Published online Mar 15, 1997. doi: 10.3748/wjg.v3.i1.6
Revised: October 12, 1996
Accepted: January 21, 1997
Published online: March 15, 1997
AIM: To probe the effect of γ-IFN on hepatic fibrosis in schistosomiasis japonica.
METHODS: The amount and distribution of γ-IFN and extracellular matrix in the liver of 60 S. japonicum infected mice and 30 healthy mice at different stages, and their dynamics in 20 infected mice after administration of recombinant γ-interferon were determined by immunohistochemical streptavidin biotin peroxidase complex method.
RESULTS: The amount of γ-IFN in liver peaked at the 16th week after infection (3 mice respectively reached 2+, 3+ and 4+ grade), which was higher than the levels of infected mice at the 8th-12th week (P < 0.01), and γ-IFN was mostly distributed around egg granuloma. Fibronectin, laminin, type I and III collagens in liver of most infected mice reached 1+ grade and individual 2+ grade at the 8th week after infection, which were higher than those of healthy controls (P < 0.01), and were linearly distributed around egg granuloma . With chronicity and decrease of γ-interferon, however, the matrix proteins and collagens gradually increased, peaked respectively at the 20th and 24th week (over 70% infected mice with 3+ to 4+ grade), became wide and thick, and deposited in band like or retiform shape around and in egg granuloma. After administration of γ-IFN, only 3 infected mice had 2+ grade of fibronectin at the 20th week, and 2 mice had 3+ grade of type III collagen at the 24th week, and none of them reached 4+ grade, which were significantly less than the untreated group at the same stage (P < 0.01-0.05).
CONCLUSION: γ-interferon may play an important role in opposing the inflammatory response of egg granuloma, decreasing secretion and deposition of extracellular matrix in the liver and suppressing hepatic fibrosis.
- Citation: He YW, Liu W, Zen LL, Luo DD. Effects of γ-interferon on hepatic fibrosis of schistosoma japonicum-infected mice. World J Gastroenterol 1997; 3(1): 6-8
- URL: https://www.wjgnet.com/1007-9327/full/v3/i1/6.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i1.6
Some cytokines play important roles during egg-induced immune response, leading to the granuloma formation and hepatic fibrosis after schistosoma infection[1,2]. For example, studies in schistosomiasis mansoni[3,4] and japonica[5] showed that γ-interferon (γ-IFN) can affect the granuloma formation and inflammatory response. However, these studies just focused on the changes of γ-IFN in peripheral blood or induced in vitro. The amount and distribution of γ-IFN in the liver and its relationship with hepatic extracellular matrix were not involved. Therefore, we chose a sensitive immunohistochemical technique, streptavidin biotin peroxidase complex (SABC) method to measure γ-IFN in the liver of S. japonicum infected mice, as well as laminin (LN), fibronectin (FN), types I and III collagen in the liver which were used for the markers of hepatic fibrosis, and gave exogenous γ-IFN to infected mice to find out the relationship between γ-IFN and hepatic fibrosis.
One hundred and ten 8-wk-old Kun Ming mice were randomly divided into infected group, control group and γ-IFN injected group. Sixty mice in infected group were equally subdivided into 6 groups, of which each mouse was infected with 20 cercariae of S. japonica, and then were killed at the 8th, 12th, 16th, 20th, 24th and 28th week after infection, respectively; 30 healthy mice in control group were subdivided into 3 groups, and were killed at the 8th, 16th, and 24th week after infection respectively; and 20 infected mice were subdivided into 2 groups, each of which was treated with murine recombinant γ-IFN (Gibco BRL, United States) 50000 units daily I.M. for 20 d, starting at the 16th week after infection, and then were killed at the 20th and 24th week after infection, respectively. All fresh livers were stored in liquid nitrogen.
SABC is a modified ABC immunohistochemical technique, which means that avidin is replaced by streptavidin to reduce background stain and to increase specificity and sensitivity. Briefly, frozen sections of the liver, dried after blowing, were fixed with pure acetone for 10 min. After drying at room temperature and washing with PBS (2 min × 3 times), they were incubated with 1.5% H2O2/methyl for 10 min at room temperature, and blocked with sheep serum diluted at 1:50 in PBS for 15 min at room temperature. Then, first antibodies diluted 1:1000 in PBS, including monoclonal antibodies against murine γ-IFN (GIBCO BRL, United States), and antisera against FN, LN, types I and III (WTB1) collagen (Beijing Friendship Hospital), were added and incubated for 1 h at 37 °C. After 3 washings, biotin conjugated anti-IgG (Sigma, United States) diluted 1:100 in PBS was added and incubated for 20 min at 37 °C. Then, 10 μL of solution A and 10 μL of solution B of SABC kit, diluted in 1 mL of PBS, were added to the sections that had been washed as above, and incubated for 20 min at 37 °C. After that, 3-amino-9-ethylcarbazole (AEC) was colorized for 30 min and then washed with water. Finally, the sections were examined under light microscope after covering with glycol gelatin.
The amounts of γ-IFN, LN, FN, types I and III collagen were divided into 4 grades, according to the shade of color, range and density of distribution. Red and sporadic stain was defined as 1+ grade, red and fine lineal shape as 2+, red and flaky or band like shape as 3+, dark red, band like or retiform shape as 4+. Statistical analysis was performed by the Chi-square test.
γ-IFN and extracellular matrix in the liver of healthy mice
In the liver of healthy mice, γ-IFN was sporadically scattered near sinusoid wall, and extracellular matrix in portal tracts and sinusoid wall, whose amount was very low and seldom reached 1+ grade. Changes in the amount and distribution of γ-IFN and extracellular matrix at different stages (8th, 16th and 24th weeks) were insignificant (Table 1).
| Groups(wk) | Cases | γ-IFN | FN | LN | Type I coll. | Type III coll. | |||||||||||||||
| 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | ||
| Control | |||||||||||||||||||||
| 8 | 10 | 1 | 2 | 2 | 2 | 1 | |||||||||||||||
| 16 | 10 | 2 | 2 | 1 | 0 | 2 | |||||||||||||||
| 24 | 10 | 1 | 1 | 0 | 1 | 2 | |||||||||||||||
| Infected | |||||||||||||||||||||
| 8 | 10 | 9 | 1 | 9 | 1 | 10 | 10 | 9 | 1 | ||||||||||||
| 12 | 10 | 3 | 7 | 8 | 2 | 7 | 3 | 5 | 5 | 5 | 5 | ||||||||||
| 16 | 10 | 1 | 3 | 3 | 3 | 6 | 4 | 6 | 1 | 3 | 8 | 2 | 6 | 4 | |||||||
| 20 | 10 | 5 | 4 | 1 | 3 | 4 | 3 | 2 | 6 | 2 | 6 | 4 | 5 | 2 | 3 | ||||||
| 24 | 10 | 8 | 2 | 5 | 5 | 4 | 4 | 2 | 3 | 5 | 2 | 2 | 5 | 3 | |||||||
| 28 | 10 | 8 | 8 | 2 | 7 | 3 | 4 | 3 | 3 | 4 | 4 | 2 | |||||||||
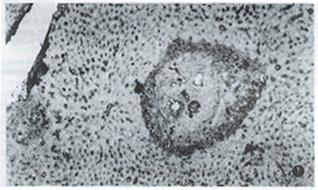
Table 1 shows the dynamics of γ-IFN and extracellular matrix in livers of infected mice. γ-IFN in livers of 9 infected mice reached 1+ grade and 1 mouse 2+ grade at the 8th week after infection when granuloma began to form and its inflammatory response was present, which was significantly higher than that of healthy control (P < 0.01), and was mainly distributed around egg granuloma and a little on sinusoid wall. The level of γ-IFN in the liver of infected mice at the 12th week after infection was higher than that at the 8th week (P < 0.01) , and peaked at the 16th week after infection (3 mice reached 2+, 3+ and 4+ grad e respectively), which was significantly higher than that at the 12th week (P < 0.01). The more severe the inflammatory response was, the higher the amount of γ-IFN was. γ-IFN started to decrease at the 20th week when inflammatory response diminished. FN, LN, type I and III collagens in the liver of most infected mice reached 1+ grade and 2+ grade at the 8th and 12th week after infection, which were higher than those of healthy controls (P < 0.01), and were lineally scattered in the portal tracts and around egg granuloma, but a little on the sinusoid wall. In contrast to γ-IFN, at the 16th week after infection when severe inflammatory response appeared and γ-IFN reached its peak, the amount of hepatic extracellular matrix lowered, although they continually rose. Then, the extracellular matrix increased steadily along with the chronicity of infection, the relief of inflammatory response and the reduction of the amount of γ-IFN. FN and LN peaked at the 20th week after infection (7 and 8 mice with 3+ grade or over); type I and III collagens peaked at the 24th week after infection (7 and 8 mice with 3+ grade or over) (Figure 1). Among them, the level of type collagen at the 24th week was higher than that at the 16th week (P < 0.05). After 20th week, extracellular matrix gradually became wide and thick, and deposited in band-like or retiform shape in and around egg granuloma and portal tracts.
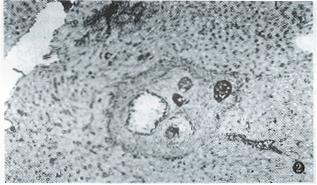
γ-IFN treatment kept the hepatic γ-IFN at a high level in the 20th week after infection, which was significantly higher than that of the non-treated group (P < 0.01), and close to that of non-treated group at the 16th week (Table 2). At the same time, the extracellular matrix in portal tracts, in and around granuloma diminished obviously. Only 3 mice had 2+ grade or over of FN in the 20th week, while 10 mice in the non-treated group (P < 0.01). At the 24th week, respectively, 2 mice had 3+ grade of type I collagen (Figure 2) and III collagen, whereas, respectively, 7 and 8 mice in non-treated group (P < 0.05). None of mice in treated group reached 4+ grade of extracellular matrix at the 20th or 24th week after infection.
| Groups(wk) | Cases | γ-IFN | FN | LN | Type I collagen | Type III collagen | |||||||||||||||
| 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | 1+ | 2+ | 3+ | 4+ | ||
| Non-treated | |||||||||||||||||||||
| 20 | 10 | 5 | 4 | 1 | 3 | 4 | 3 | 2 | 6 | 2 | 6 | 4 | 5 | 2 | 3 | ||||||
| 24 | 10 | 8 | 2 | 5 | 5 | 4 | 2 | 2 | 3 | 5 | 2 | 2 | 5 | 3 | |||||||
| Treated | |||||||||||||||||||||
| 20 | 10 | 5 | 3 | 2 | 7 | 3 | 4 | 6 | 7 | 3 | 5 | 5 | |||||||||
| 24 | 10 | 5 | 5 | 3 | 6 | 1 | 4 | 6 | 1 | 7 | 2 | 2 | 6 | 2 | |||||||
Present data showed that γ-IFN in the liver of healthy mice was very low. After S. japonicum infection, the amount and distribution of γ-IFN was associated with inflammatory response during egg granuloma formation. The more severe the inflammatory response was, the more γ-IFN, which may be caused by the increase of lymphocyte infiltration and γ-IFN release in order to oppose inflammatory response of egg granuloma.
In this study, the change of the amount of γ-IFN was contrary to that of extracellular matrix, i.e. the amounts of FN, LN, type I and III collagens were lowered while γ-IFN had reached its peak. However, extracellular matrix increased gradually when hepatic γ-IFN decreased, and collagens began to deposit in the portal tracts in and around the egg granuloma. After a repeated injection of recombinant γ-IFN to the infected mice, the amount of extracellular matrix in the liver reduced obviously, as compared with the non-treated infected mice. This indicated that γ-IFN may inhibit the synthesis and excretion of extracellular matrix and the deposition of collagen[6], which was mediated through the effect of γ-IFN on other cytokines. For example, the injection of monoclonal antibody to γ-IFN to the S. mansoni infected murine can enhance the capacity of T cell of excreting IL-2 and IL-4, which promote the formation of pulmonary granuloma. Repeated injection of enough recombinant γ-IFN can restrain the excretion of IL-2 and IL-4 and lighten the pulmonary granuloma formation. It is suggested that γ-IFN can abate the granuloma formation by inhibitng T cells from excreting IL-4 and IL-2[4].
However, the pathogenesis of liver fibrosis is very complicated. It was reported that matrix proteins in liver and collagen may come mainly from Ito cells, secondly from hepatocyte, endothelial cell of sinusoids, and fibroblast of portal tracts[7]. Our study revealed that the distribution or γ-IFN in the liver of infected mice was associated with the distribution of the above cells. Therefore, in addition to its effect on above mentioned T cells, it is necessary to clarify further whether γ-IFN inhibit directly the synthesis and excretion of extracellular matrix from the above cells and the deposition of collagen. Besides, it is still a question why the infected mice do not go on secreting γ-IFN to resist the synthesis and excretion of extracellular matrix leading to hepatic fibrosis, after the granuloma-induced inflammatory response weakened.
Original title:
S- Editor: Ma JY L- Editor: Ma JY E- Editor: Liu WX
| 1. | Cheever AW, Xu Y, Macedonia JG, Cox T, Hieny S, Sher A. The role of cytokines in the pathogenesis of hepatic granulomatous disease in Schistosoma mansoni infected mice. Mem Inst Oswaldo Cruz. 1992;87 Suppl 4:81-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Chensue SW, Terebuh PD, Warmington KS, Hershey SD, Evanoff HL, Kunkel SL, Higashi GI. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992;148:900-906. [PubMed] [Cited in This Article: ] |
| 3. | Henderson GS, Lu X, McCurley TL, Colley DG. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. II. Quantification of IL-4 mRNA, IFN-gamma mRNA, and IL-2 mRNA levels in the granulomatous livers, mesenteric lymph nodes, and spleens during the course of modulation. J Immunol. 1992;148:2261-2269. [PubMed] [Cited in This Article: ] |
| 4. | Lukacs NW, Boros DL. Lymphokine regulation of granuloma formation in murine schistosomiasis mansoni. Clin Immunol Immunopathol. 1993;68:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Liu L, Sheng Y, Guan X, Zhang Z, Sun B. [Dynamics of IL-2 and IFN-gamma levels induced by sea or Con A in spleen cells of Schistosoma japonicum-infected mice]. Zhongguo Jishengchongxue Yu Jishengchongbing Zazhi. 1995;13:35-38. [PubMed] [Cited in This Article: ] |
| 6. | Czaja MJ, Weiner FR, Takahashi S, Giambrone MA, van der Meide PH, Schellekens H, Biempica L, Zern MA. Gamma-interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology. 1989;10:795-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 144] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 263] [Article Influence: 7.7] [Reference Citation Analysis (0)] |