that EV delivered nucleic acids, proteins, autoantigens, cytokines, and surface receptors can serve as significant regulators in SLE[92,93].
Microvesicles purified from SLE patients have been identified to contain higher concentrations of immunoglobulins and complements[94,95]. Circulating exosomes from patients with SLE have been shown to induce a proinflammatory immune response, which is characterized by high levels of TNF-α, IL-1β, IL-6, and other inflammatory mediators[93]. The study by Asami et al[96] supports that MSCs may confer immunosuppressive effects in SLE[96]. Previously published studies have elucidated that the EVs produced from MSCs, can also contribute to immunosuppressive function in SLE[97]. Accordingly, EVs can be used as drug carriers because they are less immunogenic. Umbilical cord derived MSCs have been used in the treatment of SLE patients, which shows good tolerance and few adverse events associated with transplantation[98]. Therefore, MSCs and MSC-EVs can effectively control the active SLE and be used as a therapeutic strategy, particularly for the treatment of refractory SLE. Ichii and the colleagues have found that exosomal miR-26a is positively associated with urinary protein level, which suggests that exosomal miR-26a in urine of LN patients can be used as a potential biomarker for predicting podocyte injury[99]. In addition, Perez-Hernandez et al[100] have shown that urinary exosomal miRNA-146a is significantly up-regulated in active LN patients[100]. Therefore, testing urinary exosomal miRNA can be a non-invasive method for the detection and monitoring of LN. Nevertheless, the specific molecular mechanism of EVs in regulating autoimmunity in SLE is still unclear, which warrants further investigation by more future studies.
pSS is a systemic autoimmune disease that is characterized by chronic lymphocyte infiltration in the exocrine glands, primarily the lacrimal and salivary glands[101,102]. The primary target organs are the lacrimal and salivary glands, and dry eyes and dry mouth are often caused[103]. EVs purified from saliva[104,105] and tear fluid[106,107] have been identified to be potential biomarkers for the diagnosis and treatment of pSS in previous studies. Those differentially expressed proteins isolated from EVs of saliva and tear fluid from patients with pSS can contribute to pSS by regulating TNF-α signaling and B cell survival, including neutrophil gelatinase-associated lipocalin, adipocyte plasma membrane-associated protein, and copine[107]. The increase of platelet-derived microvesicles, soluble CD40 ligand (sCD40L), and soluble P-selectin (sCD62P) in pSS patients reflects platelet activation, which can serve as disease biomarkers[108]. Currently, studies on MSC-EV mediated immune responses in pSS are rare. More studies are needed to elucidate the role and underlying mechanisms of EVs in pSS.
IBD is a common digestive disease characterized by chronic, relapsing gastrointestinal tract inflammatory reactions, including two main forms, Crohn’s disease and ulcerative colitis[109,110]. To the best of our knowledge, the pathogenic mechanisms and pathogenesis of IBD are complicated, and many factors contribute to the occurrence of this disease, like autoimmune disorder, genetics, and environment[111]. Macrophages have been seen as important immune cells inducing IBD[112]. Experimental studies showed that inflammatory responses are significantly restrained by inducing the production of M2 macrophages in the dextran sulphate sodium induced mouse model of colitis[113]. Moreover, higher levels of immunosuppressive factors (IL-10 and TGF-β) were observed in mice treated with MSC-EVs, promoting repair and regeneration of damaged epithelial cells[113]. Studies have confirmed that MSC-EVs down-regulate the production of IL-1β, NO, and IL-18 by depressing NF-κB and iNOS-driven signaling in 2,4,6-trinitrobenzene sulfonic acid induced colitis[114,115]. Therefore, MSC-EVs, as an important regulator, can suppress inflammatory responses and promote injured tissue repair. That delineates the potential of MSC-EVs as biomarkers for IBD treatment.
Tumor immunity is critical in the processes of immune response, immune escape, and immune surveillance in cancer[116,117]. Previous research findings show that EVs play an critical role in anti-tumor immune reaction and inflammatory response during carcinogenesis and cancer progression[118]. In the last decade, exosomes have attracted more and more attention in cancer immunity, particularly as tumor suppressors[119]. Some bioactive factors encapsulated in EVs promote immune and inflammatory responses and thus lead to tumorigenesis, while some exert immune suppressive effects by inducing Tregs and M1 polarization.
