Published online Jun 15, 2001. doi: 10.3748/wjg.v7.i3.363
Revised: February 3, 2001
Accepted: February 12, 2001
Published online: June 15, 2001
AIM: To observe the inhibition of antisense oligonucleotides (asON) phosphorthioate to the tissue inhibitors metalloproteinase-1 (TIMP-1) gene and protein expression in the liver tissue of immunologically induced hepatic fibrosis rats. The possibility of reversing hepatic fibrosis through gene therapy was observed.
METHODS: Human serum albumin (HSA) was used to attack rats, as hepatic fibrosis model, in which asONs were used to block the gene and protein expressing TIMP-1. According to the analysis of modulator, structure protein, coding series of TIMP-1 genome, we designed four different asONs. These asONs were injected into the hepatic fibrosis models through coccygeal vein. The results was observed by RT-PCR for measuring TIMP-1 mRNA expression, immunohistochemistry and in situ hybridization for collagen I, III, special staining of collagen fiber, and electron microscopic examination.
RESULTS: Hepatic fibrosis could last within 363 days in our modified model. The expressing level of TIMP-1 was high during hepatic fibrosis process. It has been proved by the immunohistochemical and the electron microscopic examination that the asON phosphorthioate of TIMP-1 could exactly express in vivo. The effect of colchicine was demonstrated to inhibit the expressing level of mRNA and the content of collagen I, III in the liver of experimental hepatic fibrosis rats. However, the electron microscopy research and the pathologic grading of hepatic fibrosis showed that there was no significant difference between the treatment group and the model group (P > 0.05).
CONCLUSION: The experimental rat model of hepatic fibrosis is one of the preferable models to estimate the curative effect of anti-hepatic fibrosis drugs. The asON phosphorthioate of TIMP-1 could block the gene and protein expression of TIMP-1 in the liver of experimental hepatic fibrosis rats at the mRNA level. It is possible to reverse hepatic fibrosis, and it is expected to study a new drug of anti-hepatic fibrosis on the genetic level. Colchicine has very limited therapeutic effect on hepatic fibrosis, furthermore, its toxicity and side effects are obvious.
- Citation: Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol 2001; 7(3): 363-369
- URL: https://www.wjgnet.com/1007-9327/full/v7/i3/363.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i3.363
In China, the incidence of liver cirrhosis is still high for quite a long period in the future[1-5]. The pathological basis of hepatic cirrhosis is fibrosis[6-14]. Many factors inducing liver injury and inflammation will lead to chronic liver disease, and hepatic fibrosis is inevitable[15-25]. Researchers have paid more attention to reversing the hepatic fibrosis[26,27].
Recently, studies on the role of tissue inhibitors of metalloproteinase-1 (TIMP-1) and tissue inhibitors of metalloproteinase-2 (TIMP-2) in the process of hepatic fibrosis have attracted more attentions[28-33]. The main aim of our study is to seek for an approach of reversing hepatic fibrosis, to investigate the role of TIMPs in the pathogenesis of hepatic fibrosis and to observe the fluctuation of TIMPs in patients with various liver diseases in order to set up a new laboratory diagnotic index of hepatic fibrosis. It had been considered as the target gene and the antisense oligonucleotides (asON) phosphorothioate was used to inhibit the gene and protein expressing in experimental rat hepatic fibrosis.
In 1973, Miller et al[34] reported that non-ionic oligonucleotide and its derivant could specifically inhibit cellular DNA/RNA replication, transcription, translation. In 1978, Stepherson et al[35] obtained favorable anti-virus effect in infected fibroblast by Rous sarrcoma virus using 13-polyoligonucleotide. Since then attention has been made to the studies of the application of antisense oligonucleotide technology in antineoplastic and antiviral therapy[36,37]. This technology has been carried out under clinical trial stage[38-40]. In present study, we attacked the rats with HSA in order to establish experimental immune hepatic fibrosis model, and tried to block the gene and protein expression of TIMP-1 in rats with asON, so as to investigate the possible mechanism of reversing hepatic fibrosis.
According to the whole TIMP-1 cDNA sequence in rats[41], we analysed the sequences of modulator, structural protein and coding region and then designed four different antisence oligonucleotide as follows: DNA Seq1 5'-GGCGCCATCGTGGTATCTGC-3', Seq2 5'-GCTCTAGCGTGTCTCTAGGA-3', Seq3 5'-GATAAACAGTGTTCAGGCTTC-3', Seq4 5'-GTTCAGGCTTCAGCTTTTGC-3'.
Twenty-polyligodeoxyribonucleotide phosphorthi-oate was automatically synthesized by the 391A PCRMATE EP DNA synthesis machine (ABI Company, USA). AsON was modified by phosphorthioate using TETD/acetonitrile method and purified by high-performance thin layer chromatography (purity > 99%), which was accomplished by Wang SQ and Wang XH (Chinese Academy of Military Medical Sciences).
Forty healthy adult female Wistar rats, weighing 120-150 g (provided by Experimental Animal Center of FMMU), were employed in the study. Immune hepatic fibrosis rat model was produced by immunological attacking with HSA, a method introduced by Wang et al[42]. Anti-mouse monoclonal antibody IgG was bought from Coulter Company (France).
The animals survived from the experimental attack were randomly allocated as follows. Model group: animals with hepatic fibrosis did not receive any interventional factors. Treatment group: animal models in this group received colchicine (0.28 mg·kg-1) treatment, six times a week, lasting 3 months. Experimental group: animals in this group received asON phosphorthioate through coccygeal vein injection (20 μg·g-1), every other day, 15 times in all. Control group (normal group): animals in this group were injected with same quantity of N.S. Three months after the beginning of injection, all animals were killed under narcosis, and their liver samples were kept in N2, 100 mL·L-1 formalin or glutaraldehyde for designed investigations.
Pathologic observation Some hepatic sections were stained with hematoxylin and eosin, while other sections for Von Gieson and Masson special staining.
Transmission electron microscope The liver samples were fixed with glutaraldehyde, and examined with electron microscope.
Immunohistochemical staining of TIMP-1 The liver samples were embedded with paraffin, and serial sections at 4 μm thickness were prepared. SP immunostaining was performed as described by streptomycin avidin-peroxidase immunochemistry kit (purchased from Maxim Biological Technology Company). Paraffin was removed from the sections with xylene and rehydrated with graded ethanol. After repairing the antigens, nonspecific binding sites were blocked by a 20 min preincubation with 100 mL·L-1 normal human serum. The sections were incubated with monoclonal antibody against TIMP-1 at 4 °C overnight, and then secondary antibody at 37 °C for 30-40 min, avidin-peroxidase at 37 °C for 20 min, finally added DAB to be colorated. After several washings, the sections were counterstained with hematoxylin, dehydrated with ethanol, rinsed in xylene, and the sections mounted with gum for microscopic examination and photography.
Immunohistochemical staining of collagen I, III Immunohistochemical kits of collagen I, III were purchased from Bo-Shi-De Biological Technology Limited Company (Wuhan, China). The numbers of antibodies of collagen I, III were BA0325, BA0326 respectively. The immunostaining was performed as described by the kit.
Procollagenase I, III in situ hybridization We used digoxin-labeled probes to detect the mRNA expression of procollagen I, III. The in situ hybridization kit was purchased from Bo-Shi-De Biological Technology Limited Company (Wuhan, China, No. MK1171). In situ hybidization was performed according to the manufacturer's directions. Briefly, the paraffin embedded serial sections(thickness 4 μm), were dried at 80 °C, paraffin was then removed by xylene and rehydrated in graded ethanol. The sections were acidified in HCl for 30 min, and blocked in 300 mL·L-1 3 mL, H2O2, for 10 min before digestion in proteinase K for 30 min, and then dehydrated with graded ethanol. After prehybridization at 37 °C-40 °C for 2 h, the labeled cDNA probes were denatured in hybridization buffer at 95 °C for 10 min, then -20 °C for 10 min, added on tissues which had been prehybridized at 37 °C overnight. Sections were washed with 2 × SSC, 1 × SSC, 0.2 × SSC, added Buffer I, and blocking water at room temperature for 20 min, and then rabbit anti-digoxin at 37 °C for 60 min, biotinylated goat anti-rabbit at 37 °C for 30 min, SABC at 37 °C for 30 min, finally added DAB to be colorated. After several washings, the sections were counterstained with hematoxylin, dehydrated with ethanol, rinsed in xylene, and mounted the sections with gum for microscopic examination and photography.
Image pattern analysis and data processing After gray scale scanning and quantification the statistical analysis was performed. Comparison among groups was analyzed by t test, and the P value was used to judge the significant difference.
The PCR primers of TIMP-1 are listed in Table 1.
| Primer | Nucleic acid sections | Position(bp) | |
| TIMP-1 | Positive strand | 5'-TTCGTGGGGACACCAGAAGTC-3' | |
| Antisence strand | 5'-TATCTGGGACCGCAGGGACTG-3' | 482 | |
| β-actin | Positive strand | 5'-GGAGAAGATGACCCAGATCA-3' | |
| Antisence strand | 5'-GATCTTCATGAGGTAGTCAG-3' | 234 | |
RNA extraction The total RNA of liver was extracted with total RNA isolation system (produced by Promega Co.).
PCR amplification PCR was performed in 20 μL reactive volume containing 2 μL cDNA, 2 μL 10 × PCR buffer, 2 μL (2 mmol·L-1) 4 × dNTP, 10 mmol·L-1 primer (2 μL TIMP-1, 2 μL β-actin), and 1 U Taq DNA polymerase. The samples were subjected to 30 thermal cycles of 2 min at 97 °C for predenaturation, 30 s at 94 °C for denaturing, 30 s at 56 °C for annealing, 50 s at 72 °C for extension, and 7 min at 72 °C for final extension after the last cycle.
Quantitative analysis of PCR product Ten μL samples of PCR product was subjected to electrophoresis in 20 g·L-1 agarose gel with TAE buffer at 50 V for 1 h. After colorating with ethidium bromide and image forming, the quantitative analysis was performed. TIMP-1/β-actin quotient is the indication of TIMP-1 expression level.
The pathologic grading of hepatic fibrosis used in our present paper was reported by Wang et al[43].
0: Normal liver without hyperplasia of collagenous fibers.
I: Slight extension of collagenous fibers from portal area or central veins.
II: Remarkable extension of collagenous fibers, without connecting each other and encysting the whole hepatic lobules.
III: Remarkable extension of collagenous fibers, connecting each other, and encysting the whole hepatic lobules.
IV: The hepatic lobules are encysted and separated by collagenous fibers. The normal structure of hepatic lobules is destroyed. The pseudolobules are formed, and it is dominant by big-square pseudolobuli.
V: The structure of hepatic lobules is fully destroyed, and the big square pseudolobules and small round ones occupy 50% respectively.
VI: The small round hepatic lobuli occupy almost the whole liver and the hyperplatic thick collagenous fibers are visible.
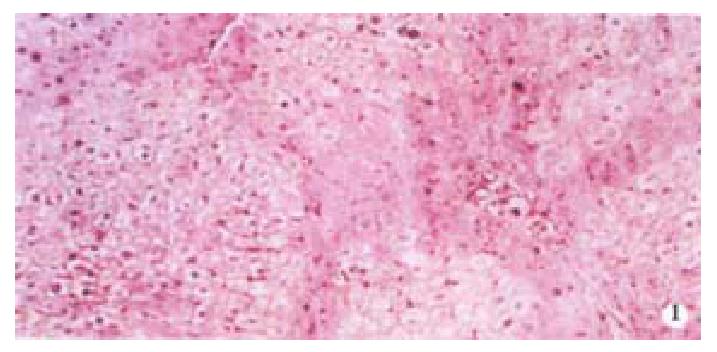
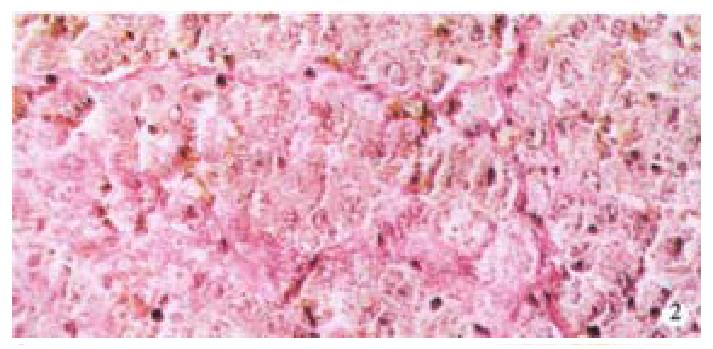
Image pattern analysis showed that the positive density value of the model group was the highest, and the colchicine treatment group was in the second place. There is no significant difference (P > 0.05) between the above-mentioned two groups, and the density value of the experimental group is low (P < 0.001, Table 2, Figure 1, Figure 2).
| Group | n | TIMP-1 |
| Normal group | 10 | 59.8 ± 20.3 |
| Experimental group | 6 | 98.7 ± 25.7 |
| Treatment group | 6 | 396.1 ± 58.4 |
| Model group | 6 | 481.1 ± 61.0 |
The gene expression level of the model group was the highest, and the colchicine treatment group was the next. There was no significant difference (P > 0.05) between the two above mentioned groups. The gene expression level of the experimental group was low (P < 0.001, Table 3).
| Group | n | TIMP-1 |
| Normal group | 10 | 0.3 ± 0.1 |
| Experimental group | 6 | 0.6 ± 0.1 |
| Treatment group | 6 | 1.7 ± 0.4 |
| Model group | 6 | 1.9 ± 0.5 |
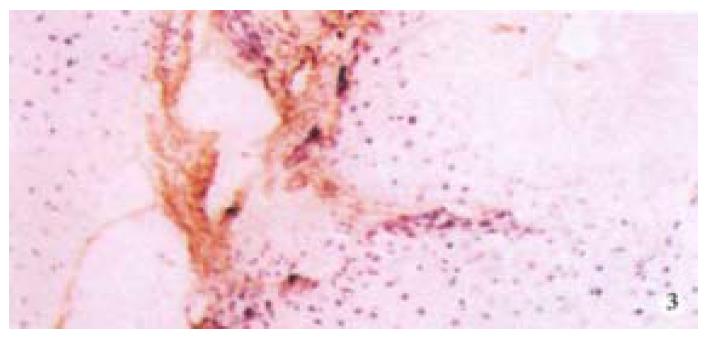
The hepatic content of collagen I, III of all the experimental animals was remarkably higher than that of the normal control group (P < 0.05, P < 0.001). Moreover, in all experimental animals, the hepatic content of collagen I, III of the model group was the highest, but that in the experimental group was the lowest (Table 4, Figure 3).
| Group | n | Collagen I | Collagen III |
| Normal group | 10 | 62 ± 17 | 100 ± 19 |
| Experimental group | 6 | 165 ± 47 | 349 ± 48 |
| Treatment group | 6 | 314 ± 65 | 516 ± 72 |
| Model group | 6 | 441 ± 87 | 699 ± 102 |
There was distinct decrease of procollagen I, III expression in the liver of rats of the experimental group, it was significantly different compared with the model group (P < 0.05). But there was no statistical difference (Table 5), between the experimental group and the treatment group.
| Group | n | Procollagen I | Procollagen III |
| Normal group | 10 | 245.6 ± 88.7 | 228.3 ± 57.5 |
| Experimental group | 6 | 917.9 ± 206.9 | 1050.9 ± 271.4 |
| Treatment group | 6 | 896.8 ± 198.1 | 977.8 ± 221.4 |
| Model group | 6 | 1217.3 ± 202.9 | 1484.5 ± 249.1 |
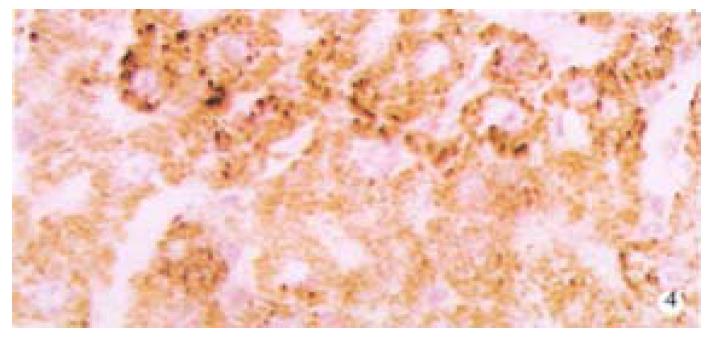
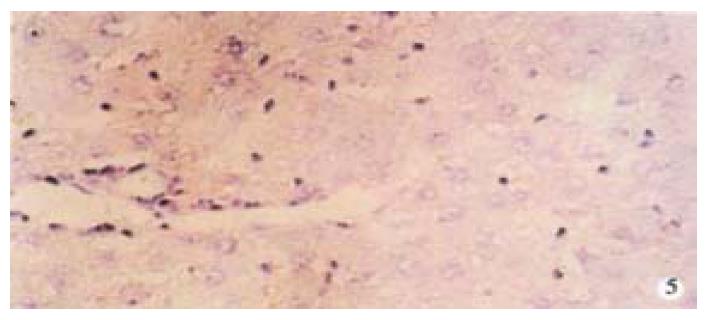
From Table 6, we can see the significant difference of pathologic grading between the control and other groups (P < 0.05, P < 0.001). The pathologic grading status was better in the experimental group than that in the treatment group (Figure 4, Figure 5). There was no significant difference between the treatment group and the model group.
| Group | n | Pathologic grading of hepatic fibrosis | ||||||
| 0 | I | II | III | IV | V | VI | ||
| Normal group | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Experimental group | 6 | 0 | 2 | 3 | 1 | 0 | 0 | 0 |
| Treatment group | 6 | 0 | 0 | 0 | 1 | 2 | 2 | 1 |
| Model group | 6 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
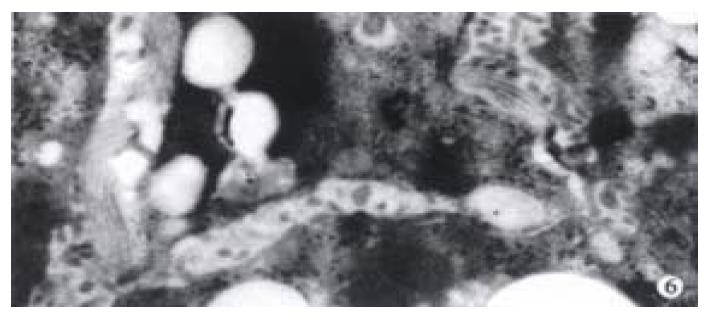
The activated hepatic stellate cell (HSC) and surrounding collagen fibers in the experimental group and the treatment group were observed. Neither deposition of collagen at the portal area in the experimental group, nor a lot of collagen deposition at the portal area were found in the treatment group. Compared with the model group, there was little change in the treatment group (Figure 6).
Nucleic acid complemented with DNA/RNA of genome is called antisense nucleic acid, including antisense DNA, antisense RNA, and ribozyme. According to the complementary principle, oligonucleotide can specifically bind to DNA/RNA of genome, therefore, the replication, transcription, translation of definite gene will be specifically inhibited and blocked. Such oligonucleotide is called antisense oligonucleotide (asON)[44]. AsON has the advantage of specificity, high potency and easy to be synthesized artificially. If asON is properly modified, it is possible to enhance the penetrating power, stability and bioavailability. Therefore, the purpose of this study mainly focused on exploring a possible way of reversing hepatic fibrosis and try to manufacture a new drug for anti-hepatic fibrosis through specifically inhibiting the gene and protein expression of TIMP-1 by asON phosphorthioate.
In our study, the expression level of TIMP-1 in the injured liver was found, and the expression appeared early with a major amplitude, especially in hepatic fibrosis and cirrhosis. The promoting effect of TIMP-1 in hepatic fibrosis and cirrhosis is stronger than TIMP-2[45-50], and 38% sequences of TIMP-2 are identical with TIMP-1, 12 cysteines of TIMP-2 are situated at the same sites, having similar spatial structure with TIMP-1, but they are non-glycosylated. There is no cross immunity reaction between TIMP-1 and TIMP-2[51-56]. TIMP-1 was chosen as the target gene. In our study, we found that the expression of TIMP-1 in the model group was four times higher than that in the normal control group. However, after asON phosphorthioate was injected through coccygeal vein, the gene and protein expression of TIMP-1 was remarkably lower than those in the other experimental groups (P < 0.001). This result was demonstrated by the detection of TIMP-1 in liver by the immunohistochemical method and PCR. Our study clearly indicate that the asON phosphorthioate can be expressed in vivo, and is able to block the gene and protein expression of TIMP-1 in experimental hepatic fibrosis rats so as to enable the reversion of hepatic fibrosis.
In healthy human liver, the collagen type I, III accounts for about 80% of the total collagen of liver, while it rises up to more than 95% in fibrotic livers. The collagen type I covers about 60%-70% of the total collagen of fibrotic livers, and type III only 20%-30%[57-60]. MMP-1 is the main protease which can be inhibited by TIMP-1. Collagen I, III is the main target of MMP-1. MMP-1 has similar capacity of degrading collagen I, III. Therefore, collagen I, III are regarded as the important parameters to reflect the metabolism of collagen. Through the observation of the quantity of the collagen, we can judge the therapeutic effect of the anti-fibrotic drugs of the liver[61-63].
It was found that, in a same experimental animal treated with a same factor, the change of the hepatic content of collagen I, III showed a high uniformity (r = 0.904, P < 0.01). The hepatic content of collagen I, III was lower in the experimental group (treated with asON) and the treatment group with Colchicine than that in the model group. This suggests that the therapeutic factors have some effect in reversing the hepatic fibrosis. Immunohistological examination revealed that the content of collagen I, III was significantly lower in the experimental group than that in the treatment group with chlocoichne, while the mRNA levels of procollagen I, III were similar. In the liver of all experimental animals, both the hepatic content of collagen I, III and the mRNA level were significantly higher than the normal control group, which suggests the intervening factors do not reverse the hepatic fibrosis to normal within a period of time. This also shows the limitation of the therapeutic factor at present, which is demonstrated by the pathologic grading of hepatic fibrosis and the electron microscopic findings.
Histological examination revealed that asON therapy had better effect on hepatic fibrosis than colchicine. The asON is efficacious to reverse the hepatic fibrosis. However, colchicine had extremely limited effect on the therapy of hepatic fibrosis. The pathologic grading of hepatic fibrosis showed no remarkable difference as compared with the model group, although chlocoichne had some inhibitory effect on the rat hepatic content of collagen I, III and mRNA level.
Until now, the study on gene therapy for hepatic fibrosis and cirrhosis is limited to the animal models, and not yet applied to human beings[14,64]. The target gene is HGF[65] or TGF-β[66]. It has not yet been reported to make TIMP as the target gene. These developments are noteworthy, but there are many works to be done before it can be used in clinical practice[67].
To sum up, the animal experiment, pathological examination and electronmicroscopic observation have proved that the asON phosphorthioate directed to TIMP-1 has some anti-hepatic fibrosis effect in the experimental immune hepatic fibrosis rat models. This result is quite heartening, but many studies should be done, especially the study of internalization of asON in vivo.
Dr. Qing-He Nie, graduated from Qinghai Medical College as a doctor in 1983, got master degree at Beijing 302 Army Hospital in 1993, got doctor degree at the Third Military Medical University in 1998, engaged in postdoctoral research at the Fourth Military Medical University from 1998 to 2000, now an associate professor, specialized in clinical and experimental research of infectious diseases, had more than 90 papers published, coauthor of ten books, first author of one book.
Edited by Wu XN and Ma JY
| 1. | Zhu YH, Hu DR, Nie QH, Liu GD, Tan ZX. Study on activation and c-fos, c-jun expression of in vitro cultured human hepatic stellate cells. Shijie Huaren Xiaohua Zazhi. 2000;8:299-302. [Cited in This Article: ] |
| 2. | Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I,III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397-403. [PubMed] [Cited in This Article: ] |
| 3. | Huang ZG, Zhai WR, Zhang YE, Zhang XR. Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J Gastroenterol. 1998;4:206-209. [PubMed] [Cited in This Article: ] |
| 4. | Jia JB, Han DW, Xu RL, Gao F, Zhao LF, Zhao YC, Yan JP, Ma XH. Effect of endotoxin on fibronectin synthesis of rat primary cultured hepatocytes. World J Gastroenterol. 1998;4:329-331. [PubMed] [Cited in This Article: ] |
| 5. | Cheng ML, Wu YY, Huang KF, Luo TY, Ding YS, Lu YY, Liu RC, Wu J. Clinical study on the treatment of liver fibrosis due to hepatitis B by IFN-alpha(1) and traditional medicine preparation. World J Gastroenterol. 1999;5:267-269. [PubMed] [Cited in This Article: ] |
| 6. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 793] [Cited by in F6Publishing: 876] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 7. | Liu XS, Li DG, Lu HM, Xu QF. Effects of tetrandrine and verapamil on fibroblastic growth and proliferation. China Natl J New Gastroenterol. 1997;3:68. [Cited in This Article: ] |
| 8. | Qiu DK, Li H, Zeng MD, Li JQ. Effect of cordyceps polysaccharides-liposome on the expression of interstitial mRNA in rats with hepatic fibrosis. Xin Xiaohuabingxue Zazhi. 1997;5:417-418. [Cited in This Article: ] |
| 9. | Yan JP, Liu JC, Ma XH, Jia JB, Zhao YC, Xu RL, Li CM, Han DW. Immunohistochemical study on basic fibroblast growth factor in experimental liver fibrosis. Xin Xiaohuabingxue Zazhi. 1997;5:642-644. [Cited in This Article: ] |
| 10. | Wang X, Chen YX, Xu CF, Zhao GN, Huang YX, Wang QL. Relationship between tumor necrosis factor-α and liver fibrosis. Huaren Xiaohua Zazhi. 1998;6:106-108. [Cited in This Article: ] |
| 11. | Xu LX, Xie XC, Jin R, Ji ZH, Wu ZZ, Wang ZS. Effect of selenium in rat experimental liver fibrosis. Huaren Xiaohua Zazhi. 1998;6:133-135. [Cited in This Article: ] |
| 12. | Li DG, Lu HM, Chen YW. Studies on anti liver fibrosis of tetrandrine. Shijie Huaren Xiaohua Zazhi. 1999;7:171-172. [Cited in This Article: ] |
| 13. | Wu YA, Kong XT. Anti-hepatic fibrosis effect of pentoxifylling. Shijie Huaren Xiaohua Zazhi. 1999;7:265-266. [Cited in This Article: ] |
| 14. | Liu F, Liu JX, Cao ZC, Li BS, Zhao CY, Kong L, Zhen Z. Relationship between TGF-β1, serum indexes of liver fibrosis and hepatic-tissue pathology in patients with chronic liver diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:519-521. [Cited in This Article: ] |
| 15. | Yang YX, Kang JY. Mechanism and serum diagnosis of liver fibrosis. Xin Xiaohuabingxue Zazhi. 1997;5:119-120. [Cited in This Article: ] |
| 16. | Gu SW, Luo KX, Zhang L, Wu AH, He HT, Weng JY. Relationship between ductule proliferation and liver fibrosis of chronic liver disease. Shijie Huaren Xiaohua Zazhi. 1999;7:845-847. [Cited in This Article: ] |
| 17. | Li BS, Wang J, Zhen YJ, Liu JX, Wei MX, Sun SQ, Wang SQ. Experimental study on serum fibrosis markers and liver tissue pathology and hepatic fibrosis in immuno-damaged rats. Shijie Huaren Xiaohua Zazhi. 1999;7:1031-1034. [Cited in This Article: ] |
| 18. | Wang Y, Gao Y, Huang YQ, Yu JL, Fang SG. Gelatinase A proenzyme expression in the process of experimental liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:165-167. [Cited in This Article: ] |
| 19. | Wang FS, Wu ZZ. Current situation in studies of gene therapy for liver cirrhosis and liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:371-373. [Cited in This Article: ] |
| 20. | Wang GQ, Kong XT. Action of cell factor and Decorin in tissue fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:458-460. [Cited in This Article: ] |
| 21. | Liu F, Wang XM, Liu JX, Wei MX. Relationship between serum TGF-β1 of chronic hepatitis B and hepatic tissue pathology and hepatic fibrosis quantity. Shijie Huaren Xiaohua Zazhi. 2000;8:528-531. [Cited in This Article: ] |
| 22. | Yao XX. Diagnosis and treatment of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:681-689. [Cited in This Article: ] |
| 23. | Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 204] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2-13. [PubMed] [Cited in This Article: ] |
| 25. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Iredale JP. Tissue inhibitors of metalloproteinases in liver fibrosis. Int J Biochem Cell Biol. 1997;29:43-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Brenner DA. Therapeutic strategy for liver fibrosis. J Gastroenterol Hepatol. 1999;14:A279-280. [Cited in This Article: ] |
| 28. | Torres L, García-Trevijano ER, Rodríguez JA, Carretero MV, Bustos M, Fernández E, Eguinoa E, Mato JM, Avila MA. Induction of TIMP-1 expression in rat hepatic stellate cells and hepatocytes: a new role for homocysteine in liver fibrosis. Biochim Biophys Acta. 1999;1455:12-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | George DK, Ramm GA, Walker NI, Powell LW, Crawford DH. Elevated serum type IV collagen: a sensitive indicator of the presence of cirrhosis in haemochromatosis. J Hepatol. 1999;31:47-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Arthur MJ, Iredale JP, Mann DA. Tissue inhibitors of metalloproteinases: role in liver fibrosis and alcoholic liver disease. Alcohol Clin Exp Res. 1999;23:940-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Murawaki Y, Ikuta Y, Idobe Y, Kawasaki H. Serum matrix metalloproteinase-1 in patients with chronic viral hepatitis. J Gastroenterol Hepatol. 1999;14:138-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Sakaida I, Uchida K, Hironaka K, Okita K. Prolyl 4-hydroxylase inhibitor (HOE 077) prevents TIMP-1 gene expression in rat liver fibrosis. J Gastroenterol. 1999;34:376-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Murawaki Y, Ikuta Y, Kawasaki H. Clinical usefulness of serum tissue inhibitor of metalloproteinases (TIMP)-2 assay in patients with chronic liver disease in comparison with serum TIMP-1. Clin Chim Acta. 1999;281:109-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Peng CH. Gene therapy-basic and clinical. Beijing: Chinese Science and Technology Publishing Company 1994; 143-161. [Cited in This Article: ] |
| 35. | Stephenson ML, Zamecnik PC. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci USA. 1978;75:285-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 568] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 37. | Crooke ST. Therapeutic applications of oligonucleotides. Annu Rev Pharmacol Toxicol. 1992;32:329-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 329] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Whitton JL. Antisense treatment of viral infection. Adv Virus Res. 1994;44:267-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Wagner RW. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994;372:333-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 605] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 40. | Nichols WG, Boeckh M. Recent advances in the therapy and prevention of CMV infections. J Clin Virol. 2000;16:25-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Okada A, Garnier JM, Vicaire S, Basset P. Cloning of the cDNA encoding rat tissue inhibitor of metalloproteinase 1 (TIMP-1), amino acid comparison with other TIMPs, and gene expression in rat tissues. Gene. 1994;147:301-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Wang BE, Wang ZF, Yin WY. Study on experimental immunity model of hepatic fibrosis. Zhonghua Yixue Zazhi. 1998;69:503-505. [Cited in This Article: ] |
| 43. | Wang BE, Wang HJ, Zhu JX. Experimental study and clinical observation of hepatic fibrosis using different dosage form of compound Dan-Shen root. Zhonghua Gangzhangbing Zazhi. 1993;1:69-72. [Cited in This Article: ] |
| 44. | Sun ZX. Modern Biochemistry Theory and Investigate Technique. Beijing: Military Medical Science Publishing Company 1995; 435-437. [Cited in This Article: ] |
| 45. | Iredale JP, Murphy G, Hembry RM, Friedman SL, Arthur MJ. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J Clin Invest. 1992;90:282-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 152] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL, Phillips BW, Zhang Y, Urbanski SJ. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153:1895-1902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Arthur MJ, Mann DA, Iredale JP. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J Gastroenterol Hepatol. 1998;13 Suppl:S33-S38. [PubMed] [Cited in This Article: ] |
| 48. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 789] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 49. | Roeb E, Purucker E, Breuer B, Nguyen H, Heinrich PC, Rose-John S, Matern S. TIMP expression in toxic and cholestatic liver injury in rat. J Hepatol. 1997;27:535-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Murawaki Y, Ikuta Y, Idobe Y, Kitamura Y, Kawasaki H. Tissue inhibitor of metalloproteinase-1 in the liver of patients with chronic liver disease. J Hepatol. 1997;26:1213-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647-1659. [PubMed] [Cited in This Article: ] |
| 52. | Kasahara A, Hayashi N, Mochizuki K, Oshita M, Katayama K, Kato M, Masuzawa M, Yoshihara H, Naito M, Miyamoto T. Circulating matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 as serum markers of fibrosis in patients with chronic hepatitis C. Relationship to interferon response. J Hepatol. 1997;26:574-583. [PubMed] [Cited in This Article: ] |
| 53. | Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 248] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 54. | Arai M, Niioka M, Maruyama K, Wada N, Fujimoto N, Nomiyama T, Tanaka S, Okazaki I. Changes in serum levels of metalloproteinases and their inhibitors by treatment of chronic hepatitis C with interferon. Dig Dis Sci. 1996;41:995-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Arthur MJ. Role of Ito cells in the degradation of matrix in liver. J Gastroenterol Hepatol. 1995;10 Suppl 1:S57-S62. [PubMed] [Cited in This Article: ] |
| 56. | Roeb E, Rose-John S, Erren A, Edwards DR, Matern S, Graeve L, Heinrich PC. Tissue inhibitor of metalloproteinases-2 (TIMP-2) in rat liver cells is increased by lipopolysaccharide and prostaglandin E2. FEBS Lett. 1995;357:33-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 263] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Louis H, Le Moine A, Quertinmont E, Peny MO, Geerts A, Goldman M, Le Moine O, Devière J. Repeated concanavalin A challenge in mice induces an interleukin 10-producing phenotype and liver fibrosis. Hepatology. 2000;31:381-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 935] [Cited by in F6Publishing: 955] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 60. | Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 61. | Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990;10:56-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 209] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Arthur MJ. Collagenases and liver fibrosis. J Hepatol. 1995;22:43-48. [PubMed] [Cited in This Article: ] |
| 63. | Arthur MJ. Degradation of matrix proteins in liver fibrosis. Pathol Res Pract. 1994;190:825-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Rockey DC. Gene therapy for hepatic fibrosis-bringing treatment into the new millennium. Hepatology. 1999;30:816-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 483] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 66. | Qi Z, Atsuchi N, Ooshima A, Takeshita A, Ueno H. Blockade of type beta transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc Natl Acad Sci USA. 1999;96:2345-2349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 246] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 67. | Fujimoto J, Kaneda Y. Reversing liver cirrhosis: impact of gene therapy for liver cirrhosis. Gene Ther. 1999;6:305-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |