Published online Oct 15, 2002. doi: 10.3748/wjg.v8.i5.923
Revised: November 4, 2001
Accepted: November 8, 2001
Published online: October 15, 2002
AIM: To determine the in vivo effects of phagocytic blockade of Kupffer cell (KC) on the release of proinflammatory cytokines in small intestinal lesion and on the integrity of intestinal tract by using gadolinium chloride (GdCl3) during early endotoxemia.
METHODS: Wistar rats were divided into three groups: Group A, rats were injected with endotoxin (E. coli O111:B4, a dose of 12 mg•kg⁻¹) only; Group B, rats were pretreated intravenously with 25 mg of GdCl3 per kg 24 h are given endotoxin; and Group C, sham operation only. All animals were sacrificed 4 h after endotoxin injection. In portion of the rats of three groups, bile duct was cannulated, which the bile was collected externally. Morphological changes of ileum were observed under light microscopy and electronic microscopy. The KC were isolated from rats by collagenase perfusion and in KC, expression of TNF-α and IL-6 mRNA were determined by RT-PCR analysis. Plasma and bile TNF-α and IL-6 Levels were determined by enzyme-linked immunosorbent assay (ELISA).
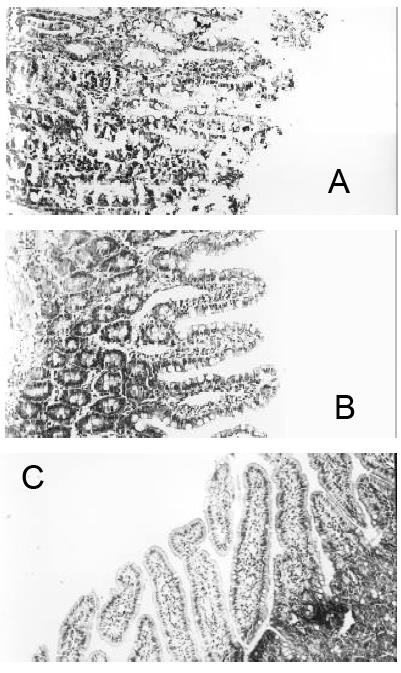
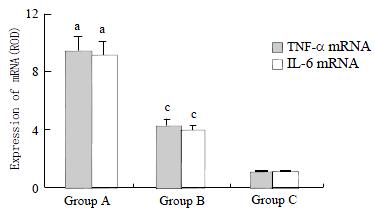
RESULTS: In group A, there were neutrophil infiltration and superficial epithelial necrosis of the ileal villi, sloughing of mucosal epithelium, and disappearance of some villi. In group B, the ileal mucosal damage was much reduced. Which in group C, no significant morphological changes were seen. GdCl3 pretreatment decreased significantly the expression of TNF-α and IL-6 mRNA in group B (4.32 ± 0.47 and 4.05 ± 0.43) when compared to group A (9.46 ± 1.21 and 9.04 ± 1.09) (P < 0.05). There was no significant expression of TNF-α and IL-6 mRNA in group C (1.03 ± 0.14 and 10.4 ± 0.13). In rats of group A, the levels of TNF-α and IL-6 in bile and plasma were 207 ± 29 ng·L-1, 1032 ± 107 ng·L-1, 213 ± 33 ng·L-1, and 1185 ± 127 ng·L-1, respectively. In group B, they were 113 ± 18 ng·L-1, 521 ± 76 ng·L-1, 147 ± 22 ng·L-1, and 572 ± 54 ng·L-1, respectively. In group C, they were 67 ± 10 ng·L-1, 72 ± 13 ng·L-1, 109 ± 18 ng·L-1, and 118 ± 22 ng·L-1 respectively. There were significant difference between the three group (P < 0.05).
CONCLUSION: KC release cytokines TNF-α and IL-6 causing damage to the integrity of intestinal epithelium and play a crucial role in the initiation and progression of intestinal mucosal damage during early endotoxemia.
- Citation: Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, Li Y, Li XH. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol 2002; 8(5): 923-927
- URL: https://www.wjgnet.com/1007-9327/full/v8/i5/923.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i5.923
LPS (lipopolysaccharide) is now considered to be a potent inducer of a series of inflammatory mediators[1-6]. In the early as infection, the main symptoms can be ascribed in part to the inflammatory cytokines as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-6 and platelet-activating factor[7-9]. Recently, Jackson et al[10] reported that intestinal damage is mediated by factors derived from the bile. KC (Kupffer cell) constitute 80%-90% of the fixed tissue macrophages of the reticuloendothelial system (RES) and their activation and subsequent release of cytokines have great influence on the systemic response during sepsis[11-14]. KC has been shown to be a major source of released IL-6 following traumatic or hemorrhagic shock and after resuscitation[15]. Since LPS has been removed from the blood by the liver, we speculated the cytokines from KC in the bile may likely cause the damage to the intestinal during endotoxemia. Therefore, the aim of this study was to determine whether the in vivo phagocytic blockade of KC by using gadolinium chloride (GdCl3) had any effects on intestinal integrity during early endotoxemia and whether the proinflammatory cytokines played crucial role in small intestinal lesions.
Wistar rats 10 wk old, weighing 220 g to 250 g were used in this experiment. The rats were obtained from Laboratory Animal Center of Chongqing University of Medical Science. These animals were divided into three groups: Group A, rats injected endotoxin only; Group B, rats pretreated with GdCl3 and given endtoxin; and group C, sham operation only.
In group B, animals were treated intravenously with 25 mg of GdCl3 per kg 24 h prior to the operation as described by Koo et al[15]. In the preliminary experiment, the optimum dose and time of administration of GdCl3 were determined by assessment of loss of KC labeled with India ink. In some of animals, in order to collect the bile, the animals were fasted overnight with free access to water. Bile duct was cannulated in rats of the three groups under ether anesthesia as described by Jackson et al[10]. Briefly, the abdomen was opened through a midline incision of 1.5-2.0 cm immediately below the sternum. The bile duct was located, and approximately 1 to 1.5 cm of a 25 cm polyethylene cannulation tube (external diameter, 0.8 mm; internal diameter, 0.4 mm) was inserted into bile duct and tied in place, the other end of the tube was exteriorized through an opening in the right flank by an 18-gauge needle, allowing bile to be collected externally. The abdominal incision was then sutured, and the rats were held in restraining cages. Rats from group A and B were then injected iv with LPS from E. coli serotype O111: B4 (Sigma, St. Louis, Mo. USA), using a dose of 12 mg·kg-1. Animals from group C were infused with some dose of pyrogen-free sterile saline. Plasma were separated from tail vein blood, and bile was collected 4 h after LPS administration when these rats were killed and the macroscopic appearance of the whole small instestine was assessed.
One-to two-centimeter segments of the ileum from each rats were fixed in 100 mL·L-1 buffered formalin or 25 g·L-1 glutaraldehyde immediately. For light microscopy, the tissue blocks were embedded in paraffin, and the 5-um sections were stained with HE. For electronic microscopy, the tissue blocks were embedded in Epon 618 resin and ultrathin sections stained with uranyl acetate and lead citrate. A H-2000 transmission electronmicroscope was used.
KC were isolated as described previously[16,17]. In brief, the livers were removed after a portal vein perfusion with Hanks' balanced salt solution (HBSS) and the homogenate was digested in a solution of 5 g·L-1 collagenase (Type IV, Sigma). The digest was washed thoroughly and plated on plastic dishes in RPMI medium containing 50 mL·L-1 fetal calf serum (FCS). After 3 h incubation at 37 °C in O2 and CO2 (0.95/0.05), nonadherent cells were removed with pipet. The adherent cells were collected with a rubber policeman.70% to 90% viable KC was obtained in this manner. GdCl3-treated and control cells exhibited similar viability, morphology, and in vitro phagocytosis, although the livers from GdCl3-treated rats yielded 10%-20% fewer KC than saline-injected rats.
RNA isolation and C-complementary DNA synthesis Total RNA was isolated from KC by using the Trizol Reagent (Life Technologies, USA). The quality of RNA was controlled by the intactness of ribosomal RNA bands. A total of 0.5 mg of each intact total RNA samples was reverse-transcribed to complementary DNA (cDNA) by using the reverse transcription polymerase chain reaction (RT-PCR) kit (Roche, USA). cDNA was stored at -70 °C till polymerase chain reaction (PCR) analysis.
Determination of TNF-α and IL-6 mRNA by RT-PCR The PCR primers used were TNF-α: sense (5'-CACCATGAGCACGGAAAGCA-3'), antisense (5'-GCAATGACTCCAAAGTAGAC-3'); IL-6: sense (5'-CTTCCAGCCAGTTGCCTTCT-3'), anti-sense (5'-AGCCAGAGTCATTCAGAGCA-3'); β-actin: sense (5'-ACCACAGCTGAGAGGGAAATCG-3'), antisense (5'-AGAGGTCTTTACGGATGTCAACG-3'). The sizes of the amplified PCR products were 692 bp for TNF-α, 309 bp for IL-6, and 281 bp for β-actin. The conditions for amplification were as follows: denaturation at 94 °C for 1 min, annealing at 57 °C for 2 min, and extension at 72 °C for 2 min for 31 cycles. The PCR products were electrophoresed in 15 g•L-1% agarose gels, and the gels were ethidium bromide stained and video photographed on an ultraviolet transilluminator.
Plasma and bile levels of TNF-α and IL-6 were determined by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacture's instructions and guidelines (Biosource International, Camarillo, CA).
All data were expressed as ¯x ± s and compared by one-way analysis of variance (ANOVA). Differences in values were considered significant if P≤ 0.05.
We investigated the effect of phagocytotic blockade of Kupffer cells by GdCl3 on the histological appearance of the ileum. Four hours after inoculation with LPS, macroscopic examination showed marked hyperemia, edema, and fluid accumulation within the lumen of small bowel in rats of group A. In contrast, in animals of group B, there was substantial reduction in hyperemia and no fluid accumulation. There was neither hyperemia nor fluid accumulation in group C.
In group A, there was marked tissue damage. Under light microscope, there were neutrophil infiltration in the villi, severely damaged epithelial layer with superficial necrosis and sloughing, some villi completely disappeared (Figure 1A). In contrast, the rats of group B, intestinal mucosal damage were much reduced, there was no evidence of hyperemia, vasodilatation or edema in the lamina propria, and the epithelial layer was intact, only modest hyperemia in the submucosa, only occasional edematous apex at the villi (Figure 1B). In group C, The histological appearance was normal (Figure 1C).
GdCl3 clearly resulted in sustained loss of phagocytic activity of KCs diminishing concentrations of TNF-α and IL-6 in bile and plasma. We found that GdCl3 pretreatment decreased KCs cytokines transcripts after injection of LPS so that expression of TNF-α and IL-6 mRNA in group B were significantly less than those in group A (P < 0.05). There were no expression of TNF-α and IL-6 genes in rats of group C (Figure 2).
TNF-α and IL-6 in the bile and plasma of above three groups were measured by bioassay (Table 1). In rats of group A, there were significant increase of TNF-α and IL-6 in bile and plasma, the concentration of the two cytokines in the bile were over 5-fold and 4-fold higher than those at in the plasma, respectively. In animals of group B, GdCl3 given 24 h prior to LPS markedly reduced the levels of TNF-α and IL-6 in bile and plasma when compared to those in group A (P < 0.05). Low levels of TNF-α and IL-6 were detectable in bile and plasma in rats of group C.
The role of cytokines in the bile causing gastrointestinal damage during sepsis has received little attention to date, and the bile continues to be viewed upon as having a purely digestive function. Other investigators reported after the relief of obstructive jaundice by internal drainage, endotoxemia could be reversed. But, the ratio of villous height to crypt depth in the mucosa of the terminal ileum was deceased in the rats with external drainage. The absence of bile from the gut may promote bacterial translocation to visceral organs[10,18]. These findings implicate the use of bile to prevent intestinal mucosal damage and reduce postoperative complications of jaundiced patient with endotoxemia. In fact, a growing body of evidence points to the presence of inflammatory mediators and other factors in bile. These factors include cytokines IL-1, IL-6, TNF-α, and epidermal growth factor, complement components, and acute-phase proteins, which are released by KC and other macrophages[13,19-27]. Recently, Jackson et al[10] reported the involvement of bile in gastrointestinal tract damage in endotoxemia. External drainage of bile or bile duct occlusion markedly reduced the effects of LPS to the intestine and prolonged survival of the animals[18]. These studies indicate that after treatment with LPS, the bile contains substances that are capable of mediating intestinal damage. Our results showed there were higher concentrations of TNF-α and IL-6 in rats with endotoxemia than those in the control rats, and there were 5-fold and 6-fold higher levels of TNF-α and IL-6 in bile than in plasma. The intestinal mucosal epithelia of these animals were severely damaged. These findings suggest the luminal cytokines derived from the bile, contribute to the intestinal damage during early endotoxemia.
Where do these cytokines in bile come from The precise molecular mechanism of the cytokines in bile is uncertain. There are several reasons to believe that cytokines in the bile originate from synthesis by KC in the bile rather than extracted from the circulating blood. First, The KC are the main site for the clearance of endotoxin, they constitute about 80%-90% of sessile macrophages of the RES. LPS is taken up by KC and leads to production of cytokines by these cells[12,15-16,19,21,28,29]. Second, the concentration of TNF-α and IL-6 in the bile was more markedly increased than those in the plasma. Finally, the levels of these cytokines were significantly reduced by treatment with GdCl3 which preferentially inhibited the KC function, again supporting the concept of production of cytokines by the hepatic KC. A number of studies had been conducted to examine the role of KC function during endotoxemia and other circulatory conditions. Studies dealing with the blockade of KCs phagocytosis in lethal endotoxic shock models had shown increased animal survival that underwent GdCl3 pretreatment[30-32]. Moreover, utilizing KC blockade by GdCl3 revealed the mediators released by KC during sepsis played a significant role[33]. Although studies had shown that intestinal damage occured during sepsis, it remained unknown whether blockade of KC prior to the onset of endotoxemia could prevent the occurrence of intestinal damage. The present study was designed to determine whether the in vivo phagocytic blockade of KC by GdCl3 prior to the onset of sepsis had any salutary effects on intestinal integrity and expression of proinflammatory cytokines such as TNF-α and IL-6, their genes and proteins. Our results indicated that expression of TNF-α and IL-6 mRNA significantly increased 4 h after the induction of LPS. However, rats that underwent GdCl3 treatment prior to LPS demonstrated significantly reduced expression of the genes and proteins of these cytokines in bile and plasma. Thus, KC can play a crucial role in the initiation and progression of intestinal damage following endotoxemia[34-38].
The role of cytokines in intestinal pathology has not been welle lucidated. But, cytokines in intestinal tract could promote bacterial association with the epithelium, or might act upon luminal microorganism to enhance their invasive characteristics[39-43]. There are evidences that TNF-α is able to affect directly the virulent properties of some organisms. For example, TNF-α enhances the invasion of cultured cells, promoted by cytokines. IL-1 has been shown to act as a growth factor for pathogenic E. coli. Fluid accumulation in the intestine of LPS-treated rats indicates presence of altered permeability. Furthermore, TNF-α can affect directly the permeability of epithelial barriers, and loosening of the tight junction so as to facilitate the early entry of bacteria into the mesenteric lymph nodes and other target organs. TNF-α can induce expression of adhesion molecules ICAM-1 and LFA-3 in human intestinal epithelial cell lines. It can also influence cytokines production and proliferation in intestinal cell lines[44-48]. These findings reveals the presence of specific receptors on such cells, the TNF-α receptors are predominantly located on the basolateral aspect of intestinal epithelial cells, as are receptors for IL-1. After LPS stimulation, macrophages release cytokines damaging the intestinal epithelial integrity, allowing luminal TNF-α and IL-6 to gain assiss to their receptors on the basolateral aspect of the epithelial cells and increase epithelial permeability, finally cause the intestinal damage.
Edited by Wu XN
| 1. | Parker SJ, Watkins PE. Experimental models of gram-negative sepsis. Br J Surg. 2001;88:22-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5802] [Cited by in F6Publishing: 5806] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 3. | Fan K. Regulatory effects of lipopolysaccharide in murine macrophage proliferation. World J Gastroenterol. 1998;4:137-139. [PubMed] [Cited in This Article: ] |
| 4. | Liu BH, Chen HS, Zhou JH, Xiao N. Effects of endotoxin on endothelin receptor in hepatic and intestinal tissues after endotoxemia in rats. World J Gastroenterol. 2000;6:298-300. [PubMed] [Cited in This Article: ] |
| 5. | Zhang SC, Dai Q, Wang JY, He BM, Zhou K. Gut derived endotoxemia: one of the factors leading to production of cytokines in liver diseases. World J Gastroenterol. 2000;6:16. [Cited in This Article: ] |
| 6. | Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 376] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Bone-Larson CL, Simpson KJ, Colletti LM, Lukacs NW, Chen SC, Lira S, Kunkel SL, Hogaboam CM. The role of chemokines in the immunopathology of the liver. Immunol Rev. 2000;177:8-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT, Thurman RG. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278:G652-G661. [PubMed] [Cited in This Article: ] |
| 9. | Le Roy D, Di Padova F, Adachi Y, Glauser MP, Calandra T, Heumann D. Critical role of lipopolysaccharide-binding protein and CD14 in immune responses against gram-negative bacteria. J Immunol. 2001;167:2759-2765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Jackson GD, Dai Y, Sewell WA. Bile mediates intestinal pathology in endotoxemia in rats. Infect Immun. 2000;68:4714-4719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 473] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 12. | Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35-46. [PubMed] [Cited in This Article: ] |
| 13. | Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Schemmer P, Bradford BU, Rivera CA. Kupffer cell-derived prostaglandin E (2) is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G100-G106. [PubMed] [Cited in This Article: ] |
| 14. | Xu MQ, Gong JP, Xue L, Han BL, Hu F. Effects of Kupffer cell NF-κB activation on liver regeneration after partial hepatectomy in biliary obstructive rats. DiSan Junyi Daxue Xuebao. 2001;23:1143-1145. [Cited in This Article: ] |
| 15. | Koo DJ, Chaudry IH, Wang P. Kupffer cells are responsible for producing inflammatory cytokines and hepatocellular dysfunction during early sepsis. J Surg Res. 1999;83:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Gong JP, Xu MQ, Li K, Zhu J, Han BL. Expression of CD14 in Kupffer's cells induced by lipopolysaccharide. DiSan Junyi Daxue Xuebao. 2001;23:425-428. [Cited in This Article: ] |
| 17. | Gong JP, Han BL. Technique of isolation, culture, and identification of liver cells. Shijie Huaren Xiaohua Zazhi. 1999;7:417-19. [Cited in This Article: ] |
| 18. | Islam AF, Moss ND, Dai Y, Smith MS, Collins AM, Jackson GD. Lipopolysaccharide-induced biliary factors enhance invasion of Salmonella enteritidis in a rat model. Infect Immun. 2000;68:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Guo X, Dudman NP. Homocysteine induces expressions of adhesive molecules on leukocytes in whole blood. Chin Med J (Engl). 2001;114:1235-1239. [PubMed] [Cited in This Article: ] |
| 20. | Enomoto N, Yamashina S, Kono H, Schemmer P, Rivera CA, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Thurman RG. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology. 1999;29:1680-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Mäck C, Jungermann K, Götze O, Schieferdecker HL. Anaphylatoxin C5a actions in rat liver: synergistic enhancement by C5a of lipopolysaccharide-dependent alpha (2)-macroglobulin gene expression in hepatocytes via IL-6 release from Kupffer cells. J Immunol. 2001;167:3972-3979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Li XH, Peng Y. Liver sinusoidal endothelial cell injury by neutrophils in rats with acute obstructive cholangitis. World J Gastroenterol. 2002;8:342-345. [PubMed] [Cited in This Article: ] |
| 23. | Han DW. The clinical sine of subsequent liver injury induced by gut derived endotoxemia. Shijie Huaren Xiaohua Zazhi. 1999;7:1055-1058. [Cited in This Article: ] |
| 24. | Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128-130. [PubMed] [Cited in This Article: ] |
| 25. | Emmanuilidis K, Weighardt H, Maier S, Gerauer K, Fleischmann T, Zheng XX, Hancock WW, Holzmann B, Heidecke CD. Critical role of Kupffer cell-derived IL-10 for host defense in septic peritonitis. J Immunol. 2001;167:3919-3927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Ling YL, Meng AH, Zhao XY, Shan BE, Zhang JL, Zhang XP. Effect of cholecystokinin on cytokines during endotoxic shock in rats. World J Gastroenterol. 2001;7:667-671. [PubMed] [Cited in This Article: ] |
| 27. | Wang LS, Zhu HM, Zhou DY, Wang YL, Zhang WD. Influence of whole peptidoglycan of bifidobacterium on cytotoxic effectors produced by mouse peritoneal macrophages. World J Gastroenterol. 2001;7:440-443. [PubMed] [Cited in This Article: ] |
| 28. | Bai XY, Jia XH, Cheng LZ, Gu YD. Influence of IFN alpha-2b and BCG on the release of TNF and IL-1 by Kupffer cells in rats with hepatoma. World J Gastroenterol. 2001;7:419-421. [PubMed] [Cited in This Article: ] |
| 29. | Gong JP, Han BL. Effects of CD14 in LPS mediating activation of Kupffer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:875-877. [Cited in This Article: ] |
| 30. | Watanabe M, Chijiiwa K, Kameoka N, Yamaguchi K, Kuroki S, Tanaka M. Gadolinium pretreatment decreases survival and impairs liver regeneration after partial hepatectomy under ischemia/reperfusion in rats. Surgery. 2000;127:456-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Takeyama O, Ikai I, Yamamoto M, Kanazawa A, Yagi T, Uesugi T, Nishitai R, Satoh S, Terajima H, Yamaoka Y. The protective role of Kupffer cells in humoral injury of xenoperfused rat livers. Transplantation. 2000;69:1283-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Arai M, Peng XX, Currin RT, Thurman RG, Lemasters JJ. Protection of sinusoidal endothelial cells against storage/reperfusion injury by prostaglandin E2 derived from Kupffer cells. Transplantation. 1999;68:440-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Roland CR, Naziruddin B, Mohanakumar T, Flye MW. Gadolinium blocks rat Kupffer cell calcium channels: relevance to calcium-dependent prostaglandin E2 synthesis and septic mortality. Hepatology. 1999;29:756-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y. Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol. 2001;7:836-840. [PubMed] [Cited in This Article: ] |
| 35. | Okada K, Marubayashi S, Fukuma K, Yamada K, Dohi K. Effect of the 21-aminosteroid on nuclear factor-kappa B activation of Kupffer cells in endotoxin shock. Surgery. 2000;127:79-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Tomioka M, Iinuma H, Okinaga K. Impaired Kupffer cell function and effect of immunotherapy in obstructive jaundice. J Surg Res. 2000;92:276-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 37. | Calne RY. Immunological tolerance--the liver effect. Immunol Rev. 2000;174:280-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Ikejima K, Enomoto N, Seabra V, Ikejima A, Brenner DA, Thurman RG. Pronase destroys the lipopolysaccharide receptor CD14 on Kupffer cells. Am J Physiol. 1999;276:G591-G598. [PubMed] [Cited in This Article: ] |
| 39. | Rocha F, Laughlin R, Musch MW, Hendrickson BA, Chang EB, Alverdy J. Surgical stress shifts the intestinal Escherichia coli population to that of a more adherent phenotype: role in barrier regulation. Surgery. 2001;130:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Echtenacher B, Weigl K, Lehn N, Männel DN. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect Immun. 2001;69:3550-3555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Téllez Gil L, Roselló AM, Collado Torres A, Moreno RL, Antonio Ferrón Orihuela J. Modulation of soluble phases of endothelial/leukocyte adhesion molecule 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 with interleukin-1beta after experimental endotoxic challenge. Crit Care Med. 2001;29:776-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001;29:1513-1518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Lehmann C, König JP, Dettmann J, Birnbaum J, Kox WJ. Effects of iloprost, a stable prostacyclin analog, on intestinal leukocyte adherence and microvascular blood flow in rat experimental endotoxemia. Crit Care Med. 2001;29:1412-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Funda DP, Tucková L, Farré MA, Iwase T, Moro I, Tlaskalová-Hogenová H. CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infect Immun. 2001;69:3772-3781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Gong JP, Liu CA, Wu CX, Li SW, Shi YJ, Li XH. Nuclear factor κB activity in patients with acute severe cholangitis. World J Gastroenterol. 2002;8:346-349. [PubMed] [Cited in This Article: ] |
| 46. | Baykal A, Iskit AB, Hamaloglu E, Guc MO, Hascelik G, Sayek I. Melatonin modulates mesenteric blood flow and TNFalpha concentrations after lipopolysaccharide challenge. Eur J Surg. 2000;166:722-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Assy N, Jacob G, Spira G, Edoute Y. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262. [PubMed] [Cited in This Article: ] |
| 48. | Li SW, Gong JP, Wu CX, Shi YJ, Liu CA. Lipopolysaccharide induced synthesis of CD14 proteins and its gene expression in hepatocytes during endotoxemia. World J Gastroenterol. 2002;8:124-127. [PubMed] [Cited in This Article: ] |