Published online Aug 15, 2002. doi: 10.3748/wjg.v8.i4.758
Revised: October 22, 2001
Accepted: October 30, 2001
Published online: August 15, 2002
AIM: To establish a successful model of heterotopic total small intestinal transplantation (SIT) in rats in order to reduce the complications and increase the survival rate.
METHODS: A total of 196 Wistar rats underwent heterotopic SIT with microsurgical technique. Technical modifications included shortening fasting time and supplying energy before surgery, administering optimal volume of crystalloid fluid to the donor and recipient during surgical procedures, reducing mechanical and ischemic injuries to donor intestine, revascularizing small intestinal graft with a combination of conventional aorta to aorta anastomosis and a cuffed portal vein to left renal vein anastomosis which resulted in an acceptably short warm ischemic time, and also an adequate blood supply and drainage of the graft.
RESULTS: The average time for the donor surgery was 86 min ± 20 min, the mean operative time for the recipient was 115 min ± 20 min and warm ischemia time was shortened to 40 min ± 5 min. There was a shorter revascularizing time of the graft, the abdominal aorta (AA) to AA anastomosis being 21 min ± 10 min, and the cuffed portal vein (PV) to the renal vein anastomosis being 5 min ± 5 min. The one-week survival rate of 98 rats with SIT was 88.78% (87/98), without thrombosis and stenosis of anastomosis. The longest survival time of recipient rats was more than 389 d after SIT, the rats were maintaining normal weight, with perfect intestinal function and intact intestinal histology.
CONCLUSION: These modified techniques for SIT would remarkably reduce the complications and improve survival rate in rats, which provided a potentially more consistent and practical model for experimental and clinical studies.
- Citation: Wu XT, Li JS, Zhao XF, Zhuang W, Feng XL. Modified techniques of heterotopic total small intestinal transplantation in rats. World J Gastroenterol 2002; 8(4): 758-762
- URL: https://www.wjgnet.com/1007-9327/full/v8/i4/758.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i4.758
Since Monchick and Russel[1] established the model of small intestinal transplantation (SIT) in 1971, much modification and development have been achieved[2-11]. However, the technical complexity and high mortality have hindered the wide use of this valuable model[12-24]. Parallel to our clinical SIT practice, we have successfully established a stable and practical model of heterotopic SIT with fewer complications and higher survival rate using the modified techniques.
One hundred and ninety-six male adult Wistar inbred strain rats weighing between 180 g and 310 g (Shanghai Laboratory Animal Center of Academy of Sciences of China) were used as donors and recipients. Housed and fed at the Animal Center of Nanjing University, the rats were put accustomed to the environment for at least 7 d before surgery. The donor and recipient were paired according to the similar body weight.
All the donor and recipient rats stayed fasting in metabolic cages with no access to waster but allowed to drink 5% glucose normal saline added with 160000 U/L gentamycin ad libitum for 10 h-12 h. The rats were anesthetized with an intraperitoneal injection of 1% ketamine (1 mL/100 g) supplemented with the 1/4 primitive dose of ketamine as required.
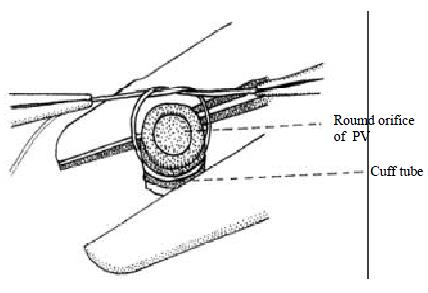
Lactated Ringer’s solution with 2.5 g/L Cefazomelin was infused via the penile vein by micropump (Perfusor Secura FT, B. Braun Melsunge AF, Germany) at 4 mL/h. The abdomen was opened using a “⊥”–shaped incision, and the jejunum was cut at 1 cm away from the Treitz's ligament and ileum at 2 cm proximal to ileocecal valve. The entire colon was removed. The portal vein (PV) was separated from pancreas. The segment of abdominal aorta (AA) containing the superior mesenteric artery (SMA) was mobilized by ligating and dividing the lumbar artery. The lumbar arteries from the AA were meticulously ligated with 8-0 nylon sutures to minimize bleeding between the celiac and left renal artery. The left renal vessels were then ligated. The dissected AA was ligated below the left renal artery. The celiac artery was ligated, followed by the ligation of the pyloric vein and splenic vein. Five to eight mL 2.5 g/L Cefazomelin in saline was injected into the small intestine through the upper end of the jejunum. The AA was cannulated with a fine polyethylene catheter and the PV was cut off near hepatic hilum. The graft was perfused in situ with 2-3 mL 4 °C lactate Ringer’s solution containing 125000 U/L heparin by micropump at 40 mL/h until the graft intestine and mesentery turned pale, and the fluid in the PV became clear. At last, the intestine and its vascular supply including a part of AA were removed en bloc. Under operational microscope and in lactated Ringer's solution ice-water bath, the PV end was placed into a polyethylene cuff tube and its end part of endothelium was turned over to cover the end of cuff tube. The PV end and cuff tube were fixed with 6-0 silk sutures. Hence, the round orifice of the PV was exactly in the center of the cuff tube (Figure 1). The small intestinal graft was stored in lactated Ringer's solution at 4 °C[25-27].
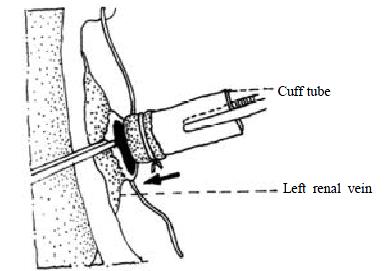
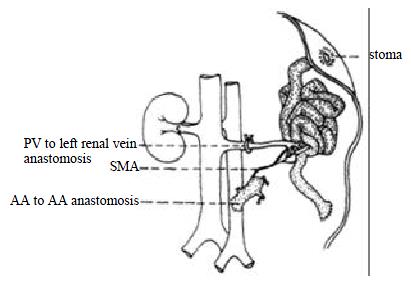
Anesthesia and intravenous infusion for the recipient were the same as for the donors. The abdomen was opened via a midline incision from the ensisternum to the bladder level. The left ureter and renal atery were ligated. The left renal vein was dissected. The pedicle near renal helium was ligated and the ligating suture was left as a tractor after removal of the left kidney. Segment of the recipient's abdominal AA (0.6-1.0 cm) was mobilized below the vessels to the left kidney. Under operating microscope (× 10 amplification), the AA of the adventitia membrance of the anterior wall was removed and opened via a longitudinal arteriotomy. The lumen was flushed with low molecular dextran solution. The donor's small intestine was picked up from the ice water, surrounded by a gauze sponge packed with ice crystals, and then placed onto the right flank of the rats. The arterial anastomosis was performed first. After ensuring that the artery was not twisted, an end-to-side anastomosis was performed using continuous 9-0 non-traumatic nylon suture. The posterior wall was anastomosed from the inside of the vessels. The anterior wall of the arterial anastomosis was sutured externally. Each lateral wall of the artery was sutured with 8 -10 sutures. The end of the left renal vein of the recipient was opened with a longitudinal incision. Two 9-0 nylon stay sutures were placed at the lateral sides of the anastomosis as a self-retaining retractor. The upper and lower sides of the incision were hauled by the pedicle ligating suture and microtweezer respectively. The cuffed PV of the small intestinal graft was inserted into the left renal vein of the recipient to revascularize the heterotopic small intestinal graft. The anastomosis was fixed with 5-0 silk suture (Figure 2). The left renal venous clamp was released first, followed by the clamps over and beneath the AA anastomosis, and the blood supply of the small intestinal graft was recovered. The arterial anastomosis was compressed lightly with a dry sponge for 1 to 2 min after reperfusion and then usually the oozing blood could be easily stopped. If blood was spouting from the arterial anastomosis, it should be quickly repaired with interruptive sutures. For the purpose of warm and flush, 20 mL warm saline was instilled in the peritoneal cavity. The small intestinal graft was put in order, and placed onto the left flank of the rats. Both ends of the graft were exteriorized as stomas. The stomas were sutured with four 7-0 silk sutures between the host perttoneum and the seromuscular layer of the graft and four 5-0 silk sutures between the skin and the everted mucosa of the graft (Figure 3). The abdomen was closed using two layers of 1-0 silk continuous sutures.
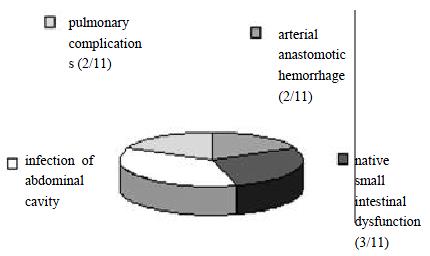
Operated by one person, the average time for the donor surgery was 86 ± 20 min, and 115 ± 20 min for the recipient and the average warm ischemic time being 40 ± 5 min. There was a shorter revascularization time of the graft, the AA to AA anastomosis was 21 ± 10 min, and the cuffed PV to the renal vein anastomosis was 5 ± 5 min. Sixteen rats which died from anesthetic accidents and hemorrhage during operation were not included in the statistical data. Among the 98 heterotopic whole small intestinal transplantation, 11 rats died in 6 d, the autopsy verified 2 cases of arterial anastomotic hemorrhage, 3 cases of the native small intestinal dysfunction, 4 cases of infection of abdominal cavity, and 2 cases of the pulmonary complications (Figure 4). There was no gross or microscopic evidence of either vascular occlusion in any of the grafts or stoma-related complications. The one-week survival rate was 88.7% (87/98). The rats recovered vigor and vitality after the operative day. The shape and color of the transplanted small intestines were nearly the same as the native intestines from the tenth day. The longest survival time of recipient rats was more than 389 d after SIT when the data were collected. They maintained normal weight, perfect intestinal function and intact intestinal histology.
SIT of the rat remains a microsurgical technique which is difficult to manage. According to the need of clinical SIT practice, we used Wistar inbred strain rats as donors and recipients to practise heterotopic SIT, so that the model would not be affected by immune responses[28-40]. Following the modified methods of Zhong et al[2] and Kiyozaki et al[3], we have accumulated a great and original experience with reducing complications and increasing survival rate in rats.
Shortening fasting time Both donor and recipient rats were placed in metabolic cages and kept fasting before surgery, without eatable cushion and dirt. The fasting time was shortened from 48 h (donors) and 24 h (recipients) respectively to less than 12 h, a satisfying result could be achieved through this management, only a little bile and bowel fluid were found in the small intestinal graft by surgery. The rats received 5% glucose normal saline ad libitum before surgery for a supplement of water, salt and energy.
Intravenous infusion Hypovolemic shock was the most common cause of postoperative death in SIT rats because of hemorrhage, evaporation and loss of bowel fluid[15]. The total blood volume of rats was about 5.5-6.5 mL/100 g body weight, if the loss of blood exceeded 3 mL, ischemic damage of small intestinal graft would occur in donors, and death would happen in recipients. Besides improving dissecting and anastomotic techniques to reduce bleeding, continuous infusion with lactated Ringer's solution via penile vein could keep blood pressure stable during surgery and increase survival rate. If there was a mass bleeding or blockage in intravenous infusion during surgical procedure, rats were given 4-5 mL (2 mL/100 g body weight) of 5% glucose normal saline via back subcutaneous injections after SIT. Fatal pulmonary edema and heart failure could be caused by overdose solution or fast infusion, so the infusion should be controled to an optimum volume, never excessive.
Improvement in the vitality of small intestinal graft The quality of donor organ affected directly the result of transplantation. This is especially true for the small intestinal graft, which is much liable to mechanical and ischemic injuries during the procedure. The recipient rat "stupor"or "no vitality" or failure to death within 1 d-2 d after SIT mostly occurred due to the quality of small intestinal graft. During the whole harvest procedure, the non-traumatic techniques should be adopted, a gauze sponge with saline was used to mobilize the graft gently instead of holding or clamping with hand and microtweezer, and not to toss and turn the graft repeatedly so as to avoid damage. Because of the “⊥”–shaped incision, the small intestinal graft dissected from the colon could be easily placed into abdominal cavity to reduce the exposure and vaporization damage. As vigorous intra-luminal irrigation and graft perfusion directly damaged the microcirculation of the graft[41-44], we changed the method of perfusion from parting body to in situ graft perfusion in living donors and significantly reduced the volume of intra-luminal irrigation from 50-70 mL[1] to 5-8 mL. The small intestine was put in order before graft perfusion, then the solution in the gut lumen could easily flowed out. The speed of irrigation was not quickened till the intra-luminal solution flowed out, avoiding over-distention of the donor small intestine. The volume of graft perfusion in situ was reduced from 12-20 mL[1] to 3-5 mL. The speed and volume of perfusion were accurately controlled by micropump instead of gravity, the graft perfusion was complete and the damage was reduced to minimum. Ischemic injury due to hypovolemic shock during the harvesting was often neglected by surgeons. For example, early ligation of the pyloric and splenic vein rapidly caused splanchnic venous congestion leading to shock[2]. Therefore, we performed this procedure just prior to perfusion and after ligation of the celiac artery to avoid graft blood flow reduction. We also minimized ischemic injury to the donor small intestine by meticulous ligation of the lumbar vessels to avoid unnecessary blood loss, early ligation of the distal AA to improve perfusion of the graft and intravenous administration of 6-8 mL of lactated Ringer's solution to supply blood volume and improve blood circulation of the small intestinal graft during the surgery. A shorter warm ischemic time was very important for the improvement of graft vitality, the warm ischemic time in our experiment was almost controlled in 40 min. If the time was too long, the normal blood circulation could not be recovered, the result of transplantation would be seriously affected, which appeared as the graft edema, segmental venous stasis, congestion and extremely thin enteric fluid.
It was critical that there was an adequate blood flow from the SMA to the graft and out through the PV smoothly. Based on Zhong[2] and Kiyozak[3] surgery procedure, we refined some techniques. The blood flow of the inferior vena cava (IVC) was not blocked during surgery, so the blood circulation was not interrupted. The cuff technique was used with a cuffed anastomosis of the donor PV to the recipient left renal vein, the whole procedure of anastomosis spent about 5 min. Since the cuff tube was sculped from a polyethylene tube with 2.3 mm in outer diameter and 1.8 mm in inner diameter, there was a larger and standard anastomosis. Furthermore, the endangium of the cuffed PV was well overturned, there was no any exposed anastomostic material in the venous lumen. So the unobstructed rate was much higher, none of rats died of the venous anastomostic complication. The AA column with SMA of the graft was anastomosed end to side to the AA of the recipient, the anastomotic bore was bigger and shapeable, so the anastomosis could be in progress smoothly without any tension. We used low molecular dextran solution without heparin to rinse the anastomosis, the damage to the endothelium was slighter[45-46], and the chance of anastomotic stenosis and thrombosis was greatly reduced[47-50]. These improved techniques resulted in an adequate blood flow to the graft without acute and chronic graft ischemia and the survival rate of the transplanted rat was obviously increased. It was true that removal of one kidney did not increase the mortality in our experiment yet. After removal of one kidney, the remained kidney usually has a capacity to compensate. The adaptation may take place within 12-24 h and reach the largest degree during 1-2 wk. There was no an obvious disadvantage effect on physiological function and some experimental researches such as the absorptive function and permeability of transplanted small intestine could be studied on the model without any inconvenient.
In conclusion, our results suggested that applying these modified techniques would remarkably reduce the complications and improve survival rate in rats, the transplanted small intestine had a long-term fine function, this provided a potentially more consistent and practical model meeting the need of experimental and clinical studies.
Edited by Ma JY
| 1. | Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery. 1971;70:693-702. [PubMed] [Cited in This Article: ] |
| 2. | Zhong R, Grant D, Sutherland F, Wang PZ, Chen HF, Lo S, Stiller C, Duff J. Refined technique for intestinal transplantation in the rat. Microsurgery. 1991;12:268-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Kiyozaki H, Kobayashi E, Toyama N, Miyata M. Segmental small bowel transplantation in the rat: comparison of lipid absorption between jejunal and ileal grafts. JPEN J Parenter Enteral Nutr. 1996;20:67-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Schraut WH, Abraham VS, Lee KK. Portal versus caval venous drainage of small bowel allografts: technical and metabolic consequences. Surgery. 1986;99:193-198. [PubMed] [Cited in This Article: ] |
| 5. | Lee KK, Schraut WH. Structure and function of orthotopic small bowel allografts in rats treated with cyclosporine. Am J Surg. 1986;151:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kimura K, Money SR, Jaffe BM. The effects of size and site of origin of intestinal grafts on small-bowel transplantation in the rat. Surgery. 1987;101:618-622. [PubMed] [Cited in This Article: ] |
| 7. | Fujiwara H, Raju S, Grogan JB, Lewin JR, Johnson WW. Total orthotopic small bowel allotransplantation in the dog. Features of atypical rejection and graft-versus-host reaction. Transplantation. 1987;44:747-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Kimura K, LaRosa CA, Money SR, Jaffe BM. Segmental intestinal transplantation in rats with resected entire small bowel, ileocecal valve, and cecum. J Surg Res. 1988;45:349-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Kaneko H, Hancock W, Schweizer RT. Progress in experimental porcine small-bowel transplantation. Arch Surg. 1989;124:587-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Kimura K, LaRosa CA, Blank MA, Jaffe BM. Successful segmental intestinal transplantation in enterectomized pigs. Ann Surg. 1990;211:158-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Li N, Li JS, Liao CX, Li YS, Wu XH. Successful segmental small bowel allotransplantation in pigs. Chin Med J (Engl). 1993;106:187-190. [PubMed] [Cited in This Article: ] |
| 12. | Zhong R, Grant D, Black R, Stiller C, Duff J. Combined small bowel and kidney transplantation in the rat. Transplant Proc. 1989;21:2907-2908. [PubMed] [Cited in This Article: ] |
| 13. | Schroeder P, Sandforth F, Gundlach M, Deltz E, Thiede A. Functional adaptation of small intestinal mucosa after syngeneic and allogeneic orthotopic small bowel transplantation. Transplant Proc. 1989;21:2887-2889. [PubMed] [Cited in This Article: ] |
| 14. | Martinelli GP, Knight RK, Kaplan S, Racelis D, Dikman SH, Schanzer H. Small bowel transplantation in the rat. Effect of pretransplant blood transfusions and cyclosporine on host survival. Transplantation. 1988;45:1021-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Zhong R, Wang P, Chen H, Sutherland F, Duff J, Grant D. Surgical techniques for orthotopic intestinal transplantation in the rat. Transplant Proc. 1990;22:2443-2444. [PubMed] [Cited in This Article: ] |
| 16. | Schweizer E, Gundlach M, Gassel HJ, Deltz E, Schroeder P. Effects of two-step small bowel transplantation on intestinal morphology and function. Transplant Proc. 1991;23:688. [PubMed] [Cited in This Article: ] |
| 17. | Frankel WL, Zhang W, Afonso J, Klurfeld DM, Don SH, Laitin E, Deaton D, Furth EE, Pietra GG, Naji A. Glutamine enhancement of structure and function in transplanted small intestine in the rat. JPEN J Parenter Enteral Nutr. 1993;17:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Harmel RP. A simplified technique of small intestinal transplantation in the rat. J Pediatr Surg. 1984;19:400-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Sigalet DL, Kneteman NN, Fedorak RN, Kizilisik T, Madsen KE, Thomson AB. Small intestinal function following syngeneic transplantation in the rat. J Surg Res. 1996;61:379-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Price BA, Cumberland NS, Clark CL, Pockley AG, Wood RF. Evidence that orthotopic transposition following rat heterotopic small bowel transplantation corrects overgrowth of potentially pathogenic bacteria. Transplantation. 1996;61:649-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Winkelaar GB, Smith LJ, Martin GR, Sigalet DL. Fat absorption after small intestinal transplantation in the rat. Transplantation. 1997;64:566-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Raofi V, Fontaine MJ, Mihalov ML, Holman DM, Dunn TB, Vitello JM, Asolati M, Kumins NH, Benedetti E. Comparison of jejunal and ileal surveillance biopsies in a porcine model of intestinal transplantation. Transplantation. 1999;68:188-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Li YS, Li JS, Li N. Surgical technique for intestinal transplantation in rats. Huaren Xiaohua Zazhi. 1998;6:667-669. [Cited in This Article: ] |
| 24. | Li YX, Li JS, Li N. Improved technique of vascular anastomosis for small intestinal transplantation in rats. World J Gastroenterol. 2000;6:259-262. [PubMed] [Cited in This Article: ] |
| 25. | Li YS, Li JS, Li N, Jiang ZW, Zhao YZ, Li NY, Liu FN. Evaluation of various solutions for small bowel graft preservation. World J Gastroenterol. 1998;4:140-143. [PubMed] [Cited in This Article: ] |
| 26. | Luther B, Lehmann C, David H, Klinnert J. Preservation of isolated intestinal segments using the University of Wisconsin solution. Transplant Proc. 1991;23:2459. [PubMed] [Cited in This Article: ] |
| 27. | Zhang S, Kokudo Y, Nemoto EM, Todo S. Biochemical evidence of mucosal damage of intestinal grafts during cold preservation in University of Wisconsin, Euro-Collins, and lactated Ringer's solutions. Transplant Proc. 1994;26:1494-1495. [PubMed] [Cited in This Article: ] |
| 28. | Hatcher PA, Deaton DH, Bollinger RR. Transplantation of the entire small bowel in inbred rats using cyclosporine. Transplantation. 1987;43:478-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Grant D, Zhong R, Gunn H, Duff J, Garcia B, Keown P, Wijsman J, Stiller C. Graft-versus-host disease associated with intestinal transplantation in the rat. Host immune function and general histology. Transplantation. 1989;48:545-549. [PubMed] [Cited in This Article: ] |
| 30. | de Bruin RW, Saat RE, Heineman E, Jeekel J, Marquet RL. The effect of cyclosporine A in small-bowel transplantation in rats is dependent on the rat strain combination used. Transplant Proc. 1990;22:2472-2473. [PubMed] [Cited in This Article: ] |
| 31. | Wang M, Qu X, Stepkowski SM, Chou TC, Kahan BD. Beneficial effect of graft perfusion with anti-T cell receptor monoclonal antibodies on survival of small bowel allografts in rat recipients treated with brequinar alone or in combination with cyclosporine and sirolimus. Transplantation. 1996;61:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Alessiani M, Spada M, Dionigi P, Arbustini E, Regazzi M, Fossati GS, Zonta A. Combined immunosuppressive therapy with tacrolimus and mycophenolate mofetil for small bowel transplantation in pigs. Transplantation. 1996;62:563-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Yin DP, Sankary HN, Williams J, Krieger N, Fathman CG. Induction of tolerance to small bowel allografts in high-responder rats by combining anti-CD4 with CTLA4Ig. Transplantation. 1996;62:1537-1539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Fryer J, Grant D, Jiang J, Metrakos P, Ozcay N, Ford C, Garcia B, Behme R, Zhong R. Influence of macrophage depletion on bacterial translocation and rejection in small bowel transplantation. Transplantation. 1996;62:553-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Toogood GJ, Rankin AM, Tam PK, Morris PJ, Dallman MJ. The immune response following small bowel transplantation: I. An unusual pattern of cytokine expression. Transplantation. 1996;62:851-855. [PubMed] [Cited in This Article: ] |
| 36. | Koide S, McVay LD, Frankel WL, Behling CA, Zhou ED, Shimada T, Zhang W, Rombeau JL. Increased expression of tissue cytokines in graft-versus-host disease after small bowel transplantation in the rat. Transplantation. 1997;64:518-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Ozcay N, Fryer J, Grant D, Freeman D, Garcia B, Zhong R. Budesonide, a locally acting steroid, prevents graft rejection in a rat model of intestinal transplantation. Transplantation. 1997;63:1220-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Toogood GJ, Rankin AM, Tam PK, Morris PJ, Dallman MJ. The immune response following small bowel transplantation. II. A very early cytokine response in the gut-associated lymphoid tissue. Transplantation. 1997;63:1118-1123. [PubMed] [Cited in This Article: ] |
| 39. | Mueller AR, Platz KP, Heckert C, Häusler M, Guckelberger O, Schuppan D, Lobeck H, Neuhaus P. The extracellular matrix: an early target of preservation/reperfusion injury and acute rejection after small bowel transplantation. Transplantation. 1998;65:770-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Johnsson C, Bengtsson M, Tufveson G. Recipient-reactive antibodies occur during development of acute graft-versus-host reaction after small bowel transplantation. Transplantation. 1996;62:343-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | van Oosterhout JM, de Boer HH, Jerusalem CR. Small bowel transplantation in the rat: the adverse effect of increased pressure during the flushing procedure of the graft. J Surg Res. 1984;36:140-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Cicalese L, Caraceni P, Nalesnik MA, Borle AB, Schraut WH. Oxygen free radical content and neutrophil infiltration are important determinants in mucosal injury after rat small bowel transplantation. Transplantation. 1996;62:161-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Sugitani A, Bauer AJ, Reynolds JC, Halfter WM, Nomoto M, Starzl TE, Todo S. The effect of small bowel transplantation on the morphology and physiology of intestinal muscle: a comparison of autografts versus allografts in dogs. Transplantation. 1997;63:186-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Kaihara S, Egawa H, Inomata Y, Uemoto S, Asonuma K, Tanaka K. Serotonin as a useful parameter for cold and warm ischemic injury in small bowel transplantation. Transplantation. 1997;64:405-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Buckley RC, Davidson SF, Das SK. The role of various antithrombotic agents in microvascular surgery. Br J Plast Surg. 1994;47:20-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Cox GW, Runnels S, Hsu HS, Das SK. A comparison of heparinised saline irrigation solutions in a model of microvascular thrombosis. Br J Plast Surg. 1992;45:345-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Davidson SF, Brantley SK, Talbot PJ, Das SK. A functional model of microvascular thrombosis. Plast Reconstr Surg. 1990;86:579-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Khouri RK, Cooley BC, Kenna DM, Edstrom LE. Thrombosis of microvascular anastomoses in traumatized vessels: fibrin versus platelets. Plast Reconstr Surg. 1990;86:110-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Davidson SF, Brantley SK, Das SK. Comparison of single-dose antithrombotic agents in the prevention of microvascular thrombosis. J Hand Surg Am. 1991;16:585-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Buckley RC, Davidson SF, Das SK. Effects of ketorolac tromethamine (Toradol) on a functional model of microvascular thrombosis. Br J Plast Surg. 1993;46:296-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |