Published online Jan 21, 2022. doi: 10.3748/wjg.v28.i3.310
Peer-review started: September 9, 2021
First decision: October 16, 2021
Revised: October 19, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: January 21, 2022
Non-alcoholic fatty liver disease (NAFLD) has emerged as the most common liver disorder worldwide mainly attributed to the epidemic spread of obesity and type 2 diabetes mellitus. Although it is considered a benign disease, NAFLD can progress to non-alcoholic steatohepatitis, liver cirrhosis and hepatocellular carcinoma (HCC). Most data regarding the epidemiology of NAFLD-related HCC are derived from cohort and population studies and show that its incidence is increasing as well as it is likely to emerge as the leading indication for liver transplantation, especially in the Western World. Although cirrhosis constitutes the main risk factor for HCC development, in patients with NAFLD, HCC can arise in the absence of cirrhosis, indicating specific carcinogenic molecular pathways. Since NAFLD as an underlying liver disease for HCC is often underdiagnosed due to lack of sufficient surveillance in this population, NAFLD-HCC patients are at advanced HCC stage at the time of diagnosis making the management of those patients clinically challenging and affecting their prognostic outcomes. In this current review, we summarize the latest literature on the epidemiology, other than liver cirrhosis-pathogenesis, risk factors and prognosis of NAFLD-HCC patients. Finally, we emphasize the prevention of the development of NAFLD-associated HCC and we provide some insight into the open questions and issues regarding the appropriate surveillance policies for those patients.
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is projected to emerge as the leading cause of hepatocellular carcinoma (HCC) worldwide. Demographic factors, genetic predisposition and behavioral parameters have been identified as independent risk factors for NAFLD-related HCC, which can arise even in the absence of cirrhosis. Currently, the most challenging issue for the scientific community worldwide is the identification of the pre-cirrhotic NAFLD patients who have increased risk for HCC. Noteworthy, the central concept for the surveillance policies in the near future should be the identification, via an individual, risk-assessment based precision screening of high-risk NAFLD patients, cirrhotic or not.
- Citation: Chrysavgis L, Giannakodimos I, Diamantopoulou P, Cholongitas E. Non-alcoholic fatty liver disease and hepatocellular carcinoma: Clinical challenges of an intriguing link. World J Gastroenterol 2022; 28(3): 310-331
- URL: https://www.wjgnet.com/1007-9327/full/v28/i3/310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i3.310
Non-alcoholic fatty liver disease (NAFLD) is defined as the presence of triglycerides ≥ 5% into the hepatic tissue (i.e. steatosis), in the absence of excessive alcohol consumption and other competing liver disorders such as chronic viral hepatitis or administration of steatogenic drugs[1]. The disease may progress to non-alcoholic steatohepatitis (NASH) which is characterized by steatosis and liver inflammation, with or without fibrosis. NAFLD is considered as the global epidemic of the 21st century in the field of liver diseases and is strongly associated with the increased prevalence of obesity[1,2]. In 2016, the World Health Organization estimated the number of overweight or obese adults to be more than 1.9 billion worldwide. NAFLD is also correlated with other metabolic comorbidities, besides obesity, namely type 2 diabetes mellitus (T2DM), hyperlipidemia, arterial hypertension and it is considered the hepatic manifestation of the metabolic syndrome (MetS)[3]. Concerning epidemiology of NAFLD, a recent large meta-analysis, including 45 studies reported an estimated global prevalence of NAFLD as high as 25.24% with highest prevalence in the South America (30.45%) and Middle East (31.8%) and lowest in Africa (13.5%)[4]. Of note, during the past decade, a consistent rise of NAFLD prevalence was observed, increasing from 15% in 2005 to 25% in 2010[4].
Concerning the advanced form of the disease, the pooled NASH prevalence among NAFLD patients with an indication for liver biopsy was 63.45% for Asian region, 69.25% for Europe and 60.64% for North America[4]. Oppositely, NASH prevalence among NAFLD patients without an indication for biopsy was 6.67% for Asia and 29.85% for North America, while no corresponding data were available for European territory[4]. Moreover, approximately 41% of NASH patients experienced fibrosis progression with an average annual progression rate of 0.09%[4]. A smaller, but significant proportion of NAFLD and mainly NASH patients will ultimately develop cirrhosis or even hepatocellular carcinoma (HCC), thus facing life-threatening liver-associated complications[5]. To our point of view, since prevalence of NAFLD is increasing rapidly globally, HCC in patients with NAFLD will become a major public health issue and will emerge as a leading cause for liver transplantation (LT) in the near future. The vast underestimation of the true burden of NAFLD, especially in the developing countries, leads to reduced and delayed access of patients to specialized medical centers for appropriate surveillance and treatment of HCC and its complications, since early diagnosis constitutes a fundamental factor for effective therapy.
In this current review, we discuss the latest data concerning epidemiology, risk factors and prognosis of NAFLD-related HCC as well as we emphasize the prevention, and the appropriate surveillance polices which shall be conducted to improve patients’ attentiveness and care.
We reviewed the current literature from the inception of this current review until March 2021. For our scope, we used “PubMed” database and we included only studies written in English. We used the following search terms: “Non-alcoholic fatty liver disease-related hepatocellular carcinoma”, “NAFLD-related HCC”, “NAFLD-HCC”, “Non-alcoholic steatohepatitis-related hepatocellular carcinoma”, “NASH-related HCC”, “NASH-HCC” and we retrieved the results of our search for the epidemiology, pathogenesis, risk factors, prognosis/outcomes, surveillance, and prevention of NAFLD/ΝΑSΗ-related HCC. Also, the references of the research articles were scrutinized for relevant studies.
HCC, as an entity, is the fifth most frequently diagnosed cancer and the second leading etiology of cancer-related mortality worldwide[6]. It is the predominant histological type of primary liver cancer accounting for 70%-85% of all liver cancer cases[6] and is estimated to be the fastest growing cause of cancer-related mortality among United States male population[7]. Although most HCC cases occur in the setting of chronic viral hepatitis or alcoholic (ALD) cirrhosis, a significant proportion of patients with NAFLD/NASH may develop HCC. According to the aforementioned recent meta-analysis, the annual incident rate of HCC in NASH patients was 5.29 per 1000 person-years (PY), whereas for NAFLD patients that percentage dropped to 0.44 per 1000 PY[4]. Noteworthy, another meta-analysis which included only studies of Asian populations reported that the incidence rate of HCC was 1.8 cases per 1000 PY on NAFLD patients, while the corresponding data for NASH patients were unavailable due to the design of that meta-analysis[8]. The prevalence of NAFLD-related HCC is rising worldwide, and data derived from studies conducted in the past decade estimated that 4%-22% of all HCC were attributed to NAFLD in Western countries with the corresponding percentage to be 1%-2% in Asian region, where viral hepatitis remains endemic[9]. However, these studies underestimate the true NAFLD-related HCC prevalence, as they ignore the impact of “cryptogenic” cirrhosis. It is widely suspected that half of the cryptogenic cirrhosis-related HCC, which accounts for 15% to 30% of all HCC cases, arise from NAFLD[10].
