Published online Feb 15, 1999. doi: 10.3748/wjg.v5.i1.15
Revised: November 30, 1998
Accepted: December 16, 1998
Published online: February 15, 1999
AIM To study the expression of proapoptotic gene Bax in human gastric carcinoma and its significance.
METHODS Using immunohistochemistry methods, the Bax protein expression in 57 specimens of gastric carcinoma and its relationship with clinical status and pathomorphological parameters were observed.
RESULTS Thirty-three (57.9%) cases were positive for Bax pr otein staining which was mainly located in the cytoplasm of tumor cells. The rate of Bax protein expression was not correlated with the tumor size, lymph node metas-tasis, depth of invasion, clinical stages of tu-mors and age and sex of patients (P < 0.05), but strongly associated with the morphological type and diff erentiation degree of tumors. It was significantly higher in intestinal type and well or moderately differentiated gastric carcinoma than in diffuse type and poorly differentiated gastric carcinoma (P < 0.05 and P < 0.01).
CONCLUSION The proapoptotic gene Bax is differently expressed in most of gastric carcinoma and may take part in the modulation of apoptosis i n gastric carcinoma. The expression of Bax might be associated with the occurrence of intestinal type gastric carcinoma and the differentiation of gastric carci noma.
-
Citation: Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene
Bax in gastric carcinoma. World J Gastroenterol 1999; 5(1): 15-17 - URL: https://www.wjgnet.com/1007-9327/full/v5/i1/15.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i1.15
Recent investigations have demonstrated that apop-tosis plays a significant role in the pathogenesis of tumors[1,2]. Emphasis has been laid on the mecha-nisms that regulate apoptosis pathways. Bcl-2 asso-ciated X protein (Bax), which has extensive amino acid homology with Bcl-2, can form heterodimers with Bcl-2 in vivo. Overexpressed Bax can counter the death repressor activity of Bcl-2, and accelerate apoptotic cell death[3]. To determine whether proapoptotic gene Bax plays a role in the regulation of apoptosis in gastric carcinoma , an immunohisto-chemical study of Bax protein expression in gastric carcinoma an d its relation to clinical status, patho-morphological parameters were carried out.
Fifty-seven cases of surgically resected gastric carci-nomas (male 39, female 16; mean age 58.6 years) were collected from the files of the Department of Pathology of our hospital. All blocks were fixed in 10% formalin and embedded in paraffin. Serial sec-tions were cut from each block in 4 μm, stained with hematoxylin and eosin and confirmed pathologi-cally.
Immunohistochemical staining for Bax protein was performed using SP technique wi th the following procedure: (1) slides were deparaffinized in xylene for 10 min each and then were hydrated in decreasing concentrations of ethanol and rinsed in phosphate-buffered saline. Endogenous peroxidase was blocked by 30 mL/L H2O2 in methanol for 5 min, and then incubated for 10 min at room temperature in normal goat serum (1:20). (2) Slides were incubated with a 1:50 dilution of the primary rabbit antihuman Bax polyclonal antibody (Santa Cruz, USA) for 30 min at 37 °C. A biotin-strep-tavidin detection system was employed with di-aminobenzidine as the chromogen. (3) Slides were washed twice wit hphosphate-buffered saline and in-cubated with the linking reagent (biotinylate d anti-immunoglobulin) for 10 min at 37 °C. After rins-ing in phosphate-buff ered saline, the slides were in-cubated with the peroxidase-conjugated streptavidin label for 10 min at37 °C,and incubated with di-aminobenzidine and H2O2 for 10 min in the dark, the sections were then counterstained with hematoxylin. With each batch of test samples, a positive control consisting of a tissue section from tonsil was evaluated. In addition, a negative control was prepar ed for each sample using an irrelevant an-tibody of the same isotype as the primary antibody.
The immunostaining of Bax protein was visually classified into negative and positive groups by observing 1000 tumor cells in the areas of the sections: no staining present in any of tumor cells or less than 10% tumor cells with staining (-); more than 10% tumor cells with positive staining. The classification was done by two senior pathologists who did not know the clinicopathological data.
Analysis of data was accomplished using Chi-square test. P values less than 0.05 were considered to be statistically significant.
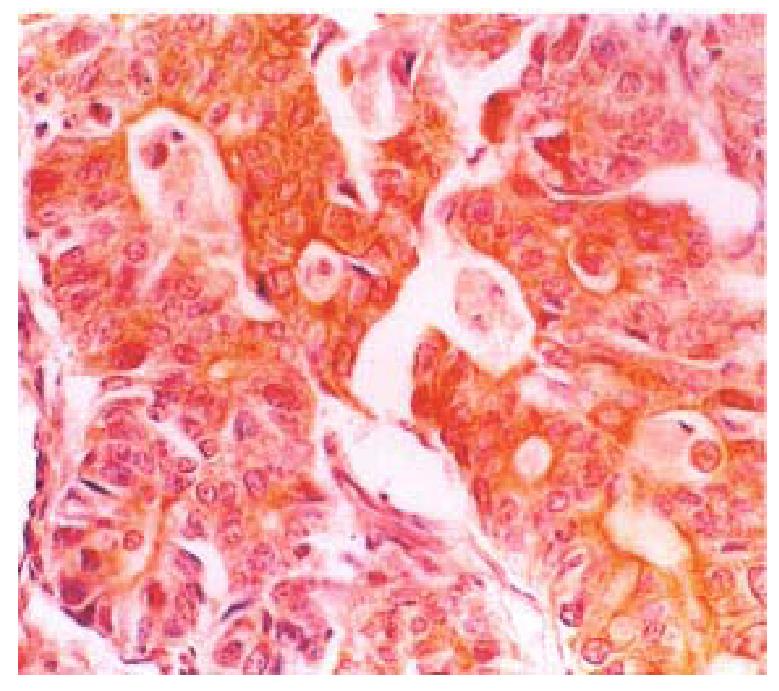
Thirty-three (57.89%) of the fifty-seven gastric carcinomas showed immunoreac tivity for Bax protein in gastric carcinoma cells. The Bax protein im-munoreactivity appeared brown or dark brown, which was mainly located in the cytoplasm (Figure 1), and a few specimens simultaneously expressed Bax protein in the cell nuclear of tumor cells. Some of the mature lymphocytes infiltrating in the stroma of gastric carcinomas also had Bax protein expression.
Correlation between Bax protein expression and clinical pathological data of gastric carcinoma is illustrated in Table 1. The rate of Bax protein expres-sion was not correlated with patient age, sex, tumor size, lymph node metastasis, depth of invasion and clinical stages (P > 0.05). The immunoreactivity of Bax wa s significantly associated with morphologic phenotype and grades of differentiation of gastric carcinoma. 20 (73.3%) of 30 gastric carcinomas of intestinal morphologic phenotype were immunoreactive versus 11 (40.7%) of 27 diffuse gastric carcino-mas (P < 0.05). 17 (81.0%) of 21 well and moderately differentiated gastric carcinomas were im-munoreactive versus 10 (38.5%) of 26 poorly differentiated gastric carcinomas(P < 0.01).
| Parameters | n | Bax protein expression | Positive rate (%) | |
| - | + | |||
| Age (year) | ||||
| ≤ 59 | 39 | 22 | 17 | 56.41 |
| ≥ 60 | 18 | 11 | 7 | 61.11 |
| Sex | ||||
| M | 39 | 24 | 15 | 61.54 |
| F | 18 | 9 | 9 | 50.00 |
| Type | ||||
| Intestinal | 30 | 22 | 8 | 73.33a |
| Diffuse | 27 | 11 | 16 | 40.74 |
| Grade of differentiation | ||||
| Well/moderate | 21 | 17 | 4 | 80.95b |
| Poor | 26 | 10 | 16 | 38.46 |
| Mucoid | 10 | 6 | 4 | 60.00 |
| Tumor size | ||||
| < 5 cm | 35 | 21 | 14 | 60.00 |
| ≥ 5 cm | 22 | 12 | 10 | 54.55 |
| Lymph-node metastasis | ||||
| Negative | 23 | 13 | 10 | 56.52 |
| Positive | 34 | 20 | 14 | 58.82 |
| Serosal invasion | ||||
| Absent | 27 | 14 | 13 | 51.85 |
| Present | 30 | 19 | 11 | 56.67 |
| Clinical stages | ||||
| I and II | 34 | 20 | 14 | 58.82 |
| III and IV | 23 | 13 | 10 | 56.52 |
Apoptosis is a highly regulated form of programmed cell death defined by distinct morphological and bio-chemical features. Apoptosis plays a major role in development, embryogenesis, regulation of the immune system, and carcinogenesis, as well as in the maintenance of tissue homeostasis. Various protein molecules or oncogenes and suppressor genes are involved in the process of apoptosis, including p53, myc, ras, Bcl-2, Bax and the Fas/Fas ligand system[4]. In recent studies, Bax protein expression has been identified in various human malignant tissues, including the prostate, colon, breast, testis and ovary[5-8]. But, little is known about Bax protein ex-pression and its relationship with the biological behavior of human gastric carcinoma.
In this study, we found that the positive rate of Bax protein staining in gastric carcinoma was 57.9%. The proapoptotic gene Bax can express to various degrees in most kinds of the gastric carcino-ma and may take part in the regulation of apoptosis of gastric carcinoma. Our findings concerning the relationship between Bax protein expression and the pathological characteristics of gastric carcinoma showed that Bax expression was associated with morphologic phenotype and grades of differentiation of gastric carcinomas. The difference in the Bax protein expression in the intestinal and diffuse types demonstrated that aberrant Bax protein expression was preferentially associated with development of intestinal type gastric carcinoma, indicating once more the different biologic mechanisms involved in the development of these two histologic subtypes. The difference in the Bax protein expression between poorly differentiated and well/ moder-ately-differentiated gastric carcinomas demonstrated that aberrant Bax protein expression was associated with differentiation or growth speed of gastric car-cinomas. There was no significant relationship between Bax protein expression and tumor size, lymph node metastasis, serosal invasion or clinical stages. Therefore, Bax prote in expression might play an important role in the early development and phenotypic differentiation of gastric carcinomas, but not in tumor progression.
Key project of the 9th 5-year plan for Medicine and Health of Army, No.96Z047.
Edited by Jing-Yun Ma
| 1. | Que FG, Gores GJ. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996;110:1238-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Solary E, Dubrez L, Eymin B. The role of apoptosis in the pathogenesis and treatment of diseases. Eur Respir J. 1996;9:1293-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4399] [Cited by in F6Publishing: 4433] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 4. | Hale AJ, Smith CA, Sutherland LC, Stoneman VE, Longthorne VL, Culhane AC, Williams GT. Apoptosis: molecular regulation of cell death. Eur J Biochem. 1996;236:1-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 460] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Krajewski S, Blomqvist C, Franssila K, Krajewska M, Wasenius VM, Niskanen E, Nordling S, Reed JC. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995;55:4471-4478. [PubMed] [Cited in This Article: ] |
| 6. | Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422-2427. [PubMed] [Cited in This Article: ] |
| 7. | Chresta CM, Masters JR, Hickman JA. Hypersensitivity of human testicular tumors to etoposide-induced apoptosis is associated with functional p53 and a high Bax: Bcl-2 ratio. Cancer Res. 1996;56:1834-1841. [PubMed] [Cited in This Article: ] |
| 8. | Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567-1576. [PubMed] [Cited in This Article: ] |