Published online Dec 15, 2003. doi: 10.3748/wjg.v9.i12.2737
Revised: May 12, 2003
Accepted: June 4, 2003
Published online: December 15, 2003
AIM: To investigate the hepatocellular apoptosis after hepatectomy in obstructive jaundice and biliary decompression rats.
METHODS: After bile duct ligation for 7 days, rats were randomly divided into OB group in which the rats underwent 70% hepatectomy, OB-CD group in which the rats underwent hepatectomy accompanied by choledochoduodenostomy, CD-Hx group in which the rats underwent choledochoduodenostomy and then received 70% hepatectomy on the fifth day after biliary decompression. The control group (Hx group) only underwent hepatectomy.
RESULTS: The level of total serum bilirubin and serum enzymes was significantly lower in CD-Hx group than in OB-CD and OB groups on day 1, 3 and 5 after hepatectomy. The apoptotic index was significantly lower in CD-Hx group than in OB-CD and OB groups on day 3 and 5. The oligonucleosomal DNA fragments and Caspase-3 activity were also lower in CD-Hx group than in OB-CD and OB groups 3 days after hepatectomy, without differences between CD-Hx and Hx groups.
CONCLUSION: Hepatocellular apoptosis plays vital roles in jaundice rats, and biliary decompression is more effective in treatment of patients with severe jaundice before operation.
- Citation: Wang DS, Dou KF, Li KZ, Gao ZQ, Song ZS, Liu ZC. Hepatocellular apoptosis after hepatectomy in obstructive jaundice in rats. World J Gastroenterol 2003; 9(12): 2737-2741
- URL: https://www.wjgnet.com/1007-9327/full/v9/i12/2737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i12.2737
Obstructive jaundice is often a clinical manifestation of the disease of extrahepatic biliary system or pancreas. In jaundiced patients with hilar bile duct carcinoma or gallbladder carcinoma, hepatectomy is one of the surgical regimens, but surgical procedures are associated with increases of morbidity and mortality rates, mainly due to postoperative complications such as hepatic failure, sepsis, bleeding and renal failure[1,2]. The significance of biliary drainage before surgery is controversial. Preoperative biliary drainage decreased the mortality and morbidity rates in patients with obstructive jaundice in some studies but not in others[3-5]. Experimental and clinical studies have identified several etiological factors including hypotension, impaired nutritional status, depressed immune function, hepatic dysfunction and the presence of toxic bile salts in circulation[6-8]. A high serum bilirubin concentration in jaundiced patients undergoing operation has been recognized as a predictor of mortality[3].
Hepatocyte injury and progression of liver disease are due to direct chemical damage to hepatocytes by toxic hydrophobic bile salts[9]. Although toxic bile salts are known to cause hepatocyte toxicity by inducing apoptosis, the precise mechanisms responsible for bile salt-mediated apoptosis remain to be established. This information is important because it could help provide rational strategies for the treatment of cholestatic liver disorders and decreasing postoperative complications. The aim of this study was to investigate the hepatocellular apoptosis after hepatectomy in obstructive jaundice and biliary decompression rats.
Male Wistar rats (Shionogi Aburabi Laboratory, Shiga, Japan) weighing 190-240 g were used for this study. Animals were housed in a controlled environment with a 12 h light/dark cycle. Rats had free access to normal rat chow and water before surgery. The care and handling of the animals were made in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The rats were divided into four groups each containing 6 animals. Hx group in which normal rats underwent 70% hepatectomy, OB group in which the rats underwent 70% hepatectomy after bile duct ligation for 7 days, OB-CD group in which the rats underwent 70% hepatectomy accompanied by choledochoduodenostomy after bile duct ligation for 7 days, CD-Hx group in which the rats underwent choledochoduodenostomy after bile duct ligation for 7 days and then received 70% hepatectomy on the fifth day after biliary decompression, as described below.
All surgical procedures were carried out under ether anesthesia. All rats were weighed before surgery and at the end of the study.
Laparotomy was performed through an upper midline incision. After ligation of the proximal and distal bile ducts, the common bile duct was divided to prevent recanalization[10]. The ligatures were placed in the same position in all rats. The 70% hepatectomy was done according to the method of Higgins and Anderson[11] on the seventh day after biliary obstruction in OB and OB-CD groups and on the fifth days after the biliary decompression in CD-Hx group.
Choledochoduodenostomy was performed according to the method of Ryan et al[12] with minor modifications. After biliary obstruction for 7 days, the abdomen was reopened through the previous incision. The dilated common hepatic bile duct was freed from its surrounding tissues and the contents were aspirated. A small incision was made in the bile duct and a silicone tube with an outer diameter of 0.9 mm was inserted and secured in position with a 7-0 silk suture. The free end of the tube was inserted into duodenum approximately 1.5 cm from the pylorus, and then tied in position with a 6-0 PDS purse-string suture. Then the suture was carried out between the bile duct and the duodenal wall to protect the separation of the anastomosis.
Blood samples were collected from the tail vein with a 27-gauge needle. Serum total bilirubin, activities of alkaline phosphatase (ALP) and aspartate aminotransferase (AST) levels were measured enzymatically using a commercial kit (Spotchem Co., Kyoto, Japan).
An aliquot from the liver homogenate was centrifuged at 13000×g. The supernatant was diluted 1000-fold and subjected to a sandwich ELISA designed to detect DNA/histone fragments using a test kit from Boehringer Mannheim, according to the manufacturer’s instructions. Briefly, a 96-well microtiter plate was coated with antibody against histones. Diluted samples were then applied to the wells for 90 min, followed by incubation with peroxidase-conjugated anti-DNA antibody for 90 min. Color substrate was added and absorbance was measured at 405 nm at 5, 10, and 15 min using a 96-well microtiter plate reader.
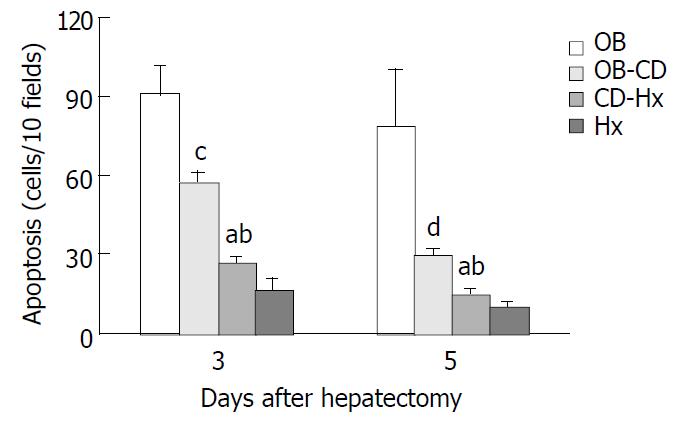
On the third and fifth day after hepatectomy, a section of liver tissue measuring approximately 5 mm in thickness was cut from the right lateral lobe and fixed in 10% buffered formalin, processed by standard techniques and embedded in paraffin. Apoptotic change in the liver was analyzed by in situ TUNEL method using an apoptosis detection kit (ApopTag, Oncor S7100, Gaithersburg, MD) according to the manufacturer’s instructions. Briefly, after deparaffinization and rehydration, 4 µm thick tissue sections were incubated in PBS, and then preincubated with equilibration buffer for 15 min, and digoxigenin-labelled dUTP and dATP in TdT buffer were added to cover the section, which was then incubated in a humidified chamber at 37 °C for 60 min. The reaction was stopped by immersing the slides in a stop/wash buffer. Following washing in PBS, the sections were incubated in anti-digoxigenin-peroxidase for 30 min at room temperature. The slides were incubated with DAB and hydrogen peroxide and counterstained with hematoxylin. Apoptotic cells were counted from at least 10 randomly chosen high-power fields at ×400 magnification. Cells with clear brown nuclear labelling were defined as TUNEL-positive. Apoptotic index was defined as the total number of apoptotic cells seen in every ten high-power field sections.