Regarding the prevalence of HCC among NASH patients (cirrhotic or not), as compared to other liver diseases, a recent meta-analysis demonstrated that it was 22.5% for all NASH and 13.6% among all other non-NASH patients[11]. More recently, a large health-care database study in the United States identified NAFLD or NASH as the most predominant underlying risk factor for HCC, being present in 59% of cases[12]. In addition, NAFLD accounted for 34.8% of HCC events in England, while a year-by-year increase of HCC attributed to NAFLD between 2000 and 2010 was identified[12,13]. In a recent analysis of the Scientific Registry of Transplant Recipients including data from 158347 candidates for LT in the United States between 2002 and 2016, the proportion of NAFLD/NASH in HCC was increased 7.7-fold (from 2.1% to 16.2%), while the corresponding proportion for other-etiologies HCC remained relatively stable[14]. Of note, during this period the prevalence of HCC in LT candidates with NASH increased 11.8-fold[14]. Consistent with that, during a 20-year period, among a French cohort of histologically confirmed HCC patients who underwent surgical resection, the prevalence of NAFLD-related HCC increased from 2.6% in the period of 1995-1999 to 19.5% in 2010-2014, while the corresponding hepatitis C virus (HCV)-related fraction was decreased from 43.5% to 19.5%[15]. Along this line, Hester et al[16] in their recent cross-sectional study including 13648 HCC patients, identified NAFLD as the predominant cause of HCC in both inpatient and outpatient population, accounting for 32.07% and 20.22% of all cases respectively, followed by HCV infection[16].
Of note, a major cause for concern is the incidence of HCC among T2DM patients considering both the high prevalence of T2DM globally and the fact that > 70% of T2DM patients have NAFLD[17]. A large observational study revealed that HCC was the most incident malignancy among 457473 T2DM patients (Hazard Ratio: 3.31), while a large population-control study further confirmed that T2DM was independently associated with 2-3-fold increase of HCC risk regardless of other well-established risk factors for HCC[18]. Interestingly, Dyson et al[13] showed that the prevalence of T2DM or obesity among HCC patients was growing during a decade of follow-up (2000-2010) while, intriguingly, in one third of all HCC patients referred to this tertiary care centre, metabolic dysregulation was the only identified risk factor for HCC[13]. Of importance, numerous meta-analyses have also demonstrated similar findings[19-21]. We should emphasize that throughout the above-mentioned meta-analyses, the association between T2DM and HCC was robust across different population groups, geographic areas, and a plethora of control groups while it remained significant even after adjusting for demographic and laboratory parameters.
Of cardinal importance, a distinctive feature of NAFLD/NASH, compared to other liver diseases such as HCV or ALD, is the development of HCC even in the absence of cirrhosis[22]. In a retrospective study of 1500 patients with HCC, the incidence of HCC development without cirrhosis was higher in NAFLD patients, since 34.6% of NAFLD-HCC patients were non-cirrhotic, while only 8.9% in HCV, 7.7% in hepatitis B virus (HBV) and 11.1% in ALD groups had no evidence of cirrhosis[23]. Consistently, two large independent Western studies showed that 54% and 46.2% of NAFLD and NASH-related HCCs respectively, arose in a non-cirrhotic background[12,24], while the corresponding proportion was similar (49%) in a cross-sectional multicenter Japanese study[25]. Additionally, a recent meta-analysis confirmed those findings since in non-cirrhotic NASH subjects the pooled prevalence of HCC was 38% compared to 14.2% in non-cirrhotic non-NASH[11] suggesting that the former had significantly increased odds for developing HCC[11]. Thus, NAFLD seems to be the second cause, together with HBV, where HCC can develop in non-cirrhotic liver. Studies of the last decade concerning the epidemiology of NAFLD-related HCC are summarized in Table 1[13,15,16,23,26-45], while studies including cohorts of NAFLD patients who prospectively developed HCC are summarized in Table 2[24,25,46-52].
| Ref. | Year/Country | Study design | HCC patients, n | HCC caused by NAFLD, n (%) | Non-cirrhotics among HCC-related NAFLD, n (%) | Fibrosis stage of NAFLD-HCC patients, n (%) | Tumor size |
| Yang et al[26] | 2011/United States | Retrospective | 460 | 61 (13.27) | NA | NA | NA |
| Schütte et al[27] | 2014/Germany | Retrospective | 664 | 43 (6.5) | 6 (13.95) | NA | NA |
| Chun et al[28] | 2014/United States | Retrospective | 27 | 13 (48.1) | NA | NA | NA |
| Edenvik et al[29] | 2015/Sweden | Retrospective | 616 | 69 (11.2) | 15 (21.7) | NA | NA |
| Younossi et al[30] | 2015/United States | Retrospective | 4979 | 701 (14.1) | NA | NA | NA |
| Weinmann et al[31] | 2015/Germany | Retrospective | 1119 | 45 (4) | 10 (22.2) | NA | Trend towards ↑ tumor size in NASH-HCC (6 cm) vs non-NASH-HCC (4.8 cm) (P = 0.18) |
| Mittal et al[23] | 2016/United States | Retrospective | 1500 | 107 (8) | 37 (34.6) | NA | ΝΑ |
| Wong et al[32] | 2017/United States | Retrospective | 17.664 | 5898 (33.4) | 3326 (56.4%) | NA | ↑ proportion of tumors > 5 cm in NAFLD-HCC vs non-NAFLD-HCC (P < 0.001) |
| Huang et al[33] | 2017/Australia | Prospective | 270 | 38 (14) | 9 (23.7) | NA | ΝΑ |
| Koh et al[34] | 2019/Singapore | Prospective | 996 | 152 (15.3) | 100 (65.8) | F0 = 78 (51.7); F1 = 10 (6.6); F2 = 45 (29.8); F3 = 9 (6); F4 = 9 (6) | ↓ tumor size in NAFLD-HCC (0.7 cm) vs non-NAFLD-HCC (4 cm) (P < 0.001) |
| Hassan and Gane[35] | 2019/New Zealand | Retrospective | 1985 | 159 (5.1) (Undefined cirrhosis stage in 57) | 25 (24.5) (based on well-defined stage patients) | F0 = 2 (8); F1 = 3 (14); F2 = 1 (3); F3/4 = 19 (75.5) | NA |
| Gawrieh et al[36] | 2019/United States | Retrospective | 5144 | 767 (14.9) | 159 (26.3) | NA | NA |
| Hester et al[16] | 2020/United States | Retrospective | 12471 | 3019 | 1565 | NA | NA |
| Hong et al[37] | 2018/Australia | Prospective | 272 | 39 (14.3) | NA | NA | NA |
| Jamwal et al[38] | 2020/India | Prospective | 56 | 20 (35.7) | 20 (100) | NA | NA |
| Pais et al[15] | 2017/France | Retrospective | 323 | 39 (12.1) | 30 (76.9) | F0 = 16 (40); F1 = 9 (23); F2 = 0 (0); F3 = 5 (14); F4 = 9 (23) | ↑ tumor size in NAFLD-HCC (8.7 cm) vs non-NAFLD-HCC (6.2 cm) (P = 0.002) |
| Dyson et al[13] | 2013/United Kingdom | Prospective | 632 | 136 (21.5) | 31 (22.8) | NA | NA |
| Phipps et al[39] | 2020/United States | Retrospective | 5327 | 790 (14.8) | NA | NA | NA |
| Bengtsson et al[40] | 2019/Sweden | Retrospective | 1562 | 225 (14.4) | 83 (36.9) | F0 = 1; F1 = 13; F2 = 16; F3 = 5 (Undefined fibrosis stage in 48 patients) | NSD in tumor size between NAFLD-HCC vs non-NAFLD-HCC; ↑ tumor size in non-cirrhotic vs cirrhotic NAFLD-HCC (P = 0.001) |
| Tokushige et al[41] | 2013/Japan | Retrospective | 14.530 | 292 (2) | 111 (38) | NA | ΝΑ |
| Reddy et al[42] | 2012/United States | Retrospective | 303 | 52 (NASH) (17.2) | 14 (26.9) | NA | NSD in tumor size between NAFLD-HCC (3.2 cm) vs non-NAFLD-HCC (3 cm) |
| Phan et al[43] | 2019/United States | Retrospective | 545 | 28 (5.1) | 3 (10.7) | NA | NA |
| Van Meer et al[44] | 2016/Netherlands | Retrospective | 1221 | 181 (14.8) | 67 (28) | NA | ↑ tumor size in NAFLD-HCC (6 cm) vs HCV-HCC (3 cm) (P < 0.001) |
| Yang et al[45] | 2017/United States | Retrospective | 93 | 10 (11) | 3 (27.3) | NA | NA |
| Ref. | Year/Country | Total NAFLD-HCC patients | Prevalence of NAFLD-HCC without cirrhosis, n (%) | Fibrosis stage of non-cirrhotic NAFLD-HCC patients, n (%) | Tumor characteristics in cirrhotic vs non-cirrhotic NAFLD-HCC patients (differentiation) |
| Piscaglia et al[24] | 2015/Italy | 145 patients | 67 (46) | F0 = 3 (18.75); F1-F2 = 2 (12.5); F3 = 11 (68.75) (Undefined fibrosis stage in 51 patients) | NSD in tumor size |
| Leung et al[46] | 2015/Australia | 54 patients | 8 (15) | F0 = 2 (33.3); F1-F2 = 4 (66.7) (Undefined fibrosis stage in 2 patients) | ↑ tumor diameter in non-cirrhotic (4.7 cm) vs cirrhotic (3.2 cm) (P = 0.041). NSD in median number of tumors in non-cirrhotic (2) vs cirrhotic (1). NSD in HCC differentiation |
| Kodama et al[47] | 2019/Japan | 104 patients | 58 (55.8) | F0 = 6 (5.8); F1 = 11 (10.6); F2 = 18 (17.3); F3 = 23 (22.1) | NSD in HCC differentiation |
| Mohamad et al[48] | 2015/United States of America | 83 patients | 36 (43.4) | F0 = 18 (55.9); F1 = 6 (17.6); F2 = 3 (8.8); F3 = 6 (17.6) | ↑ incidence of single nodules in non-cirrhotic (80.6%) vs cirrhotic (52.2%) (P < 0.05). ↑ proportion of large nodule size (> 5 cm) in non-cirrhotic (77.8%) vs cirrhotic (10.6%) (P < 0.05). NSD in HCC differentiation |
| Tobari et al[49] | 2020/Japan | 119 patients | 48 (40.3) | F0-F1 = 12 (32.4); F2 = 17 (46); F3 = 8 (21.6) (Undefined fibrosis stage in 11 patients) | ↑ tumor size in non-cirrhotic (46 mm) vs cirrhotic (28 mm) (P < 0.01). NSD in HCC differentiation. NSD in median number of tumors |
| Yasui et al[25] | 2011/Japan | 87 patients | 43 (49.4) | F1 = 10 (23.2); F2 = 15 (34.9); F3 = 18 (41.9) | NA |
| Thompson[50] | 2018/United States | 48 patients | 26 (54) | F0 = 10 (38.5); F1 = 8 (30.8); F2 = 5 (19.2); F3 = 1 (3.8) | ↓ tumor size in non-cirrhotic (3.3 cm) vs cirrhotic (5.7 cm) (P < 0.01). NSD in HCC differentiation |
| Cotrim et al[51] | 2016/Brazil | 110 patients | 20 (48.5) | F0 = 2 (12.5); F1-3 = 14 (87.5) | NA |
| Iannaccone et al[52] | 2007/France | 22 patients | 16 (72.3) | F0 = 7 (31.8); F1-3 = 9 (40.9) | NA |
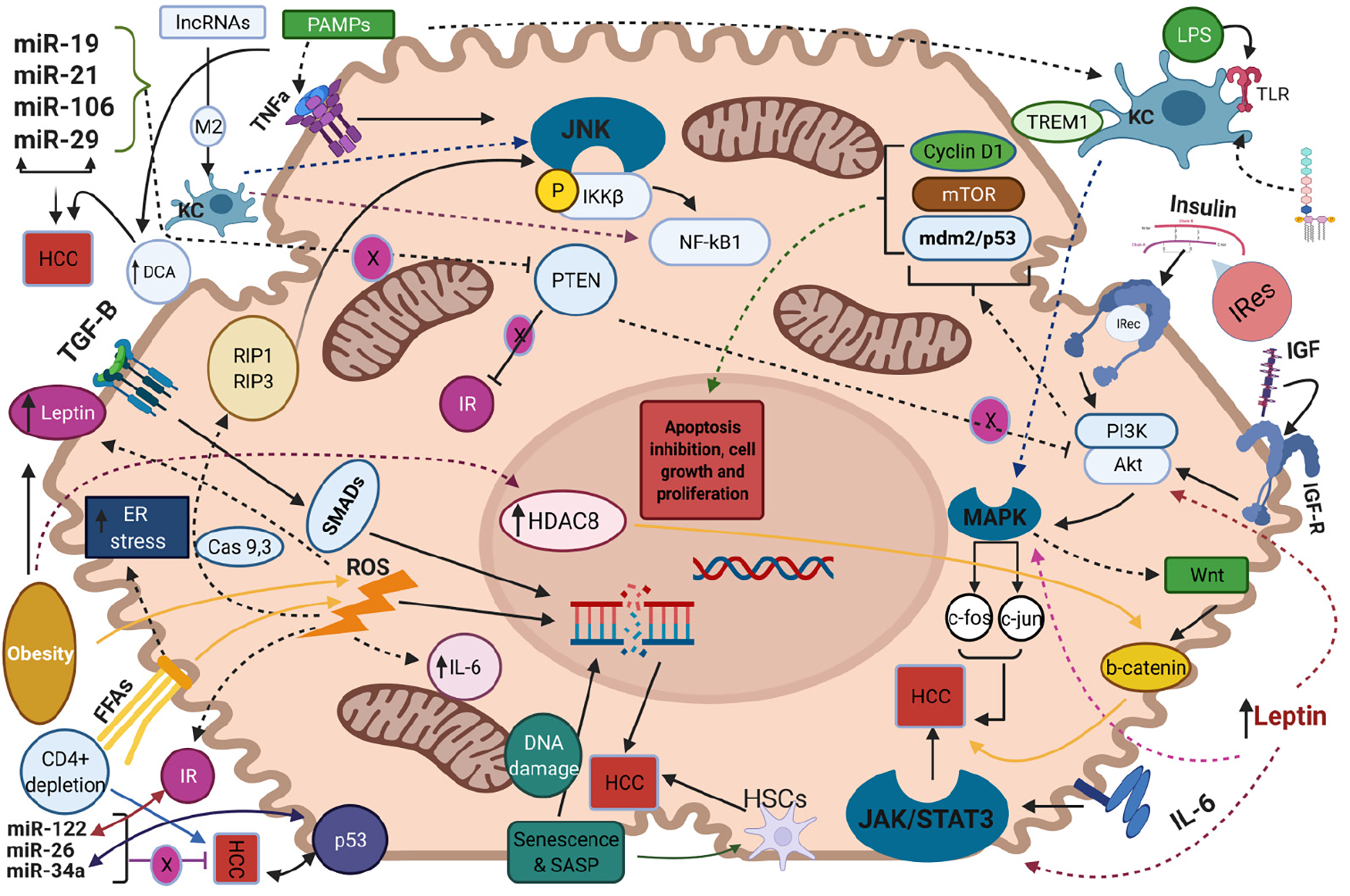
Like other malignancies, NAFLD-related HCC development is a chronic process with gradual transition from the state of NAFLD to the state of cirrhosis and HCC onset. In the setting of liver cirrhosis, the pathophysiological mechanisms of HCC arising have been well-studied. Repeated cycles of hepatocyte death and subsequent liver regeneration and tissue restoring along with cellular proliferation and constant cell growth lead to tumor development[53]. Besides hepatic cirrhosis, in this current review we shed light to several other etiologies and mechanisms that have been specifically implicated in the development of NAFLD-related HCC, since a lot of non-cirrhotic related HCC are associated with NAFLD[36]. The potential pathogenetic molecular pathways implicated in the NAFLD-related HCC development are illustrated in Figure 1 (Created with BioRender https://biorender.com/). Herein, we highlighted the major and well-established pathogenetic mechanisms whereas the remaining and relatively recently proposed ones are in detail described in the Supplementary material.
NAFLD is closely associated with insulin resistance and subsequently increased levels of insulin and insulin-like growth factor-1 (IGF-1)[54]. Binding of these two molecules to insulin receptor and insulin-like growth factor-1 receptor (IGF1R) respectively, results in activation of PI3K/AKT and Mitogen-activated protein kinase (MAPK) molecular pathways[55]. Regarding the first, exerts its action by signaling on cyclin D1, Mdm2/p53 and mTOR and leads to inhibition of apoptosis, induction of cell proliferation and excessive cell growth respectively, while the activation of MAPK mediates the transcription of proto-oncogenes c-fos and c-jun, further affecting cell growth[56]. Moreover, MAPK pathway facilitates the activation of Wnt/β-catenin signaling cascade, which leads to liver fibrosis and promotion of hepatocarcinogenesis[57]. Moreover, insulin resistance and energy imbalance drive to excessive liver lipid accumulation, metabolic reprogramming and production of free fatty acids (FFAs)[58]. Increased mitochondrial oxidation of these FFAs induces the formation of reactive oxygen species (ROS) leading to insufficient mitochondrial respiratory chain activity as well as triggering apoptotic death pathways, such as receptor interacting protein 1 (RIP1) and RIP3- activated Jun-(N)-terminal kinase (JNK),which in turn facilitate liver inflammation and fibrosis[59,60]. In addition, the increased FFA oxidation is associated with augmented levels of endoplasmic reticulum (ER) and oxidative stress in hepatocytes. The latter promotes increased calcium release from ER that leads to mitochondrial permeabilization, disrupted ER function, liver cell injury and tumorigenesis in NASH[61]. Moreover, the crosstalk between oxidative or ER stress and ROS overproduction aggravates the progression of liver disease into NASH and HCC as the aforementioned mitochondrial dysfunction leads to further overproduction of ROS, which facilitate the activation of proapoptotic paths, mediating by caspases 9 and 3[62,63].
Furthermore, the products from lipid peroxidation and the elevated levels of ROS provoke the release of several pro-inflammatory and inflammatory substances such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) as well as affects adipokines’ secretion, namely leptin and adiponectin[64]. Increased expression of IL-6 activates the oncogenic pathway of signal transducer and activator of transcription 3 (STAT-3) which mediates cell proliferation, inhibits apoptosis and contributes to HCC development, while augmented TNF-α levels mediate the activation of pro-oncogenic paths namely nuclear factor kB (NF-kB) via JNK and phosphorylation of inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta (IKKβ)[65]. Consistently, leptin, as a profibrotic and proangiogenic factor exerts its action by both stimulating an intracellular signaling cascade of the inflammatory molecules namely TNF-α and IL-6 and by activating the previously mentioned JAK2/STAT-3, MAPK and PI3K pathways upon its binding to its receptor in HCC cells[66]. Noteworthy, although adiponectin is a strong anti-inflammatory mediator which modulates apoptosis under normotropic conditions, the exacerbated insulin resistance suppresses its action while ROS-induced overproduction of leptin, as an antagonistic hormone on the field of hepatic fibro
As the activation of the immune system considered as a prerequisite for NAFLD progression to NASH, the involvement of immunological pathways in the NAFLD-related HCC is of much interest. Ma et al[69] reported that selective intrahepatic depletion of CD4+ T cells robustly induced tumor development in a methionine-choline-deficient liver specific MYC transgenic mouse showing that CD4+ T cells mediate tumor regression[69]. Moreover, stimulation of hepatocellular lymphotoxin-β receptor (LTBR) and NF-kB signaling led to HCC onset in a MCD high fat diet mouse model whereas that same dietary pattern induced activation of natural killer T (NKT) cells and intrahepatic CD8+ T cells, which in turn facilitated NASH to HCC transition[70]. Additionally, liver damage and subsequent inflammatory response leads to activation of Kupffer cells (KCs), which are the resident macrophages of the liver and their involvement into NAFLD progression is well established in both animal models and human hepatic dysregulation[71]. Yet, these cells are also implicated in the hepatocarcinogenesis since they express the pro-inflammatory myeloid cell surface receptor TREM1 which facilitates HCC development in a diethyl nitrosamine-induced HCC mouse model[72]. KCs also express Toll-like receptor 4 and binding of Lipopolysaccharides drives the activation of the above-mentioned tumorigenic pathways of NF-kB, JNK and MAPK[73]. Additionally, upon acute liver cells injury, the signaling pathway Hedgehog was triggered and reinforced the recruitment of hepatic progenitor cells at the sites of injury in order to replace the damaged hepatocytes[74]. Dysregulated signaling of this pathway leads to insufficient cell repair within the hepatic parenchyma and results in malignancy and HCC progression[75].
Although cirrhosis constitutes the major risk factor for the development of HCC in various liver diseases, including NAFLD, HCC can also occur in non-cirrhotic NAFLD individuals[76]. Demographic, behavioral, or genetic factors contribute along with cirrhosis or even more in absence of cirrhosis to HCC. Older age, male sex and Hispanic ethnicity are strongly associated with higher risk of HCC development[5]. In a cohort study of 296707 NAFLD patients, age over 65 years comprised an independent risk factor for HCC occurrence[77]. In the same study, the incidence of HCC was higher in males compared to females (0.22 vs 0.04 per 1000 PY respectively), in Latino vs White and African-American patients (0.29 vs 0.21 and 0.12 per 1000 PY, respectively) and in cirrhotic compared to non-cirrhotic patients (10.2 vs 0.02 per 1000PY, respectively)[77].
Furthermore, distinctive MetS-related features, namely obesity and T2DM have been identified as risk factors for HCC[78]. In a meta-analysis, overweight and obese patients had 17% and 89% increased relative risk for HCC respectively compared to normal-weight individuals[79]. In another study, obesity was recognized as an independent predictor for HCC development only in patients with cryptogenic [odds ratio (OR): 11.1; 95% confidence interval (CI): 1.5-87.4] and ALD-related cirrhosis (OR: 3.2; 95%CI: 1.5-6.6)[80]. Concerning the burden of T2DM, in a retrospective study including 6508 NAFLD patients, T2DM comprised an independent risk factor for the HCC (Hazard ratio: 3.21; 95%CI: 1.09-9.50)[81], while in a Mayo clinic study with 354 NASH-cirrhotic patients, T2DM along with older age and decreased serum albumin levels were identified as independent risk factors for HCC[82]. Consistently, in a case-control study of 185 HCC cases and 404 controls, T2DM (OR: 4.33, 95%CI: 1.89–9.86) and obesity (OR: 1.97, 95%CI: 1.03–3.79) were associated with increased HCC risk, while the combination of obesity and T2DM further exacerbated the hazard of HCC (OR: 4.75, 95%CI: 1.75-12.89)[83]. However, it should be noted that evaluating the exact independent pathogenetic burden of T2DM or obesity in the development of HCC can be really challenging, due to the strong association of these two entities[84].
Moreover, lifestyle modifiable factors, such as smoking and alcohol consumption seem to be implicated in NAFLD-related HCC[84]. In a meta-analysis of 81 studies, the risk for HCC development was higher for both current and former smokers[85], but no specific data were given regarding the relationship between smoking and the incidence of HCC in NAFLD patients[84]. Alcohol consumption is independently related to elevated risk for HCC in NAFLD patients[86], despite that some studies imply that the higher HCC risk is limited only on heavy alcohol use (e.g. > 50 g/d[87,88]), during which NAFLD is excluded by definition. Intriguingly, Sookoian et al[89] in their meta-analysis, showed that moderate alcohol consumption (< 30 g/d) is associated with decreased incidence of NAFLD occurrence[89], whereas a prospective study demonstrated a potential synergism between obesity and alcohol intake in increasing the risk of HCC[90]. However, the evidence from those studies could be hindered by potential biases, such as the observational study design which does not permit to ascertain causality, the implication of obesity as potential confounder and the overall insufficient alcohol intake assessment. Overall, the impact of moderate alcohol consumption in NAFLD-related HCC is still difficult to be clarified.
Noteworthy, genetic predisposition further aggravates the risk for NAFLD-related HCC. The possession of Patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409C>G and Membrane Bound O-Acyltransferase Domain Containing 7rs641738 polymorphism were independently correlated with increased risk of HCC[91], with the latter being a burdened factor particularly in non-cirrhotic NAFLD patients[92]. On the contrary, a loss of function of variant rs72613567 in 17-beta-hydroxysteroid dehydrogenase 13 has been recently identified to protect against HCC development[93].
The prognostic outcomes of NAFLD-HCC patients as compared to their non-NAFLD-HCC counterparts have been evaluated by population-based and cohort studies with controversial findings (Table 3)[15,24,30,34,40,42,94-103].
| Ref. | Year/country/type of study | Total HCC patients (underlying disease) | Features of hepatocellular carcinoma (NAFLD vs other etiologies) | Treatment | Overall survival (NAFLD vs other etiologies) | Recurrence-free survival (NAFLD vs other etiologies) |
| Younossi et al[30] | 2015/United States/Retrospective | 4979 with HCC; 701 NAFLD, 254 AH/BC, 817 ALD, 471 HBV, 2736 HCV | NAFLD: ↑ possibility of unstaged HCC vs HCV/HBV | LT | NAFLD: ↓ OS vs HCV/HBV (NAFLD: 1-yr mortality risk is 61% vs 50% for the HCV/HBV group) | NA |
| Golabi et al[94] | 2017/United States/Retrospective | 11187 total HCC patients; 1277 NAFLD, 1421 ALD, 586 HBV, 3591 HCV | Among HCC patients treated with SR: 57% had HCV vs 17% had NAFLD | LT, SR, TACE | NAFLD: ↓ OS vs HCV and/or HBV (HR: 0.82) but ↑ OS vs ALD (HR: 1.59) | NA |
| Piscaglia et al[24] | 2016/Italy/Prospective | 756 total HCC patients; 145 NALFD, 611 HCV | NAFLD: More advanced BCLC HCC stage and more commonly outside the Milan criteria vs HCV | LT, SR, PEI, Thermal ablation, TACE, BSC or trials | NAFLD: ↓ 1-yr and 3-yr OS vs HCV (1-yr and 3-yr survival; 76.4% and 48.7% in the NAFLD-HCC group and 84.2% and 61.1% in the HCV-HCC respectively) NSD among treatment choices | NA |
| Hester et al[96] | 2019/United States/Retrospective | 1051 total HCC patients; 92 NASH, 153 ALD, 87 HBV, 719 HCV | NASH and HBV HCC patients: Larger median tumor size vs HCV and ALD. NSD in BCLC staging among the groups | LT, SR or ablative techniques, TACE, yttrium 90, or TARE or radiation therapy, systemic therapy | NAFLD: ↓ OS vs ALD (HR: 1.92) NSD between NAFLD-HCC and viral-related HCC | NA |
| Giannini et al[97] | 2009/Italy/Prospective | 471 total HCC patients; 45 CC, 426 HCV | CC: ↑ prevalence of multinodular and diffuse lesions, ↑ size of the largest lesion and advanced classification according to Milano criteria (69% vs 41%) vs HCV | LT, SR, PEI, RFA, TACE | CC: ↓ OS vs HCV | NA |
| Koh et al[34] | 2019/Singapore/Prospective | 996 total HCC patients; 152 with NAFLD, 844 non-NAFLD | NAFLD: Smaller median tumor size | Total liver resection | NAFLD: ↑ 5-yr and 10-yr OS vs non-NAFLD groups (5-yr and 10-yr OS; 70.1% and 49.6% in the NAFLD-HCC group vs 60.9% and 41.0% in the non-NAFLD-HCC respectively) | NSD in RFS (P = 0.0931) |
| Reddy et al[42] | 2012/United States/Retrospective | 303 HCC patients; 52 with NAFLD vs 162 HCV/ALD | NASH: NSD in largest tumor size, tumor differentiation and presence of satellite lesions vs HCV/ALD | Resection, ablation, and LT | NASH: ↑ 3-yr OS vs HCV/ALD (60.9% vs 36.2%) | NSD |
| Benhammou et al[98] | 2020/United States/Retrospective | 454 total HCC patients; 125 NAFLD, 170 HBV, 159 HCV | NAFLD and HCV more likely to be within Milan and UCSF criteria for LT vs HBV | LT, SR, RFA, PEI, TACE/Y-90, chemotherapy, BSC | NAFLD: ↑ OS vs HBV (HR: 0.35) and HCV (HR: 0.37) | NAFLD: ↑ RFS vs HCV (HR: 0.64) and HBV (HR: 0.69) |
| Viganò et al[99] | 2015/United States/Retrospective | 1563 total HCC patients; 96 HCV, 96 MetS matched | MetS: NSD in satellite nodules and microvascular invasion vs HCV | SR, preoperative PVE, TACE | MetS: ↑ OS vs HCV (65.6% vs 61.4%) | MetS: Trend for ↑ RFS vs HCV (37.0% vs 27.5%, P = 0.077) |
| Bengtsson et al[40] | 2019/Sweden/Retrospective | 1562 total HCC patients; 225 NAFLD, 1337 non-NAFLD | NAFLD: NSD in BCLC staging, number of tumors and largest tumor size vs non-NAFLD | LT ± RFA or TACE, SR, RFA, TACE, systemic therapy or BSC | NAFLD: NSD in OS vs non-NAFLD (HR: 1.04) | NA |
| Than et al[100] | 2017/United States/Retrospective | 487 total HCC patients; 212 NAFLD, 275 HCV | NAFLD: ↑ tumor size vs HCV | TACE, RFA, SR, PEI, sorafenib, LT | NAFLD: NSD vs HCV (44% vs 56% respectively) | NA |
| Wakai et al[101] | 2011/Japan/Retrospective | 225 total HCC patients; 17 NAFLD, 61 HBV, 147 HCV | NAFLD: ↑ tumor size vs HCV & HBV | SR | NAFLD: ↑ postoperative morbidity and 30-d mortality rates (59% and 12% in NAFLD vs 31% and 0.7% in HCV respectively & 28% and 3.3% in HBV respectively) | NAFLD: ↑ RFS vs HBV & HCV |
| Jung et al[95] | 2021/South Korea/Retrospective | 426 total HCC patients; 32 NAFLD, 200 HBV, 194 HBV/NAFLD | NAFLD: ↑ average tumor size vs HBV group (4.4 cm vs 3.4 cm) | Hepatectomy | Before PSM: NAFLD: ↓ 5-yr OS vs HBV (63% vs 80%). After PSM, NSD in 5-yr OS rates | NSD in RFS or disease-specific survival before and after PSM |
| Tokushige et al[102] | 2010/Japan/Prospective | 90 total HCC patients; 34 NASH, 56 HCV | NASH: NSD in tumor size vs HCV | SR, RFA, TACE | NASH: NSD in 5-yr survival rate (55.2% in NASH vs 50.6% in all HCV) | NSD in 5-yr recurrence rate |
| Pais et al[15] | 2017/France/Retrospective | 323 total HCC patients; 39 NAFLD, 284 non-NAFLD | NAFLD: ↑ larger tumor size vs non-NAFLDNSD in other tumor characteristics | SR, TACE, PVE, PEI, LT | NSD in 2.5 post-LT OS (Mortality: 36% in NAFLD, 48% in ALD, 45% in HCV and 36% in CHB) | NSD |
| Hernandez-Alejandro et al[103] | 2012/Canada/Retrospective | 81 total HCC patients; 17 NASH, 64 HCV | NASH: ↓ proportion had poorly differentiated HCC vs HCV | LT | NA | NASH: trend of ↑ 5-yr RFS (P = 0.11) |
Based on Surveillance, Epidemiology and End Results Medicare database, Younossi et al[30] performed a large retrospective cohort study including 4979 HCC patients and showed that the one-year mortality risk was significantly higher in NAFLD-HCC compared to HCV/HBV-HCC patients[30]. On average, the former had almost 5 mo shorter survival time compared to the latter[30]. Moreover, in the multivariable model adjusted for clinical and tumour-related parameters, NAFLD was an independent factor associated with increased one-year mortality risk[30]. More recently, Golabi et al[94] based on the afore-mentioned database along with outpatients files, showed that NAFLD-HCC patients displayed markedly higher risk of 2-year mortality in comparison with patients with HCV-HCC[94]. Consistent to the abovementioned study, the magnitude of this association remained significant in the multivariable analysis[94]. The afore-mentioned findings were mainly attributed to the more advanced tumour stage at the time of diagnosis due to less intense surveillance of NAFLD patients[94]. In addition, due to the presence of increased visceral obesity, the ultrasonography, which is the current HCC screening tool, may fail to distinguish small tumours in NAFLD patients[94]. Furthermore, the increased prevalence of cardio-metabolic comorbidities along with higher age at the time of HCC diagnosis of NAFLD population may also contribute to the lower possibility of receiving LT, compared to HCC patients with viral hepatitis, and to their decreased survival time[94]. Ιn a prospective study in Italy, among 756 HCC patients the crude 1-year and 3-year overall survival (OS) was remarkably lower in the NAFLD-HCC patients compared to HCV-HCC cohort[24]. In order to account for the potential biases of less intense surveillance and later detection of the NAFLD-related malignancy, they adjusted the whole HCC cohort of patients for the lead time who were under surveillance[24]. Intriguingly, both the mean 1-year and 3-year survival time of NAFLD-HCC patients remained significantly lower than the corresponding of HCV-HCC patients[24]. Along this line, more recently, Hester et al[96] in their multivariable analysis demonstrated that the NASH-HCC was associated with worse OS compared only to the ALD-HCC[96]. Additionally, since the magnitude of NAFLD in cryptogenic cirrhosis is major, it is of interest that Giannini et al[97] using data form the ITALICA database, showed that patients with cryptogenic cirrhosis-related HCC displayed significantly shorter survival time compared to HCV-HCC patients during a median follow-up of 21 mo[97].
Of importance, we should note that a selection bias within clinical studies could be implicated concerning the worse prognostic outcomes of NAFLD-HCC patients in comparison to other-aetiologies-HCC. Patients who were eligible for radical surgical treatments or LT, were subsequently enrolled in the cohort studies whereas patients with major comorbidities, cardiovascular diseases or advanced age, aspects more common among NAFLD patients, were excluded.
On the contrary, numerous studies have emphasized the better prognosis of NAFLD-HCC patients, compared to other aetiologies of HCC. In a prospective study from Singapore, 844 non-NAFLD-HCC and 152 NAFLD-HCC patients who underwent total liver resection were enrolled and the latter displayed significantly increased 5-year OS as compared to the former, whereas NAFLD was independently associated with lower hazard for mortality in a multivariable model adjusted for clinical and epidemiological parameters[34]. Consistently, after a median follow-up of 50 mo, Reddy et al[42] evaluated HCC patients suffered from NASH compared to those from ALD and/or HCV who received curative treatment[42]. Although the postoperative mortality and the recurrence free survival (RFS) did not significantly differ between the two groups, NASH patients had longer OS, compared to ALD and HCV patients, independently of clinical factors and type of the curative treatment they received[42]. In another study, during a median follow-up of 17 mo, NAFLD-HCC patients displayed significantly improved OS and a trend towards increased RFS, compared to both HCV and HBV patients, in a model adjusted for demographic factors, Child-Pugh score and most definite treatment[98]. Notably, in order to assess the afore-mentioned long-term outcomes independently of the LT, authors omitted the LT recipients from all groups[98]. To this end, they showed that the NAFLD-HCC patients still had significantly improved OS rates compared to their HCV counterparts and a trend towards increased survival compared to HBV patients[98]. In 2015 Viganò et al[99], matched 96 HCC patients with MetS with 96 HCV-HCC patients who received liver resection during a 12-year study period[99]. Matching was based on age, prevalence of cirrhosis, Child-Pugh class, portal hypertension and HCC characteristics[99]. MetS-HCC patients had significantly better OS and lower recurrence rate compared to HCV-HCC cases whereas in the multivariate analysis MetS-HCC was an independent protective factor for both OS and early recurrence[99].
Finally, several studies have highlighted the similar long-term outcomes regarding NAFLD and non-NAFLD-HCC patients. A Swedish retrospective study revealed that although NAFLD-HCC patients had higher age, higher prevalence of comorbidities and less HCC surveillance, they had similar survival to non-NAFLD-HCC patients, mainly attributed to the poor prognosis of HCC in general[40]. Consistently, Than et al[100] compared the outcomes of 212 NAFLD-HCC and 275 HCV-HCC patients who were referred for LT and showed that the 3-year post-diagnosis OS was similar in the two groups[100] and this finding was confirmed in the subgroup-analysis of patients who eventually received LT[100]. Along this line, Wakai et al[101] upon evaluating 317 HCC patients who received hepatic resection, showed that the 5-year post-resection cumulative survival rate did not differ significantly between NAFLD and non-NAFLD groups. Yet, RFS following liver resection was markedly better in the NAFLD group[101]. Additionally, Jung et al[95] reviewed the outcomes of NAFLD-HCC and HBV-HCC patients who underwent hepatectomy over a 10-year period. After a median follow up of 74 mo, the latter had superior 5-year OS rates, compared to the former. However, when the authors performed a propensity score matching to minimize the bias of lead time, 5-year OS was similar between the two groups[95]. In a Japanese prospective study, the OS and the RFS after curative treatment of NAFLD-HCC and HCV-HCC patients were evaluated, and both were comparable between the two cohorts[102]. Similarly, Pais et al[15] in their retrospective study evaluating a 20-year period confirmed those results[15], while a Canadian study revealed only a trend for better 5-year RFS of NASH-HCC, compared to HCV-HCC patients who underwent LT[103].
However, we should emphasize that due to less intense surveillance of HCC in general, the disease is likely to be diagnosed at advanced stages and therefore even fewer patients are eligible for radical therapy or LT. Thus, the prognostic outcomes of NAFLD-HCC patients compared to non-NAFLD-HCC ones, should be interpreted with cautiousness and accordingly to each clinical study design and type of HCC-treatment that patients received.
Moreover, another factor raising concern for defining the long-term survival outcomes of NAFLD-HCC patients is that those patients seem to have a lower MELD score waiting at LT list compared to their non-NAFLD counterparts, as they tend to have a more preserved liver function. Therefore, they are less likely to receive LT in short-term. This in turn results in longer duration in the waiting list increasing the risk for severe health-related complications and morbidity negatively affecting their survival. Indeed, when Wong et al[104] retrospectively analysing UNOS registry data concerning LT waiting list registrations in the United States, demonstrated that NAFLD patients as compared to their HCV or ALD counterparts, were markedly less likely to receive a liver transplant within 90 d and one year after their registration, ending in higher mortality while on the waiting list[104].
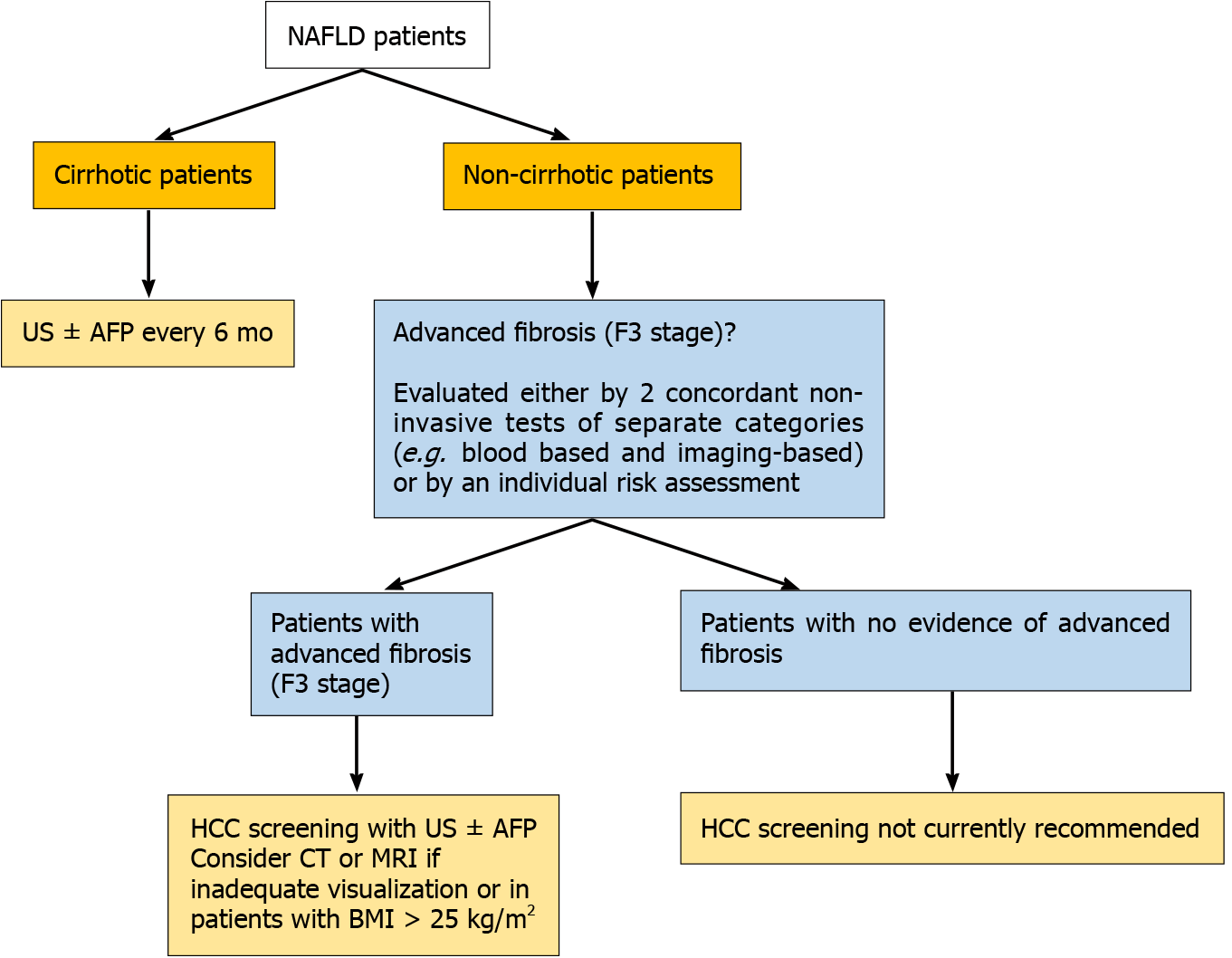
Since NAFLD patients seem to be frequently diagnosed in advanced tumor stages, their surveillance for HCC development represents quite the most challenging issue among professional societies worldwide. As for the cirrhotic-NAFLD patients, since they appear to have an expected HCC incidence of approximately 1.5% per year, they should follow the screening guidelines for cirrhotic patients of any cause, consisted of abdominal examination with liver ultrasonography with or without alpha-fetoprotein (AFP) every 6 mo[105] (Figure 2).
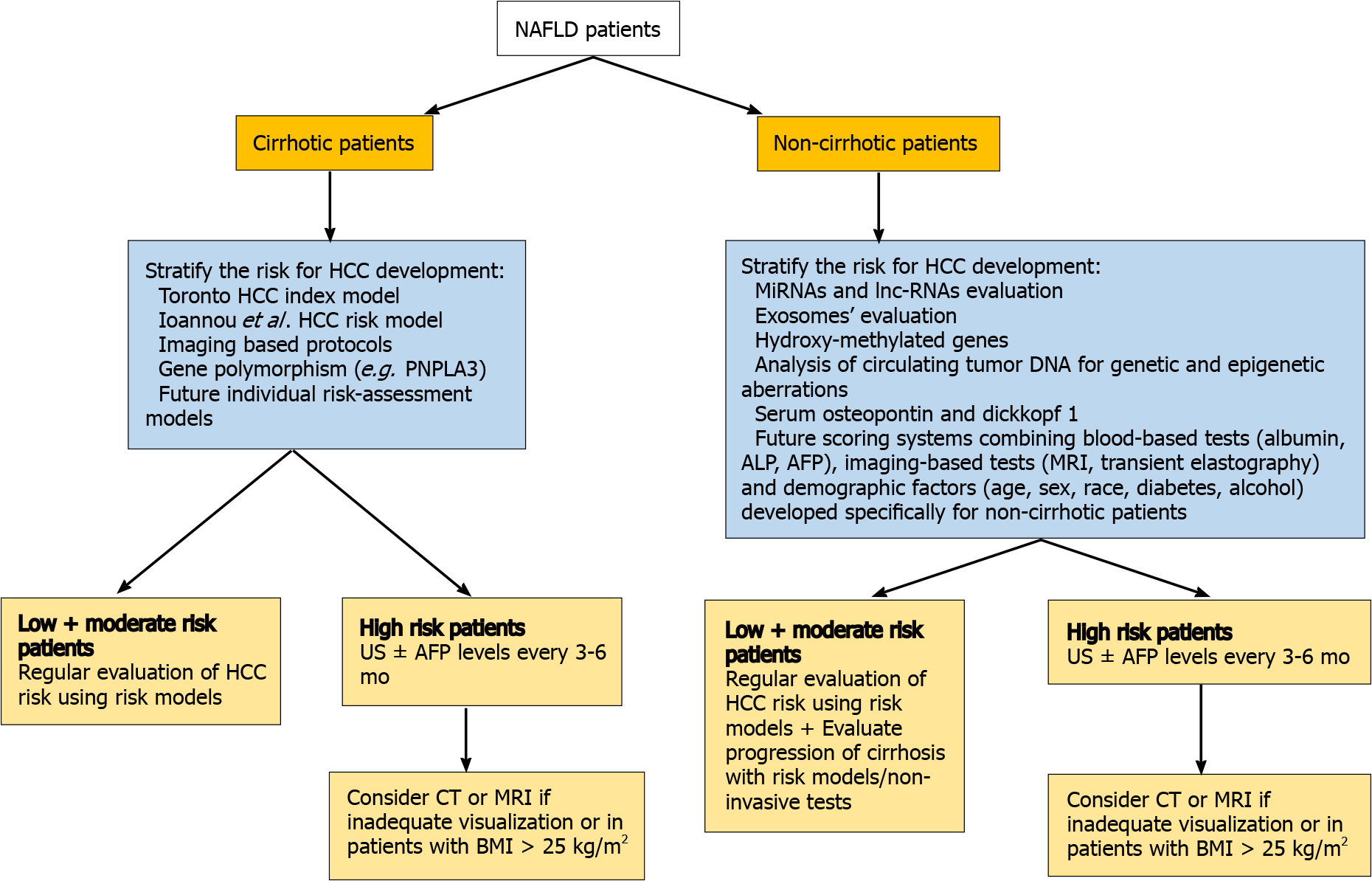
Regarding non-cirrhotic NAFLD patients, there is a lack of consensus whether NAFLD patients with F3 fibrosis should undergo screening. The updated recommendation of EASL guidelines suggest that patients with F3-fibrosis might be eligible for HCC surveillance based on an individual risk stratification[106], while the clinical update of the American Gastroenterology Association also recommend the screening for patients with findings indicative of advanced fibrosis (F3), as evaluated by two or more concordant non-invasive fibrosis tests of separate categories[107]. AASLD guidelines recommend HCC surveillance only in cirrhotic (not advanced fibrosis-F3) patients[108]. Finally, the Asian guidelines do not provide specific recommendation for non-cirrhotic NAFLD patients[109] (Figure 2). However, some concerns are raised. Even if a consensus for screening of F3 patients was reached, a large proportion of HCC cases that occur in F0-F2 NAFLD patients would still be missed. Moreover, the diagnosis of F3 fibrosis based on a broad spectrum of non-invasive tests mitigates the utility of screening since HCC risk would not still be the same for all F3-patients, while screening all patients with F3 fibrosis would drastically increase the cost of the surveillance strategy. Moreover, it should be mentioned that even among cirrhotic patients, there are differences in risk for HCC and therefore they should not be aggregated into a single category. Ioannou et al[110] developed a predictive model that estimates HCC risk in cirrhotic-NAFLD patients by implicating demographic, clinical and laboratory parameters[110]. Based on this model, patients were categorized into low-risk (annual risk: < 1%), medium-risk (annual risk: 1%–3%) and high-risk (annual risk: > 3%), suggesting that individualized screening for HCC is associated with standardized benefit compared with screening of all cirrhotic-NAFLD patients[110,111]. Moreover, the identification of PNPLA3rs738409C>G or other polymorphisms might be of value for screening since it would improve prediction accuracy, but its evaluation in multi-factorial risk models would decrease the cost-effectiveness of patients’ surveillance[112]. The future surveillance policies should focus on identification of prognostic factors, consisting of imaging modalities, serum biomarkers and genetic variants, testing for which would need to become cheaper, that will stratify the risk of HCC in both cirrhotic and non-cirrhotic NAFLD patients promoting the cost-effectiveness of the programmes. The afore-mentioned parameters along with well-established traditional risk factors of HCC may be incorporated in future risk-assessment models and could result in more accurate prediction of NAFLD-related HCC and optimized surveillance strategies. A proposed algorithm of NAFLD-related HCC surveillance based on future perspectives is illustrated in Figure 3.
As yet, circulating micro-RNAs (miRNAs) and long non-coding-RNAs have been shown promising results since they were associated with HCC progression in NAFLD patients and may constitute potential non-invasive tools for NAFLD-related HCC screening[113,114]. Several micro-RNAs, such as miR-29 and miR-199, mainly expressed in NASH, are associated with fibrosis progression and HCC development[113]. Furthermore, hydroxy-methylated genes are strongly related to the involvement of chromatin in the progression of HCC and form promising genetic factors for the risk classification of AFP-negative HCC patients[115]. Results derived from animal models examining the progression of HCC in NASH mice, suggested that serum osteopontin and dikkopf-1 could be possible novel biomarkers for the early detection of HCC[116]. Finally, the identification and amplification of circulating tumor-DNA can reveal critical HCC-related genetic mutations and therefore could be used for the screening of HCC patients[117]. However, the incorporation of those prognostic biomarkers in screening programmes would significantly increase the cost with ambiguous results. As until now, they comprise mostly future perspectives rather than clinical point-of-care practice.
Although weight loss is considered fundamental for the management of NAFLD, there is no current evidence to directly indicate that weight loss leads to reduction of NAFLD-related HCC. Importantly, a recent large multi-national study with 467336 individuals, demonstrated that physical exercise, defined as performing at least 2 hours of vigorous activity per week, can reduce the risk of developing HCC indepen
In the 21st century, we are in the midst of an epidemic of obesity, T2DM and NAFLD. Consequently, the burden of NAFLD in HCC development is rapidly rising partially explaining the elevated incidence of HCC in both men and women globally. Although the exact pathogenetic mechanisms involved in NAFLD-related HCC onset are still not well-established especially regarding the non-cirrhotic hepatic parenchyma, specific risk factors for HCC concerning demographic, genetic and behavioral parameters have been already identified. Noteworthy, the surveillance of NAFLD-HCC patients is not standard in medical practice and therefore many patients do not undergo screening and that leads to diagnosis of HCC at advanced stages negatively affecting their survival and diminishing the therapeutic options. Concerning systemic treatment for HCC, the latest data[130,131] do not support the hypothesis that the therapeutic decisions should be based on the underlying HCC etiology and therefore, HCC systemic therapy was not in the field of our review. However, noteworthy, in a recent meta-analysis of 3 trials[132-134], authors suggested that immunotherapy might be less efficacious in NASH-HCC patients as compared to their viral-HCC counterparts, presumably owing to the NASH-provoked aberrant T-cells activation and subse
We are grateful to Dr. Panagiotis Lembessis for the language polishing of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lu XL, Tunsophon S, Zhou J S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3544] [Cited by in F6Publishing: 4051] [Article Influence: 675.2] [Reference Citation Analysis (7)] |
| 2. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2383] [Cited by in F6Publishing: 3032] [Article Influence: 505.3] [Reference Citation Analysis (2)] |
| 3. | Cholongitas E, Pavlopoulou I, Papatheodoridi M, Markakis GE, Bouras E, Haidich AB, Papatheodoridis G. Epidemiology of nonalcoholic fatty liver disease in Europe: a systematic review and meta-analysis. Ann Gastroenterol. 2021;34:404-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6259] [Article Influence: 782.4] [Reference Citation Analysis (0)] |
| 5. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 787] [Article Influence: 262.3] [Reference Citation Analysis (0)] |
| 6. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 20827] [Article Influence: 2314.1] [Reference Citation Analysis (2)] |
| 7. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2800] [Cited by in F6Publishing: 3370] [Article Influence: 561.7] [Reference Citation Analysis (3)] |
| 8. | Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, Li X, Chien N, Wei M, Ogawa E, Zhao C, Wu X, Stave CD, Henry L, Barnett S, Takahashi H, Furusyo N, Eguchi Y, