Published online Sep 28, 2022. doi: 10.3748/wjg.v28.i36.5324
Peer-review started: May 2, 2022
First decision: June 19, 2022
Revised: June 22, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: September 28, 2022
At the diagnosis of hepatocellular carcinoma (HCC), more than 90% of HCC patients present cirrhosis, a clinical condition often associated to malnutrition. Sarcopenia is an indirect marker of malnutrition assessable on computed tomography (CT).
To evaluate the prognostic value of sarcopenia in patients with HCC treated by trans-arterial (chemo)-embolization.
Patients with HCC treated by a first session of trans-arterial (chemo)embolization and an available CT scan before treatment were included. Sarcopenia was assessed using skeletal muscle index at baseline and at the first radiological assessment. Radiological response was recorded after the first session of treatment using mRECIST.
Of 225 patients treated by trans-arterial bland embolization (n = 71) or trans-arterial chemoembolization (n = 154) for HCC between 2007 and 2013, Barcelona Clinic of Liver Cancer stage was A, B, and C in 27.5%, 55%, and 16.8% of cases, respectively. Sarcopenia was present in 57.7% of the patients. Patients with sarcopenia presented a higher rate of progressive disease (19% vs 8%, P = 0.0236), a shorter progression-free survival (8.3 vs 13.2 mo, P = 0.0035), and a shorter median overall survival (19.4 mo vs 35.5 mo, P = 0.0149) compared with non-sarcopenic patients. Finally, patients whose sarcopenia appeared after first transarterial treatment had the worst prognosis (P = 0.0004).
Sarcopenia is associated with tumor progression and poor survival outcomes after trans-arterial (chemo)-embolization for HCC.
Core Tip: This work evaluated the predictive value of sarcopenia for tumor response and survival outcomes in hepatocellular carcinoma patients treated by transarterial chemoembolization or transarterial embolization. In this study, sarcopenia at imaging was observed in 57.7% of patients. It was associated with a higher rate of progressive disease and a decreased overall survival after adjustment with usual risk factors of death. Sarcopenia is an easy-to-assess radiological biomarker of poor prognosis that should be measured in order to assess prognosis and test a targeted intervention mixing nutritional support and physical activity.
- Citation: Roth G, Teyssier Y, Benhamou M, Abousalihac M, Caruso S, Sengel C, Seror O, Ghelfi J, Seigneurin A, Ganne-Carrie N, Gigante E, Blaise L, Sutter O, Decaens T, Nault JC. Impact of sarcopenia on tumor response and survival outcomes in patients with hepatocellular carcinoma treated by trans-arterial (chemo)-embolization. World J Gastroenterol 2022; 28(36): 5324-5337
- URL: https://www.wjgnet.com/1007-9327/full/v28/i36/5324.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i36.5324
Liver cancer is the second cause of cancer-related deaths worldwide[1], mostly represented by hepatocellular carcinoma (HCC). At the diagnosis, 70% of HCC patients have only access to palliative treatments, and among intermediate HCC, classified as Barcelona Clinic of Liver Cancer (BCLC)-B[2], transarterial chemoembolization (TACE) and transarterial embolization (TAE) are the best therapeutic options to offer[3-5]. Nonetheless, despite a good level of tumor response, around 50%-55% of patients receiving these treatments suffer from a high level of relapse[6]. It is well demonstrated that TACE presents the best results on patients with a good general status and a low level of liver insufficiency[7], but additional reliable predictive markers are needed to better define which patients will take full benefit of this procedure, and which ones present an increased risk of low efficacy and liver deterioration. At the diagnosis, more than 90% of HCC patients present with cirrhosis, a clinical condition often associated to malnutrition with sarcopenia. Indeed, sarcopenia, defined as the “progressive loss of muscle mass and strength with a risk of adverse outcomes such as disability, poor quality of life, and death”[8], is a consequence of chronic inflammation, hypercatabolism, and anorexia found in cirrhosis and advanced tumor stages. Sarcopenia has already been described as a poor prognostic factor in HCC patients undergoing surgical resection or treated by systemic therapies[9-12]. Further studies are needed to clarify the predictive value of sarcopenia in other HCC treatment settings, such as TAE or TACE. Indeed, several studies with small numbers of patients showed interesting results on the predictive value of sarcopenia regarding survival outcomes of patients treated by TACE but without clear impact on tumor response[13]. This study aimed to evaluate the predictive value of sarcopenia for tumor response and survival outcomes in a bicentric cohort of HCC patients treated by TACE or TAE.
Patients were retrospectively included from December 1, 2007 to November 1, 2013 in Jean Verdier Hospital and from June 1, 2011 to December 1, 2014 in Grenoble-Alpes University Hospital. The inclusion criteria were as follow: Age > 18 years, HCC diagnosed by histology or non-invasive criteria[7], first treatment using TAE or TACE, and available pre- and post-therapeutic computed tomography (CT) scan. Transarterial procedures performed for acute bleeding of HCC were excluded.
Patients were treated with transarterial therapy following standard local protocol[14]. Each indication of TACE or TAE was validated during multidisciplinary tumor board including a hepatologist, an interventional radiologist, and a liver surgeon. In case of TACE, chemotherapy was either doxorubicin or idarubicin according to institutional standards of care, as previously described[15]. The choice of chemotherapy was left to the investigator’s discretion. Every TACE or TAE was performed by an expert interventional radiologist.
As recommended by European guidelines, TACE could be repeated 2 mo after the first treatment in case of partial response on postoperative scan and preserved liver function after rediscussion in multidisciplinary tumor board[7].
Written consent was obtained for every patient before transarterial procedures and the study protocol respects the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). Study ethics was also approved by the independent French ethic committee CERIM (Comité d’éthique de la recherche en imagerie médicale; No. CRM-2004-084).
Clinical and biological data were recorded before the treatment: Demographic data, body mass index, liver function, platelets, presence of cirrhosis, etiology of the underlying liver disease, alpha-fetoprotein (AFP) level, tumor size, number of nodules, tumor portal invasion, and esophageal varices at the last upper endoscopy.
After the first treatment, all patients were prospectively followed until death or the last recorded visit until June 30, 2018.
Anthropometric measurements were assessed on the pre-therapeutic CT scan and the first follow-up CT scan realized 1-3 mo after the transarterial treatment by two radiologists (OSu and YT) using the software Image J®. Skeletal muscle and psoas muscle area were measured on a cross-sectional CT image at the level of the 3rd lumbar vertebra. Skeletal muscle index (SMI) was calculated using the total muscle area on the L3 CT slice divided by squared height as previously described[16]. Sarcopenia was defined by SMI < 50 cm2/m2 in male and < 39 cm2/m2 in female patients as defined by the North American expert statement on sarcopenia in liver transplantation[17]. Psoas muscle index (PMI) defined as the psoas muscle area on height ratio was also assessed as previously described[17].
The following data were recorded on imaging: Liver volume, spleen volume, presence of ascites at imaging, presence of para-umbilical vein, presence of esophageal varices, and presence of splenomegaly defined by a spleen’s cranio-caudal diameter superior to 12 cm.
All imaging examinations were archived in a picture archiving and communication system and blindly read by two radiologists (OSu and YT). Clinical and paraclinical parameters were extracted from the patient’s electronic medical records.
Radiological response was assessed by two radiologists (OSu and YT) comparing the baseline imaging and the imaging available 1-3 mo after the first session of trans-arterial treatment as recommended[7]. Radiological response was classified into complete response, partial response, stable disease, and progressive disease as defined by the modified RECIST criteria[7].
Progression-free survival was defined as the time between the date of the first treatment and the date of death, radiological progression, or the last recorded visit. Patients were censured at the date of liver transplantation. Overall survival (OS) was defined as the time between the date of the first treatment and the date of death or the last recorded visit, with censoring at the date of liver transplantation in transplanted patients.
Categorical variables were compared using the Fisher exact test for two groups and Chi squared test for three groups and more. Continuous variables were compared using the Mann-Whitney test. Logistic regression was used to compare the association with radiological response and baseline variables.
Survival outcomes as OS and progression free survival (PFS) were computed using the Kaplan-Meier method, and the Log-rank test was used to compare survival rates. The association between baseline variable and OS and PFS was assessed in univariate analysis using the Cox model. Variables with a P value < 0.05 in the univariate analysis were computed in multivariate analysis using the Cox model. Statistical analyses were performed using Graph Pad (PRISM) and R software.
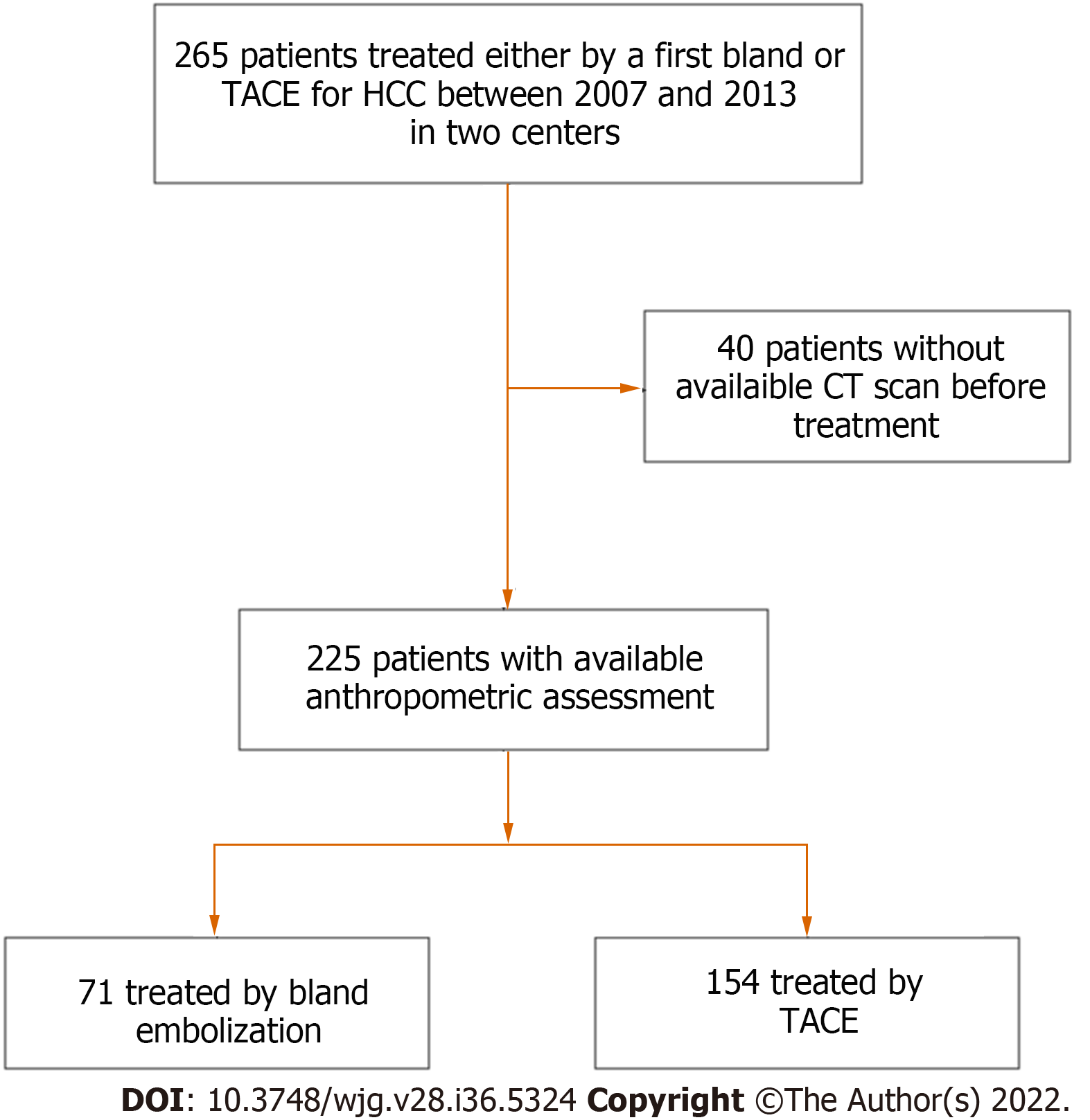
A total of 225 patients were included in the analysis, including 93 from Jean Verdier Hospital and 132 from Grenoble-Alpes University Hospital (Figure 1). Patients were mainly male (88.8%) with a median age of 65 (58-75) years old. The underlying liver diseases were mainly related to chronic alcohol intake (61%), hepatitis C (28%), and non-alcoholic steatohepatitis (29%) with 81.6% of patients classified as Child-Pugh A.
