Published online Jun 15, 1998. doi: 10.3748/wjg.v4.i3.222
Revised: April 2, 1998
Accepted: May 16, 1998
Published online: June 15, 1998
AIM: To study the peripheral mechanism of the inhibitory effect of intra-third ventricular administration (icv) of histamine (HA) on gastric acid secretion in rats.
METHODS: Gastric acid was continuously washed with 37 °C saline by a perfusion pump in male adrenalectomized SD rats. Drugs were injected intravenously (iv) by a syringe pump and their effect on pentagastrin-induced (10 μg·kg·h, iv) gastric acid secretion was observed.
RESULTS: The inhibitory effect of HA (1 μg, icv) on gastric acid secretion was blocked by subdiaphragmatic vagotomy, and pretreatment with atropine (0.005 mg·kg·h, iv). Pretreatment with somatostatin antagonist, cyclo-[7-aminoheptanoyl-Phe-D-Trp-Lys-Thr(Bzl)], (2 μg-4 μg·kg·100 min, iv) could also block the inhibitory effect of HA on gastric acid secretion in a dose dependent manner.
CONCLUSION: The inhibitory effect of centrally administrated HA on gastric acid secretion may be mediated by vagi, acetylcholine M receptor and somatostatin.
- Citation: Zhang ZF, Wang ZL, Lu GQ. Peripheral mechanism of inhibitory effect of centrally administrated histamine on gastric acid secretion. World J Gastroenterol 1998; 4(3): 222-224
- URL: https://www.wjgnet.com/1007-9327/full/v4/i3/222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i3.222
It has been reported by our laboratory that intra-third-ventricular administration (icv) of histamine (HA) or 2-pyridylethylamine (PEA), a H1-receptor agonist, inhibits gastric acid secretion induced by intravenous (iv) pentagastrin (G-5) in rats. The inhibitory effect of HA or PEA on gastric acid secretion was mediated in turn by corticotropin-releasing factor (CRF) and β-endorphin in central nervous system and abolished by subdiaphragmatic vagotomy (SV)[1-3]. The aim of the present study was designed to determine the peripheral mechanism of inhibitory effect of centrally administrated histamine on gastric acid secretion.
Male Sprague-Dawley (SD) rats weighing between 200 g-300 g were used. The animals were deprived of food for 24 h, but were allowed free access to water prior to anesthesia.
The rats were anesthetized with a single intraperitoneal injection of pentobarbital (50 mg/kg). Intra-third-ventricular implantation and acute gastric lumen perfusion were carried out as described previously by our laboratory[1]. Gastric perfusion samples were collected every 10 min and were titrated by 0.01 mol/L NaOH to neuter. Total acid output per 10 min was calculated. The anus temperature of rats was kept at 37 °C by electric light during the experiment. Sufficient pentobarbital was given subcutaneously before G-5 was injected.
In has been reported by our laboratory[2] that adrenal gland was associated with stimulating effect of icv PEA on gastric acid secretion in SV rats. To remove this effect, the following experiments were done in adrenalectomized rats.
After gastric acid secretion was kept at base level (0.8-3.5 μmol/10 min) for 20 min, G-5 was injected iv by a syringe pump to increase gastric acid secretion. After the gastric acid secretion was increased and kept stable for 30 min, other experimental drugs were given. HA was administered by a syringe pump in a silicon tube, which was equally long and connected with the implanted cannula. The injected volume of HA solution or vehicle was 5 μL. The acute SV was performed as described previously[4].
G-5 and HA were purchased from Shanghai Dongfeng Biochemistry Reagent Factory, Chinese Academy of Sciences. Cyclo-[7-aminoheptanoyl-Phe-D-Trp-Lys-Thr (Bzl)] (c-PTLT), from Sigma Company, U.S.A. and atropine sulfate, from Guangzhou Qiaoguang Pharmaceutical Factory.
Experimental data was expressed in % change of total acid output, which was calculated as follows: % change of total acid output = (E2-E1)/E1 × 100%, in which E1 represents total acid output per 10 min before experimental drugs were given (it is the mean of total acid output of 30 min before experimental drugs were given); E2, the total acid output (TAO) per 10 min after experimental drugs were given; “+”, the TAO increase and “-’, TAO decrease. Significance was assessed by Student’s paired t test. The results were expressed in x-±sx-.
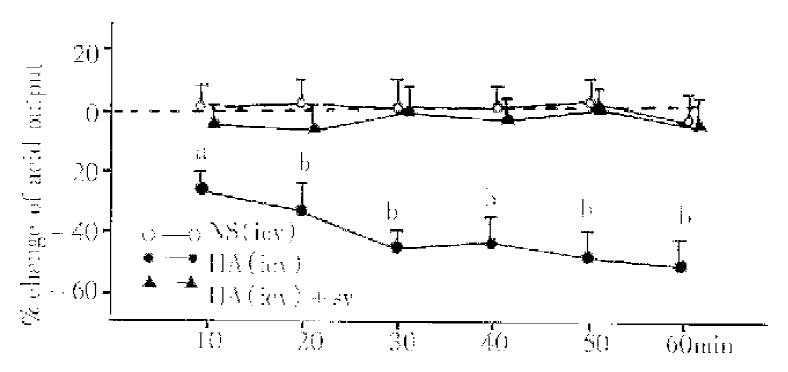
All the experiments were done on the basis of iv injection of G-5 in a dosage of 10 μg·kg·h. HA, injected icv in the dosage of 1 μg/5 μL (n = 9), decreased significantly the TAO (the maximum reached 44%). When HA group was compared with NS group (5 μL, n = 9), P values were less than 0.05, 0.01 or 0.001 at 10 min, 20 min, 30 min, 40 min, 50 min and 60 min (Figure 1). In SV rats (n = 8), icv injection of HA in the same dosage had no significant effect on the TAO (Figure 1).
