Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.1920
Revised: November 5, 2002
Accepted: November 16, 2002
Published online: September 15, 2003
AIM: Using a monoclonal antibody against gastric cancer antigen named MGb1 to screen a phage-displayed random peptide library fused with coat protein pIII in order to get some information on mimotopes.
METHODS: Through affinity enrichment and ELISA screening, positive clones of phages were amplified. 10 phage clones were selected after three rounds of biopanning and the ability of specific binding of the positive phage clones to MGb1-Ab were detected by ELISA assay (DNA sequencing was performed and the amino acid sequences were deduced) By blocking test, specificity of the mimic phage epitopes was identified.
RESULTS: There were approximately 200 times of enrichment about the titer of bound phages after three rounds of biopanning procedures. DNA of 10 phage clones after the third biopanning was assayed and the result showed that the positive clones had a specific binding activity to MGb1-Ab and a weak ability of binding to control mAb or to mouse IgG. DNA sequencing of 10 phage clones was performed and the amino acid sequences were deduced. According to the homology of the amino acid sequences of the displayed peptides, most of the phage clones had motifs of H(x)Q or L(x)S. And these 10 phage clones could also partly inhibit the binding of MGb1-Ab to gastric cancer cell KATO-III. The percentage of blocking was from (21.0 ± 1.6)% to (39.0 ± 2.7)%.
CONCLUSION: Motifs of H(x)Q and L(x)S selected and identified show a high homology in the mimic epitopes of gastric cancer associated antigen. There may be one or more clones which can act as candidates of tumor vaccines.
- Citation: Han ZY, Wu KC, He FT, Han QL, Nie YZ, Han Y, Liu XN, Zheng JY, Xu MH, Lin T, Fan DM. Screening and identification of mimotope of gastric cancer associated antigen MGb1-Ag. World J Gastroenterol 2003; 9(9): 1920-1924
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/1920.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.1920
Filamentous bacteriophages have been used extensively in recent years for the display large repertoires of peptides on their surface[1-3]. These peptides can be expressed by cloning random oligonucleotides at the end of the genes encoding the phage coat proteins[4]. Phage display system has many advantages such as efficacy of biopanning, ability of amplification and linkage of the displayed peptide with its encoding DNA sequence within the phage particle, enabling quick and simple elucidation of binding sequences. Numerous papers have shown that this approach is successful with an extraordinarily wide range of protein targets, such as structural proteins, signal transduction proteins, receptors, serum proteins, oncoproteins and so on. It is also possible to select phage display peptides that mimic the original characteristics of antigen binding to specific antibodies without previous knowledge of the antigen structure[5-9]. In this respect, phage display technology has been well established as an important experimental approach in the development of novel vaccines and drugs. Antigens and epitopes play important roles in immune responses to tumors. To select the possible mimotopes of gastric cancer, a random phage display peptide library constructed on pIII was screened by biopanning with MGb1-Ab, a monoclonal antibody against gastric cancer, as a selective molecule.
Monoclonal antibody The hybridoma cell line which secretes mouse mAb MGb1 (its immunoglobulin type is IgG) against gastric carcinoma-associated antigens was established by our laboratory. This hybridoma was prepared by taking human gastric carcinoma cell line KATOIII as antigen, and the immunized mouse spleen cells were fused with SP2/0 according to routine procedures for mAb preparation[10-15]. The mAb in ascitic fluid was prepared by intraperitoneal injection of hybridoma cells into Balb/c mouse and collected at the aseptic environment. Then the mAb was purified by saturated ammonium sulfate precipitation and DEAE-52 anion exchange chromatography[16,17].
Random peptide library and bacteria The library used was purchased from New England Biolabs Company, containing approximately 2.8 × 109 different phage clones, which composed of the genome of the filamentous phage. The phages in this library were engineered to express a recombinant form of gene III containing a degenerated DNA insert encoding random 7-mer peptide. The recombinant gene III was under the control of LacZ promoter. And all the sub-major coat proteins pIII were recombinants. The ER2537 strain of E. coli was used for culture of phage. Bacteria were cultured in LB medium without any antibiotics.
Amplification of phages Infections were carried out by incubating phages for 10-15 min at room temperature with ER2537 at the number ratio of 3-5:1(bacteria: phages). And the bacteria were cultured for another 4.5 h at 37 °C. The supernatants from the culture where phages existed were collected. The culture supernatants of infected bacteria were collected, where bacteria secreted phages. The solution of 200 g·L-1 PEG 2.5 mol·L-1 NaCl was added into the supernatant at the volume ratio to supernatant of 1:5. And the mixture was incubated for 1 h at 4 °C. After centrifuged at 10000 g, the precipitated phages were pelleted, and then resuspended in Tris-buffered saline (TBS). The protocols of PEG precipitation were repeated. Phages from a culture supernatant volume of 5 mL were usually resuspended in a final volume of 150 μL TBS. The titer of the phages was then assayed.
Assay of phage titer The prepared phages were 10-fold serial diluted in LB with the suggested dilution ranges: 108-1011 for amplified phage culture supernatants and101-104 for unamplified biopanning eluates. A single colony of ER2537 in 5 mL LB was cultured with shaking until mid-log phase. 200 μL of bacteria culture reached mid-log phase was dispensed into microfuge tubes and then 10 μL of diluted phages per tube was vortexed quickly and incubated at room temperature for 1-5 min. Bacteria infected with phages were transfered to a culture tube containing 3 mL 45 °C LB/agarose at the top and immediately poured onto a pre-warmed LB/IPTG/X-gal plate and spread evenly after vortexed. Plates were cooled for 5 min, inverted and incubated overnight at 37 °C. Plaques on plates were counted.
Biopanning Three rounds of biopanning were carried out with mAb-MGb1. During the first round, a ELISA well was used as the solid phage. It was coated by incubating overnight with aliquots of mAb (100 mg·L-1) in the coating buffer (carbonate-bicarbonate buffer, pH9.6) at 4 °C in a humid atmosphere, then washed with TBS for 6 times, blocked in TBS-10 g·L-1 BSA. One aliquot of the library containing 2 × 1011 pfu phages in 100 μL TBS-0.5 g·L-1 BSA was added to the mAb coated well, incubated for 1 h at 37 °C. Unbound phages were removed and the well was washed 6 times with TBS-1 g·L-1 Tween-20. Eluting buffer (0.2 mol·L-1 glycine with its pH adjusted to 2.2 by HCl) was added for 10 min, then removed and neutralized by adding of Tris-HCl (pH9.0). Phages eluted from each round were used to infect exponential phase ER2537. After cultured for 4.5 h, phages in the supernatant were precipitated by PEG. The second and third rounds of biopanning were followed as described above except that unbound phages were washed 20 times with TBS-0.5% Tween-20. After neutralization in the third round of biopanning, the eluted phages were planted on the LB medium directly and cultured overnight at 37 °C.
ELISA to identify positive phage clones ELISA was carried out as routine procedures[18-26]. ELISA wells were coated by incubating overnight with mAb-MGb1 (100 mg·L-1) in the coating buffer (carbonate-bicarbonate buffer, pH9.6) at 4 °C in a humid atmosphere, washed 6 times with TBS, blocked with TBS-50 g·L-1 BSA. Single clone of phage expressing the recombinant form of gene III containing the peptide insert was incubated in LB for 4.5 h. The coated wells were incubated for 1h at 37 °C with supernatant from such cultures and then the horseradish peroxidase (HRP) labeled mouse-anti-M13 antibody diluted to a volume ratio of 1:5000 in blocking buffer was added. TMB was used as a substrate for HRP and the absorbance of each well was read at 450 nm.
Extraction of single strand DNA of phages and DNA sequencing Single strand DNA was prepared from 1.5 mL overnight cultures by extraction and purification by using a single strand of M13 extraction and purification kit (Huashun Bio. Com., China). The culture supernatant of infected bacteria containing phages at the volume of 1.2 mL was transferred to sterile 1.5 mL Eppendorf tubes. 200 μL precipitate buffer was added. The mixture was cooled on ice for 15 min. After centrifugation for 5 min at 10000 g, the supernatant was discarded and the precipitate was preserved for DNA extraction. Phages were lysed in 500 μL lysis buffer for 2 min at room temperature. The product was transfered to a column containing resin that could absorb DNA. After centrifugation for 15 s, DNA absorbed in resin was washed twice with ethanol and eluted with 20 μL Tris buffer. Single strand DNA of phages was extracted and 5 μL of product was run on a 10 g·L-1 agarose gel in 1 × TAE. DNA bands were detected by ethidium bromide staining and visualized by UV light photography. Sequencing procedures were carried out by using a auto-sequence apparatus ABI PRISMTM310 (PE Com. U.S.A.). 100 ng of such single strand DNA was used as a template. The oligonucleotide 5’-CCCTCATAGTTAGCGTAACG-3’, complementary to the genomic DNA of the phage and the downstream of the insert, was used as a primer.
Blocking test ELISA wells were coated with KATOIII (1 × 105 cells per well), washed 6 times with PBS, blocked for 2 h. MAb MGb1 was mixed with 1 × 1010 pfu individual phages for 1 h at 37 °C and then the mixture was added into the KATOIII coated wells, incubated for another 1 h. The HRP labeled goat-anti-mouse IgG diluted to a volume ratio of 1:5000 in the blocking buffer was added then. TMB was used as a substrate for HRP and the absorbance of each well was read at 450 nm[27].
The ratios of output/input phages in three biopanning rounds are listed in Table 1. The ratio of the last round was about 100 times higher than that of the first round. It indicated that the specific phages were enriched.
| Round | Input phages (pfu) | Output phages (pfu) | Yields/% |
| 1 | 2.0 × 1011 | 5.8 × 104 | 2.9 × 10-5 |
| 2 | 2.0 × 1011 | 3.2 × 105 | 1.6 × 10-4 |
| 3 | 2.0 × 1011 | 4.0 × 106 | 2.0 × 10-3 |
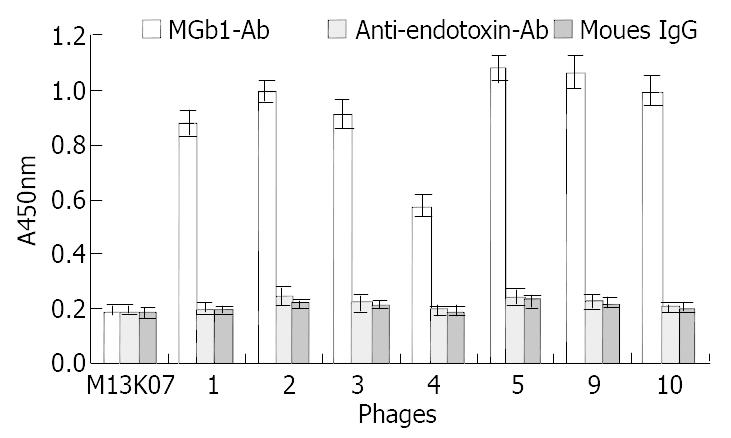
MGb1 mAb with specificity to gastric carcinoma was used to screen the library of phages containing random 7-mer peptide inserts. After three rounds of biopanning, some individual phage clones were isolated and screened by ELISA to identify those of interests which bound strongly to the antibody, and 10 clones of them which gave a clear positive signal and had the highest absorbance at 450 nm were selected.
MGb1 mAb was immobilized on the ELISA wells in which the selected phage clones were added. HRP labeled anti-M13 mAb was then used to detect the captured phages. As negative control, anti-endotoxin mAb and mouse IgG were used to replace the coating and the binding antibodies. The results revealed that the selected phages could react to mAb MGb1 specifically, and had weak ability of binding to unrelated antibodies such as anti-endotoxin mAb and mouse IgG (Figure 1).
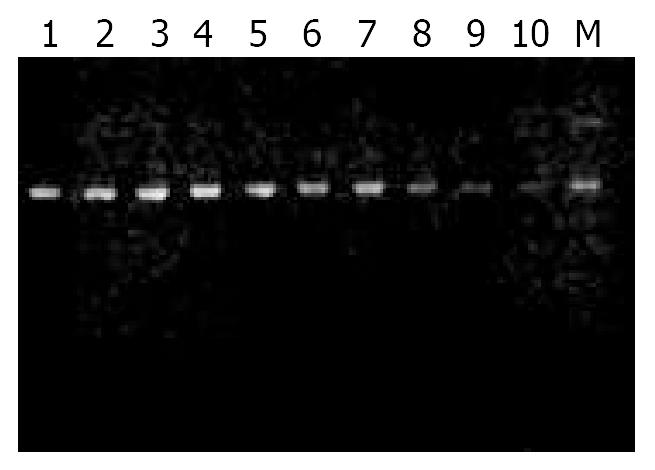
The length of all phage clones was the same as M13 single strand DNA marker, which indicated that single strand DNA of phages was prepared well and there were no other kinds of phages contaminated (Figure 2).
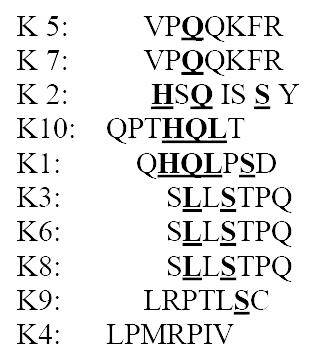
The selected 10 clones were sequenced and motifs could be identified amongst the deduced amino sequences of phage clone inserts for mAb MGb1. According to the homology of amino acid sequences of the displayed peptides, some preserved mimotope information was obtained. Most phage clones had motifs of H(x)Q and L(x)S (Figure 3).
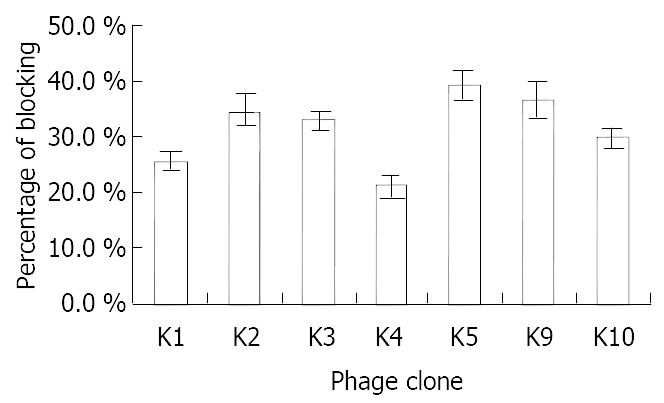
If the selected clones contained epitopes or mimotopes of the native antigen, then they should be presented to block the binding of mAb MGb1 to gastric cancer cell-line KATOIII. To test this hypothesis, 7 of representative phage clones were used in a blocking test. Wild-type phage M13 was used as a negative control (whose absorbance at 450 nm was represented by B) to ensure that blocking of binding of the mAb to gastric cancer cell-line KATOIII was due to the phage insert (whose absorbance at 450 nm was represented by A) rather than the mere physical presence of phages. The blocking percentage of each phage was (25.6 ± 1.3)%, (34.6 ± 2.5)%, (32.6 ± 1.8)%, (21.0 ± 1.6)%, (39.0 ± 2.7)%, (36.3 ± 3.2)%, (29.5 ± 1.9)%, respectively (Figure 4), compared with wells to which no phage was added (whose absorbance at 450 nm was represented by C), which was calculated using the formula: blocking% = [(C - B) - (C - A)]/A× 100%.
Gastric cancer is one kind of malignant tumors with complicated mechanism and is the leading cause of death in China[27-51]. Biotherapy is a new way for human to combat gastric cancer[52-63]. Tumor antigens play important roles in the induction of immune responses[64-66], which are predominantly focused on specific antigenic sites, known as epitopes. Identification of B-cell epitopes can be used to design peptide vaccines against tumor and is actively pursued in many laboratories. Since antibodies recognize B-cell epitopes that are mainly located on the surface of molecules, the native conformation of the antigen is a critical parameter for this interaction to occur. Early studies on the antigenicity of globular proteins suggested that antibodies recognized amino acids which either constituted a short linear stretch along the polypeptide chain (continuous or linear B-cell epitopes), or were brought together by the juxtaposition of the polypeptide chains when the protein was in its native conformation (discontinuous or conformational B-cell epitopes)[67].
Considerable attention has been paid to the difficulties in identifying the structural characteristics of B-cell epitopes. A number of empirical approaches have been employed to predict B-cell epitopes from the primary amino acid sequence of the protein including identification of regions of hydrophilicity, solvent accessibility, protrusion, atomic mobility and secondary structures. But many of them identify linear B-cell epitopes instead of discontinuous epitopes. B-cell epitopes are entities that can be defined only by their mutual complementarity. Phage display random peptide library offers a convenient way to select phage display peptides that mimic the original characteristics of antigen binding to specific antibodies[68,69]. One of the advantages of screening random peptide libraries to identify B-cell epitopes is that there is no need to know the amino acid sequence of the protein against which the antibody has been elicited. Since peptide libraries provide a rich source of mimic antigens, identification of B-cell epitopes has been greatly simplified.
In the present study, to select the possible mimotopes of gastric cancer, a random phage display peptide library constructed on pIII was screened by biopanning using MGb1-Ab as a selective molecule. After three biopanning procedures, there was a remarkable enrichment in the titer of bound phages. Then, 10 phage clones were selected from the third biopanning and ELISA. Their single strand DNA was sequenced and the amino acid sequences were deduced. According to the homology of amino acid sequences of the displayed peptides, some preserved epitope information was obtained. Most phage clones had motifs of H(X)Q or L(X)S. The results of ELISA showed that the positive phage clones had a specific binding activity with MGb1-Ab and could inhibit the binding of MGb1-Ab to gastric cancer cell line KATO-III.
Since the binding site of an antibody is not unique for a single antigen, several mimotopes with different amino acid sequences can be recognized by binding to different subsites within the binding site[70-72]. To date, little is known about the structure and amino acid sequence of parental antigen MGb1, so we can not tell the exact characteristics of the peptides selected from the 7-mer random peptide library. Research should be done to further characterize the mimotopes recognized by mAb MGb1.
Edited by Xu XQ and Wang XL
| 1. | Li R, Hoess RH, Bennett JS, DeGrado WF. Use of phage display to probe the evolution of binding specificity and affinity in integrins. Protein Eng. 2003;16:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Korpimäki T, Rosenberg J, Virtanen P, Lamminmäki U, Tuomola M, Saviranta P. Further improvement of broad specificity hapten recognition with protein engineering. Protein Eng. 2003;16:37-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Hsiao KC, Brissette RE, Wang P, Fletcher PW, Rodriguez V, Lennick M, Blume AJ, Goldstein NI. Peptides identify multiple hotspots within the ligand binding domain of the TNF receptor 2. Proteome Sci. 2003;1:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Willats WG. Phage display: practicalities and prospects. Plant Mol Biol. 2002;50:837-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, Walsh K, Fiore S, Koch AE, Firestein GS. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunol. 2003;170:838-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Baker AH. Development and use of gene transfer for treatment of cardiovascular disease. J Card Surg. 2002;17:543-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO. Biomimetic synthesis and patterning of silver nanoparticles. Nat Mater. 2002;1:169-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 890] [Cited by in F6Publishing: 648] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 8. | Pichurin PN, Guo J, Estienne V, Carayon P, Ruf J, Rapoport B, McLachlan SM. Evidence that the complement control protein-epidermal growth factor-like domain of thyroid peroxidase lies on the fringe of the immunodominant region recognized by autoantibodies. Thyroid. 2002;12:1085-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Taylor PC. Anti-TNFalpha therapy for rheumatoid arthritis: an update. Intern Med. 2003;42:15-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Yang LJ, Wang WL. Preparation of monoclonal antibody against apoptosis-associated antigens of hepatoma cells by subtractive immunization. World J Gastroenterol. 2002;8:808-814. [PubMed] [Cited in This Article: ] |
| 11. | Ji Y, Ling MY, Li Y, Xie H. Effect of cell fusion on metastatic ability of mouse hepatocarcinoma cell lines. World J Gastroenterol. 1999;5:22-24. [PubMed] [Cited in This Article: ] |
| 12. | Liu JW, Li KZ. Pancreatic cancer, oncogene and anti oncogene. Shijie Huaren Xiaohua Zazhi. 2001;9:72-73. [Cited in This Article: ] |
| 13. | Xu HY, Song JD. Application of TAA-LEA in the diagnosis of precancer and early stage cancer of colon. Shijie Huaren Xiaohua Zazhi. 1999;7:992. [Cited in This Article: ] |
| 14. | Shi YQ, Xiao B, Miao JY, Li MF, Qiao TD, Chen BJ, Chen Z, Han JL, Zhou SJ, Fan DM. A novel cDNA fragment associated with gastric cancer drug resistance screened from a library by mAb MGr1. Huaren Xiaohua Zazhi. 1998;6:656-659. [Cited in This Article: ] |
| 15. | Chen ZN, Bian HJ, Jiang JL. Kecent progress in anti-hepatoma monoclonal antibody and its application. Huaren Xiaohua Zazhi. 1998;6:461-462. [Cited in This Article: ] |
| 16. | Si XH, Yang LJ. Extraction and purification of TGFbeta and its effect on the induction of apoptosis of hepatocytes. World J Gastroenterol. 2001;7:527-531. [PubMed] [Cited in This Article: ] |
| 17. | Sun K, Jin BQ, Feng Q, Zhu Y, Yang K, Liu XS, Dong BQ. Identification of CD226 ligand on colo205 cell surface. World J Gastroenterol. 2002;8:108-113. [PubMed] [Cited in This Article: ] |
| 18. | Zheng PY, Hua J, Ng HC, Yeoh KG, Bow H. Expression of Lewis(b) blood group antigen in Helicobacter pylori does not interfere with bacterial adhesion property. World J Gastroenterol. 2003;9:122-124. [PubMed] [Cited in This Article: ] |
| 19. | Du DW, Jia ZS, Li GY, Zhou YY. HBV DNA vaccine with adjuvant cytokines induced specific immune responses against HBV infection. World J Gastroenterol. 2003;9:108-111. [PubMed] [Cited in This Article: ] |
| 20. | Tang NH, Chen YL, Wang XQ, Li XJ, Yin FZ, Wang XZ. Construction of IL-2 gene-modified human hepatocyte and its cultivation with microcarrier. World J Gastroenterol. 2003;9:79-83. [PubMed] [Cited in This Article: ] |
| 21. | Shi M, Wang FS, Wu ZZ. Synergetic anticancer effect of combined quercetin and recombinant adenoviral vector expressing human wild-type p53, GM-CSF and B7-1 genes on hepatocellular carcinoma cells in vitro. World J Gastroenterol. 2003;9:73-78. [PubMed] [Cited in This Article: ] |
| 22. | Wang KX, Li CP, Wang J, Tian Y. Cyclospore cayetanensis in Anhui, China. World J Gastroenterol. 2002;8:1144-1148. [PubMed] [Cited in This Article: ] |
| 23. | Zhao CY, Liu JX, Tang HH, Feng ZJ, Zhen Z, Zhang SH. Significance of IL-2 and related indexes in patients with hepatitis and hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:479-481. [Cited in This Article: ] |
| 24. | Ren JM, Zou QM, Wang FK, He Q, Chen W, Zen WK. PELA microspheres loaded H. pylori lysates and their mucosal immune response. World J Gastroenterol. 2002;8:1098-1102. [PubMed] [Cited in This Article: ] |
| 25. | Jiao XY, Shi JS, Ren H, Chen WK, Pan ML, Chang DM, He JJ, Hao XY, Zhou LS, Han Y. Effects of radical cholecystectomy on nutritional and immune status of patients with gallbladder carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:394-396. [Cited in This Article: ] |
| 26. | Wu YD, Song XQ, Zhou DN, Hu XH, Gan YQ, Li ZG, Liao P. Expermental and clinical study on targeting treatment of liver cancer using radionuclide anti AFP antibody MMC double bomb. Shijie Huaren Xiaohua Zazhi. 1999;7:387-390. [Cited in This Article: ] |
| 27. | Liu HJ, Guo XL, Dong M, Wang L, Yuan Y. Association between pepsinogen C gene polymorphism and genetic predisposition to gastric cancer. World J Gastroenterol. 2003;9:50-53. [PubMed] [Cited in This Article: ] |
| 28. | Yin T, Ji XL, Shen MS. Relationship between lymph node sinuses with blood and lymphatic metastasis of gastric cancer. World J Gastroenterol. 2003;9:40-43. [PubMed] [Cited in This Article: ] |
| 29. | Xin Y, Zhao FK, Zhang SM, Wu DY, Wang YP, Xu L. Relationship between CD44v6 expression and prognosis in gastric carcinoma patients. Shijie Huaren Xiaohua Zazhi. 1999;7:210-214. [Cited in This Article: ] |
| 30. | Liu HF, Liu WW, Fang DC, Men RF, Wang ZH. Apoptosis and its relationship with Fas ligand expression in gastric carcinoma and its precancerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:561-563. [Cited in This Article: ] |
| 31. | Cui DX, Yan XJ, Zhang L, Zhao JR, Jiang M, Guo YH, Zhang LX, Bai XP, Su CZ. Screening and its clinical significance of 6 fragments of highly expressing genes in gastric cancer and precancerous mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:770-772. [Cited in This Article: ] |
| 32. | Yang L, Kuang LG, Zheng HC, Li JY, Wu DY, Zhang SM, Xin Y. PTEN encoding product: a marker for tumorigenesis and progression of gastric carcinoma. World J Gastroenterol. 2003;9:35-39. [PubMed] [Cited in This Article: ] |
| 33. | Wang GS, Wang MW, Wu BY, You WD, Yang XY. A novel gene, GCRG224, is differentially expressed in human gastric mucosa. World J Gastroenterol. 2003;9:30-34. [PubMed] [Cited in This Article: ] |
| 34. | Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103-1107. [PubMed] [Cited in This Article: ] |
| 35. | Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, Chen JP, Wang L, Wang CH, Chen HY, Li YP. Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol. 2002;8:1023-1028. [PubMed] [Cited in This Article: ] |
| 36. | Fu QG, Meng FD, Shen XD, Guo RX. Efficacy of intraperitoneal thermochemotherapy and immunotherapy in intraperitoneal recurrence after gastrointestinal cancer resection. World J Gastroenterol. 2002;8:1019-1022. [PubMed] [Cited in This Article: ] |
| 37. | Chen Y, Wu Q, Song SY, Su WJ. Activation of JNK by TPA promotes apoptosis via PKC pathway in gastric cancer cells. World J Gastroenterol. 2002;8:1014-1018. [PubMed] [Cited in This Article: ] |
| 38. | Guo DL, Dong M, Wang L, Sun LP, Yuan Y. Expression of gastric cancer-associated MG7 antigen in gastric cancer, precancerous lesions and H. pylori -associated gastric diseases. World J Gastroenterol. 2002;8:1009-1013. [PubMed] [Cited in This Article: ] |
| 39. | Zhang FX, Deng ZY, Zhang XY, Kang SC, Wang Y, Yu XL, Wang H, Bian XH. Telomeric length associated with prognosis in human primary and metastatic gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:153-15. [Cited in This Article: ] |
| 40. | Zhou LY, Chen CY, Liang P, Chen LY. ICAM-1 and VCAM-1 expressions on benign gastric mucosa and gastric adenocarcinoma associated with Helicobacter pylori infection. Shijie Huaren Xiaohua Zazhi. 2000;8:279-281. [Cited in This Article: ] |
| 41. | Liu HF, Liu WW, Fang DC, Yang SM, Wang RQ. Bax gene expression and its relationship with apoptosis in human gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:665-668. [Cited in This Article: ] |
| 42. | Wang B, Shi LC, Zhang WB, Xiao CM, Wu JF, Dong YM. Expression and significance of p16 gene in gastric cancer and its precancerous lesions.. Shijie Huaren Xiaohua Zazhi. 2001;9:39-42. [Cited in This Article: ] |
| 43. | Ning XX, Wu KC, Shi YQ, Wang X, Zhao YQ, Fan DM. Construction and expression of gastric cancer MG7 mimic epitope fused to heat shock protein 70. Shijie Huaren Xiaohua Zazhi. 2001;9:892-896. [Cited in This Article: ] |
| 44. | Jiang YA, Zhang YY, Luo HS, Xing SF. Mast cell density and the context of clinicopathological parameters and expression of p185, estrogen receptor, and proliferating cell nuclear antigen in gastric carcinoma. World J Gastroenterol. 2002;8:1005-1008. [PubMed] [Cited in This Article: ] |
| 45. | Liu JR, Chen BQ, Yang YM, Wang XL, Xue YB, Zheng YM, Liu RH. Effect of apoptosis on gastric adenocarcinoma cell line SGC-7901 induced by cis-9, trans-11-conjugated linoleic acid. World J Gastroenterol. 2002;8:999-1004. [PubMed] [Cited in This Article: ] |
| 46. | Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994-998. [PubMed] [Cited in This Article: ] |
| 47. | Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987-993. [PubMed] [Cited in This Article: ] |
| 48. | Wu K, Li Y, Zhao Y, Shan YJ, Xia W, Yu WP, Zhao L. Roles of Fas signaling pathway in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:982-986. [PubMed] [Cited in This Article: ] |
| 49. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] [Cited in This Article: ] |
| 50. | Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. 2002;8:787-791. [PubMed] [Cited in This Article: ] |
| 51. | Zhao Y, Wu K, Xia W, Shan YJ, Wu LJ, Yu WP. The effects of vitamin E succinate on the expression of c-jun gene and protein in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:782-786. [PubMed] [Cited in This Article: ] |
| 52. | Li N, Xu CP, Song B, Liu WW, Wang X, Zhang CS, Xu YJ, Feng DX. Studies on the anti invasive character of TIMP2 gene transfected gastric carcinoma cells. Huaren Xiaohua Zazhi. 1998;6:663-666. [Cited in This Article: ] |
| 53. | Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of fas gene or bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. Huaren Xiaohua Zazhi. 1998;6:675-679. [Cited in This Article: ] |
| 54. | Shi YQ, Xiao B, Miao JY, Zhao YQ, You H, Fan DM. Construction of eukaryotic expression vector pBK fas and MDR reversal test of drug resistant gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:309-312. [Cited in This Article: ] |
| 55. | Pan X, Ke CW, Pan W, He X, Cao GW, Qi ZT. Killing effect of DT/VEGF system on gastric carcinoma cell. Shijie Huaren Xiaohua Zazhi. 2000;8:393-396. [Cited in This Article: ] |
| 56. | Guo JC, Li JC, Fan DM, Qiao TD, Zhang XY. Regulation of HSP70 expression in human gastric cancer cell line SGC7901 by gene transfection. Shijie Huaren Xiaohua Zazhi. 1999;7:773-776. [Cited in This Article: ] |
| 57. | Luo ZB, Luo YH, Lu R, Jin HY, Zhang BP, Xu CP. Immunohistochemical study on dendritic cells in gastric mucosa of patients with gastric cancer and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:400-402. [Cited in This Article: ] |
| 58. | Guo SY, Gu QL, Liu BY, Zhu ZG, Yin HR, Lin YZ. Experimental study on the treatment of gastric cancer by TK gene combined with mIL-2 gene. Shijie Huaren Xiaohua Zazhi. 2000;8:974-978. [Cited in This Article: ] |
| 59. | Liu DH, Zhang W, Su YP, Zhang XY, Huang YX. Constructions of eukaryotic expression vector of sense and antisense VEGF-165 and its expression regulation. Shijie Huaren Xiaohua Zazhi. 2001;9:886-891. [Cited in This Article: ] |
| 60. | Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421-425. [PubMed] [Cited in This Article: ] |
| 61. | Chen B, Zhang XY, Zhang YJ, Zhou P, Gu Y, Fan DM. Antisense to cyclin D1 reverses the transformed phenotype of human gastric cancer cells. World J Gastroenterol. 1999;5:18-21. [PubMed] [Cited in This Article: ] |
| 62. | Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430-432. [PubMed] [Cited in This Article: ] |
| 63. | Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6:664-670. [PubMed] [Cited in This Article: ] |
| 64. | Xiao LF, Luo LQ, Zou Y, Huang SL. Study of the phenotype of PBLs activated by CD28/CD80 and CD2/CD58 and acting with hepatoma cells and the restricted usage of TCR Vb gene subfamily. Shijie Huaren Xiaohua Zazhi. 1999;7:1044-1046. [Cited in This Article: ] |
| 65. | Zheng CX, Zhan WH, Zhao JZ, Zheng D, Wang DP, He YL, Zheng ZQ. The prognostic value of preoperative serum levels of CEA, CA19-9 and CA72-4 in patients with colorectal cancer. World J Gastroenterol. 2001;7:431-434. [PubMed] [Cited in This Article: ] |
| 66. | Li XW, Ding YQ, Cai JJ, Yang SQ, An LB, Qiao DF. Studies on mechanism of Sialy Lewis-X antigen in liver metastases of human colorectal carcinoma. World J Gastroenterol. 2001;7:425-430. [PubMed] [Cited in This Article: ] |
| 67. | Messmer BT, Sullivan JJ, Chiorazzi N, Rodman TC, Thaler DS. Two human neonatal IgM antibodies encoded by different variable-region genes bind the same linear peptide: evidence for a stereotyped repertoire of epitope recognition. J Immunol. 1999;162:2184-2192. [PubMed] [Cited in This Article: ] |
| 68. | Sanderson SD, Cheruku SR, Padmanilayam MP, Vennerstrom JL, Thiele GM, Palmatier MI, Bevins RA. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. Int Immunopharmacol. 2003;3:137-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Zhang WY, Wan Y, Li DG, Tang Y, Zhou W. A mimotope of pre-S2 region of surface antigen of viral hepatitis B screened by phage display. Cell Res. 2001;11:203-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Bracci L, Pini A, Lozzi L, Lelli B, Battestin P, Spreafico A, Bernini A, Niccolai N, Neri P. Mimicking the nicotinic receptor binding site by a single chain Fv selected by competitive panning from a synthetic phage library. J Neurochem. 2001;78:24-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Scherf T, Kasher R, Balass M, Fridkin M, Fuchs S, Katchalski-Katzir E. A beta -hairpin structure in a 13-mer peptide that binds alpha -bungarotoxin with high affinity and neutralizes its toxicity. Proc Natl Acad Sci USA. 2001;98:6629-6634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | MacDonald NJ, Shivers WY, Narum DL, Plum SM, Wingard JN, Fuhrmann SR, Liang H, Holland-Linn J, Chen DH, Sim BK. Endostatin binds tropomyosin. A potential modulator of the antitumor activity of endostatin. J Biol Chem. 2001;276:25190-25196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |