Published online Aug 26, 2019. doi: 10.4252/wjsc.v11.i8.452
Peer-review started: February 27, 2019
First decision: June 5, 2019
Revised: July 4, 2019
Accepted: July 16, 2019
Article in press: July 16, 2019
Published online: August 26, 2019
Ischemic stroke is a critical disease which causes serious neurological functional loss such as paresis. Hope for novel therapies is based on the increasing evidence of the presence of stem cell populations in the central nervous system (CNS) and the development of stem-cell-based therapies for stroke patients. Although mesenchymal stem cells (MSCs) represented initially a promising cell source, only a few transplanted MSCs were present near the injured areas of the CNS. Thus, regional stem cells that are present and/or induced in the CNS may be ideal when considering a treatment following ischemic stroke. In this context, we have recently showed that injury/ischemia-induced neural stem/progenitor cells (iNSPCs) and injury/ischemia-induced multipotent stem cells (iSCs) are present within post-stroke human brains and post-stroke mouse brains. This indicates that iNSPCs/iSCs could be developed for clinical applications treating patients with stroke. The present study introduces the traits of mouse and human iNSPCs, with a focus on the future perspective for CNS regenerative therapies using novel iNSPCs/iSCs.
Core tip: Ischemic stroke is a critical disease that is accompanied by serious symptoms, such as paresis. Until recently, it was believed that areas affected by stroke mainly consist of necrotic and inflammatory cells. However, we have recently demonstrated that novel ischemia-induced stem cells can be isolated from not only mouse brains after stroke but also human brains after stroke. These stem cells exhibited the multipotency and differentiated into electrophysiologically functional neurons. In this article, we introduce the future perspectives for patients suffering from ischemic stroke using these regionally derived stem cells.
- Citation: Nakagomi T, Takagi T, Beppu M, Yoshimura S, Matsuyama T. Neural regeneration by regionally induced stem cells within post-stroke brains: Novel therapy perspectives for stroke patients. World J Stem Cells 2019; 11(8): 452-463
- URL: https://www.wjgnet.com/1948-0210/full/v11/i8/452.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i8.452
Cerebrovascular diseases, including stroke, are a leading cause of death worldwide. Owing to recent therapeutic advances such as reperfusion therapies by intravenous administration of recombinant tissue plasminogen activator (IV t-PA) and neuroendovascular treatment, including mechanical thrombectomy[1-3], some patients can recover from stroke without sequelae. With the increased implementation of these therapies, it is speculated that more stroke patients can benefit from them. In addition, the therapeutic time window of IV t-PA was extended to 4.5 h[4]. Moreover, there is a possibility, when guided by imaging, for the IV t-PA indication to be expanded in patients with acute ischemic stroke of unknown onset[5]. As for mechanical thrombectomy, the therapeutic time window was expanded up to 16 h from onset or to 24 h if the acute stroke patients had a mismatch between the ischemic core and hypoperfusion area[6,7]. However, many patients with stroke are not eligible for these therapies because of excluding factors (e.g., time after onset and portion of vascular obstruction). Currently, approximately 13%-20% of acute ischemic stroke patients are potentially eligible for mechanical thrombectomy[7,8]. In patients who had mechanical thrombectomy, the rate of good clinical outcome was below 50%[3]. Alternatively, patients receive rehabilitation, but many continue to suffer from various sequelae such as paresis.
Thus, more attention is paid to reparative medicines, particularly to those based on stem cell therapies. Various types of stem cells, including neural stem/progenitor cells (NSPCs)[9-12], mesenchymal stem cells (MSCs)[13,14] (e.g., bone marrow-derived MSCs, adipose-derived MSCs [15,16]), embryonic stem (ES) cell-derived NSPCs[17], and induced pluripotent stem (iPS) cell-derived NSPCs[18], are considered as candidates for cell transplantation following ischemic stroke.
Although the central nervous system (CNS), brain and spinal cord, was long considered not to have regeneration potential after injury, accumulating evidence indicate that the adult CNS contains NSPCs[19,20]. Therefore, CNS repair might be achieved through endogenous stem cells. However, no concrete evidence showing that stem-cell-based therapies by NSPCs are clinically useful for patients with various CNS diseases, including stroke, was reported. Although the reason remains unclear, increasing evidence shows that the traits of not only stem cells themselves but also a stem-cell niche surrounding stem cells (e.g., endothelial cells) alter after ischemia/hypoxia and differ among the developing ages of mice in the CNS[21-24]. Thus, the lack of data may be due to the NSPCs being derived not from pathological but from normal conditions (e.g., developmental fetal NSPCs)[9,10] and investigation having focused on the reparative mechanism not emerging from the pathological CNS.
In our laboratory, we aimed to develop a method to isolate and utilize endogenous NSPCs specifically induced by brain injury such as ischemic stroke (injury/ischemia-induced NSPC; iNSPC). We used a mouse model of cerebral infarction whose post-ischemic areas were highly reproducible[25,26]. As a result, we demonstrated for the first time that, although mature neural cells such as neurons, astrocytes, and oligodendrocytes underwent cell death within ischemic regions, iNSPCs that had the potential to differentiate into these cells developed within the same areas[27]. In addition, we have shown that activation of iNSPCs promoted neural repair and functional recovery following ischemic stroke[22,28].
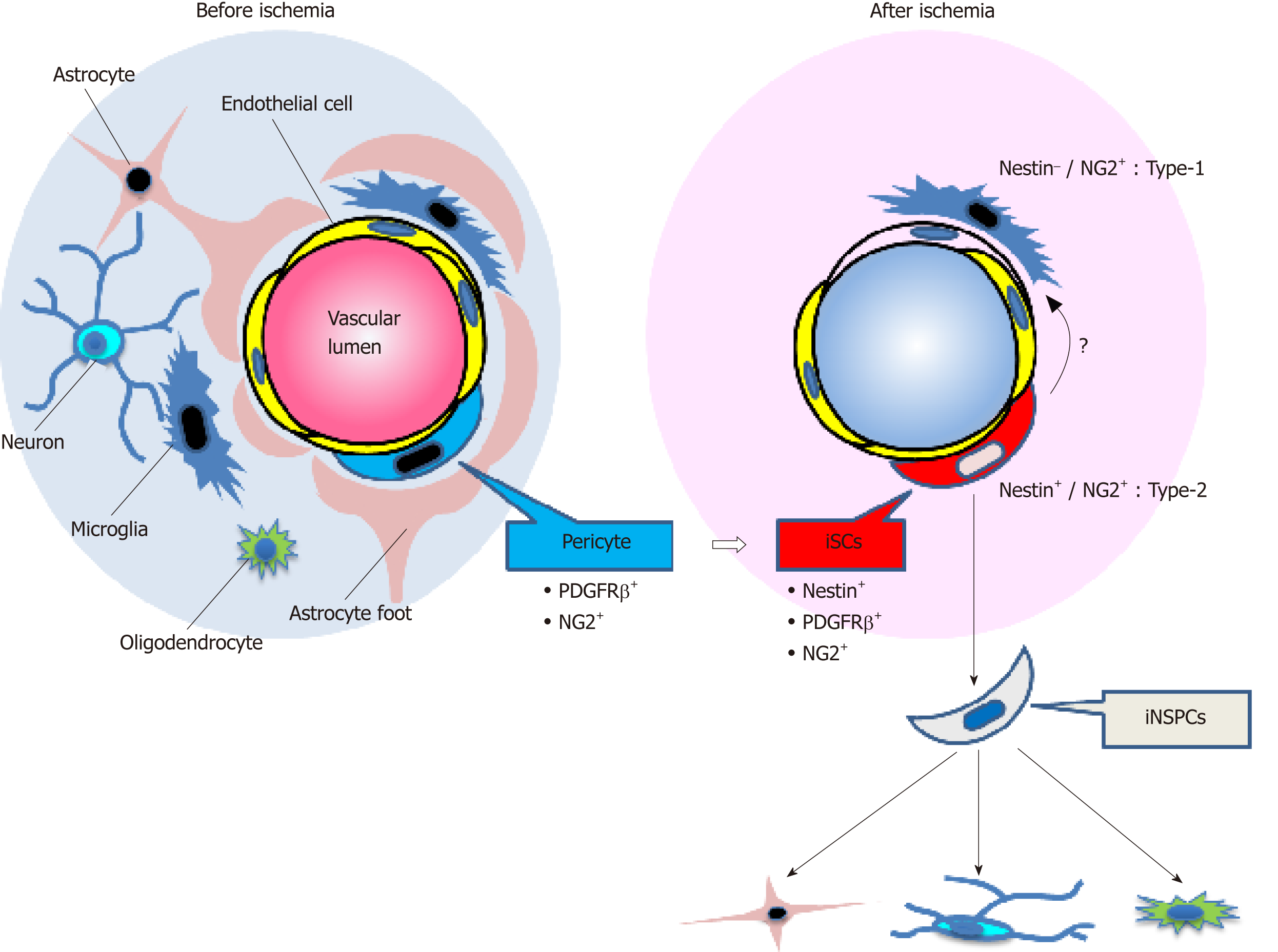
Many types of cells, including astrocytes in the subventricular zone (SVZ)[29,30], reactive astrocytes[31], resident glia[32], oligodendrocyte precursor cells (OPCs)[33,34], and ependymal cells[35,36], have been reported as NSPC candidates. Although the origin of iNSPCs remains unclear, previous studies showed that several types of NSPCs such as SVZ astrocytes[37,38] and OPCs[39,40] reside near blood vessels, in close association with endothelial cells. We have previously shown that nestin+ iNSPCs within ischemic areas express various pericyte markers such as platelet-derived growth factor receptor beta (PDGFRβ), neuronal/glial 2 (NG2), and alpha smooth muscle actin (αSMA)[21,24,41]. Importantly, nestin+ cells were absent from non-ischemic areas in the cortex of adult mice, indicating that normal pericytes in the adult brain do not express nestin. Thus, we proposed that brain pericytes, localized near blood vessels, are potentially giving rise to iNSPCs after injuries such as ischemic stroke[24,42].
Pericytes are localized near blood vessels and form a neurovascular unit (NVU) together with endothelial cells and neural lineage cells (neurons and astrocytes). Pericytes are heterogeneous cells: although PDGFRβ, NG2, nestin,αSMA, CD146, Glast, Tbx18, and regulator of G protein signaling 5[24,43-51] are expressed on pericytes, none of those are specific markers. Birbrair et al[44] divided skeletal-muscle-derived pericytes into two subtypes (nestin−/NG2+ type-1 pericytes and nestin+/NG2+ type-2 pericytes). Using their proposed categorization, iNSPCs would be classified as type-2 pericytes as they express both nestin and NG2. In addition, Birbrair et al[52] reported that nestin+/NG2+ type-2 pericytes have NG2+ glia-like traits. However, NG2+ glia is identical to OPCs[53], and both pericytes and OPCs express common markers, including NG2 and PDGFRα[54]. Thus, the precise connection between iNSPCs and resident glia should be determined in further studies (Figure 1).
Brain pericytes are a key component of the NVU and play an important role in maintaining this unit[55]. Even after severe stress such as ischemic stroke, cells forming the NVU, including pericytes[42] and endothelial cells[23], survive, suggesting that these cells play an essential role under pathological conditions as well as under normal conditions.
Besides endothelial cells[56-59], pericytes possess plasticity[54,60] and function as multipotent stem cells as well[43,44,47,61-67]. Therefore, we investigated whether iNSPCs maintain their multipotency under pathological conditions. We found out that iNSPCs can differentiate into not only neural but also mesenchymal lineages, including osteoblasts, adipocytes, and chondrocytes[21,41]. Thus, under ischemic conditions following stroke, brain pericytes might convert into injury/ischemia-induced multipotent stem cells (iSCs) by acquiring the stemness, thereby producing iNSPCs (Figure 1). Consistent with our previous reports[21,41], using a mouse model of cerebral infarction, other groups have also shown that brain pericytes following ischemia display the potential to differentiate into multilineage cells[68]. We also showed that iSCs share angioblast features and give rise to hematopoietic cell lineages such as microglia[21,41]. Consistent with these reports, a recent study showed that brain pericytes and endothelial cells share certain traits[69]. Interestingly, a subtype of pericytes was reported to be derived from hematopoietic lineages, including microglia[70-72]. Thus, the relationship among iSCs, pericytes, and hematopoietic lineages remains to be elucidated in future studies.
It remains unclear whether brain pericytes behave as multipotent stem cells in vivo. Ideally, this should be clarified in mice using pericyte markers. A recent study using genetic mapping by the Cre-loxP system failed to demonstrate that Tbx18+ brain pericytes function as multipotent stem cells in vivo following mild injury, although they behave as multipotent stem cells in vitro[50]. However, phenotypes of cells expressing certain genes (e.g., nestin) in transgenic mice differ depending on the intron regions in which a tag (e.g., green fluorescent protein) is inserted[73-75]. Accumulating evidence also shows that genetic mapping techniques by the Cre-loxP system present several pitfalls[76-78]. For example, gene expression patterns and localizations of certain genes (e.g., nestin) are different depending on the reporter mice used for crossbreeding[78]. Additionally, recombination efficiency following tamoxifen treatment differs among the developing stages of mice[77]. Furthermore, we have previously demonstrated that induction of iNSPCs/iSCs varies with the degree of ischemic stimuli and that a severe injury is essential for inducing iNSPCs/iSCs[42]. Therefore, whether brain pericytes function as multipotent stem cells following injury in vivo should be carefully investigated in further studies.
Moreover, to confirm that iSCs are multipotent, it is necessary to show that iSCs derived from a single-cell type can differentiate into multiple cell types. We previously proposed that iSCs might be composed of subpopulations each specifically differentiating into neural or mesenchymal lineages[79]. If so, these subpopulations once isolated could be useful for clinical applications. For example, the sub-population that can predominantly differentiate into neuronal lineages would be used for neural repair following CNS injuries. However, the precise relations between iNSPCs and iSCs should be clarified in further studies (Figure 1).
Although the mechanism by which brain pericytes acquire multipotency under ischemic conditions remains unclear, we have previously demonstrated that brain pericytes display up-regulated expression of various stem cell and undifferentiated cell markers when they are incubated under oxygen–glucose deprivation (OGD) that mimics ischemia/hypoxia[21,41]. In general, pericytes have the characteristics of mesenchymal lineages, and NSPCs have traits of epithelial lineages. Following OGD stimuli, we showed that the mesenchymal-epithelial transition (MET) was facilitated in brain pericytes as demonstrated by the up-regulated expression of the Sox2 gene[21,41].
These findings suggest that iNSPCs/iSCs are derived from brain PCs having developed stemness through cellular reprogramming and MET. In support of this viewpoint, accumulating evidence shows that brain PCs reprogrammed by gene transduction (e.g., Sox2 gene) acquire neural lineage traits, including NSPC and neuron phenotypes[48,80].
In addition to the NSPC marker nestin, iNSPCs/iSCs express various stem cell and undifferentiated cell markers, including Sox2, Nanog, c-myc, and Klf4. However, iNSPCs/iSCs lack Oct 3/4 gene expression, which is essential in producing iPS cells[21,24,81], even though iNSPCs/iSCs can differentiate into neural and mesenchymal lineages. Therefore, iNSPCs/iSCs differ from pluripotent stem cells such as iPS cells and ES cells. We also found out that it is not easy for somatic adult pericytes to be reprogrammed into a pluripotent state even when subjected to severe stress such as ischemia[21]. However, a recent study showed that an injury stimulus did convert skeletal muscle cells into a pluripotent state[82]. Thus, whether injury stimuli can induce somatic cells to become pluripotent cells should be carefully investigated in future studies.
Akin to pericytes, previous studies showed that multipotent stem cells such as MSCs[83-87] and neural crest stem cells (NCSCs)[88] reside in the perivascular regions of multiple organs. These cells also differentiate into various lineages, including neural and mesenchymal lineages, consistent with the traits of iNSPCs/iSCs.
Comparing iNSPCs/iSCs with other types of multipotent stem cells such as bone-marrow-derived MSCs, iNSPCs/iSCs differentiate into mesenchymal lineages, including osteoblasts and adipocytes as well as MSCs. Using multi-electrode arrays[89], we recently reported that iNSPCs/iSCs, but not MSCs, have the potential to differentiate into electrophysiologic-functional neurons[90]. On the basis of their developmental origin in multiple organs, the majority of non-CNS pericytes originate from the mesoderm. However, brain pericytes are likely neural crest derivatives[91,92].
The cells of the neural crest originate from the neural tube through the epithelial-mesenchymal transition. The cells of the neural crest are multipotent stem cells (NSCs) that share both neural and mesenchymal traits[79,93,94].
Considering their origin, iNSPCs/iSCs have a stronger neural phenotype than MSCs. Thus, it is likely that iNSPCs/iSCs are stem cells which differ from previously reported ones. However, recent studies show that the traits of MSCs vary among organs[87]. Thus, brain MSCs might have features differing from those of MSCs derived from other organs (e.g., bone-marrow-derived MSCs)[95], and further investigations are necessary regarding the relations among iNSPCs/iSCs, brain pericytes, and brain MSCs.
To translate the non-clinical findings obtained in mouse iNSPCs/iSCs into clinical applications, it is essential to understand the traits of human iNSPCs/iSCs obtained from patients with stroke.
Using brain samples obtained from stroke patients who needed both decompressive craniectomy and partial lobectomy as a life-saving therapy for diffuse cerebral infarction, we attempted to isolate human iNSPCs/iSCs. We detected iNSPCs/iSCs within post-stroke areas of the human brains, consistent with those of mouse brains[21,24,41,90].
Recently, we have reported the traits of iNSPCs/iSCs obtained from two patients with cerebral infarction[96]. The samples obtained from two elderly patients displayed gross necrosis and histological cell death. Immunohistochemical analysis showed that, although mature neural cells disappear within post-stroke areas, nestin+ cells were present within these areas. The nestin+ cells localized near blood cells and expressed pericyte markers such as NG2 and αSMA. After the cells isolated from post-ischemic human tissues were incubated in medium with basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF), many proliferative cells emerged, and they expressed the dividing cell marker Ki67. The cells isolated from post-ischemic human tissues expressed not only nestin but also the pericyte markers NG2, PDGFRβ, and αSMA. However, these nestin+ cells did not express endothelial cells and astrocytes markers. These findings indicate that brain pericytes convert into nestin+ iNSPCs/iSCs within post-stroke human brains, consistent with mouse brains[21].
Next, we examined the multipotency of human iNSPCs/iSCs. Even after several passages, nestin+ iNSPCs/iSCs retained the expression of various stem cell and undifferentiated cell markers, including Sox2, c-myc, and Klf4. When they were incubated under conditions to promote the differentiation into mesoderm lineages such as osteoblasts, adipocytes, and chondrocytes, they differentiated into these cells, respectively. They also formed neurosphere-like cells under floating cultures and differentiated into Tuj-1+ and MAP2+ neuronal cells. These findings demonstrate that iNSPCs/iSCs are present within post-stroke human brains as well as in post-stroke mouse brains.
However, more precise traits of human iNSPCs/iSCs remain unclear, including their multipotency potential to differentiate into functional neurons. To address this question, we are now investigating the features of human iNSPCs/iSCs obtained from additional post-ischemic cerebral samples. Our preliminary study shows that human iNSPCs/iSCs expanded from a single-cell lineage mainly differentiated into Tuj1+ neurons under neuronal differentiation conditions, and they differentiated into fatty acid binding protein 4 (FABP4)+ adipocytes under adipogenic differentiation conditions. Our recent study also reveals that human iNSPCs/iSCs have the potential to differentiate into functional neurons[97]. These results indicate that iNSPCs/iSCs (at least a sub-population) function as multipotent stem cells that differentiate into neuronal cells. Therefore, these cells should be renamed iSCs rather than iNSPCs because they can differentiate into various cell lineages other than neural.
Other questions remain. For example, the traits of iNSPCs/iSCs may differ from the time of injury onset to surgery. Also, iNSPC/iSC features may vary among CNS regions (e.g., cerebrum, cerebellum, brainstem, spinal cord). Regarding the latter question, our recent study demonstrated that iNSPCs/iSCs could be isolated from the cerebellum[97] as well as the cerebrum[96]. Comparative gene expression profiles showed that although the cerebellar iNSPCs/iSCs resembled cerebral iNSPCs/iSCs, they expressed certain cerebellum-specific genes[97]. Thus, further studies are needed using additional samples to identify comprehensively the traits of iNSPCs/iSCs.
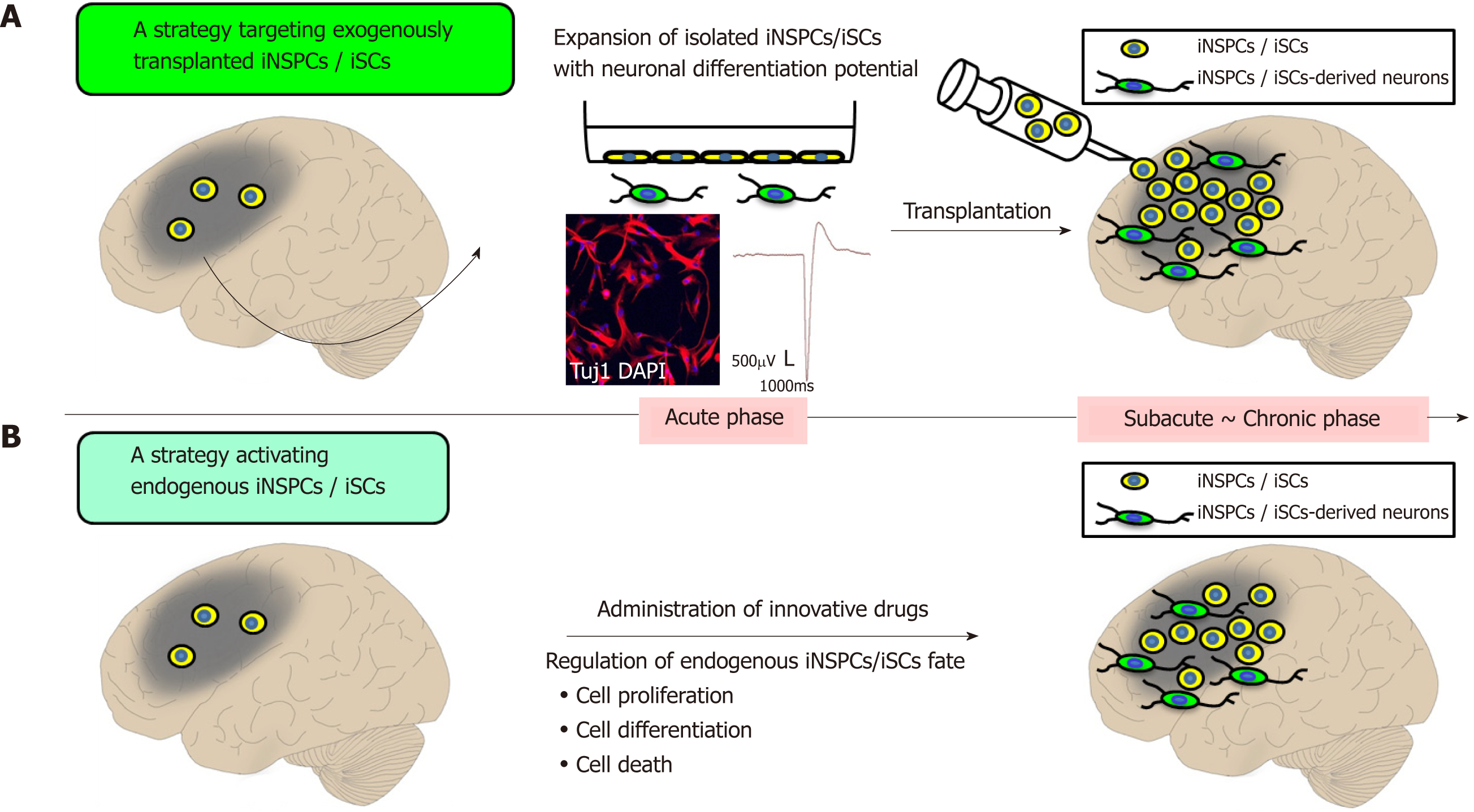
Evidence showing that iNSPCs/iSCs are present within post-stroke human brains suggests that stem-cell-based therapies using iNSPCs/iSCs could contribute to neural repair in patients with stroke in the future. Two strategies for clinical applications using iNSPCs/iSCs could be implemented as follows.
The first strategy implies to transplant exogenous iNSPCs/iSCs within or near post-ischemic areas (Figure 2A). iNSPCs/iSCs isolated from ischemic areas exhibit high proliferative activities in a medium containing bFGF and EGF[96]. Thus, after a satisfactory expansion of iNSPCs/iSCs, the autologous transplantation of iNSPCs/iSCs could be performed during subacute and chronic periods. This therapy presents the advantage to repeatedly transplant iNSPCs/iSCs that satisfy certain cell profiles. Another advantage is that the cell number (e.g., low dose of cells and high dose of cells) and the transplant location (e.g., within ischemic areas, around ischemic areas, and non-ischemic areas) can be chosen.
On the other hand, there are several disadvantages. For example, several weeks are required to prepare enough iNSPCs/iSCs in vitro, not allowing iNSPC/iSC transplantation in stroke patients during acute phases. Furthermore, iNSPCs/iSCs cannot be obtained from any stroke patients. Currently, iNSPCs/iSCs can only be obtained from patients who needed both decompressive craniectomy and partial lobectomy as a life-saving therapy for diffuse cerebral infarction. It is ethically impossible to get iNSPCs/iSCs from patients with small infarcted areas (e.g., lacunar infarction). Therefore, only a small portion of stroke patients would be eligible for this treatment in the future.
Currently, we are investigating the safety (e.g., tumorigenesis onset and formation) and efficiency (e.g., cell survival, neuronal differentiation, and functional improvement) upon transplantation of human iNSPCs/iSCs in mice post-stroke. Theoretically, the above-mentioned problems would be solved if iNSPCs/iSCs are expandable in allograft and autograft transplantations. However, we have to carefully evaluate whether iNSPCs/iSCs can be utilized as an allograft because iNSPCs/iSCs are stem cells that originated from brains that differ from stem cells derived from non-CNS (e.g., bone marrow-derived MCS).
These problems may be solved using iNSPCs/iSCs derived from iPS cells. For example, using iPS-cell-derived iNSPCs/iSCs obtained from skin fibroblasts of stroke patients, patients may receive an autologous transplantation therapy using iNSPCs/iSCs. However, when making iPS cells, new problems could emerge, such as tumor formation.
The second strategy involves identifying the factors regulating the fate of iNSPCs/iSCs (e.g., factors promoting cell proliferation and differentiation, and factors inhibiting cell death) and to develop those as innovative drugs (Figure 2B).
Using a mouse model of cerebral infarction, we previously showed that iNSPCs/iSCs isolated from ischemic areas differentiated into electrophysiologic-functional neurons and did express mature neuronal markers[27]. In vivo, the number of nestin+ iNSPCs/iSCs peaked around post-stroke day 3 and then gradually decreased. In addition, immature newly born neurons were identified within and near ischemic areas at post-stroke day 3, and their numbers decreased thereafter as well[24,42,49].
This suggests that, although iNSPCs/iSCs are present within ischemic areas, several factors regulate their survival, proliferation, and differentiation. In support of this viewpoint, we have previously demonstrated that the endothelial cells residing around iNSPCs/iSCs promote their survival, proliferation, and neuronal differentiation[22,28]. This suggests that endothelial-derived trophic factors exhibit a positive effect on iNSPCs/iSCs. Alternatively, endothelial cells and/or the extracellular matrix produced by endothelial cells[98] may function as a niche for iNSPCs/iSCs, as it is the case with NSPCs[99].
Further investigations are needed to understand the factors involved in the regulation of iNSPCs/iSCs. However, our previous studies indicated that a subset of lymphocytes that infiltrated into ischemic areas during acute phases inhibited the survival of iNSPCs/iSCs[100,101]. In addition, our preliminary study showed that inflammatory cells such as microglia/macrophages rapidly increase at the time when nestin+ iNSPCs/iSCs disappear. These findings indicate that iNSPC/iSC regulation also relies on environmental factors surrounding them (e.g., inflammatory cells), and both intrinsic and extrinsic factors play an essential role in neural regeneration.
Our studies showed that iNSPCs/iSCs are present within post-stroke areas of mouse and human brains. Further studies are needed to identify the traits, fate, proliferation, and differentiation factors of iNSPCs/iSCs for their clinical applications. However, iNSPCs/iSCs represent a cornerstone in contributing to CNS repair because they are stem cells that develop within ischemic areas following CNS injuries. Evidence of the presence of iNSPCs/iSCs within post-ischemic human brains is encouraging for the development of new stem-cell-based therapies for stroke patients.
We would like to thank members of Institute for Advanced Medical Sciences and Department of Neurosurgery at Hyogo College of Medicine for helpful assistance.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Perez-Campo FM, Zhang GL S-Editor: Dou Y L-Editor: A E-Editor: Xing YX
| 1. | Mokin M, Kass-Hout T, Kass-Hout O, Dumont TM, Kan P, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. Intravenous thrombolysis and endovascular therapy for acute ischemic stroke with internal carotid artery occlusion: a systematic review of clinical outcomes. Stroke. 2012;43:2362-2368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Zaidat OO, Suarez JI, Sunshine JL, Tarr RW, Alexander MJ, Smith TP, Enterline DS, Selman WR, Landis DM. Thrombolytic therapy of acute ischemic stroke: correlation of angiographic recanalization with clinical outcome. AJNR Am J Neuroradiol. 2005;26:880-884. [PubMed] [Cited in This Article: ] |
| 3. | Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4230] [Cited by in F6Publishing: 4498] [Article Influence: 562.3] [Reference Citation Analysis (0)] |
| 4. | Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4614] [Cited by in F6Publishing: 4402] [Article Influence: 275.1] [Reference Citation Analysis (0)] |
| 5. | Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, Ford I, Galinovic I, Gellissen S, Golsari A, Gregori J, Günther M, Guibernau J, Häusler KG, Hennerici M, Kemmling A, Marstrand J, Modrau B, Neeb L, Perez de la Ossa N, Puig J, Ringleb P, Roy P, Scheel E, Schonewille W, Serena J, Sunaert S, Villringer K, Wouters A, Thijs V, Ebinger M, Endres M, Fiebach JB, Lemmens R, Muir KW, Nighoghossian N, Pedraza S, Gerloff C; WAKE-UP Investigators. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med. 2018;379:611-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 704] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 6. | Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3099] [Cited by in F6Publishing: 3245] [Article Influence: 540.8] [Reference Citation Analysis (0)] |
| 7. | Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG; DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2796] [Cited by in F6Publishing: 2910] [Article Influence: 485.0] [Reference Citation Analysis (0)] |
| 8. | Chia NH, Leyden JM, Newbury J, Jannes J, Kleinig TJ. Determining the Number of Ischemic Strokes Potentially Eligible for Endovascular Thrombectomy: A Population-Based Study. Stroke. 2016;47:1377-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci. 2007;26:605-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839-11844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 453] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 11. | Hicks AU, Hewlett K, Windle V, Chernenko G, Ploughman M, Jolkkonen J, Weiss S, Corbett D. Enriched environment enhances transplanted subventricular zone stem cell migration and functional recovery after stroke. Neuroscience. 2007;146:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Kameda M, Shingo T, Takahashi K, Muraoka K, Kurozumi K, Yasuhara T, Maruo T, Tsuboi T, Uozumi T, Matsui T, Miyoshi Y, Hamada H, Date I. Adult neural stem and progenitor cells modified to secrete GDNF can protect, migrate and integrate after intracerebral transplantation in rats with transient forebrain ischemia. Eur J Neurosci. 2007;26:1462-1478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 834] [Cited by in F6Publishing: 827] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 15. | Huang H, Lin F, Jiang J, Chen Y, Mei A, Zhu P. Effects of intra-arterial transplantation of adipose-derived stem cells on the expression of netrin-1 and its receptor DCC in the peri-infarct cortex after experimental stroke. Stem Cell Res Ther. 2017;8:223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Zhao K, Li R, Gu C, Liu L, Jia Y, Guo X, Zhang W, Pei C, Tian L, Li B, Jia J, Cheng H, Xu H, Li L. Intravenous Administration of Adipose-Derived Stem Cell Protein Extracts Improves Neurological Deficits in a Rat Model of Stroke. Stem Cells Int. 2017;2017:2153629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kimura H, Yoshikawa M, Matsuda R, Toriumi H, Nishimura F, Hirabayashi H, Nakase H, Kawaguchi S, Ishizaka S, Sakaki T. Transplantation of embryonic stem cell-derived neural stem cells for spinal cord injury in adult mice. Neurol Res. 2005;27:812-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Salewski RP, Mitchell RA, Li L, Shen C, Milekovskaia M, Nagy A, Fehlings MG. Transplantation of Induced Pluripotent Stem Cell-Derived Neural Stem Cells Mediate Functional Recovery Following Thoracic Spinal Cord Injury Through Remyelination of Axons. Stem Cells Transl Med. 2015;4:743-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1022] [Cited by in F6Publishing: 966] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 20. | Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027-2033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2237] [Cited by in F6Publishing: 2141] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 21. | Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, Kawahara M, Taguchi A, Matsuyama T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33:1962-1974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 22. | Nakano-Doi A, Nakagomi T, Fujikawa M, Nakagomi N, Kubo S, Lu S, Yoshikawa H, Soma T, Taguchi A, Matsuyama T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells. 2010;28:1292-1302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Nakano-Doi A, Sakuma R, Matsuyama T, Nakagomi T. Ischemic stroke activates the VE-cadherin promoter and increases VE-cadherin expression in adult mice. Histol Histopathol. 2018;33:507-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 24. | Nakagomi T, Molnár Z, Nakano-Doi A, Taguchi A, Saino O, Kubo S, Clausen M, Yoshikawa H, Nakagomi N, Matsuyama T. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev. 2011;20:2037-2051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Kasahara Y, Ihara M, Nakagomi T, Momota Y, Stern DM, Matsuyama T, Taguchi A. A highly reproducible model of cerebral ischemia/reperfusion with extended survival in CB-17 mice. Neurosci Res. 2013;76:163-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Taguchi A, Kasahara Y, Nakagomi T, Stern DM, Fukunaga M, Ishikawa M, Matsuyama T. A Reproducible and Simple Model of Permanent Cerebral Ischemia in CB-17 and SCID Mice. J Exp Stroke Transl Med. 2010;3:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Nakagomi T, Taguchi A, Fujimori Y, Saino O, Nakano-Doi A, Kubo S, Gotoh A, Soma T, Yoshikawa H, Nishizaki T, Nakagomi N, Stern DM, Matsuyama T. Isolation and characterization of neural stem/progenitor cells from post-stroke cerebral cortex in mice. Eur J Neurosci. 2009;29:1842-1852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, Yoshikawa H, Stern DM, Matsuyama T, Taguchi A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185-2195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2818] [Cited by in F6Publishing: 2914] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 30. | Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 31. | Shimada IS, Peterson BM, Spees JL. Isolation of locally derived stem/progenitor cells from the peri-infarct area that do not migrate from the lateral ventricle after cortical stroke. Stroke. 2010;41:e552-e560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Yokoyama A, Sakamoto A, Kameda K, Imai Y, Tanaka J. NG2 proteoglycan-expressing microglia as multipotent neural progenitors in normal and pathologic brains. Glia. 2006;53:754-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Gaughwin PM, Caldwell MA, Anderson JM, Schwiening CJ, Fawcett JW, Compston DA, Chandran S. Astrocytes promote neurogenesis from oligodendrocyte precursor cells. Eur J Neurosci. 2006;23:945-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754-1757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 661] [Cited by in F6Publishing: 623] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 35. | Carlén M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabé-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisén J. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 36. | Moreno-Manzano V, Rodríguez-Jiménez FJ, García-Roselló M, Laínez S, Erceg S, Calvo MT, Ronaghi M, Lloret M, Planells-Cases R, Sánchez-Puelles JM, Stojkovic M. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells. 2009;27:733-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 38. | Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 752] [Cited by in F6Publishing: 802] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 39. | Maki T, Maeda M, Uemura M, Lo EK, Terasaki Y, Liang AC, Shindo A, Choi YK, Taguchi A, Matsuyama T, Takahashi R, Ihara M, Arai K. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett. 2015;597:164-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, Pham LD, Suwa F, Taguchi A, Matsuyama T, Ihara M, Kim KW, Lo EH, Arai K. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS One. 2014;9:e103174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Sakuma R, Kawahara M, Nakano-Doi A, Takahashi A, Tanaka Y, Narita A, Kuwahara-Otani S, Hayakawa T, Yagi H, Matsuyama T, Nakagomi T. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation. 2016;13:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 42. | Nakata M, Nakagomi T, Maeda M, Nakano-Doi A, Momota Y, Matsuyama T. Induction of Perivascular Neural Stem Cells and Possible Contribution to Neurogenesis Following Transient Brain Ischemia/Reperfusion Injury. Transl Stroke Res. 2017;8:131-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 44. | Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013;10:67-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 45. | Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 711] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 46. | Mitchell TS, Bradley J, Robinson GS, Shima DT, Ng YS. RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis. 2008;11:141-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307:C25-C38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 48. | Karow M, Sánchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascón S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Götz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 49. | Nakagomi T, Molnár Z, Taguchi A, Nakano-Doi A, Lu S, Kasahara Y, Nakagomi N, Matsuyama T. Leptomeningeal-derived doublecortin-expressing cells in poststroke brain. Stem Cells Dev. 2012;21:2350-2354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Guimarães-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell. 2017;20:345-359.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 329] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 51. | Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 578] [Cited by in F6Publishing: 608] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 52. | Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res. 2013;319:45-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Moyon S, Liang J, Casaccia P. Epigenetics in NG2 glia cells. Brain Res. 2016;1638:183-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Santos GSP, Magno LAV, Romano-Silva MA, Mintz A, Birbrair A. Pericyte Plasticity in the Brain. Neurosci Bull. 2019;35:551-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1701] [Cited by in F6Publishing: 1787] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 56. | Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1508] [Cited by in F6Publishing: 1603] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 57. | Yu W, Liu Z, An S, Zhao J, Xiao L, Gou Y, Lin Y, Wang J. The endothelial-mesenchymal transition (EndMT) and tissue regeneration. Curr Stem Cell Res Ther. 2014;9:196-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Susienka MJ, Medici D. Vascular endothelium as a novel source of stem cells for bioengineering. Biomatter. 2013;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012;125:1795-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 60. | Berthiaume AA, Grant RI, McDowell KP, Underly RG, Hartmann DA, Levy M, Bhat NR, Shih AY. Dynamic Remodeling of Pericytes In Vivo Maintains Capillary Coverage in the Adult Mouse Brain. Cell Rep. 2018;22:8-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 61. | Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Péault B. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 62. | Kabara M, Kawabe J, Matsuki M, Hira Y, Minoshima A, Shimamura K, Yamauchi A, Aonuma T, Nishimura M, Saito Y, Takehara N, Hasebe N. Immortalized multipotent pericytes derived from the vasa vasorum in the injured vasculature. A cellular tool for studies of vascular remodeling and regeneration. Lab Invest. 2014;94:1340-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22:2298-2314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 64. | Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. 2014;6:245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 65. | Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226-2232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 66. | Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 67. | Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 448] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 68. | Gouveia A, Seegobin M, Kannangara TS, He L, Wondisford F, Comin CH, Costa LDF, Béïque JC, Lagace DC, Lacoste B, Wang J. The aPKC-CBP Pathway Regulates Post-stroke Neurovascular Remodeling and Functional Recovery. Stem Cell Reports. 2017;9:1735-1744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Smyth LCD, Rustenhoven J, Park TI, Schweder P, Jansson D, Heppner PA, O'Carroll SJ, Mee EW, Faull RLM, Curtis M, Dragunow M. Unique and shared inflammatory profiles of human brain endothelia and pericytes. J Neuroinflammation. 2018;15:138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 70. | Yamamoto S, Muramatsu M, Azuma E, Ikutani M, Nagai Y, Sagara H, Koo BN, Kita S, O'Donnell E, Osawa T, Takahashi H, Takano KI, Dohmoto M, Sugimori M, Usui I, Watanabe Y, Hatakeyama N, Iwamoto T, Komuro I, Takatsu K, Tobe K, Niida S, Matsuda N, Shibuya M, Sasahara M. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci Rep. 2017;7:3855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, Yamamoto S, Ema M, Mukouyama YS. Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-β Signaling in Developing Skin Vasculature. Cell Rep. 2017;18:2991-3004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 72. | Fujita Y, Ihara M, Ushiki T, Hirai H, Kizaka-Kondoh S, Hiraoka M, Ito H, Takahashi R. Early protective effect of bone marrow mononuclear cells against ischemic white matter damage through augmentation of cerebral blood flow. Stroke. 2010;41:2938-2943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 2010;58:721-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 74. | Namiki J, Suzuki S, Masuda T, Ishihama Y, Okano H. Nestin protein is phosphorylated in adult neural stem/progenitor cells and not endothelial progenitor cells. Stem Cells Int. 2012;2012:430138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Bernal A, Arranz L. Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci. 2018;75:2177-2195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 76. | Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Gonçalves R, Mintz A, Delbono O. How Plastic Are Pericytes? Stem Cells Dev. 2017;26:1013-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 77. | Liang H, Hippenmeyer S, Ghashghaei HT. A Nestin-cre transgenic mouse is insufficient for recombination in early embryonic neural progenitors. Biol Open. 2012;1:1200-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Sun MY, Yetman MJ, Lee TC, Chen Y, Jankowsky JL. Specificity and efficiency of reporter expression in adult neural progenitors vary substantially among nestin-CreER(T2) lines. J Comp Neurol. 2014;522:1191-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Takagi T, Yoshimura S, Sakuma R, Nakano-Doi A, Matsuyama T, Nakagomi T. Novel Regenerative Therapies Based on Regionally Induced Multipotent Stem Cells in Post-Stroke Brains: Their Origin, Characterization, and Perspective. Transl Stroke Res. 2017;8:515-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Karow M, Camp JG, Falk S, Gerber T, Pataskar A, Gac-Santel M, Kageyama J, Brazovskaja A, Garding A, Fan W, Riedemann T, Casamassa A, Smiyakin A, Schichor C, Götz M, Tiwari VK, Treutlein B, Berninger B. Direct pericyte-to-neuron reprogramming via unfolding of a neural stem cell-like program. Nat Neurosci. 2018;21:932-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 81. | Nakagomi T, Nakano-Doi A, Narita A, Matsuyama T. Concise Review: Are Stimulated Somatic Cells Truly Reprogrammed into an ES/iPS-Like Pluripotent State? Better Understanding by Ischemia-Induced Multipotent Stem Cells in a Mouse Model of Cerebral Infarction. Stem Cells Int. 2015;2015:630693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Vojnits K, Pan H, Mu X, Li Y. Characterization of an Injury Induced Population of Muscle-Derived Stem Cell-Like Cells. Sci Rep. 2015;5:17355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Paul G, Özen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, Jansson K, Dannaeus K, Henriques-Oliveira C, Roybon L, Anisimov SV, Renström E, Svensson M, Haegerstrand A, Brundin P. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One. 2012;7:e35577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 84. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2900] [Cited by in F6Publishing: 2763] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 85. | Esteves CL, Sheldrake TA, Dawson L, Menghini T, Rink BE, Amilon K, Khan N, Péault B, Donadeu FX. Equine Mesenchymal Stromal Cells Retain a Pericyte-Like Phenotype. Stem Cells Dev. 2017;26:964-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Ozen I, Boix J, Paul G. Perivascular mesenchymal stem cells in the adult human brain: a future target for neuroregeneration? Clin Transl Med. 2012;1:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Vezzani B, Pierantozzi E, Sorrentino V. Not All Pericytes Are Born Equal: Pericytes from Human Adult Tissues Present Different Differentiation Properties. Stem Cells Dev. 2016;25:1549-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Kubota Y, Takubo K, Hirashima M, Nagoshi N, Kishi K, Okuno Y, Nakamura-Ishizu A, Sano K, Murakami M, Ema M, Omatsu Y, Takahashi S, Nagasawa T, Shibuya M, Okano H, Suda T. Isolation and function of mouse tissue resident vascular precursors marked by myelin protein zero. J Exp Med. 2011;208:949-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Dranias MR, Ju H, Rajaram E, VanDongen AM. Short-term memory in networks of dissociated cortical neurons. J Neurosci. 2013;33:1940-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Sakuma R, Takahashi A, Nakano-Doi A, Sawada R, Kamachi S, Beppu M, Takagi T, Yoshimura S, Matsuyama T, Nakagomi T. Comparative Characterization of Ischemia-Induced Brain Multipotent Stem Cells with Mesenchymal Stem Cells: Similarities and Differences. Stem Cells Dev. 2018;27:1322-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Morse DE, Cova JL. Pigmented cells in the leptomeninges of the cat. Anat Rec. 1984;210:125-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059-1068. [PubMed] [Cited in This Article: ] |
| 93. | Nagoshi N, Shibata S, Nakamura M, Matsuzaki Y, Toyama Y, Okano H. Neural crest-derived stem cells display a wide variety of characteristics. J Cell Biochem. 2009;107:1046-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Nakagomi T, Nakano-Doi A, Kawamura M, Matsuyama T. Do Vascular Pericytes Contribute to Neurovasculogenesis in the Central Nervous System as Multipotent Vascular Stem Cells? Stem Cells Dev. 2015;24:1730-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Appaix F, Nissou MF, van der Sanden B, Dreyfus M, Berger F, Issartel JP, Wion D. Brain mesenchymal stem cells: The other stem cells of the brain? World J Stem Cells. 2014;6:134-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | Tatebayashi K, Tanaka Y, Nakano-Doi A, Sakuma R, Kamachi S, Shirakawa M, Uchida K, Kageyama H, Takagi T, Yoshimura S, Matsuyama T, Nakagomi T. Identification of Multipotent Stem Cells in Human Brain Tissue Following Stroke. Stem Cells Dev. 2017;26:787-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 97. | Beppu M, Nakagomi T, Takagi T, Nakano-Doi A, Sakuma R, Kuramoto Y, Tatebayashi K, Matsuyama T, Yoshimura S. Isolation and Characterization of Cerebellum-Derived Stem Cells in Poststroke Human Brain. Stem Cells Dev. 2019;28:528-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 98. | Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol. 2011;71:1018-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 99. | Kazanis I, ffrench-Constant C. Extracellular matrix and the neural stem cell niche. Dev Neurobiol. 2011;71:1006-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 100. | Saino O, Taguchi A, Nakagomi T, Nakano-Doi A, Kashiwamura S, Doe N, Nakagomi N, Soma T, Yoshikawa H, Stern DM, Okamura H, Matsuyama T. Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke. J Neurosci Res. 2010;88:2385-2397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Takata M, Nakagomi T, Kashiwamura S, Nakano-Doi A, Saino O, Nakagomi N, Okamura H, Mimura O, Taguchi A, Matsuyama T. Glucocorticoid-induced TNF receptor-triggered T cells are key modulators for survival/death of neural stem/progenitor cells induced by ischemic stroke. Cell Death Differ. 2012;19:756-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |