Published online Jun 15, 1998. doi: 10.3748/wjg.v4.i3.219
Revised: January 10, 1998
Accepted: February 21, 1998
Published online: June 15, 1998
AIM: To study the expression and significance of laminin in human colorectal carcinoma.
METHODS: Using the monoclonal antibody to laminin and streptavidin-peroxidase immunohistochemical method, the expression of laminin in 63 cases of human colorectal carcinoma tissues was determined.
RESULTS: In normal marge intestinal mucosa adjacent to carcinoma, laminin was largely restricted to basement membrane in continuous linear pattern. In contrast, human colorectal carcinomas exhibited a progressive loss of an intact basement membrane that was correlated with decreasing differentiation degree. Well and moderately differentiated tumors exhibited a thin basement membrane with intermittent disruptions, and poorly differentiated tumors exhibited no areas of intact basement membrane. An association was found between lack of basement membrane laminin immunohistochemical staining in colorectal carcinoma and poorly differentiated tumor (P < 0.01).
CONCLUSION: Immunohistochemical staining for laminin could provide a very useful indexfor the determination of the differentiation degree of colorectal carcinoma.
- Citation: Feng S, Wang YY, Song JD. Relationship between expression of laminin and pathological features in human colorectal carcinoma. World J Gastroenterol 1998; 4(3): 219-221
- URL: https://www.wjgnet.com/1007-9327/full/v4/i3/219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i3.219
Normal epithelial tissues secretes, assembles, and adhere to well-defined basement membranes. Loss of a well-defined basement membrane is one of the features of neoplastic proliferation for epithelial-derived tumors[1]. Laminin, a major glycoprotein of basement membrane which has been shown to regulate a variety of biological phenomena including cell attachment, growth, morphology and cell migration by specific high affinity receptors, plays an important role in the interaction of tumor cells with the basement membrane, the differentiation, invasion and metastasis of tumors[2,3]. Immunohistological studies, by using antibodies specific for laminin and other basement membrane components, such ast type-IV collagen, have revealed disruption or loss of basement membrane staining in a number of malignant tumors, including breast, pancreatic, bladder, prostatic and colorectal carcinomas[4]. In this study, basement membrane protein laminin was assessed by streptavidin-peroxidase immunohistochemical method to facilitate the understanding of the relationship between laminin expression in human colorectal carcinoma and the pathological features of tumor
Sixty-three cases of human colorectal carcinoma specimens were obtained from the Department of Pathology, China Medical University. Thirty-three were male and 30 were female with ages ranging from 26 to 72 years. The tissues were fixed in 10% nautral buffered formalin and embedded in paraffin wax. Five μm paraffin sections were cut and used for HE staining and immunohistochemistry.
Laminin expression was determined by using streptavidin-peroxidase (SP) immunohistochemical method with anti-laminin monoclonal antibody (SIGMA, 1:1000; SP kit ZYMED). Both negative and positive controls were set. The staining was scored according to both intensity and continuity of the basement membrane. they were scored as negative (-), fragment pattern (+), interrupted linear pattern (+ +), and continuous linear pattern (+ + +). Negative and fragment patterns were classified as discontinuity and the other two patterns as continuity. Statistical analyses were conducted by χ2 test. Results were considered significantly different when P value was less than 0.05.
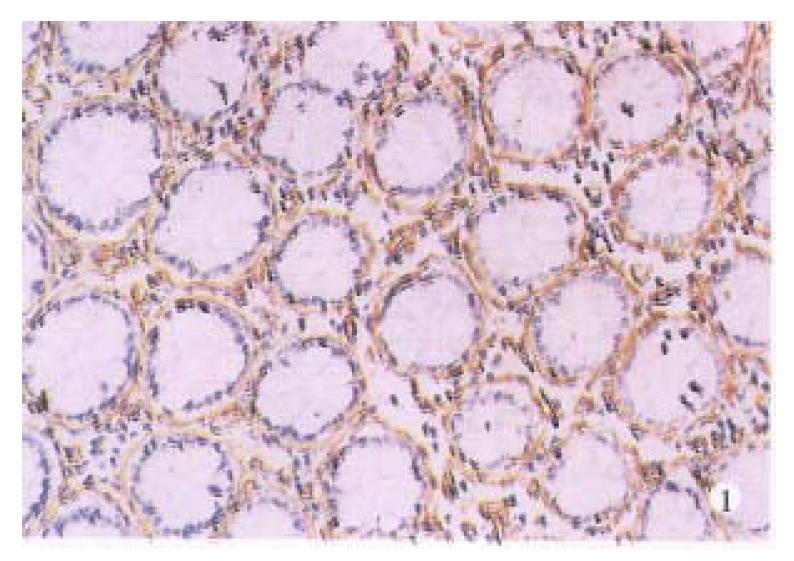
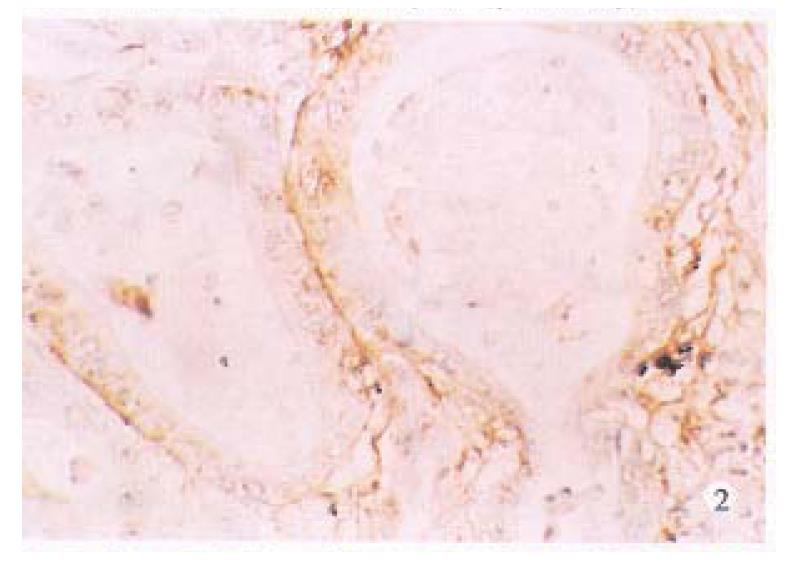
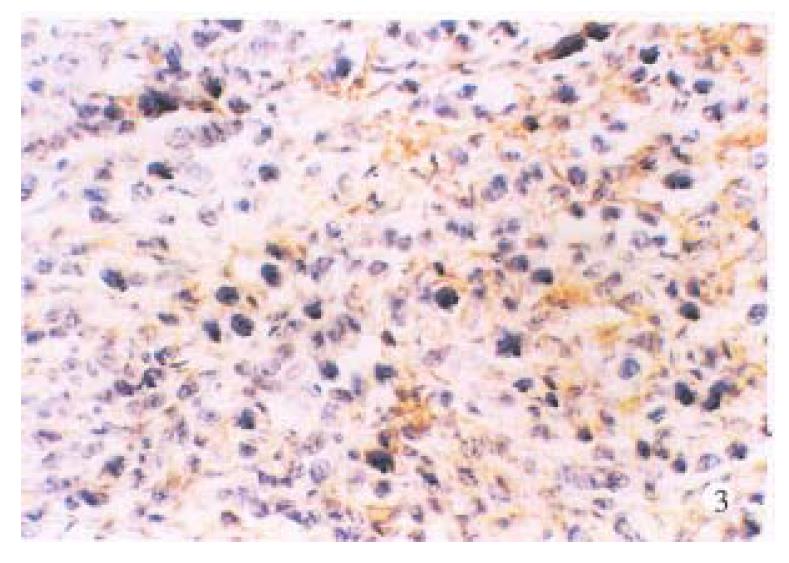
Normal large intestinal mucosa adjacent to carcinoma exhibited an organized glandular epithelium overlying a well-defined continuous linear basement membrane that stained for laminin (+ + +), (Figure 1).Well and moderately differentiated colorectal carcinomas differed from normal colon in which periglandular basement membrane staining was thin and intermittently discontinuous (+ + to + + +), (Figure 2). Poorly differentiated tumors consisted of solid nonglandular islets of cells. Patches of laminin staining was scatered throughout the tumor. Nonpolarized cytoplasmic laminin staining without a surrounding basement membrane (- to +) (Figure 3) was found sometimes in the nests of cells in these tumors.
Relationship between tumor grade and basement membrane laminin staining pattern in the tumors is shown in Table 1. Of the well and moderately differentiated cases, 80% and 57.1% showed continuous basement membrane laminin staining respectively, compared with 14.6% of poorly differentiated ones. When the staining patterns for poorly differentiated tumors were compared statistically with well and moderately differentiated tumors, a significant difference was found (χ2: P < 0.01). Hence, discontinuous basement membrane laminin staining is associated with poor differentiation of tumors.
Comparison between basement membrane laminin staining pattern in tumor and tumor stage is given in Table 2. When regional lymph nodes were involved (Dukes’s stage C), there was no linear staining of basement membrane laminin in 13/21 (61%) cases of primary tumors, whereas the majority (23/42) of stages A and B tumors showed continuous basement membrane laminin staining. However, there was no statistical significance (χ2: P > 0.05).
With the increasing maximum depth of tumor invasion, discontinuous basement membrane laminin staining became more prominent in tumors (Table 3), but without statistical significance (χ2: P > 0.05).
Human colorectal carcinomas are comprised, in part, of malignant cells of varying degrees of differentiation from well to poorly differentiated. At present, tumor histology is the primary criterion used to determine the degree of differentiation of a given tumor. It is becoming increasingly apparent, however that the morphological characteristics of tumors that relate to their degree of differentiation result from alterations in the biological properties of the cells[5]. Moreover, these biological properties probably contribute to the clinical be havior associated with tumors of a particular differentiation state. For example, poorly differentiated colorectal carcinomas are considered, in general, to be more aggressive and to offer a worse progress than well and moderately differentiated tumors[6].
Laminin, the major basement membrane component, was determined by using immunohistochemical method. We demonstrated a defined, continuous basement membrane underlying normal colonic epithelium. In contrast, human colorectal carcinomas exhibited a progressive loss of an intact basement membrane that was correlated with decreasing differentiation degree. Well and moderately differentiated tumors showed a thin basement membrane with intermittent disruptions, and poorly differentiated tumors exhibited no areas of intact basement membrane. An association was found between lack of basement membrane laminin immunohistochemical staining in colorectal carcinoma and poorly differentiated (P < 0.01).
We also found an association between discontinuous basement membrane laminin immunostaining in colorectal carcinomas and more advanced tumor stage; and with increasing depth of invasion, tumors more frequently show discontinous basement membrane laminin staining. An important underlying factor may be the basement membrane laminin discontinuity in tumor is associated with poor tumor differentiation, which itself is linked with a more invasive phenotype.
There is an evidence for a number of mechanisms explaining basement membrane discontinuity in malignant tumors. Daneker et al[7] reported that in poorly differentiated colon carcinoma cells, newly synthesized laminin was secreted more slowly than in well differentiated cells. Basement membrane degradation by tumor-derived proteinases may also be important. Proteinase specific for the basement membrane component type-IV collagen has been identified in invasive tumors. Increased amount of the degradative enzymes cathepsin B, urokinas-type plasmignogen activator and β-glucuronidase has been demonstrated in colorectal carcinomas[4,8-9].
Up to now, the genetic and biochemical factors that under the tumore differentiation and other biological properties are poorly understood. Studies aimed at definding these factors would not only contribute to an understanding of differentiation at molecular and cellular level but also would provide new reagents and criteria for a more precise determination of the degree of tumor differentiation, thus faciliate the understanding of the biological behaviors of tumors. The results of this study confirmed that immunohistochemical staining of laminin could provide a very useful index for the determination of the differentiation degree of human colorectal carcinoma.
Project supported by the National Natural Science Foundation of China, No. 3904005.
| 1. | Grover A, Andrews G, Adamson ED. Role of laminin in epithelium formation by F9 aggregates. J Cell Biol. 1983;97:137-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Terranova VP, Hujanen ES, Martin GR. Basement membrane and the invasive activity of metastatic tumor cells. J Natl Cancer Inst. 1986;77:311-316. [PubMed] [Cited in This Article: ] |
| 3. | Liotta LA. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1-7. [PubMed] [Cited in This Article: ] |
| 4. | Liotta LA. Cancer cell invasion and metastasis. Sci Am. 1992;266:54-59, 62-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 171] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Wewer UM, Taraboletti G, Sobel ME, Albrechtsen R, Liotta LA. Role of laminin receptor in tumor cell migration. Cancer Res. 1987;47:5691-5698. [PubMed] [Cited in This Article: ] |
| 6. | Klimpfinger M, Beham A, Denk H. [Pathologic findings in colorectal cancers and discussion of their significance for tumor therapy according to stage]. Wien Klin Wochenschr. 1987;99:488-493. [PubMed] [Cited in This Article: ] |
| 7. | Daneker GW, Mercurio AM, Guerra L, Wolf B, Salem RR, Bagli DJ, Steele GD. Laminin expression in colorectal carcinomas varying in degree of differentiation. Arch Surg. 1987;122:1470-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Boyd D, Florent G, Kim P, Brattain M. Determination of the levels of urokinase and its receptor in human colon carcinoma cell lines. Cancer Res. 1988;48:3112-3116. [PubMed] [Cited in This Article: ] |
| 9. | Feng Shu, Song Jindan. Study on the relationship between invasiveness and β-glucuronidase secreted by human colorectal carcinoma cell line. Chin J Cell Biol. 1996;18:138-140. [Cited in This Article: ] |