A previous study has demonstrated the specific binding capacity of exosomal PD-L1 to its receptor PD-1 to depress the anti-tumor effect of T cells in breast cancer[120]. MSCs also express and release PD-L1 to regulate T cell activity, and thus both MSCs and exosomes possess immunosuppressive effect[45]. Besides, it has been documented that EVs play a critical in anti-tumor immune response by regulating macrophages polarization. It has been found that EVs-delivering miR-103a contributes to lung cancer by targeting PTEN and inducing M2 polarization[121]. Similarly, exosomal miR-301a-3p purified from pancreatic cancer cells was found to induce M2 macrophage polarization via the PTEN/PI3Kγ signaling pathway[122]. Taken together, EVs, particularly MSC-EVs, exert immunomodulatory effects on cancer and mediate intercellular communications between cancer cells and immune cells through EVs harboring bioactive molecules, including proteins and ncRNAs.
Kidney transplantation is the current preferred treatment for end stage renal disease. However, the long-term survival rate of the transplanted kidney is still low because the transplanted recipients often suffer from acute or chronic rejection for a long period of time[123], which finally leads to graft-versus-host disease. Biopsy is still the gold standard for the diagnosis of rejection of kidney transplantation[124,125], but it is risky and traumatic. EVs in urine can be a potential biomarker for monitoring kidney transplant rejection[126]. T cells infiltrate the renal tubule during acute inflammatory response, which is a major cause for transplanted renal damage. MSC-EVs possess potential of inhibition of T cell activity and proliferation and thus EVs tend to gather in damaged renal tissues and are more likely to enter the urine. Consequently, using urine for detecting rejection of kidney transplantation is more likely to operate and promising. In addition, a previous report has showed that MSC derived exosomes provide a novel and effective clinical treatment for graft-versus-host disease[127]. Nonetheless, the role of MSC-EVs in transplantation immunity needs to be further investigated in the future.
MSC-EVs are a hot topic in current molecular biology. Accumulated data have implicated their immunomodulatory effects on many immune cells, including T cells, B cells, macrophages, NK cells, and DCs. Increasing studies have confirmed that MSC-EVs can serve as regulators in the pathogenesis of autoimmune related diseases. In particular, MSC-EVs and the encapsulated bioactive molecules are potential targets for the diagnosis and treatment of autoimmune disease, cancer, and other diseases. MSC-EVs can serve as new medicines in the suppression of inflammatory responses. Increasing experimental results show that application of MSC-EVs can effectively inhibit immune reactions and promote the survival and regeneration of injured cells. However, there is still a long way for investigating the therapeutic strategy for autoimmunity related diseases based on MSC-EVs. More in-depth research is warranted in the future, particularly regarding the molecular mechanism of MSC-EVs in autoimmunity.
Mesenchymal stem cells (MSCs) have been reported to possess immune regulatory effects in innate and adaptive immune reactions. MSCs can mediate intercellular communications by releasing extracellular vesicles (EVs), which deliver functional molecules to targeted cells. MSC derived EVs (MSC-EVs) confer altering effects on many immune cells, including T lymphocytes, B lymphocytes, natural killer cells, dendritic cells, and macrophages. A large number of studies have suggested that MSC-EVs participate in regulating autoimmunity related diseases. This characteristic of MSC-EVs makes them be potential biomarkers for the diagnosis and treatment of autoimmunity related diseases.
This article describes and focuses on the identification, characteristics, immunomodulatory function, and underlying mechanism of MSC-EVs in autoimmunity related diseases. Understanding the immunomodulation effects of MSC-EVs better will help us to investigate the pathogenesis of diseases and develop novel targeted medicines.
The immune modulation of MSC-EVs play a key role in disease initiation, maintenance, and progression. This article provides a new direction for us to understand the precise mechanisms of action of autoimmunity related diseases, which will promote the improvement of therapeutic regimen.
Literature search was conducted in PubMed to retrieve articles published between 2010 and 2020 in the English language. The keywords, such as “MSCs,” “EVs,” “autoimmune responses,” “immune cells,” and “autoimmunity related diseases,” and Boolean operator “AND” and “NOT” coalesced admirably to be used for searching in vitro studies on the specific molecular mechanisms of MSC-EVs in many immune cell types and many autoimmunity related diseases. Studies that did not investigate the molecular mechanisms of MSC-EVs in the occurrence and development of autoimmune diseases were excluded.
A large number of articles were retrieved and their abstracts were skimmed. When analyzing the publications, we found that it has been well documented that MSC-EVs have the ability to induce multiple immune cells, like T lymphocytes, B lymphocytes, natural killer cells, dendritic cells, and macrophages, to regulate immune responses in innate immunity and adaptive immunity. Many validated EVs-delivered molecules have been identified as key biomarkers, such as proteins, lipids, and nucleotides. Some EVs-encapsulated functional molecules can serve as promising therapeutic targets particularly for autoimmune disease.
MSC-EVs play an important part in the differentiation, activation, and proliferation of immune cells, and they may become potential biomarkers for the diagnosis and treatment of autoimmunity related diseases.
MSC-EVs can serve as regulators in the pathogenesis of autoimmune related diseases. In particular, MSC-EVs and the encapsulated bioactive molecules are potential targets for the diagnosis and treatment of autoimmune disease, cancer, and other diseases. However, there is still a long way for investigating the therapeutic strategy for autoimmunity related diseases based on MSC-EVs. More in-depth research is warranted in the future, particularly regarding the molecular mechanism of MSC-EVs in autoimmunity
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pelagalli A, Khan I, Kuo FC S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li X
| 1. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1715] [Cited by in F6Publishing: 1652] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 2. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15372] [Cited by in F6Publishing: 14771] [Article Influence: 590.8] [Reference Citation Analysis (0)] |
| 3. | De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-1942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 4. | De Bari C, Dell'Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 326] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 6. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4817] [Cited by in F6Publishing: 4866] [Article Influence: 221.2] [Reference Citation Analysis (0)] |
| 7. | Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, Noël D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8:1399-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 301] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 8. | Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 608] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 9. | Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 578] [Cited by in F6Publishing: 593] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 10. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1009] [Cited by in F6Publishing: 1011] [Article Influence: 168.5] [Reference Citation Analysis (0)] |
| 11. | Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 841] [Cited by in F6Publishing: 937] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 12. | Trento C, Bernardo ME, Nagler A, Kuçi S, Bornhäuser M, Köhl U, Strunk D, Galleu A, Sanchez-Guijo F, Gaipa G, Introna M, Bukauskas A, Le Blanc K, Apperley J, Roelofs H, Van Campenhout A, Beguin Y, Kuball J, Lazzari L, Avanzini MA, Fibbe W, Chabannon C, Bonini C, Dazzi F. Manufacturing Mesenchymal Stromal Cells for the Treatment of Graft-versus-Host Disease: A Survey among Centers Affiliated with the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24:2365-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1035] [Cited by in F6Publishing: 1088] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 14. | Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1088] [Cited by in F6Publishing: 1163] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 15. | Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98-110. [PubMed] [Cited in This Article: ] |
| 16. | Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 392] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 17. | Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, Doevendans PA, Pasterkamp G, de Kleijn DP. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6:206-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 436] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 19. | Chavez-Muñoz C, Morse J, Kilani R, Ghahary A. Primary human keratinocytes externalize stratifin protein via exosomes. J Cell Biochem. 2008;104:2165-2173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 641] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 21. | Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna). 2010;117:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 22. | Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175:2237-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1347] [Cited by in F6Publishing: 1444] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 24. | Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3409] [Cited by in F6Publishing: 3679] [Article Influence: 229.9] [Reference Citation Analysis (0)] |
| 25. | Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106:3794-3799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 513] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 26. | Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4900] [Cited by in F6Publishing: 5526] [Article Influence: 502.4] [Reference Citation Analysis (0)] |
| 27. | Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 454] [Cited by in F6Publishing: 453] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 28. | Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev. 2015;34:474-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 304] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 29. | Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 794] [Cited by in F6Publishing: 855] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 30. | Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3453] [Cited by in F6Publishing: 3442] [Article Influence: 382.4] [Reference Citation Analysis (0)] |
| 31. | Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1410] [Cited by in F6Publishing: 1524] [Article Influence: 152.4] [Reference Citation Analysis (0)] |
| 32. | Distler JH, Huber LC, Gay S, Distler O, Pisetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39:683-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Turpin D, Truchetet ME, Faustin B, Augusto JF, Contin-Bordes C, Brisson A, Blanco P, Duffau P. Role of extracellular vesicles in autoimmune diseases. Autoimmun Rev. 2016;15:174-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Tofiño-Vian M, Guillén MI, Alcaraz MJ. Extracellular vesicles: A new therapeutic strategy for joint conditions. Biochem Pharmacol. 2018;153:134-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95:2229-2234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 36. | Ansboro S, Roelofs AJ, De Bari C. Mesenchymal stem cells for the management of rheumatoid arthritis: immune modulation, repair or both? Curr Opin Rheumatol. 2017;29:201-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Cosenza S, Ruiz M, Maumus M, Jorgensen C, Noël D. Pathogenic or Therapeutic Extracellular Vesicles in Rheumatic Diseases: Role of Mesenchymal Stem Cell-Derived Vesicles. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, Kandeel F, Mullen Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37:604-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 39. | Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou X, Zhu X, Lu C, Liang W, Liao L, Zenke M, Zhao RC. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 40. | Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells. 2009;27:693-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1552] [Cited by in F6Publishing: 1473] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 42. | Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119:9433-9443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 43. | Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980-1991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 44. | Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826-4835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 45. | Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017;35:766-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 46. | Serejo TRT, Silva-Carvalho AÉ, Braga LDCF, Neves FAR, Pereira RW, Carvalho JL, Saldanha-Araujo F. Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22:369-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 49. | Bolego C, Cignarella A, Staels B, Chinetti-Gbaguidi G. Macrophage function and polarization in cardiovascular disease: a role of estrogen signaling? Arterioscler Thromb Vasc Biol. 2013;33:1127-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2742] [Cited by in F6Publishing: 2843] [Article Influence: 203.1] [Reference Citation Analysis (0)] |
| 51. | Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 393] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 52. | Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3696] [Cited by in F6Publishing: 4215] [Article Influence: 351.3] [Reference Citation Analysis (1)] |
| 53. | Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4154] [Cited by in F6Publishing: 4458] [Article Influence: 234.6] [Reference Citation Analysis (0)] |
| 54. | Martinez FO. Regulators of macrophage activation. Eur J Immunol. 2011;41:1531-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 55. | Domenis R, Cifù A, Quaglia S, Pistis C, Moretti M, Vicario A, Parodi PC, Fabris M, Niazi KR, Soon-Shiong P, Curcio F. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018;8:13325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 56. | Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1382] [Cited by in F6Publishing: 1312] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 57. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5683] [Cited by in F6Publishing: 5906] [Article Influence: 347.4] [Reference Citation Analysis (1)] |
| 58. | Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821-1830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 2450] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 59. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 3491] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 60. | Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 491] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 61. | Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes. 2018;67:235-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 387] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 62. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1678] [Cited by in F6Publishing: 1742] [Article Influence: 108.9] [Reference Citation Analysis (1)] |
| 63. | Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1593] [Cited by in F6Publishing: 1551] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 64. | Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci Rep. 2017;7:4323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 65. | Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, Park JW, Kim TW, An SY, Prockop DJ, Lee RH. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Reports. 2017;8:1214-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 66. | Abumaree MH, Bahattab E, Alsadoun A, Al Dosaimani A, Abomaray FM, Khatlani T, Kalionis B, El-Muzaini MF, Alawad AO, AlAskar AS. Characterization of the interaction between human decidua parietalis mesenchymal stem/stromal cells and natural killer cells. Stem Cell Res Ther. 2018;9:102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Fan Y, Herr F, Vernochet A, Mennesson B, Oberlin E, Durrbach A. Human Fetal Liver Mesenchymal Stem Cell-Derived Exosomes Impair Natural Killer Cell Function. Stem Cells Dev. 2019;28:44-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 68. | Favaro E, Carpanetto A, Caorsi C, Giovarelli M, Angelini C, Cavallo-Perin P, Tetta C, Camussi G, Zanone MM. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59:325-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 69. | Ji J, Sundquist J, Sundquist K. Gender-specific incidence of autoimmune diseases from national registers. J Autoimmun. 