Published online Dec 26, 2020. doi: 10.4252/wjsc.v12.i12.1652
Peer-review started: July 30, 2020
First decision: September 17, 2020
Revised: October 1, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 26, 2020
Impaired wound healing can be associated with different pathological states. Burn wounds are the most common and detrimental injuries and remain a major health issue worldwide. Mesenchymal stem cells (MSCs) possess the ability to regenerate tissues by secreting factors involved in promoting cell migration, proliferation and differentiation, while suppressing immune reactions. Preconditioning of MSCs with small molecules having cytoprotective properties can enhance the potential of these cells for their use in cell-based therapeutics.
To enhance the therapeutic potential of MSCs by preconditioning them with isorhamnetin for second degree burn wounds in rats.
Human umbilical cord MSCs (hU-MSCs) were isolated and characterized by surface markers, CD105, vimentin and CD90. For preconditioning, hU-MSCs were treated with isorhamnetin after selection of the optimized concentration (5 µmol/L) by cytotoxicity analysis. The migration potential of these MSCs was analyzed by the in vitro scratch assay. The healing potential of normal, and preconditioned hU-MSCs was compared by transplanting these MSCs in a rat model of a second degree burn wound. Normal, and preconditioned MSCs (IH + MSCs) were transplanted after 72 h of burn injury and observed for 2 wk. Histological and gene expression analyses were performed on day 7 and 14 after cell transplantation to determine complete wound healing.
The scratch assay analysis showed a significant reduction in the scratch area in the case of IH + MSCs compared to the normal untreated MSCs at 24 h, while complete closure of the scratch area was observed at 48 h. Histological analysis showed reduced inflammation, completely remodeled epidermis and dermis without scar formation and regeneration of hair follicles in the group that received IH + MSCs. Gene expression analysis was time dependent and more pronounced in the case of IH + MSCs. Interleukin (IL)-1β, IL-6 and Bcl-2 associated X genes showed significant downregulation, while transforming growth factor β, vascular endothelial growth factor, Bcl-2 and matrix metallopeptidase 9 showed significant upregulation compared to the burn wound, showing increased angiogenesis and reduced inflammation and apoptosis.
Preconditioning of hU-MSCs with isorhamnetin decreases wound progression by reducing inflammation, and improving tissue architecture and wound healing. The study outcome is expected to lead to an improved cell-based therapeutic approach for burn wounds.
Core Tip: In this study, we propose an improved cell-based therapeutic approach using a cytoprotective chemical compound, isorhamnetin to precondition human umbilical cord mesenchymal stem cells (MSCs) for second degree burn wounds. The findings of this study suggest that transplantation of preconditioned MSCs in the rat burn wound model decreases wound progression by the downregulation of inflammatory cytokines and restoration of tissue architecture. The study outcome may lead to an effective cell-based treatment strategy for burn wounds to accelerate wound healing and promote skin regeneration.
- Citation: Aslam S, Khan I, Jameel F, Zaidi MB, Salim A. Umbilical cord-derived mesenchymal stem cells preconditioned with isorhamnetin: potential therapy for burn wounds. World J Stem Cells 2020; 12(12): 1652-1666
- URL: https://www.wjgnet.com/1948-0210/full/v12/i12/1652.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i12.1652
Burn wounds caused by thermal injuries are the most common and devastating injuries, constituting approximately 86% of the burn victims admitted to hospitals. Burn injuries cause high morbidity and mortality with approximately 300000 deaths per year worldwide due to multiple organ dysfunction and abnormalities[1-4]. These injuries occur by exposure to intolerable heat, particularly hot surfaces, liquids, steam and flames[5,6]. Burn injuries are classified on the basis of severity and depth of the wound. In the case of superficial burns, heat penetrates only the epidermis and causes skin redness with slight inflammation. Partial thickness burns involve the epidermis and part of the dermis, causing pain and hypersensitivity. If, however, the burn completely damages the dermis, it causes red and white coloration of the tissue and reduces tissue sensitivity. Full thickness burns damage the subcutaneous layer causing white coloration of the tissue with no sensitivity. To improve burn prognosis, efforts are being made to use improved cellular therapy and tissue engineering approaches using biological skin replacements[7,8].
The process of wound healing is dynamic and involves regeneration of skin tissue after injury. The human body functions in a manner that any disruption to the first line of defense i.e., skin immediately activates a cascade of orchestrated events with three main phases: inflammation, proliferation and remodeling. The inflammatory phase begins as the cytokines and chemokines reinforce different cell types at the wound site by stimulating a signaling cascade, initiating cell migration and proliferation as well as elimination of cell debris[9]. Proliferation serves as the crucial phase of wound healing and occurs as the inflammation subsides. It promotes healing by inducing proliferation of various cell types, including those of the epidermal and endothelial layers and of the connective tissue[10]. It further leads to the formation of granulation tissue also termed fibroplasia which helps in the reorganization of the dermal matrix[11]. The final stage of wound healing termed tissue remodeling is responsible for a dynamic change in the tissue framework that continues for a long period as the cells develop matrix structure, which sometimes results in scar formation as the poorly oriented collagen is extensively constituted; however, the wound seems to be healed[12,13].
Impaired wounds and their aberrant healing are a major healthcare issue worldwide and a liability in patients and their families. Current treatment options used in clinics such as skin grafting are not very efficient and often do not completely promote wound closure. Efforts are being made to develop skin substitutes through bioengineering which is quite costly. Cell therapy has emerged as a promising strategy, with the ability to repair the tissue to the level of its original architecture[14]. Stem cells have the remarkable potential to differentiate into any cell type which makes them an attractive choice for the treatment of degenerative diseases[15]. Mesenchymal stem cells (MSCs) represent adult stem cells that belong to the population of non-hematopoietic stem cells originating from the mesodermal germ layer. These are multipotent cells with the ability to differentiate into multiple lineages. MSCs enhance wound healing by moderating the immune response and increasing angiogenesis[16-18]. Human umbilical cord tissue (Wharton’s jelly) serves as a potent and rich source of MSCs. However, these cells can also be isolated from bone marrow, adipose tissues, dental pulp etc., and can be easily expanded in culture[19-21]. A major obstacle in advancing stem cell therapy into successful clinical trials is their poor survival in the inflammatory environment of the impaired tissue, which reduces the efficiency of these cells[22]. Therefore, preconditioning strategies can be adopted by treating stem cells with small molecules having cytoprotective properties.
For preconditioning of stem cells, bioactive components of plants that have been traditionally used for the treatment of wounds could be beneficial. Various medicinal plants and plant products have been used in traditional medicine for healing of wounds[22,23]. The shrub Hippophae rhamnoides L commonly known as Sea buckthorn (SBT) which belongs to the family Elaeagnaceae has been extensively used for the treatment of skin diseases, asthma and lung disorders. This plant is a good source of a number of bioactive compounds like flavonoids such as quercetin, isorhamnetin, kaempferol etc.[24,25]. A study conducted on the aqueous extract of SBT showed enhanced wound healing due to a significant increase in wound contraction[26]. Due to its significant medicinal properties and beneficial role in wound healing, we selected isorhamnetin for our study. Isorhamnetin is a methylated derivative of quercetin, known for its anti-microbial and anti-inflammatory properties[27-29]. It has been used for the treatment of cutaneous wound healing[30,31]. We propose that this compound could enhance the migration efficiency and survival of MSCs at the wound site and increase their therapeutic potential for effective wound healing.
