Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.492
Revised: August 16, 1999
Accepted: September 3, 1999
Published online: December 15, 1999
AIM: To study the therapeutical effectiveness, dosage range an d toxic adverse effects of domestic phosphorus 32 glass microsphere and evaluate its clinical significance.
METHODS: I. Fifty-two BALB/c tumor bearing male nude mice w ere allocated into treatment group ( n = 38) and control group ( n = 14). In the former group different doses of 32P-GMS were injected into the tumor mass, while in the latter 31P-GMS or no treatment was given. The experimental animals were sacrificed in batches, and then the tumors and their nearby tissues were examined by light and electron microscopy. II. Through selective catheterizati on of hepatic artery, 32P-GMS was infused to 5 healthy domestic pigs in a dosage equivalent to the therapeutic dose for human being, and 31P-GMS was infused to another 5 healthy domestic pigs. Two pigs infused with con trast medium served as whole course blank controls. One pig from each group was surrendered to euthanasia at week 1, 4, 8 and 16 respectively. The ultrastructur al histopath-ological changes in liver tissues taken from different sites were evaluated semiquan-titatively. III. One hundred and twenty-seven times of 32P-GMS intrahepatic artery interventional therapies were performed on 93 patients with hepatic carcinoma, including 79 cases of primary hepatic carcinoma and 14 cases of secondary hepati c carcinoma. 32P-GMS ( n = 30), and group B, 32P-GMS and half-dose of trans-hepatic artery embolization ( TAE ) ( n = 49), and 18 patients with HCC by TAE only as control group C. Fourteen patients with secondary hepatic carcinoma were treated in the same way as group B or C.
RESULTS: I. Comparing with the control group, the treatment group of tumor bearing nude mice attained the tumor inhibition rates of 59.7%-93.7% (F = 579.62, P < 0.01) at 14d. At an absorbed dose of 7320Gy, the tumor cells were completely destroyed. When the absorbed doses ranged from 1830Gy to 3660Gy, most of the tumor cells showed the evidences of injury or necrosis, but there appeared some well-differentiated tumor cells and enhanced effect of the autoimmunocytes. At an absorbed dose of 366Gy or less, some tumor cells still remained active proliferative ability. The definite anticancer effect appeared as early as 3d after intratumoral injection of 32P-GMS. II. The cumulative amount of 32P-GMS in the target tissue after trans-hepatic artery instillation attained more than 90% of the tot al dose administrated. Semiquantitative analysis of ultrastructral morphology in the experimental group showed no statistical difference between the nuclear abnormality ( nabn) and mitochondrial variability (Mvar) at week 1 or 2 , but revealed prominent difference ( χ2 = 6.70-9.68, P < 0.01, χ2 = 65.09-115.09, P < 0.001) as compared with those in the other groups. In the experimental group the nabn in tissues showed no significant difference between week 8 and week 16. no apparent changes were found in the stomach, spleen, kidney and lung tissues of the experimental pigs. III. The therapeutical results of HCC patients in group A were closely approximated to those of group C, no hematological toxic side effects were noted, and the systemic reaction was mild. In some patients 2 mos-3 mos after treatment some secondary foci appeared around the periphery of the primary lesion. In general better effectiveness was obtained in patients with small lesion. After analyzing by RIDIT method, the therapeutic result in group B was significantly better than that in group C, and secondary foci around the original lesion were rarely seen at 3mos after treatment. In group C the collateral circulation was reestablished along the periphery of primary foci and the secondary foci appeared more frequently, and were required to undergo several courses of treatment. In group B, 4 cases of HCC were treated surgically as their mass decreased in size after 32P-GMS treatment. Resected specimens showed that the tumor was encapsulated by fibrotic tissue and most of the tumor cells necrosed. The 3-year survival rates were 43.3%-51.0% after A and B regimen treatment. In 14 cases of secondary HCC, the foci were well controled within one year after-treatment.
CONCLUSION: When the experimental model of implanted human liver cancer cells received 32P-GMS of 1830Gy-3660Gy, it produced excellent anticancer effect without any injury to the normal neighboring tissues and the prominent anticancer effect was shown within 3d after intratumoral injec tion. Intrahepatic arterial administration of 32P-GMS at the macrocosmic absorbed dosage less than 190 Gy/dose exerted reversible sub-lethal injury to domestic pig liver tissues. It took more than 8 weeks to repair the injured liver tissue and restore its function. 32P-GMS trans-hepatic artery embolization is an effective and safe regimen in treating hepatic carcinoma.
- Citation: Liu L, Jiang Z, Teng GJ, Song JZ, Zhang DS, Guo QM, Fang W, He SC, Guo JH. Clinical and experimental study on regional administration of phosphorus 32 glass microspheres in treating hepatic carcinoma. World J Gastroenterol 1999; 5(6): 492-505
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/492.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.492
Trans-hepatic artery embolization (TAE)[1] is the main regimen for trea ting unresectable hepatic carcinoma (HCC). The experimental investigation using microsphere carriers such as colloidal microsphere, artificial cell membrane-liposome etc, in treating malignant tumors had been carried out for more than a decade with advanced development[2]. The microspheres mainly conjugated with anticancer drugs released slowly into the cancer tissue. Up to now, a novel anticancer microsphere preparation has been evolved, i.e. incorporation of radionuclide (32P of 90Y) to the glass microspheres forming a nontoxic, unde gradable radioactive radiation source through regional medication, which aroused the interest and notice of investigators in this field[3-5].
We report the results of evaluating the pharmacology, toxicology and clinical effect of 32-phosphorus-glass microspheres (32P-GMS) in three parts. I. By using human liver cancer cell bearing nude mouse model to explore the experimental anti cancer effect of intratumoral injection of 32P-GMS and investigate the appropriate dose range, time course and the influence on the neighboring tissues. II. By administrating 32P -GMS to the whole liver or certain liver lobes of domestic pig model and observing the local irradiative reaction and systemic toxic effect on the normal liver tissue to provide the experimental basis for determining the appropriate tolera ble dosage and treatment course of 32P-GMS internal irradiation in normal human liver tissues. III. From 1996 to 1998, 93 patients with liver cancer received 127 times of interventional 32P-GMS internal irradiation.
By activation of standardized glass microspheres with nonradioactive 31P (31P-GMS, cold sphere) through nuclear-chemical reaction [31P (n,γ)32P] transformed into radioactive 32P glass microsphere (provided by nuclear Power Rese arch Institute of China, nPIC)[6], having the properties as follows: dia meter of glass sphere 46 μm-76 μm, radioactive nuclide purity > 99%, radioactivity per unit 550 MBq·g-1 3700 MBq·g-1 (15 mCi·g-1-100 mCi·g-1), 32P elution rate < 0.1% within 30 days; 32P physical half-life 1428 days, average β ray energy per disintegration: 0.695 MeV ( maximum energy 1.711 MeV); and soft tissue pen etration distance, max. 8.0 mm, averaging 3.2 mm. 32P-GMS suspension was prepared by mixing 32P-GMS with super-liquidized iodized oil or 50% glucose solution to the concentration of 100 mg·mL-1 on oscillator.
Loevinger’s formula[7] for calculating the absorbed dose of β emitter radionuclide:
Dβ∝ = 73.8EβC0Teff
where Dβ∝ the total absorbed beta particle dose (cGy), Eβ, the average beta ray energy per disintegration (MeV), C0, the initial tissue concentration of radioactivity ( mCi/kg ) and Teff, the effective hal f-life (days).
Based on the pharmacokinetic characteristics of regional administration of 32P-GMS and the related parameters, the following formulae were established[8]:
D (cGy) = 20A (MBq)·m (kg)-1
D (cGy)=732A (mCi)·m (kg)-1
where A: the cumulative activity of radioactive nuclide, D: the total dose of absorbed β particles in tissue, m: the tissue weight.