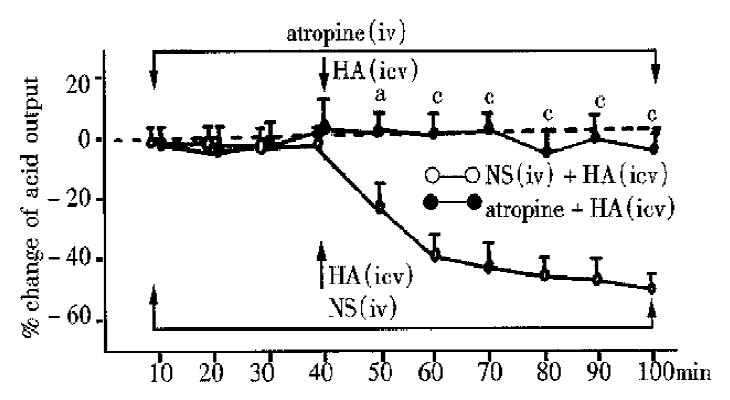
HA (icv 1 μg/5 μL, n = 8) was injected 40 min after the atropine (iv, 0.05 mg·kg·100 min) was administered, the total acid output, had no significant change (Figure 2) compared with that before HA. If HA (icv, 1 μg/5 μL, n = 9) was injected 40 min after iv NS was given, the total acid output, decreased significantly (the maximum to -44%) compared with that before HA. Compared with NS group, atropine group had significant changes at 50 min, 60 min, 70 min, 80 min, 90 min and 100 min (P < 0.05, 0.01 or 0.001), (Figure 2).
There were no significant changes (P > 0.05) in the total acid output at 10 min, 20 min, 30 min, 40 min, 50 min, 60 min, 70 min, 80 min, 90 min, and 100 min with pretreatment of c-PTLT (4 μg·kg·100 min, iv). These results suggest c-PTLT in the dosage of 4 μg·kg·100 min had no significant effect on gastric acid secretion induced by iv G-5. The following experiments were divided into three groups according to the difference of c-PTLT dosage: group 1, 4 μg·kg·100 min; group 2, 3 μg·kg ·100 min; and group 3, 2 μg·kg·100 min.
In group 1 (n = 9), 40 min after c-PTLT was given HA (icv, 1 μg/5 μL), had no significant effect on the TAO (Figure 3), suggesting the inhibitory effect of HA (icv) on gastric acid secretion was blocked. In the control group (iv NS), HA (icv, 1 μg/5 μL), injected 40 min after NS was injected, could still inhibit gastric acid secretion. Compared between the two groups, P values were less than 0.01 or 0.001 at 60 min,70 min, 80 min, 90 min and 100 min.
In group 2 (n = 9), the inhibitory effect of HA (icv,1 μg/5 μL) on gastric acid secretion was partly blocked (Figure 3). Compared with the control group, P values were less than 0.05, 0.01 or 0.001 at 50 min, 60 min, 70 min, 80 min, 90 min and 100 min.
In group 3 (n = 8), the inhibitory effect of HA (icv, 1 μg/5 μL) on gastric acid secretion was not changed. Compared with the control group, P values were more than 0.05.
The present study demonstrated that HA has central inhibitory effect on gastric acid secretion in adrenalectomized rats. This result is consistent with the results reported by Wang, Sun and Li[1-3]. The central inhibitory effect of HA on gastric acid secretion could be blocked by pretreatment with either SV, or atropine and c-PTLT, an antagonist of somatostatin, suggesting that the central inhibitory effect of HA on gastric acid secretion may be mediated by vagi, acetylcholine M receptor and somatostatin.
It is D cell that synthetises and secrets somatostatin in mammals. D cell is located near G cell in gastric antrum, and along gastric gland, particularly near the parietal cell in oxyntic mucosa. Somatostatin, mediated by its receptor in the membrane of parietal cells, inhibits gastric acid secretion induced by gastrin and acetylcholine. In addition, somatostatin inhibits the gastrin secretion in the basal condition or the gastrin secretion induced by feeding, acetylcholine and bombesin. In stomach, somatostatin inhibits the histamine secretion in the basal condition or induced by gastrin. The c-PTLT, as an artificial antagonist of somatostatin, completely blocked the inhibitory effects of exogenous somatostatin on growth hormone, insulin, and glucagon release. The efficiency of c-PTLT was demonstrated by the almost complete and sustained reversal of acid inhibition after exogenous infusion of somatostatin. Therefore, c-PTLT should have effectively reversed inhibition by endogenously released somatostatin throughout the period of the intraduodenal fat perfusion[5]. In the present study, c-PTLT, dose-dependently inhibited central inhibitory effect of HA on gastric acid secretion, which might be mediated by somatostatin in the periphery. The blocked central inhibitory effect of HA on gastric acid secretion by SV suggests that this effect was accomplished by the vagi. That vagi was involved in the control of somatostatin secretion was proved by some early studies. Vagotomy reduced the somatostatin responses to feeding during the first 30-min period following the ingestion of the meal. Atropine sulfate in the dosage of 0.02 mg·kg·h, iv decreased the somatostatin responses to the meal, while the dosage of 0.05 mg·kg·h blocked such responses[6]. This suggests that vagus and cholinergic mechanisms play important roles in the control of somatostatin secretion responses to the meal. After vagotomy, atropine sulfate still decreased the somatostatin secretion[6], indicating that local factors are involved in the control of somatostatin secretion. CRF, injected icv, increased blood level of somatostatin. This effect was blocked by vagotomy or pretreatment with atropine[7]. Li et al[3] in our laboratory reported that the central inhibitory effect of PEA on gastric acid secretion was blocked by antiserum of CRF, suggesting that the effect of PEA is mediated by CRF in the central nervous system. According to this report and the present study, vagi or cholinergic mechanisms are involved in the control of somatostatin secretion. Holst[8] reported that vagus stimulation or atropine mediated by GRP (gastrin-releasing polypeptide)-containing fibers stimulated somatostatin secretion in the isolated perfused porcine antrum. Schubert[9] reported that methacholine exerted dual inhibitory and stimulatory effects on somatostatin cells of mucosal segments from the fundus and antrum of rat or the isolated luminally perfused mouse stomach. There are some contradictory reports about somatostatin secretion of vagal control. For example, somatostatin secretion was deoreased by vagus stimulation and this effect was abolished by atropine 10-9 M[10]. As there are lots of fibers in vagi, the above contradictory reports may be related with too much fibers stimulation when vagi was stimulated electrically. In summary, the mechanism that somatostatin secretion is controlled by vagi still remains unclear and more studies are needed.
Project supported by the Doctoral Program Fund for Institutions of Higher Education. This subject was awarded the first prize of outstanding thesis by the Guangdong Association of Physiological Sciences.
| 1. | Wang ZL, Lu GQ. Effect of intra ventricular administration of histamine and its receptor agonists on pentagastrin-induced gastric acid secretion. Acta Physiologica Sinica. 1992;44:261-268. [Cited in This Article: ] |
| 2. | Sun CG, Wang ZL, Wang TZ, Lu GQ. Mechanism of effect of intra ventricular administration of histamine H-1-receptor agonists on gastric acid secretion in rats. Acta Physiologica Sinica. 1993;45:581-586. [Cited in This Article: ] |
| 3. | Li MY, Wang ZL, Lu GQ. Central mechanism of inhibitory effect of hista-mine H-1-receptor agonists PEA on gastric acid secretion. Acta Physiologica Sinica. 1995;47:259-263. [Cited in This Article: ] |
| 4. | Tache Y, Goto Y, Gunion MW, Rivier J, Debas H. Inhibition of gastric acid secretion in rats and in dogs by corticotropin-releasing factor. Gastroenterol Jpn. 1984;86:281-286. [Cited in This Article: ] |
| 5. | Fung L, Pokol-Daniel S, Greenberg GR. Cholecystokinin type A receptors mediate intestinal fat-induced inhibition of acid secretion through somatostatin-14 in dogs. Endocrinology. 1994;134:2376-2382. [PubMed] [Cited in This Article: ] |
| 6. | Woussen-Colle MC, Lalieu C, Simoens C, De-Graef J. Effect of vagotomy and atropine on plasma somatostatin responses to a meal in conscious dogs. Regul Pept. 1988;21:29-36. [Cited in This Article: ] |
| 7. | Smedh U, Uvnäs-Moberg K. Intracerebroventricularly administered corticotropin-releasing factor releases somatostatin through a cholinergic, vagal pathway in freely fed rats. Acta Physiol Scand. 1994;151:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Holst JJ, Skak-Nielsen T, Orskov C, Seier-Poulsen S. Vagal control of the release of somatostatin, vasoactive intestinal polypeptide, gastrin-releasing peptide, and HCl from porcine non-antral stomach. Scand J Gastroenterol. 1992;27:677-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Schubert ML, Hightower J. Functionally distinct muscarinic receptors on gastric somatostatin cells. Am J Physiol. 1990;258:G982-G987. [PubMed] [Cited in This Article: ] |
| 10. | Madaus S, Bender H, Schusdziarra V, Kehe K, Munzert G, Weber G, Classen M. Vagally induced release of gastrin, somatostatin and bombesin-like immunoreactivity from perfused rat stomach. Effect of stimulation frequency and cholinergic mechanisms. Regul Pept. 1990;30:179-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |