Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2353
Revised: June 4, 2003
Accepted: June 11, 2003
Published online: October 15, 2003
AIM: To determine whether or not a low dose of HB vaccine can be effectively used in the rapid vaccination.
METHODS: Rapid vaccination (0, 1, 2 months) with low dose (5 μg) or routine dose (10 μg) HB vaccine was studied in 250 subjects (130 school children and 120 university students). Serum from all the participants was tested for HBsAg, anti-HBs and anti-HBc at 1, 3 and 7 months after the first dose of vaccination and all subjects were serum HBV marks negative before the vaccination. Non-responders to a complete initial vaccination from university students were given an additional vaccination with 10 μg of HB vaccine and their serum anti-HBs was tested again one month later.
RESULTS: One month after the third dose of vaccination (third month) sero-conversion rates and geometric mean titer (GMTs) were significantly (P < 0.01) higher in the routine dose (resp. 89% and 106.8) than in the low dose group (resp. 72% and 59.5). Sero-conversion rates and GMTs were maintained stable for another 4 months in both groups. After an additional vaccination to non-responders with 10 μg HB vaccine, 17/23 subjects (13/15 from those vaccinated with 5 μg vaccine and 4/8 from those vaccinated with 10 μg vaccine) became anti-HBs positive, yielding similar sero-conversion rates for both dose groups.
CONCLUSION: Higher sero-conversion rates and GMTs were reached in those vaccinated with 10 μg HB vaccine than in those vaccinated with 5 μg HB vaccine after a complete vaccination with a 0, 1, 2 month scheme. But the subjects vaccinated with 5 μg vaccine can also reach the similar sero-conversion rate after an additional vaccination.
- Citation: Wang RX, Boland G, Guo Y, Lei SP, Yang CH, Chen J, Tian J, Wen JY, Du KH, Hattum JV, Gast GC. Is a low dose of hepatitis B vaccine enough for a rapid vaccination scheme? World J Gastroenterol 2003; 9(10): 2353-2355
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2353
Although hepatitis B vaccine has been available for 20 years, hepatitis B nowadays remains prevalent in the world, especially in a majority of the developing countries. In China, 5 μg of vaccine (Merck) has been used in the HB vaccination for several years and acquired acceptable results. The long-term effectiveness of low-dose HB vaccine immunization in the infancy has been observed, but that in school children or adults remains to be determined. It is known that protective anti- HBs antibody titers can generally be reached in 80%-90% of individuals vaccinated with 10 μg vaccine according to a 0, 1, 6 month vaccination scheme. Considering of the relative length of the current HB vaccination scheme and the availability of low dose HB vaccine in the market, it seemed, therefore, worthwhile to test a routine dose (10 μg) vaccination in a rapid scheme (0, 1, 2 months) compared to a low dose (5 μg) vaccination. We here report that significantly higher sero-conversion rates and GMTs are reached in those vaccinated with a 10 μg dose vaccination than those vaccinated with 5 μg dose vaccination after 3 months from the initial dose. School children and university students showed no significant differences apart from a more rapid response in school children.
Two hundred and fifty subjects (130 school children aged 8-10 years old and 120 university students aged 18-20 years old) with negative HB marks (HBsAg, anti-HBs, anti- HBc) were included in the study. One hundred and thirty subjects (72 school children and 58 university students) nominated as low dose group and were vaccinated with 5 μg HB vaccine, 120 subjects (55 school children and 65 university students) named as routine dose group received 10 μg HB vaccine (5 μg dose x 2) respectively according to a 0, 1, 2 month vaccination scheme. All non-responding university students after a complete initial vaccination series were given a fourth dose with 10 μg HB vaccine at the 8th month. All injections were given intramuscularly at the site of deltoid muscle. The HB vaccine used in this study is 5 μg yeast recombinant HB vaccine (Lot no: 2990104-1, from Merck), provided by Kangtai Biological Pharmaceutical Company, China.
Blood samples that were collected from all vaccinees for three times performed were used to detect serum anti-HBs (Ausab EIA, No 642841 M401) at month 1, 3 and 7 after the first dose of vaccination respectively. The third month serum specimen from all non responders after the initial vaccination was tested for HBsAg and anti-HBc by ELISA (Lizon Kit, China) in order to make sure that they are real no-responders to HB vaccine and not HBV carriers. Those with negative tests received a fourth dose of vaccine at 8th month after vaccination and were again tested for serum anti- HBs one month later.