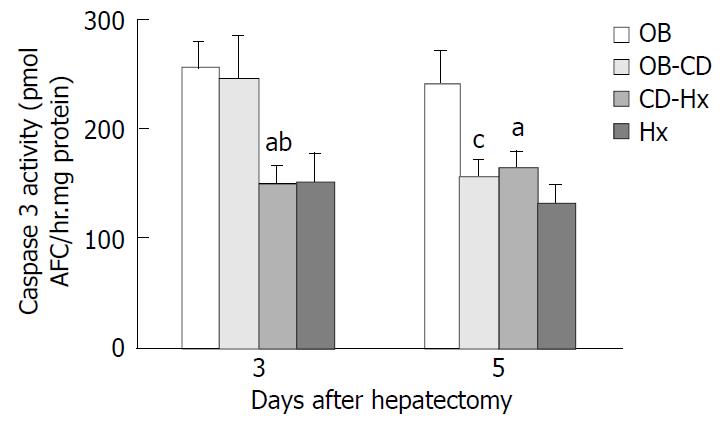
Liver tissues were immediately frozen by liquid nitrogen and stored at -80 °C on the third and fifth days after hepatectomy. The Caspase 3-like activity was measured using a commercially available kit (Clontech Laboratories, Palo Alto, CA) according to the manufacturer’s instructions. Caspase enzymatically cleaved the substrate and released free 7-amino-4-trifluoromethyl coumarin (AFC) that produced a blue-green fluorescence, which was measured fluorometrically. Liver samples were homogenized in 500 µl of lysis buffer. Samples were centrifuged at 16000×g in a microfuge for 10 min at 4 °C and normalized to protein using the Bradford assay (Bio-Rad). Fluorescent substrate, DEVD- AFC was incubated with 50 µl supernatants for liver extracts at 37 °C for 60 min. The fluorescence of cleaved substrates was measured using a fluorometer (Molecular Dynamics Inc.) at an excitation wavelength of 400 nm and an emission wavelength of 505 nm. An AFC calibration curve was established by serial dilution of a pure AFC solution provided in the kit.
All data were expressed as mean ± SEM. Statistical analysis was done by statistical package of SPSS for Window release 9.0. Differences between mean were tested with the Mann-Whitney test. A probability value less than 0.05 was considered statistically significant.
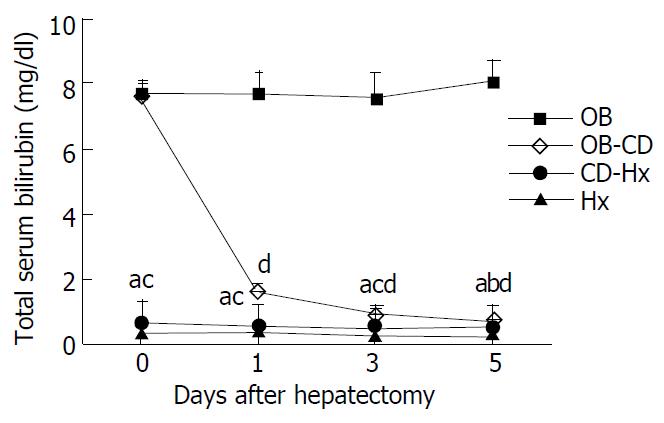
The level of total serum bilirubin reached more than 7 mg/dl after 7 days’ biliary obstruction, and the high bilirubin level rapidly decreased to 0.8 mg/dl only on day 1 after biliary decompression. We therefore thought that our models functioned well for biliary decompression. The total serum bilirubin concentration on the first day after hepatectomy in OB-CD group was 1.6 mg/dl, which was higher than that in CD-Hx group. The level of bilirubin in OB group was increased, while there was no statistically significant differences among other three groups on day 5 after hepatectomy (Figure 1).
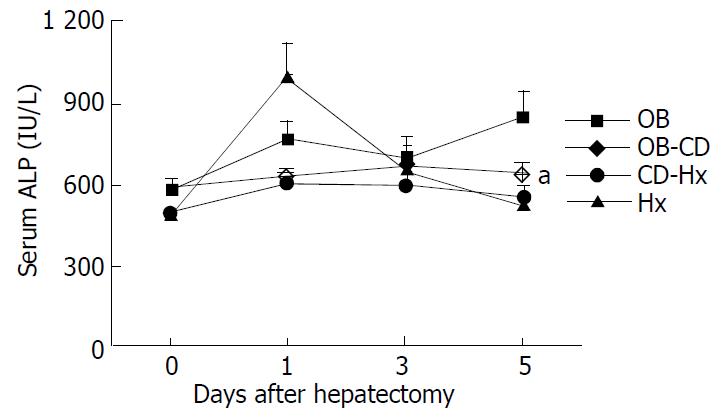
The ALP concentration of OB group was significantly higher than that of other groups, but no differences of serum ALP level were found among the other groups after hepatectomy (Figure 2).
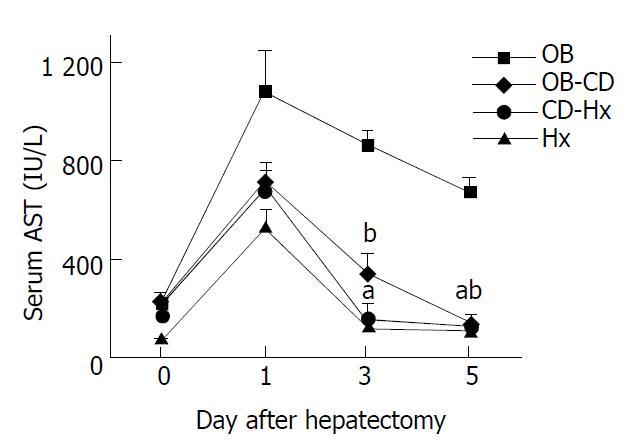
The level of serum AST peaked on the first day after hepatectomy and decreased gradually (Figure 3). There was no difference of AST concentration between CD-Hx and Hx groups on the third and fifth days after hepatectomy. The serum AST concentration in OB group on the fifth day was nearly 6 times that of the concentration in CD-Hx group.
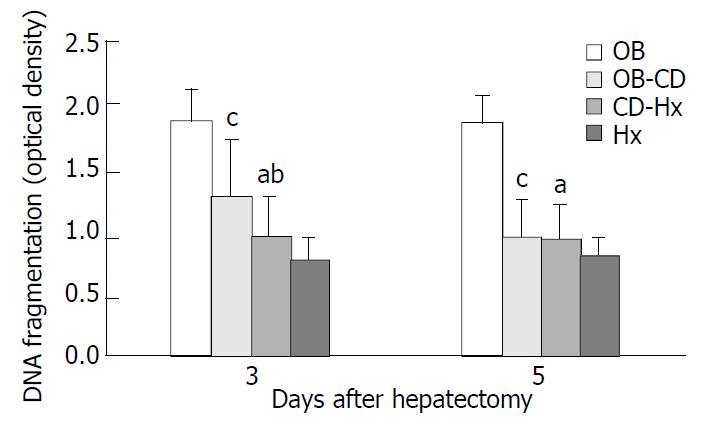
The cytoplasmic levels of DNA (histone-associated) oligonucleosomal fragments in hepatocytes after hepatectomy were determined. The presence of these fragments in the cytoplasm reflected the extent of DNA fragmentation and nuclear disruption that are characteristic of apoptosis. The level of DNA fragments in CD-Hx group was significantly lower than that in OB and OB-CD groups on the third day after hepatectomy (Figure 4). There was no significant difference between CD-Hx and OB groups.
TUNEL signal was defined by distinct nuclear staining. The number of hepatocellular apoptosis was high on day 3 and decreased on day 5 when the liver was harvested on days 3 and 5 from rats after hepatectomy. The data shown in Figure 5 indicate that apoptosis positive cells were significantly higher in OB group than in other groups. A very low rate of apoptosis was found in CD-Hx group, with no difference between CD-Hx and Hx groups.
Cleavage of peptide substrate DEVD-AFC was used as an indicator of Caspase-3-like protease activity. The level of Caspase-3 activity in CD-Hx group was significantly lower than that in OB and OB-CD groups on the third day after hepatectomy. But on day 5, the level of Caspase-3 activity in OB-CD group decreased rapidly, without differences between OB-CD and CD-Hx groups (Figure 6).
The perioperative mortality rate in patients with obstructive jaundice was reported to be 8% to 28%[13]. Perioperative complications in patients with obstructive jaundice included infections, endotoxemia, renal failure, stress ulceration, disseminated intravascular coagulation, and wound dehiscence[14,15]. Numerous reports suggested that in obstructive jaundice there was not only impairment of hepatocellular function but also depression of Kupffer cell activity and ultimately hepatic structural damage, leading to the development of portal hypertension[16,17]. In addition, it could result in depressed wound healing, significant portal and systemic endotoxaemia, decreased reticuloendothelial cell function, reduced T cell responses, depressed non-specific cell-mediated immunity, decreased bacterial clearance and increased bacterial translocation[18,19].
This animal model was designed to simulate the clinical treatment procedures used for patients with obstructive jaundice undergoing hepatectomy. Choledochoduodenal drainage was performed before or at the same time as 70% hepatectomy. Obstruction at the choledochoduodenostomy occurred in about 2% rats. These rats were excluded from further studies. The mortality rate was about 20% following choledochoduodenostomy or hepatectomy in jaundice rats. No rats died during the procedure and all deaths occurred in the first 3 days postoperatively, usually secondary to bleeding.
During obstructive jaundice, the retention of biliary constituents and high biliary pressure caused hepatocellular injury[20]. This is supported by the fact that serum AST and ALP levels increased during obstructive jaundice and decreased after choledochoduodenostomy (data not shown). Kanno[21] reported that biliary obstruction for even 5 days suppressed rat liver function, as assessed with aminopyrine breath test and galactose tolerance test. These results suggested that obstructive jaundice could cause serious impairment of pivotal liver functions, consequently resulting in poor prognosis after partial hepatectomy. The present study clearly showed that biliary drainage before hepatectomy significantly improved liver functions and decreased hepatocellular apoptosis. Serum liver function tests showed that choledochoduodenal drainage was effective in both OB-CD and CD-Hx groups. Furthermore, serum enzyme levels, apoptotic index, DNA fragments and Caspase-3 activity were significantly lower in CD-Hx group than in other groups. These data suggested that biliary drainage before hepatectomy had beneficial effects in severe jaundice patients.
Accumulation of these toxic bile salts within hepatocytes has been thought to play a key role in liver injury during obstructive jaundice. Indeed, hepatic levels of toxic bile salts chenodeoxycholate and deoxycholate correlated with the degree of liver damage[22]. Because widespread necrosis was not prominent in most cholestatic liver diseases, it has become apparent that the occurrence of hepatocyte death during cholestasis was rather due to apoptosis than due to necrosis[23]. The mechanisms of apoptosis by toxic bile salts could been partially elucidated in recent years. Toxic bile salts could directly cause apoptosis in cultured rodent hepatocytes[24]. Bile acid-induced toxicity is typically characterized by hepatocyte swelling, disruption of plasma membrane integrity, and release of intracellular constituents. The apoptotic pathway in hepatocytes could be initiated by a ligand-independent activation of Fas by bile salt[25]. Apoptosis was triggered by the Fas pathway, at least in part, by the activation of intracellular enzymes called Caspases. Caspases have been found to be cysteine-containing, aspartic acid-specific proteases which exist as zymogens in the soluble cytoplasm, mitochondrial intermembrane space, and nuclear matrix of virtually all cells[26]. Upon activation of the apoptotic pathway, initiator Caspases (i.e., Caspases 8 and 9) were converted to their active forms, which in turn activated downstream Caspase 3, which could activate other Caspases and ultimately cleave various substrates[27]. One of these substrates has been found to be a Caspase-dependent endonuclease that is freed from its inhibitor by Caspase-3 in the cytoplasm, and subsequently enters the nucleus, where it cuts DNA into oligonuclesomal (180 bp) fragments[28]. Additionally, Bid, a protein responsible for the release of mitochondrial cytochrome C and amplification of the apoptotic cascade, could also be activated by Caspases[29]. Ultimately, this pathway would ensure the self-destruction of cells with the characteristic morphological features of apoptosis, including nuclear fragmentation, cellular shrinkage, and acidophilic staining of the cytoplasm[30]. Bile acid induced apoptosis also appeared to require an activation and translocation of protein kinase C, which then could lead to an increase of intracellular magnesium[31,32]. This would cause an activation of Mg2+-dependent endonucleases, leading to DNA cleavage.
In conclusion, hepatocellular apoptosis plays a vital role in jaundice rats. Biliary drainage is preferable, as evidenced in rats by significantly better liver function and decrease of hepatocellular apoptosis. Biliary decompression is an effective preoperative treatment for patients with severe jaundice.
We would express our sincere thanks to Prof. Monden and Dr. Umeshita for their technical and financial support.
Supported in part by a Grant-in-aid for Cancer Research and Scientific Research From the Osaka University of Japan
Edited by Ma JY and Wang XL
| 1. | Su CH, Tsay SH, Wu CC, Shyr YM, King KL, Lee CH, Lui WY, Liu TJ, P'eng FK. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 209] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003;9:385-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 109] [Cited by in F6Publishing: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Dixon JM, Armstrong CP, Duffy SW, Davies GC. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut. 1983;24:845-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 194] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Cherqui D, Benoist S, Malassagne B, Humeres R, Rodriguez V, Fagniez PL. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135:302-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | McPherson GA, Benjamin IS, Hodgson HJ, Bowley NB, Allison DJ, Blumgart LH. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71:371-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 285] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Greve JW, Gouma DJ, Soeters PB, Buurman WA. Suppression of cellular immunity in obstructive jaundice is caused by endotoxins: a study with germ-free rats. Gastroenterology. 1990;98:478-485. [PubMed] [Cited in This Article: ] |
| 7. | Ito Y, Machen NW, Urbaschek R, McCuskey RS. Biliary obstruction exacerbates the hepatic microvascular inflammatory response to endotoxin. Shock. 2000;14:599-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Rodrigues CM, Steer CJ. Mitochondrial membrane perturbations in cholestasis. J Hepatol. 2000;32:135-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Lee E. The effect of obstructive jaundice on the migration of reticulo-endothelial cells and fibroblasts into early experimental granulomata. Br J Surg. 1972;59:875-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Fukuhara Y, Hirasawa A, Li XK, Kawasaki M, Fujino M, Funeshima N, Katsuma S, Shiojima S, Yamada M, Okuyama T. Gene expression profile in the regenerating rat liver after partial hepatectomy. J Hepatol. 2003;38:784-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Ryan CJ, Than T, Blumgart LH. Choledochoduodenostomy in the rat with obstructive jaundice. J Surg Res. 1977;23:321-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Friedman LS. The risk of surgery in patients with liver disease. Hepatology. 1999;29:1617-1623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Li XH, Peng Y. Liver sinusoidal endothelial cell injury by neutrophils in rats with acute obstructive cholangitis. World J Gastroenterol. 2002;8:342-345. [PubMed] [Cited in This Article: ] |
| 15. | Plusa S, Webster N, Primrose J. Obstructive jaundice causes reduced expression of polymorphonuclear leucocyte adhesion molecules and a depressed response to bacterial wall products in vitro. Gut. 1996;38:784-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Daglar GO, Kama NA, Atli M, Yuksek YN, Reis E, Doganay M, Dolapci M, Kologlu M. Effect of 5-lipoxygenase inhibition on Kupffer cell clearance capacity in obstructive jaundiced rats. J Surg Res. 2001;96:158-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Zimmermann H, Reichen J, Zimmermann A, Sägesser H, Thenisch B, Höflin F. Reversibility of secondary biliary fibrosis by biliodigestive anastomosis in the rat. Gastroenterology. 1992;103:579-589. [PubMed] [Cited in This Article: ] |
| 18. | Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Megison SM, Dunn CW, Horton JW, Chao H. Effects of relief of biliary obstruction on mononuclear phagocyte system function and cell mediated immunity. Br J Surg. 1991;78:568-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Trauner M, Meier PJ, Boyer JL. Molecular regulation of hepatocellular transport systems in cholestasis. J Hepatol. 1999;31:165-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Kanno Y, Miyazaki M, Udagawa I, Koshikawa H, Ito H, Teramoto O, Okui K. [Relation of hepatic protein synthesis and hepatic functional mass in obstructive jaundiced rats]. Nihon Geka Gakkai Zasshi. 1991;92:160-166. [PubMed] [Cited in This Article: ] |
| 22. | Schmucker DL, Ohta M, Kanai S, Sato Y, Kitani K. Hepatic injury induced by bile salts: correlation between biochemical and morphological events. Hepatology. 1990;12:1216-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 147] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Patel T, Gores GJ. Apoptosis and hepatobiliary disease. Hepatology. 1995;21:1725-1741. [PubMed] [Cited in This Article: ] |
| 24. | Takikawa Y, Miyoshi H, Rust C, Roberts P, Siegel R, Mandal PK, Millikan RE, Gores GJ. The bile acid-activated phosphatidylinositol 3-kinase pathway inhibits Fas apoptosis upstream of bid in rodent hepatocytes. Gastroenterology. 2001;120:1810-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 430] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Hatano E, Bennett BL, Manning AM, Qian T, Lemasters JJ, Brenner DA. NF-kappaB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis. Gastroenterology. 2001;120:1251-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Cong B, Li SJ, Yao YX, Zhu GJ, Ling YL. Effect of cholecystokinin octapeptide on tumor necrosis factor alpha transcription and nuclear factor-kappaB activity induced by lipopolysaccharide in rat pulmonary interstitial macrophages. World J Gastroenterol. 2002;8:718-723. [PubMed] [Cited in This Article: ] |
| 28. | Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1194] [Cited by in F6Publishing: 1180] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 29. | Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2665] [Cited by in F6Publishing: 2608] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 30. | Hoglen NC, Hirakawa BP, Fisher CD, Weeks S, Srinivasan A, Wong AM, Valentino KL, Tomaselli KJ, Bai X, Karanewsky DS. Characterization of the caspase inhibitor IDN-1965 in a model of apoptosis-associated liver injury. J Pharmacol Exp Ther. 2001;297:811-818. [PubMed] [Cited in This Article: ] |
| 31. | Liu XJ, Yang L, Wu HB, Qiang O, Huang MH, Wang YP. Apoptosis of rat hepatic stellate cells induced by anti-focal adhesion kinase antibody. World J Gastroenterol. 2002;8:734-738. [PubMed] [Cited in This Article: ] |
| 32. | Jones BA, Rao YP, Stravitz RT, Gores GJ. Bile salt-induced apoptosis of hepatocytes involves activation of protein kinase C. Am J Physiol. 1997;272:G1109-G1115. [PubMed] [Cited in This Article: ] |