HCCs were classified as BCLC-A in 27.5%, BCLC-B in 55.5%, and BCLC-C due to segmental portal vein thrombosis in 16.8% of patients. One hundred and fifty-four (68.4%) of the patients were treated by TACE (127 with doxorubicin and 27 with idarubicin; including 87 by lipiodol TACE and 40 by TACE using drug eluting beads). Seventy-one (31.5%) patients were treated by TAE using lipiodol with gelatin sponge. Patients’ characteristics are detailed in Table 1.
| Overall cohort (n = 225) | Sarcopenia (n = 130) | No sarcopenia (n = 95) | P value | |
| Clinical and biological features | ||||
| Age (years old)1 | 65 (58-75) | 67.5 (59.25-76) | 62.5 (57-72.5) | 0.0338 |
| Gender2 | 200 (88.8%) | 120 (92%) | 80 (84%) | 0.0841 |
| Alcohol2 | 138 (61%) | 72 (55%) | 66 (69%) | 0.04 |
| NASH2 | 66 (29%) | 28 (22%) | 38 (40%) | 0.003 |
| HCV2 | 64 (28%) | 42 (32%) | 22 (23%) | 0.14 |
| HBV2 | 21 (9%) | 11 (8%) | 10 (11%) | 0.65 |
| Cirrhosis2 | 206 (91.5%) | 116 (89.2%) | 90 (94.7%) | 0.1554 |
| Platelets (103/mm3)1 | 123 (83-180) | 131 (83.5-198.3) | 113 (82-169) | 0.1561 |
| Creatinine (μmol/L) | 77 (64-89) | 77 (64-90) | 77 (65-89) | 0.93 |
| Total bilirubin (μmol/L) | 16 (9.9-22) | 15.8 (9-22) | 16 (10-22) | 0.55 |
| Albumin (g/dL) | 36 (32-39) | 35 (32-38) | 36 (32-40) | 0.11 |
| AFP (ng/mL)1 | 10 (4.3-49) | 11.5 (5.135-76.5) | 7.7 (4-25.5) | 0.0152 |
| Child-Pugh A2 | 184 (81.57%) | 105 (80.7%) | 79 (83.2%) | 0.728 |
| MELD | 8.7 (7-11) | 8.5 (7.6-10) | 9.1 (7.3-11) | 0.53 |
| BCLC-A2 | 62 (27.5%) | 33 (25.3%) | 29 (30.5%) | 0.1796 |
| BCLC-B2 | 125 (55.5%) | 70 (53.8%) | 55 (57.89%) | |
| BCLC-C2 | 38 (16.8%) | 27 (20.76%) | 11 (11.57%) | |
| Segmental portal vein thrombosis2 | 34 (15.1%) | 24 (18.46%) | 10 (10.5%) | 0.1314 |
| Type of treatment | ||||
| TACE2 | 154 (68.4%) | 86 (66.6%) | 68 (71.5%) | 0.4681 |
| Bland embolization2 | 71 (31.5%) | 43 (33.3%) | 27 (28.4%) | 0178 |
| Number of procedures per patient1 | 2 (1-3) | 2 (1-2) | 2 (1-3) | 0.0178 |
| Body mass index1 | 26.4 (23.5-29.18) | 24.3 (22.3-26.9) | 28.7 (26.4-32.33) | < 0.0001 |
| Oesophageal varices2 | 107 (49.3%) | 61 (48.4%) | 46 (50.5%) | 0.784 |
| Radiological features | ||||
| Size of the largest nodule (mm)1 | 37 (24-61) | 40 (24-70) | 34 (23-53) | 0.0665 |
| Multiples nodules2 | 169 (75.1%) | 99 (76.1%) | 70 (73.7%) | 0.7552 |
| Skeletal muscle index (cm2/m2)1 | 47.12 (41.75-53.2) | 42.85 (38.61-46.79) | 54.19 (51.38-58.27) | < 0.0001 |
| Psoas muscle index (cm2/m2)1 | 10 (8.575-11.94) | 9.205. (8.087-10.97) | 11.51 (9.679-14.26) | < 0.0001 |
| Psoas L3 (cm2)1 | 17 (14.72-37.27) | 15.95 (13.95-18.7) | 19.8 (16.6-23.8) | < 0.0001 |
| Muscle L3 (cm2)1 | 137 (119.3-155.4) | 125.8 (113.8-138.1) | 156.2 (145-176) | < 0.0001 |
| Umbilical vein repermeabilization2 | 82 (36.4%) | 51 (39.2%) | 31 (32.6%) | 0.3292 |
| Oesophageal varices2 | 160 (71.1%) | 91 (70%) | 69 (72%) | 0.7661 |
| Ascites2 | 53 (23.5%) | 29 (22.3%) | 24 (25.2%) | 0.6355 |
| Liver/spleen volume ratio1 | 4.346 (2.885-6.798) | 4.562 (2.927-7.205) | 3.797 (2.605-6.187) | 0.1085 |
| Liver volume (cm3)1 | 1715 (1430-2071) | 1701 (1407-1978) | 1800 (1461-2142) | 0.0833 |
| Spleen volume (cm3)1 | 414 (252-606) | 382 (230.5-573.3) | 480 (308-634) | 0.0283 |
| Splenomegaly2 | 129 (57.6%) | 71 (55%) | 58 (61%) | 0.4127 |
| Treatments following TAE/TACE | ||||
| Curative-intent | ||||
| Liver resection | 2 (0.9%) | 1 (0.8%) | 1 (1.1%) | |
| RFA/Microwaves/Alcoholization | 27 (12.0%) | 14 (10.8%) | 13 (13.7%) | |
| Liver transplantation | 46 (20.4%) | 19 (14.6%) | 27 (28.4%) | |
| Palliative-intent | ||||
| SIRT | 2 (0.9%) | 2 (1.5%) | 0 | |
| Sorafenib | 44 (19.6%) | 28 (21.5%) | 16 (16.8%) | |
| Other systemic treatments | 8 (3.6%) | 4 (3.1%) | 4 (4.2%) | |
| No additional treatment | 82 (36.4%) | 50 (38.5%) | 32 (33.7%) | |
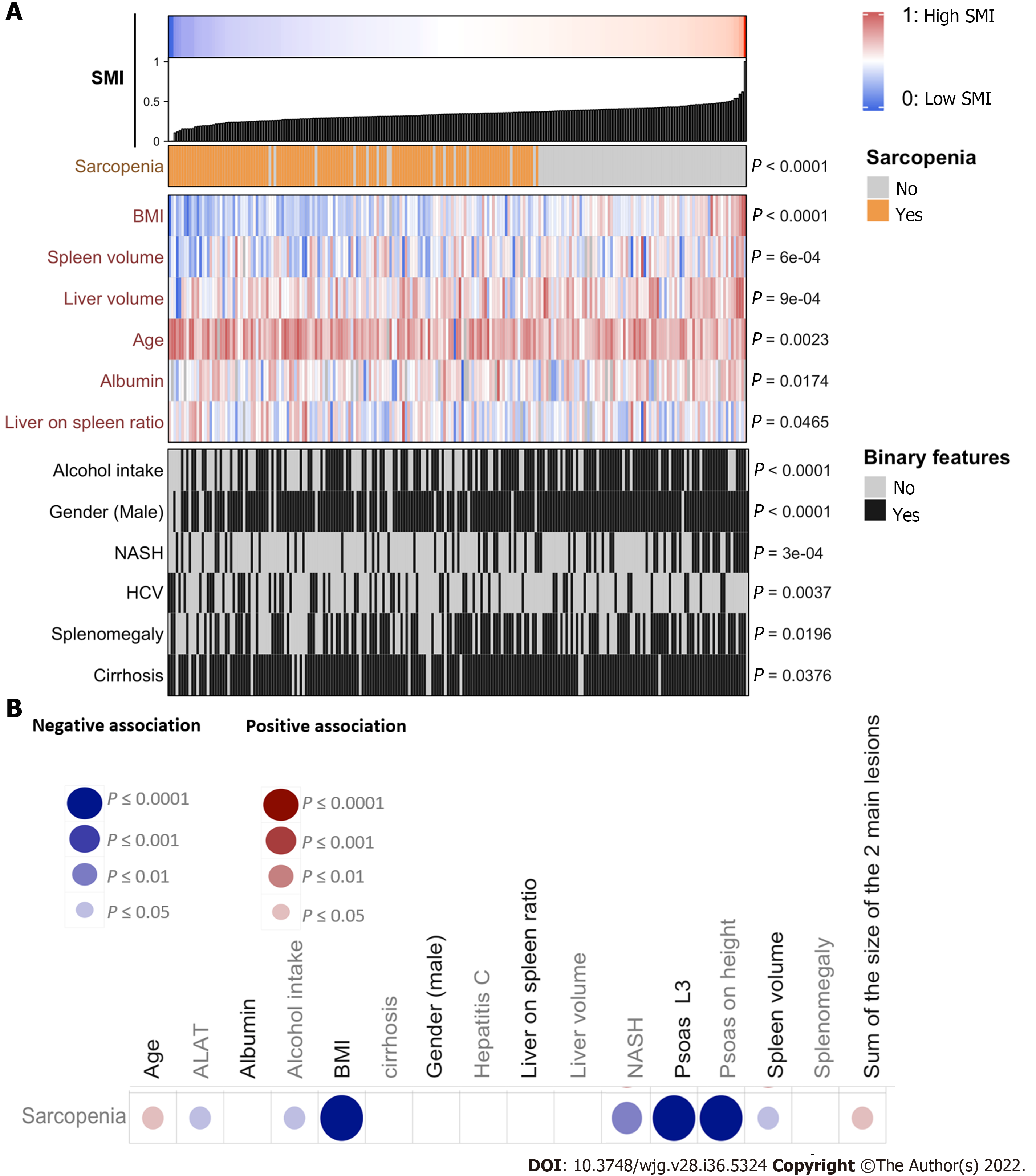
Anthropometric measurements including the value of total muscle area in L3 and psoas area in L3 are detailed in Table 1. Ascites was identified in 23.5% of the cases at imaging as well as splenomegaly in 57% of the cases, umbilical vein repermeabilization in 36.4%, and esophageal varices in 71.1%. About 57.7% (n = 130) of the patients had sarcopenia based on the SMI. The value of psoas area/squared height (cm2/m2) was significantly positively associated with body mass index (BMI), liver volume, albumin, alcohol intake, male, and non-alcoholic steatohepatitis (NASH), and negatively associated with age and hepatitis C virus (Figure 2A). Sarcopenia at imaging (defined by SMI < 50 cm2/m2 in male and < 39 cm2/m2 in female patients) was observed in 130 (57.7%) patients.
Sarcopenic patients were significantly older (67.5 years old vs 62.5 years old; P = 0.0338), with a lower BMI (24.3 vs 28.7 P < 0.0001) and a higher median serum AFP level (11.5 ng/mL vs 7.7 ng/mL; P = 0.0152) compared to non-sarcopenic patients at imaging (Table 1 and Figure 2B). Sarcopenia was also associated with a higher tumor burden (sum of the size of the 2 main tumors) and a lower BMI, ALAT level, and spleen volume, and was less frequent in alcohol-related cirrhosis and in NASH patients (Figure 2B). At the first radiological assessment, 28.7% of the patients with sarcopenia harbored an increase in Child-Pugh Class (A to B/C or B to C) compared to 24.53% of patients without sarcopenia (P = 0.6862, Fisher exact test). Patients’ baseline features as well as treatments following TACE or TAE, according to the presence or not of sarcopenia, are detailed in Table 1.
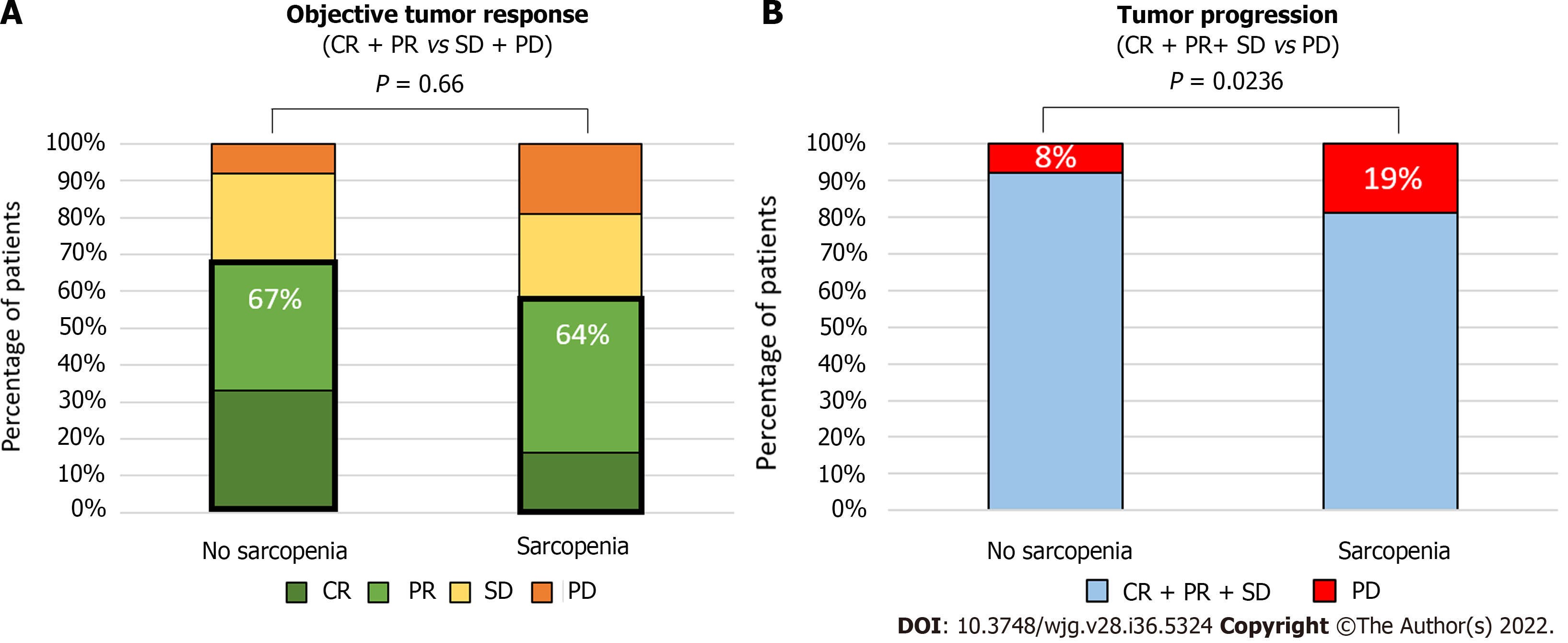
At the first radiological evaluation after TACE or TAE, 23% (n = 52) of patients harbored a complete response, 39% a partial response (n = 88), 23% a stable disease (n = 52), and 15% (n = 33) a progressive disease based on mRECIST criteria. Presence of portal hypertension signs on endoscopy or imaging was not encountered as predictive markers of radiological response. Presence of sarcopenia had a significant impact on progression proportions after TACE (P = 0.0084, Chi square test Figure 3A). Whereas objective tumor response (complete and partial radiological response) was not statistically different in sarcopenic (64%) compared to non-sarcopenic patients (67%, P = 0.66, Fisher exact test), a higher rate of progressive disease was observed in patients with sarcopenia compared to patients without (19% vs 8%, P = 0.0236, Fisher exact Test, Figure 3B). In univariate analysis, sarcopenia [odds ratio (OR): 2.59; 95%CI: 1.16-6.40, P = 0.0274], serum AFP level (OR: 1.00029; 95%CI: 1.00008-1.0006, P = 0.0226), and BCLC-C stage (OR: 2.22; 95%CI: 1.25-4.05; P = 0.00758) were related to progressive disease at imaging. In multivariate analysis, only BCLC-C stage (OR: 1.98; 95%CI: 1.038-3.88, P = 0.0416) remained independently associated with a higher rate of progressive disease.
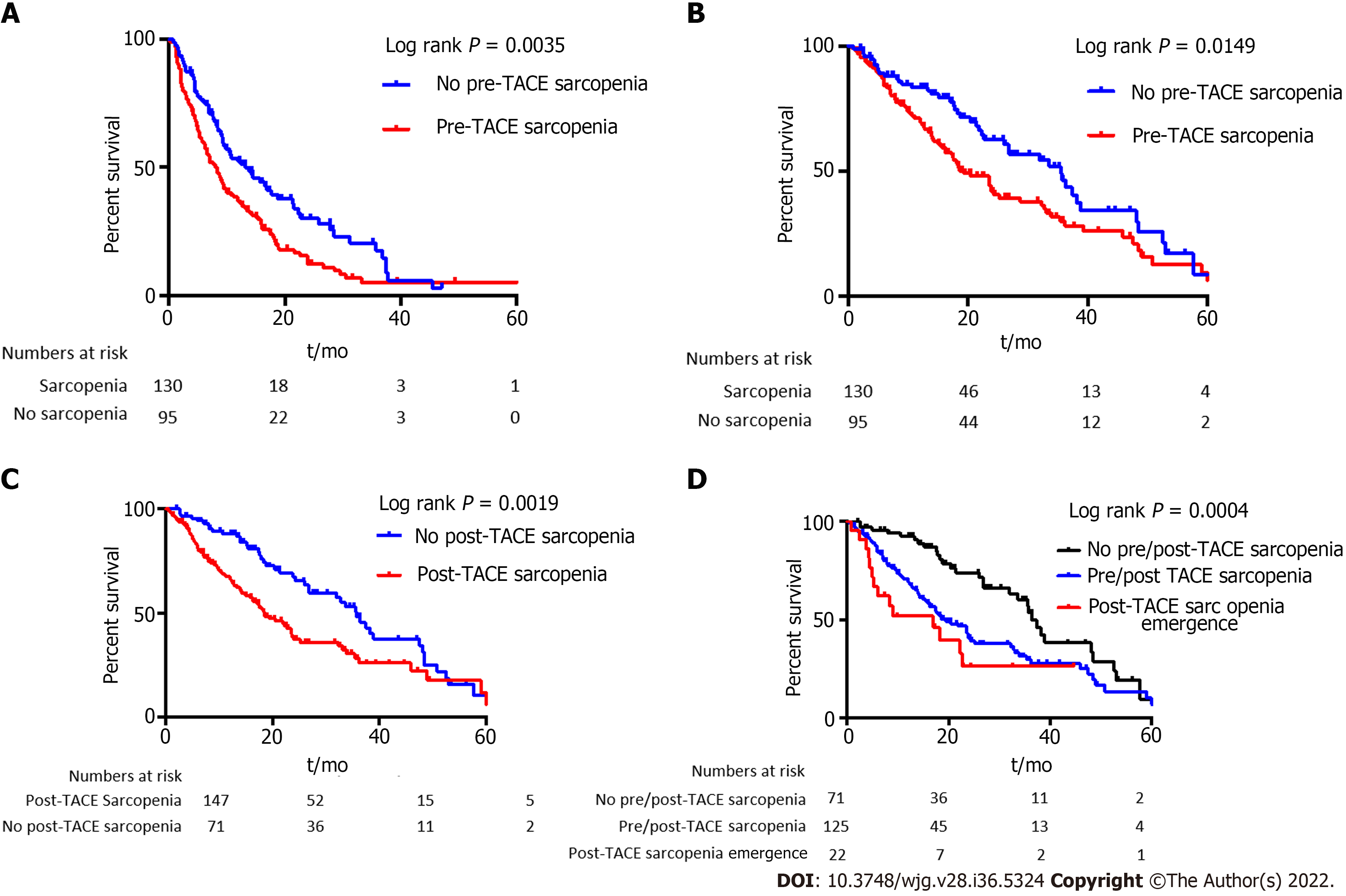
The median progression-free survival was 9.4 mo. Patients with sarcopenia had a lower median PFS compared to patients without (8.3 mo vs 13.2 mo, P = 0.0035, log rank test, Figure 4A). In univariate analysis, sarcopenia, tumor portal vein thrombosis, size of the largest nodule, serum AFP level, and platelet level were significantly associated with a lower PFS (Table 2). In multivariate analysis, sarcopenia [hazard ratio (HR): 1.62; 95%CI: 1.15-2.28], tumor portal vein thrombosis (HR: 1.77; 95%CI: 1.11-2.83), size of the largest nodule (HR: 1.008; 95%CI: 1.002-1.013), and platelet level (HR: 1.002; 95%CI: 1.002; 95%CI: 1.0001-1.005) remained independently associated with a lower PFS (Table 2).
| Variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sarcopenia (yes vs no, imaging) | 1.58 | 1.17-2.15 | 0.003 | 1.62 | 1.15-2.28 | 0.006 |
| Skeletal muscle index (imaging)1 | 0.91 | 0.98-1.01 | 0.306 | |||
| Psoas muscle index (imaging)1 | 0.97 | 0.92-1.02 | 0.18 | |||
| Paraombilical vein (imaging) | 1.21 | 0.88-1.65 | 0.24 | |||
| Esophageal varices (imaging) | 0.94 | 0.68-1.30 | 0.72 | |||
| Ascites (imaging) | 1.38 | 0.97-1.97 | 0.07 | |||
| Liver/spleen ratio (imaging)1 | 1.02 | 0.98-1.07 | 0.21 | |||
| Liver volume (imaging)1 | 1.00 | 1.00-1.00 | 0.062 | |||
| Spleen volume (imaging)1 | 0.99 | 0.99-1.00 | 0.54 | |||
| Splenomegaly (imaging) | 1.18 | 0.87-1.60 | 0.28 | |||
| BCLC stage | 1.24 | 0.98-1.58 | 0.068 | |||
| Multiple nodules | 1.03 | 0.73-1.45 | 0.88 | |||
| Portal vein thrombosis | 1.58 | 1.05-2.39 | 0.03 | 1.77 | 1.11-2.83 | 0.02 |
| Size of the largest nodule1 | 1.01 | 1.00-1.01 | 0.0003 | 1.008 | 1.002-1.013 | 0.006 |
| Esophageal varices at endoscopy | 0.98 | 0.72-1.33 | 0.88 | |||
| Serum AFP1 | 1.00 | 1.00-1.00 | 0.0007 | 1.0001 | 1.00001-1.00002 | 0.07 |
| Platelets1 | 1.00 | 1.00-1.01 | 0.002 | 1.002 | 1.0001-1.005 | 0.04 |
| Child Pugh B7 | 1.02 | 0.67-1.56 | 0.93 | |||
| Creatinin1 | 1.00 | 0.99-1.01 | 0.097 | |||
| Prothrombin time1 | 1.00 | 0.99-1.01 | 0.68 | |||
| Total bilirubin1 | 0.99 | 0.99-1.01 | 0.99 | |||
| Albumin1 | 0.99 | 0.97-1.03 | 0.84 | |||
| Clinical ascites | 1.00 | 0.63-1.59 | 0.99 | |||
| Cirrhosis | 0.87 | 0.53-1.45 | 0.60 | |||
| NASH | 1.21 | 0.60-1.14 | 0.24 | |||
| HCV | 0.85 | 0.61-1.18 | 0.32 | |||
| HBV | 0.73 | 0.41-1.28 | 0.27 | |||
| Alcohol intake | 1.013 | 0.75-1.37 | 0.93 | |||
| Body mass index1 | 0.98 | 0.95-1.01 | 0.21 | |||
| Gender (Male) | 1.37 | 0.86-2.19 | 0.19 | |||
| Age (years old)1 | 1.00 | 0.99-1.02 | 0.68 | |||
The median OS was 24.3 mo. OS was shorter in patients with sarcopenia compared to patients without (19.4 mo vs 35.5 mo, P = 0.0149, log rank test, Figure 4B). Sarcopenia, ascites at imaging, size of the largest nodule, serum AFP level, Child-Pugh score, and BMI were associated with OS at univariate analysis (Table 3). In multivariate analysis, sarcopenia (HR: 1.68; 95%CI: 1.04-2.72), AFP level (HR: 1.0001; 95%CI: 1.00001-1.002), and size of the largest nodule (HR: 1.007, 95%CI: 1.0015-1.013) were independently associated with a higher risk of death (Table 3).
| Variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sarcopenia (yes vs no, imaging) | 1.57 | 1.09-2.27 | 0.016 | 1.68 | 1.04- 2.72 | 0.03 |
| Skeletal muscle index (imaging)1 | 0.99 | 0.97-1.01 | 0.19 | |||
| Psoas muscle index (imaging)1 | 0.95 | 0.89-1.01 | 0.14 | |||
| Paraombilical vein (imaging) | 1.16 | 0.80-1.66 | 0.43 | |||
| Esophageal varices (imaging) | 1.09 | 0.75-1.61 | 0.63 | |||
| Ascites (imaging) | 1.74 | 1.16-2.60 | 0.007 | 1.59 | 0.97-2.60 | 0.07 |
| Liver/spleen ratio (imaging)1 | 1.03 | 0.98-1.08 | 0.13 | |||
| Liver volume (imaging)1 | 1.00 | 0.99-1.00 | 0.86 | |||
| Spleen volume (imaging)1 | 0.99 | 0.99-1.00 | 0.19 | |||
| Splenomegaly (imaging) | 0.95 | 0.66-1.35 | 0.77 | |||
| BCLC stage | 1.28 | 0.97-1.68 | 0.08 | |||
| Multiple nodule | 0.83 | 0.56-1.22 | 0.34 | |||
| Portal vein thrombosis | 1.52 | 0.98-2.38 | 0.06 | |||
| Size of the largest nodule1 | 1.01 | 1.00-1.01 | 0.003 | 1.007 | 1.0015-1.013 | 0.014 |
| Esophageal varices (endoscopy) | 1.17 | 0.82-1.69 | 0.38 | |||
| AFP1 | 1.00 | 1.00-1.00 | 0.00018 | 1.0001 | 1.00001-1.0002 | 0.006 |
| Platelet1 | 1.00 | 0.99-1.00 | 0.170 | |||
| Child-Pugh B7 | 2.04 | 1.21-3.45 | 0.007 | 1.59 | 0.86-2.96 | 0.14 |
| Creatinin1 | 1.00 | 0.99-1.00 | 0.18 | |||
| Prothrombin time1 | 1.01 | 0.98-1.01 | 0.45 | |||
| Total bilirubin1 | 1.02 | 1.00-1.03 | 0.035 | |||
| Albumin1 | 0.94 | 0.91-0.98 | 0.003 | |||
| Clinical ascites | 1.46 | 0.88-2.42 | 0.143 | |||
| Cirrhosis | 1.27 | 0.71-2.26 | 0.42 | |||
| NASH | 0.70 | 0.47- 1.04 | 0.075 | |||
| HCV | 1.32 | 0.89-1.95 | 0.17 | |||
| HBV | 0.71 | 0.33-1.54 | 0.39 | |||
| Alcohol intake | 1.05 | 0.67-1.37 | 0.80 | |||
| Age (years old)1 | 1.00 | 0.99-1.02 | 0.79 | |||
| BMI1 | 0.96 | 0.93-0.99 | 0.026 | 0.99 | 0.95-1.040 | 0.75 |
| Gender (Male) | 1.08 | 0.63-1.85 | 0.79 | |||
Post-TACE SMI was assessed by CT scan at the first radiological assessment in 218 patients in order to assess the evolution of sarcopenia after treatment. Among the patients without sarcopenia at baseline, 71 were still non-sarcopenic, and 22 became sarcopenic. Among the 22 patients who became sarcopenic, 19 had a progressive disease at the first radiological assessment.
Patients with post-TACE sarcopenia presented a shorter median OS (n = 147, 18.15 mo) compared with non-sarcopenic patients (n = 71, 35.7 mo, P = 0.0019) (Figure 4C).
In non-sarcopenic patients at baseline, emergence of a post-TACE sarcopenia was associated with a significant shorter median OS of 17 mo when compared with already sarcopenic patients before TACE (19.3 mo) and patients who stayed non-sarcopenic along TACE procedure (36.43 mo, P = 0.0004) (Figure 4D).
This study is the largest multicentric cohort study exploring the impact of sarcopenia on tumor response and survival outcomes in patients with HCC treated by TACE or TAE. Sarcopenia represents a major challenge in chronic diseases and especially in the treatment of cancers in which the general status is classically altered, and aggressive treatments with poor tolerance profiles are frequent. Sarcopenia in cirrhosis has already been described as impacting survival, confirming the need of a global approach with a close nutritional support of these patients. Sarcopenia measures were assessed on the open-access software Image J®, which has been proved as equivalently efficient as other commercial programs, meaning that radiological assessment of sarcopenia is accessible to every center[18]. The use of SMI was preferred to methods only based on the measurement of PMI or the transverse psoas muscle thickness. Even though the two latter are simple to assess and showed interesting results in term of survival in HCC patients treated by TACE in previous studies, SMI seems to offer a more robust and complete measurement of the muscle mass in cirrhotic patients. Besides, PMI may identify fewer patients at risk of an increased mortality and presents a higher inter-observer variability. To finish, SMI is easy to use and recommended by the North American Expert Opinion Statement on Sarcopenia in Liver Transplantation[17,19,20]. In this study, measures were performed by only one radiologist per center, which limits bias induced by potential interobserver variability. This also constitutes a limit as interobserver variability should be studied to improve result exportability and better evaluate their reproducibility.
In this study, a high prevalence of sarcopenia (57%) in patients with HCC treated by transarterial treatment was observed despite the predominance of Child-Pugh A patients and intermediate stage tumors (BCLC-B). This is consistent with a recent HCC cohort study exploring the impact of sarcopenia on survival in patients treated by hepatectomy where 54% of patients were sarcopenic, as well as published data in liver transplantation[17]. Even if patients with sarcopenia have a lower BMI than patients without, most of patients with sarcopenia harbored a normal BMI (median value of 24). Moreover, with the increase of overweight and obesity which represents 15%-20% of the worldwide population[21], BMI is even less sensitive to detect malnutrition in patients with chronic diseases and especially liver diseases. These elements suggest that sarcopenia measured by performant radiological methods reflect more precisely the nutritional state and the protein catabolism of these patients. Nonetheless, other markers that could impact the outcomes after trans-arterial (chemo)embolization such as patient’s daily activities and diet are not collected in this study due to its retrospective character. As well, this study lacked of other frailty parameters such as grip strength or walking speed. It constitutes another limit that cannot be addressed due to the retrospective character of the study.
One of the main strengths of this study is the radiological reviewing of radiological response using mRECIST criteria. This analysis revealed that sarcopenia was associated with a higher risk of progressive disease after trans-arterial treatment but without any difference on objective tumor response. Nevertheless, in multivariate analysis, only BCLC-C (segmental portal thrombosis) was independently associated with progressive disease. Sarcopenia was also associated with a shorter PFS together with tumor size in multivariate analysis. These data suggest that a subgroup of patients with advanced tumor stage and sarcopenia have a more aggressive disease which is more prone to resist to trans-arterial treatments. Indeed, sarcopenia may reflect the consequences of an intense hypercatabolism due to a particularly aggressive disease. In these patients, transarterial treatment may be deleterious in addition to be less effective as it could lead to liver failure and decrease the possibility of using systemic treatments after progression. Besides, the increased rate of radiological response obtained with the recent combination atezolizumab-bevacizumab forces us to better select the optimal treatment for patients between TACE and systemic treatments[22]. However, as sarcopenia has also been associated with poorer survival outcomes in patients under systemic therapies[10], it remains to be studied if sarcopenic patients with a high tumor burden benefit more from systemic treatments than trans-arterial procedures. In any case, sarcopenia needs to be detected as early as possible to initiate a medical intervention using nutritional support and physical activity to reverse sarcopenia and potentially improve survival. Indeed, muscle restoration before starting these treatments showed interesting results in terms of survival[10,23], and this type of intervention should be tested in a randomized controlled trial as up to 60% of patients treated by TACE harbored sarcopenia.
Sarcopenia was also associated with a shorter OS independently of tumor burden, suggesting that undernutrition and loss of muscle are key prognostic factors in patients with HCC treated by TACE or TAE. As sarcopenia is easily assessable using a CT scan in clinical practice, it could be useful to stratify patients in clinical trials. This study showed that sarcopenia assessed at the first radiological evaluation after TACE was also associated with a shorter OS, underlying the robustness of this association. Moreover, a subset of patients rapidly developed sarcopenia at the first radiological assessment which was particularly associated with a poor OS. Almost all these patients presented a progressive disease at the first evaluation, suggesting that they harbored an aggressive tumoral disease potentially responsible for the fast development of sarcopenia.
In conclusion, sarcopenia is associated with a higher rate of tumor progression and shorter survival in patients with HCC treated by TACE or TAE. Moreover, sarcopenia is an easy-to-assess radiological biomarker of poor prognosis that should be measured in order to better estimate prognosis and test a targeted intervention mixing nutritional support and physical activity.
At the diagnosis of hepatocellular carcinoma (HCC), more than 90% of HCC patients present a cirrhosis, a clinical condition often associated to malnutrition. Sarcopenia has been associated with a lower tumor response or poorer survival of patients undergoing various treatments such as surgery or systemic therapies. Transarterial chemoembolization is the treatment of choice for intermediate HCC and is largely used worldwide, but the impact of sarcopenia on its results was poorly studied.
Finding easy ways to detect sarcopenia within daily practice should benefit to patients by better characterizing their prognosis and taking their nutritional status into account in therapeutic decisions.
This study aimed to evaluate the prognostic value of sarcopenia in patients with HCC treated by trans-arterial (chemo)-embolization based on baseline computed tomography (CT) findings and study its impact on objective tumor response and survival outcomes.
Sarcopenia is easy to assess on CT by measuring the skeletal muscle index. A skeletal muscle index (SMI) < 50 cm2/m2 in male and < 39 cm2/m2 in female patients corresponding to sarcopenia was observed in 57.7% of the patients.
Based on SMI analysis measured on baseline imaging, sarcopenia was observed in 57.7% of the patients. After full review of radiological response using mRECIST criteria, sarcopenia was associated with a higher rate of progressive disease. It was also associated with a decrease overall survival even after adjustment with usual risk factors of death.
Sarcopenia is an easy-to-assess radiological biomarker of poor prognosis that should be measured in order to better assess prognosis of HCC patients.
Sarcopenia should be systematically detected at baseline, and induce a targeted intervention mixing nutritional support and physical activity. Further studies are needed to assess the benefit of these strategies in HCC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Posa A, Italy; Xi D, China; Zhao G, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 50764] [Article Influence: 8460.7] [Reference Citation Analysis (44)] |
| 2. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 1311] [Article Influence: 655.5] [Reference Citation Analysis (41)] |
| 3. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2207] [Cited by in F6Publishing: 2190] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 4. | Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62:1187-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 5. | Blanc JF, Debaillon-Vesque A, Roth G, Barbare JC, Baumann AS, Boige V, Boudjema K, Bouattour M, Crehange G, Dauvois B, Decaens T, Dewaele F, Farges O, Guiu B, Hollebecque A, Merle P, Selves J, Aparicio T, Ruiz I, Bouché O; Thésaurus National de Cancérologie Digestive (TNCD); Société Nationale Française de Gastroentérologie (SNFGE); Fédération Francophone de Cancérologie Digestive (FFCD); Groupe Coopérateur multidisciplinaire en Oncologie (GERCOR); Fédération Nationale des Centres de Lutte Contre le Cancer (UNICANCER); Société Française de Chirurgie Digestive (SFCD); Société Française d’Endoscopie Digestive (SFED); Société Française de Radiothérapie Oncologique (SFRO); Association Française pour l’Etude du Foie (AFEF). Hepatocellular carcinoma: French Intergroup Clinical Practice Guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, AFEF, SIAD, SFR/FRI). Clin Res Hepatol Gastroenterol. 2021;45:101590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 433] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3934] [Cited by in F6Publishing: 4873] [Article Influence: 812.2] [Reference Citation Analysis (0)] |
| 8. | Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1860] [Cited by in F6Publishing: 2045] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 9. | Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, Salloum C, Luciani A, Azoulay D. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg. 2015;261:1173-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 10. | Nishikawa H, Nishijima N, Enomoto H, Sakamoto A, Nasu A, Komekado H, Nishimura T, Kita R, Kimura T, Iijima H, Nishiguchi S, Osaki Y. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol Lett. 2017;14:1637-1647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 473] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 12. | Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, Dajti E, Ravaioli F, Golfieri R, Cescon M, Festi D, Colecchia A. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. 2020;55:927-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Loosen SH, Schulze-Hagen M, Bruners P, Tacke F, Trautwein C, Kuhl C, Luedde T, Roderburg C. Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Roth GS, Benhamou M, Teyssier Y, Seigneurin A, Abousalihac M, Sengel C, Seror O, Ghelfi J, Ganne-Carrié N, Blaise L, Sutter O, Decaens T, Nault JC. Comparison of Trans-Arterial Chemoembolization and Bland Embolization for the Treatment of Hepatocellular Carcinoma: A Propensity Score Analysis. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Roth GS, Teyssier Y, Abousalihac M, Seigneurin A, Ghelfi J, Sengel C, Decaens T. Idarubicin vs doxorubicin in transarterial chemoembolization of intermediate stage hepatocellular carcinoma. World J Gastroenterol. 2020;26:324-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 16. | Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1822] [Cited by in F6Publishing: 2077] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 17. | Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, Tsien C, Kallwitz ER, Ng V, Dasarathy S, Kappus M, Bashir MR, Montano-Loza AJ. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology. 2019;70:1816-1829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Long DE, Villasante Tezanos AG, Wise JN, Kern PA, Bamman MM, Peterson CA, Dennis RA. A guide for using NIH Image J for single slice cross-sectional area and composition analysis of the thigh from computed tomography. PLoS One. 2019;14:e0211629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Lanza E, Masetti C, Messana G, Muglia R, Pugliese N, Ceriani R, Lleo de Nalda A, Rimassa L, Torzilli G, Poretti D, D'Antuono F, Politi LS, Pedicini V, Aghemo A; Humanitas HCC Multidisciplinary Group. Sarcopenia as a predictor of survival in patients undergoing bland transarterial embolization for unresectable hepatocellular carcinoma. PLoS One. 2020;15:e0232371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 20. | Beer L, Bastati N, Ba-Ssalamah A, Pötter-Lang S, Lampichler K, Bican Y, Lauber D, Hodge J, Binter T, Pomej K, Simbrunner B, Semmler G, Trauner M, Mandorfer M, Reiberger T. MRI-defined sarcopenia predicts mortality in patients with chronic liver disease. Liver Int. 2020;40:2797-2807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Tao W, Lagergren J. Clinical management of obese patients with cancer. Nat Rev Clin Oncol. 2013;10:519-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2542] [Cited by in F6Publishing: 3308] [Article Influence: 827.0] [Reference Citation Analysis (1)] |
| 23. | Cheng TY, Lee PC, Chen YT, Chao Y, Hou MC, Huang YH. Pre-sarcopenia determines post-progression outcomes in advanced hepatocellular carcinoma after sorafenib failure. Sci Rep. 2020;10:18375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |