Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.515
Revised: June 30, 1999
Accepted: July 13, 1999
Published online: December 15, 1999
AIM: To observe the effect of acupuncture and moxibustion on the expression of IL-1β and IL-6 mRNA in ulcerative colitis rats.
METHODS: The SD rat ulcerative colitis model was created by immunological method associated with local stimulation. Colonic mucosa was prepared from human fresh surgical colonic specimens, homogenized by adding appropriate amount of normal saline and centrifuged at 3000 r/min. The supernatant was collected for measurement of protein conentration and then mixed with Freund adjuvant. This antigen fluid was first injected into the plantae of the model group rats, and then into their plantae, dorsa, inguina and abdominal cavities (no Freund adjuvant for the last injection) again on the 10th, 17th, 24th and 31st day. When a certain titer of serum anti-colonic anti body was reached, 2% formalin and antigen fluid ( no Freund adjuvant ) were administered separately by enema. The ulcerative colitis rat model was thus set up. The animals were randomly divided into four groups: model control group ( MC, n = 8 ), electro-acupuncture group (EA, n = 8), herbs-partition moxibustion group (HPM 8), normal control group ( NC, n = 8 ). HPM: Moxa cones made of refined mugwort floss were placed on the medicinal pad (medicinal pad dispensing: Radix Aconiti praeparata, cortex Cinnamomi, etc) for Qihai (RN 6) and Tianshu (S T 25, bilateral) and ignited. Two moxa cones were used for each acupoint once a day and 14 times in all. EA: Tianshu (bilateral) and Qihai were stimulated by the intermittent pulse with 2 Hz frequency, 4mA intensity for 20 min once a day and 14 times in all. After treatment, rats of all four groups were killed simultaneously. The spleen was separated and the distal colon was dissected. Total tissue RNA was isolated by the guanidinium thiocyanate phenol-chloroform extraction method. RT-PCR technique was used to study the expression of IL-1β and IL-6 mRNA.
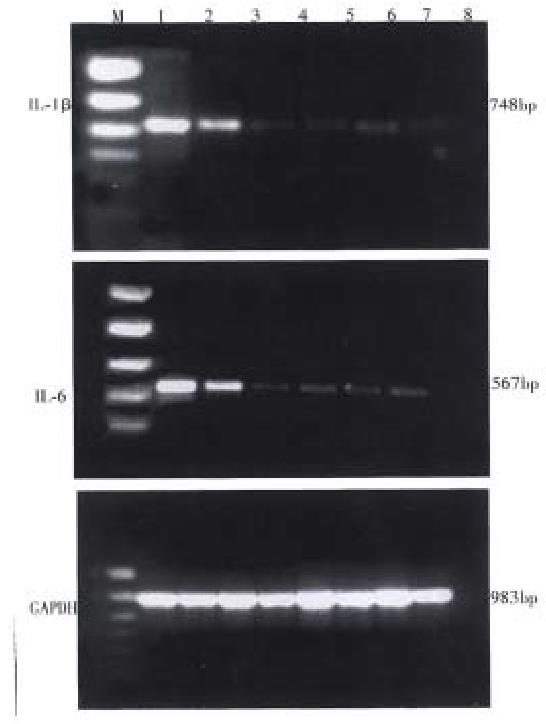
RESULTS: IL-1β and IL-6 mRNAs were not detected in the spleen and colonic mucosa of the NC rats, whereas they were significantly expressed in that of the MC rats. IL-1β and IL-6 mRNAs we re markedly lower in the EA and HPM rats than that in MC rats. There was no significant difference between the levels of IL-1β and IL-6 mRNAs in the EA and HPM rats. The expressions of IL-1β and IL-6 mRNAs were nearly the same in the spleen and colon of all groups.
CONCLUSION: Acupuncture and moxibustion greatly inhibited the expression of IL-1β and IL-6 mRNA in the experimental ulcerative colitis rats.
- Citation: Wu HG, Zhou LB, Pan YY, Huang C, Chen HP, Shi Z, Hua XG. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol 1999; 5(6): 515-517
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/515.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.515
Ulcerative colitis ( UC ) is a nonspecific inflammatory bowel disorder of unknown etiology but associated with immunological abnormalities[1]. The cytokines, involved in the regulation of the immune response, play important roles in the pathogenesis of UC. Especially the interleukin-1β (IL-1β ) and interleukin -6 (IL-6), inflammatory mediators released by lymphocytes, monocytes and macro phages, are intricately linked with the initiation and propagation of the inflammatory reaction in UC[2]. Both clinical and experimental researches indicated that acupuncture and moxibustion had good therapeutic effects on UC. The mechanism of such effects may be related to its immunoregulation, but the role of cytokines in it has not been reported. In this study, a UC rat model was established by immunological method to observe the effect of acupuncture and moxibustion on the expression of IL-1β and IL-6 mRNA in spleen and colonic mucosa of model rats, in order to clarify the possible mechanism of acupuncture and moxibustion on UC.
Male SD rats weighing, 140 g ± 20 g, were provided by The Experimental Animal Center of Shanghai University of TCM. The rats were randomly divided into the model group (n = 24) and normal control group ( NC, n = 8 ). We consulted the Methodology of Pharmacy and created the rat model by immunological method associated with local stimulation (refer to the Abstract). These models were randomly subdivided into three groups after being created: model control group (MC, n = 8), electro-acupuncture group (EA, n = 8) and herbs-partition moxibustion group ( HPM, n = 8 ). The points Qihai (CV6) and bilateral Tianshu (ST25) were located on analogy of person’s points. HPM: Moxa cones made of refined mug wort floss were placed on the medicinal pad (medicinal pad dispensing: Radix A coniti praeparata, cortex Cinnamomi, etc ) for Qihai ( RN 6 ) and Tianshu (ST 25, bilateral) and ignited. Two moxa cones were used for each acupoint once a day and 14 times in all. EA: Tianshu (bilateral) and Qihai were stimulated by the intermittent pulse with 2 Hz frequency, 4 mA intensity for 20 min once a day and 14 times in all. After treatment, all rats of the four groups were killed simula taneously. The spleen was separated and the distal colon 6cm long was dissected and reserved in liquid nitrogen.
According to the reference[3], total tissue RNA was isolated by the guanidinium thiocyanate phenol chloroform extraction method. The concentration of sample RNA was measured with ultraviolet spectrophotometer OD260; the integrity of RNA was identified by agarose (sepharose) gel (10 g/L) eletrophoresis.
Two µg total RNA was reverse transcribed to cDNA, 20 µL reverse transcription reaction system (Promega ), which comprised 10 × reverse transcription buffer solution 2 µL, 25 mmol/L MgCl2 4 µL, 4 × dNTPs (10 mmol/L for each) 2 µL, RNAase inhibitor 0.5 µL ( 20 U ), AMV reverse transcriptase 0.65 µL ( 15 U ), oligomer ( dT )15 and primer 1 µL ( 0.5 µg ); added DEPC up to 20 µL, well mixed, placed in 42 °C water for 40 min, heated in 95 °C water for 5 min to deactivate reverse transcriptase, and then preserved at -20 °C.
According to the reference[4], the primer was synthesized in the oncogene laboratory of the Cell Institute of the Chinese Academy of Sciences. The sequence of IL-1β was ATAGCAGCTTTCGACAGTGAG (sense chain), GTCAACTATGTCCCGACCATT (antisence chain) 748 bp; IL-6, TTCCCTACTTCACAAGTC (sense chain), CTAGGTTTGCCGAGTAGA (antisense chain) 567 bp; glyceraldehyde dehydrogenase (GAPDH) triphos-phate, TGAAGGTCGGTGTCAACGGATTTGTC ( sense chain ), CAGTAGGCCATGAGGTCCACCAC (antisense chain) 983 bp. GAPDH as house keeping gene monitor s the consumption of RNA and eliminates the errors among samples.
The total 50 µL PCR reaction system consisted of 10 × amp lification buffer solution 5 µL, 4 × dNTPs ( 2.5 mmol/L for each ) 4 µL, primers of sense chain and antisense chain 50 pmol for each, 2 µL reverse transcripti on product, Taq- DNA polymerase 2.5 U, and added ddH2O up to 50 µL, to mix them together. After 10 second centrifugation, 50 µL liquid paraffin was added and placed in PCR Gene Amp Machine (Pharmacia). The amplification condition: IL-1β:94 °C pre-denature 4 min, 94 °C 30 s, 50 °C 45 s, 72 °C 90 s for 30 cycles; IL-6 and GAPDH: 94 °C pre-denature 4 min, 94 °C 1 min, 52 °C 1 min, 72 °C 1 min for 30 cycles.
Ten µL PCR product was added to 6 × electrophoresis buffer solution 2 µL, and underwent agarose gel (1.5 g/L, containing Ethdium bromide 0.5 mg/L) electrophoresis at 100 V for 1 h and photographed under ultraviolet lamp.
The ratios of OD260/OD280 of the total RNA samples were between 1.70-2.00 and two bands, 18 s and 28 s, were shown in electrophoresis, indicating that the total RNA was not polluted and degraded.
IL-1β and IL-6 mRNA expressions of spleen and colon mucosa were observed in model control group, electro-acupuncture group and herbs-partition moxibustion group, but the degrees of expression in EA or HPM were lower than that in MC. There was no significant difference between EA and HPM, but the degree of expression in HPM as a whole tended to be less. The expressions of spleen and colon mucosa in all groups were nearly the same. Neither IL-1β nor IL-6 mRNA expression of spleen and colon mucosa could be observed in the normal control group.
The pathogenesis of UC may involve in both local and systemic immunological abnormalities. Cytokines now have attracted special attention by virtue of their participation in the intestinal inflammation and immune reactions. IL-1β and Il-6, as important inflammatory factors and immune regulators, play a fundamental role in the pathogenesis of UC. Many studies showed increased IL-1β and IL-6 levels in the colonic mucosa and peripheral blood of patients with UC, and indicated that IL-6 was possibly correlated with the severity of the manifestations in UC patients[5,6]. IL-1β and IL-6, produced mainly by activated phagocytes and lymphocytes, show a wide variety of biological functions. They can influence secretion of other cytokines and inflammatory mediators in an autocrine or paracrine fashion, induce expression of surface immune molecules of antigen-presenting cells to serve as an activation factor and differentiati on factor on T cells and B cells, mediate immunoglobulin secretion, activate the complements, killer cells and phagocytes, and enhance tissue injury mediated by cellular and humoral immune reactions. In addition, IL-1β and IL-6 can promote the expression of adhesion molecules on endothelial-leukocyte and are regarded as a chemoattractant of circulating neutrophils to migrate in to the inflammed site, thus causing a series of long lasting intestinal inflammatory reaction and tissue injury[7-9].
This study demonstrated that IL-1β and IL-6 were undetectable in the spleen and colonic mucosa of the normal control rats, whereas they were significantly expressed in that of the model control rats. Lymphocytes and monocytes/macrophages in the rats were activated by persistent stimulation of exogenous antigens which might contribute to the expression of cytokines. The amount of IL-1β and IL-6 mRNA was nearly the same between the spleen and colon in different groups, suggesting that immunocytes in the spleen and colon responded to the antigen in a similar way and interacted through the pathway of cytokines. In our study the markedly decreased expressions of IL-1β and Il-6 mRNA in EA and HPM suggested that acupuncture and moxibustion could inhibit the expression of inflammatory cytokines in UC model rats, regulate the immunological abnormalities, reduce immunocyte response to inflammation, and then contribute to the elimination of inflammation and repair of tissue.
The pathogenesis of UC may be due to an imbalance between inflammatory cytokines on one hand and anti inflammatory immune factors such as IL-4, IL-1ra, IL-10, etc on the other. Our results suggest that in addition to inhibit the expression of inflammatory cytokines, acupuncture and moxibustion can activate the anti-inflammatory factors, but further studies are needed.
Edited by Han-Ming Lu
Proofread by Jing-Yun Ma
| 1. | Kusugami K, Fukatsu A, Tanimoto M, Shinoda M, Haruta J, Kuroiwa A, Ina K, Kanayama K, Ando T, Matsuura T. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig Dis Sci. 1995;40:949-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. 1993;104:1285-1292. [PubMed] [Cited in This Article: ] |
| 3. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [PubMed] [Cited in This Article: ] |
| 4. | Murphy PG, Grondin J, Altares M, Richardson PM. Induction of interleukin-6 in axotomized sensory neurons. J Neurosci. 1995;15:5130-5138. [PubMed] [Cited in This Article: ] |
| 5. | Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, Harada K, Tateishi H, Nishiyama T, Tanikawa K. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Stevens C, Walz G, Singaram C, Lipman ML, Zanker B, Muggia A, Antonioli D, Peppercorn MA, Strom TB. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992;37:818-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 228] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Hogaboam CM, Snider DP, Collins SM. Cytokine modulation of T-lymphocyte activation by intestinal smooth muscle cells. Gastroenterology. 1997;112:1986-1995. [PubMed] [Cited in This Article: ] |
| 8. | Nassif A, Longo WE, Mazuski JE, Vernava AM, Kaminski DL. Role of cytokines and platelet-activating factor in inflammatory bowel disease. Implications for therapy. Dis Colon Rectum. 1996;39:217-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Schreiber S, Raedler A, Stenson WF, MacDermott RP. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol Clin North Am. 1992;21:451-502. [PubMed] [Cited in This Article: ] |