Published online Nov 26, 2018. doi: 10.4252/wjsc.v10.i11.146
Peer-review started: August 13, 2018
First decision: August 24, 2018
Revised: September 7, 2018
Accepted: October 11, 2018
Article in press: October 12, 2018
Published online: November 26, 2018
Adipose-derived mesenchymal stem cells (ADSCs) are a treatment cell source for patients with chronic liver injury. ADSCs are characterized by being harvested from the patient’s own subcutaneous adipose tissue, a high cell yield (i.e., reduced immune rejection response), accumulation at a disease nidus, suppression of excessive immune response, production of various growth factors and cytokines, angiogenic effects, anti-apoptotic effects, and control of immune cells via cell-cell interaction. We previously showed that conditioned medium of ADSCs promoted hepatocyte proliferation and improved the liver function in a mouse model of acute liver failure. Furthermore, as found by many other groups, the administration of ADSCs improved liver tissue fibrosis in a mouse model of liver cirrhosis. A comprehensive protein expression analysis by liquid chromatography with tandem mass spectrometry showed that the various cytokines and chemokines produced by ADSCs promote the healing of liver disease. In this review, we examine the ability of expressed protein components of ADSCs to promote healing in cell therapy for liver disease. Previous studies demonstrated that ADSCs are a treatment cell source for patients with chronic liver injury. This review describes the various cytokines and chemokines produced by ADSCs that promote the healing of liver disease.
Core tip: We previously showed that conditioned medium of adipose-derived mesenchymal stem cells (ADSCs) promoted hepatocyte proliferation and improved the liver function in a mouse model of acute liver failure. Furthermore, as reported by many other groups, the administration of ADSCs improved liver tissue fibrosis in a mouse model of liver cirrhosis. A comprehensive protein expression analysis by liquid chromatography with tandem mass spectrometry showed that the various cytokines and chemokines produced by ADSCs have the ability to promote the healing of liver disease. In this review, we examine the ability of the expressed protein components of ADSCs to promote healing in cell therapy for liver disease.
- Citation: Nahar S, Nakashima Y, Miyagi-Shiohira C, Kinjo T, Toyoda Z, Kobayashi N, Saitoh I, Watanabe M, Noguchi H, Fujita J. Cytokines in adipose-derived mesenchymal stem cells promote the healing of liver disease. World J Stem Cells 2018; 10(11): 146-159
- URL: https://www.wjgnet.com/1948-0210/full/v10/i11/146.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i11.146
We and others have conducted clinical studies in patients with symptoms of autoimmune liver disease[1], hepatitis C[2,3], bacterial infection[4], acute liver failure liver[5], nonalcoholic fatty liver disease[6] and cirrhosis. We have also conducted translational research that bridges basic research using hematopoietic cells[7-14], hepatic stellate cells (HSCs)[15-18], embryonic stem cell-derived hepatocytes[19-22], bioartificial livers[23-26], animals[27-33] and clinical research in humans.
Thanks to their expected therapeutic efficacy, mesenchymal stem cells[34] are currently under clinical evaluation as a cell source for cell therapy in trials of regenerative medicine for a broad spectrum of diseases. Since adipose-derived mesenchymal stem cells (ADSCs)[35,36] are obtained from the patient’s abdomen by liposuction, cell procurement is relatively easy (large numbers of cells can be obtained by minimally invasive treatment). ADSCs can avoid immune rejection if they are autografted[37-39]; however, similarly to other cell sources, they are subject to immune rejection if allogeneic or xeno cell transplantation is performed. Mesenchymal stem cells are used for medical treatment worldwide. Since ADSCs are not a mainstream therapeutic cell, we have been performing clinical studies of treatments using ADSCs. For this reason, cell therapy using ADSCs is now performed in many public and private hospitals worldwide. In this review, we examine the effects of ADSCs in cell therapy for liver diseases, focusing on the proteins secreted by ADSCs. We previously reported that the proteins expressed by human ADSCs cultured using Dulbecco’s Modified Eagle’s medium [10% fetal bovine serum (FBS)] and clinical-grade chemically-defined medium showed 98% similarity, demonstrating that the proteins expressed by ADSCs cultured in media for research and clinical use largely coincide[40,41]. However, using animal-derived components in the process of culturing cells for treatment is associated with a risk of transmitting pathogenic infections derived from animals (e.g., bovine spongiform encephalopathy or swine fever). Furthermore, when animal-derived ingredients are used the risks and quality may vary in individual lots. Thus, the use of chemically defined media with recombinant protein is recommended for large-scale culture conditions, such as the manufacturing of therapeutic cells for industrial use. In this background, we use the medium containing FBS as the control medium for the cultivation of research cells, while chemically-defined media is the first choice for culturing therapeutic cells. In addition, we do not deny the option of using a supplement that uses infectious and highly safe virus-tested human serum as a raw material for culturing therapeutic cells.
Gene ontology (GO) facilitates[42,43] the development of a common vocabulary to describe biological concepts. Terms defined in GO are called GO terms (GO is a classification of biological phenomena that associates genes with their known [reported in the existing literature] biological role structured based on given criteria), which are divided into three categories: Biological processes, cellular components, and molecular functions. The Gene Ontology Consortium (http://www.geneontology.org/) is a database of functional information that aims to describe biological phenomena in standardized terms. In recent years, liquid chromatography with tandem mass spectrometry (LC-MS/MS) has been used to perform a GO classification of comprehensive expression data using protein analysis software programs. Both a comprehensive expression analysis of proteins using LC-MS/MS and a protein GO analysis were performed according to methods that we reported previously[40,41,44].
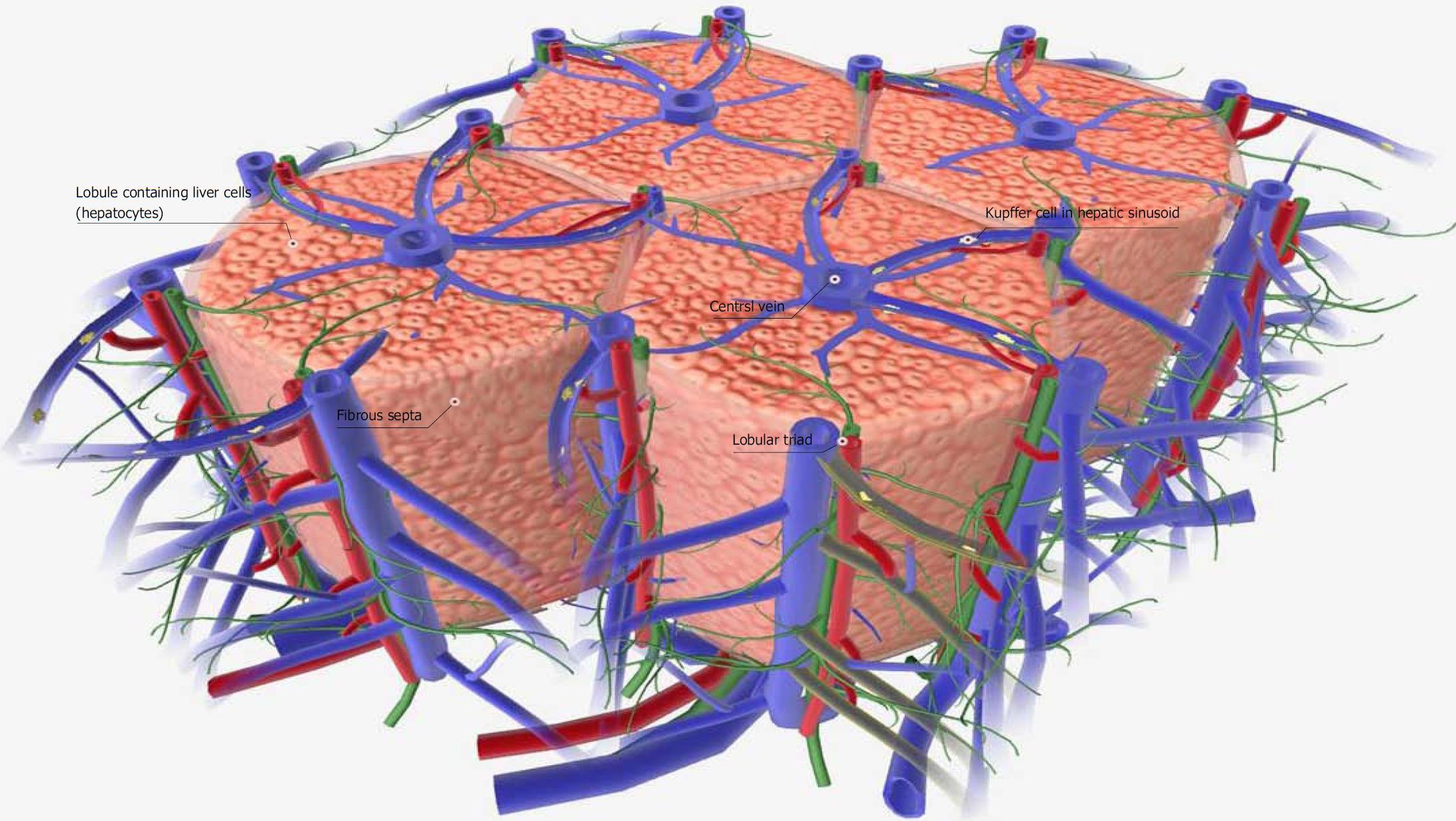
In normal liver tissue, blood flows from the portal vein through the central veins, each of which supplies sufficient blood to the hepatocytes of a single hepatic lobule (Figure 1). The blood supply to hepatocytes remains sufficient in carbon tetrachloride (CCL4: 0.5 mL/kg)-administered acute liver failure model in mice[40,41]. Liver failure is a defined as a group of diseases associated with the development of symptoms such as jaundice, ascites, hepatic encephalopathy, bleeding tendency, or the like, due to a decrease in the number of hepatocytes or a decrease in their function. Acute liver failure is defined by the presence of necrosis and inflammation in normal liver tissue, with the period until the onset of symptoms of hepatic insufficiency being within 8 wk. Cases in which the onset of symptoms is 8-24 wk or > 24 wk are classified as delayed liver failure (late onset hepatic failure; LOHF) and chronic liver failure, respectively. The American Association for the Study of Liver Diseases published a position paper on acute liver failure in 2005 to unify the terms and disease concepts[45].
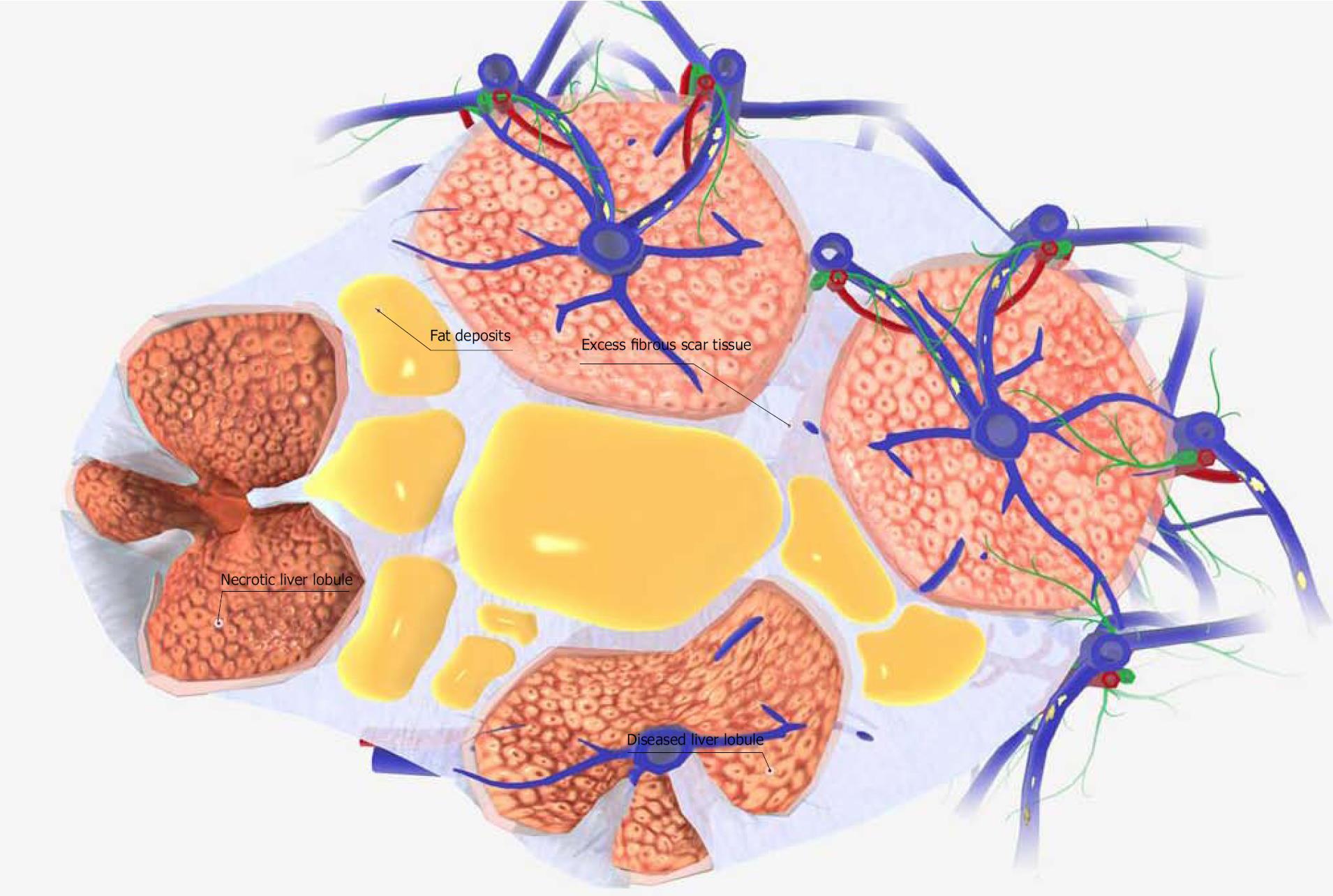
In contrast to this acute model, the preparation of the CCL4 (2.0 mL/kg)-administered cirrhosis model mouse requires more than 6 wk. The liver tissue in this mouse model of liver cirrhosis shows a marked increase in the fiber component of the fibrous septa. This results in the separation of some lobules by fiber components, and the creation of pseudo-lobules. At the same time, the blood supply to the lobules decreases and liver cell necrosis occurs (Figure 2). Chronic liver injury also results in the differentiation of astrocytes into myofibroblastoid cells, in turn causing the pathogenesis of fibrotic liver injury[46]. Given this background, the factor most necessary for the improvement of acute liver failure symptoms is hepatocyte growth factor (HGF). In contrast, the most important factors for the improvement of the symptoms of chronic liver failure (i.e., liver cirrhosis) are: (1) growth factors; (2) inhibition of the inflammation of hepatic stellate cells; and (3) angiogenic factors.
When the disordered repair process is delayed or inhibited after liver damage from drugs, trauma, inflammation, or other insults, liver regeneration is insufficient and hepatic failure develops. In hepatic tissue repair, in addition to growth factors that promote hepatocyte proliferation, angiogenic factors that promote hepatic microvascular remodeling are important. In addition, the extracellular matrix in the liver is mainly produced in hepatic stellate cells. The ability of hepatic stellate cells to produce extracellular matrix is low in the normal liver. However, in the fibrous liver, it is known that hepatic stellate cells are activated to differentiate into myofibroblasts and their ability to produce extracellular matrix markedly increases. Interleukin-6 (IL-6), tumor necrosis factor-α, HGF, and other factors secreted from HSCs, hepatic sinusoidal endothelial cells and Kupffer cells are thought to have the greatest influence on hepatocyte proliferation[47]. Angiogenesis is a physiological phenomenon in which a new blood vessel branch is branched from an existing blood vessel to construct a vascular network. The various factors involved in angiogenesis include fibroblast growth factor, vascular endothelial cell growth factor (VEGF), angiopoietin, and platelet derived growth factor (PDGF).
HGF acts as a HGF and metabolic regulator and promotes hepatocyte proliferation[48]. The hepatic development of the liver is the origin of the gut tube, which is formed by the accumulation of hematopoietic cells[49]. Thus, the idea that HGF expressed by hematopoietic cells promotes the regeneration of hepatocytes is plausible. Liver tissue regeneration using HGF may accordingly be considered a treatment method that reproduces the original development of the liver. HGF is expressed by both HSCs and ADSCs.
Our group previously investigated the clinical application of organ preservation solution[50-56]. We found that the expression level of HGF mRNA did not decrease in ADSCs, even when they were stored in preservation solution for 16 h after separation from adipose tissue. In addition, we found no difference between the expression levels of HGF using glucose-free University of Wisconsin and glucose-containing (5.6 mmol/L) Hank’s Balanced Salt Solution. This result shows that the expression of HGF by ADSCs does not decrease after separation from adipose tissue. Moreover, the expression is not affected by the glucose concentration. In addition, the expression of VEGF showed a similar tendency. In short, ADSCs constantly express HGF and VEGF both in vivo and in vitro[57]. A recent theory suggests that the biliary tree functions as a source of liver and pancreatic stem cells and progenitor cells. VEGF is secreted by the biliary tree as a response to stress[58]. From these developmental perspectives, HGF and VEGF secreted by ADSCs appear to have a marked promoting effect on hepatocyte proliferation.
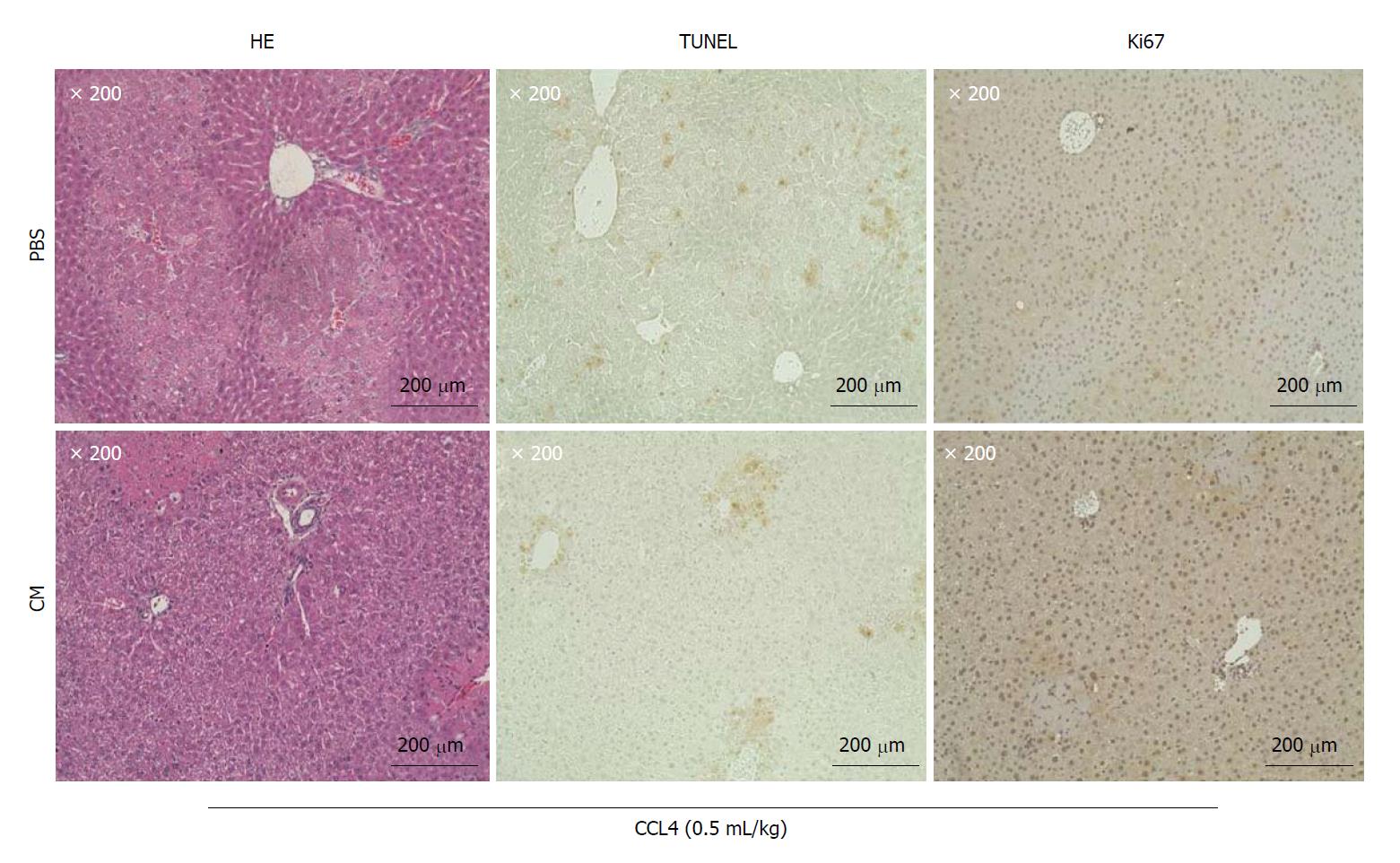
Our experiments showed that the administration of ADSC conditioned medium (CM) from a single vein rapidly promotes the cellular proliferation of mouse hepatocytes (Figure 3). The proteins associated with a growth function (GO analysis), identified by the presence of ADSC-CM, were Periostin (POSTN), P component (SAP), semaphorin 7A (SEM7A), and Inactive tyrosine-protein kinase 7[40,41]. Periostin, which is encoded by the POSTIN gene, has been reported to be an extracellular factor that promotes hepatosteatosis[59,60]. Nevertheless, much remains unknown about proteins with the ability to promote the cellular proliferation of hepatocytes. For example, SAP, a protein that is expressed in hepatocytes and secreted into serum, is known to be involved in processes associated with immune regulation, such as the action of opsonins[61], but whether SAP is involved in the cellular proliferation of hepatocytes is unknown. Further, SEM7A is known to contribute to transforming growth factor (TGF)-β-mediated hepatic fibrosis[62], but whether it promotes hepatocyte cell proliferation is unknown. Future studies should therefore investigate whether the growth-associated proteins that are newly identified by GO analyses promote the cellular proliferation of hepatocytes in CCL4-induced liver impairment. What is certain is that HGF and VEGF secreted by ADSCs are among the key factors promoting the proliferation of hepatocytes.
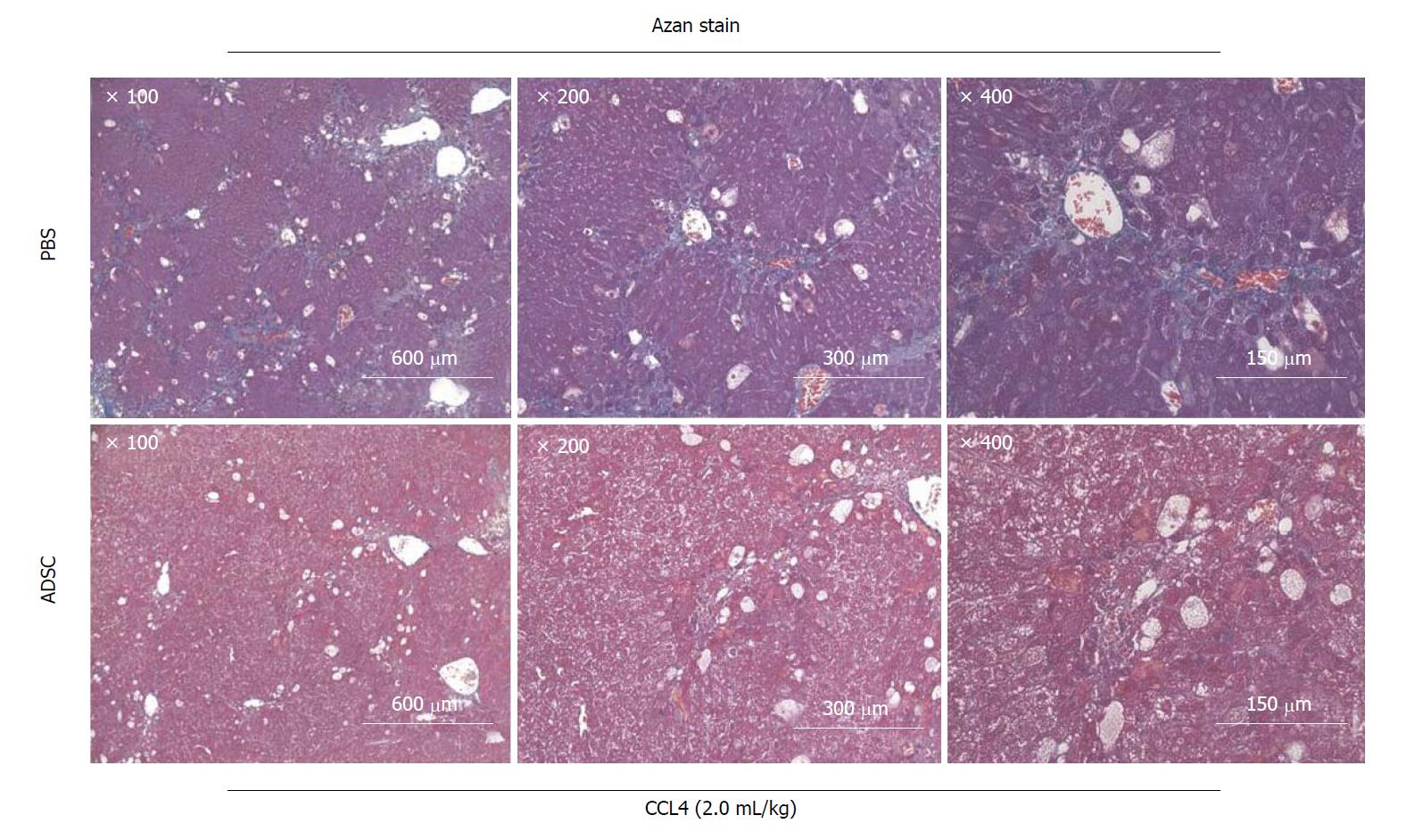
The Jun amino-terminal kinases (JNK) signaling pathway is involved in the activation of HSCs[63,64]. JNK1 plays a major role in the upregulation of the α-SMA expression in HSCs under the stress conditions induced by TGF-β during liver fibrosis[65]. We previously reported the clinical application of organ preservation solution with a JNK inhibitory peptide (11R-JNKI)[66-68] and 8R-sJNKI(-9)[69]. The design of these cell-permeable inhibitory peptides is not only significant for in vivo studies, but also for future attempts to design inhibitors of liver fibrosis for the clinical treatment of liver cirrhosis. In addition, we previously reported that Arg-Gly-Asp (RGD) peptide[70] and Rho-kinase inhibitor[71] suppresses liver fibrosis. Our experiments show that the administration of ADSCs (1 × 106 cells) from a total of three veins at a twice weekly interval rapidly improves the fibrosis of excessive mesenchyme around mouse hepatocytes (Figure 4). When ADSCs are administered via the mouse tail vein, they cause pulmonary embolism, which has a high probability of causing the death of the mouse. Our group developed a method to safely administer ADSCs using heparin[72]. The proteins associated with the immune system process (GO analysis) identified by the presence of ADSC-CM were FINC, CO1A2, CO1A1, CATB, TSP1, CFAH, GAS6, LEG1, PTX3, C1S, SEM7A, CLUS, G3P, PXDN, SRCRL, CD248, SPON2, ENPP2, CD109, CFAB, CATL1, MFAP5, MIF, CXCL5, ADA M9, and CATK (Table 1)[40,41]. Among these ADSC-secreted proteins, we found no studies reporting a relationship in the field of liver cirrhosis and hepatic stellate cells for FINC, CO1A2, CATB, CFAH, LEG1, C1S, SEM7A, CLUS, G3P, PXDN, SRCRL, SPON2, ENPP2, CD109, CFAB, CATL1, MFAP5, ADAM9, or CATK. It is expected that these proteins will be investigated in future studies.
| UniProt/SWISS- | |||
| PROT ID | Description | Reference | |
| FINC | Fibronectin | Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro | [74] |
| CO1A2 | Collagen alpha-2(I) chain | N/A | |
| CO1A1 | Collagen alpha-1(I) chain | Type I collagen has also been reported to be one of the components of hepatic fibrosis | [73] |
| CATB | Cathepsin B | N/A | |
| TSP1 | Thrombospondin-1 | TSP1 might contribute to liver fibrosis not only as an activator of TGF-β, but also as an modulator of angiogenesis | [75] |
| CFAH | Complement factor H | N/A | |
| GAS6 | Growth arrest-specific protein 6 | Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation | [77] |
| LEG1 | Galectin-1 | N/A | |
| PTX3 | Pentraxin-related protein PTX3 | The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling | [78] |
| C1S | Complement C1s subcomponent | N/A | |
| SEM7A | Semaphorin-7A | N/A | |
| CLUS | Clusterin | N/A | |
| G3P | Glyceraldehyde-3-phosphate dehydrogenase | N/A | |
| PXDN | Peroxidasin homolog | N/A | |
| SRCRL | Soluble scavenger receptor cysteine-rich domain-containing protein SSC5D | N/A | |
| CD248 | Endosialin | CD248 reduces susceptibility to liver fibrosis via an effect on PDGF signalling | [79] |
| SPON2 | Spondin-2 | N/A | |
| ENPP2 | Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 | N/A | |
| CD109 | CD109 antigen | N/A | |
| CFAB | Complement factor B | N/A | |
| CATL1 | Cathepsin L1 | N/A | |
| MFAP5 | Microfibrillar-associated protein 5 | N/A | |
| MIF | Macrophage migration inhibitory factor | MIF exerts antifibrotic effects in experimental liver fibrosis via CD74 | [80] |
| CXCL5 | C-X-C motif chemokine 5 | Plasma CXCL5 levels in patients with liver cirrhosis were lower than in healthy controls | [81,82] |
| ADAM9 | Disintegrin and metalloproteinase domain-containing protein 9 | N/A | |
| CATK | Cathepsin K | N/A |
Type I collagen and fibronectin are also reported to be components of hepatic fibrosis[73,74]. It is therefore unlikely that CO1A1 and CO1A2, which are secreted by ADSCs, suppress the excess activity of HSCs. Thrombospondin-1, a matricellular glycoprotein that is secreted by many cell types, modulates a variety of cellular functions by binding to extracellular proteins and/or cell surface receptors. Thrombospondin-1 might contribute to liver fibrosis not only as an activator of TGF-β, but also as a modulator of angiogenesis[75]. In the normal liver, growth arrest-specific gene 6 (Gas6) is mainly expressed in Kupffer cells. The expression of Gas6 increases in activated HSCs and macrophages after acute CCL4 administration[76]. Given that Gas6 and Axl are reported to be necessary for HSC activation[77], Gas6 secreted by ADSCs seems to have no effect in inhibiting the activity of HSCs. Pentraxin 3 (PTX3) is expressed and released by hematopoietic cells and stromal cells and is an essential component of innate immunity. IL-1 induces the production of PTX3 by Kupffer cells, endothelial cells and biliary duct epithelial cells. PTX3 is reported to be a biomarker of liver fibrosis in response to hepatic injury[78]. These reports indicate that PTX3 secreted by ADSCs is unlikely to suppress the activation of HSCs. CD248 (endosialin) is a stromal cell marker expressed on fibroblasts and pericytes. During liver injury, myofibroblasts are the main source of fibrotic matrix. Liver fibrosis was reported to be suppressed in CD248 knockout mice[79], suggesting that it is unlikely that CD248 secreted by ADSCs inhibits hepatic fibrosis. Macrophage migration inhibitory factor (MIF) is a pleiotropic inflammatory cytokine that has been implicated in various inflammatory diseases. MIF knockout mice were reported to have strongly increased fibrosis in a mouse model of chronic liver injury model. This phenomenon was accompanied by no change in the infiltration of intrahepatic immune cells. MIF has an anti-fibrotic effect on the liver via the MIF receptor (CD74). In addition, recombinant MIF protein has a similar anti-fibrotic effect[80]. These results indicate that MIF secreted by ADSCs is a major component in the suppression of liver fibrosis. CXCL5 is the best known for its function as a neutrophil chemotactic factor and activator, with a molecular structure similar to that of IL-8, 4, 5. CXCL5 is released from monocytes, neutrophils, epithelial cells, fibroblasts and smooth muscle during inflammation. Interestingly, CXCL5 has a proliferative effect on rat hepatocytes. The use of a neutralizing antibody of CXCL5 slowed the liver regeneration rate after partial hepatectomy[81]. The plasma CXCL5 levels are low in patients with chronic liver disease, and CXCL5 may be involved in the pathogenesis of chronic liver disease[82]. These results strongly indicate the possibility that CXCL5 secreted by ADSCs also promotes hepatocyte proliferation. These findings indicated that MIF is one of the components that suppress liver fibrosis among the ADSC-secreted proteins that we identified. In addition, CXCL5 was identified as a component that promotes hepatocyte proliferation. Of course, the function of these proteins has been previously reported. Table 1 shows 26 different proteins classified as immunomodulatory (GO analysis). The 18 proteins indicated by N/A have not been reported in the liver field, and further research into them is anticipated. Note that proteins that were not classified as immunomodulatory (by a GO analysis) may also have effects on the liver.
EGF, VEGF and HGF, which are expressed by ADSCs, have a strong angiogenic effect on liver tissue[83-85]. Table 2 lists eight types of proteins [Plasminogen activator inhibitor 1 (PAI1), follistatin-related protein 1 (FSTL1), periostin (POSTN), matrix metalloproteinases 2 (MMP2), TSP1, metalloproteinase inhibitor 1 (TIMP1), Fibulins 3 (FBLN3) and Lactadherin (MFGM)] affecting angiogenesis from among 101 types of proteins secreted by ADSCs. PAI1 is a member of a family of proteins that inhibit plasminogen activators[86]. Although the binding of VEGF to vitronectin induces strong angiogenic signaling, this is inhibited by competitive binding to PAI1[87]. PAI1 secreted from ADSCs is therefore thought to inhibit angiogenesis by VEGF in liver tissue. FSTL1 is a secretory glycoprotein belonging to the follistatin and SPARC family. FSTL1 was reported to be highly expressed in fibrotic human liver tissue and activated HSCs[88]. FSTL1 has the effect of promoting the activity of HSCs. It is therefore unlikely that FSTL1 secreted by ADSCs affects angiogenesis. POSTN, an extracellular matrix (ECM) molecule of the fasciclin family, has roles in vascular cell differentiation and migration[89]. We therefore hypothesize that POSTN secreted by ADSCs may promote angiogenesis of the liver. MMPs are a family of over 24 zinc-dependent endopeptidases capable of degrading virtually any component of the ECM[90]. MMP2 plays an important role in the preservation of liver vascular homeostasis via its participation in the TGF-β activation process[91]. MMP2 secreted by ADSCs-and many other cell types-is therefore considered to be one of the main factors. TSP1 was reported to be increased in HSCs isolated from the liver in a mouse model of CCL4-induced cirrhosis. In liver samples of patients with alcohol cirrhosis and non-alcoholic steatohepatitis-related cirrhosis, TSP1 levels were reported to be increased[92]. It is thought that TSP1 expressed in the liver has the effect of promoting liver fibrosis. If so, it would be unlikely that TSP1 secreted by ADSCs promotes liver angiogenesis. TIMP1 is a widely expressed inhibitor of MMPs. Given that the inhibition of TIMP1 promotes angiogenesis by increasing cell motility during fibrovascular invasion[93], TIMP1 secreted by ADSCs may inhibit liver angiogenesis. FBLNs, a versatile family of extracellular matrix proteins, comprise a small family of widely expressed ECM proteins[94]. FBLN3 has been reported to be an angiogenesis antagonist that regulates cell morphology, growth, adhesion and motility[95]. It is therefore unlikely that FBLN3 secreted by ADSCs promotes liver angiogenesis. MFGM interacts with alphaV-beta3 and alphaV-beta5 integrins and alters both VEGF-dependent Akt phosphorylation and neovascularization[96]. MFGM was reported to promote angiogenesis via enhanced PDGF-PDGFRβ signaling mediated by cross-talk of the integrin growth factor receptor[97]. Thus, MFGM secreted by ADSCs is considered to be one of the main components promoting liver angiogenesis. We recently reported that ADSCs strongly express MFGM and that human MFGM protected dopamine neurons in a rat model of Parkinson’s disease model[98]. At the present stage, there are no reports on a therapeutic method for the disease-specific selection of therapeutic cells that reference to the list of protein components expressed by therapeutic stem cells.
| UniProt/SWISS- | |||
| PROT ID | Description | Reference | |
| PAI1 | Plasminogen activator inhibitor 1 | PAI-1 regulates angiogenesis via effects on extracellular matrix proteolysis and cell adhesion | [87] |
| FSTL1 | Follistatin-related protein 1 | Knockdown of Fstl1 attenuates hepatic stellate cell activation through the TGFβ1/Smad3 signaling pathway | [88] |
| POSTN | Periostin | POSTN, a ligand of αvβ3/5 integrins, as an effector protein in SULF2-induced angiogenesis | [89] |
| MMP2 | 72 kDa type IV collagenase | MMP2 has an important role in the preservation of liver vascular homeostasis | [91] |
| TSP1 | Thrombospondin-1 | TSP1 was reported to be increased in HSCs isolated from the liver of CCl4-induced cirrhosis model mice | [92] |
| TIMP1 | Metalloproteinase inhibitor 1 | Inhibition of TIMP1 was reported to promote angiogenesis by increasing cell motility during fibrovascular invasion | [93] |
| FBLN3 | EGF-containing fibulin-like extracellular matrix protein 1 | FBLN3 has been reported as an angiogenesis antagonist regulating cell morphology, growth, adhesion and motility | [95] |
| MFGM | Lactadherin | MFGM promote angiogenesis via enhanced PDGF-PDGFRβ signaling mediated by cross-talk of the integrin growth factor receptor | [97] |
Regarding cell therapy using ADSCs, we believe that excellent effects on immune response control by cell adhesion can be expected based on reports in the literature. For treatment with ADSC-CM, we used a method to concentrate ADSC-CM 20 times using a 10k filter. ADSC-CM is a liquid and has the advantage of being able to pass through both a 0.22-μm sterilizing filter and a 0.10-μm virus removal filter. We think that lowering the hurdles to cell therapy by taking advantage of the simple adjustment of the solution will contribute to a wide range of medical needs.
These results suggest that VEGF, HGF, EGF, MMP2, POSTN, and MFGM secreted by ADSCs promote hepatic angiogenesis. Among the 101 proteins expressed by ADSCs, we identified three proteins that promote angiogenesis from among eight proteins reported to be involved in angiogenesis. The further development of research into the 93 other proteins is expected.
A study of one-shot stem cell therapy reported the effects of bone marrow-derived mesenchymal stem cell treatment in 53 cirrhosis patients[99]. Although study period was sufficient to observe short-term symptomatic improvement over a period of days to weeks, it has been reported that there is no improvement in symptoms over the longer term (more than a few months). Although this study did not involve the use of ADSCs, it showed that stem cell treatment improves the symptoms of cirrhosis over the short term. However, it shows that one-shot stem cell therapy does not reset the pathological state of cirrhosis through natural healing power leading to recovery. In this paper, we reported that ADSC has three angiogenesis-inducing effects, which promoted the proliferation of hepatocytes, which suppressed the fibrosis of the liver tissue. We believe that medical stakeholders and patients will be more likely to challenge clinical studies of ADSCs in the treatment of cirrhosis. However, it is unlikely that ADSCs play a direct role in controlling all of the pathological conditions of cirrhosis in the body. Thus, we are of the opinion that cell therapy using ADSCs and treatment using ADSC-CM will be useful as supplementary treatments.
The factors necessary for improvement of the symptoms of chronic liver failure (i.e., liver cirrhosis) are: (1) growth factors; (2) inhibitors of hepatic stellate cell inflammation; and (3) angiogenic factors. (1) It is certain that ADSC-secreted HGF and VEGF are among the factors that promote the proliferation of hepatocytes. In addition, CXCL5 was identified as a component that promotes hepatocyte proliferation; (2) MIF, which was one of the ADSC-secreted proteins that we identified that suppressed liver fibrosis; and finally (3) ASDC-secreted VEGF, HGF, EGF, MMP2, POSTN and MFGM are factors that promote hepatic angiogenesis. It seems that the therapeutic effect on the symptoms of cirrhosis is based on ADSC-secreted growth factors, anti-inflammatory effects on stellate cells, and the anti-fibrotic and angiogenic effects of ADSC-secreted proteins.
We thank Naomi Kakazu (University of the Ryukyus) for clerical assistance and Maki Higa, Yuki Kawahira and Saori Adaniya (University of the Ryukyus) for providing technical support.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Grawish ME, Labusca S, Song YH, Tanabe S S- Editor: Wang JL L- Editor: A E- Editor: Tan WW
| 1. | Aoyama H, Hirata T, Sakugawa H, Watanabe T, Miyagi S, Maeshiro T, Chinen T, Kawane M, Zaha O, Nakayoshi T. An inverse relationship between autoimmune liver diseases and Strongyloides stercoralis infection. Am J Trop Med Hyg. 2007;76:972-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Hoshino K, Sugiyama M, Date T, Maruwaka S, Arakaki S, Shibata D, Maeshiro T, Hokama A, Sakugawa H, Kanto T. Phylogenetic and phylodynamic analyses of hepatitis C virus subtype 1a in Okinawa, Japan. J Viral Hepat. 2018;25:976-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Maeshiro T, Arakaki S, Watanabe T, Aoyama H, Shiroma J, Yamashiro T, Hirata T, Hokama A, Kinjo F, Nakayoshi T. Different natural courses of chronic hepatitis B with genotypes B and C after the fourth decade of life. World J Gastroenterol. 2007;13:4560-4565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Hibiya K, Utsunomiya K, Yoshida T, Toma S, Higa F, Tateyama M, Fujita J. Pathogenesis of systemic Mycobacterium avium infection in pigs through histological analysis of hepatic lesions. Can J Vet Res. 2010;74:252-257. [PubMed] [Cited in This Article: ] |
| 5. | Tanaka K, Kobayashi N, Gutierrez AS, Rivas-Carrillo JD, Navarro-Alvarez N, Chen Y, Narushima M, Miki A, Okitsu T, Noguchi H. Prolonged survival of mice with acute liver failure with transplantation of monkey hepatocytes cultured with an antiapoptotic pentapeptide V5. Transplantation. 2006;81:427-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Kobayashi N, Noguchi H, Westerman KA, Watanabe T, Matsumura T, Totsugawa T, Fujiwara T, Leboulch P, Tanaka N. Successful Retroviral Gene Transfer of Simian Virus 40 T Antigen and Herpes Simplex Virus-Thymidine Kinase into Human Hepatocytes 1. Cell Transplant. 2001;10:377-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kobayashi N, Noguchi H, Westerman KA, Watanabe T, Matsumura T, Totsugawa T, Fujiwara T, Leboulch P, Tanaka N. Cre/loxP-Based Reversible Immortalization of Human Hepatocytes 1. Cell Transplant. 2001;10:383-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Shahid JM, Iwamuro M, Sasamoto H, Kubota Y, Seita M, Kawamoto H, Nakaji S, Noguchi H, Yamamoto K, Kobayashi N. Establishment of an immortalized porcine liver cell line JSNK-1 with retroviral transduction of SV40T. Cell Transplant. 2010;19:849-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Miyamoto Y, Ikeuchi M, Noguchi H, Yagi T, Hayashi S. Spheroid Formation and Evaluation of Hepatic Cells in a Three-Dimensional Culture Device. Cell Med. 2015;8:47-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Miyamoto Y, Ikeuchi M, Noguchi H, Yagi T, Hayashi S. Three-Dimensional In Vitro Hepatic Constructs Formed Using Combinatorial Tapered Stencil for Cluster Culture (TASCL) Device. Cell Med. 2014;7:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Kobayashi N, Westerman KA, Tanaka N, Fox IJ, Leboulch P. A reversibly immortalized human hepatocyte cell line as a source of hepatocyte-based biological support. Addict Biol. 2001;6:293-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kobayashi N, Tanaka N. Engineering of Human Hepatocyte Lines for Cell Therapies in Humans: Prospects and Remaining Hurdles. Cell Transplant. 2002;11:417-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Sawada S, Kinjo T, Makishi S, Tomita M, Arasaki A, Iseki K, Watanabe H, Kobayashi K, Sunakawa H, Iwamasa T. Downregulation of citrin, a mitochondrial AGC, is associated with apoptosis of hepatocytes. Biochem Biophys Res Commun. 2007;364:937-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Watanabe T, Shibata N, Westerman KA, Okitsu T, Allain JE, Sakaguchi M, Totsugawa T, Maruyama M, Matsumura T, Noguchi H. Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation. 2003;75:1873-1880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Shibata N, Watanabe T, Okitsu T, Sakaguchi M, Takesue M, Kunieda T, Omoto K, Yamamoto S, Tanaka N, Kobayashi N. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12:499-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Higashi N, Kohjima M, Fukushima M, Ohta S, Kotoh K, Enjoji M, Kobayashi N, Nakamuta M. Epigallocatechin-3-gallate, a green-tea polyphenol, suppresses Rho signaling in TWNT-4 human hepatic stellate cells. J Lab Clin Med. 2005;145:316-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Nakamuta M, Higashi N, Kohjima M, Fukushima M, Ohta S, Kotoh K, Kobayashi N, Enjoji M. Epigallocatechin-3-gallate, a polyphenol component of green tea, suppresses both collagen production and collagenase activity in hepatic stellate cells. Int J Mol Med. 2005;16:677-681. [PubMed] [Cited in This Article: ] |
| 19. | Soto-Gutiérrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24:1412-1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Soto-Gutierrez A, Navarro-Alvarez N, Rivas-Carrillo JD, Chen Y, Yamatsuji T, Tanaka N, Kobayashi N. Differentiation of human embryonic stem cells to hepatocytes using deleted variant of HGF and poly-amino-urethane-coated nonwoven polytetrafluoroethylene fabric. Cell Transplant. 2006;15:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Soto-Gutiérrez A, Navarro-Alvarez N, Zhao D, Rivas-Carrillo JD, Lebkowski J, Tanaka N, Fox IJ, Kobayashi N. Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines. Nat Protoc. 2007;2:347-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Basma H, Soto-Gutiérrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 23. | Kobayashi N, Okitsu T, Tanaka N. Cell choice for bioartificial livers. Keio J Med. 2003;52:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Kobayashi N, Okitsu T, Nakaji S, Tanaka N. Hybrid bioartificial liver: establishing a reversibly immortalized human hepatocyte line and developing a bioartificial liver for practical use. J Artif Organs. 2003;6:236-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Kobayashi N. Life support of artificial liver: development of a bioartificial liver to treat liver failure. J Hepatobiliary Pancreat Surg. 2009;16:113-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Iwamuro M, Shiraha H, Nakaji S, Furutani M, Kobayashi N, Takaki A, Yamamoto K. A preliminary study for constructing a bioartificial liver device with induced pluripotent stem cell-derived hepatocytes. Biomed Eng Online. 2012;11:93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Yonekawa Y, Okitsu T, Wake K, Iwanaga Y, Noguchi H, Nagata H, Liu X, Kobayashi N, Matsumoto S. A new mouse model for intraportal islet transplantation with limited hepatic lobe as a graft site. Transplantation. 2006;82:712-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Totsugawa T, Yong C, Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, Okitsu T, Westerman KA, Kohara M, Reth M. Survival of liver failure pigs by transplantation of reversibly immortalized human hepatocytes with Tamoxifen-mediated self-recombination. J Hepatol. 2007;47:74-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Yuasa T, Yamamoto T, Rivas-Carrillo JD, Chen Y, Navarro-Alvarez N, Soto-Guiterrez A, Noguchi H, Matsumoto S, Tanaka N, Kobayashi N. Laparoscopy-assisted creation of a liver failure model in pigs. Cell Transplant. 2008;17:187-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Yukawa H, Noguchi H, Oishi K, Takagi S, Hamaguchi M, Hamajima N, Hayashi S. Cell transplantation of adipose tissue-derived stem cells in combination with heparin attenuated acute liver failure in mice. Cell Transplant. 2009;18:611-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Yamamoto T, Navarro-Alvarez N, Soto-Gutierrez A, Yuasa T, Iwamuro M, Kubota Y, Seita M, Kawamoto H, Javed SM, Kondo E. Treatment of acute liver failure in mice by hepatocyte xenotransplantation. Cell Transplant. 2010;19:799-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Maruyama M, Totsugawa T, Kunieda T, Okitsu T, Shibata N, Takesue M, Kurabayashi Y, Oshita M, Nakaji S, Kodama M. Hepatocyte isolation and transplantation in the pig. Cell Transplant. 2003;12:593-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Chen Y, Kobayashi N, Suzuki S, Soto-Gutierrez A, Rivas-Carrillo JD, Tanaka K, Navarro-Alvarez N, Fukazawa T, Narushima M, Miki A. Transplantation of human hepatocytes cultured with deleted variant of hepatocyte growth factor prolongs the survival of mice with acute liver failure. Transplantation. 2005;79:1378-1385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 856] [Cited by in F6Publishing: 757] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 35. | Szöke K, Brinchmann JE. Concise review: therapeutic potential of adipose tissue-derived angiogenic cells. Stem Cells Transl Med. 2012;1:658-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Miyagi-Shiohira C, Kurima K, Kobayashi N, Saitoh I, Watanabe M, Noguchi Y, Matsushita M, Noguchi H. Cryopreservation of Adipose-Derived Mesenchymal Stem Cells. Cell Med. 2015;8:3-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Seki A, Sakai Y, Komura T, Nasti A, Yoshida K, Higashimoto M, Honda M, Usui S, Takamura M, Takamura T. Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology. 2013;58:1133-1142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35:e00191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 715] [Cited by in F6Publishing: 813] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 39. | Miyagi-Shiohira C, Kobayashi N, Saitoh I, Watanabe M, Noguchi Y, Matsushita M, Noguchi H. Evaluation of Serum-Free, Xeno-Free Cryopreservation Solutions for Human Adipose-Derived Mesenchymal Stem Cells. Cell Med. 2016;9:15-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Nakashima Y, Nahar S, Miyagi-Shiohira C, Kinjo T, Kobayashi N, Saitoh I, Watanabe M, Fujita J, Noguchi H. A Liquid Chromatography with Tandem Mass Spectrometry-Based Proteomic Analysis of Cells Cultured in DMEM 10% FBS and Chemically Defined Medium Using Human Adipose-Derived Mesenchymal Stem Cells. Int J Mol Sci. 2018;19:2042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Nakashima Y, Nahar S, Miyagi-Shiohira C, Kinjo T, Toyoda Z, Kobayashi N, Saitoh I, Watanabe M, Fujita J, Noguchi H. A liquid chromatography with tandem mass spectrometry-based proteomic analysis of the proteins secreted by human adipose-derived mesenchymal stem cells. Cell Transplantation. 2018;2:1469-1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26268] [Cited by in F6Publishing: 25945] [Article Influence: 1081.0] [Reference Citation Analysis (1)] |
| 43. | Huntley RP, Sawford T, Martin MJ, O’Donovan C. Understanding how and why the Gene Ontology and its annotations evolve: the GO within UniProt. Gigascience. 2014;3:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Nakashima Y, Miyagi-Shiohira C, Kobayashi N, Saitoh I, Watanabe M, Noguchi H. A proteome analysis of pig pancreatic islets and exocrine tissue by liquid chromatography with tandem mass spectrometry. Islets. 2017;9:159-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 602] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 46. | Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997;430:195-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 211] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 47. | Zimmers TA, McKillop IH, Pierce RH, Yoo JY, Koniaris LG. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology. 2003;38:326-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1126] [Cited by in F6Publishing: 1152] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 49. | Soto-Gutierrez A, Navarro-Alvarez N, Caballero-Corbalan J, Tanaka N, Kobayashi N. Endoderm induction for hepatic and pancreatic differentiation of ES cells. Acta Med Okayama. 2008;62:63-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 50. | Hamada E, Ebi N, Miyagi-Shiohira C, Tamaki Y, Nakashima Y, Kobayashi N, Saitoh I, Watanabe M, Kinjo T, Noguchi H. Comparison Between Modified Extracellular-Type Trehalose-Containing Kyoto Solution and University of Wisconsin Solution in 18-Hour Pancreas Preservation for Islet Transplantation. Pancreas. 2018;47:e46-e47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Nakashima Y, Miyagi-Shiohira C, Ebi N, Hamada E, Tamaki Y, Kuwae K, Kobayashi N, Saitoh I, Watanabe M, Kinjo T. A Comparison of Pancreatic Islet Purification using Iodixanol with University of Wisconsin Solution and with Na-lactobionate and Histidine Solution. Cell Med. 2018;10:1-7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Miyagi-Shiohira C, Nakashima Y, Ebi N, Hamada E, Tamaki Y, Kuwae K, Kobayashi N, Saitoh I, Watanabe M, Kinjo T. Comparison of tissue loading before and after the creation of a continuous density gradient in porcine islet purification. Cell Med. 2018;10:1-7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Miyagi-Shiohira C, Kobayashi N, Saitoh I, Watanabe M, Noguchi Y, Matsushita M, Noguchi H. Comparison of Purification Solutions With Different Osmolality for Porcine Islet Purification. Cell Med. 2016;9:53-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Takesue M, Maruyama M, Shibata N, Kunieda T, Okitsu T, Sakaguchi M, Totsugawa T, Kosaka Y, Arata A, Ikeda H. Maintenance of cold-preserved porcine hepatocyte function with UW solution and ascorbic acid-2 glucoside. Cell Transplant. 2003;12:599-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Kunieda T, Maruyama M, Okitsu T, Shibata N, Takesue M, Totsugawa T, Kosaka Y, Arata T, Kobayashi K, Ikeda H. Cryopreservation of primarily isolated porcine hepatocytes with UW solution. Cell Transplant. 2003;12:607-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Arata T, Okitsu T, Fukazawa T, Ikeda H, Kobayashi K, Yong C, Kosaka Y, Narushima M, Matsuoka J, Yamamoto I. Maintenance of glucose-sensitive insulin secretion of cryopreserved human islets with University of Wisconsin solution and ascorbic acid-2 glucoside. Artif Organs. 2004;28:529-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Nahar S, Nakashima Y, Miyagi-Shiohira C, Kinjo T, Toyoda Z, Kobayashi N, Saitoh I, Watanabe M, Fujita J, Noguchi H. Tissue-derived mesenchymal stem cells using the university of wisconsin solution and hank’s balanced salt solution. Stem Cells Int. 2018;2018:1625464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | de Jong IEM, van Leeuwen OB, Lisman T, Gouw ASH, Porte RJ. Repopulating the biliary tree from the peribiliary glands. Biochim Biophys Acta. 2018;1864:1524-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Wu T, Wu S, Ouyang G. Periostin: a new extracellular regulator of obesity-induced hepatosteatosis. Cell Metab. 2014;20:562-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Lu Y, Liu X, Jiao Y, Xiong X, Wang E, Wang X, Zhang Z, Zhang H, Pan L, Guan Y. Periostin promotes liver steatosis and hypertriglyceridemia through downregulation of PPARα. J Clin Invest. 2014;124:3501-3513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 61. | Zhou Z, Xu MJ, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol. 2016;13:301-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 62. | De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Trozzi L, Candelaresi C, Bataller R, Millán C, Brenner DA, Vivarelli M. Semaphorin 7A contributes to TGF-β-mediated liver fibrogenesis. Am J Pathol. 2013;183:820-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Kluwe J, Pradere JP, Gwak GY, Mencin A, De Minicis S, Osterreicher CH, Colmenero J, Bataller R, Schwabe RF. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 64. | Poulos JE, Weber JD, Bellezzo JM, Di Bisceglie AM, Britton RS, Bacon BR, Baldassare JJ. Fibronectin and cytokines increase JNK, ERK, AP-1 activity, and transin gene expression in rat hepatic stellate cells. Am J Physiol. 1997;273:G804-G811. [PubMed] [Cited in This Article: ] |
| 65. | Hong IH, Park SJ, Goo MJ, Lee HR, Park JK, Ki MR, Kim SH, Lee EM, Kim AY, Jeong KS. JNK1 and JNK2 regulate α-SMA in hepatic stellate cells during CCl4 -induced fibrosis in the rat liver. Pathol Int. 2013;63:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Noguchi H, Nakai Y, Ueda M, Masui Y, Futaki S, Kobayashi N, Hayashi S, Matsumoto S. Activation of c-Jun NH2-terminal kinase (JNK) pathway during islet transplantation and prevention of islet graft loss by intraportal injection of JNK inhibitor. Diabetologia. 2007;50:612-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Noguchi H, Nakai Y, Matsumoto S, Kawaguchi M, Ueda M, Okitsu T, Iwanaga Y, Yonekawa Y, Nagata H, Minami K. Cell permeable peptide of JNK inhibitor prevents islet apoptosis immediately after isolation and improves islet graft function. Am J Transplant. 2005;5:1848-1855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Noguchi H. Activation of c-Jun NH2-terminal kinase during islet isolation. Endocr J. 2007;54:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Noguchi H, Miyagi-Shiohira C, Nakashima Y, Ebi N, Hamada E, Tamaki Y, Kuwae K, Kobayashi N, Saitoh I, Watanabe M. Modified cell-permeable JNK inhibitors efficiently prevents islet apoptosis and improves the outcome of islet transplantation. Sci Rep. 2018;8:11082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Kotoh K, Nakamuta M, Kohjima M, Fukushima M, Morizono S, Kobayashi N, Enjoji M, Nawata H. Arg-Gly-Asp (RGD) peptide ameliorates carbon tetrachloride-induced liver fibrosis via inhibition of collagen production and acceleration of collagenase activity. Int J Mol Med. 2004;14:1049-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Fukushima M, Nakamuta M, Kohjima M, Kotoh K, Enjoji M, Kobayashi N, Nawata H. Fasudil hydrochloride hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver Int. 2005;25:829-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Baba Y, Hayashi S. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012;33:2177-2186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Koilan S, Hamilton D, Baburyan N, Padala MK, Weber KT, Guntaka RV. Prevention of liver fibrosis by triple helix-forming oligodeoxyribonucleotides targeted to the promoter region of type I collagen gene. Oligonucleotides. 2010;20:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Liu XY, Liu RX, Hou F, Cui LJ, Li CY, Chi C, Yi E, Wen Y, Yin CH. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol Med Rep. 2016;14:3669-3675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Li Y, Turpin CP, Wang S. Role of thrombospondin 1 in liver diseases. Hepatol Res. 2017;47:186-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 76. | Lafdil F, Chobert MN, Couchie D, Brouillet A, Zafrani ES, Mavier P, Laperche Y. Induction of Gas6 protein in CCl4-induced rat liver injury and anti-apoptotic effect on hepatic stellate cells. Hepatology. 2006;44:228-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Bárcena C, Stefanovic M, Tutusaus A, Joannas L, Menéndez A, García-Ruiz C, Sancho-Bru P, Marí M, Caballeria J, Rothlin CV. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J Hepatol. 2015;63:670-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 78. | Bottazzi B, Inforzato A, Messa M, Barbagallo M, Magrini E, Garlanda C, Mantovani A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J Hepatol. 2016;64:1416-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 79. | Wilhelm A, Aldridge V, Haldar D, Naylor AJ, Weston CJ, Hedegaard D, Garg A, Fear J, Reynolds GM, Croft AP. CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism. Gut. 2016;65:1175-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 80. | Heinrichs D, Knauel M, Offermanns C, Berres ML, Nellen A, Leng L, Schmitz P, Bucala R, Trautwein C, Weber C. Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis via CD74. Proc Natl Acad Sci U S A. 2011;108:17444-17449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 81. | Colletti LM, Green M, Burdick MD, Kunkel SL, Strieter RM. Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock. 1998;10:248-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Tacke F, Zimmermann HW, Trautwein C, Schnabl B. CXCL5 plasma levels decrease in patients with chronic liver disease. J Gastroenterol Hepatol. 2011;26:523-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Elpek GÖ. Angiogenesis and liver fibrosis. World J Hepatol. 2015;7:377-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 84. | Amarapurkar AD, Amarapurkar DN, Vibhav S, Patel ND. Angiogenesis in chronic liver disease. Ann Hepatol. 2007;6:170-173. [PubMed] [Cited in This Article: ] |
| 85. | Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 413] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 86. | Huber K, Christ G, Wojta J, Gulba D. Plasminogen activator inhibitor type-1 in cardiovascular disease. Status report 2001. Thromb Res. 2001;103 Suppl 1:S7-S19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Wu J, Strawn TL, Luo M, Wang L, Li R, Ren M, Xia J, Zhang Z, Ma W, Luo T. Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-αVβ3 integrin cross talk. Arterioscler Thromb Vasc Biol. 2015;35:111-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Shang H, Liu X, Guo H. Knockdown of Fstl1 attenuates hepatic stellate cell activation through the TGFβ1/Smad3 signaling pathway. Mol Med Rep. 2017;16:7119-7123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3192] [Cited by in F6Publishing: 3489] [Article Influence: 249.2] [Reference Citation Analysis (0)] |
| 91. | Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44-46:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 309] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 92. | Smalling RL, Delker DA, Zhang Y, Nieto N, McGuiness MS, Liu S, Friedman SL, Hagedorn CH, Wang L. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G364-G374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Reed MJ, Koike T, Sadoun E, Sage EH, Puolakkainen P. Inhibition of TIMP1 enhances angiogenesis in vivo and cell migration in vitro. Microvasc Res. 2003;65:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4:479-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 95. | Wang R, Zhang YW, Chen LB. Aberrant promoter methylation of FBLN-3 gene and clinicopathological significance in non-small cell lung carcinoma. Lung Cancer. 2010;69:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 96. | Silvestre JS, Théry C, Hamard G, Boddaert J, Aguilar B, Delcayre A, Houbron C, Tamarat R, Blanc-Brude O, Heeneman S. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 97. | Uchiyama A, Yamada K, Ogino S, Yokoyama Y, Takeuchi Y, Udey MC, Ishikawa O, Motegi S. MFG-E8 regulates angiogenesis in cutaneous wound healing. Am J Pathol. 2014;184:1981-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Nakashima Y, Miyagi-Shiohira C, Noguchi H, Omasa T. The Healing Effect of Human Milk Fat Globule-EGF Factor 8 Protein (MFG-E8) in A Rat Model of Parkinson’s Disease. Brain Sci. 2018;8:167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 537] [Article Influence: 41.3] [Reference Citation Analysis (0)] |