2016;69:102-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33:197-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 480] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 71. | Gershwin LJ. Current and Newly Emerging Autoimmune Diseases. Vet Clin North Am Small Anim Pract. 2018;48:323-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Autoimmune disease and the environment. Environ Health Perspect. 1998;106:A592-A593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 73. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 830] [Cited by in F6Publishing: 949] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 74. | Xu H, Jia S, Xu H. Potential therapeutic applications of exosomes in different autoimmune diseases. Clin Immunol. 2019;205:116-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 974] [Cited by in F6Publishing: 968] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 76. | MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K, Silman AJ. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000;43:30-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 77. | Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50:3085-3092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 464] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 78. | Guo W, Zheng X, Zhang W, Chen M, Wang Z, Hao C, Huang J, Yuan Z, Zhang Y, Wang M, Peng J, Wang A, Wang Y, Sui X, Xu W, Liu S, Lu S, Guo Q. Mesenchymal Stem Cells in Oriented PLGA/ACECM Composite Scaffolds Enhance Structure-Specific Regeneration of Hyaline Cartilage in a Rabbit Model. Stem Cells Int. 2018;2018:6542198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Kamei N, Ochi M, Adachi N, Ishikawa M, Yanada S, Levin LS, Kamei G, Kobayashi T. The safety and efficacy of magnetic targeting using autologous mesenchymal stem cells for cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2018;26:3626-3635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic Potential of Mesenchymal Cell-Derived miRNA-150-5p-Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J Immunol. 2018;201:2472-2482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 81. | Wang Y, Zheng F, Gao G, Yan S, Zhang L, Wang L, Cai X, Wang X, Xu D, Wang J. MiR-548a-3p regulates inflammatory response via TLR4/NF-κB signaling pathway in rheumatoid arthritis. J Cell Biochem. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 82. | Xu D, Song M, Chai C, Wang J, Jin C, Wang X, Cheng M, Yan S. Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4. J Cell Physiol. 2019;234:1502-1511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 83. | Song J, Kim D, Han J, Kim Y, Lee M, Jin EJ. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med. 2015;15:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 84. | Wang L, Wang C, Jia X, Yu J. Circulating Exosomal miR-17 Inhibits the Induction of Regulatory T Cells via Suppressing TGFBR II Expression in Rheumatoid Arthritis. Cell Physiol Biochem. 2018;50:1754-1763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, McInnes IB. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci. 2011;108:11193-11198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 438] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 86. | Nakasa T, Miyaki S, Okubo A, Hashimoto M, Nishida K, Ochi M, Asahara H. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58:1284-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 455] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 87. | Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481-12486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3121] [Cited by in F6Publishing: 3382] [Article Influence: 187.9] [Reference Citation Analysis (0)] |
| 88. | Yoo J, Lee SK, Lim M, Sheen D, Choi EH, Kim SA. Exosomal amyloid A and lymphatic vessel endothelial hyaluronic acid receptor-1 proteins are associated with disease activity in rheumatoid arthritis. Arthritis Res Ther. 2017;19:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 89. | Al-Shobaili HA, Al Robaee AA, Alzolibani AA, Rasheed Z. Antibodies against 4-hydroxy-2-nonenal modified epitopes recognized chromatin and its oxidized forms: role of chromatin, oxidized forms of chromatin and 4-hydroxy-2-nonenal modified epitopes in the etiopathogenesis of SLE. Dis Markers. 2012;33:19-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 90. | Colasanti T, Maselli A, Conti F, Sanchez M, Alessandri C, Barbati C, Vacirca D, Tinari A, Chiarotti F, Giovannetti A, Franconi F, Valesini G, Malorni W, Pierdominici M, Ortona E. Autoantibodies to estrogen receptor α interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2012;64:778-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1314] [Cited by in F6Publishing: 1266] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 92. | Perez-Hernandez J, Redon J, Cortes R. Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 93. | Lee JY, Park JK, Lee EY, Lee EB, Song YW. Circulating exosomes from patients with systemic lupus erythematosus induce an proinflammatory immune response. Arthritis Res Ther. 2016;18:264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 94. | Nielsen CT, Østergaard O, Stener L, Iversen LV, Truedsson L, Gullstrand B, Jacobsen S, Heegaard NH. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012;64:1227-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 95. | Østergaard O, Nielsen CT, Iversen LV, Tanassi JT, Knudsen S, Jacobsen S, Heegaard NH. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum. 2013;65:2680-2690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 96. | Asami T, Ishii M, Fujii H, Namkoong H, Tasaka S, Matsushita K, Ishii K, Yagi K, Fujiwara H, Funatsu Y, Hasegawa N, Betsuyaku T. Modulation of murine macrophage TLR7/8-mediated cytokine expression by mesenchymal stem cell-conditioned medium. Mediators Inflamm. 2013;2013:264260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 97. | Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1435] [Cited by in F6Publishing: 1559] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 98. | Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16:R79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 99. | Ichii O, Otsuka-Kanazawa S, Horino T, Kimura J, Nakamura T, Matsumoto M, Toi M, Kon Y. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS One. 2014;9:e110383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 100. | Perez-Hernandez J, Forner MJ, Pinto C, Chaves FJ, Cortes R, Redon J. Increased Urinary Exosomal MicroRNAs in Patients with Systemic Lupus Erythematosus. PLoS One. 2015;10:e0138618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 101. | Jonsson R, Bolstad AI, Brokstad KA, Brun JG. Sjögren's syndrome--a plethora of clinical and immunological phenotypes with a complex genetic background. Ann N Y Acad Sci. 2007;1108:433-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 102. | Jonsson R, Vogelsang P, Volchenkov R, Espinosa A, Wahren-Herlenius M, Appel S. The complexity of Sjögren's syndrome: novel aspects on pathogenesis. Immunol Lett. 2011;141:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 103. | Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X, Tzioufas AG. Topical and systemic medications for the treatment of primary Sjögren's syndrome. Nat Rev Rheumatol. 2012;8:399-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 104. | Hu S, Vissink A, Arellano M, Roozendaal C, Zhou H, Kallenberg CG, Wong DT. Identification of autoantibody biomarkers for primary Sjögren's syndrome using protein microarrays. Proteomics. 2011;11:1499-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 105. | Delaleu N, Mydel P, Kwee I, Brun JG, Jonsson MV, Jonsson R. High fidelity between saliva proteomics and the biologic state of salivary glands defines biomarker signatures for primary Sjögren's syndrome. Arthritis Rheumatol. 2015;67:1084-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 106. | Tomosugi N, Kitagawa K, Takahashi N, Sugai S, Ishikawa I. Diagnostic potential of tear proteomic patterns in Sjögren's syndrome. J Proteome Res. 2005;4:820-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 107. | Aqrawi LA, Galtung HK, Vestad B, Øvstebø R, Thiede B, Rusthen S, Young A, Guerreiro EM, Utheim TP, Chen X, Utheim ØA, Palm Ø, Jensen JL. Identification of potential saliva and tear biomarkers in primary Sjögren's syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res Ther. 2017;19:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 108. | Sellam J, Proulle V, Jüngel A, Ittah M, Miceli Richard C, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM, Mariette X. Increased levels of circulating microparticles in primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11:R156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 109. | Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett. 2014;161:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 110. | Hodson R. Inflammatory bowel disease. Nature. 2016;540:S97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 111. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1299] [Cited by in F6Publishing: 1364] [Article Influence: 80.2] [Reference Citation Analysis (1)] |
| 112. | Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16:26-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 296] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 113. | Cao L, Xu H, Wang G, Liu M, Tian D, Yuan Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharmacol. 2019;72:264-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 114. | Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y, Deng SJ, Liu YJ. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS One. 2015;10:e0140551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 115. | Wu H, Fan H, Shou Z, Xu M, Chen Q, Ai C, Dong Y, Liu Y, Nan Z, Wang Y, Yu T, Liu X. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 116. | de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1633] [Cited by in F6Publishing: 1589] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 117. | Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 118. | Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 432] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 119. | Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17:959-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 120. | Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia W, Cha JH, Hou J, Hsu JL, Sun L, Hung MC. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28:862-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 304] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 121. | Hsu YL, Hung JY, Chang WA, Jian SF, Lin YS, Pan YC, Wu CY, Kuo PL. Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN. Mol Ther. 2018;26:568-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 122. | Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018;78:4586-4598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 440] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 123. | Srinivas TR, Meier-Kriesche HU. Minimizing immunosuppression, an alternative approach to reducing side effects: objectives and interim result. Clin J Am Soc Nephrol. 2008;3 Suppl 2:S101-S116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 124. | Halawa A. The early diagnosis of acute renal graft dysfunction: a challenge we face. The role of novel biomarkers. Ann Transplant. 2011;16:90-98. [PubMed] [Cited in This Article: ] |
| 125. | Williams WW, Taheri D, Tolkoff-Rubin N, Colvin RB. Clinical role of the renal transplant biopsy. Nat Rev Nephrol. 2012;8:110-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 126. | Park J, Lin HY, Assaker JP, Jeong S, Huang CH, Kurdi T, Lee K, Fraser K, Min C, Eskandari S, Routray S, Tannous B, Abdi R, Riella L, Chandraker A, Castro CM, Weissleder R, Lee H, Azzi JR. Integrated Kidney Exosome Analysis for the Detection of Kidney Transplant Rejection. ACS Nano. 2017;11:11041-11046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 127. | Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 755] [Article Influence: 75.5] [Reference Citation Analysis (0)] |