Human umbilical cord samples were obtained from Zainab Panjwani Memorial Hospital after approval of the Independent Ethics Committee of International Center for Chemical and Biological Sciences (IEC, ICCBS) with formal consent from the donor. Cord samples (8-10 cm) were collected in sterile phosphate-buffered saline (PBS) and processed in the tissue culture facility using aseptic conditions. Cord samples were dissected and cut into small pieces of 1-3 mm in size. The explants were then cultured in DMEM supplemented with 10% FBS, 100 U penicillin/streptomycin, 1 mmol/L sodium pyruvate and 4 mmol/L L-glutamine and incubated in a CO2 incubator at 37°C. The medium was changed every third day to remove non-adherent cells. After 10-15 d of culture, the cells were released from the explants and adhered to the flask surface. The cells at this stage were considered passage zero (P0) cells. Once the cells achieved 60%-80% confluence, they were subcultured by adding 1× trypsin-EDTA (0.25%). The cells were centrifuged for 8 min at 1000 rpm and the cell pellet was suspended in fresh DMEM and cultured in two flasks and incubated in a CO2 incubator at 37°C. These cultures were designated as passage 1 (P1) cells. Further subculturing of the cells was performed in the same manner. All experiments were conducted using P3-P5 cells.
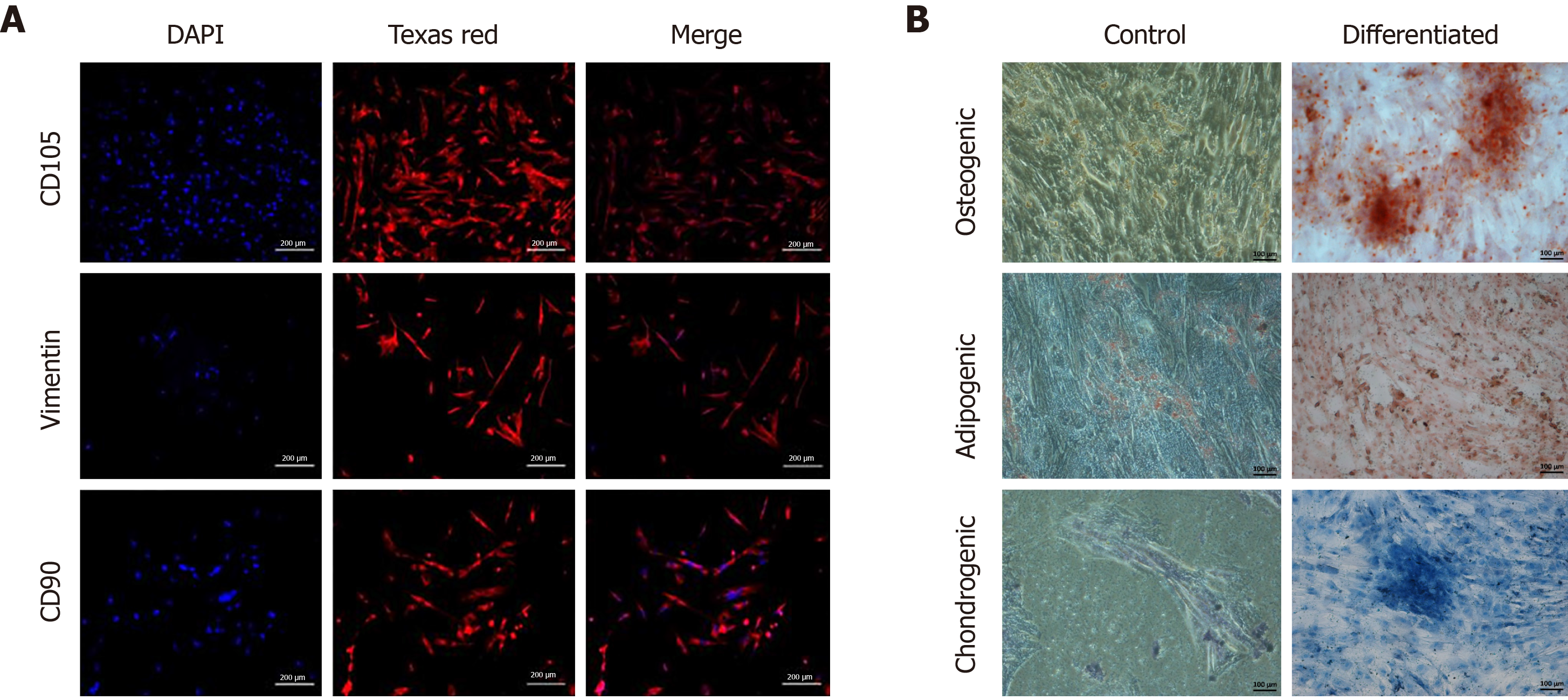
Human umbilical cord MSCs (hU-MSCs) were characterized by immunocytochemistry to determine specific surface markers. Approximately, 10000 cells per well were seeded in a 24-well plate and incubated in a CO2 incubator. Once the cells adhered, the medium was discarded and cells were washed with 1× PBS. The cells were fixed using 4% paraformaldehyde for 10 min. To prevent non-specific binding, blocking solution was added and the cells were incubated for 1 h. Specific monoclonal primary antibodies against CD105, vimentin and CD90 (1:100 dilution) were then added, respectively, and the cells were left overnight at 4°C. Alexa Fluor 546 goat anti-mouse IgG (1:200 dilution) was added for 60 min at 37°C. DAPI solution (0.5 μg/mL) was added for 15 min at 37°C to stain cell nuclei and the cells were observed under a fluorescence microscope (TE2000-S, Nikon, Japan).
hU-MSCs were characterized for trilineage differentiation as chondrogenic, osteogenic and adipogenic lineages. Approximately, 40000 cells were seeded per well in a 6 well plate and incubated at 37°C in a CO2 incubator for 24 h. Once the cells achieved 60%-70% confluence, they were washed with 1× PBS and sterile chondro-induction medium was added to each well and the plate was incubated again under the same conditions. The medium was changed every 4th d for approximately 21 d. After 21 d, the induction medium was removed followed by cell fixation with 4% PFA for 15 min. After fixation, the cells were stained with 1% Alcian Blue stain for 60 min followed by washing with 1× PBS. Osteogenic and adipogenic characterization was performed using a similar methodology. Staining was performed using 25% Alizarin Red stain for 50 min and Oil Red O stain for 60 min, respectively. Stained cells were observed under a phase contrast microscope.
For preconditioning, hU-MSCs were treated with isorhamnetin obtained commercially (Leap Chem, China). Calculated volumes for a range of concentrations (2-100 µmol/L) were used to determine the least cytotoxic concentration. The cytotoxicity analysis was performed using the MTT colorimetric assay according to manufacturer’s instructions. The optimized concentration (5 µmol/L) of isorhamnetin obtained from the cytotoxicity analysis was used in all subsequent experiments.
Cell migration potential was analyzed by performing the scratch assay to determine the effect of preconditioning. A T-25 culture flask was seeded with 3 × 106 cells/mL and treated with the optimized concentration (5 µmol/L) of isorhamnetin once the cells attained 80% confluence. After 24 h of treatment, the medium was discarded and the monolayer of cells was gently scratched in a straight line using a 200 μL sterile tip. Cell migration was evaluated by determining the distance covered by the cells, migrating towards the scratch area at time intervals of 24 and 48 h.
Adult Wistar rats of both sexes weighing 170-200 g were housed in the institutional animal resource facility and maintained at 21 ± 1ºC with relative humidity of 55% ± 5% and 12 h light/dark cycle with ad libitum access to food and water. All experiments were performed in accordance with the international guidelines for the care and use of laboratory animals recommended by NIH (NIH Publication No. 85-23, revised 1996) and approved by the Institutional Animal Care and Use Committee (IACUC, animal study protocol number: 2018-0018). The in vivo burn wound model was developed following a protocol of consistent burn injury[32]. Five animals were used for the development of the burn model. Intraperitoneal injections of 60 mg/kg of ketamine hydrochloride (50 mg/mL) and 7 mg/kg of xylazine hydrochloride (100 mg/mL) were given to the rats to induce anesthesia. Hair was removed from the dorsal surface of the skin at the wound site using depilatory cream. The skin was thoroughly cleaned and disinfected with an alcohol swab. A 1.5 cm diameter steel rod weighing 150 g was placed in a beaker containing water and heated on a hot plate until the temperature reached 100°C. The heated rod was kept on the stretched and pulled skin for 10 s without applying any external pressure to form a consistent burn wound. To minimize pain or discomfort, the rats were injected subcutaneously with diclofenac sodium (25 mg/mL) and streptomycin/penicillin (10000 U/mL). The animals were euthanized with a dose of 200 mg/kg body weight of sodium pentobarbital for tissue harvesting.
Cells were prepared for transplantation after 24 h of treatment while untreated cells were used as the control. Cells were trypsinized and the pellet was suspended in 1× PBS. Localized transplantation of one million cells using a sterile insulin syringe was performed at the wound periphery with multiple injections at different sites after 72 h of burn infliction. Five animals were used for each transplantation group.
Control and treated burn wounds were macroscopically visualized for rate of skin contraction and detachment of the scab. Images of control and treated burns were captured over a period of 2 wk at defined time points of day 1, 3, 7 and 14 with reference to phases of wound healing.
Histological analysis was performed by harvesting tissue samples on day 1, 3, 7 and 14. About 6 μmol/L thin paraffin sections of embedded tissue blocks were collected on the slides, and were stained using hematoxylin-eosin stain for microscopic analysis.
For gene expression analysis, tissues were harvested and homogenized in the presence of the One Step-RNA reagent (Bio Basic, Canada). RNA was extracted according to the recommendations provided by the manufacturer. RNA was quantified by measuring the absorbance at 260 nm and the presence of protein contamination was determined by calculating the ratio of absorbance at 260/280 nm. cDNA was synthesized using the RevertAid First Strand cDNA synthesis kit (Fermentas, ThermoFisher Scientific, United States) according to the manufacturer’s protocol.
Real-time polymerase chain reaction (qPCR) was performed to determine the changes in gene expression. The genes were selected corresponding to the cytokines involved at different phases of wound healing. Amplification was performed using qPCR (CXF96 Touch Real-Time PCR Detection System, Bio-Rad, United States) under the following conditions; denaturation at 95°C for 1 min, annealing at 58°C for 1 min (40 cycles) and extension at 72°C for 1 min. GAPDH was taken as an internal control. The genes analyzed are presented in Table 1.
| No. | Genes | Primer sequence (5’-3’) | Annealing temperatures (ºC) | Product sizes (bp) |
| 1 | Interleukin 1 beta (IL-1β) | F: TCATCTTTGAAGAAGAGCCCGT | 60 | 170 |
| R: GTTCTGTCCATTGAGGTGGAGA | ||||
| 2 | Interleukin 6 (IL-6) | F: GATGGATGCTTCCAAACTGGATA | 58 | 140 |
| R: TGAATGACTCTGGCTTTGTCTTT | ||||
| 3 | Transforming growth factor (TGF-β) | F: TCGCCAGTCCCCCGA | 60 | 101 |
| R: TCGGGCTCCGGGTCA | ||||
| 4 | Vascular endothelial growth factor (VEGF) | F: CCAATTGAGACCCTGGTGGA | 60 | 195 |
| R: TCCTATGTGCTGGCTTTGGT | ||||
| 5 | Tumor necrosis factor (TNF-α) | F: CCTCTTCTCATTCCTGCTCGT | 60 | 144 |
| R: GATCTGAGTGTGAGGGTCTGG | ||||
| 6 | Bcl-2 associated X (Bax) | F: CTTCTTCCGTGTGGCAGC | 58 | 172 |
| R: CAGACAAACAGCCGCTCC | ||||
| 7 | Apoptosis regulator (Bcl-2) | F: ACCCCTGGCATCTTCTCC | 59 | 172 |
| R: AGAAGTCATCCCCAGCCC | ||||
| 8 | Matrix metallopeptidase 9 (MMP-9) | F: TACCAGCTACTCGAACCAATCA | 59 | 187 |
| R: AAATAAAAGGGCCGGTAAGGTG |
The statistical analysis was performed by One-way ANOVA with the Bonferroni post-hoc test using IBM SPSS Statistics 21. All values were represented as mean ± SE with the number of observations (n) as 3-5 and level of confidence as aP < 0.05; where aP < 0.05, bP < 0.01 and cP < 0.001.
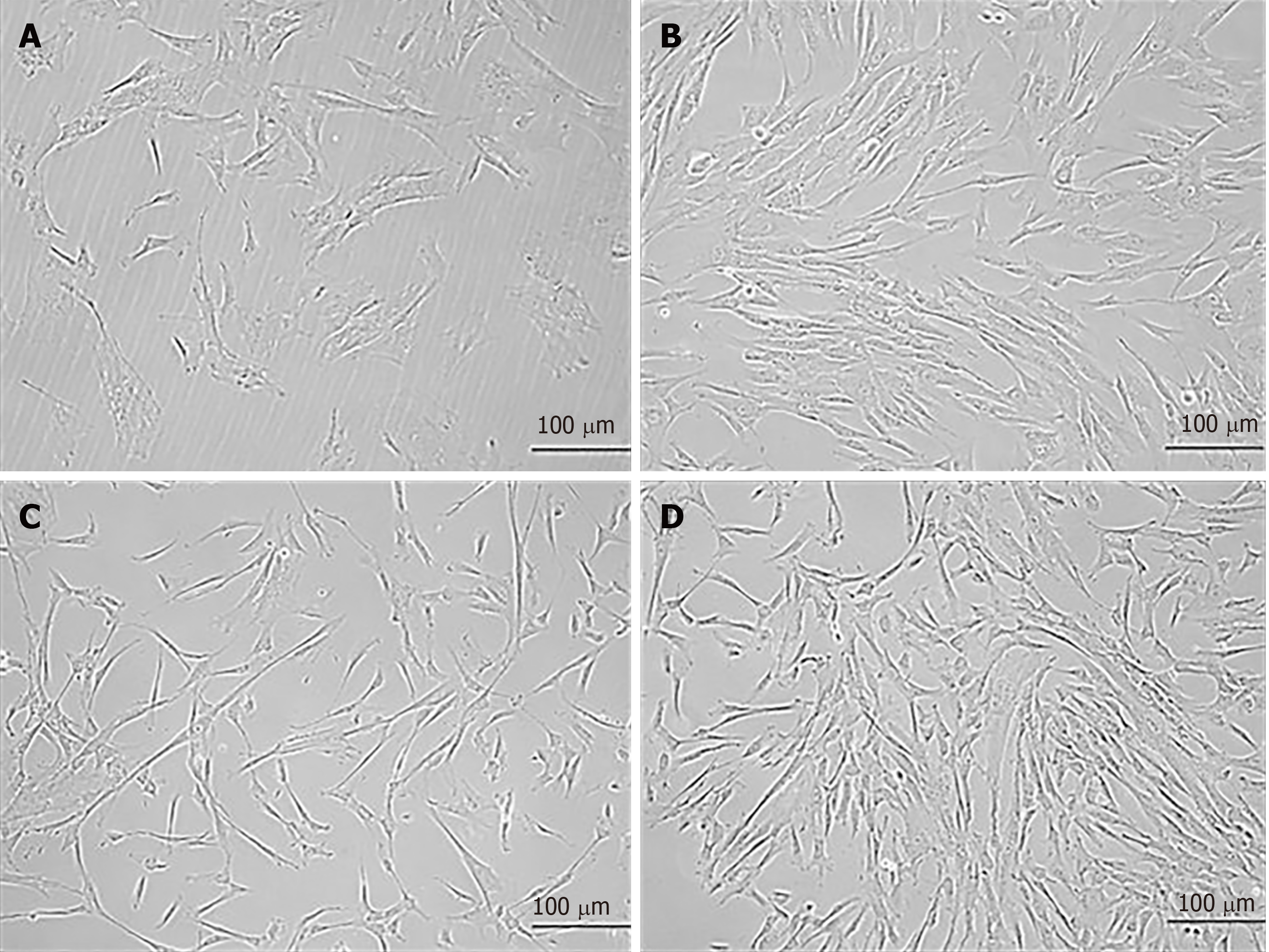
Cells released from the explant were termed as P0, which proliferated up to 70% confluence, and formed a monolayer by the 12th d. Later, the cells were subcultured to P1 and left for 12 d to achieve the required confluence for further expansion (Figure 1). Isolated cells were positive for CD105, vimentin and CD90 surface markers. This confirmed the single and homogenous population of hU-MSCs (Figure 2A). In the case of trilineage differentiation, cells grown in the selective medium showed mineral deposits after Alizarin Red staining, prominent oil droplets after Oil Red O staining and proteoglycans after Alcian Blue staining, confirming that the cells differentiated into osteocytes, adipocytes and chondrocytes, respectively (Figure 2B).
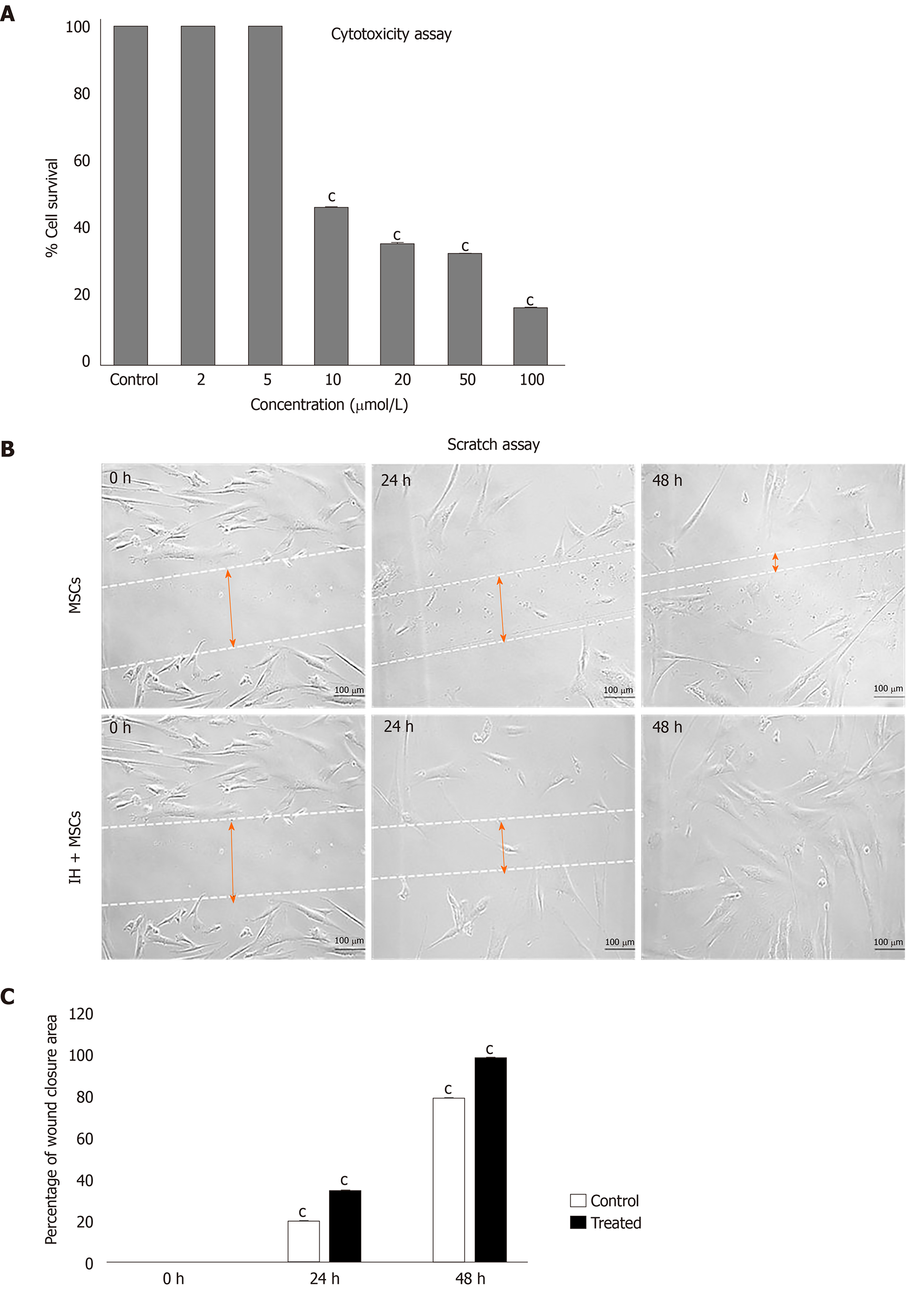
The cytotoxicity analysis showed that the percentage of cell survival decreased with increasing concentration of the compound. Less than 5 µmol/L of isorhamnetin was confirmed as non-cytotoxic (Figure 3A). For preconditioning, hU-MSCs were treated with 5 µmol/L concentration for 24 h. Isorhamnetin-treated MSCs are referred to as preconditioned MSCs (IH + MSCs) in subsequent sections.
Quantitative analysis of the scratch test showed that IH + MSCs migrated towards the scratch better than normal MSCs. The scratch area in IH + MSCs was significantly reduced compared to normal MSCs at 24 h and complete closure of the scratch was observed at 48 h (Figure 3B).
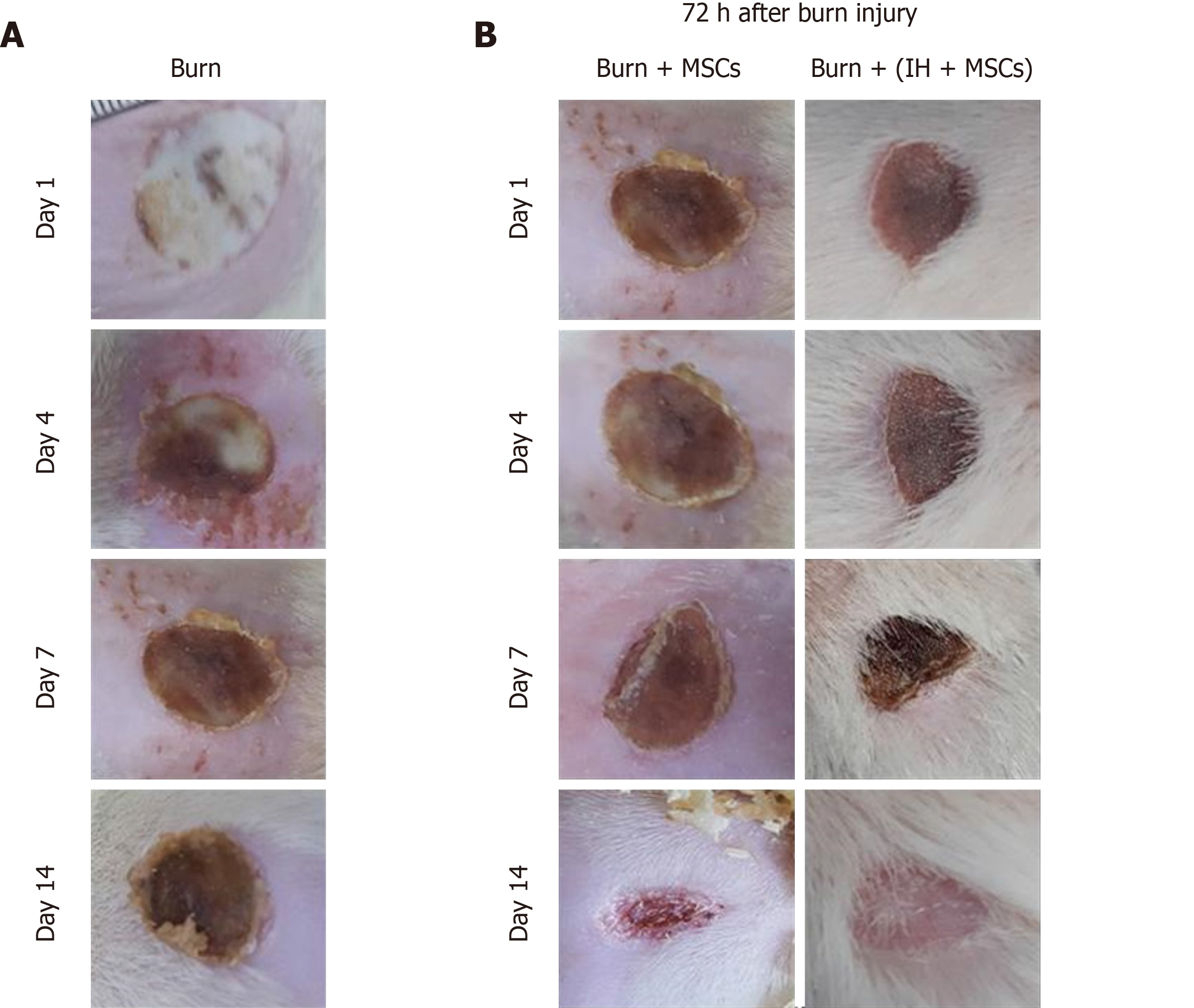
All groups were macroscopically observed at time points corresponding to the phases of wound healing that is day 1, 4, 7 and 14. Detachment of the scab indicates initiation of wound healing. In the case of the burn wound, the scab was not detached until day 14. Burn wound treated with normal MSCs showed scab detachment at day 14. The wound area was also reduced as compared to the burn wound at the same time point. The IH + MSCs treated burn wound showed scab detachment as well as re-epithelialization of the wound at day 14. Slight hair growth was also observed indicating hair follicle regeneration with complete healing of the wound (Figure 4).
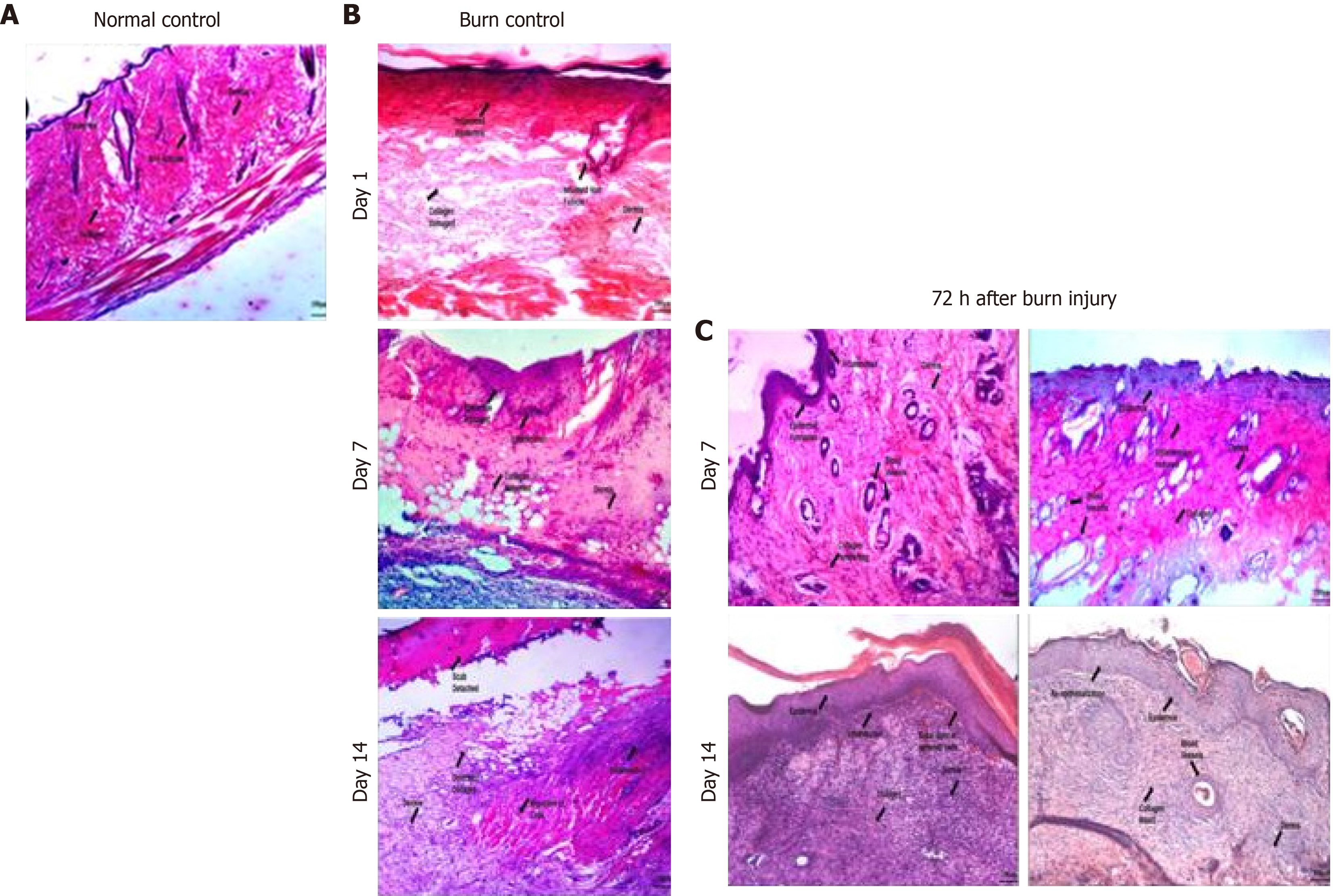
The histology of burn wound after day 1 of burn injury showed that both the epidermis and dermis were damaged due to inflammation with distorted collagen matrix and dermis. Hair follicles were also inflamed with increased infiltration of inflammatory cells which confirmed second degree burn. At day 7 and 14, angiogenesis and scab formation were observed which indicated progression towards healing but with a deformed tissue framework (Figure 5B). In comparison to the burn control, histological analysis of the MSCs treated wound showed increased angiogenesis and reduced inflammation of the hair follicles and initiation of epidermis formation at day 7. Fourteen days after transplantation, reduced angiogenesis, formation of epidermis and dermis with extracellular matrix deposition and infiltration of inflammatory cells were observed. The IH + MSCs treated burn wound showed significant healing and remodeling without scar formation as compared to the burn control and normal MSCs treated burn wound. At day 7, epidermis formation was observed along with increased angiogenesis and reduced inflammation. It was observed that the dermis formation and collagen deposition were similar to the natural tissue framework. Later, at day 14, complete re-epithelialization was observed with intact collagen, healthy blood vessel formation and completely remodeled tissue framework with no sign of inflammation and scar formation (Figure 5C).
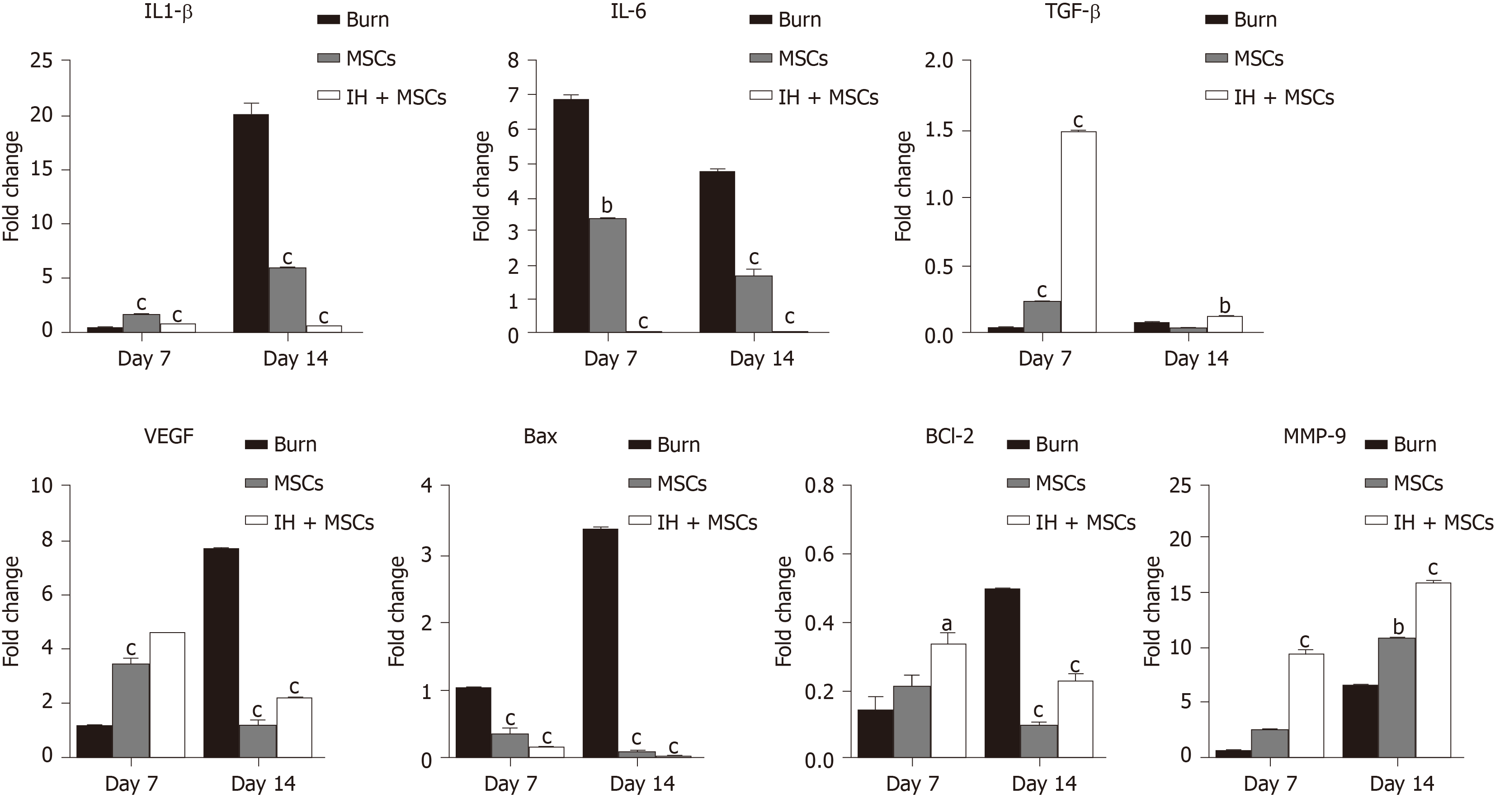
The gene expression analysis of the burn control, normal MSCs, and IH + MSCs treated burn wound showed significant modulation which was time dependent in some cases (Figure 6). The inflammatory genes interleukin (IL)-1β and IL-6 were significantly downregulated in the case of both normal MSCs and IH + MSCs treated burn wounds as compared to the burn control, but this decrease was observed at day 14 in the case of IL-1β. The anti-inflammatory gene, transforming growth factor β (TGF-β) showed significant upregulation in the case of normal MSCs at day 7, and at both time points in the case of IH + MSCs. The angiogenic gene, vascular endothelial growth factor (VEGF) was also upregulated in both treatment groups as compared to the burn control at day 7 and its expression was reduced at day 14. Apoptosis was reduced as shown by the decreased levels of Bcl-2 associated X (Bax) gene in both treatment groups, while anti-apoptotic Bcl-2 gene was upregulated in the case of IH + MSCs at day 7. Matrix metallopeptidase 9 (MMP-9) levels were found to be significantly upregulated in the normal MSCs and IH + MSCs treated burn wounds as compared to the burn control which showed improved tissue architecture.
In this study, we used a small molecule, isorhamnetin (a flavonoid), to precondition hU-MSCs, in order to improve the survival and migration potential of MSCs. This could lead to complete wound healing without scar formation, thus enhancing the therapeutic potential of these MSCs. Migration potential corresponds to the rate of wound healing. In the in vitro scratch assay, we observed that the preconditioned MSCs showed significant migration potential as compared to normal MSCs after 24 and 48 h. These preconditioned cells migrated towards the scratch resulting in the complete closure of the scratch compared to the normal MSCs. This confirms the improved migration potential of preconditioned MSCs necessary for rapid wound healing.
To analyze the effect of preconditioning in vivo, we developed rat burn wound model with a consistent second degree burn. Macroscopic analysis after burn injury showed severe edema with a pale appearance on the burn site. After 24 h, the wound appeared yellowish brown with a well-defined margin of erythema and no signs of blistering, which is in agreement with previous studies[33,34]. The wound progression led to a thick scab which completely detached at day 21, leaving behind granulation tissue. This indicates the initiation of wound healing through angiogenesis which is responsible for restoring skin structure at the epidermal level[35]. Macroscopic observation of the wound tissue transplanted with normal MSCs and IH + MSCs showed no apparent change in the wound morphology compared to the burn wound at day 7. Detachment of the scab was observed at day 14 in both groups. In addition, the formation of granulation tissue was observed in normal MSCs, while complete wound closure and re-epithelialization with no granulation tissue and scar formation was seen in the case of IH+MSCs.
Histological analysis of the wound tissues further confirmed our macroscopic findings. Inflammation of the epidermis after 24 h corresponded to the edema and erythema observed macroscopically. Slow progress of the burn wound is indicated by an increase in loose connective tissues and infiltration of lymphocytes and plasma cells after 72 h. Disrupted epidermis and dermis with extreme inflammation and conservation of hypodermis persisted even after 1 wk (day 7), which is in agreement with previous studies[36,37]. Microscopic analysis of the burn wound at day 14 revealed adequate tissue autolysis with distinct neovascularization, formation of granulation tissue and moderate fibrosis with no signs of remodeling. Normal MSCs and IH + MSCs treated wound tissues revealed significant improvement in wound healing as compared to the burn control. Normal MSCs treated wounds at day 7 showed increased angiogenesis, regeneration of epidermis, and extensive infiltration of inflammatory cells along the epidermal layer and within the lumen of newly formed vessels. Significant fibroblast proliferation was also observed forming granulation tissue under the scab. In contrast, IH + MSCs treated wound tissues showed reduced inflammation, increased neovascularization and granulation tissue formation at day 7, forming the dermis architecture. At day 14, complete scab detachment and regeneration of epidermis along with a keratin layer was observed. Initiation of dermis formation with collagen deposition and an increase in fibroblast proliferation indicates the presence of granulation tissue[38]. Vascularization was decreased at this stage by stimulating vessel regression. This may progress towards remodeling[39]. Moreover, the IH + MSCs treated burn wound showed re-epithelialization and remodeling without scar formation, which also supports our macroscopic data.
Modulation of gene expression was time dependent in some cases. Burn wound tissue transplanted with normal, and preconditioned MSCs showed significantly reduced gene expression of both the inflammatory genes, IL-1β and IL-6, which confirmed the anti-inflammatory effect of the compound particularly at day 14. It is also evident in the case of our histological analysis of the corresponding wound tissues. The expression of TGF-β, an anti-inflammatory cytokine, in the wound bed of both the normal MSCs and IH + MSCs treated groups showed increased levels at day 7, and the level persisted until day 14 in the latter group. It was shown in an earlier study that TGF-β promotes angiogenesis by stimulating the release of IL-1β and tumor necrosis factor-α[40]. As TGF-β expression is essential for dermal wound healing, its increased expression can lead to scar formation following fibrosis[41]. The expression of VEGF after transplantation of normal MSCs and IH + MSCs was found to be significantly elevated at day 7, which correlated with our histological findings which showed increased angiogenesis. These results are consistent with an earlier study which showed that the time point of VEGF expression is crucial for the progression of wound healing; during proliferation which lasts for about 3-7 d, neovascularization is at its maximum and the VEGF level increases[42-44]. At day 14, we observed that VEGF expression was reduced in both treatment groups. This provides evidence of tissue maturation and remodeling[45]. The expression of apoptotic protein Bax was significantly reduced after transplantation of normal MSCs and IH + MSCs at days 7 and 14. Bcl-2, an anti-apoptotic protein, was increased at day 7; however, at day 14, it was downregulated. This is supported by our histological analysis which showed that the tissue framework was regenerated at day 14, leading to re-epithelialization and no scar formation during remodeling. With regard to MMP-9 expression, the IH + MSCs group showed significant upregulation of its expression. MMP-9 is observed in several damaged epithelia including gut and eye along with the skin during wound healing[46,47]. The elevated expression of MMP-9 suggested an increase in re-epithelialization leading to restoration of the tissue framework similar to that of uninjured tissue.
In burn wounds, inflammation is the major contributor to tissue injury and pathological remodeling. One of the reasons for using isorhamnetin is to reduce the inflammation in burn tissue in combination with MSCs. Previously, it was reported that isorhamnetin possesses anti-inflammatory properties[48]. It has also been reported to have anti-oxidant properties. During burn wounds, inflammatory cells, neutrophils and macrophages concentrate at the site of injury, and reactive oxygen species are generated increasing oxidative stress. With its anti-oxidant properties, isorhamnetin has been shown to inhibit hydrogen peroxide-induced reactive oxygen species production[49]. Results obtained from our in vivo experiments correlated well with the anti-inflammatory potential of isorhamnetin, which led to the efficient preconditioning of MSCs for improved wound healing.
It is concluded that preconditioning of human umbilical cord-derived MSCs with the anti-inflammatory compound, isorhamnetin, heals the burn wound causing re-epithelialization and remodeling without scar formation. The skin tissue framework was repaired to the level of normal tissue with regeneration of the hair follicles. Preconditioning reduces the inflammatory reaction after burn injury, which not only decreases wound progression by downregulating inflammatory cytokines but also leads to restoring tissue architecture by maintaining the balance in the expression of cytokines in the respective phases of wound healing.
Impaired wound healing can be associated with different pathological states. Burn wounds are the most common and detrimental injuries that remain a major health issue worldwide. Mesenchymal stem cells (MSCs) possess the ability to regenerate tissues by secreting factors involved in promoting cell migration, proliferation and differentiation, while suppressing immune reactions. Preconditioning of MSCs with small molecules having cytoprotective properties can enhance the potential of these cells for their use in cell-based therapeutics.
Mesenchymal stem cells are a promising source for cell-based treatment to achieve skin regeneration. Preconditioning approaches using treatment with cytoprotective agents can lead to improvement in the wound healing potential of these stem cells and would be a crucial step in defining efficient therapy following burn injuries.
The objective of this study was to enhance the therapeutic potential of human umbilical cord MSCs (hU-MSCs) by preconditioning them with the cytoprotective flavonoid compound, isorhamnetin in a second degree burn wound model.
hU-MSCs were isolated and characterized by specific surface markers. hU-MSCs were treated with isorhamnetin to precondition these cells. The migration potential of MSCs was analyzed by the in vitro scratch assay, while the healing potential of normal, and preconditioned MSCs was compared by transplanting them into a rat model of a second degree burn wound 72 h after burn injury and observed for 2 wk. Histological and gene expression analyses were performed on day 7 and 14 after cell transplantation to determine complete wound healing.
The in vitro scratch assay analysis showed that preconditioned hU-MSCs significantly reduced the scratch area at 24 h, and completely closed the scratch area at 48 h as compared to normal MSCs. The preconditioned MSCs showed reduced inflammation, remodeled epidermis and dermis without scar formation and regeneration of hair follicles as observed following histological analysis. Preconditioning of MSCs promoted angiogenesis and remodeling, and decreased apoptosis as observed by gene expression studies of the corresponding burn wounds.
Preconditioning of hU-MSCs with isorhamnetin decreases wound progression by reducing inflammation, and improving tissue architecture and wound healing. The study outcome is expected to lead to an improved cell-based therapeutic approach for burn wounds.
The current study will be useful for developing an effective treatment strategy for burn patients based on the synergistic effect of isorhamnetin and MSCs to accelerate wound healing with complete skin regeneration without scar formation.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casado-Diaz A, Jurjus A S-Editor: Gao CC L-Editor: Webster JR P-Editor: Xing YX
| 1. | Brink C, Isaacs Q, Scriba MF, Nathire MEH, Rode H, Martinez R. Infant burns: A single institution retrospective review. Burns. 2019;45:1518-1527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Jones CD, Ho W, Gunn E, Widdowson D, Bahia H. E-cigarette burn injuries: Comprehensive review and management guidelines proposal. Burns. 2019;45:763-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN. Emerging infections in burns. Surg Infect (Larchmt). 2009;10:389-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Williams FN, Herndon DN, Hawkins HK, Lee JO, Cox RA, Kulp GA, Finnerty CC, Chinkes DL, Jeschke MG. The leading causes of death after burn injury in a single pediatric burn center. Crit Care. 2009;13:R183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Kraft R, Herndon DN, Al-Mousawi AM, Williams FN, Finnerty CC, Jeschke MG. Burn size and survival probability in paediatric patients in modern burn care: a prospective observational cohort study. Lancet. 2012;379:1013-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Jeschke MG, Gauglitz GG, Finnerty CC, Kraft R, Mlcak RP, Herndon DN. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg. 2014;259:814-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Ramos-e-Silva M, Ribeiro de Castro MC. New dressings, including tissue-engineered living skin. Clin Dermatol. 2002;20:715-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26:812-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 10. | Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg. 1998;25:341-356. [PubMed] [Cited in This Article: ] |
| 11. | Falanga V. Wound healing and chronic wounds. J Cutan Med Surg. 1998;3 Suppl 1:S1-1. [PubMed] [Cited in This Article: ] |
| 12. | Lawrence WT, Diegelmann RF. Growth factors in wound healing. Clin Dermatol. 1994;12:157-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 146] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Martin P, Hopkinson-Woolley J, McCluskey J. Growth factors and cutaneous wound repair. Prog Growth Factor Res. 1992;4:25-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 162] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Kosaric N, Kiwanuka H, Gurtner GC. Stem cell therapies for wound healing. Expert Opin Biol Ther. 2019;19:575-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 15. | Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs. 2009;24:98-103; quiz 104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Granero-Molto F, Weis JA, Longobardi L, Spagnoli A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther. 2008;8:255-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 652] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 18. | Bianchi G, Borgonovo G, Pistoia V, Raffaghello L. Immunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells. Histol Histopathol. 2011;26:941-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 51] [Reference Citation Analysis (0)] |
| 19. | In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 824] [Cited by in F6Publishing: 790] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 20. | Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1210] [Cited by in F6Publishing: 1083] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 22. | Gupta A, Upadhyay NK, Sawhney RC, Kumar R. A poly-herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regen. 2008;16:784-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Shetty S, Udupa S, Udupa L. Evaluation of Antioxidant and Wound Healing Effects of Alcoholic and Aqueous Extract of Ocimum sanctum Linn in Rats. Evid Based Complement Alternat Med. 2008;5:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Gupta A, Kumar R, Pal K, Banerjee PK, Sawhney RC. A preclinical study of the effects of seabuckthorn (Hippophae rhamnoides L.) leaf extract on cutaneous wound healing in albino rats. Int J Low Extrem Wounds. 2005;4:88-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Zu Y, Li C, Fu Y, Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharm Biomed Anal. 2006;41:714-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Upadhyay NK, Kumar R, Siddiqui MS, Gupta A. Mechanism of Wound-Healing Activity of Hippophae rhamnoides L. Leaf Extract in Experimental Burns. Evid Based Complement Alternat Med. 2011;2011:659705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Lee HJ, Lee HJ, Lee EO, Ko SG, Bae HS, Kim CH, Ahn KS, Lu J, Kim SH. Mitochondria-cytochrome C-caspase-9 cascade mediates isorhamnetin-induced apoptosis. Cancer Lett. 2008;270:342-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Jaramillo S, Lopez S, Varela LM, Rodriguez-Arcos R, Jimenez A, Abia R, Guillen R, Muriana FJ. The flavonol isorhamnetin exhibits cytotoxic effects on human colon cancer cells. J Agric Food Chem. 2010;58:10869-10875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Kim JE, Lee DE, Lee KW, Son JE, Seo SK, Li J, Jung SK, Heo YS, Mottamal M, Bode AM, Dong Z, Lee HJ. Isorhamnetin suppresses skin cancer through direct inhibition of MEK1 and PI3-K. Cancer Prev Res (Phila). 2011;4:582-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Park BK, Lee S, Seo JN, Rhee JW, Park JB, Kim YS, Choi IG, Kim YE, Lee Y, Kwon HJ. Protection of burn-induced skin injuries by the flavonoid kaempferol. BMB Rep. 2010;43:46-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Hosnuter M, Melikoglu C, Aslan C, Saglam G, Sutcu R. The Protective Effects of Epigallocatechin Gallate Against Distant Organ Damage After Severe Skin Burns--Experimental Study Using a Rat Model of Thermal Trauma. Adv Clin Exp Med. 2015;24:409-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1012] [Cited by in F6Publishing: 1025] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 33. | Cai EZ, Ang CH, Raju A, Tan KB, Hing EC, Loo Y, Wong YC, Lee H, Lim J, Moochhala SM, Hauser CA, Lim TC. Creation of consistent burn wounds: a rat model. Arch Plast Surg. 2014;41:317-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Guo HF, Ali RM, Hamid RA, Zaini AA, Khaza'ai H. A new model for studying deep partial-thickness burns in rats. Int J Burns Trauma. 2017;7:107-114. [PubMed] [Cited in This Article: ] |
| 35. | Busuioc CJ, Mogoşanu GD, Popescu FC, Lascăr I, Pârvănescu H, Mogoantă L. Phases of the cutaneous angiogenesis process in experimental third-degree skin burns: histological and immunohistochemical study. Rom J Morphol Embryol. 2013;54:163-171. [PubMed] [Cited in This Article: ] |
| 36. | Chandran PK, Kuttan R. Effect of Calendula officinalis Flower Extract on Acute Phase Proteins, Antioxidant Defense Mechanism and Granuloma Formation During Thermal Burns. J Clin Biochem Nutr. 2008;43:58-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Tavares Pereira Ddos S, Lima-Ribeiro MH, de Pontes-Filho NT, Carneiro-Leão AM, Correia MT. Development of animal model for studying deep second-degree thermal burns. J Biomed Biotechnol. 2012;2012:460841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns. 2014;40:1375-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Bodnar RJ. Chemokine Regulation of Angiogenesis During Wound Healing. Adv Wound Care (New Rochelle). 2015;4:641-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Ganapathy N, Venkataraman SS, Daniel R, Aravind RJ, Kumarakrishnan VB. Molecular biology of wound healing. J Pharm Bioallied Sci. 2012;4:S334-S337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 2006;20:692-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 729] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 43. | Frank S, Hübner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607-12613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 502] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 44. | Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445-1452. [PubMed] [Cited in This Article: ] |
| 45. | Bodnar RJ, Yates CC, Rodgers ME, Du X, Wells A. IP-10 induces dissociation of newly formed blood vessels. J Cell Sci. 2009;122:2064-2077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Fini ME, Parks WC, Rinehart WB, Girard MT, Matsubara M, Cook JR, West-Mays JA, Sadow PM, Burgeson RE, Jeffrey JJ, Raizman MB, Krueger RR, Zieske JD. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol. 1996;149:1287-1302. [PubMed] [Cited in This Article: ] |
| 47. | Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065-2072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Iannuzzi AM, Moschini R, De Leo M, Pineschi C, Balestri F, Cappiello M, Braca A, Del-Corso, A. Chemical profile and nutraceutical features of Salsola soda (agretti): Anti-inflammatory and antidiabetic potential of its flavonoids. Food Bioscience. 2020;37:100713. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Choi YH. The cytoprotective effect of isorhamnetin against oxidative stress is mediated by the upregulation of the Nrf2-dependent HO-1 expression in C2C12 myoblasts through scavenging reactive oxygen species and ERK inactivation. Gen Physiol Biophys. 2016;35:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |