修回日期: 2002-10-20
接受日期: 2002-11-25
在线出版日期: 2003-05-15
探讨组织特异性胞嘧啶脱氨基酶(cytosine deaminase, CD)/5-氟胞嘧啶(5-fluorocytosine, 5-FC)系统对不同分泌癌胚抗原(carcinoembryonic antigen, CEA)的大肠癌细胞LoVo和SW480的靶向杀伤作用.
脂质体法将CEA基因顺式转录调控序列(TRS)驱动CD基因的组织特异性逆转录病毒载体G1CEACDNa及非组织特异性逆转录病毒载体pCD2分别转导入大肠癌细胞LoVo和SW480, 以G418筛选阳性克隆扩增后给予前药5-FC进行敏感试验.
LoVo-CEACD及LoVo-CD比LoVo对5-FC的敏感性明显提高(P<0.01, t = 5.688, n = 9; P<0.01, t = 3.136, n = 9), SW480-CEACD及SW480-CD比SW480对5-FC的敏感性明显提高(P<0.01, t = 3.437, n = 9; P<0.01, t = 3.516, n = 9), LoVo-CEACD比LoVo-CD对5-FC的敏感性明显增强(P<0.05, t = 2.183, n = 9), 而SW480-CEACD对5-FC的敏感性小于SW480-CD, SW480-CEACD对前药5-FC的敏感性低于LoVo-CEACD (P<0.05, t = 2.504, n = 9), 转CD基因之LoVo和SW480细胞体外实验均可观察到明显的旁观者效应.
组织特异性CD/5-FC系统对LoVo和SW480细胞均有明显的靶向杀伤效果, 但对SW480细胞的杀伤作用小于LoVo细胞.
引文著录: 黎成金, 马庆久, 赖大年, 鲁建国, 王小军, 王青, 潘伯荣, 武永忠, 李金茂. CD/5-FC系统对结肠癌细胞的杀伤作用. 世界华人消化杂志 2003; 11(5): 535-539
Revised: October 20, 2002
Accepted: November 25, 2002
Published online: May 15, 2003
To investigate the killing effect of carcinoembryonic antigen (CEA) and tissue-specific cytosine deaminase (CD)/5-fluorocytosine (5-FC) system on human colorectal carcinoma cell lines LoVo and SW480 in vitro.
Recombinant retroviral vector G1CEACDNa was constructed, in which the CD gene was controlled under the CEA promoter, and retroviral vector pCD2 were introduced through liposome technique respectively to the human colorectal carcinoma cell lines LoVo and SW480. Expression of CEA was high and low in both the cell lines respectively. The cells were selectively cultured in G418. The proliferative colonies were treated with 5-FC.
After the transfection, LoVo-CEACD cells and LoVo-CD cells were more sensitive to 5-FC than their parental cells (P<0.01, t = 5.688, n = 9; P<0.01, t = 3.136, n = 9), and SW480-CEACD cells and SW480-CD cells were more sensitive than their parental cells as well (P<0.01, t = 3.437, n = 9; P <0.01, t = 3.516, n = 9). Furthermore, the LoVo-CEACD cells were more sensitive to 5-FC than the LoVo-CD cells (P <0.05, t =2.183, n =9) while the SW480-CEACD cells were less sensitive than SW480-CD cells.TheLoVo-CEACD cells displayed a higher anti-tumor effect than SW480-CEACD cells in vitro. The bystander effect in all cells transfected with CD gene were observed in this study.
The CEA tissue-specific CD/5-FC system displays an obvious targeting anti-tumor effect on human colorectal carcinoma cell lines LoVo and SW480, but the killing effect on the LoVo-CEACD cells is higher than that on the SW480-CEACD cells in vitro.
- Citation: Li CJ, Ma QJ, Lai DN, Lu JG, Wang XJ, Wang Q, Pan BR, Wu YZ, Li JM. Killing effect of CD/5-FC system on human colon cancer cell lines SW 480 and LoVo. Shijie Huaren Xiaohua Zazhi 2003; 11(5): 535-539
- URL: https://www.wjgnet.com/1009-3079/full/v11/i5/535.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i5.535
CD/5-FC系统作为前药转换基因系统之一, 近年来对其在肿瘤治疗中的作用进行了广泛研究[1-4], 已进行了少量Ⅰ期临床实验[1,5-8]. 研究表明[9], 癌胚抗原(CEA)转录调控序列可控制CD基因在CEA阳性的大肠癌组织中高效表达, 在前药5-FC作用下, 产生选择性杀伤肿瘤细胞的作用. 然而, CEA启动子控制的CD基因对低表达CEA的大肠癌细胞是否具有靶向性杀伤作用?为此, 我们进行了初步研究.
含CD基因逆转录病毒载体pCD2由美国Anderson癌症研究中心的Mullen博士惠赠; CEA启动子调控CD基因表达的逆转录病毒载体G1CEACDNa由第二军医大学长海医院崔龙教授惠赠; 大肠癌LoVo细胞株由上海医科大学瑞金消化外科研究所提供; 大肠癌SW480细胞株由第四军医大学动物研究所提供; 感受态菌由第四军医大学遗传与发育教研室制备; 脂质体liopfectamineTM2000 (LF2000)为Invitrogen公司产品; G418, DMEM为Gibco公司产品; 5-FC, MTT为Sigma公司产品; RPMI 1640培养基, 胎牛血清, BamHⅠ酶, EcoRⅠ酶, SalⅠ酶为Promega公司产品; 大量柱离心式质粒抽提纯化试剂盒及小量柱离心式组织和细胞基因组DNA抽提试剂盒为上海华舜公司产品; PCR扩增Premix TaqTM酶为Biotech公司产品, CD基因引物由Biotech公司合成, 引物序列(扩增产物1.5 kb)为: 正义链 5'-ATAGAATTCAGGCTAACAATGTCGAATTAACGCTT-3', 反义链 5'-TATGGATCCTCAACGTTTGTAATCCATGGCTT-3'.
1.2.1 质粒扩增和酶切鉴定 将质粒pCD2, G1CEACDNa在感受态中大量扩增后, 按质粒抽提试剂盒说明书提取并纯化质粒, 主要步骤: 在细菌沉淀中加入P1液3.5 mL, 振荡后加入P2液3.5 mL并混匀, 室温静置4 min, 加入PN液5 mL并混匀, 12000 g离心30 min, 将上清液移入黏附柱, 离心2 min, 加入W1液12 mL, 静置1 min, 离心2 min, 在黏附膜中央加入T1液500 μL, 室温静置2 min后, 离心5 min.质粒pCD2用BamHⅠ及EcoRⅠ酶切鉴定, 质粒G1CEACDNa用SalⅠ酶切鉴定, 用20 g/L琼脂糖凝胶电泳.
1.2.2 大肠癌细胞的转染 在24孔培养板中分别接种3×105个SW480、LoVo细胞, 待细胞生长至95%融合时, 用无血清RPMI 1640培养基轻洗细胞并用之置换加宁L (含1 μg质粒DNA和2.5 μL LF 2000) 培养24 h, 以含400 mg/LG 418及100 mL/L胎牛血清的RPMI 1 640培养基传代培养转染细胞, 选择培养14 d, 其中原传代2次, 筛选抗性克隆并扩增.
1.2.3 细胞基因组DNA的提取及PCR检测 按细胞DNA提取试剂盒说明书操作, 主要步骤如下: 将107细胞移入离心管中, 10000 g离心10 s, 弃上清, 加入RNase A 20 μL和DT液200 μL, 混匀, 65 ℃温浴5 min, 加入DL液400 μL和Proteinase K 25 μL并混匀后65 ℃温浴20 min, 离心5 min, 将上清液移入另一离心管中, 加入异丙醇200 μL混匀后, 取样品液至黏附柱中, 离心30 s, 加入W1液500 μL, 静置1 min, 离心30 s, 加入W1液500 μL, 离心30 s, 在黏附柱中央加入T1液100 μL, 65 ℃静置5 min, 离心1 min, -20 ℃保存. PCR用50 μL反应体系, 反应条件: 94 ℃, 3 min, 94 ℃, 30 s, 60 ℃, 30 s, 72 ℃, 40 s, 72 ℃, 10 min, 40个循环, PCR产物用20 g / L琼脂糖凝胶电泳.
1.2.4 前药5-FC对转基因大肠癌细胞的杀伤作用 将转染G1CEACDNa、 pCD2之LoVo、SW480细胞(分别命名为: LoVo-CEACD, LoVo-CD, SW480-CEACD, SW480-CD)及末转基因之LoVo、SW480细胞以5×104个/孔接种到96孔细胞培养板中, 接种同时加入含各种梯度浓度的前药5-FC, 每种浓度设3个复孔, 同时设置对照孔及调零孔. 在37 ℃、50 mL / L CO2孵箱中培养, 每 2 d换液1次, 第8天去除培养液, 以MTT法测定活细胞比率并计算杀伤率: 加入MTT 20 μL(5g / L), 37 ℃孵育4 h后弃上清加入二甲基亚砜(Sigma) 150 μL , 10 min后振荡, 以490为测定波长, 上酶标仪测定吸光度值(A). 根据下列公式计算细胞存活率: 存活率 = 实验组A值/对照组A值×100%. 将转CD基因SW480, LoVo细胞与未转基因之SW480、LoVo细胞分别以100, 80, 50, 30, 20, 10, 0之比率(%)混合接种至24孔板中, 每种混合细胞设3个复孔, 同时设置对照孔及调零孔, 以含2.0 mmol/L 5-FC的RPMI 1 640完全培养基中培养8 d后, 以MTT法检测活细胞比率并计算旁观者效应杀伤率.
统计学处理 在SPLM软件中输入药敏实验中各浓度前药下每组细胞生存率, 作线图并计算IC50, 采用未配对计量资料的t检验进行统计学处理.
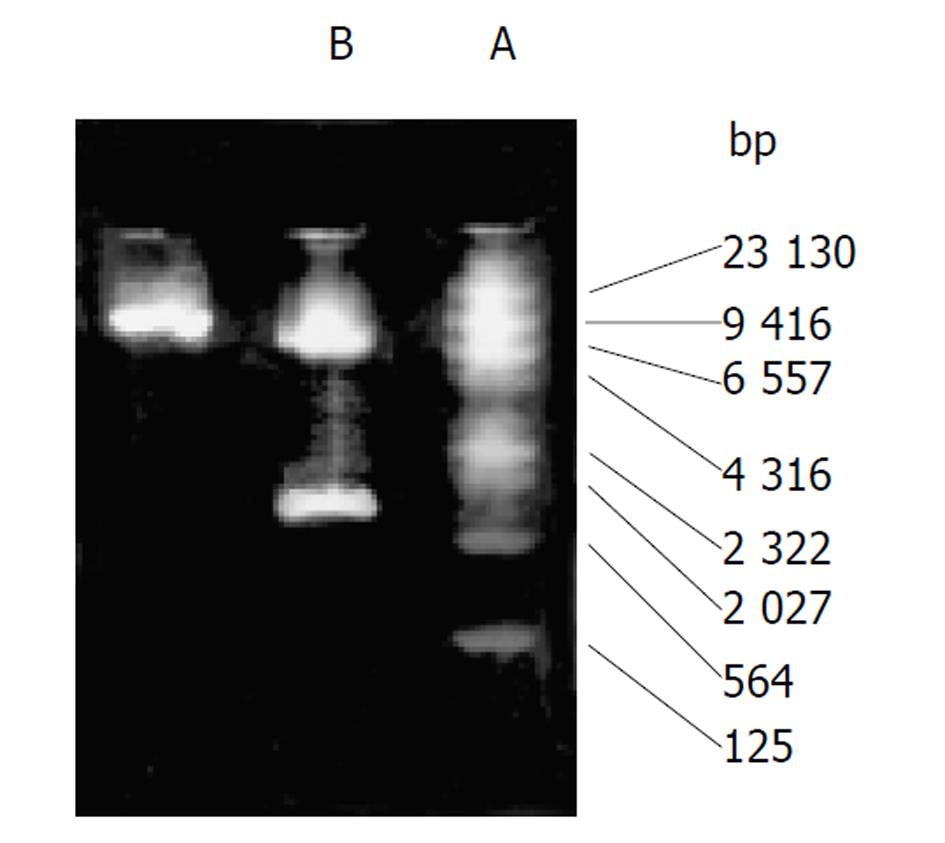
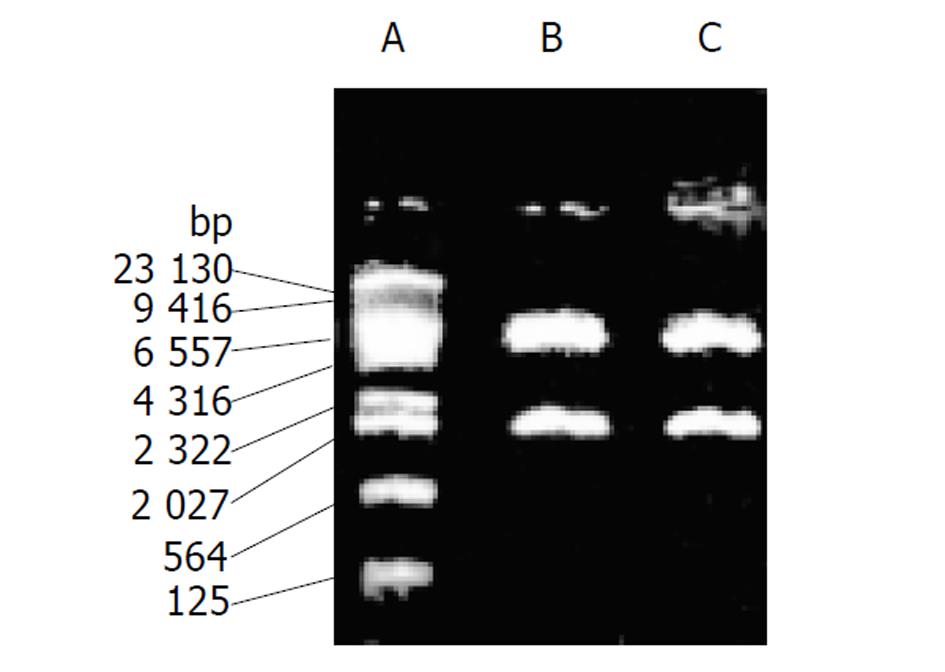
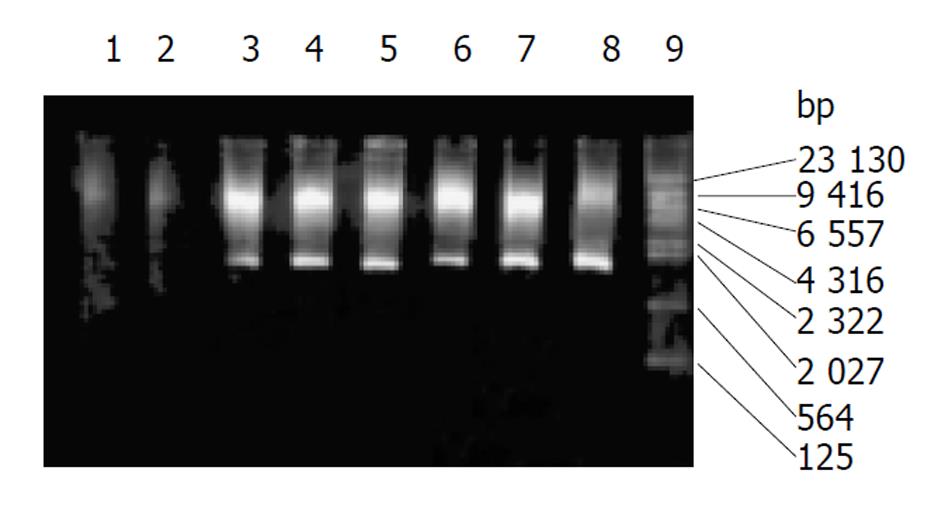
质粒pCD2用BamHⅠ及EcoRⅠ酶切可见1.5 kb酶切片段(图1), 质粒G1CEACDNa用SalⅠ酶切可见1.9 kb酶切片段(图2). 转pCD2及G1CEACDNa之SW480、LoVo细胞PCR产物电泳均可见1.5 kb片段(图3) .
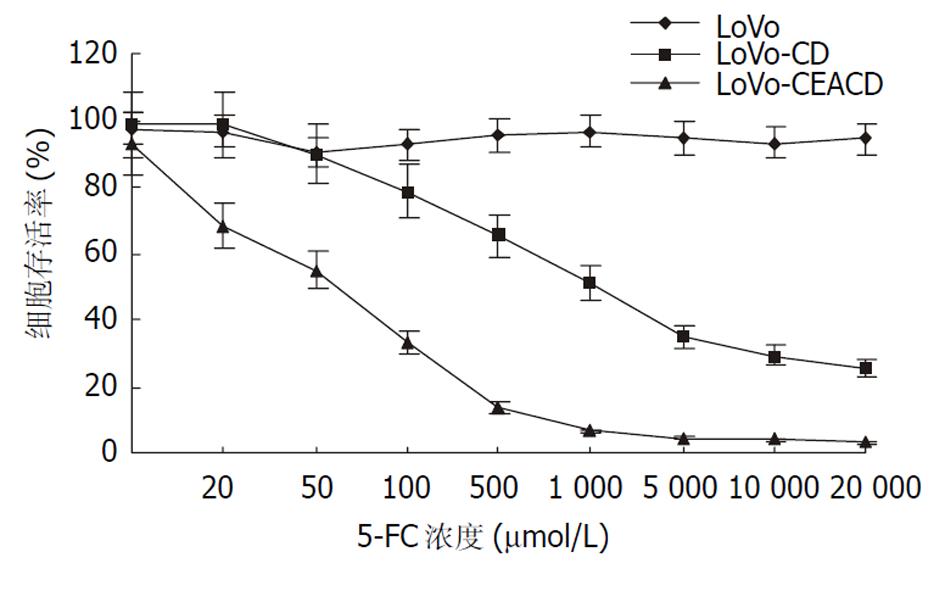
LF2 000转染pCD2、G1CEACDNa, 以含400 mg/L G418的1 640培养基选择培养14 d, 对照组细胞在6 d后全部死亡. 5-FC对转基因大肠癌细胞LoVo, SW480有杀伤作用(图4, 图5), 转CD基因之LoVo, SW480细胞对5-FC的敏感性明显提高, 转G1CEACDNa之LoVo细胞较转pCD2之LoVo细胞对5-FC的敏感性明显增强, 其IC50分别为0.1 mmol/L和0.8 mmol/L, 而转G1CEACDNa之SW480细胞对5-FC的敏感性小于转pCD2之SW480细胞, 其IC50分别为1.0 mmol/L和 0.8 mmol/L. 细胞的杀伤率与转基因细胞的比例并不一致, 当转基因细胞达到30%时, 对混合细胞的杀伤作用就与100%转基因细胞的杀伤作用相似(旁观者效应, 表1).
| 细胞 | 转基因细胞比率 | ||||||
| 0 | 10 | 20 | 30 | 50 | 80 | 100 | |
| LoVo-CD | 0 | 31.5 | 44.5 | 58.2 | 61.3 | 63.6 | 71.5 |
| LoVo-CEACD | 0 | 45.6 | 79.4 | 88.5 | 91.3 | 92.8 | 96.5 |
| SW480-CD | 0 | 32.5 | 41.3 | 53.5 | 60.2 | 61.2 | 63.2 |
| SW480-CEACD | 0 | 33.3 | 41.3 | 52.0 | 53.6 | 55.5 | 59.3 |
胞嘧啶脱氨基酶(cytosine deaminase, CD)基因是来源于某些细菌和真菌的一种自杀基因, 其编码的CD酶能将对真核细胞相对无毒的5-氟胞嘧啶(5-FC)转换成细胞毒性化疗药5-氟尿嘧啶(5-FU), 抑制细胞RNA和DNA的合成而致细胞死亡[10]. 5-FU作为结肠癌的一线化疗药物, 对机体正常组织的毒副作用限制了其在结肠癌化疗的临床应用[11-13], 靶向基因治疗可将治疗基因在肿瘤组织中特异地表达, 使正常组织免受损害. CEA基因属于组织特异性表达基因, 利用CEA基因的转录调控序列来调控CD基因的表达, 可特异性杀死CEA阳性分泌的大肠癌细胞[14-16], 对结肠癌肝转移也有特异性治疗作用及旁杀伤效应[17-21], 对结肠癌的腹膜转移亦能进行有效治疗[22]. 临床资料显示, 虽然CEA作为结肠癌疗效及转移的监测指标有其特异性[23], 但在大肠癌患者中, 近2/3血清CEA正常[24], Dukes A期患者血清CEA水平仅5.3±1.8 μg/L[25], 表明临床有相当一部分大肠癌患者其CEA表达水平较低. 我们所采用LoVo和SW480分别是高表达CEA和低表达CEA的人大肠癌细胞株, CEA分泌水平分别为49 fg/cell 和3.1 fg/ cell.
本研究用逆转录病毒载体G1CEACDNa及pCD2分别转染LoVo细胞和SW480细胞, G1CEACDNa由组织特异性CEA基因顺式转录调控序列(TRS)调控CD基因的表达, pCD2为非组织特异性CD基因逆转录病毒载体, 我们用脂质体LF2000直接转染大肠癌细胞, 经G418选择培养14 d后, 用前药5-FC进行实验. 结果显示, 目的基因均成功整合到靶细胞中, LoVo-CEACD及LoVo-CD细胞比未转基因之LoVo细胞对5-FC的敏感性明显提高(P<0.01, t = 5.688, n = 9; P<0.01, t = 3.136, n = 9), SW480-CEACD及SW480-CD细胞比未转基因之SW480细胞对5-FC的敏感性明显提高(P<0.01, t = 3.437, n = 9 ; P<0.01, t = 3.516, n = 9), LoVo-CEACD较LoVo-CD细胞对5-FC的敏感性明显增强(P<0.05, t = 2.183, n = 9), 其IC50提高了8倍, 而SW480-CEACD细胞对5-FC的敏感性小于SW480-CD细胞, 后者的IC50是前者的1.25倍; 转染CEA组织特异性CD基因后, 低表达CEA的大肠癌细胞SW480对前药5-FC的敏感性低于高表达CEA的人大肠癌细胞LoVo(P<0.05, t = 2.504, n = 9), 后者IC50是前者的10倍; 转G1CEACDNa及pCD2之LoVo和SW480细胞均可观察到明显的旁观者效应. 我们的实验结果表明, 用组织特异性CEA启动子调控CD基因的表达, 对高表达CEA的大肠癌细胞LoVo和低表达CEA的大肠癌细胞SW480均有明显的靶向杀伤作用, 提高了细胞对前药5-FC的敏感性, 但对SW480的杀伤作用不如对LoVo细胞的杀伤作用理想, 甚至低于普通型CD基因, 分析可能是由于肿瘤细胞CEA的低表达导致CEA启动子调控的CD基因的低表达所致, 其机制有待进一步实验证实.
靶向性和有效性是基因治疗的两大瓶颈, 前者可使正常组织细胞免受损害, 后者保证了治疗的效率. 我们的实验提示, CEA组织特异性CD/5FC系统对低表达CEA大肠癌的治疗效率将受到影响, 有研究表明, 用CEA启动子和增强子共同调节CD 基因的表达, 能明显提高肿瘤细胞CD基因的表达[26], 放疗、免疫因子与CD/5FC系统的联合应用、双自杀基因的共同转导[27-38]等都能提高CD/5FC系统的治疗效果, 这样可以在保证治疗靶向性的前提下提高治疗效率.
| 1. | Greco O, Dachs GU. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J Cell Physiol. 2001;187:22-36. [DOI] |
| 2. | Sandalon Z, Fusenig NE, McCutcheon J, Taichman LB, Garlick JA. Suicide gene therapy for premalignant disease: a new strategy for the treatment of intraepithelial neoplasia. Gene Ther. 2001;8:232-238. [PubMed] [DOI] |
| 3. | Dilber MS, Gahrton G. Suicide gene therapy: possible applications in haematopoietic disorders. J Intern Med. 2001;249:359-367. [PubMed] [DOI] |
| 4. | Beltinger C, Uckert W, Debatin KM. Suicide gene therapy for pediatric tumors. J Mol Med. 2001;78:598-612. [DOI] |
| 5. | Harvey BG, Maroni J, O Donoghue KA, Chu KW, Muscat JC, Pippo AL, Wright CE, Hollmann C, Wisnivesky JP, Kessler PD. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002;13:15-63. [PubMed] [DOI] |
| 6. | Crystal RG, Harvey BG, Wisnivesky JP, O'Donoghue KA, Chu KW, Maroni J, Muscat JC, Pippo AL, Wright CE, Kaner RJ. Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum Gene Ther. 2002;13:65-100. [PubMed] [DOI] |
| 7. | Harvey BG, Worgall S, Ely S, Leopold PL, Crystal RG. Cellular immune responses of healthy individuals to intradermal administration of an E1-E3- adenovirus gene transfer vector. Hum Gene Ther. 1999;10:2823-2837. [PubMed] [DOI] |
| 8. | pencer DM. Developments in suicide genes for preclinical and clinical applications. Curr Opin Mol Ther. 2000;2:433-440. [PubMed] |
| 9. | Cui L, Lin YZ, Zhu ZG, Chen XH, Gu QL, Liu BY, Yu BM. Killing effect of 5-FC/CD system on nude mice bearing human colorectal carcinoma. ShijieHuarenXiaohuaZazhi. 1999;7:473-475. |
| 10. | Nakamura H, Mullen JT, Chandrasekhar S, Pawlik TM, Yoon SS, Tanabe KK. Multimodality therapy with a replication-conditional herpes simplex virus 1 mutant that expresses yeast cytosine deaminase for intratumoral conversion of 5-fluorocytosine to 5-fluorouracil. Cancer Res. 2001;61:5447-5452. [PubMed] |
| 11. | Gennatas C, Mouratidou D, Androulakis G, Georgoulias V, Tsavaris N, Philippakis M, Michailakis E, Kalofonos C, Mpesmpeas S, Katsos J. Adjuvant systemic therapy protocol for Dukes B2 and C resectable colon carcinoma. Tumori. 2002;88:32-36. [PubMed] |
| 12. | Ravaioli A, Marangolo M, Pasquini E, Rossi A, Amadori D, Cruciani G, Tassinari D, Oliverio G, Giovanis P, Turci D. Bolus fluorouracil and leucovorin with oxaliplatin as first-line treatment in metastatic colorectal cancer. J ClinOncol. 2002;20:2545-2550. [PubMed] |
| 13. | Baars A, Claessen AM, Wagstaff J, Giaccone G, Scheper RJ, Meijer S, Schakel MJ, Gall HE, Meijer CJ, Vermorken JB. A phase II study of active specific immunotherapy and 5-FU/Leucovorin as adjuvant therapy for stage III colon carcinoma. Br J Cancer. 2002;86:1230-1234. [PubMed] [DOI] |
| 14. | Shen LZ, Wu WX, Xu DH, Zheng ZC, Liu XY, Ding Q, Hua YB, Yao K. Specific CEA-producing colorectal carcinoma cell killing with recombinant adenoviral vector containing cytosine deaminase gene. World J Gastroenterol. 2002;8:270-275. [PubMed] [DOI] |
| 15. | Cao G, Kuriyama S, Gao J, Kikukawa M, Cui L, Nakatani T, Zhang X, Tsujinoue H, Pan X, Fukui H. Effective and safe gene therapy for colorectal carcinoma using the cytosine deaminase gene directed by the carcinoembryonic antigen promoter. Gene Ther. 1999;6:83-90. [PubMed] [DOI] |
| 16. | Cao G, Kuriyama S, Cui L, Nagao S, Pan X, Toyokawa Y, Zhang X, Nishiwaki I, Qi Z. Analysis of the human carcinoembryonic antigen promoter core region in colorectal carcinoma-selective cytosine deaminase gene therapy. Cancer Gene Ther. 1999;6:572-580. [PubMed] [DOI] |
| 17. | Humphreys MJ, Ghaneh P, Greenhalf W, Campbell F, Clayton TM, Everett P, Huber BE, Richards CA, Ford MJ, Neoptolemos JP. Hepatic intra-arterial delivery of a retroviral vector expressing the cytosine deaminase gene, controlled by the CEA promoter and intraperitoneal treatment with 5-fluorocytosine suppresses growth of colorectal liver metastases. Gene Ther. 2001;8:1241-1247. [PubMed] [DOI] |
| 18. | Block A, Freund CT, Chen SH, Nguyen KP, Finegold M, Windler E, Woo SL. Gene therapy of metastatic colon carcinoma: regression of multiple hepatic metastases by adenoviral expression of bacterial cytosine deaminase. Cancer Gene Ther. 2000;7:438-445. [PubMed] [DOI] |
| 19. | Pierrefite-Carle V, Baque P, Gavelli A, Benchimol D, Bourgeon A, Milano G, Saint-Paul MC, Rossi B. Regression of experimental liver tumor after distant intra-hepatic injection of cytosine deaminase-expressing tumor cells and 5-fluorocytosine treatment. Int J Mol Med. 2000;5:275-278. [DOI] |
| 20. | Nyati MK, Symon Z, Kievit E, Dornfeld KJ, Rynkiewicz SD, Ross BD, Rehemtulla A, Lawrence TS. The potential of 5-fluorocytosine/cytosine deaminase enzyme prodrug gene therapy in an intrahepatic colon cancer model. Gene Ther. 2002;9:844-849. [PubMed] [DOI] |
| 21. | Baque P, Pierrefite-Carle V, Gavelli A, Brossette N, Benchimol D, Bourgeon A, Staccini P, Saint-Paul MC, Rossi B. Naked DNA injection for liver metastases treatment in rats. Hepatology. 2002;35:1144-1152. [PubMed] [DOI] |
| 22. | Bentires AM, Hellin AC, Lechanteur C, Princen F, Lopez M, Fillet G, Gielen J, Merville MP, Bours V. Cytosine deaminasesuicide gene therapy for peritoneal carcinomatosis. Cancer Gene Ther. 2000;7:20-26. [PubMed] [DOI] |
| 23. | Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH, Yen CC, Chen WS, Jiang JK, Yang SH, Wang HS. Tumor marker CEA in monitoring of response to tegafur-uracil and folinic acid in patients with metastatic colorectal cancer. Hepatogastroenterology. 2002;49:388-392. [PubMed] |
| 24. | Perez CA, Ravindranath MH, Gupta RK, Tollenaar RA, van de Velde CJ, Wood TF, Soh D, Morton DL, Bilchik AJ. Serum total gangliosides and TA90-IC levels: novel immunologic markers in colorectal cancer. Cancer J. 2002;8:5561. [DOI] |
| 25. | Wichmann MW, Muller C, Hornung HM, Lau-Werner U, Schildberg FW. Results of long-term follow-up after curative resection of dukes a colorectal cancer. World J Surg. 2002;26:732-736. [PubMed] [DOI] |
| 26. | Nyati MK, Sreekumar A, Li S, Zhang M, Rynkiewicz SD, Chinnaiyan AM, Rehemtullaalawrence TS. High and selective expression of yeast cytosine deaminase under a carcinoembryonic antigen promoter-enhancer. Cancer Res. 2002;62:2337-2342. [PubMed] |
| 27. | Ueno M, Koyama F, Yamada Y, Fujimoto H, Takayama T, Kamada K, Naito A, Hirao S, Mukogawa T, Hamada H. Tumor-specific chemo-radio-gene therapy for colorectal cancer cells using adenovirus vector expressing the cytosine deaminase gene. Anticancer Res. 2001;21:2601-2608. [PubMed] |
| 28. | Sun W, Tang Z, Wei D, Tang Y, Chen S. Combined gene therapy for murine liver cancer with interleukin-18 and cytosine deaminase genes. ZhonghuaGanzangbingZazhi. 2001;9:300-302. |
| 29. | Cao X, Huang X, Ju DW, Zhang W, Hamada H, Wang J. Enhanced antitumoral effect of adenovirus-mediated cytosine deaminase gene therapy by induction of antigen-presenting cells through stem cell factor/granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Gene Ther. 2000;7:177-186. [PubMed] [DOI] |
| 30. | Lee YJ, Lee H, Borrelli MJ. Gene transfer into human prostate adenocarcinoma cells with an adenoviral vector: Hyperthermia enhances a double suicide gene expression, cytotoxicity and radiotoxicity. Cancer Gene Ther. 2002;9:267-274. [PubMed] [DOI] |
| 31. | Ueda K, Iwahashi M, Nakamori M, Nakamura M, Matsuura I, Yamaue H, Tanimura H. Carcinoembryonic antigen-specific suicide gene therapy of cytosine deaminase/5-fluorocytosine enhanced by the cre/loxP system in the orthotopic gastric carcinoma model. Cancer Res. 2001;61:6158-6162. [PubMed] |
| 32. | Kievit E, Nyati MK, Ng E, Stegman LD, Parsels J, Ross BD, Rehemtulla A, Lawrence TS. Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluorocytosine of human colorectal cancer xenografts. Cancer Res. 2000;60:6649-6655. [PubMed] |
| 33. | Ju DW, Yang Y, Tao Q, Song WG, He L, Chen G, Gu S, Ting CC, Cao X. Interleukin-18 gene transfer increases antitumor effects of suicide gene therapy through efficient induction of antitumor immunity. Gene Ther. 2000;7:1672-1679. [PubMed] [DOI] |
| 34. | Koyama F, Sawada H, Hirao T, Fujii H, Hamada H, Nakano H. Combined suicide gene therapy for human colon cancer cells using adenovirus-mediated transfer of escherichia coli cytosine deaminase gene and Escherichia coli uracil phosphoribosyltrans ferase gene with 5-fluorocytosine. Cancer Gene Ther. 2000;7:1015-1022. [PubMed] [DOI] |
| 35. | Stackhouse MA, Pederson LC, Grizzle WE, Curiel DT, Gebert J, Haack K, Vickers SM, Mayo MS, Buchsbaum DJ. Fractionated radiation therapy in combination with adenoviral delivery of the cytosine deaminase gene and 5-fluorocytosine enhances cytotoxic and antitumor effects in human colorectal and cholangiocarcinoma models. Gene Ther. 2000;7:1019-1026. [PubMed] [DOI] |
| 36. | Rogulski KR, Wing MS, Paielli DL, Gilbert JD, Kim JH, Freytag SO. Double suicide gene therapy augments the antitumor activity of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum Gene Ther. 2000;11:67-76. [PubMed] [DOI] |
| 37. | Moriuchi S, Wolfe D, Tamura M, Yoshimine T, Miura F, Cohen JB, GloriosoJC . Double suicide gene therapy using a replication defective herpes simplex virus vector reveals reciprocal interference in a malignant glioma model. Gene Ther. 2002;9:584-591. [PubMed] [DOI] |
| 38. | Chen G, Li S, Yu B, An P, Cai H, Guo W. X-ray combined with cytosine deaminase suicide gene therapy enhances killing of colorectal carcinoma cells in vitro. ZhonghuaWaikeZazhi. 2002;40:136-138. |