Published online Dec 15, 1997. doi: 10.3748/wjg.v3.i4.228
Revised: August 26, 1996
Accepted: September 13, 1996
Published online: December 15, 1997
AIM: To establish a new experimental model system of human fetal hepatocytes to study the mechanisms underlying the protective effect of silybin and polyporus umbellalus polysaccharides (PSP) on the cellular ultrastructure.
METHODS: Human fetal hepatocytes were obtained from the liver of a human fetus that resulted from a medically necessary induced labor; the mother provided informed consent for sampling, experimental use and publication of findings. The hepatocytes were cultured and then pretreated with silybin or PSP or without either (control), after which the treated cells were exposed to CCl4 for 4 h. Changes in cellular ultrastructure were observed by scanning electron microscopy and transmission electron microscopy, and changes in alanine aminotransferase (ALT), aspartate aminotransferase (AST) and superoxide dismutase (SOD) were assayed.
RESULTS: Levels of ALT and AST were significantly decreased, and level of SOD was elevated in the two pretreatment groups following CCl4 exposure, as compared to the control group. The cellular integrity and ultrastructure were well preserved in the two pretreatment groups but were seriously damaged in the control group.
CONCLUSION: The CCl4-induced hepatotoxic cell model system of human fetal hepatocytes is an effective tool for studying the hepatoprotective effect of drugs and may be applicable for studies to screen medicines for treatment of hepatitis.
- Citation: Wang MR, Le MZ, Xu JZ, He CL. Establishment and application of an experimental model of human fetal hepatocytes for investigation of the protective effects of silybin and polyporus umbellalus polysaccharides. World J Gastroenterol 1997; 3(4): 228-230
- URL: https://www.wjgnet.com/1007-9327/full/v3/i4/228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i4.228
The protective effects of drugs against hepatic injury, such as that induced by viruses or chemical substances, were traditionally studied by using animal model systems. The animals — most frequently dogs or rats — were pretreated with the drugs under investigation either by oral administration or injection and then administered hepatotoxic substances — usually CCl4 or D-galactosamine[1]. The protective effects of the drug were then determined by biochemical assay of serum and the pathological changes observed in the liver. However, there are some drawbacks to the animal-based approach, including a long experimental period and complicated procedure and inconsistent results due to the inherent individual differences of the animals and animal-human interspecies differences. Effects of a drug on an animal’s liver may not precisely or comprehensively reflect effects on the human liver. Moreover, the animal models are limited in their ability to indicate whether any protective effects of a drug result from a direct or an indirect action, specifically because the protective effects of a drug on liver can be produced by stimulating the humoral factors. In order to overcome each of these challenges of the animal model system approach, we established a model system based on primary culture of human fetal hepatocytes and the applied it to the study of the protective effects of silybin and polyporus umbellalus polysaccharides (PSP) on liver using scanning and transmission electron microscopy (SEM and TEM, respectively).
Hepatocytes were obtained from the liver of a human fetus that had resulted from a medically necessary induced labor; the mother provided written informed consent for sampling, experimentation and publication of the related findings. The hepatocytes were isolated by means of the modified collagenase method[2]. The viability of the final parenchymal cell suspension was determined by trypan blue exclusion assay, and found to be > 90% with a < 5% contamination of non-parenchymal cells. The cells were seeded at a density of 1 × 1012·L-1 into 24-well plates (Nuclon) and cultured at 37 °C in a humidified atmosphere of 5% CO2 in 2 mL RPMI-1640 (pH 7.0, Gibco). Each well was then supplemented with 4 mmol·L-1- L-glutamine, 10% fetal calf serum, penicillin (at 100 IU/mL) and streptomycin (at 100 μg/mL).
The medium was exchanged at 4 h after plating, and levels of alpha-fetoprotein (aFP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the medium were measured at 1, 4 and 16 h of culture.
Ten hours after the first medium change, a co-culture of silybin or PSP (or normal saline, for a control group) was started with hepatocytes at a final concentration of 0.5 g·L-1. The hepatotoxic injury was then induced at 1, 4 and 16 h by addition of 1 mol·L-1 CCl4 directly into the medium, with hepatocytes at a final concentration of 20 μg·L-1. After 4 h, the levels of ALT, AST and superoxide dismutase (SOD) in culture medium were measured and the cells were collected for SEM and TEM.
The primarily cultured hepatocytes were collected for SEM and TEM observation after 4 h exposure to CCl4 for the groups pretreated with silybin or PSP and the normal saline control group. The methods for the hepatocyte preparation were carried out as previously described[3]. The changes of hepatocytes were observed under scanning (Philips SEM-50) and transmission electron (Japanese H-300) microscopes.
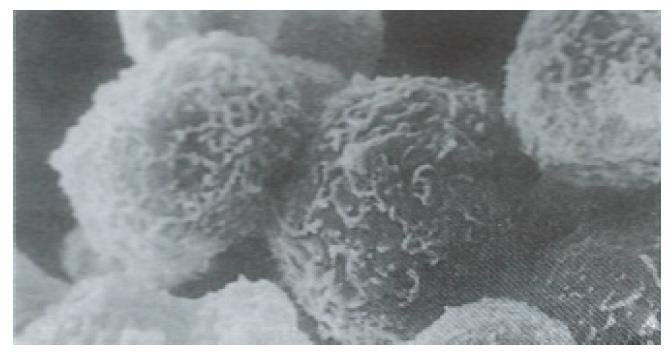
The isolated human fetal hepatocytes possessed good bioactivities, as evidenced by the detection of aFP in medium after 24 h of culture. The levels of ALT and AST were lower in the culture medium (Table 1) and the hepatocytes were less damaged than in the control group, and showed normal integrity of cellular membrane under SEM (Figures 1 and 2).
| n | Activity (U) | |||
| 1 h | 16 h | 24 h | ||
| aFP/ng·L-1 | 3 | 18.3 ± 2.0 | ||
| ALT/IU·L-1 | 3 | 8.2 ± 2.3 | 10.0 ± 1.6 | 12.0 ± 2.1 |
| AST/IU·L-1 | 3 | 10.3 ± 1.6 | 12.4 ± 1.8 | 13.5 ± 1.5 |
As shown in Tables 2 and 3, the activity of ALT and AST in the medium was significantly decreased and the level of SOD was elevated in the silybin and PSP pretreatment groups as compared with the control. The affects peaked at 16 h for the cultured hepatocytes pretreated with silybin and PSP.
| Group | n | ALT activity (U) | AST activity (U) | ||||
| 1 h | 4 h | 16 h | 1 h | 4 h | 16 h | ||
| Control | 3 | 40 ± 3.1 | 63 ± 2.5 | 71 ± 3.0 | 108 ± 5.6 | 165 ± 4.5 | 178 ± 2.4 |
| PSP (0.5 g·L-1) | 3 | 48 ± 2.2 | 35 ± 2.5 | 27 ± 3.4 | 95 ± 2.8 | 126 ± 2.3 | 101 ± 3.1 |
| Silybin (0.5 g·L-1) | 3 | 40 ± 2.5 | 46 ± 3.0 | 34 ± 3.3 | 93 ± 3.2 | 143 ± 4.0 | 123 ± 3.8 |
| Group | n | SOD (nmol/L) | ||
| 1 h | 4 h | 16 h | ||
| Control | 3 | 2.3 ± 0.8 | 3.0 ± 0.6 | 3.3 ± 1.2 |
| PSP (0.5 g·L-1) | 3 | 3.1 ± 1.8 | 4.8 ± 1.6 | 7.9 ± 2.1 |
| Silybin (0.5 g·L-1) | 3 | 2.4 ± 1.6 | 3.7 ± 1.1 | 5.9 ± 0.7 |
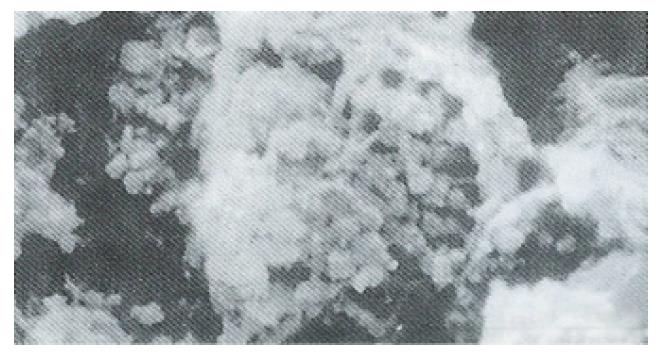
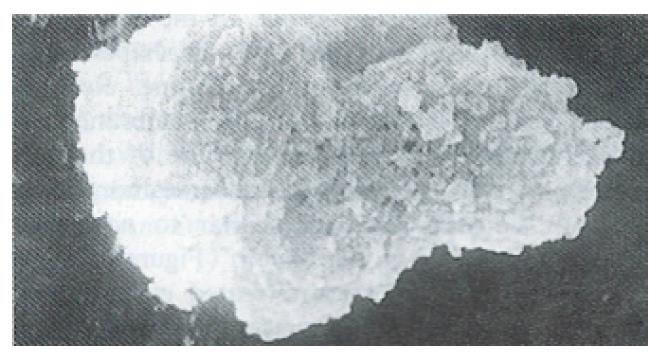
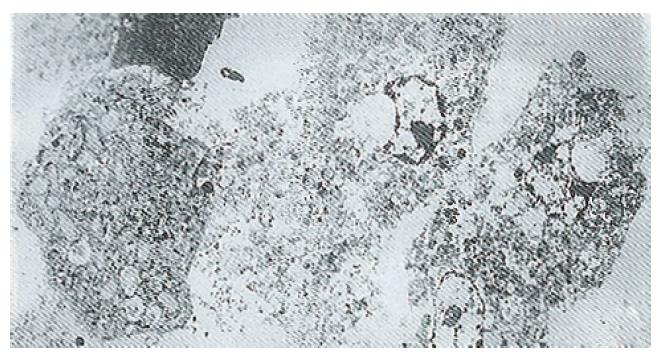
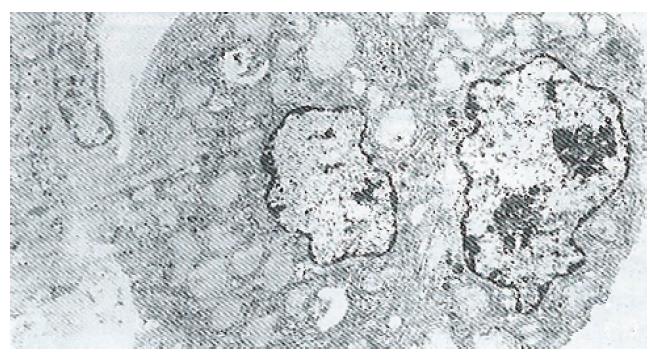
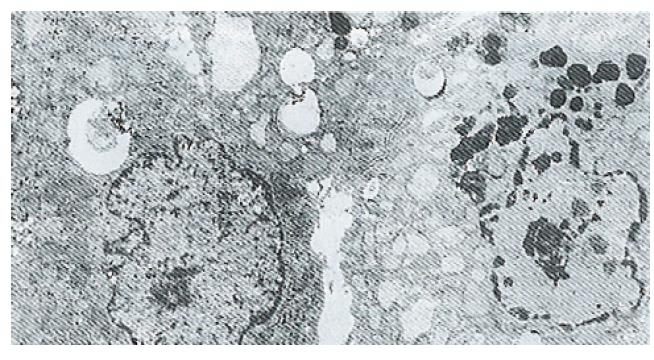
Ultrastructure of the human fetal hepatocytes that had been pretreated with silybin or PSP remained well preserved following exposure to CCl4, as compared with that of the control group. The control group showed a remarkable amount of dead cells and irregularly shaped hepatocytes (Figure 3), with the cellular membranes showing mesh-like appearance. In the silybin (Figure 4) and PSP pretreatment groups (Figure 5), the surface structure of the cellular membrane was slightly damaged and the morphological integrity was indistinguishable from that of the normal fetal hepatocytes (Figure 1). In the control group, most hepatocytes showed necrosis and serious damage to the cellular membranes (Figure 6). In contrast, the hepatocytes pretreated with silybin or PSP appeared generally similar to the normal fetal hepatocytes (Figure 2), with only a few showing swelling of the mitochondria, increased chromatin, reduced glycogen granules and slight dilatation of endoplasmic reticulum (Figures 7 and 8).
In this study, the hepatoprotective effects of silybin and PSP were demonstrated using the newly established experimental model system based on human fetal hepatocytes with toxic injury induced by CCl4 exposure. In particular, pretreatment with silybin or PSP protected the ultrastructure of the hepatocytes CCl4-induced damage. Moreover, the pretreated cells showed lower transaminases and higher SOD in the culture medium. Thus, these two drugs were able to effectively protect the cultured primary human fetal hepatocytes from chemical injury of CCl4. The lower transaminase activity in the culture medium may have been due to the preserved integrity of the cellular membrane system and/or of the mitochondria. The preserved integrity itself may have been brought about by the elevated SOD, which normally acts to prevent cellular membrane damage caused by free radicals.
To date, there has been no report of an experimental model system using primary cultured human fetal hepatocytes to study the hepatoprotective effects of drugs using assessment by SEM and TEM. The design of this model system was thought to be reasonable. The parenchymal hepatocytes were isolated from the liver of a human fetus by using collagenase digestion, and this process provided a high purity and good bioactivity, with sensitivity to CCl4. Cell systems offer many advantages as compared to whole animal model systems. The experimental time in the cell system is shorter, the procedures are simpler, and the cost is lower. Additionally, there exists no cellular individual difference between the hepatocytes used in experimental group and control group because they are of the same origin; thus, the results are more reliable. Moreover, the results of silybin and PSP treatment of the human fetal hepatocytes in this study were similar to those observed with silybin and PSP treatment of whole animals[4,5]. Therefore, this model system will be useful for studying the effects of drugs on human liver cells.
The model system established in this study could also be applied to the following studies: comparative analysis of several drugs on human hepatocytes at the same time, due to large amounts of hepatocytes being obtained from a single liver from a human fetus; study of the mechanisms and the localization of the action of medicines against hepatitis by observation of the ultrastructural changes of hepatocytes combined with biochemical and enzymological changes in culture medium; and screening of new drugs against viral hepatitis or alcoholic hepatitis or cirrhosis[2].
The authors thank Prof. Huang JS for providing the scanning electron microscope and Prof. Zhou XJ for the preparation of hepatocytes for transmission electron microscopy.
Original title:
S- Editor: A L- Editor: Filipodia E- Editor: Li RF
| 1. | Ju JL, Xu JZ, Ding Q, Liu GZ, Zhang DQ, Wang MR. Therapeutic effects of BHGF on acute hepatic failure induced by D-galactosamine in rats. Zhongguo Yaolixue Tongbao. 1991;7:222-224. [Cited in This Article: ] |
| 2. | Wang MR, Jia KM. Umbilicus vein perfusion enhancing the purity of human fetal hepatocytes isolated by trypsin digestion. Wannan Yixueyuan Xuebao. 1992;11:153-155. [Cited in This Article: ] |
| 3. | Wang MR, Le MZ, Xu JZ, He CL, Xia TY, Wang GX, et al. Foundation and application of model system for sieving drugs protecting hepatocytes with human fetal hepatocytes injured by CCla-4. Zhongguo Yaolixue Tongbao. 1996;12:55-59. [Cited in This Article: ] |
| 4. | Qiu ZY. Clinical study of Silybin on chronic viral hepatitis. Beifang Yaoxue Zazhi. 1984;4:43-44. [Cited in This Article: ] |
| 5. | Liu YF. Protective effects of polyporus umbellalus polysaccharides on the liver of mice injured by CCla-4. Zhongguo Yiyao Zazhi. 1988;6:345-347. [Cited in This Article: ] |