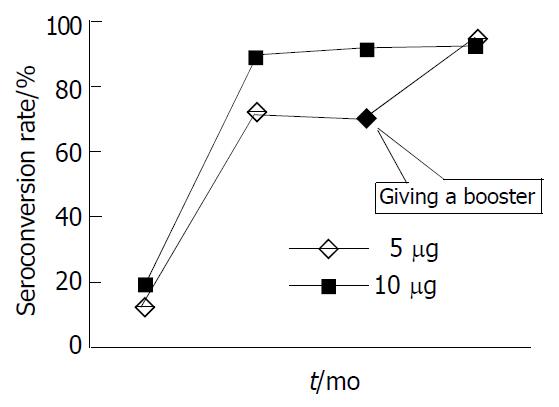
128/130 subjects in low dose group were tested for serum anti-HBs levels one month after the first dose of vaccination and only 114 subjects were available for the test one month after the third vaccination. The anti-HBs sero-conversion rate and GMT in this group was 71.9% and 9.5 respectively (Table 1). 116/120 subjects in the routine group were tested for anti-HBs levels one month after the first dose of vaccination and only 110 subjects were available for the test one month after the third dose of vaccination. A significantly higher anti-HBs sero-conversion rate (89.1%, P < 0.01) and GMT (106.8, P < 0.01) in routine dose group were observed after the third dose of vaccination compared with that in low dose group. At the seventh month the sero-conversion rate and GMT persisted at similar level to that after the second dose of vaccination.
Twenty three non-responding university students (15 vaccinated with 5 μg vaccine and 8 with 10 μg vaccine) were given a fourth dose of 10 μg HB vaccine after completion of the initial vaccination. Of these individuals, 13/15 non- responders in the low dose group and 4/8 non-responders in the routine dose group responded with anti-HBs one month after the additional dose. As a result of this additional vaccination, serum conversion rate in the low dose group was nearly similar to that in the routine dose group (95.2% vs 92.9%, Figure 1).
Since the introduction of hepatitis B vaccination in the early 1980s, the safety and immunogenicity of the plasma-derived and yeast-derived hepatitis B vaccines have been well demonstrated[1-12]. However, the disease remains a global problem. The failure of obtaining a complete 3 doses HB vaccination due to the length of the scheme probably contributes to the current prevalence of hepatitis B in the developing countries. Clearly, the protection against hepatitis B could be improved if a shorter vaccination regimen of achieving protective anti-HBs levels in > 90% of vaccinees was available. Our results clearly showed that with a rapid vaccination scheme (0, 1, 2 months) using 10 μg HB vaccine, serum anti-HBs levels can be induced, while it can not be reached by using 5 μg of HB vaccine. The similar results were also reported previously by Carlsson et al[13] who vaccinated some medical staff and compared the effects of intramuscular (I m, 20 μg.) vaccination with the low-dose intradermal (i d, 2 μg) vaccination on their early sero-conversion rates according to a 0, 4, 8 week scheme or to an accelerated 0, 2, 6 week scheme. He concluded that when a rapid protection against hepatitis B virus (HBV) infection was needed, such as the post-exposure prophylaxis, low-dose i.d. vaccination schedule could not be used.
A study on evaluating the potential for developing rapid seroprotection was performed by Young et al[14], beneficial results of a one month/two dose regimen with a novel triple antigen vaccine (Hepacare) have been achieved[14]. These results confirmed that an accelerated vaccination could be achieved with appropriate vaccinating protocols.
Low-dose vaccination has been proposed as a cost-saving strategy to implement mass vaccination of neonates with HB vaccine world-wide, particularly in developing countries. In a previous study, Shokri F compared the effectiveness of three doses of recombinant HB vaccine (Heberbiovac) in three different doses, among three groups of healthy Iranian neonates. His results showed that there were no significant differences in sero-protection rate and GMTs between the 10 and 5 μg dose recipients. Both parameters, however, were significantly lower in neonates vaccinated with a 2.5 μg vaccine dose[12]. Leroux-Roels G observed the immunogenicity and reactogenicity profiles of different doses of Engerix-B (R) (10 microg hepatitis B surface antigen) and Recombivax (R) (5 microg hepatitis B surface antigen), on a 0, 1, 6 month schedule in healthy adolescents. One month following the third dose of vaccination, seroprotection rates of Engerix-B and Recombivax vaccination were similar but the geometric mean titer was significantly higher in those vaccinated with Engerix-B than that with Recombivax[15]. Similar results were also reported that higher immunogenicity is usually related with higher vaccine dosages, especially in older adult population[13-18]. Our results showed that a higher dose (10 μg) of vaccine can induce much higher sero- conversion rate and higher GMTs compared with the reduced dose (5 μg) vaccination whereas the GMTs can be sustained for at least 5 months in both groups after the primary vaccination.
In most studies on immunocompetent subjects, 5% to 10% vaccinees failed to respond to HB vaccination. The possible reasons for lack of adequate antibody response have been well reported[16-22]. In order to confirm if the reduced dose of HB vaccination could cause a higher rate of non- or hypo-response to HB vaccine, we gave all non-responders an additional dose after the initial HB vaccination scheme and one month later, the majority of them converted into anti-HBs positive. The final sero-conversion rates of non-responders in low dose group and routine dose group were 95.2% and 92.9% respectively, showing that it is rather a hyporesponse to a lower dose of HB vaccine than a non-response as found earlier[23-31]. Based on the results, we conclude that it is better for adults to choose 10 μg HB vaccine, especially when a rapid vaccination program is needed.
Edited by Wang XL
| 1. | Kojouharova M, Teoharov P, Bahtchevanova T, Maeva I, Eginlian A, Deneva M. Safety and immunogenicity of a yeast-derived recombinant hepatitis B vaccine in Bulgarian newborns. Infection. 2001;29:342-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Liao SS, Li RC, Li H, Yang JY, Zeng XJ, Gong J, Wang SS, Li YP, Zhang KL. Long-term efficacy of plasma-derived hepatitis B vac-cine among Chinese children: a 12-year follow-up study. World J Gastroenterol. 1999;5:165-166. [Cited in This Article: ] |
| 3. | Li H, Li RC, Liao SS, Yang JY, Zeng XJ, Wang SS. Persistence of hepatitis B vaccine immune protection and response to hepatitis B booster immunization. World J Gastroenterol. 1998;4:493-496. [PubMed] [Cited in This Article: ] |
| 4. | Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, Wiesinger K, Kollaritsch H. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19:2055-2060. [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Ozaki T, Mochizuki H, Ichikawa Y, Fukuzawa Y, Yoshida S, Morimoto M. Persistence of hepatitis B surface antibody levels after vaccination with a recombinant hepatitis B vaccine: a 3-year follow-up study. J Oral Sci. 2000;42:147-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Jain A, Mathur US, Jandwani P, Gupta RK, Kumar V, Kar P. A multicentric evaluation of recombinant DNA hepatitis B vaccine of Cuban origin. Trop Gastroenterol. 2000;21:14-17. [PubMed] [Cited in This Article: ] |
| 7. | Al-Faleh FZ, Al-Jeffri M, Ramia S, Al-Rashed R, Arif M, Rezeig M, Al-Toraif I, Bakhsh M, Mishkkhas A, Makki O. Seroepidemiology of hepatitis B virus infection in Saudi children 8 years after a mass hepatitis B vaccination programme. J Infect. 1999;38:167-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Li H, Li RC, Liao SS, Gong J, Zeng XJ, Li YP. Long-term effectiveness of infancy low-dose hepatitis B vaccine immunization in Zhuang minority area in China. World J Gastroenterol. 1999;5:122-124. [PubMed] [Cited in This Article: ] |
| 9. | Liu HB, Meng ZD, Ma JC, Han CQ, Zhang YL, Xing ZC, Zhang YW, Liu YZ, Cao HL. A 12-year cohort study on the efficacy of plasma-derived hepatitis B vaccine in rural newborns. World J Gastroenterol. 2000;6:381-383. [PubMed] [Cited in This Article: ] |
| 10. | Li H, Wang L, Wang SS, Gong J, Zeng XJ, Li RC, Nong Y, Huang YK, Chen XR, Huang ZN. Research on optimal immunization strategies for hepatitis B in different endemic areas in China. World J Gastroenterol. 2000;6:392-394. [PubMed] [Cited in This Article: ] |
| 11. | Zeng XJ, Yang GH, Liao SS, Chen AP, Tan J, Huang ZJ, Li H. Survey of coverage, strategy and cost of hepatitis B vaccination in rural and urban areas of China. World J Gastroenterol. 1999;5:320-323. [PubMed] [Cited in This Article: ] |
| 12. | Shokri F, Jafarzadeh A. High seroprotection rate induced by low doses of a recombinant hepatitis B vaccine in healthy Iranian neonates. Vaccine. 2001;19:4544-4548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Carlsson T, Struve J, Sönnerborg A, Weiland O. The anti-HBs response after 2 different accelerated intradermal and intramuscular schemes for hepatitis B vaccination. Scand J Infect Dis. 1999;31:93-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Young MD, Rosenthal MH, Dickson B, Du W, Maddrey WC. A multi-center controlled study of rapid hepatitis B vaccination using a novel triple antigen recombinant vaccine. Vaccine. 2001;19:3437-3443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Leroux-Roels G, Abraham B, Fourneau M, De Clercq N, Safary A. A comparison of two commercial recombinant vaccines for hepatitis B in adolescents. Vaccine. 2000;19:937-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Treadwell TL, Keeffe EB, Lake J, Read A, Friedman LS, Goldman IS, Howell CD, DeMedina M, Schiff ER, Jensen DM. Immunogenicity of two recombinant hepatitis B vaccines in older individuals. Am J Med. 1993;95:584-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | de Rave S, Heijtink RA, Bakker-Bendik M, Boot J, Schalm SW. Immunogenicity of standard and low dose vaccination using yeast-derived recombinant hepatitis B surface antigen in elderly volunteers. Vaccine. 1994;12:532-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Bennett RG, Powers DC, Remsburg RE, Scheve A, Clements ML. Hepatitis B virus vaccination for older adults. J Am Geriatr Soc. 1996;44:699-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Yucesoy B, Sleijffers A, Kashon M, Garssen J, de Gruijl FR, Boland GJ, van Hattum J, Simeonova PP, Luster MI, van Loveren H. IL-1beta gene polymorphisms influence hepatitis B vaccination. Vaccine. 2002;20:3193-3196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Jabaaij L, van Hattum J, Vingerhoets JJ, Oostveen FG, Duivenvoorden HJ, Ballieux RE. Modulation of immune response to rDNA hepatitis B vaccination by psychological stress. J Psychosom Res. 1996;41:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Sleijffers A, Yucesoy B, Kashon M, Garssen J, De Gruijl FR, Boland GJ, Van Hattum J, Luster MI, Van Loveren H. Cytokine polymorphisms play a role in susceptibility to ultraviolet B-induced modulation of immune responses after hepatitis B vaccination. J Immunol. 2003;170:3423-3428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Goldwater PN. Randomized, comparative trial of 20 micrograms vs 40 micrograms Engerix B vaccine in hepatitis B vaccine non-responders. Vaccine. 1997;15:353-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Wismans P, van Hattum J, Stelling T, Poel J, de Gast GC. Effect of supplementary vaccination in healthy non-responders to hepatitis B vaccination. Hepatogastroenterology. 1988;35:78-79. [PubMed] [Cited in This Article: ] |
| 24. | Wismans PJ, van Hattum J, Mudde GC, Endeman HJ, Poel J, de Gast GC. Is booster injection with hepatitis B vaccine necessary in healthy responders? A study of the immune response. J Hepatol. 1989;8:236-240. [PubMed] [Cited in This Article: ] |
| 25. | Zàruba K, Grob PJ, Bolla K. Thymopentin as adjuvant therapy to hepatitis B vaccination in formerly non-or hyporesponding hemodialysis patients. Surv Immunol Res. 1985;4 Suppl 1:102-106. [PubMed] [Cited in This Article: ] |
| 26. | Maruyama N, Sata M, Ishii K, Atono Y, Ono K, Matuda T, Suzuki H, Muraoka H, Nakano H, Hino K. [Hepatitis B vaccination in alcoholics]. Kansenshogaku Zasshi. 1989;63:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Fabrizi F, Andrulli S, Bacchini G, Corti M, Locatelli F. Intrader-mal versus intramuscular hepatitis b re-vaccination in non-re-sponsive chronic dialysis patients: a prospective randomized study with cost-effectiveness evaluation. Nephrol Dial Transplant. 1997;12:1204-1211. [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Hollinger FB. Factors influencing the immune response to hepatitis B vaccine, booster dose guidelines, and vaccine protocol recommendations. Am J Med. 1989;87:36S-40S. [PubMed] [Cited in This Article: ] |
| 29. | Erensoy S, Bilgiç A, Arda B, Ozer O. Low-dose intramuscular hepatitis B vaccination in medical students: 4-year follow-up. Infection. 2002;30:303-305. [PubMed] [Cited in This Article: ] |
| 30. | Huang LM, Chiang BL, Lee CY, Lee PI, Chi WK, Chang MH. Long-term response to hepatitis B vaccination and response to booster in children born to mothers with hepatitis B e antigen. Hepatology. 1999;29:954-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Clemens R, Sänger R, Kruppenbacher J, Höbel W, Stanbury W, Bock HL, Jilg W. Booster immunization of low- and non-responders after a standard three dose hepatitis B vaccine schedule--results of a post-marketing surveillance. Vaccine. 1997;15:349-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |