修回日期: 2002-11-05
接受日期: 2002-11-16
在线出版日期: 2003-02-15
目的: 探讨苦参素对实验性大鼠肝纤维化的预防与治疗作用.
方法: 采用人血清白蛋白免疫损伤Wistar大鼠造成肝纤维化模型, 60只Wister大鼠, 分为5组: 造模组、秋水仙碱治疗组、试验组I(苦参素预防组)、试验组II(苦参素治疗组), 另设正常对照组. 动态观察大鼠肝组织病例变化, 采用HE染色观察肝组织改变及Von-Gieson胶原纤维特殊染色观察肝纤维化程度, 并采用免疫组织化学染色及肝组织原位杂交检测I型、III胶原含量及I型、III型胶原mRNA的表达.
结果: 试验组肝组织结构明显好转, 纤维组织增生程度明显低于肝纤维化模型组; I型、III型胶原含量及I型、III型胶原的mRNA表达均低于模型组免疫诱导型肝纤维化大鼠, 其效果为试验组I(苦参素预防组)效果最好, 试验组II(苦参素治疗组)次之, 秋水仙碱治疗组再次之, 三组均优于造模组.
结论: 苦参素可减轻肝脏炎症活动、抑制肝内胶原合成, 对实验性大鼠肝纤维化具有防治作用.
引文著录: 康文臻, 谢玉梅, 聂青和, 张岩, 郝春秋, 王久平, 陈伟红. 苦参素对实验性大鼠肝纤维化防治作用的研究. 世界华人消化杂志 2003; 11(2): 195-198
Revised: November 5, 2002
Accepted: November 16, 2002
Published online: February 15, 2003
AIM: To study the effect of Oxymatrine on liver fibrosis in immunogenic liver fibrosis rat model.
METHODS: Rat liver fibrosis model was induced by human serum albumin (HSA), 60 Wistar rats were randomly divided into 5 groups, control group without any treatment, liver fibrosis model group, oxymatrine preventive group, oxymatrine therapeutic group, and cochicine therapeutic group. The pathological changes of liver were observed by HE and Von-Gieson staining. The expressions of mRNA and proteins of collagen I/III in liver were determined by in situ hybridization and immunohistochemistry.
RESULTS: The liver fibrosis degree and level of mRNA and proteins of collagen I/III in the liver were significantly reduced in the decreasing order in oxymatrine preventive group, oxymatrine therapeutic group, and cochicine therapeutic group.
CONCLUSION: Oxymatrine may inhibit hepatic inflammation and hepatic synthesis of collagen I/III, and thus prevent and inhibit hepatic fibrosis.
- Citation: Kang WZ, Xie YM, Nie QH, Zhang Y, Hao CQ, Wang JP, Chen WH. Effect of Oxymatrine on experimental liver fibrosis. Shijie Huaren Xiaohua Zazhi 2003; 11(2): 195-198
- URL: https://www.wjgnet.com/1009-3079/full/v11/i2/195.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i2.195
肝纤维化是慢性肝损伤向肝硬化发展的基础, 是由于肝细胞外基质增生与降解失衡所至, 其主要病理变化是肝细胞外基质在肝脏的过渡沉积[1-4]. 目前, 人们普遍认为, 此阶段是可逆的病理过程[5-8]. 目前国内外对于肝纤维化尚无理想的治疗方法, 因此, 寻找有效药物, 阻断、延缓及逆转肝纤维化的发生和发展是急待解决的问题, 是治疗肝硬化的关键. 曾有文献[9-12]报道秋水仙碱具有抑制肝细胞外基质生成的作用, 并曾应用于少数病例, 但由于疗效不佳, 毒副作用较大, 临床应用受到限制. 苦参素是从中药苦豆子和苦参根中提取的有效成分, 具有抗炎、免疫调节、稳定细胞膜及阻断肝细胞凋亡等作用[13-17]. 有文献报道, 苦参素临床应用具有抗纤维化作用[18,19], 本研究应用免疫损伤性大鼠肝纤维化模型, 初步探讨了苦参素抗肝纤维化的作用机制, 以期为其临床应用提供可靠的理论依据.
Wister大鼠♀, 体重140±20 g, 60只, 清洁级(购自第四军医大学实验动物中心). 取54只Wister大鼠参照文献[20,21]应用人血白蛋白免疫攻击方法制备免疫性肝纤维化大鼠模型(人血白蛋白, 选自兰州生物制品研究所, 不完全福氏佐剂系sigma公司产品). 抗大鼠IgG单克隆抗体购自法国Coulter公司. 另6只Wister大鼠设为正常对照组.
试验组I(10只): 尾静脉攻击同时, 开始予苦参素(氧化苦参碱, 博尔泰力注射液, 由宁夏绿谷药业公司生产)50 mg/kg腹腔内注射1次/d, 维持3 mo, 造膜结束后存活8只; 44只实验攻击动物存活29只, 按随机分组法随机分为4组: 模型组(9只): 尾静脉攻击结束后不予任何其他干扰因素; 治疗组(10只): 尾静脉攻击结束后予秋水仙碱(系昆明制药股份有限公司生产)0.28 mg/kg 灌胃治疗, 6次/wk, 维持3 mo; 试验组II(10只): 尾静脉攻击结束后, 予苦参素50 mg/kg腹腔内注射1次/d, 维持3 mo; 正常对照组(6只): 等量无菌生理盐水腹腔内注射, 1次/d, 维持3 mo. 所有动物均于给药3 mo后处死, 打开腹腔, 取肝脏方叶小块组织置10%中性甲醛固定, 留作病理检查.
1.3.1 病理学观察: 常规HE染色观察肝组织学改变; Von Gieson胶原纤维特殊颜色.
1.3.2 Ⅰ型、Ⅲ型胶原的免疫组化染色: Ⅰ型、Ⅲ型胶原免疫组化试剂盒购自武汉博士德生物工程有限公司, 兔抗Ⅰ型、Ⅲ型胶原单克隆抗体试剂编号分别为BA0325和BA0326.具体方法按试剂盒说明书常规进行操作.
1.3.3 肝组织原位杂交: Ⅰ型、Ⅲ型前胶原mRNA表达检测采用地高辛标记探针原位杂交法. Ⅰ型、Ⅲ前胶原原位杂交试剂盒均购自武汉博士德生物工程有限公司, 试剂编号分别为MK1171和MK1549.按试剂盒说明书常规进行操作.
1.3.4 图像分析与数据处理: 免疫组化及杂交结果经计算机图像灰度扫描数字转换后, 进行统计数字处理, 各组间行t检验, 以P值<0.05判断有显著性差异.
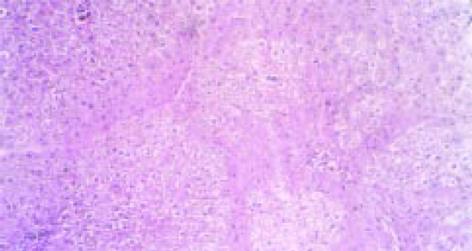
正常大鼠肝脏颜色鲜红, 表面光滑, 质脆柔软. 造模组大鼠肝脏体积缩小, 质地较韧, 边缘较钝, 颜色呈暗红色, 表面粗糙. 肝组织汇管区中可见纤维组织增生, 同时, 纤维组织于中央静脉周围增生并向肝小叶内延伸, 但纤维间隔较纤细, 汇管区及中央静脉周围可见炎细胞浸润, 沿界板及其他部位有肝细胞浓染、溶解及小双核细胞. 造模组V-G染色胶原纤维沿汇管区及中央静脉周围向肝小叶内延伸, 交联成网状结构(图1). 试验组I、试验组II与治疗组大鼠肝脏在色泽、质地、大小及表面光滑度方面较造模组均有明显改善. 光镜检查可见肝脏组织内炎性细胞浸润、肝细胞坏死和纤维组织增生等方面均较造模组大鼠为轻, 纤维隔较薄.
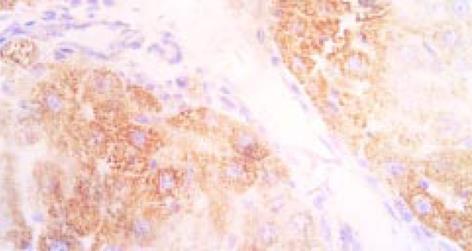
免疫组化检测的阳性信号呈现为棕黄色颗粒状, 正常肝脏I型胶原阳性染色只见于汇管区和中央静脉管壁, III型胶原主要存在于大血管周围; 造模组肝脏中Ⅰ型、Ⅲ型胶原广泛存在于纤维间隔及肝细胞内; 试验组I、试验组II与治疗组大鼠肝脏中Ⅰ型、Ⅲ型胶原明显减少, 纤维间隔较薄. 将胶原免疫组化结果进行图像分析, 表明试验组I、试验组II与治疗组肝脏中Ⅰ型、Ⅲ型胶原较造模组明显减少. 杂交结果经计算机图像灰度扫描数字转换值见表1.
多种病因所致的慢性肝损伤, 由于肝细胞的变性坏死、纤维组织增生, 最终可发展成肝硬变, 因此, 肝纤维化是慢性肝炎向肝硬变发展的必经阶段. 肝纤维化是继发于肝脏炎症或损伤后组织修复过程中的代偿反应. 目前认为, 肝纤维化的发生机制主要是细胞外基质(EMC)的过度增生并在肝内异常沉积[22-30]. 细胞外基质包括胶原、糖蛋白及蛋白多糖等, 其中胶原是最主要的成分. 肝纤维化的形成过程主要取决于胶原的合成、沉积、降解和吸收的动态平衡. 当胶原合成与沉积大于降解和吸收时, 肝内胶原纤维增加, 逐渐形成肝纤维化. 胶原在正常肝组织中只占蛋白成分的5-10%, 但在肝硬化组织中可增高到50%以上, 而I型胶原可占硬化肝脏胶原总量的60%-70%, III型胶原可占硬化肝脏胶原总量的20%-30%[31-33], 因而I型、III型胶原可作为反应胶原代谢的重要参数[34-38], 观察I型、III型胶原合成的多少, 可以判断抗纤维化药物的疗效[39,40]. 原位杂交是一项分子病理学技术, 主要检测的是正在表达的基因[45,46], 定位性强, 敏感性高, 有利于对肝纤维化发病机制的研究; 地高辛是近年应用较广的非同位素标记物, 其敏感性高, 具有特异性好, 储存稳定等特点, 同时避免了同位素的污染, 易被人们接受使用[47-49]. 在肝纤维化的形成过程中, 前胶原mRNA的增高先于或同步于胶原的增加, 前胶原的转录过程是胶原合成的限速步骤. 与组织学检测相比, 前胶原mRNA的测定能更好地反映当时肝脏胶原合成的状况, 并可提示肝纤维化的发展趋势[41-44].
目前, 人们普遍认为, 肝纤维化是可逆的病理过程[5-8]. 因此, 寻找有效药物, 阻断延缓及逆转肝纤维化的发生和发展是急待解决的问题. 抗肝纤维化的治疗, 国内外虽陆续有过一些报道, 但因种种原因尚未找到十分理想的药物, 众多学者仍在进行有益的探索. 近些年来, 国内学者报道以中药为主或中西药物联合应用治疗肝纤维化, 动物实验和临床应用均显示出较好的苗头[47-55]. 苦参素(oxymatrine)又名氧化苦参碱, 系由中药苦豆子中提取的生物碱类, 大量研究表明, 苦参素有抗HBV作用, 能阻断肝细胞凋亡、稳定细胞膜、消除自由基、保护肝细胞, 调节免疫功能及防治肝纤维化等作用[13-19].
本实验采用人血白蛋白注射所致免疫诱导性大鼠肝纤维化模型, 用苦参素治疗大鼠肝纤维化. 应用苦参素预防和治疗的大鼠, 其肝表面色泽、质地较肝纤维化模型组明显改善, Ⅰ型、Ⅲ型前胶原mRNA均较造模组明显降低, 治疗效果依次为: 试验组I>试验组II>治疗组>模型组. Ⅰ型、Ⅲ型胶原的免疫组化也显示同样的结果. 表明苦参素能有效地抑制大鼠肝内Ⅰ型、Ⅲ型胶原的累积; 降低肝纤维化大鼠肝内Ⅰ型、Ⅲ型前胶原mRAN的表达, 因而推测苦参素可能通过抑制肝脏胶原合成而实现抗纤维化作用, 也可通过抗炎、抗病毒、抑制免疫等环节起到抗肝纤维化作用. 而其抑制成纤维细胞的增生及原胶原mRNA的表达可能是其抗肝纤维化的主要环节. 另外, 鉴于苦参素无明显毒副作用, 有望成为很有前途的抗肝纤维化药物, 其临床疗效还有待进一步深入研究.
编辑: N/A
| 2. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [PubMed] [DOI] |
| 3. | Oberti F, Valsesid E, Pilette C. Noninasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609-1616. [DOI] |
| 4. | Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351-372. [PubMed] [DOI] |
| 6. | Okazaki I, Watanabe T, Hozawa S, Niioka M, Arai M, Maruyama K. Reversibility of hepatic fibrosis: from the first report of collagenase in the liver to the possibility of gene therapy for recovery. Keio J Med. 2001;50:58-65. [PubMed] [DOI] |
| 7. | Garcia-Bañuelos J, Siller-Lopez F, Miranda A, Aguilar LK, Aguilar-Cordova E, Armendariz-Borunda J. Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors. Evidence of cirrhosis reversion. Gene Ther. 2002;9:127-134. [PubMed] [DOI] |
| 8. | Bai WY, Tao XX, Feng LY. The advance of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:1267-1268. [DOI] |
| 9. | Rodríguez L, Cerbón-Ambriz J, Muñoz ML. Effects of colchicine and colchiceine in a biochemical model of liver injury and fibrosis. Arch Med Res. 1998;29:109-116. [PubMed] |
| 10. | Rhoden EL, Pereira-Lima J, Rhoden CR, Mauri M, Pereira-Lima JC, Zettler CG, Barros EG. The role of colchicine in prevention of hepatic cirrhosis induced by carbon tetrachloride. Hepatogastroenterology. 1999;46:1111-1115. [PubMed] |
| 11. | Montasser MF, Younis TA, Fahmy IA, Sabry NM, el-Monayerhy MS, Rashad MS. Histopathological evidences for the antifibrogenic effect of colchicine in schistosomiasis mansoni in mice. J Egypt Soc Parasitol. 1994;24:155-165. [PubMed] |
| 12. | Yang CQ, Hu GL, Zhou WH, Tan DM, Zhang Z. Effects of colchicines on the expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in the livers of rats with hepatic fibrosis. Zhonghua Chuanranbing Zazhi. 2000;18:176-179. |
| 13. | Xiang XX, Wang GJ, Cai X, Li YL. The protective effect of oxymatrine on the fulminant hepatic failure in mice. Linchuang Gandanbing Zazhi. 2000;16:92-93. |
| 14. | Yu Y, Si C, Zeng Z, Wang Q, Zhou X, Zhang Q, Huang Z, Zhang L, Qiao G. [A clinical trial of oxymatrine in treating chronic viral hepatitis type B]. Zhonghua Nei Ke Za Zhi. 2001;40:843-846. [PubMed] |
| 15. | Wang JX, Wang GJ, Cai X, He P. Effect of oxymatrin and glycyrrhizin on hepatocyte apoptosis induced by cessation of phenobabital treatment in mice. Dier Junyidaxue Xuebao. 1999;20:222-224. |
| 16. | L iu T, Hu JH, Cai Q. Study on the mechanism of Matrine on hepatic fibrosis in vitro. Jiefangjun Yaoxue Xuebao. 2002;16:119-122. |
| 17. | Hu ZL, Zhang JP, Qian DH, Lin W, Xie WF, Zhang XR, Chen WZ. Effects of matrine on mouse splenocyte proliferation and release of interleukin-1 and -6 from peritoneal macrophages in vitro. Zhongguo Yao Li Xue Bao. 1996;17:259-261. [PubMed] |
| 18. | Tao SC, Wang JZ. Pharmacology of Alkaloids of Sophora Alopecuroides. Zhongguo Yaoxue Zazhi. 1992;27:201-202. |
| 19. | Chen WZ, Zhang JP, Xu Q. effect of matrine on rat liver fibrosis model. Dier Junyidaxue Xuebao. 1996;17:424-426. |
| 21. | Nyberg A, Engström-Laurent A, Lööf L. Serum hyaluronate in primary biliary cirrhosis--a biochemical marker for progressive liver damage. Hepatology. 1988;8:142-146. [PubMed] [DOI] |
| 22. | Isbrucker RA, Oeferson TC. Platelet-derived growth factor and pentoxifylline modulation of collagen synthesis in myofibroblasts Toxicology and Applied. Pharmacology. 1998;149:120. |
| 23. | Jiang HQ, Zhang XL. Pathogenesis of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:687-689. [DOI] |
| 24. | Lamireau T, Desmoulière A, Bioulac-Sage P, Rosenbaum J. [Mechanisms of hepatic fibrogenesis]. Arch Pediatr. 2002;9:392-405. [PubMed] [DOI] |
| 25. | Murawaki Y, Kawasaki H. Pathophysiology of hepatic fibrosis. Nippon Shokakibyo Gakkai Zasshi. 1999;96:1143-1152. |
| 26. | Okazaki I, Watanabe T, Inagaki Y. [Recent advance in understanding mechanisms of fibrogenesis and fibrolysis in hepatic fibrosis]. Nihon Shokakibyo Gakkai Zasshi. 2002;99:353-364. [PubMed] |
| 27. | Housset C, Guéchot J. [Hepatic fibrosis: physiopathology and biological diagnosis]. Pathol Biol (Paris). 1999;47:886-894. [PubMed] |
| 28. | Valkova M. Hepatic fibrogenesis. Bratisl Lek Listy. 2002;103:76-85. [PubMed] |
| 29. | Murawaki Y, Kawasaki H. [Pathophysiology of hepatic fibrosis]. Nihon Shokakibyo Gakkai Zasshi. 1999;96:1143-1152. [PubMed] |
| 30. | Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001;5:315-334, v-vi. [PubMed] [DOI] |
| 31. | Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1-10. [PubMed] [DOI] |
| 32. | Pierce RA, Glaug MR, Greco RS, Mackenzie JW, Boyd CD, Deak SB. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987;262:1652-1658. [PubMed] |
| 33. | Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373-384. [PubMed] [DOI] |
| 34. | Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710-719. [PubMed] |
| 35. | Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397-403. [PubMed] [DOI] |
| 36. | Gagliano N, Arosio B, Grizzi F, Masson S, Tagliabue J, Dioguardi N, Vergani C, Annoni G. Reduced collagenolytic activity of matrix metalloproteinases and development of liver fibrosis in the aging rat. Mech Ageing Dev. 2002;123:413-425. [PubMed] [DOI] |
| 37. | Suzuki C, Kayano K, Uchida K, Sakaida I, Okita K. Characteristics of the cell proliferation profile of activated rat hepatic stellate cells in vitro in contrast to their fibrogenesis activity. J Gastroenterol. 2001;36:322-329. [PubMed] [DOI] |
| 38. | Shiba M, Shimizu I, Yasuda M, Ii K, Ito S. Expression of type I and type III collagens during the course of dimethylnitrosamine-induced hepatic fibrosis in rats. Liver. 1998;18:196-204. [PubMed] [DOI] |
| 39. | Bickel M, Baringhaus KH, Gerl M, Günzler V, Kanta J, Schmidts L, Stapf M, Tschank G, Weidmann K, Werner U. Selective inhibition of hepatic collagen accumulation in experimental liver fibrosis in rats by a new prolyl 4-hydroxylase inhibitor. Hepatology. 1998;28:404-411. [PubMed] [DOI] |
| 40. | Wang Y, Gao Y, Yang JZ. Expression of interstitial collagenase in rats with experimental liver fibrosis. Shijie Huaren Xiaohua Zazhi. 1999;7:1004-1006. [DOI] |
| 41. | Goddard CRJ, Smith A, Hoyland JA. Localization and semiquatitative assessment of hepatic procollangen mRNA in primary biliary cirrhosis. Gut. 1998;43:433-440. |
| 42. | Du WD, Zhang YE, Zhai WR, Zhou XM, Zhou XH. Study on the collogen producing cells in experimental liver fibrosis. Zhonghua Ganzangbing Zazhi. 1998;6:238-240. |
| 43. | Kauschke SG, Knorr A, Heke M, Kohlmeyer J, Schauer M, Theiss G, Waehler R, Burchardt ER. Two assays for measuring fibrosis: reverse transcriptase-polymerase chain reaction of collagen alpha(1) (III) mRNA is an early predictor of subsequent collagen deposition while a novel serum N-terminal procollagen (III) propeptide assay reflects manifest fibrosis in carbon tetrachloride-treated rats. Anal Biochem. 1999;275:131-140. [PubMed] [DOI] |
| 44. | Goddard CJ, Smith A, Hoyland JA, Baird P, McMahon RF, Freemont AJ, Shomaf M, Haboubi NY, Warnes TW. Localisation and semiquantitative assessment of hepatic procollagen mRNA in primary biliary cirrhosis. Gut. 1998;43:433-440. [PubMed] [DOI] |
| 45. | Shimizu I, Mizobuchi Y, Yasuda M, Shiba M, Ma YR, Horie T, Liu F, Ito S. Inhibitory effect of oestradiol on activation of rat hepatic stellate cells in vivo and in vitro. Gut. 1999;44:127-136. [PubMed] [DOI] |
| 46. | Cho JJ, Lee YS. Enzyme-linked immunosorbent assay for serum procollagen type III peptide in rats with hepatic fibrosis. J Vet Med Sci. 1998;60:1213-1220. [PubMed] [DOI] |
| 47. | Kayano K, Sakaida I, Uchida K, Okita K. Inhibitory effects of the herbal medicine Sho-saiko-to (TJ-9) on cell proliferation and procollagen gene expressions in cultured rat hepatic stellate cells. J Hepatol. 1998;29:642-649. [PubMed] [DOI] |
| 48. | Yang H, Chen Y, Xu R, Shen W, Chen G. Clinical observation on the long-term therapeutic effects of traditional Chinese medicine for treatment of liver fibrosis. J Tradit Chin Med. 2000;20:247-250. [PubMed] |
| 49. | Sun YF, Tao XX, Jiang SL. Treatment of Chinese medicine in liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:686-687. [DOI] |
| 50. | Yao X, Yao XX, Xiu HM, Gao JP, Zhang YZ. Blood-activating and stasis-eliminating effects of concentrated Yigan decoction of Chinese herb on hepatic fibrosis in rats. Shijie Huaren Xiaohua Zazhi. 2002;10:544-548. [DOI] |
| 51. | Yang Q, Yan YC, Gao YX. Inhibitory effect of Quxianruangan Capsulae on l iver fibrosis in rats and chronic hepatitis atients. Shijie Huaren Xiaohua Zazhi. 2001;9:1246-1249. [DOI] |
| 52. | Liu YK, Shen W. Effect of Cordyceps sinensis on hepatocytic proliferation of experimental hepatic fibrosis in rats. Shijie Huaren Xiaohua Zazhi. 2002;10:388-391. [DOI] |
| 53. | Yao YX, Tang YW, Yao DM, Xiu HM. Effect of Yigan Decoction on the expression of type Ⅰ、Ⅲ collagen proteins in experimental hepatic fibrosis in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:263-267. [DOI] |
| 54. | Shen BS, Wong XG, Qiao HC, Lu CH, Han Q, Wang W, Guan HL. Preventive effect of Sanjia Yigan granule in hepatofibrosis in rats. Huaren Xiaohua Zazhi. 1998;6:386-388 10.3748/wjg.v11.i47.7413. [DOI] |
| 55. | Sun YF, Yao XX, Jiang SL. Therapeutic effects of traditional Chinese medicine for treatment of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:686-687. [DOI] |