Published online Apr 15, 2002. doi: 10.3748/wjg.v8.i2.346
Revised: October 15, 2001
Accepted: October 22, 2001
Published online: April 15, 2002
AIM: To determine the NF-κB activity in peripheral blood mononuclear cells (PBMC) in patients with acute cholangitis of severe type (ACST) and correlate the degree of NF-κB activation with severity of biliary tract infection and clinical outcome.
METHODS: Twenty patients with ACST were divided into survivor group (13 cases) and nonsurvivor group (7 cases). Other ten patients undergoing elective gastrectomy or inguinal hernia repair were selected as control group. Peripheral blood samples were taken 24 h postoperatively. PBMC were separated by density gradient centrifugation, then nuclear proteins were isolated from PBMC, and Electrophoretic Mobility Shift Assay (EMSA) used determined. The results were quantified by scanning densitometer of a Bio-Image Analysis System and expressed as relative optical density (ROD). The levels of TNF-α, IL-6, and IL-10 in the plasma of patients with ACST and healthy control subjects were determined by using an enzyme-linked immunoassay (ELISA).
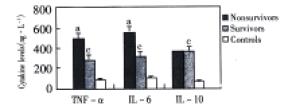
RESULTS: The NF-κB activity was 5.02 ± 1.03 in nonsurvivor group, 2.98 ± 0.51 in survivor group and 1.06 ± 0.34 in control group. There were statistical differences in three groups (P < 0.05). The levels of TNF-α and IL-6 in plasma were (498 ± 53) ng·L-1 and (587 ± 64) ng·L-1 in nonsurvivor group, (284 ± 32) ng·L-1 and (318 ± 49) ng·L-1 in survivor group and (89 ± 11) ng·L-1 and (102 ± 13) ng·L-1 in control group. All patients with ACST had increased levels of TNF-α and IL-6, which were manyfold greater than those of control group, and there was an evidence of significantly higher levels in those of nonsurvivor group than that in survivor group (P < 0.05). The levels of IL-10 in plasma were (378 ± 32) ng·L-1, (384 ± 37) ng·L-1 and (68 ± 11) ng·L-1 in three groups, respectively. All patients had also increased levels of IL-10 when compared with control group (P < 0.05), but the IL-10 levels were not significantly higher in nonsurvivors than in survivors (P > 0.05).
CONCLUSION: NF-κB activity in PBMC in patients with ACST increases markedly and the degree of NF-κB activation is correlated with severity of biliary tract infection and clinical outcome.
- Citation: Gong JP, Liu CA, Wu CX, Li SW, Shi YJ, Li XH. Nuclear factor κB activity in patients with acute severe cholangitis. World J Gastroenterol 2002; 8(2): 346-349
- URL: https://www.wjgnet.com/1007-9327/full/v8/i2/346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i2.346
Acute cholangitis of severe type (ACST) is a common problem facing today's surgeons[1-3]. Despite a multitude of advances in the area of surgical infection and surgical or nonsurgical interventions to treat biliary tract diseases, ACST and biliary sepsis remain a significant cause of morbidity and mortality[4-9]. Many reports have focused on aspects of the proinflammatory cytokines which are believed to be central to the pathophysiology of the sepsis syndrome[10-11]. Recent investigations have shown that expression of many cytokines is closely linked to the activation of transcriptional factors[12-13]. Among several transcriptional regulatory factors involved in immuno-regulatory genes expression, nuclear factor kappa B (NF-κB) acts as a critical step for directing the transcription of many proinflammatory cytokine genes in animal models of sepsis or endotoxemia[14-16]. Investigations regarding the role of NF-κB in human inflammatory diseases are scarce[17-20]. So far, no study has aimed to examine in patients with ACST the relationship among NF-κB activity in peripheral blood mononuclear cells (PBMC), the concentrations of the pro-inflammatory cytokines in plasma, and clinical outcome. The purpose of this study was to determine the NF-κB DNA binding activity in circulating blood cells and the cytokines tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-10 profile in patients with ACST. Attempts were made also to correlate degree of NF-κB activation with severity of biliary tract infection and clinical outcome.
The study population was recruited from a series of 20 patients with a clinical diagnosis of ACST. Among them, 13 were male, and 7 female, ranging in age from 27 to 78 yr. All patients had manifestations of fever, chill, jaundice, and right upper quadrant pain. Other manifestations included two or more of the following clinical conditions: Blood cultures were positive; Core body temperature > 39 °C or < 36 °C; Heart rate > 120 beats/min; Hypotension: A systolic blood pressure of < 12.0 kPa or a reduction of > 5.33 kPa from baseline in the absence of other causes of hypotension; White blood cell count > 1.5 × 109 L-1. These patients were divided into nonsurvivor group (7 cases) and survivor group (13 cases). Ten patients undergoing elective gastrectomy or inguinal hernia repair were selected as control group. Peripheral blood samples were taken 24 h postoperatively.
PBMC were separated by density gradient centrifugation, as previously described[18]. In brief, PBMC were isolated grom blood freshly collected on sodium citrate by centrifugation on Ficoll-Hypaue. Before Ficoll, a fraction of the blood was centrifuged 5 min at 1500 r·min-1 and 1 mL of plasma was collected and put immediately at -20 °C for further cytokine measurements.
Nuclear proteins were isolated from PBMC extract by placing the sample in 0.8 mL of ice-cold hypotonic buffer [10 mmol•L⁻¹HEPES (pH7.9), 10 mL KCl, 0.1 mmol•L⁻¹ EDTA, 0.1 mmol•L⁻¹ ethylene glycol tetraacetic acid, 1 mmol•L⁻¹ DTT; Protease inhibitors (aprotinin, pepstatin, and leupeptin, 10 mg•L⁻¹ each)]. The homogenates were incubated on ice for 20 min, vortexed for 20 s after adding 50 μL of 10 per cent Nonidet P-40, and then centrifuged for 1 minute at 4 °C in an Eppendorf centrifuge. Supernatants were decanted, the nuclear pellets after a single wash with hypotonic buffer without Nonidet P-40 were suspended in an ice-cold hypertonic buffer [20 mmol•L⁻¹ HEPES (pH7.9), 0.4 mol•L⁻¹ NaCl, 1 mmol•L⁻¹ EDTA, 1 mmol•L⁻¹ DTT; Protease inhibitors], incubated on ice for 30 min at 4 °C, mixed frequently, and centrifuged for 15 min at 4 °C. The supernatants were collected as nuclear extracts and stored at -70 °C. Concentrations of total proteins in the samples were determined according to the method of Bradford.
NF-κB binding activity was performed in a 10-uL binding reaction mixture containing 1 × binding buffer [50 mg•L⁻¹ of double-stranded poly (dI-dC), 10 mmmol•L⁻¹ Tris-HCl (pH7.5), 50 mmmol•L⁻¹ NaCl, 0.5 mmmol•L⁻¹ EDTA, 0.5 mmmol•L⁻¹ DTT, 1 mmmol•L⁻¹ MgCl2, and 100 mL•L⁻¹ glycerol], 5 μg of nuclear protein, and 35 fmol of double-stranded NF-κB consensus oligonucleotide (5'-AGTTGAGGGGACTTTCCCAGG-3') that was endly labeled with γ- 32P (111 TBq mmol-1 at 370 GBq L-1) using T4 polynucleotide kinase. The binding reaction mixture was incubated at room temperature for 20 min and analyzed by electrophoresis on 7 per cent nondenaturing polyacrylamide gels. After electrophoresis the gels were dried by Gel-Drier (Bio-Rad Laboratories, Hercules, CA) and exposed to Kodak X-ray films at -70 °C.
The binding bands were quantified by scanning densitometer of a Bio-Image Analysis System. The results were expressed as relative optical density (ROD).
TNF-α, IL-6, and IL-10 levels in the plasma of patients with ACST and healthy control subjects were determined with using an enzyme-linked immunoassay (ELISA). The detection limits of the assays were 50 ng•L⁻¹ (TNF-α), 49 ng•L⁻¹ (IL-6), and 49 ng•L⁻¹ (IL-10). All cytokines assays were performed in duplicate and had intra- and interassay variations lower than 8% and 11%, respectively.
Data were analyzed with using Microsoft Excel with Astute statistical add-in and were expressed as median ± standard error. A value of P≤ 0.05 was considered statistically significant.
The patients of nonsurvivor group died within 35 d, all from complications of SIRS, sepsis or MOF. The patients of survivor group were well discharged from hospital postoperatively in 28 d. The median age in nonsurvivor group was 54 yr, which was not significantly different from that of survivor group (53 yr) (P > 0.05). An admission APACHE II score in nonsurvivor group was 25, which was also not different from that of survivor group (24) (P > 0.05).
The NF-κB activity was 5.02 ± 1.03 in nonsurvivor group, 2.98 ± 0.51 in survivor group and 1.06 ± 0.34 in control group. Therewere statistical differences in three groups (P < 0.05). The NF-κBactivity increased in all patients with ACST, versus the control group (P < 0.05), and the patients of nonsurvivor group had higher levels of NF-κB activation than those of survivor group (P < 0.05, Figure 1).
The levels of TNF-α and IL-6 in plasma were (498 ± 53) ng·L-1 and (587 ± 64) ng·L-1 in nonsurvivor group, (284 ± 32) ng·L-1 and (318 ± 49) ng·L-1 in survivor group and (89 ± 11) ng·L-1 and (102 ± 13) ng·L-1 in control group. All patients with ACST had increased levels of TNF-α and IL-6, which were many-fold greater than those of control group, and there was an evidence of significantly higher levels in those of nonsurvivor group than that in survivor group (P < 0.05). The levels of IL-10 in plasma were (378 ± 32) ng·L-1, (384 ± 37) ng·L-1 and (68 ± 11) ng·L-1 in three groups, respectively. All patients had also increased levels of IL-10 when compared with control group (P < 0.05), but the IL-10 levels were not significantly higher in nonsurvivors than in survivors (P > 0.05). (Figure 2).
The overwhelming inflammatory response in patients with ACST is a major cause which induces systemic inflammatory response syndrome (SIRS) and MOF[1-4]. Mortality in patients with ACST reflects a multifactorial pathology, and neither cytokine concentrations in plasma nor even the APACHE II score can be expected to accurately predict patients' outcomes[5-9]. NF-κB is a protein found in inflammatory cells such as lymphocytes, monocytes, and macrophages. NF-κB activation is stimulated by LPS, TNF-α, and IL-1, the very early mediators or factors in the inflammatory cascade[21,22]. Once stimulated NF-κB activates various parts of the inflammatory resposes: TNF-α, IL-6 and IL-10, and adhesion molecules such as selectins and integrin[23-25]. These mediators and factors then promote further activity of the inflammatory cascade and “off” goes the SIRS and MOF[2,8,9,24]. Therefore, we chose to focus on NF-κB as an important regulatory factor to regulate the expression of multiple cytokine genes. NF-κB is a ubiquitous transcription factor involved in the signal transduction pathway of many inducers of the inflammatory response and is therefore a potentially attractive target for immunomodulatiom to reduce sepsis and organ dysfunction[26], but we are not yet clear about changes of NF-κB and relation between NF-κB and cytokines in patients with ACST. Foulds et al[20] reported that levels of nuclear-bound NF-κB (activated NF-κB) were greater in patients who developed organ dysfunction after surgery, and patients with lower levels of nuclear NF-κB who recovered from surgery without organ dysfunction. Bohrer et al investigated activity of NF-κB in nuclear extracts from PBMC of 15 patients with sepsis, of whom 10 survived. NF-κB activity was measured on days 1, 2, 3, 4, 5, 6, 8, 10, and 14 after admission where available. All patients with NF-κB binding activty exceeding 200% of day 1 died. This small study concluded that NF-κB activation might be an important event in clinical sepsis. But, Adib-Conquy et al[27] found the expression of NF-κB was signifcantly reduced for all patients with sepsis and trauma as compared with control subjects. In our study, by comparing the predictive value of measuring NF-κB activity in PBMC and the concentrations of some pro- and anti-inflammatory mediators in plasma in patients with ACST, we found that the NF-κB activity measured in the PBMC was a better overall predictor of mortality than the balance and time course of pro- and anti-inflammatory cytokines released in plasma. On the basis of these results NF-κB would seem to be a more sensitive molecular marker assay when compared with cytokines used as an indicator of sepsis.The expressions of many genes involved in the inflammatory and immune processes are regulated by NF-κB, TNF-α, IL-6, and IL-10, and they possess NF-κB binding sites in the promoter region, enabling messenger ribonucleic acid to express in response to extracellular stimuli[28-31]. TNF-α, IL-6, and IL-10 have all been implicated in the pathogenesis of SIRS and MOF that results from trauma, injury, infection, and sepsis[32-43]. In the present study, we found that TNF-α and IL-6 were elevated in the patients with ACST and there were higher levels of TNF-α and IL-6 in patients who survived than in patients who died, which is in agreement with previous reports. These previous studies also showed that levels were higher in patients with sepsis when compared with trauma patients. We found significant relationship between NF-κB activation and circulating concentrations of TNF-α and IL-6. IL-10, as an anti-inflammtory mediator, was elevated in the patients with ACST but reduced in patients who died. There might be other transcription factor as AP-1 involved in the signal transduction pathway of the inflammatory response[16,21,23,44-48] and its mechanism is going on.
In conclusion, we have shown NF-κB activation in PBMCs in patients with ACST increased markedly before death and was related to plasma TNF-αand IL-6 concentrations. These findings have important implications for the development of future therapeutic interventions in the critically ill and support the need for further study of the role of NF-κB activation in mortality from ACST and MOF.
Edited by Hu DK
| 1. | Zhi QH. New development of biliary surgery in China. World J Gastroenterol. 2000;6:187-192. [PubMed] [Cited in This Article: ] |
| 2. | Kimmings AN, van Deventer SJ, Rauws EAJ K, Gouma DJ. Systemic inflammatory response in acute cholangitis and after subsequent treatment. Eur J Surg. 2000;166:700-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Lillemoe KD. Surgical treatment of biliary tract infections. Am Surg. 2000;66:138-144. [PubMed] [Cited in This Article: ] |
| 5. | Parker SJ, Watkins PE. Experimental models of gram-negative sepsis. Br J Surg. 2001;88:22-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Tabrizi AR, Zehnbauer BA, Freeman BD, Buchman TG. Genetic markers in sepsis. J Am Coll Surg. 2001;192:106-107; quiz 145-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Jackson GD, Dai Y, Sewell WA. Bile mediates intestinal pathology in endotoxemia in rats. Infect immun. 2000;68:4714-4719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Islam AF, Moss ND, Dai Y, Smith MS, Collins AM, Jackson GD. Lipopolysaccharide-induced biliary factors enhance invasion of Salmonella enteritidis in a rat model. Infect immun. 2000;68:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Erwin PJ, Lewis H, Dolan S, Tobias PS, Schumann RR, Lamping N, Wisdom GB, Rowlands BJ, Halliday MI. Lipopolysaccharide binding protein in acute pancreatitis. Crit Care Med. 2000;28:104-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kordzaya DJ, Goderdzishvili VT. Bacterial translocation in obstructive jaundice in rats: role of mucosal lacteals. Eur J Surg. 2000;166:367-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Ohtsuka M, Miyazaki M, Kubosawa H, Kondo Y, Ito H, Shimizu H, Shimizu Y, Nozawa S, Furuya S, Nakajima N. Role of neutrophils in sinusoidal endothelial cell injury after extensive hepatectomy in cholestatic rats. J Gastroenterol Hepatol. 2000;15:880-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ito Y, Machen NW, Urbaschek R, McCuskey RS. Biliary obstruction exacerbates the hepatic microvascular inflammatory response to endotoxin. Shock. 2000;14:599-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Han SJ, Choi JH, Ko HM, Yang HW, Choi IW, Lee HK, Lee OH, Im SY. Glucocorticoids prevent NF-kappaB activation by inhibiting the early release of platelet-activating factor in response to lipopolysaccharide. Eur J immunol. 1999;29:1334-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | West MA, Clair L, Kraatz J, Rodriguez JL. Endotoxin tolerance from lipopolysaccharide pretreatment induces nuclear factor-kappaB alterations not present in C3H/HeJ mice. J Trauma. 2000;49:298-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Reddy SA, Huang JH, Liao WS. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-kappa B activation. J immunol. 2000;164:1355-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Paterson RL, Galley HF, Dhillon JK, Webster NR. Increased nuclear factor kappa B activation in critically ill patients who die. Crit Care Med. 2000;28:1047-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Arnalich F, Garcia-Palomero E, López J, Jiménez M, Madero R, Renart J, Vázquez JJ, Montiel C. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect immun. 2000;68:1942-1945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Pennington C, Dunn J, Li C, Ha T, Browder W. Nuclear factor kappaB activation in acute appendicitis: A molecular marker for extent of disease. Am Surg. 2000;66:914-918; discussion 914-918;. [PubMed] [Cited in This Article: ] |
| 20. | Foulds S, Galustian C, Mansfield AO, Schachter M. Transcription factor NF kappa B expression and postsurgical organ dysfunction. Ann Surg. 2001;233:70-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT, Thurman RG. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278:G652-G661. [PubMed] [Cited in This Article: ] |
| 22. | Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 955] [Cited by in F6Publishing: 938] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 23. | Hedin KE, Kaczynski JA, Gibson MR, Urrutia R. Transcription factors in cell biology, surgery, and transplantation. Surgery. 2000;128:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 24. | Stärkel P, Horsmans Y, Sempoux C, De Saeger C, Wary J, Lause P, Maiter D, Lambotte L. After portal branch ligation in rat, nuclear factor kappaB, interleukin-6, signal transducers and activators of transcription 3, c-fos, c-myc, and c-jun are similarly induced in the ligated and nonligated lobes. Hepatology. 1999;29:1463-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Strieter RM, Kunkel SL. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J immunol. 2000;164:2738-2744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J immunol. 2000;165:3541-3544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 259] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, Dhainaut JF, Cavaillon JM. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162:1877-1883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Heumann D, Adachi Y, Le Roy D, Ohno N, Yadomae T, Glauser MP, Calandra T. Role of plasma, lipopolysaccharide-binding protein, and CD14 in response of mouse peritoneal exudate macrophages to endotoxin. Infect immun. 2001;69:378-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Belich MP, Salmerón A, Johnston LH, Ley SC. TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105. Nature. 1999;397:363-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 187] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Bone-Larson CL, Simpson KJ, Colletti LM, Lukacs NW, Chen SC, Lira S, Kunkel SL, Hogaboam CM. The role of chemokines in the immunopathology of the liver. Immunol Rev. 2000;177:8-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Han DW. The clinical sine of subsequent liver injury induced by gut derived endotoxemia. Shijie Huaren Xiaohua Zazhi. 1999;7:1055-1058. [Cited in This Article: ] |
| 32. | Zhang SC, Dai Q, Wang JY, He BM, Zhou K. Gut-derived endotoxemia: one of the factors leading to production of cytokines in liver diseases. World J Gastroenterol. 2000;6:16. [Cited in This Article: ] |
| 33. | Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 376] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 34. | Gong JP, Xu MQ, Li K, Zhu J, Han BL. Expression of CD14 in Kupffer cells induced by lipopolysaccharide. Acta Acad Med Mil Tert. 2001;23:425-428. [Cited in This Article: ] |
| 35. | Koo DJ, Chaudry IH, Wang P. Kupffer cells are responsible for producing inflammatory cytokines and hepatocellular dysfunction during early sepsis. J Surg Res. 1999;83:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Hardaway RM. A review of septic shock. Am Surg. 2000;66:22-29. [PubMed] [Cited in This Article: ] |
| 37. | Sindram D, Porte RJ, Hoffman MR, Bentley RC, Clavien PA. Synergism between platelets and leukocytes in inducing endothelial cell apoptosis in the cold ischemic rat liver: A Kupffer cell-mediated injury. FASEB J. 2001;15:1230-1232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Deaciuc IV, D'Souza NB, Sarphie TG, Schmidt J, Hill DB, McClain CJ. Effects of exogenous superoxide anion and nitric oxide on the scavenging function and electron microscopic appearance of the sinusoidal endothelium in the isolated, perfused rat liver. J Hepatol. 1999;30:213-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128-130. [PubMed] [Cited in This Article: ] |
| 40. | Neubauer K, Ritzel A, Saile B, Ramadori G. Decrease of platelet-endothelial cell adhesion molecule 1-gene-expression in inflammatory cells and in endothelial cells in the rat liver following CCl (4)-administration and in vitro after treatment with TNFalpha. Immunol Lett. 2000;74:153-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Assy N, Jacob G, Spira G, Edoute Y. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262. [PubMed] [Cited in This Article: ] |
| 42. | Blunck R, Scheel O, Müller M, Brandenburg K, Seitzer U, Seydel U. New insights into endotoxin-induced activation of macrophages: involvement of a K+ channel in transmembrane signaling. J immunol. 2001;166:1009-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J immunol. 2000;164:549-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 223] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Ling YL, Meng AH, Zhao XY, Shan BE, Zhang JL, Zhang XP. Effect of cholecystokinin on cytokines during endotoxic shock in rats. World J Gastroenterol. 2001;7:667-671. [PubMed] [Cited in This Article: ] |
| 45. | Gordon H. Detection of alcoholic liver disease. World J Gastroenterol. 2001;7:297-302. [PubMed] [Cited in This Article: ] |
| 46. | Bai XY, Jia XH, Cheng LZ, Gu YD. Influence of IFN alpha-2b and BCG on the release of TNF and IL-1 by Kupffer cells in rats with hepatoma. World J Gastroenterol. 2001;7:419-421. [PubMed] [Cited in This Article: ] |
| 47. | Wang LS, Zhu HM, Zhou DY, Wang YL, Zhang WD. Influence of whole peptidoglycan of bifidobacterium on cytotoxic effectors produced by mouse peritoneal macrophages. World J Gastroenterol. 2001;7:440-443. [PubMed] [Cited in This Article: ] |
| 48. | Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y. Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol. 2001;7:836-840. [PubMed] [Cited in This Article: ] |