修回日期: 2021-03-13
接受日期: 2021-03-26
在线出版日期: 2021-04-28
胰腺癌是预后最差的实体恶性肿瘤, 其中约90%是胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDAC). 尽管手术切除是唯一可能治愈PDAC的手段, 但术后总体生存率不容乐观. 因此, 以吉西他滨(gemcitabine, GEM)为基础的化疗仍是PDAC最重要的治疗选择之一. 然而, GEM单药治疗晚期PDAC的生存期改善十分有限, 其关键原因在于GEM耐药. GEM耐药的机制复杂且不明确. 肿瘤微环境(tumor microenvironment, TME)中广泛而致密的纤维间质是PDAC的重要特征. 越来越多的证据显示, 纤维化TME不仅是肿瘤生长、扩散的积极参与者, 更是诱导GEM耐药的贡献者. 本文将重点从PDAC细胞外途径对GEM化疗耐药的主要细胞和分子机制研究进展进行综述, 讨论潜在的GEM化疗增敏策略, 以期提高化疗有效率, 改善PDAC的总体预后.
核心提要: 吉西他滨(gemcitabine, GEM)耐药是影响胰腺癌化疗疗效的关键原因. GEM耐药的机制复杂且不明确, 近年来细胞外调控机制的研究取得众多新进展, 这将为GEM化疗增敏策略提供了新的潜在靶点, 对进一步提高化疗有效率, 改善胰腺癌的总体预后具有重要意义.
引文著录: 顾宗廷, 李宗泽, 王成锋. 胰腺癌细胞外吉西他滨耐药机制的研究进展. 世界华人消化杂志 2021; 29(8): 421-434
Revised: March 13, 2021
Accepted: March 26, 2021
Published online: April 28, 2021
Pancreatic cancer is a solid malignant tumor with the worst prognosis worldwide, and about 90% of cases are pancreatic ductal adenocarcinoma (PDAC). Although surgical resection is the only potential way to cure PDAC, the overall survival rate after surgery is still not optimistic. Consequently, gemcitabine (GEM)-based chemotherapy is still one of the most important treatment options for PDAC. However, the survival improvement by GEM monotherapy for advanced PDAC is very limited, and GEM resistance is the key reason. The mechanism underlying gemcitabine resistance is complex and still unclear in PDAC. The extensive and dense fibrous mesenchyme in the tumor microenvironment (TME) is an important feature of PDAC. More and more evidence has shown that TME is not only an active participant in tumor growth and spread, but also a contributor to the induction of GEM resistance. This article will review the recent advances in the understanding of the cellular and molecular mechanisms underlying GEM resistance in PDAC, and discuss potential GEM chemosensitization strategies, in order to improve the effective rate of chemotherapy and the outcome.
- Citation: Gu ZT, Li ZZ, Wang CF. Advances in research of extracellular mechanisms underlying gemcitabine resistance in pancreatic cancer. Shijie Huaren Xiaohua Zazhi 2021; 29(8): 421-434
- URL: https://www.wjgnet.com/1009-3079/full/v29/i8/421.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v29.i8.421
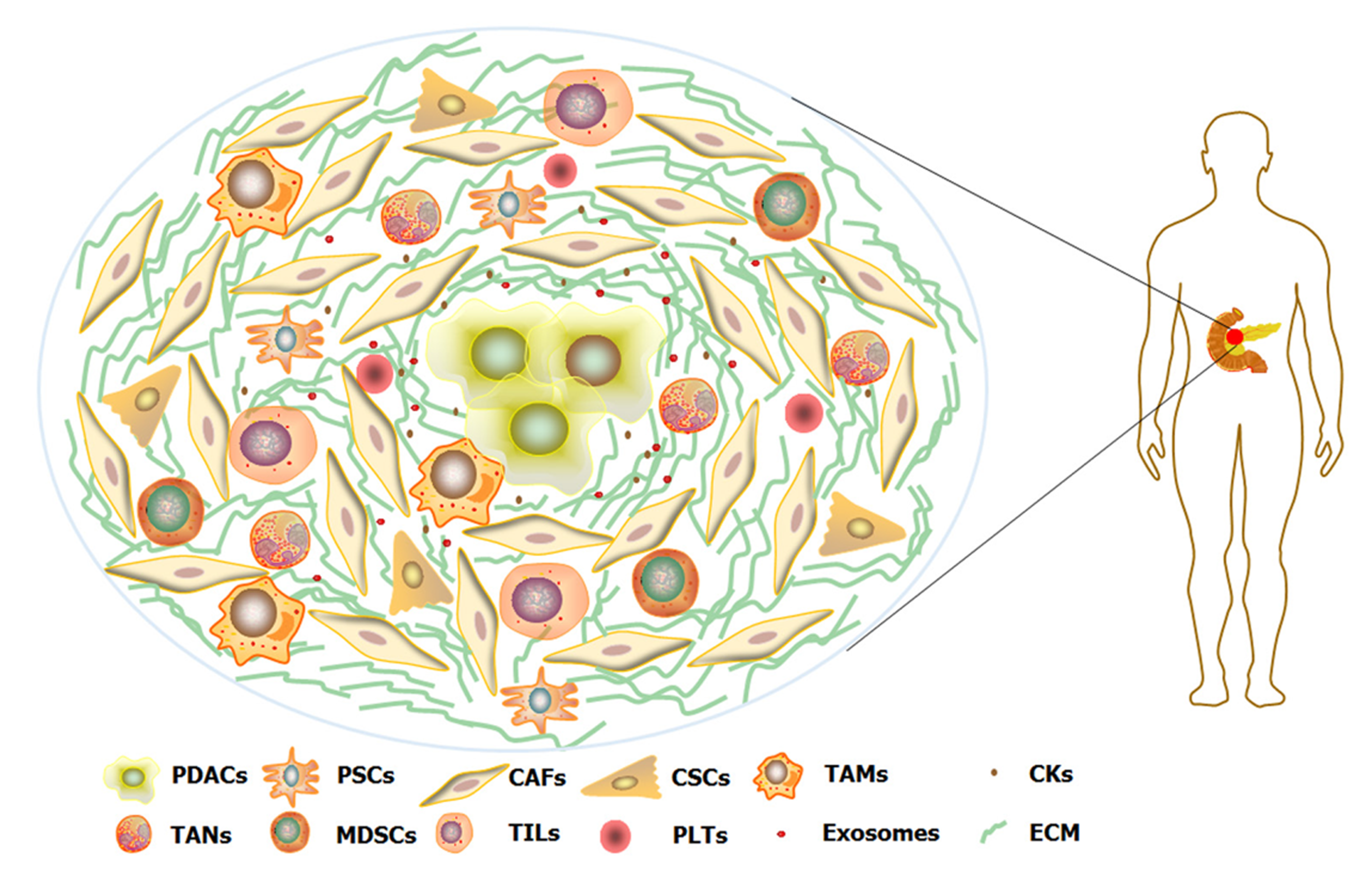
胰腺癌是目前人类预后最差的实体恶性肿瘤, 其中约90%是胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDAC). 在中国, PDAC的5年生存率仅为7.2%, 且无明显改善趋势[1]. 尽管手术切除是唯一可能治愈PDAC的手段, 但术后5年生存率也仅为10%-25%, 且有手术指征的患者仅占15%-20%[2]. 因此, 化疗仍是PDAC最重要的治疗选择之一. 改良FOLFIRINOX方案(奥沙利铂、伊立替康、5-FU和亚叶酸钙)对PDAC的疗效已证实优于吉西他滨(gemcitabine, GEM), 但更高的毒性作用发生率限制了其应用[3]. 因此, GEM仍是目前PDAC化疗的基石. GEM是一种脱氧胞苷核苷类似物, 可作为竞争性底物掺入DNA链并终止其复制, 最终导致细胞死亡[4]. 然而, GEM单药治疗晚期PDAC的中位无进展生存期仅为3.7个月[5], GEM耐药是其中的关键原因. 与其他恶性肿瘤相比, 广泛而致密的纤维间质是PDAC的重要特征, PDAC细胞周围的增生结缔组织约占肿瘤总体积的90%, 严重扭曲了正常的胰腺结构[6]. 增生结缔组织主要由间质细胞成分、细胞外基质(extracellular matrix, ECM)、血管和淋巴管组成, 共同构建成肿瘤微环境(tumor microenvironment, TME)(图1). PDAC细胞与TME之间的相互作用是刺激广泛纤维增生的主要原因[7]. TME不仅是PDAC生长、扩散的积极参与者, 更是诱导GEM耐药的贡献者[8]. TME通过ECM的物理屏障作用和改变细胞外信号调节激酶(extracellular regulated protein kinases, ERK)、丝/苏氨酸蛋白激酶(serine/threonine protein kinases, Akt)、转录激活因子3(signal transducer and activator of transcription 3, STAT3)等通路活化状态, 影响PDAC细胞中相关基因的表达, 诱导GEM抵抗[9]. 因此, PDAC细胞外途径并非孤立事件, 而是与PDAC细胞共同促进GEM耐药的发生. 本文将重点从PDAC细胞外途径讨论GEM化疗耐药的主要细胞和分子机制研究进展(表1). 细胞外TME各组分与PDAC细胞间相互作用机制的进一步研究, 有助于发现GEM耐药的潜在治疗靶点, 为化疗增敏和改善PDAC总体预后提供新的策略.
| 潜在靶点 | 耐药机制 | 临床试验 | 文献 |
| Hypoxia/HIF-1α | 诱导EMT; 增加Glycolysis, 提高dCTP水平; 活化Akt/Notch1通路, 诱导CSCs表型 | NCT01746979 | [11-15] |
| CA9/Acidosis | 增加Glycolysis, 改变细胞内、外PH; 诱导EMT | [17,18] | |
| MT1-MMP/HMGA2/HATs | 基质物理屏障; 增加H3K9、H3K27乙酰化, 修复受损DNA | [8,21-24] | |
| TIMP1 | 活化PI3K/Akt促生存通路 | [25] | |
| HA/CD44 | 增加IFP; 上调MDR1表达; 诱导EMT | NCT01839487; NCT02715804; HALO109-301 | [27-35] |
| TG2/LN/FAK | 基质物理屏障; 活化PI3K/Akt、NF-κB促生存通路 | [39-41] | |
| FN/ERK | 基质物理屏障; 活化ERK通路, 抑制Apoptosis | [43] | |
| CTGF/XIAP | 抑制Caspase-3/7/9介导的凋亡小体形成 | NCT02210559 | [45-47] |
| TGF-β/CYR61/VAV1 | TGF-β/ALK5/Smad2/3通路活化, 下调 hENT1、hCNT3表达; 诱导EMT | [48-51] | |
| HAb18G/EGFR/STAT3 | 活化EGFR/STAT3促生存通路 | CONKO005; ISRCTN96397434; NCT00471146 | [53-59] |
| IGF-1R | 活化PI3K/Akt/mTOR、MEK/ERK促生存通路 | NCT00769483; NCT01231347 | [61-64] |
| PDGFR | 增加IFP; 活化PI3K/Akt、mTOR、NF-κB、ERK、MAPK、Notch通路诱导EMT | BAYPAN | [65-69] |
| CXCL12/CXCR4 | 活化FAK、ERK、Akt促生存通路, 上调Survivin的表达 | [72-74] | |
| Hes1/Notch | 基质物理屏障; 活化Notch通路, 诱导 EMT和CSCs表型 | [77-79] | |
| HGF/c-Met | 活化PI3K/Akt/mTOR促生存通路 | NCT00874042 | [80-82] |
| Periostin | 抑制Apoptosis | [83] | |
| dC | 细胞内dCK竞争作用 | [84] | |
| α-SMA-CAFs | 增加Hypoxia; 诱导EMT和CSCs表型; 基质物理屏障 | [86,87] | |
| FAP-CAFs | 重塑ECM, 基质物理屏障 | [89,90] | |
| SHH | 活化HH促生存通路 | NCT01064622; NCT01088815 | [91-94] |
| IL-6 | 活化促炎症通路 | [95,96] | |
| Exosome | 激活Snail, 诱导EMT | [98,99] | |
| CSCs/ncRNA | 诱导EMT; 抑制Apoptosis; 活化PI3K/Akt/mTOR促生存通路 | [101-103] | |
| TAMs/STAT3 | 活化STAT3, 诱导 CSCs表型; 抑制Apoptosis; 上调CDA表达; M2型极化 | [105-112] | |
| GM-CSF | 活化MAPK、NF-κB促生存通路, 促MDSCs的分化 | ISRCTN4382138 | [120,122] |
| PD-1/L1, CTLA-4 | 抑制CTLs毒性作用 | [126,127] | |
| HEATR1/Nrf2 | 负性调控Akt促生存通路, 活化效应CTLs; 负性调控下游抗氧化和细胞保护基因转录 | [128,129] | |
| ADP-P2Y12 | 上调Slug、ZEB1的表达,诱导EMT; 下调hENT1、 CDA的表达; 活化EGFR通路 | NCT02404363 | [133-135] |
纤维结缔组织增生和供血血管减少削弱了PDAC的组织灌注, 造成了乏氧的TME[10](图1). 低氧通过缺氧诱导因子-1α(hypoxia-inducible factor-1α, HIF-1α)介导上皮-间充质转化(epithelial-mesenchymal transition, EMT)、糖酵解通路的活化, 导致GEM耐药[11,12]. 体外试验亦证实, 下调HIF-1α可增加GEM的敏感性[11,13]. 另外, 低氧也可通过Akt/Notch1信号通路增强GEM诱导的细胞干性并促进化疗抵抗[14]. 因此, 缺氧赋予肿瘤组织对GEM的抵抗力. 但遗憾的是, 一项关于GEM联合靶向低氧TME的细胞毒性药(TH-302)治疗晚期PDAC的Ⅲ期临床试验(NCT01746979)结果并未显示出总生存期(overall survival, OS)的统计学差异[15]. PDAC自身及所属TME的异质性增加了缺氧诱发GEM耐药机制的复杂性, 这可能是临床试验失败的原因之一. 酸中毒是TME的另一重要特征. HIF-1α介导的糖酵解乳酸生成和碳酸酐酶(carbonic anhydrase, CA)介导的碳酸生成, 是PDAC细胞外H+的主要来源[16]. 一项最新的研究显示, 缺氧诱导HIF-1α通过上调CA9的表达, 介导PDAC糖酵解增加和细胞内、外pH的变化, 增加GEM抵抗. 相反, 沉默或抑制CA9则可逆转这一过程[17]. 此外, 酸性TME也可能通过诱导EMT介导GEM抵抗[18]. 因此, 靶向改变细胞外pH值有望成为GEM增敏的新策略. 另外, TME应激也参与了细胞代谢的重塑, 但其代谢影响与GEM耐药的直接关系仍待进一步明确[19].
PDAC的TME中存在大量致密的基质成分(图1), 一方面致密的纤维组织压迫肿瘤组织内的血管, 阻止GEM进入组织, 另一方面基质成分与PDAC细胞间相互作用, 共同促进了GEM耐药.
PDAC的基质主要由I型胶原蛋白组成, 其正常亚型是一种可被胶原酶降解的异型三聚体, 但PDAC细胞可分泌特有的同型三聚体, 后者对所有胶原溶解性基质金属蛋白酶(matrix metalloproteinases, MMPs)均具有抗性[20]. 临床前研究证实, 基质胶原蛋白可形成物理屏障影响GEM渗透和治疗反应[8]. 此外, 胶原蛋白通过上调PDAC中膜型1-基质金属蛋白酶(membrane type 1-matrix metalloproteinase , MT1-MMP)的表达, 增加ERK1/2磷酸化, 并进一步上调高迁移率族蛋白A2(high mobility group A2, HMGA2)的表达[21]. HMGA2是DNA碱基末端连接修复机制的一部分, 可从DNA中去除小的受损碱基, 并具有嘌呤/嘧啶裂解酶活性, 可削弱GEM在富含胶原的TME中的作用[22]. 另外, HMGA2过表达还促进PDAC细胞组蛋白乙酰转移酶(histone acetyltransferases, HATs)上调, 增加组蛋白H3K9和H3K27乙酰化, 促进染色质松弛和最终的DNA修复, 介导GEM抗性[23]. 由此可见, 靶向MT1-MMP/HMGA2/HATs信号通路可能是新的GEM增敏策略. 遗憾的是, 针对MMPs广谱抑制剂的所有临床试验, 与单独使用GEM相比均未显示临床优势, 缺乏选择性地MMPs抑制剂一度被认为是试验失败的原因之一[24]. 但有趣的是, 一项最新的研究显示, MMP1组织抑制剂(tissue inhibitor of metalloproteinases 1, TIMP1)可通过激活PI3K/Akt促生存信号通路参与GEM抵抗, 下调TIMP1表达则可逆转这一过程[25]. 因此, PDAC基质胶原蛋白在GEM耐药机制中可能发挥双重作用, 同时也提示TIMP1可能是潜在的GEM增敏靶点.
透明质酸(hyaluronic acid, HA)是一种非硫酸化糖胺聚糖, 组织中HA在生理状态下受到合成和降解动态平衡的调节[9]. HA在PDAC基质成分中含量最高, HA高表达是PDAC患者的独立预后因素, 并在GEM抵抗中发挥关键作用[26]. 高吸水特性的HA累积导致肿瘤中的间质液压力(interstitial fluid pressure, IFP)显著增加, 后者限制了对流, 从而降低了灌注血管的溶质通量, 并最终导致肿瘤的低灌注, 这是GEM抵抗的流体力学机制[27]. 除此之外, HA与CD44等细胞表面受体结合, 通过酪氨酸激酶受体诱导的重要信号通路介导化疗抵抗, 下调 CD44可逆转GEM耐药[28]. 另外, HA-CD44轴亦可通过增加肿瘤干细胞(cancer stem cells, CSCs)标记分子Nanog的磷酸化, 促进多药耐药蛋白1(multidrug resistance protein 1, MDR1)的上调, 增加GEM外排, 或通过β-连环蛋白(β-catenin)的Wnt信号通路诱导EMT, 介导GEM耐药[29,30]. 因此, 靶向HA-CD44信号轴可能是有希望的GEM增敏策略. 对此, 一项最新的研究设计了一种靶向CD44的新型纳米GEM载药脂质体, 并在体外实验中证实了疗效[31]. PEGPH20是一种新型聚乙二醇重组透明质酸酶, 能够有效清除基质HA, 且在PDAC动物模型中可有效增敏GEM[32]. 目前, PEGPH20联合GEM治疗晚期PDAC的部分临床试验(NCT01839487, NCT02715804)已获得积极的结果[33,34]. 遗憾的是, 最近一项有关转移性PDAC的Ⅲ期临床试验(HALO 109-301)则提示, PEGPH20联合GEM并未显示额外的优势, 且毒副作用明显增加[35]. 值得一提的是, 最近两项研究表明, 动态增强(dynamic contrast-enhanced, DCE)-MRI可用于识别肿瘤微血管的灌注变化[36], SPECT/CT可用于识别新型荧光分子标记的HA-CD44肿瘤细胞[37] , 这便为PDAC靶向HA治疗反应评价提供了可能.
层粘连蛋白(laminin, LN)是肿瘤ECM组成蛋白中的另一关键成分, 其与整联蛋白结合组成PDAC细胞黏附分子, 并与不良预后显著相关[38]. PDAC细胞与LN的粘附以及随后信号通路的激活参与了GEM抵抗. 局灶性粘附激酶(focal adhesion kinase, FAK)是ECM向细胞传递信号的关键细胞内分子, LN通过诱导FAK/Akt磷酸化, 促进生存蛋白(survivin)的表达, 抵抗GEM诱导的细胞毒性和凋亡[39]. 另外, 一项最近的异种移植模型研究显示, PDAC细胞分泌的组织转谷氨酰胺酶2(transglutaminase 2, TG2)可刺激间质肿瘤相关成纤维细胞(cancer-associated fibroblasts, CAFs)分泌LN-1, 进而增加GEM抵抗, 下调TG2则可逆转这一趋势[40]. 除了诱导LN分泌构成基质物理屏障之外, TG2也可通过激活细胞内FAK/PI3K/Akt和NF-κB促生存通路参与GEM抵抗[41]. 因此, TG2/LN/FAK可能是GEM增敏的潜在靶点, 但由于缺少特异性抑制剂, 目前相关的研究仍十分有限.
纤连蛋白(fibronectin, FN)也是ECM的主要组成成分之一, 可与PDAC细胞表面的黏附受体整联蛋白结合, 参与细胞内外的信号传递, 并在GEM抵抗中发挥重要作用[42]. FN除了与其他基质蛋白一样参与形成基质物理屏障之外, 还可通过诱导ERK1/2磷酸化抵抗GEM诱导的细胞凋亡, 介导GEM耐药. 体外抑制ERK则可恢复GEM的敏感性[43]. 因此, 靶向FN/ERK有望成为PDAC克服GEM耐药的潜在策略.
细胞因子和趋化因子是PDAC细胞与ECM之间的调节介质, 同样在GEM抵抗中发挥关键作用. 其中, 结缔组织生长因子(connective tissue growth factor, CTGF)可介导间质纤维形成并在PDAC中过表达[44]. CTGF还可通过诱导细胞凋亡抑制蛋白(X-linked inhibitor of apoptosis protein, XIAP)磷酸化, 直接抑制caspase-3/7/9介导的凋亡小体形成, 参与GEM抵抗[45]. 体外和异种模型研究表明, 靶向抑制CTGF(FG-3019)或XIAP(AZD5582)可通过诱导PDAC凋亡进一步逆转GEM耐药[45,46]. 因此, CTGF/XIAP轴是抗GEM耐药的潜在靶点. 目前, 靶向阻断CTGF的单克隆抗体(pamrevlumab)联合GEM治疗局部晚期PDAC的新辅助方案正在进行临床试验(NCT02210559), 初步结果显示联合方案可增加R0切除率且耐受性良好[47]. 此外, 转化生长因子-β(transforming growth factor β, TGF-β)也在PDAC中过表达, 其通过经典的TGF-β/ALK5/Smad2/3信号通路诱导CYR61表达, 后者负调控核苷转运蛋白hENT1和hCNT3, 增加吉西他滨的细胞摄取. 最新的多项研究证实, PDAC中TGF-β信号的激活或CYR61的上调促进了GEM耐药, 靶向抑制TGF-β/CYR61则可实现GEM增敏[48-50]. 另外, TGF-β还通过调控VAV1基因甲基化来促进EMT并介导GEM耐药, 靶向抑制TGF-β/VAV1轴则可显著增强GEM的疗效[51]. 因此, 靶向TGF-β/CYR61/VAV1也是GEM增敏的潜在策略.
众所周知, 表皮生长因子受体(epidermal growth factor receptor, EGFR)和血管内皮生长因子受体(vascular endothelial growth factor receptor, VEGFR)通路在癌症的发生发展中具有关键作用, 其表达与不良预后密切相关[52]. 其中, EGFR是生长因子受体酪氨酸激酶ErbB的家族成员, 可在40%-60%的PDAC中表达. 临床前研究显示, GEM可通过激活HAb18G(CD147)/EGFR/STAT3促生存信号通路, 诱导PDAC对GEM获得性耐药[53,54]. 靶向抑制HAb18G(CD147)或EGFR/STAT3信号则可逆转GEM抗性[54,55]. 因此, HAb18G(CD147)/EGFR/STAT3轴是克服GEM耐药的潜在靶点. 遗憾的是, EGFR-酪氨酸激酶抑制剂(EGFR-tyrosine kinase inhibitors, EGFR-TKIs)联合GEM较GEM单药治疗可切除或晚期PDAC的临床试验均未显示无病生存期(disease-free survival, DFS)或OS的改善[56-58], VEGFR-TKIs联合GEM的Ⅲ期临床试验(NCT00471146)也宣告失败[59]. TKIs诱导的细胞快速反馈代偿机制可能是试验失败的原因之一[60].
此外, 胰岛素样生长因子-1(insulin-like growth factor 1, IGF-1)与其受体IGF-1R的结合可激活PI3K/Akt/mTOR、MEK/ERK通路, 促进PDAC增殖并诱导GEM耐药[61]. 靶向抑制IGF-1R可增强GEM在PDAC异种移植物的抗肿瘤效果[62]. 目前, 人源IGF-1R单克隆抗体已经在几种癌症中应用, 但针对转移性PDAC联合GEM治疗的III期临床试验(NCT01231347)并未取得成功[63]. 令人振奋的是, 最近一项针对进展期PDAC的I/II期临床试验(NCT00769483)显示, IGF-1R抑制剂联合GEM+EGFR-TKIs较GEM+EGFR-TKIs可显著改善OS[64]. 另外, 血小板衍生生长因子-D(platelet-derived growth factor-D, PDGF-D)是诱导PDAC间质纤维化和IFP递增并参与GEM耐药的关键因子, 这一过程可能是通过PI3K/Akt、mTOR、NF-κB、ERK、MAPK、Notch通路触发EMT实现的[65]. 虽然PDGFR抑制剂联合GEM在临床前研究中可显示疗效[66], 但遗憾的是, 最近完成的临床试验均宣告失败[67-69].
除了细胞因子之外, 趋化因子配体(CXC ligand, CXCL)与其受体(CXC chemokine receptor, CXCR)结合通过PDAC间质细胞的旁分泌和癌细胞自分泌信号传导参与GEM抵抗, 其中一个关键的轴心是CXCL12/CXCR4[70]. CXCR4是细胞表面G蛋白偶联受体家族成员, 其在PDAC中高表达且与不良预后密切相关[71]. CXCL12/CXCR4轴可激活FAK、ERK和Akt促生存通路, 增强β-catenin和NF-κB的转录活性, 促进Survivin的表达, 诱导GEM耐药[72]. 临床前研究显示, CXCR4拮抗剂可显著增强GEM的疗效[72-74]. 近年来, 不断有新的CXCR通路被揭示, 并且相关通路的体外抑制亦显示了类似的效果[75,76]. 可以预见, 靶向CXCR将是未来GEM增敏研究的热点之一.
间质细胞是TME的重要成员(图1), 其通过直接细胞间接触和细胞旁分泌(ECM蛋白)途径与PDAC细胞相互作用, 共同参与GEM耐药.
胰腺星状细胞(pancreatic stellate cells, PSCs)是PDAC中最重要间质细胞成分之一, 其可被TNF-α、TGF-β、IL-1、IL-2、IL-10和PDGF激活从静止的表型转变成CAFs, 后者通过分泌ECM蛋白构建纤维化间质和乏氧的TME, 促进GEM耐药[77,78]. PSCs除了参与构建GEM耐药的物理屏障之外, 还可通过与PDAC细胞直接接触和旁分泌细胞因子途径诱导GEM抗性. 体外共培养研究证实, PSCs可通过增加PDAC细胞Hes1(Notch信号通路组成部分)的表达激活Notch信号通路, 后者通过增强EMT和CSCs表型诱导GEM耐药. 当抑制Hes1基因表达或Notch通路时, PSCs诱导的化疗耐药被有效地逆转[79]. 因此, 靶向Hes1/Notch信号通路可能是逆转GEM耐药的策略之一. 体外三维(three-dimensional, 3D)共培养研究显示, PSCs可通过分泌肝细胞生长因子(hepatocyte growth factor, HGF)与PDAC细胞受体酪氨酸激酶c-Met结合并诱导其磷酸化, 后者通过PI3K/Akt/mTOR促生存通路的激活诱导GEM抗性[80]. 临床前c-Met靶向抑制剂联合GEM可显著增强其疗效[80,81], Ⅰ期临床试验(NCT00874042)亦证实两者联合应用有良好的安全性和耐受性[82]. 因此, 靶向抑制HGF/c-Met通路将是有希望的GEM增敏策略. 此外, PSCs分泌的骨膜蛋白(Periostin)赋予GEM抵抗力, 沉默其表达可逆转GEM抗性, 这一过程可能与GEM诱导凋亡的抑制有关[83]. 最近一项研究显示, PSCs可通过分泌脱氧胞苷(deoxycytidine, dC)保护PDAC免受GEM的毒性, 但具体机制仍不清楚, 可能与GEM细胞内代谢的脱氧胞苷激酶(deoxycytidine kinase, dCK)竞争作用有关[84]. 因此, Periostin或基质dC也可能是GEM增敏的潜在靶点.
在PDAC中, CAFs主要来源于PSCs的活化, 少部分由静止的成纤维细胞、间充质干细胞或肿瘤细胞EMT衍生而来[85]. 以α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA)、成纤维细胞活化蛋白(fibroblast activation protein, FAP)和成纤维细胞特异性蛋白1(fibroblast specific protein 1, FSP1)表达为特征表型的活化CAFs对GEM天然耐药, 并可通过多种机制诱导获得性耐药[86]. 遗憾的是, 转基因小鼠模型研究显示, 靶向耗竭α-SMA表型的CAFs并未增加GEM对PDAC的疗效, 但却上调了细胞毒性T淋巴细胞相关抗原-4(cytotoxic T lymphocyte associated antigen-4, CTLA-4)的表达, 且靶向CTLA-4的抗体疗法可提高整体生存率[87]. 有趣的是, 最近的一项研究亦显示, 预先GEM处理可促进程序性细胞死亡蛋白-1(programmed death-1, PD-1)的表达, 并随后对联合PD-1免疫疗法敏感[88]. 因此, GEM可能通过重塑PDAC的TME增加了免疫靶点的表达, 联合免疫疗法可能是GEM耐药二线治疗的潜在策略. 尽管在临床前模型中, 靶向抑制FAP可提高化疗疗效[89], 但较早的一项II期临床试验却显示, FAP抑制剂与GEM联合对转移性PDAC的疗效改善仍十分有限[90]. 此外, Hedgehog(HH)信号通路及其配体之一Sonic Hedgehog(SHH)是CAFs活化的有效调节因子, 参与PDAC的增殖和损伤修复, 但其在诱导GEM耐药中的作用仍存有争议[24]. 尽管临床前研究显示, 小分子抑制剂阻断SHH通路可提高GEM敏感性[91,92], 但随后与GEM联合治疗晚期PDAC的II期临床试验却均告失败[93,94]. 另外, 基因组分析表明, 白细胞介素-6(interleukin-6, IL-6)等炎症介质在耐GEM的CAFs中过表达且耐药性与IL-6水平呈正相关[95], 这提示CAFs诱导的GEM抗性可能有促炎症通路的参与[96]. 有趣的是, 一项表征CAFs的3D共培养研究揭示了在PDAC中两种α-SMA、IL-6表达程度高低不同且作用相反的CAFs亚型存在, 该研究强调了间质CAFs的异质性[97], 这也可能是上述临床试验失败的原因之一[86]. 最近一项研究显示, 耐GEM的CAFs细胞外囊泡即外泌体(exosomes)的释放显著增加, 这些外泌体通过激活间充质转录因子Snail, 促进EMT并最终诱导GEM抗性[98]. 其中, 外泌体中的miRNA-106b可能在这一过程中起重要作用[99]. 用外泌体释放抑制剂或miRNA-106b抑制剂处理耐GEM的CAFs显著降低了共培养的PDAC细胞存活率[98,99], 这表明外泌体可能是克服GEM耐药的潜在靶点.
CSCs是PDAC中一类具有干细胞特征的特殊细胞群, 维持肿瘤的形成和生长. CSCs在GEM耐药中的作用逐渐引发关注, 但确切的细胞和分子机制尚未完全阐明[100]. 体外研究显示, CSCs可通过EMT表型转化逃避GEM细胞毒性作用[101]. HOX转录反义RNA(HOX transcript antisense RNA, HOTAIR)是一类由GEM诱导CSCs产生的长非编码RNA(long non-coding RNAs, lncRNA), 其可通过促进增殖和抑制凋亡, 诱导GEM抗性[102]. 最近一项研究亦显示, CSCs可上调非编码微小RNA(microRNA, miRNA)中的miR-210表达并增加外泌体miR-210的释放, 进一步通过PI3K/Akt/mTOR促生存通路活化, 促进PDAC细胞的GEM抵抗[103]. 因此, 靶向CSCs及相关非编码RNA(non-coding RNA, ncRNA)有望成为新的GEM增敏策略.
炎症细胞浸润和免疫抑制是PDAC的特征之一[104], 参与的免疫细胞包括肿瘤相关巨噬细胞(tumor-associated macrophages, TAMs)、肿瘤相关嗜中性粒细胞(tumor-associated neutrophils, TANs)、骨髓来源抑制细胞(myeloid-derived suppressor cells, MDSCs)、肿瘤浸润T淋巴细胞(tumor infiltrating lymphocytes, TILs)和血小板(platelets, PLTs)(图1). PDAC中的免疫细胞并非GEM耐药的孤立观察者, 而是这一过程的重要参与者.
消除凋亡细胞是TAMs最重要的功能之一, M2型极化的TAMs可通过激活转录因子STAT3直接增强CSCs的肿瘤起始能力[105], 并通过下调caspase-3通路活化抑制GEM诱导的细胞凋亡[106], 促进GEM抵抗. 体外试验显示, 靶向抑制集落刺激因子-1受体(colony stimulating factor-1 receptor,CSF-1R)或趋化因子(CC基序)受体2(C-C motif chemokine receptor 2, CCR2)可选择性清除TAMs, 进一步提高GEM的疗效[105,106]. 此外, 活化的STAT3还可通过上调胞苷脱氨酶(cytidine deaminase, CDA; 一种可将GEM由活性形式代谢为非活性的酶)的表达和基质重塑促进GEM耐药[106,107]. 动物模型研究显示, 联合STAT3靶向抑制剂可明显提高GEM的疗效[107]. 因此, 靶向TAMs/STAT3可能是有潜力的GEM增敏策略[108,109]. 值得关注的是, TAMs具有高度可塑性, 既可以极化成致瘤且对化疗耐药的M2型, 也可以极化成抗瘤且对化疗敏感的M1型[110]. 因此, TAMs定向转化将是除TAMs耗竭之外另一重要的GEM增敏路径[111,112].
TANs是炎症和免疫状态的关键调节剂, 在PDAC的发生发展中起重要作用[113]. 同TAMs类似, TANs亦可由TME中不同的趋化因子诱导极化成抗瘤(N1)或促瘤(N2)表型[114]. 尽管TANs的数量和比例是包括PDAC在内的实体肿瘤公认的预后生物标志物[115], 但TANs与GEM耐药的关系目前仍知之甚少. 尽管如此, TANs的可塑性研究已逐渐成为热点领域之一[116], 并可能成为GEM增敏的潜在靶点. MDSCs是源自骨髓祖细胞的异质免疫细胞群, 以粒细胞样MDSCs(granulocytic myeloid de-rived suppressor cells, G-MDSCs)亚型在肿瘤中分布最为广泛[117], 其可通过抑制T细胞免疫和促进血管生成加速肿瘤进展[118]. 因此, MDSCs又被称为抑制T细胞的嗜中性粒细胞. 同样, 循环中的MDSCs水平已证实与PDAC的不良预后密切相关[119]. 一项最近的体外研究显示, GEM通过诱导MAPK信号通路的活化和NF-κB启动子活性, 进一步增加与KRAS突变相关的主要细胞因子-粒细胞巨噬细胞集落刺激因子(granulocyte-macrophage colony-stimulating factor, GM-CSF)的释放, 进而增强PDAC中MDSCs的分化并促进其耐药. 用抗体靶向中和GM-CSF可以有效降低MDSCs的比例, 并有助于T细胞功能的恢复, 逆转GEM耐药[120]. 但值得注意的是, GM-CSF还可通过刺激树突状细胞来诱导或激活抗肿瘤的T细胞免疫, 并基于这一原理设计了GM-CSF基因转染的胰腺肿瘤疫苗(GVAX)[121], 这表明GM-CSF在PDAC的TME中可能发挥双向的作用. 这或许是一项针对GM-CSF+端粒酶疫苗GV1001联合/不联合GEM治疗晚期PDAC的Ⅲ期临床试验(ISRCTN4382138)失败的原因之一[122]. 遗憾的是, 由于缺乏特异性的表型标志, 目前还无法有效的区分TANs和MDSCs, 因此相关研究之间的可比性和可靠性尚待进一步确认[116,117].
TILs包含细胞毒性T淋巴细胞(cytotoxic T lymphocytes , CTLs)、辅助T细胞(helper T cell, Th)、调节T细胞(regulatory T cells, Tregs)等多种亚型, 是参与抗肿瘤免疫和调控免疫检查点的关键介质. 其中, PD-1和CTLA-4是TILs备受关注的抑制性免疫检查点受体, 可负性调控其抗肿瘤免疫[123]. 研究证实, CTLs的高浸润水平与PDAC良好预后呈正相关[124], 这也是目前免疫检查点靶向阻断治疗的理论基础. 遗憾的是, 目前针对PD-1/L1和CTLA-4免疫检查点的单一抑制剂疗法均未显示对PDAC有效[125]. 一项新的异种模型研究表明, GEM可通过诱导T细胞免疫增加对PD-1和CTLA-4抑制剂的敏感性, GEM联合免疫检查点抑制剂可提高PDAC的生存率[126], 但实际疗效仍需临床试验进一步验证[127]. 此外, HEAT重复序列的蛋白1(HEAT repeat-containing protein 1, HEATR1)可负性调控Akt信号通路诱导效应CTLs发挥抗肿瘤免疫效应, 但其在PDAC中普遍下调且与GEM耐药密切相关[128]. 一项最新的研究显示, HEATR1下调还能通过激活核因子E2相关因子2(nuclear factor erythroid-2-related factor 2, Nrf2)信号传导, 后者进一步促进其下游抗氧化和细胞保护性基因的转录, 最终导致GEM耐药[128,129]. 因此, HEATR1/Nrf2可能是GEM化疗增敏的潜在靶点.
血液高凝和静脉血栓栓塞(venous thromboembolism, VTE)风险是PDAC的特征之一, 这提示PDAC发生发展与PLTs密切相关[130]. PDAC细胞可以诱导PLTs活化和聚集, PDGF有助于肿瘤进展和GEM耐药[131-134]. 对具有缺氧特征TME的PDAC, 二磷酸腺苷(adenosine diphosphate, ADP)是重要的PLT激动剂. 最近, 靶向PLT的ADP-P2Y12受体逐渐引发了研究人员的兴趣[135]. P2Y12属于嘌呤能P2(purinergic receptor P2)G蛋白偶联受体(G protein-coupled receptors, GPCRs), ADP与P2Y12受体结合可激活PLT, 进一步上调EMT相关转录因子Slug、ZEB1的表达, 并下调GEM转运蛋白hENT1和GEM代谢酶CDA的表达, 诱导GEM耐药[133,134]. 临床前研究证实, ADP-P2Y12轴抑制剂(ticagrelor)联合GEM治疗PDAC有明显的协同作用, 但单一使用则未显示疗效[134]. 最近, 一项氯吡格雷(P2Y12抑制剂)联合GEM治疗PDAC的临床试验(NCT02404363)正在进行, 结果令人期待. 此外, 由于P2Y12-EGFR的信号串扰, 抑制P2Y12信号还可显著增强抗EGFR治疗的疗效[134], 但具体机制尚不明确, 这提示P2Y12抑制剂+GEM联合EGFR-TKIs可能是治疗PDAC的潜在策略.
随着研究的不断深入, 以结缔组织广泛增生为特征的PDAC细胞外TME在GEM耐药中的关键作用逐渐被揭示(表1). 但遗憾的是, 针对TME相关分子途径的靶向治疗并未取得令人满意的结果, 甚至基质的物理屏障作用也受到质疑[136]. 其中, 关键的原因包括: PDAC自身及所属TME的异质性; TME成分可能的双向作用; 缺乏有效的选择性抑制剂, 以及替代代偿途径的快速上调. 此外, 目前多数的研究证据均来自体外试验模型, PDAC细胞与TME间复杂生物学行为仍不能在临床前模型中完全重现, 这一原因同样不可忽视[137]. 因此, 要提高GEM化疗有效率, 改善PDAC的总体预后, 上述原因都将是未来要迫切解决的关键问题. 值得关注的是, GEM对TME重塑作用的深入研究也有望指导多靶点的联合治疗, 最终可能改变未来PDAC的治疗格局.
学科分类: 胃肠病学和肝病学
手稿来源地: 北京市
同行评议报告学术质量分类
A级 (优秀): A
B级 (非常好): B
C级 (良好): C
D级 (一般): 0
E级 (差): 0
科学编辑:张砚梁 制作编辑:张砚梁
| 1. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [PubMed] [DOI] |
| 2. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [PubMed] [DOI] |
| 3. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI-PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [PubMed] [DOI] |
| 4. | Saif MW, Lee Y, Kim R. Harnessing gemcitabine metabolism: a step towards personalized medicine for pancreatic cancer. Ther Adv Med Oncol. 2012;4:341-346. [PubMed] [DOI] |
| 5. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [PubMed] [DOI] |
| 6. | Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179-187. [PubMed] [DOI] |
| 7. | Neesse A, Algül H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64:1476-1484. [PubMed] [DOI] |
| 8. | Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68:159-171. [PubMed] [DOI] |
| 9. | Liang C, Shi S, Meng Q, Liang D, Ji S, Zhang B, Qin Y, Xu J, Ni Q, Yu X. Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going. Exp Mol Med. 2017;49:e406. [PubMed] [DOI] |
| 10. | Lohse I, Lourenco C, Ibrahimov E, Pintilie M, Tsao MS, Hedley DW. Assessment of hypoxia in the stroma of patient-derived pancreatic tumor xenografts. Cancers (Basel). 2014;6:459-471. [PubMed] [DOI] |
| 11. | Wang R, Cheng L, Xia J, Wang Z, Wu Q, Wang Z. Gemcitabine resistance is associated with epithelial-mesenchymal transition and induction of HIF-1α in pancreatic cancer cells. Curr Cancer Drug Targets. 2014;14:407-417. [PubMed] [DOI] |
| 12. | Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, Illies AL, Gebregiworgis T, Dai B, Augustine JJ, Murthy D, Attri KS, Mashadova O, Grandgenett PM, Powers R, Ly QP, Lazenby AJ, Grem JL, Yu F, Matés JM, Asara JM, Kim JW, Hankins JH, Weekes C, Hollingsworth MA, Serkova NJ, Sasson AR, Fleming JB, Oliveto JM, Lyssiotis CA, Cantley LC, Berim L, Singh PK. MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer Cell. 2017;32:71-87.e7. [PubMed] [DOI] |
| 13. | Hou XF, Li S, Wu C, Li K, Xu SN, Wang JF. Effects of obatoclax combined with gemcitabine on the biological activity of pancreatic cancer cells under hypoxic conditions. Mol Med Rep. 2018;18:495-501. [PubMed] [DOI] |
| 14. | Zhang Z, Han H, Rong Y, Zhu K, Zhu Z, Tang Z, Xiong C, Tao J. Hypoxia potentiates gemcitabine-induced stemness in pancreatic cancer cells through AKT/Notch1 signaling. J Exp Clin Cancer Res. 2018;37:291. [PubMed] [DOI] |
| 15. | Cutsem EV, Lenz HJ, Furuse J, Tabernero J, Heinemann V, Ioka T, Bazin I, Ueno M, Csõszi T, Wasan H, Melichar B, Karasek P, Macarulla TM, Guillen C, Kalinka-Warzocha E, Horvath Z, Prenen H, Schlichting M, Ibrahim A, Bendell JC. MAESTRO: A randomized, double-blind phase III study of evofosfamide (Evo) in combination with gemcitabine (Gem) in previously untreated patients (pts) with metastatic or locally advanced unresectable pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol. 2016;34:4007-4007. [DOI] |
| 16. | Corbet C, Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer. 2017;17:577-593. [PubMed] [DOI] |
| 17. | McDonald PC, Chafe SC, Brown WS, Saberi S, Swayampakula M, Venkateswaran G, Nemirovsky O, Gillespie JA, Karasinska JM, Kalloger SE, Supuran CT, Schaeffer DF, Bashashati A, Shah SP, Topham JT, Yapp DT, Li J, Renouf DJ, Stanger BZ, Dedhar S. Regulation of pH by Carbonic Anhydrase 9 Mediates Survival of Pancreatic Cancer Cells With Activated KRAS in Response to Hypoxia. Gastroenterology. 2019;157:823-837. [PubMed] [DOI] |
| 18. | Riemann A, Rauschner M, Gießelmann M, Reime S, Haupt V, Thews O. Extracellular Acidosis Modulates the Expression of Epithelial-Mesenchymal Transition (EMT) Markers and Adhesion of Epithelial and Tumor Cells. Neoplasia. 2019;21:450-458. [PubMed] [DOI] |
| 19. | Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, Zhao F, You L, Wang W, Zhao Y. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19:50. [PubMed] [DOI] |
| 20. | Makareeva E, Han S, Vera JC, Sackett DL, Holmbeck K, Phillips CL, Visse R, Nagase H, Leikin S. Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer Res. 2010;70:4366-4374. [PubMed] [DOI] |
| 21. | Dangi-Garimella S, Krantz SB, Barron MR, Shields MA, Heiferman MJ, Grippo PJ, Bentrem DJ, Munshi HG. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 2011;71:1019-1028. [PubMed] [DOI] |
| 22. | Summer H, Li O, Bao Q, Zhan L, Peter S, Sathiyanathan P, Henderson D, Klonisch T, Goodman SD, Dröge P. HMGA2 exhibits dRP/AP site cleavage activity and protects cancer cells from DNA-damage-induced cytotoxicity during chemotherapy. Nucleic Acids Res. 2009;37:4371-4384. [PubMed] [DOI] |
| 23. | Dangi-Garimella S, Sahai V, Ebine K, Kumar K, Munshi HG. Three-dimensional collagen I promotes gemcitabine resistance in vitro in pancreatic cancer cells through HMGA2-dependent histone acetyltransferase expression. PLoS One. 2013;8:e64566. [PubMed] [DOI] |
| 24. | Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527-540. [PubMed] [DOI] |
| 25. | D'Costa Z, Jones K, Azad A, van Stiphout R, Lim SY, Gomes AL, Kinchesh P, Smart SC, Gillies McKenna W, Buffa FM, Sansom OJ, Muschel RJ, O'Neill E, Fokas E. Gemcitabine-Induced TIMP1 Attenuates Therapy Response and Promotes Tumor Growth and Liver Metastasis in Pancreatic Cancer. Cancer Res. 2017;77:5952-5962. [PubMed] [DOI] |
| 26. | Nakazawa H, Yoshihara S, Kudo D, Morohashi H, Kakizaki I, Kon A, Takagaki K, Sasaki M. 4-methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;57:165-170. [PubMed] [DOI] |
| 27. | Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer. 2013;108:1-8. [PubMed] [DOI] |
| 28. | Zhao S, Chen C, Chang K, Karnad A, Jagirdar J, Kumar AP, Freeman JW. CD44 Expression Level and Isoform Contributes to Pancreatic Cancer Cell Plasticity, Invasiveness, and Response to Therapy. Clin Cancer Res. 2016;22:5592-5604. [PubMed] [DOI] |
| 29. | Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323-2331. [PubMed] [DOI] |
| 30. | Skandalis SS, Karalis TT, Chatzopoulos A, Karamanos NK. Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell Signal. 2019;63:109377. [PubMed] [DOI] |
| 31. | Serri C, Quagliariello V, Iaffaioli RV, Fusco S, Botti G, Mayol L, Biondi M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J Cell Physiol. 2019;234:4959-4969. [PubMed] [DOI] |
| 32. | Wong KM, Horton KJ, Coveler AL, Hingorani SR, Harris WP. Targeting the Tumor Stroma: the Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20). Curr Oncol Rep. 2017;19:47. [PubMed] [DOI] |
| 33. | Thompson B, Lee J, Clift R, Taverna D, Garrovillo S, Blouw B, Kang D, Thompson C, Maneval D. PO-262 Remodelling of the tumour microenvironment by pegvorhyalurondiase alfa (PEGPH20): a novel, first-in-class biologic that enzymatically degrades tumour hyaluronan (HA) to improve anti-tumour efficacy. ESMO Open. 2018;3:A329-A330. [DOI] |
| 34. | Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359-366. [PubMed] [DOI] |
| 35. | Van Cutsem E, Tempero MA, Sigal D, Oh DY, Fazio N, Macarulla T, Hitre E, Hammel P, Hendifar AE, Bates SE, Li CP, Hingorani SR, de la Fouchardiere C, Kasi A, Heinemann V, Maraveyas A, Bahary N, Layos L, Sahai V, Zheng L, Lacy J, Park JO, Portales F, Oberstein P, Wu W, Chondros D, Bullock AJ; HALO 109-301 Investigators. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J Clin Oncol. 2020;38:3185-3194. [PubMed] [DOI] |
| 36. | Cao J, Pickup S, Clendenin C, Blouw B, Choi H, Kang D, Rosen M, O'Dwyer PJ, Zhou R. Dynamic Contrast-enhanced MRI Detects Responses to Stroma-directed Therapy in Mouse Models of Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2019;25:2314-2322. [PubMed] [DOI] |
| 37. | Dubey RD, Klippstein R, Wang JT, Hodgins N, Mei KC, Sosabowski J, Hider RC, Abbate V, Gupta PN, Al-Jamal KT. Novel Hyaluronic Acid Conjugates for Dual Nuclear Imaging and Therapy in CD44-Expressing Tumors in Mice In Vivo. Nanotheranostics. 2017;1:59-79. [PubMed] [DOI] |
| 38. | Takahashi S, Hasebe T, Oda T, Sasaki S, Kinoshita T, Konishi M, Ochiai T, Ochiai A. Cytoplasmic expression of laminin gamma2 chain correlates with postoperative hepatic metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma. Cancer. 2002;94:1894-1901. [PubMed] [DOI] |
| 39. | Huanwen W, Zhiyong L, Xiaohua S, Xinyu R, Kai W, Tonghua L. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Mol Cancer. 2009;8:125. [PubMed] [DOI] |
| 40. | Lee J, Yakubov B, Ivan C, Jones DR, Caperell-Grant A, Fishel M, Cardenas H, Matei D. Tissue Transglutaminase Activates Cancer-Associated Fibroblasts and Contributes to Gemcitabine Resistance in Pancreatic Cancer. Neoplasia. 2016;18:689-698. [PubMed] [DOI] |
| 41. | Verma A, Mehta K. Tissue transglutaminase-mediated chemoresistance in cancer cells. Drug Resist Updat. 2007;10:144-151. [PubMed] [DOI] |
| 42. | Topalovski M, Brekken RA. Matrix control of pancreatic cancer: New insights into fibronectin signaling. Cancer Lett. 2016;381:252-258. [PubMed] [DOI] |
| 43. | Amrutkar M, Aasrum M, Verbeke CS, Gladhaug IP. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer. 2019;19:596. [PubMed] [DOI] |
| 44. | Wenger C, Ellenrieder V, Alber B, Lacher U, Menke A, Hameister H, Wilda M, Iwamura T, Beger HG, Adler G, Gress TM. Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene. 1999;18:1073-1080. [PubMed] [DOI] |
| 45. | Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci USA. 2013;110:12325-12330. [PubMed] [DOI] |
| 46. | Moon JH, Shin JS, Hong SW, Jung SA, Hwang IY, Kim JH, Choi EK, Ha SH, Kim JS, Kim KM, Hong DW, Kim D, Kim YS, Kim JE, Kim KP, Hong YS, Choi EK, Lee JS, Hattersley M, Jin DH, Kim TW. A novel small-molecule IAP antagonist, AZD5582, draws Mcl-1 down-regulation for induction of apoptosis through targeting of cIAP1 and XIAP in human pancreatic cancer. Oncotarget. 2015;6:26895-26908. [PubMed] [DOI] |
| 47. | Picozzi V, Alseidi A, Winter J, Pishvaian M, Mody K, Glaspy J, Larson T, Matrana M, Carney M, Porter S, Kouchakji E, Rocha F, Carrier E. Gemcitabine/nab-paclitaxel with pamrevlumab: a novel drug combination and trial design for the treatment of locally advanced pancreatic cancer. ESMO Open. 2020;5. [PubMed] [DOI] |
| 48. | Hesler RA, Huang JJ, Starr MD, Treboschi VM, Bernanke AG, Nixon AB, McCall SJ, White RR, Blobe GC. TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3. Carcinogenesis. 2016;37:1041-1051. [PubMed] [DOI] |
| 49. | Xian G, Zhao J, Qin C, Zhang Z, Lin Y, Su Z. Simvastatin attenuates macrophage-mediated gemcitabine resistance of pancreatic ductal adenocarcinoma by regulating the TGF-β1/Gfi-1 axis. Cancer Lett. 2017;385:65-74. [PubMed] [DOI] |
| 50. | Pei Y, Chen L, Huang Y, Wang J, Feng J, Xu M, Chen Y, Song Q, Jiang G, Gu X, Zhang Q, Gao X, Chen J. Sequential Targeting TGF-β Signaling and KRAS Mutation Increases Therapeutic Efficacy in Pancreatic Cancer. Small. 2019;15:e1900631. [PubMed] [DOI] |
| 51. | Huang PH, Lu PJ, Ding LY, Chu PC, Hsu WY, Chen CS, Tsao CC, Chen BH, Lee CT, Shan YS, Chen CS. TGFβ promotes mesenchymal phenotype of pancreatic cancer cells, in part, through epigenetic activation of VAV1. Oncogene. 2017;36:2202-2214. [PubMed] [DOI] |
| 52. | Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5:521-530. [PubMed] [DOI] |
| 53. | Dosch AR, Dai X, Reyzer ML, Mehra S, Srinivasan S, Willobee BA, Kwon D, Kashikar N, Caprioli R, Merchant NB, Nagathihalli NS. Combined Src/EGFR Inhibition Targets STAT3 Signaling and Induces Stromal Remodeling to Improve Survival in Pancreatic Cancer. Mol Cancer Res. 2020;18:623-631. [PubMed] [DOI] |
| 54. | Xu BQ, Fu ZG, Meng Y, Wu XQ, Wu B, Xu L, Jiang JL, Li L, Chen ZN. Gemcitabine enhances cell invasion via activating HAb18G/CD147-EGFR-pSTAT3 signaling. Oncotarget. 2016;7:62177-62193. [PubMed] [DOI] |
| 55. | Venkatasubbarao K, Peterson L, Zhao S, Hill P, Cao L, Zhou Q, Nawrocki ST, Freeman JW. Inhibiting signal transducer and activator of transcription-3 increases response to gemcitabine and delays progression of pancreatic cancer. Mol Cancer. 2013;12:104. [PubMed] [DOI] |
| 56. | Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330-3337. [PubMed] [DOI] |
| 57. | Evans TRJ, Van Cutsem E, Moore MJ, Bazin IS, Rosemurgy A, Bodoky G, Deplanque G, Harrison M, Melichar B, Pezet D, Elekes A, Rock E, Lin C, Strauss L, O'Dwyer PJ. Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer. Ann Oncol. 2017;28:354-361. [PubMed] [DOI] |
| 58. | Middleton G, Palmer DH, Greenhalf W, Ghaneh P, Jackson R, Cox T, Evans A, Shaw VE, Wadsley J, Valle JW, Propper D, Wasan H, Falk S, Cunningham D, Coxon F, Ross P, Madhusudan S, Wadd N, Corrie P, Hickish T, Costello E, Campbell F, Rawcliffe C, Neoptolemos JP. Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial. Lancet Oncol. 2017;18:486-499. [PubMed] [DOI] |
| 59. | Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262. [PubMed] [DOI] |
| 60. | Lakkakula BVKS, Farran B, Lakkakula S, Peela S, Yarla NS, Bramhachari PV, Kamal MA, Saddala MS, Nagaraju GP. Small molecule tyrosine kinase inhibitors and pancreatic cancer-Trials and troubles. Semin Cancer Biol. 2019;56:149-167. [PubMed] [DOI] |
| 61. | Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M, Milella M, Tafuri A, Bonati A, Bäsecke J, Cocco L, Evangelisti C, Martelli AM, Montalto G, Cervello M, McCubrey JA. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY). 2011;3:192-222. [PubMed] [DOI] |
| 62. | Camblin AJ, Pace EA, Adams S, Curley MD, Rimkunas V, Nie L, Tan G, Bloom T, Iadevaia S, Baum J, Minx C, Czibere A, Louis CU, Drummond DC, Nielsen UB, Schoeberl B, Pipas JM, Straubinger RM, Askoxylakis V, Lugovskoy AA. Dual Inhibition of IGF-1R and ErbB3 Enhances the Activity of Gemcitabine and Nab-Paclitaxel in Preclinical Models of Pancreatic Cancer. Clin Cancer Res. 2018;24:2873-2885. [PubMed] [DOI] |
| 63. | Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H, Szczylik C, Moore MJ, Peeters M, Bodoky G, Ikeda M, Melichar B, Nemecek R, Ohkawa S, Świeboda-Sadlej A, Tjulandin SA, Van Cutsem E, Loberg R, Haddad V, Gansert JL, Bach BA, Carrato A. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26:921-927. [PubMed] [DOI] |
| 64. | Abdel-Wahab R, Varadhachary GR, Bhosale PR, Wang X, Fogelman DR, Shroff RT, Overman MJ, Wolff RA, Javle M. Randomized, phase I/II study of gemcitabine plus IGF-1R antagonist (MK-0646) versus gemcitabine plus erlotinib with and without MK-0646 for advanced pancreatic adenocarcinoma. J Hematol Oncol. 2018;11:71. [PubMed] [DOI] |
| 65. | Wu Q, Hou X, Xia J, Qian X, Miele L, Sarkar FH, Wang Z. Emerging roles of PDGF-D in EMT progression during tumorigenesis. Cancer Treat Rev. 2013;39:640-646. [PubMed] [DOI] |
| 66. | Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L, Mizumoto K, Tanaka M. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013;73:2345-2356. [PubMed] [DOI] |
| 67. | Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X, Esterni B, Genre D, Moureau-Zabotto L, Giovannini M, Seitz JF, Delpero JR, Turrini O, Viens P, Raoul JL. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799-2805. [PubMed] [DOI] |
| 68. | Moss RA, Moore D, Mulcahy MF, Nahum K, Saraiya B, Eddy S, Kleber M, Poplin EA. A Multi-institutional Phase 2 Study of Imatinib Mesylate and Gemcitabine for First-Line Treatment of Advanced Pancreatic Cancer. Gastrointest Cancer Res. 2012;5:77-83. [PubMed] |
| 69. | Kindler HL, Wroblewski K, Wallace JA, Hall MJ, Locker G, Nattam S, Agamah E, Stadler WM, Vokes EE. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs. 2012;30:382-386. [PubMed] [DOI] |
| 70. | Sleightholm RL, Neilsen BK, Li J, Steele MM, Singh RK, Hollingsworth MA, Oupicky D. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol Ther. 2017;179:158-170. [PubMed] [DOI] |
| 71. | Maréchal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, Closset J, Devière J, Salmon I, Van Laethem JL. High expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinoma. Br J Cancer. 2009;100:1444-1451. [PubMed] [DOI] |
| 72. | Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 2010;103:1671-1679. [PubMed] [DOI] |
| 73. | Morimoto M, Matsuo Y, Koide S, Tsuboi K, Shamoto T, Sato T, Saito K, Takahashi H, Takeyama H. Enhancement of the CXCL12/CXCR4 axis due to acquisition of gemcitabine resistance in pancreatic cancer: effect of CXCR4 antagonists. BMC Cancer. 2016;16:305. [PubMed] [DOI] |
| 74. | Costa MJ, Kudaravalli J, Ma JT, Ho WH, Delaria K, Holz C, Stauffer A, Chunyk AG, Zong Q, Blasi E, Buetow B, Tran TT, Lindquist K, Dorywalska M, Rajpal A, Shelton DL, Strop P, Liu SH. Optimal design, anti-tumour efficacy and tolerability of anti-CXCR4 antibody drug conjugates. Sci Rep. 2019;9:2443. [PubMed] [DOI] |
| 75. | Lee S, Heinrich EL, Li L, Lu J, Choi AH, Levy RA, Wagner JE, Yip ML, Vaidehi N, Kim J. CCR9-mediated signaling through β-catenin and identification of a novel CCR9 antagonist. Mol Oncol. 2015;9:1599-1611. [PubMed] [DOI] |
| 76. | Timaner M, Letko-Khait N, Kotsofruk R, Benguigui M, Beyar-Katz O, Rachman-Tzemah C, Raviv Z, Bronshtein T, Machluf M, Shaked Y. Therapy-Educated Mesenchymal Stem Cells Enrich for Tumor-Initiating Cells. Cancer Res. 2018;78:1253-1265. [PubMed] [DOI] |
| 77. | Nielsen MF, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol. 2016;22:2678-2700. [PubMed] [DOI] |
| 78. | Liu SL, Cao SG, Li Y, Sun B, Chen D, Wang DS, Zhou YB. Pancreatic stellate cells facilitate pancreatic cancer cell viability and invasion. Oncol Lett. 2019;17:2057-2062. [PubMed] [DOI] |
| 79. | Cao F, Li J, Sun H, Liu S, Cui Y, Li F. HES 1 is essential for chemoresistance induced by stellate cells and is associated with poor prognosis in pancreatic cancer. Oncol Rep. 2015;33:1883-1889. [PubMed] [DOI] |
| 80. | Firuzi O, Che PP, El Hassouni B, Buijs M, Coppola S, Löhr M, Funel N, Heuchel R, Carnevale I, Schmidt T, Mantini G, Avan A, Saso L, Peters GJ, Giovannetti E. Role of c-MET Inhibitors in Overcoming Drug Resistance in Spheroid Models of Primary Human Pancreatic Cancer and Stellate Cells. Cancers (Basel). 2019;11. [PubMed] [DOI] |
| 81. | Xu Z, Pang TCY, Liu AC, Pothula SP, Mekapogu AR, Perera CJ, Murakami T, Goldstein D, Pirola RC, Wilson JS, Apte MV. Targeting the HGF/c-MET pathway in advanced pancreatic cancer: a key element of treatment that limits primary tumour growth and eliminates metastasis. Br J Cancer. 2020;122:1486-1495. [PubMed] [DOI] |
| 82. | Pant S, Saleh M, Bendell J, Infante JR, Jones S, Kurkjian CD, Moore KM, Kazakin J, Abbadessa G, Wang Y, Chen Y, Schwartz B, Camacho LH. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann Oncol. 2014;25:1416-1421. [PubMed] [DOI] |
| 83. | Liu Y, Li F, Gao F, Xing L, Qin P, Liang X, Zhang J, Qiao X, Lin L, Zhao Q, Du L. Periostin promotes the chemotherapy resistance to gemcitabine in pancreatic cancer. Tumour Biol. 2016;37:15283-15291. [PubMed] [DOI] |
| 84. | Dalin S, Sullivan MR, Lau AN, Grauman-Boss B, Mueller HS, Kreidl E, Fenoglio S, Luengo A, Lees JA, Vander Heiden MG, Lauffenburger DA, Hemann MT. Deoxycytidine Release from Pancreatic Stellate Cells Promotes Gemcitabine Resistance. Cancer Res. 2019;79:5723-5733. [PubMed] [DOI] |
| 85. | Nair N, Calle AS, Zahra MH, Prieto-Vila M, Oo AKK, Hurley L, Vaidyanath A, Seno A, Masuda J, Iwasaki Y, Tanaka H, Kasai T, Seno M. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci Rep. 2017;7:6838. [PubMed] [DOI] |
| 86. | Huang H, Brekken RA. Recent advances in understanding cancer-associated fibroblasts in pancreatic cancer. Am J Physiol Cell Physiol. 2020;319:C233-C243. [PubMed] [DOI] |
| 87. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell. 2015;28:831-833. [PubMed] [DOI] |
| 88. | Principe DR, Narbutis M, Kumar S, Park A, Viswakarma N, Dorman MJ, Kamath SD, Grippo PJ, Fishel ML, Hwang RF, Thummuri D, Underwood PW, Munshi HG, Trevino JG, Rana A. Long-Term Gemcitabine Treatment Reshapes the Pancreatic Tumor Microenvironment and Sensitizes Murine Carcinoma to Combination Immunotherapy. Cancer Res. 2020;80:3101-3115. [PubMed] [DOI] |
| 89. | Li M, Li M, Yin T, Shi H, Wen Y, Zhang B, Chen M, Xu G, Ren K, Wei Y. Targeting of cancerassociated fibroblasts enhances the efficacy of cancer chemotherapy by regulating the tumor microenvironment. Mol Med Rep. 2016;13:2476-2484. [PubMed] [DOI] |
| 90. | Nugent FW, Cunningham C, Barve MA, Fisher W, Patel H, Meiri E, Oza YV, Yang Z, Jurkowski EC, Uprichard MJ. Phase 2 study of talabostat/gemcitabine in Stage IV pancreatic cancer. J Clin Oncol. 2007;25:4616-4616. [DOI] |
| 91. | Chun SG, Zhou W, Yee NS. Combined targeting of histone deacetylases and hedgehog signaling enhances cytoxicity in pancreatic cancer. Cancer Biol Ther. 2009;8:1328-1339. [PubMed] [DOI] |
| 92. | Khan MA, Srivastava SK, Zubair H, Patel GK, Arora S, Khushman M, Carter JE, Gorman GS, Singh S, Singh AP. Co-targeting of CXCR4 and hedgehog pathways disrupts tumor-stromal crosstalk and improves chemotherapeutic efficacy in pancreatic cancer. J Biol Chem. 2020;295:8413-8424. [PubMed] [DOI] |
| 93. | Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:4284-4292. [PubMed] [DOI] |
| 94. | De Jesus-Acosta A, Sugar EA, O'Dwyer PJ, Ramanathan RK, Von Hoff DD, Rasheed Z, Zheng L, Begum A, Anders R, Maitra A, McAllister F, Rajeshkumar NV, Yabuuchi S, de Wilde RF, Batukbhai B, Sahin I, Laheru DA. Phase 2 study of vismodegib, a hedgehog inhibitor, combined with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic adenocarcinoma. Br J Cancer. 2020;122:498-505. [PubMed] [DOI] |
| 95. | Neumann CCM, von Hörschelmann E, Reutzel-Selke A, Seidel E, Sauer IM, Pratschke J, Bahra M, Schmuck RB. Tumor-stromal cross-talk modulating the therapeutic response in pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2018;17:461-472. [PubMed] [DOI] |
| 96. | Toste PA, Nguyen AH, Kadera BE, Duong M, Wu N, Gawlas I, Tran LM, Bikhchandani M, Li L, Patel SG, Dawson DW, Donahue TR. Chemotherapy-Induced Inflammatory Gene Signature and Protumorigenic Phenotype in Pancreatic CAFs via Stress-Associated MAPK. Mol Cancer Res. 2016;14:437-447. [PubMed] [DOI] |
| 97. | Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579-596. [PubMed] [DOI] |
| 98. | Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770-1778. [PubMed] [DOI] |
| 99. | Fang Y, Zhou W, Rong Y, Kuang T, Xu X, Wu W, Wang D, Lou W. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp Cell Res. 2019;383:111543. [PubMed] [DOI] |
| 100. | Gzil A, Zarębska I, Bursiewicz W, Antosik P, Grzanka D, Szylberg Ł. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol Biol Rep. 2019;46:6629-6645. [PubMed] [DOI] |
| 101. | Yin T, Wei H, Gou S, Shi P, Yang Z, Zhao G, Wang C. Cancer stem-like cells enriched in Panc-1 spheres possess increased migration ability and resistance to gemcitabine. Int J Mol Sci. 2011;12:1595-1604. [PubMed] [DOI] |
| 102. | Wang L, Dong P, Wang W, Huang M, Tian B. Gemcitabine treatment causes resistance and malignancy of pancreatic cancer stem-like cells via induction of lncRNA HOTAIR. Exp Ther Med. 2017;14:4773-4780. [PubMed] [DOI] |
| 103. | Yang Z, Zhao N, Cui J, Wu H, Xiong J, Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell Oncol (Dordr). 2020;43:123-136. [PubMed] [DOI] |
| 104. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [PubMed] [DOI] |
| 105. | Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128-1141. [PubMed] [DOI] |
| 106. | Weizman N, Krelin Y, Shabtay-Orbach A, Amit M, Binenbaum Y, Wong RJ, Gil Z. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33:3812-3819. [PubMed] [DOI] |
| 107. | Nagathihalli NS, Castellanos JA, Shi C, Beesetty Y, Reyzer ML, Caprioli R, Chen X, Walsh AJ, Skala MC, Moses HL, Merchant NB. Signal Transducer and Activator of Transcription 3, Mediated Remodeling of the Tumor Microenvironment Results in Enhanced Tumor Drug Delivery in a Mouse Model of Pancreatic Cancer. Gastroenterology. 2015;149:1932-1943.e9. [PubMed] [DOI] |
| 108. | Beltraminelli T, De Palma M. Biology and therapeutic targeting of tumour-associated macrophages. J Pathol. 2020;250:573-592. [PubMed] [DOI] |
| 109. | Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in Cancer Immunotherapy. Mol Cancer. 2020;19:145. [PubMed] [DOI] |
| 110. | Ireland L, Santos A, Ahmed MS, Rainer C, Nielsen SR, Quaranta V, Weyer-Czernilofsky U, Engle DD, Perez-Mancera PA, Coupland SE, Taktak A, Bogenrieder T, Tuveson DA, Campbell F, Schmid MC, Mielgo A. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factors. Cancer Res. 2016;76:6851-6863. [PubMed] [DOI] |
| 111. | Bulle A, Dekervel J, Deschuttere L, Nittner D, Libbrecht L, Janky R, Plaisance S, Topal B, Coosemans A, Lambrechts D, Van Cutsem E, Verslype C, van Pelt J. Gemcitabine Recruits M2-Type Tumor-Associated Macrophages into the Stroma of Pancreatic Cancer. Transl Oncol. 2020;13:100743. [PubMed] [DOI] |
| 112. | Yao L, Wang M, Niu Z, Liu Q, Gao X, Zhou L, Liao Q, Zhao Y. Interleukin-27 inhibits malignant behaviors of pancreatic cancer cells by targeting M2 polarized tumor associated macrophages. Cytokine. 2017;89:194-200. [PubMed] [DOI] |
| 113. | Lianyuan T, Gang L, Ming T, Dianrong X, Chunhui Y, Zhaolai M, Bin J. Tumor associated neutrophils promote the metastasis of pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2020;21:937-945. [PubMed] [DOI] |
| 114. | Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183-194. [PubMed] [DOI] |
| 115. | Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e98259. [PubMed] [DOI] |
| 116. | Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28:187-196. [PubMed] [DOI] |
| 117. | Bergenfelz C, Leandersson K. The Generation and Identity of Human Myeloid-Derived Suppressor Cells. Front Oncol. 2020;10:109. [PubMed] [DOI] |
| 118. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [PubMed] [DOI] |
| 119. | Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419-1430. [PubMed] [DOI] |
| 120. | Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, Nakanishi S, Usui Y, Higuchi K, Takahashi M, Inoko K, Sato S, Takano H, Shichinohe T, Seino K, Hirano S. Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer. Cancer Res. 2015;75:2629-2640. [PubMed] [DOI] |
| 121. | Nemunaitis J. Vaccines in cancer: GVAX, a GM-CSF gene vaccine. Expert Rev Vaccines. 2005;4:259-274. [PubMed] [DOI] |
| 122. | Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, Cunningham D, Falk S, Wadd N, Harrison M, Corrie P, Iveson T, Robinson A, McAdam K, Eatock M, Evans J, Archer C, Hickish T, Garcia-Alonso A, Nicolson M, Steward W, Anthoney A, Greenhalf W, Shaw V, Costello E, Naisbitt D, Rawcliffe C, Nanson G, Neoptolemos J. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15:829-840. [PubMed] [DOI] |
| 123. | Zhou Q, Tao X, Xia S, Guo F, Pan C, Xiang H, Shang D. T Lymphocytes: A Promising Immunotherapeutic Target for Pancreatitis and Pancreatic Cancer? Front Oncol. 2020;10:382. [PubMed] [DOI] |
| 124. | Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914-923. [PubMed] [DOI] |
| 125. | Gong J, Hendifar A, Tuli R, Chuang J, Cho M, Chung V, Li D, Salgia R. Combination systemic therapies with immune checkpoint inhibitors in pancreatic cancer: overcoming resistance to single-agent checkpoint blockade. Clin Transl Med. 2018;7:32. [PubMed] [DOI] |
| 126. | Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res. 2015;3:399-411. [PubMed] [DOI] |
| 127. | Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A, Mulcahy M. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncologist. 2020;25:e808-e815. [PubMed] [DOI] |
| 128. | Liu T, Fang Y, Zhang H, Deng M, Gao B, Niu N, Yu J, Lee S, Kim J, Qin B, Xie F, Evans D, Wang L, Lou W, Lou Z. HEATR1 Negatively Regulates Akt to Help Sensitize Pancreatic Cancer Cells to Chemotherapy. Cancer Res. 2016;76:572-581. [PubMed] [DOI] |
| 129. | Zhou Y, Wang K, Zhou Y, Li T, Yang M, Wang R, Chen Y, Cao M, Hu R. HEATR1 deficiency promotes pancreatic cancer proliferation and gemcitabine resistance by up-regulating Nrf2 signaling. Redox Biol. 2020;29:101390. [PubMed] [DOI] |
| 130. | Delluc A, Rousseau A, Delluc C, Le Moigne E, Le Gal G, Mottier D, Van Dreden P, Lacut K. Venous thromboembolism in patients with pancreatic cancer: implications of circulating tissue factor. Blood Coagul Fibrinolysis. 2011;22:295-300. [PubMed] [DOI] |
| 131. | Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775-7783. [PubMed] [DOI] |
| 132. | Battinelli EM, Markens BA, Italiano JE. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359-1369. [PubMed] [DOI] |
| 133. | Elaskalani O, Falasca M, Moran N, Berndt MC, Metharom P. The Role of Platelet-Derived ADP and ATP in Promoting Pancreatic Cancer Cell Survival and Gemcitabine Resistance. Cancers (Basel). 2017;9. [PubMed] [DOI] |
| 134. | Elaskalani O, Domenichini A, Abdol Razak NB, E Dye D, Falasca M, Metharom P. Antiplatelet Drug Ticagrelor Enhances Chemotherapeutic Efficacy by Targeting the Novel P2Y12-AKT Pathway in Pancreatic Cancer Cells. Cancers (Basel). 2020;12. [PubMed] [DOI] |
| 135. | Ballerini P, Dovizio M, Bruno A, Tacconelli S, Patrignani P. P2Y12 Receptors in Tumorigenesis and Metastasis. Front Pharmacol. 2018;9:66. [PubMed] [DOI] |
| 136. | Ramu I, Buchholz SM, Patzak MS, Goetze RG, Singh SK, Richards FM, Jodrell DI, Sipos B, Ströbel P, Ellenrieder V, Hessmann E, Neesse A. SPARC dependent collagen deposition and gemcitabine delivery in a genetically engineered mouse model of pancreas cancer. EBioMedicine. 2019;48:161-168. [PubMed] [DOI] |
| 137. | Frappart PO, Hofmann TG. Pancreatic Ductal Adenocarcinoma (PDAC) Organoids: The Shining Light at the End of the Tunnel for Drug Response Prediction and Personalized Medicine. Cancers (Basel). 2020;12. [PubMed] [DOI] |