Human liver cancer cell-bearing nude mouse model and anti-cancer effect of 32P-GMS Human liver cancer cell line subset (H-CS)[9] with higher oncogenicity and liability of metastasis was implanted into the dorsal subcutaneous tissue of 52BALB/c nu/nu nude mice (male, body we ight 16.8 g-21.3 g, mean 19.2 g, aged 4 weeks, derived from Shanghai Experimental Animal Center, Chinese Academy of Sciences) at the dosage of 0.1 mL-0.2 mL (1 × 107 tumor cells for each animal).
Experiment 1. Forty tumor-bearing nude mice with the tumor mass diameter of 0.7 cm-1.0 cm, different doses of 32P-GMS were injected to the mass center of 32 nude mice (subgroup 1-V) in the treatment group and non-radioactive 31P-GMS to mass center of 8 nude mice as the control subgroup. The animals were sacrificed on the 14th day.
Experiment 2. Twelve tumor-bearing nude mice with matched tumor size were eq ually allocated into treatment and control group, and 32P-GMS 3.7 MGq were injected to the tumor mass at points with 0.8 cm apart from each other, the total dosage being 7.4 MBq-14.8 MBq, varied with the size of tumor. no treatment was given to the control animals. The mice in the treatment group were sacrificed in batches on day 3, 6, 13, 20, and 28 after medication and the same was done for those in control group. The tumor masses were disposed similar to Experiment 1. One mouse in both treatment and control groups died on day 19 and 22 spontaneously without any difference from the survivals in appearance. All of the tumor specimens were submitted to gross inspection, light and electron microscopy to observe the morphological and ultrastructral changes and then calculate the tumor inhibition rate. Tumor inhibition rate (at the time of execution) = (tumor weight of control-tumor weight of treatment group )/ tumor weight of control group × 100%.
Experimental study on the toxicology of 32P-GMS Twelve domestic pigs (6 males, 6 females) with average body weight of 23.4 kg, were randomly divided into 3 groups: warm sphere group, 32P-GMS ( n = 5 ), cold sphere group 31P-GMS ( n = 5 ) and whole course blank control group ( n = 2 ). Under generalized anesthesia the catheter was inserted through femoral artery to the hepatic artery of the experimental animal. To the warm sphere group 32P-GMS was administered at a dose equivalent to that of man, 31P-GMS administered to the cold sphere group a nd roentgenographic contrast medium to the blank control group. For the pigs with 32P-GMS, the distribution of nuclide radioactivity was studied by SPECT. The radioactivity count rate was recorded on the body surface of hepatic, pulmonary and splenic regions of pigs for 14 consecutive days. One pig a time was surrendered to euthanasia on week 1, 2, 4, 8 and 16. The animal liver was dissected and weighed as soon as possible. From different sites of liver 8 tissue specimens were taken for light and electron microscopy. And at the same time, the major organs suspected to be involved such as lung, spleen, stomach and kidney were sampled for light microscopy. At the corresponding time point, liver biopsies were performed on the rest surviving animals for light and electron microscopy.
Venous blood specimens were taken for routine blood count, estimation of liver and renal function and for dynamic study of liver fibrosis markers, such as hyalu ronic acid (HA), human procollagen III (hPCIII), collagen IV(C-IV), laminin (LN) and glycocholate (CG) by radioimmunoassay. The specimens prepared routinely were studied under H-600 electron microscopy at 8000 folds magnification, to observe the ultrastructure and analyze morphometrically. A total of 100 hepatocyte nuclei and 100 mitochondria were observed in each sample group, and the nuclear abnormality (Nabn ) and mitochondrial variability (Mvar) were calculated respectively. The characteristics of abnormal nuclei were: nuclei irregular and deformed in shape; abnormal nuclear membrane, distension of the perinuclear gap; abnormal chromatin with peripheral condensation, and increase in intranuclear inclusion bodies with giant nucleolus. The abnormal mitochondria were characterized by swelling with disrupted external membrane and decrease in cristae; shrunken mitoch ondria, deep staining of ground matrix with decreased granules but with some vacuoles. The rates of abnormal nucleus and mitochondria variation were the percentage calculated from the number of abnormal or variation per total number of nuclei observed.
Clinical materials Seventy-nine cases of primary hepatocellula r carcinoma, male 67 and female 12 with average age of 52 years (32 years-77 years). The diagnosis was based on the evidence afforded from the results of B- mode sonography, computed tomography or angiogram and blood AFP > 400 μg/L. In some cases with negative AFP, their pathological and cytological evidence settled the diagnostic problem. The clinical types in 79 cases of HCC were single massive types (52 cases, left lobe 3, right lobe 49); multi-nodular type (24 cases); and diffuse type (3 cases). According to Child’s classification of liver function, 25 were of grade A, 40 grade B and 14 grade C. The average diameter of tumor mass was 8 cm ( 3 cm-15 cm), 62 cases (78.5%) showed positive hepatitis B antigen, 52 cases (65.8%) were complicated with cirrhosis, 4 cases (5.1%) portal veine mbolization, 6 (7.6%) pulmonary metastasis, 7 (8.9%) peritoneal lymph node met astasis and 8 (10.1%) had family histories of gastrointestinal tumors. In 14 cases of secondary hepatic carcinoma, 6 were primary colonic tumors, 5 gastric, 1 pulmonary and 2 esophageal cancers.
Treatment regimen and grouping Superselective catheterization w as performed by Seldinger’s procedure to the distal end of hepatic artery proper for 127 times in 93 cases of hepatic carcinoma. No hepatic A-V fistula was found in all of the cases as confirmed by DSA, then the 32P-GMS suspension prepared by occillation of super-liquidized iodized oil 4 mL-10 mL and 32P-GMS with calculated tumor tissue absorbed dose of 50Gy-100Gy and activity range from 370 MBq-470 MBq was instilled. HCC patients were allocated randomly into 3 groups. Group A: 32P-GMS internal irrad iation embolization therapy (30 cases); Group B: 32P-GMS and half dose TAE [Adriamycin (Adr) 30 mg/m2 + cis-diammine dichloroplatinum (CDDP) 50 mg/m2 + iodized oil] combined therapy (49 cases); Group C: TAE (18 cases). Fourteen cases of secondary hepatic carcinoma were tr eated by B and C regimen. After embolization the vasculature and tumors taining disappeared on DSA.
Follow-up Before treatment the average life quantity score was 65.5 (Karnofsty score)[10]. The results in liver and renal function, ECG, routine blood counts, blood AFP and CEA on day 10-14 after tre atment were compared with the corresponding basal data. SPECT liver images were conducted in 20 cases before treatment. Distribution of nuclide radioactivity in chest and abdomen within 80 h after treatment was studied using bremsstrah lung conducted by SPECT. Plain film of liver region showed the foci of condensed iodized oil shadow within 5 days after treatment. B-mode sonogram, or computed tomogram or plain film of abdomen was taken at the scheduled time of follow-up.
Evaluation of therapeutical results According to the modified WHO[10] criteria for tumor therapy, the effectiveness of grades A, B and C was the product of two perpendicular diameters which were decreased > 50%, 50%-25% and 25%-10%, respectively, while the product decreased < 10% was defined as stable. When the product was increased, it meant ineffective.
Chi-square test and RIDIT method were used for analyzing categorical data, t test and ANOVA were used for analyzing numerical data and survival rate was calculated by life-table method.
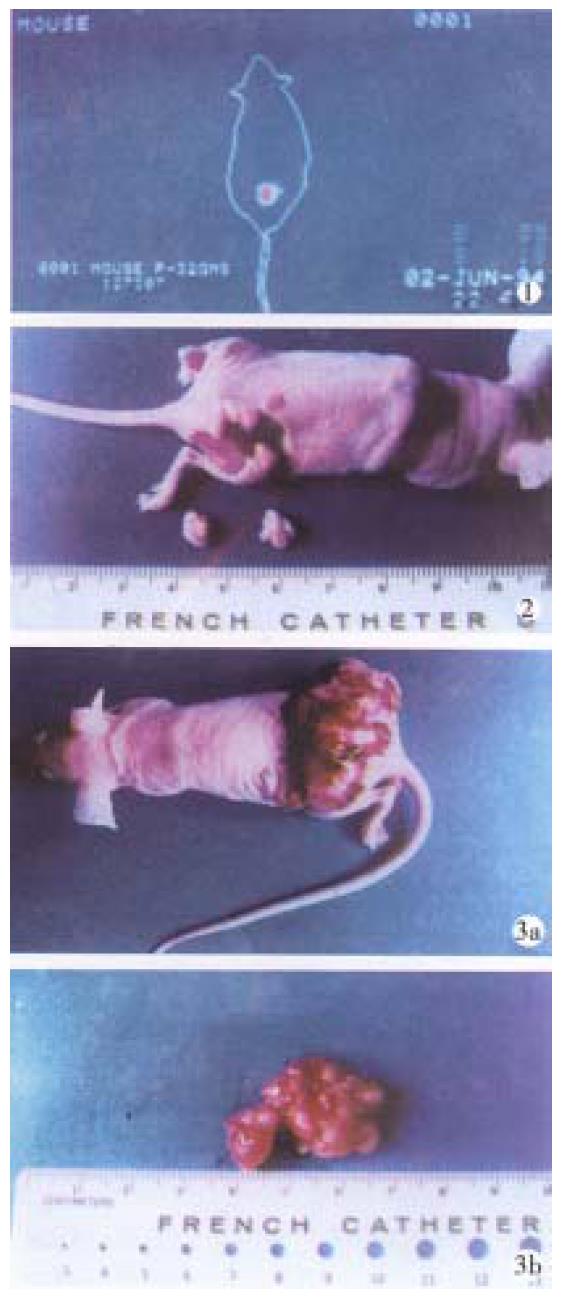
Gross inspection ( Figure 1 ) The similar manifestation in Experi ments I, II and controls was the rapid progressive growth of tumor mass, beginn ing from the dorsal injection points extended to the contralateral side, eventually distributed to the whole dorsa and buttocks. At the time of execution, the tumor mass presented nodular or lobular in shape with axial diameter of 2.3 cm-4.0 cm, hard and firm in consistency on palpitation with thin intact reddish covering epiderma without ulceration. After the covered epiderma was incised, there was plenty of blood vessels on the mass surface, bleeding readily, and the section showed light reddish in color, dense in consistency, rich in vessels, and sometimes with central necrosis and focal liquification. In the treatment group the growth of tumor mass was evidently inhibited, and the inhibiting rate was directly proportional to the dosage administered and time elapsed (Tables 1 and 2). About 5 days after medication, the tumor mass began with uleration, bleeding or petech ia, liquification and cystic degeneration. These changes might result in increas e in tumor size, but that was qualitatively different from the growth of tumor in control group.The tumor of subgroups I and II shrank with scars or cystic degeneration. The section of tumor showed grayish white color with poor vascularity. In subgroups III-IV, most of the implanted tumors presented with ulceration, bleeding, liquification and cystic degeneration, the section of tumor showed grayish white in color with poor vascularity, too. These changes had already appeared on 3 day after medication.
| Time of execution (d) | Weight of tumor (g) treatment group/control group | Tumor inhibiting rate (%) |
| 3 | 1.5/2.3 | 34.8 |
| 6 | 1.7/3.2 | 46.9 |
| 13 | 2.1/5.9 | 64.4 |
| 20 | 1.6/6.7 | 76.1 |
| 28 | 1.0/6.8 | 85.3 |
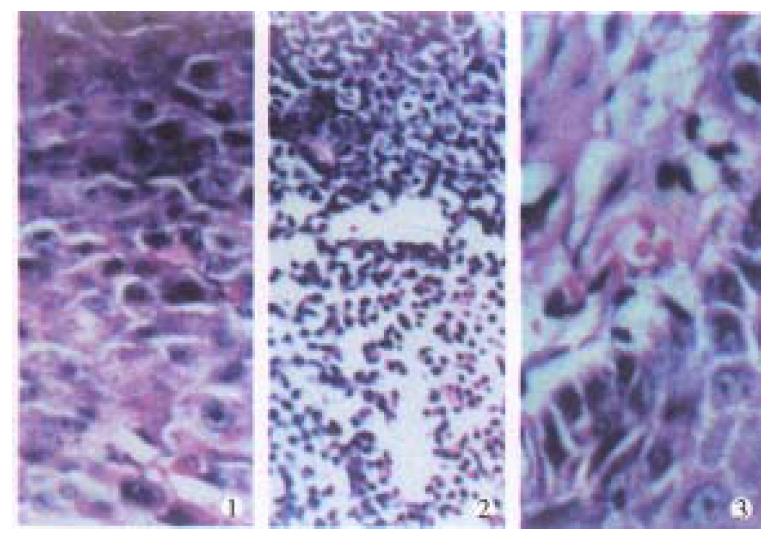
Light microscopy (Figure 2) The tumor cells of control group w ere closely arranged in the form of trabeculae with large nuclei and prominent nucleoli. Some cells showed binuclei or giant nucleus and mitosis were readily found. Plenty of blood sinusoids and concentrated bile could be found between the intercellular space of tumor cells. Some of the tumor tissues showed fatty change or scattered focal necrosis. In the treatment group the microscopic manifestat ion varied with the different activities of the 32P-GMS administrated . In the I and II subgroups the tumor cells were loosely arranged with widely d istributed coagulation necrosis. Some nuclei showed prominent shrinking degeneration. In subgroup III the tumor cells were loosely arranged and separated by thick or thin bundles of vesiculo-fibro-connective tissue forming pseudoacini, and in the nest some nuclei were broken with deeply stained scant cytoplasm. The histological characters in subgroups IV and V were that the arrangement of tumor cells transformed from dense to loose with scattered spot necrosis, blood sinuses being not found. In loosely arranged tumor cells desmosomes were fewer than those in the closely arranged tumor cells, but the degenerated necrotic cells increased. The necrosis was mainly located at the center of tumor mass and scattered among the dispersed tumor cells. In this experiment, a tumor mass with largest dose of radiation showed metaplasia in the neighboring epidermal tissues.
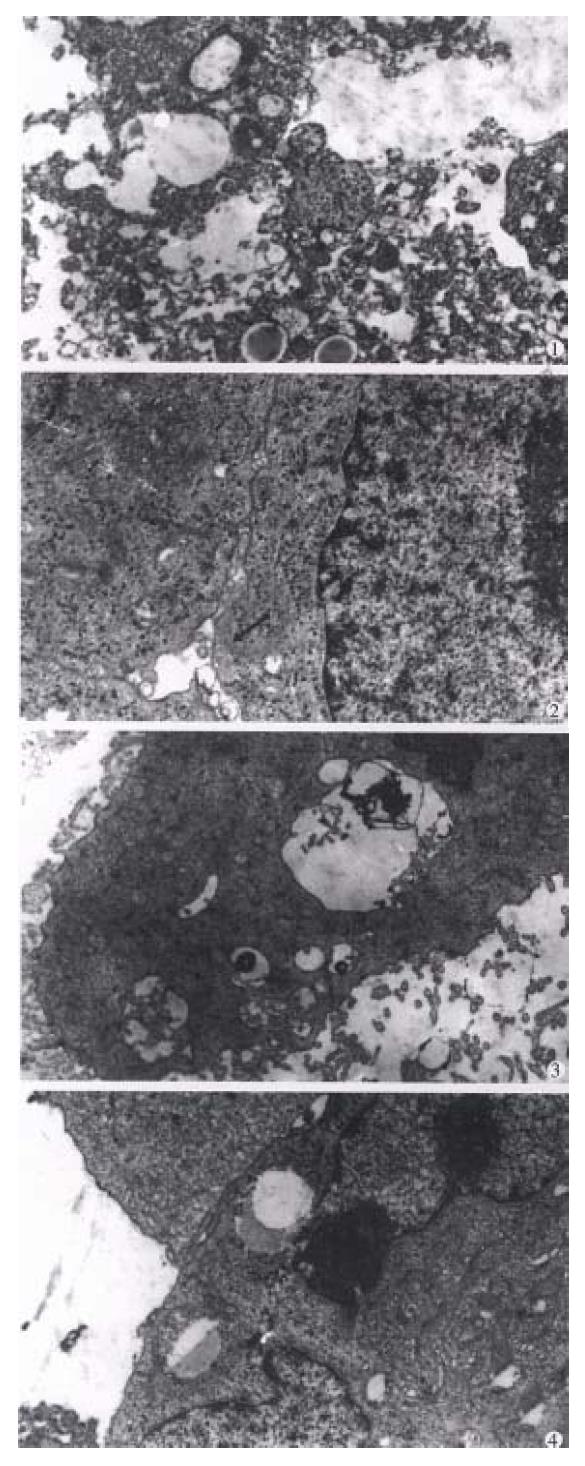
Electron microscopy (Figure 3) In the control group, most of the tumor cells were poorly differentiated and rapidly multiplicated, with the characteristics of irregular large nuclei with deep indentation, pseudo-inclusion body formation, large and prominent nucleoli, several peripherally aggregated, nucleoli with plenty of chromatin in it. In cytoplasm mainly free polyribosome presented, while mitochondria, glycogen and rough endoplasmic reticulum were scant. In the nearby interstitial tissue infiltrated tumor cells, degenerated lymph ocytes, damaged fibroblasts, loose collagen fiber, and many vesicular inclusion bodies in the nuclei were found. The tumor cells of subgroup I revealed necrotic injury, and in the severely injured cells the nuclei lysed, cell membrane disrupted and numerous debries were found. The injured tumor cells showed condensation of nuclear chromatin, peripheral aggregation of heterochromatin in pieces, dam aged organellae in cytoplasm, disappearance of mitochondrial cristae and ribosom es, appearance of many vacuoles and lipid particles. In subgroup II, many tumor cells presented histological structures similar to those in subgroup I, but with many lysosomes and mimetic secretory granules. Some were differentiated tumor cells with the characters of round nucleus with small nucleolus, evenly distributed chromatin, mainly euchromatin, mitochondria and rough endoplasmic reticula in the cytoplasm, formation of microvilli at the interface of tumor cells. The capillaries between the moderately differentiated tumor cells had thickened or loosened basal membrane with local defects, and abundant fibroblast and coll agen fibers could be found in the matrix. And there was a tendency of bile canal iculi formation somewhere in the matrix. The tumor cells in subgroup III showed different morphologic appearance, some damaged mildly and others severely, but some were moderately differentiated with plenty of cytoplasmic free polyribosomes. Plasma cells scattered among the tumor cells, and some lymphocytes protruded pseudopodia when contacted with the tumor cells, no abnormality was found in the dermal cells of adjacent skin. There were residual tumor cells showing active proliferation in subgroup IV, and some normal or degenerated lymphocytes, fibroblasts and collagen fibers presented in the interstitial tissue near the tumor. In subgroup V, the histological structure was manifested in various complicated forms, and the active multiplication of tumor cells were readily seen.
Absorbed dose of 32P-GMS Liver tissue histological parameter of domestic pigs with intrahepatic arterial administration of 32P-GMS and the absorbed dose of internal radiation in liver lobe at the time of euthanasia were estimated (Table 3). By scanning the different body surface regions of experimental pigs, the macrocosmic radioactivity counts in the target organ might attain more than 90% of total dose of 32P-GMS given through the hepatic arterial catheterization. The effect of the dispersed radiation was also included in this rate.
| Animal serial No. | Sex | Route of medication | Time of death (wk) | 32P-GMS (MPq·mg-1) | Tissue macrocosmic mean absorbed dose (Gy) | ||
| Administered activity (MBq) | Cumulated activity (MBq) | Weight (mg) | |||||
| 1 | F | Right hepatic artery | 1 | 0 | 0 | 300 | |
| 2 | M | Left hepatic artery | 2 | 0 | 0 | 260 | |
| 3 | F | Hepatic artery proper | 4 | 0 | 0 | 1353 | |
| 4 | M | Hepatic artery proper | 8 | 0 | 0 | 374 | |
| 5 | F | Right hepatic artery | 16 | 0 | 0 | 1000 | |
| 6 | M | Right hepatic artery | 1 | 925 | 266 | 313 | 48 |
| 7 | F | Left hepatic artery | 2 | 944 | 465 | 705 | 190 |
| 8 | M | Hepatic artery proper | 4 | 1070 | 825 | 375 | 104 |
| 9 | F | Left hepatic artery | 8 | 459 | 529 | 343 | 136 |
| 10 | M | Right hepatic artery | 16 | 461 | 459 | 349 | 61△ |
| 11 | F | Hepatic artery proper | 16 | 0 | 0 | ||
| 12 | M | Hepatic artery proper | 16 | 0 | 0 | ||
Serological manifestation In warm sphere group, the lactic acid dehydrogenase level attained 2 folds to the upper limit of normal in human being at the beginning, and one week later it rose to 4-6 folds. It did not decline significantly to 3-4 folds until week 4, and then it continuously declined to the initial level at week 8. As for aspartate aminotransferase (AST) or γ-glutamyl transpeptidase (γ-GT) there was an elevation in different degr ee, but for total protein (TP) and total bilirubin (TB) no changes were observed. The se items were neither found abnormal in the cold sphere group nor in blank control one. As for the markers of pig liver fibrosis, the basal level of HA was wi thin normal human range (2 µg/L-100 µg/L),in the warm sphere group and rose to the peak and then declined to normal. While in the cold sphere one, it rose slightly at week 1, then restored gradually to normal. The initial level of hPCIII was 2 folds to the upper limit of normal ( < 120 µg/L). Within two weeks of warm sphere admini stration it increased to 3 folds of the normal value and recovered to initial level within 8 weeks; for the cold sphere group, hPCIII value increased slightly at week 2, then returned to the initial level at week 4. There was no abnormality of above markers in the blank control group. In the dynamic studies of G-IV, C G and LN, no apparent alterations were found in any animal group.
Light microscopy In the portal area the debris of 32P-GMS (Figure 4) was found. In the warm sphere group, some hepatocytes show ed granulation and eosinophil granulocytes infiltration at week 2 and week 4; slight granulation of hepatocytes and some with fatty change were found at week 8; no apparent abnormalities were found at the week 16 and during the whole course in cold sphere and blank control groups. no apparent changes were seen in the lung, spleen, stomach and kidney of all the experimental animals.
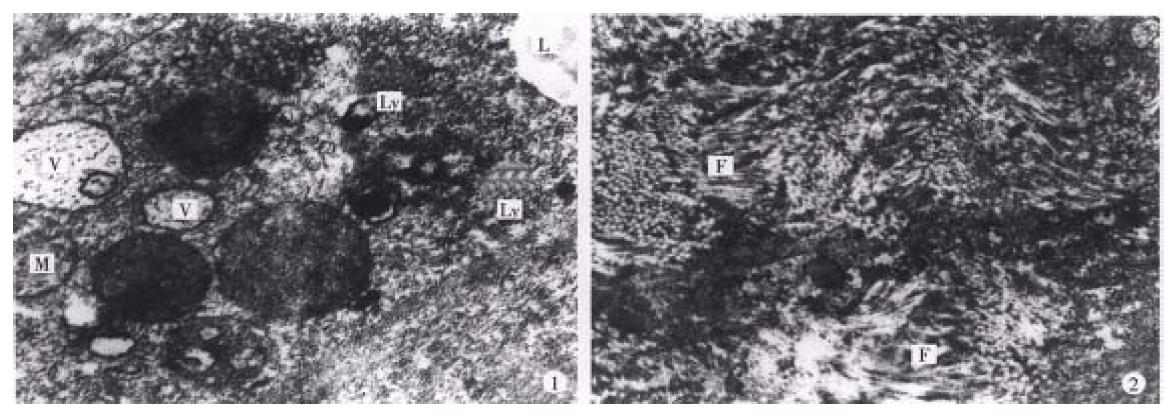
Electron microscopy (Figure 5) By clectron microscopic morphome tric analysis, the Nabn and Mvar of the hepatocytes are shown in Table 4. One to two weeks after internal irradiation, in the warm sphere group there were alterations in nuclei and mitochondria, dilatation of rough endoplasmic reticulum, local lytic injury in endothelial lining of sinusoid, and apale faint halo at the periphery of erythrocyte. no liver tissue abnormality was found in the cold sphere group. In warm sphere one at week 4 of internal irradiation, the hep atocytes still showed some abnormal features including decreased mitochondira, distended rough endoplasmic reticulum, detached ribosome, greatly increased lysos omes and myeloid bodies, bile canaliculi disrupted showing cholestasis, and vasc ular endothelium was prominently damaged. Eight weeks after irradiation, the injured hepatocytes decreased. There were plenty of organellae and glycogen particles in cytoplasm, intercellular junction among the hepatocytes showed normal configuration with regularly arranged microvilli, the matrix of mitochondria condensed, rough endoplasmic reticulum distended, and fat-storing cells of collagen fibers were prominently presented in the Disse’s spaces; and endoth elium of blood sinus was integrated and accompanied with neutrophil granulocytes infiltration. In the liver tissue of whole liver embolization with cold spheres the nuclei of hepatocytes remained normal, but the cytoplasm revealed the chang es similar to those found 4 weeks-8 weeks after internal irradiation. The liver tissue specimens taken at 16 week of internal irradiation demonstrated that most of the hepatocytes recovered almost to normal with abundant collagen fibers in the Disse’s space, while those from cold sphere group were essentially normal. The liver tissue was morphologically normal in the whole course of blank c ontrol group.
Therapeutic effectiveness (Table 5) In groups A and B, most of the HCC patients with 32P-GMS treatment revealed prominent symptomatic improvement, relief of pain in liver region, improvement of appetite, gain of body weight, decreased tumor-size and iodized oil condensed in the form of fragments or encapsulated cumulation on the film or CT ( Figure 6 ). No collateral circulation around the tumor body was found after 32P-GMS treatm ent, but in 5 cases of group A some secondary foci neighboring the primary foci which had been controlled, appeared within 2-3 months after therapy. no such pro blem was found in group B. In three cases of diffused type of HCC, the foci were not controlled effectively. Of the 79 cases of HCC, the post-treated tumor size as compared with their original sizes, was decreased more than 50%, 50%- 25%, 25%-10% and less than 10% in 24 (30.37%) cases, 25 (31.64%), 22 (27.84%) and 8 (10.1%), respectively. After 32P-GMS and TAE treatment in group B, 4 received surgical resections of tumor with fair results but the other 6 with decreased tumor size refused to be operated on. Twelve of 14 cases of metastatic hepatic carcinoma after regimen B and C treatment showed decrease in size of foci, giving an effective rate of 85.71%.
| Grade of effectiveness | Group A | Group B( n ) | Group C |
| > 50% | 9 | 15 | 4 |
| 50%-25% | 7 | 18 | 5 |
| 25%-10% | 9 | 13 | 5 |
| < 10% | 5 | 3 | 4 |
| Effective rate % | 83.33 | 93.87 | 77.77 |
Toxic or adverse effects About 2-3 days after TAE treatment in group C, some patients experienced fever of 38.5 °C and had grade IV leukocytopenia, almost all patients had nausea, vomiting and upset or pain in liver region. Serum ALT, ALP and bilirubin were slightly elevated, the markers of liver fibrosis HA and hPCIII revealed transient elevation, restored to the pretreatment level about half month later, C-IV, LN and CG did not show any fluctuation. no abn ormalities were found in renal function and ECG. As compared with group C, the a bove features in group A patients were rare and mild, among them 7 cases had pre treatment WBC < 2.0 × 10 9/L and one patient with uremia under regimen A treatment did not present significant side effects or complications. In group B patients no grade III-IV gastrointestinal reaction and no grade IV leukocytopeni a occurred after treatment (Table 6).
| Grading | Group A ( n ) | Group B ( n ) | Group C ( n ) | ||||||
| Hb | WBC | Plt | Hb | WBC | Plt | Hb | WBC | Plt | |
| 0 | 10 | 12 | 12 | 1 8 | 15 | 16 | 2 | 4 | 2 |
| I | 12 | 16 | 10 | 2 2 | 24 | 23 | 5 | 2 | 4 |
| II | 8 | 2 | 8 | 7 | 8 | 9 | 6 | 6 | 6 |
| III | 2 | 2 | 1 | 3 | 4 | 3 | |||
| IV | 2 | 2 | 3 | ||||||
Living quality and survival period The median survival period of HCC patients in groups A and B was 585 days, in group C, 455 days. The 0.5, 1, 2 and 3 year survival rates in group A, B and C were 100%, 96.7%, 56.7%, 43.3%; 97.7%, 91.8%, 61.2%, 51.0% and 96.4%, 81.8%, 41.2%, 31.0%, respec tively. The living quality of patients in groups A and B has improved prominently as evaluated by Karnofsty score, which rose from the basal level of 65.5 to 7 5.5, eventually to 80 in the recovery stage of some individual patients. A patient complicated with uremia maintained by hemodialysis survived up to 26 months after treatment, another patient committed suicide due to the cause unrelated to hisillness. Two cases complicated with cancer cell embolism of portal vein survived merely 3.5 and 6.5 months and died from upper digestive tract bleeding a nd hepatorenal syndrome respectively.
Prognostic factors affecting the survival rate Multifactorial analysis revealed that the following prognostic factors may affect the survival rate: accumulation of 32P-GMS in the tumor mass, parameters of hepatic fibrosis, the clinical types, size and its magnitude of decrease after treatment and whether intrahepatic or remote metastasis was present. The therapeutic effectiveness was not fully dependent upon the different histocyto logical types.
As the experiment demonstrated that the local internal irradiation of 32P-GMS surely had the cytocidal effect on tumor cells and exerted a potent inhibitive effect on the growth of tumor even at the third day of medication. The β-ray generated from 32P-GMS exerted injurio us effect on tumor tissue with very complicated mechanism: (1) After the tumor cells absorbed theβ-ray energy, it directly affected the ionizat ion a nd excitation of biological active macromolecules or broke its chemical bonds and destroyed the molecular structure. Since the active biological macromolecules were the main component of cell membrane, organelle and nucleus, defects in these structures apparently would reflect the impairment of their function. (2) The indirect effect ofβ-ray irradiation was to conduct ionizatio n and irradiation of the water molecule in intracellular environment and to gene rate many kinds of free radicals and superoxides such as O2, H2O2 etc. hav ing very active chemical property with high oxidative toxicity. These irradiative products injured or destroyed the biological macromolecules[12]. (3) β-ray acted on the cell DNA to induce the ce ll-death related gene expression, hence to accelerate the apoptosis of cancer cells[13]. When the cancer cells received massive dose of irradiation, the metabolic activity ceased immediately, the cell structure disrupted and lysed, resulting in cell death at metaphase; when the cancer cells received irradiation at a certain dosage, and fulfilled several times of multiplication, they would lose the ability of proliferation leading to proliferative death[14]. In addition, β-ray irradiation had the effect on occluding capillary vessels and inducing the hyperplasia of connective tissue in tumor resulti ng in structural derangement and promotion of the injury and necrosis of tumor cells.
On the 14th day of local injection of 32P-GMS to the tumor, the tumor cell death rate was 43%-82% in different treatment groups, but 4% in control group. The anticancer effect of 32P-GMS was directly proport ional to the dose administrated. In a particular time period, the rate of tumor cell death exceeded its rate of proliferation, then the tumor decreased in size; on the contrary, the death rate of tumor cell did not exceed their rate of growth, the tumor growth might be somewhat inhibited in a certain extent too. In the specimens of different treatment groups, the tumor cells might exhibit as survi ved, denatured or necrosed (in early or typical changes). This reflected essentially the whole course of tumor cell progression from denaturing to cell death after irradiation. The ultrastructural changes demonstrated that under the effect of high-dose irradiation, the tumor tissues received a lethal radiation energy in a short period, resulting in nonexistence of tumor cells which had a high ability to synthesize endogenous protein. In subgroup I, two tumor masses ne crosed thoroughly the skin neighboring to one of them showing metaplasia. Whether this was the result of radiation injury evolving to malignant change and degenerati on or not should be further investigated. Under the appropriate dose of irradiat ion (subgroup II and III), besides most of the tumor cells necrosed, the tendency of deriving to nearly normal histological picture evolved, such as plenty of microvilli on the cell surface, genesis of bile canaliculi-like structure, etc. These demonstrated that the 32P-GMS has the ability of killing the actively proliferative tumor cells and promoted the normalization of regenerative cells. These were similar to the effect of irradiation in trace amount which mig ht stimulate and enhance the local metabolism of inflammatory tissues, accelerate the death of injured cells and promote the growth of normal tissue, but this was not found in control group. It is also observed that the synergestic effect of immunocytes, cytolytic phenomena and its inhibition on the dispersion of tumor cells were enhanced in the tumor cells or nearby tissues. It denoted that the a nticancer effect of 32P-GMS was directly proportional to the time course of medication, based on the principle of after effect and cumulative effect of radioactive nuclide therapy. After intratumoral injection of 32P-GMS, it was not dispersed or displayed to the non-targeting tissue as confirmed by SPECT imaging. In comparison with intratumoral injection of ethyl alcohol[15] or acetic acid[16,17], 32P-GMS needs no repeated injection, with minimal side reaction[18]. Intratumoral injection of 32P-GMS was the best choice in treating the unresectable tumor or some metastasized lesion as well as those unsuitable for intra-arterial interventi onal therapy, it was also suitable for solid malignant tumors which could be reached anywhere on the human body.
We have got sufficient data from the dynamic study on the ultrastructural morpho metric analysis of liver tissues taken from warm sphere, cold sphere and control groups. According to the injury of normal liver after internal irradiation and its repairing process, it was allocated into 4 periods: (1) acute reactive period (within 2 weeks ), (2) subacute reactive period (2-4 weeks), (3) prerecovery period (4-8 weeks), (4) recovery period (8-16 weeks). In the acute period of warm sphere group, there was decreased proteosynthetic function and alteration of energy metabolism, decreased synthetic ability of ATP. The faint halo around the erythrocytes in the blood sinus as shown under electronmicroscopy probably was the super liquidiz ed iodized oil. The disruption of sinusoidal endothelium was closely related to the route of medication. No apparent injury of hepatic tissue was found in the control group. This suggested that the serial changes in ultrastructure which was seen in the warm sphere group might be the result of radiation injury. The sinu soidal endothelium was most prominently disrupted. All these changes represented the synergestic action of internal irradiation and embolization. At the prerecovery period, the abnormal nuclei of hepatocytes were scarcely seen, but the cytoplas mic organellae recovered more slowly than the nuclei. Cell injury was resulting in increase in myeloid bodies in the cytoplasm, which indicated the liver tissue evolved into self-repairing stage. The hepatocytes appeared essentially normal in the recovery period. Electron microscopy revealed prominent collagen fibers in the Disse’s space in some of the liver specimens, whether it indicated the te ndency of early liver fibrosis or not should be further studied.
Recent evidences[19] showed the C-IV increases prominently in the early stage of liver fibrosis, LN is closely related to the genesis of liver fibrosis, and CG is the important marker for estimating the severity of biliary cirrhosis, but the serum C-IV, LN and CG all fell into the human normal range in the whole course of the experimental animals. Therefore, the presence of collagen fibers in the Disse’s space was probably of transient local changes. The transient ch anges in HA value were similar to those of hPCIII, and the liver injury induced serum HA elevation was positively proportional to the severity of illness, and the serum hPCIII level was closely related to the extent of fibrosis. When the function of hepatocyte was damaged, hPCIII might be released to circulation and often used as the guidance for selection of therapeutic medicine[20]. Therefore, the transient changes in domestic pig serum HA and hPCIII values were the imp airment of liver function resulted from 32P-GMS administration. It was reported[21] that intra-hepatic arterial administration of cold spheres to the dosage equivalent to 12 folds of the human tolerable dose merely induced the clinical permissible intrahepatic changes and did not follow with portal fibrosis or hepatic cirrhosis after 90 days observation. The experiment demonstr ated that the cold sphere slightly injured the hepatocytes, probably being the embolization of the nutritional artery rather than irradiation. In this experiment, through hepatic artery medication, no non-target organs developed in all of the domestic pigs. no prominent ultrastructural changes were found in the hepatic lobe during the whole course in the control animals. The experiment demonstrates that intrahepatic change of clinically permissible extent might be induced in the liver tissue of domestic pigs that received 32P-GMS in the macrocosmic average absorbed dosage of 48Gy-190Gy. These changes were reversible subleth al injury[14] and essentially recovered within 8 weeks. In the observ ation of 120 days there was no evidence of portal fibrosis. This is the eviden ce that superselective intrahepatic medication may yield a high energy region and without serious injury to the nearby non-medicated tissues or organs.
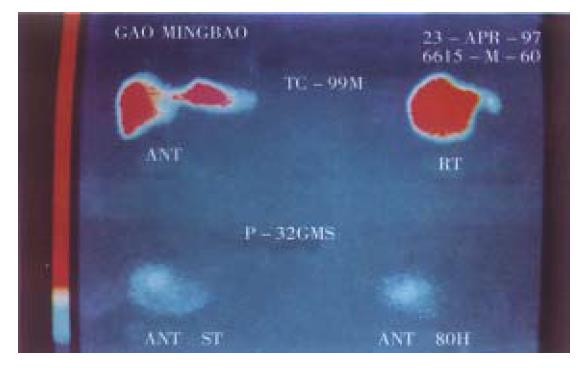
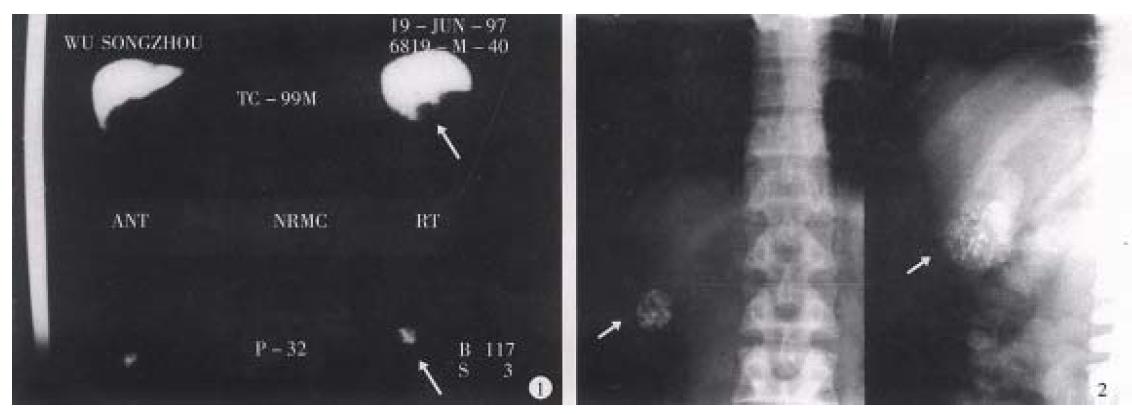
Hepatic artery embolization is the important measure in treating hepatic carcinoma[22,23]. Investigating an ideal embolizing agent is the substantial project in interventional therapy of tumor. The ideal internal radioactive nuclide should serve as a spotted source of radiation with high energy reserve and the carrier having high orientating rate, lasting longer in the target tissue, and the loaded nuclide was not easy in detaching or leaking to circulation. 32P-GMS, administrated through the hepatic artery, wedged in the terminals of this artery with its mixed iodized oil which was not absorbable could occlude the arterial capillary. In addition to the radiation obliteration of blood vessels induced by internal irradiation, the collateral circulation was not readily generated in the foci. All of the above mentioned advantages will benefit to boost ing therapeutic efficiency. Therefore 32P-GMS is an appropriate inte rnal radioactive embolizing agent with medium or long duration. It has been conf irmed in the clinical investigation, the tumor in liver showed angiogenesis at the microcirculatory level and might trap microspheres 3-4 folds to that of normal liver tissue[24-26]. Instillation of therapeutic dose of radionuclide to the nutritional arteries of tumor, particularly condensed in the tumor tissue, may exert potent cytocidal effect to fulfill the purpose of therapy. It has been reported[27-29] that Yttrium-90-GMS ( 90Y-GMS ) used in intrahepatic arterial embolization got fair result, and the absorbed dosage attained 50Gy-100Gy in the tumor may have radical effectiveness[29]. neve rtheless 90Y-has the disadvantages of short half-life, inconveniened in clinical application, and 90Y-GMS having smaller diameter, and higher hepato-pulmonary shunting index than that of 32P-GMS[30]. As reported that intratumoral injection of 90Y-GMS in 33 cases of hepatic carcinoma, the bremsstrahlung radiation conducted by SPECT showed 21.4% had development in lung, and 14.3% in intestine. In our study 32P-GMS in therapeutic dosage through superselective hepatic arterial regional instillation, particularly condensed in the tumor tissue including the central and peripheral portions, forming a high energy region and it is worth noting that extra-hepatic development was not found (Figure 7). Therefore transhepatic artery administration of anticancer agent is the first choice in regional medication to treat hepatic tumor. If the hepatic artery is severely distorted or fails in catheterization, intraparenchymal injection of 32P-GMS should be considered instead.
The therapeutical results of TAE were inconsistent in different histocytological type of HCC[31]. The clear cell type was most sensitive, the small cell and poorly or undifferential type was moderately sensitive. Based on the radio-biology, cell sensitivity to nuclide beam depended upon the functional status of the cell proper. The cytocidal effect and durability of 32P-GMS was superior to the other anticancer chemicals. The therapeutic results de pended upon the clinical classification rather than histocytological types.
The results of clinical observation revealed that the clinical classification and the specific features of angiogram might be the basis for evaluating the therapeutical effectiveness and predicting the prognosis. The clinical materials suggested that the regimen B is superior to regimen A and C in the respect of decreasing tumor size and prolonging the survival period. The follow-up results demonstrated that the excellent effect was evolved in the cases with small tumors, plenty of blood supply, intact capsule and heavily aggregated 32P-GMS in the tumor as revealed by SPECT. Analysis of the clinical classificati on showed that in average survival time of the 3 clinical types of HCC solitary mass type was longer than the multiple and diffuse type. The patients with apparent decrease in AFP level were consistently accompanied with decrease in tu mor size and necrosis in the tumor. In case of these features relapsed and eleva tion of AFP or complicated with intrahepatic or remote metastasis, all these would result in poor prognosis.
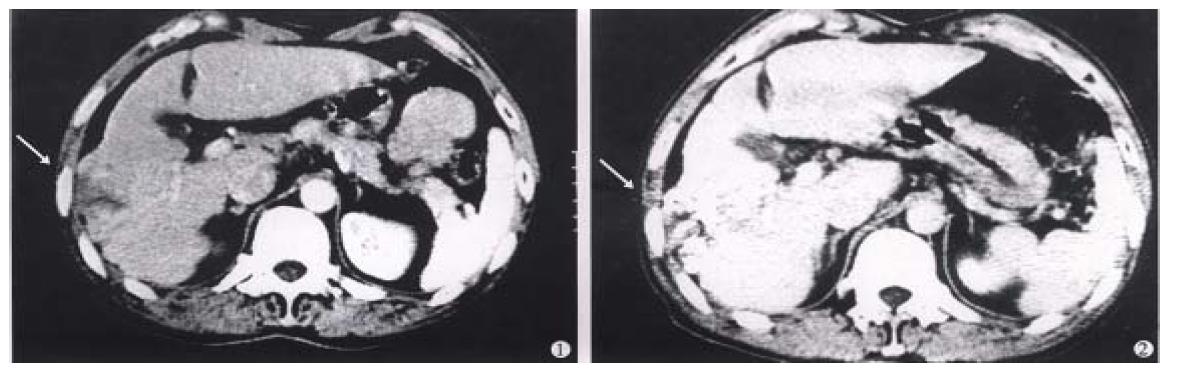
Strategy of therapy: for those hepatic carcinoma with intact capsule and diameter below 6cm treated by the regimen A for 1 or 2 courses could get satisfac tory result, and for those with occult metastatic foci, the secondary foci would be present besides the controlled original lesion several months later. It is of significance to use 32P-GMS and TAE in combination resulting in apparent synergetic effect (regimen B), which not only enhanced the anticancer and embolic effects but also lessened the side effects, and could completely cure the small liver cancer, eliminated the need of operation (Figure 8), diminished the number of medication with mild liver injury and low relapse rate. After underg oing regimen B treatment, some cases of unresectable tumor acquired the possibility of being resected (Figure 9, Figure 10). The dosage of 32P-GMS shou ld be decided by the specialists majoring in nuclear medicine and interventional therapy. The optimal schedule was to give two courses of medicine at an interval of 2 months, and as the tumor size decreased, the residual lesion should be resected as soon as possible in order to improve the survivability. There were few viable cancer cells in the 4 resected specimens on pathological examination after the regimen B treatment. Hepatic carcinoma readily invaded the portal vein with cancer cell emboli. It is apparent in such case that intrahepatic artery medication should be complemented with other measures as minimal invasive embolectomy which will be an intelligent choice. For the metastatic abdominal lymph nodes intratumoral injection of 32P-colloids under the guidance of CT is also a helpful measure. It is emphasized that we should choose reasonable regimen, strict manipulation, superselective catheterization, well controlled speed in medication to avoid regurgitation of nuclide to the non-target blood vessel, and lessen the complication, particularly depending on the individualized situation. The hepatic arterio-venous fistula should be considered as the contraindication for medication via catheterization.
In conclusion, 32P-GMS which possesses the anticancer effect through interventional medication may block the blood supply to tumor and evenly distrib ute the highly concentrated anticancer medicines in the tumor to exert the radioactive cytocidal effect with the advantage of low local radiation reaction and no apparent systemic toxic effect.32P-GMS is an effecti ve measure for the comprehensive treatment of hepatic cancer. Owing to the moder ate half-life of 32P, the dosage of 32P-GMS required to attain the same absorbed radioactivity is approximately one-fourth or one-third of 90Y-GMS dosage[8]. Therefore it will satisfy the clinical requirement in reducing the risk of radiation to the handlers, simplify ing the medical care and lowering the expenses. Thus, a hopeful prospective ther apeutic weapon will be developed and popularized in the near future.
Edited by Xie-Ning Wu and Jing-Yun Ma
Proofread by Qi-Hong Miao
| 1. | Tang YX, Jiang YD, Zhang Y, Li YJ, Nie Y, Zang H, Zhang XZ, Qiao WA, Lan FS. Observation on the therapeutic results of interventional irradiation in midand late stage hepatic carcinoma. Linchuang Yixue Yingxiang Zazhi. 1997;8:224-225. [Cited in This Article: ] |
| 2. | Zhang YH, Wu YB. Advances of anticancer target preparation. Zhongguo YaoxueZazhi. 1992;27:389-393. [Cited in This Article: ] |
| 3. | Kobayashi H, Hidaka H, Kajiya Y, Tanoue P, Inoue H, Ikeda K, Nakajo M, Shinohara S. Treatment of hepatocellular carcinoma by transarterial injection of anticancer agents in iodized oil suspension or of radioactive iodized oil solution. Acta Radiol Diagn (Stockh). 1986;27:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Herba MJ, Illescas FF, Thirlwell MP, Boos GJ, Rosenthall L, Atri M, Bret PM. Hepatic malignancies: improved treatment with intraarterial Y-90. Radiology. 1988;169:311-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 89] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Houle S, Yip TK, Shepherd FA, Rotstein LE, Sniderman KW, Theis E, Cawthorn RH, Richmond-Cox K. Hepatocellular carcinoma: pilot trial of treatment with Y-90 microspheres. Radiology. 1989;172:857-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Sun WH, Zhang LZ, Li ML. Research on radiotherapy of 32 Pglass microsphere. Hedongli Gongcheng. 1990;11:75-78. [Cited in This Article: ] |
| 7. | Loveinger R, Holt JG, Hine GJ. Internally administered radio isotopes. In: Hine GJ, Brownell GL, eds. Radiation Dosimetry. New York: Academic Press. 1956;801-873. [Cited in This Article: ] |
| 8. | Liu L, Sun WH, Wu FP, Han DQ, Teng GJ, Fan J. An experimental study of treatment of liver cancer by locally administration with phosphate 32 glass microspheres & estimation of tissue absorbed dose. Nanjing Tiedao Yixueyuan Xuebao. 1997;16:223-226. [Cited in This Article: ] |
| 9. | Bao JZ, Wang Y, Zhan RZ, Wu MC. Clonal analysis of a hepatocarcinoma cell line: an experimental model of tumor heterogeneity. Zhongliu Fangzhi Yanjiu. 1995;22:65-67. [Cited in This Article: ] |
| 10. | Common statistic form and method in the diagnosis and treat-ment of tumor. In standards for diagnosis and treatment of com-mon malignant tumor in China, Section 9, edited by Dept Medical Administration, Ministry of Public Health, PRC. Beijing: Beijing Med Univ & China Union Med College Joint Press. 1991;10-15. [Cited in This Article: ] |
| 11. | Yang SQ, eds . Health statistics. 3rd edition. Beijing: The Public Health Press. 1993;43-181. [Cited in This Article: ] |
| 12. | Edictorial board of pratical oncology. Practical Oncology. Vol. 1. Beijing: People's Medical Press. 1997;406-413. [Cited in This Article: ] |
| 13. | Zheng DX. Advance in apoptosis research. Zhonghua Binglixue Zazhi. 1996;25:50-53. [Cited in This Article: ] |
| 14. | Liu XC, Han KC. Hygienic protection and safety transportation of radioactive materials. Beijing: Zhongguo Tiedao Chubanshe. 1990;55-66. [Cited in This Article: ] |
| 15. | Ohto M, Karasawa E, Tsuchiya Y, Kimura K, Saisho H, Ono T, Okuda K. Ultrasonically guided percutaneous contrast medium injection and aspiration biopsy using a renal-time puncture transducer. Radiology. 1980;136:171-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 92] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ohnishi K, Ohyama N, Ito S, Fujiwara K. Small hepatocellular carcinoma: treatment with US-guided intratumoral injection of acetic acid. Radiology. 1994;193:747-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Zhao YF, Wen QS, Jia ZS. Local acetic acid injection in the treat-ment of transplanted tumor in mice. Shijie Huaren Xiaohua Zazhi. 1999;7:43-45. [Cited in This Article: ] |
| 18. | Liu L, Fan J, Zhang J, Du MH, Wu FP, Teng GJ. Experimental treatment carcinoma in a mouse model by local injection of phosphorus 32 glass microspheres. J Vasc Interv Rad. 1998;9:166. [Cited in This Article: ] |
| 19. | Ueno T, Inuzuka S, Torimura T, Oohira H, Ko H, Obata K, Sata M, Yoshida H, Tanikawa K. Significance of serum type-IV collagen levels in various liver diseases. Measurement with a one-step sandwich enzyme immunoassay using monoclonal antibodies with specificity for pepsin-solubilized type-IV collagen. Scand J Gastroenterol. 1992;27:513-520. [PubMed] [Cited in This Article: ] |
| 20. | Gressner AM, Tittor W, Negwer A, Pick-Kober KH. Serum concentrations of laminin and aminoterminal propeptide of type III procollagen in relation to the portal venous pressure of fibrotic liver diseases. Clin Chim Acta. 1986;161:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Wollner I, Knutsen C, Smith P, Prieskorn D, Chrisp C, Andrews J, Juni J, Warber S, Klevering J, Crudup J. Effects of hepatic arterial yttrium 90 glass microspheres in dogs. Cancer. 1988;61:1336-1344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 22. | Wang DZ, Sun WH, Zheng GY, Li ML, Wen YM. A study about the anticancer effect and the clinical application of the phosphate 32 glass microspheres (P32 GMS) by local arterial infusion. I. The ultrastructural study after internal radiation of P 32GMS in cancer cells. Huaxi Kouqiang Yixue Zazhi. 1991;9:7-10. [Cited in This Article: ] |
| 23. | Chen XL, Wu YT, Yan LN, Li L, Tan TZ, Sun WH, Li ML, Jia QB, Du JP, Shen WL. Treatment of liver cancer with 32P glass microsphere-an experimental study. Puwai Jichuyulinchuang Zazhi. 1996;3:68-70. [Cited in This Article: ] |
| 24. | BLANCHARD RJ, GROTENHUIS I, LAFAVE JW, PERRY JF. BLOOD SUPPLY TO HEPATIC V2 CARCINOMA IMPLANTS AS MEASURED BY RADIOACTIVE MICROSPHERES. Proc Soc Exp Biol Med. 1965;118:465-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Sundqvist K, Hafström L, Persson B. Measurements of total and regional tumor blood flow and organ blood flow using 99Tcm labelled microspheres. An experimental study in rats. Eur Surg Res. 1978;10:433-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Gyves JW, Ziessman HA, Ensminger WD, Thrall JH, Niederhuber JE, Keyes JW, Walker S. Definition of hepatic tumor microcirculation by single photon emission computerized tomography (SPECT). J Nucl Med. 1984;25:972-977. [PubMed] [Cited in This Article: ] |
| 27. | Yan ZP, Lin G, Zhao HY, Dong YH. An experimental study and clinical pilot trials on yttrium-90 glass microspheres through the hepatic artery for treatment of primary liver cancer. Cancer. 1993;72:3210-3215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 28. | Andrews JC, Walker SC, Ackermann RJ, Cotton LA, Ensminger WD, Shapiro B. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637-1644. [PubMed] [Cited in This Article: ] |
| 29. | Shepherd FA, Rotstein LE, Houle S, Yip TC, Paul K, Sniderman KW. A phase I dose escalation trial of yttrium-90 microspheres in the treatment of primary hepatocellular carcinoma. Cancer. 1992;70:2250-2254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 30. | Tian JH, Xu BX, Zhang JM, Dong BW, Liang P, Wang XD. Ultrasound-guided internal radiotherapy using yttrium-90-glass microspheres for liver malignancies. J Nucl Med. 1996;37:958-963. [PubMed] [Cited in This Article: ] |
| 31. | Wang YP, Zhang JS, Gao YA. Therapeutic efficacy of transcatheter arterial embolization of primary hepatocellular carcinoma: discrepancy in different histopathological types of HCC. Zhonghua Fangshexue Zazhi. 1997;31:586-588. [Cited in This Article: ] |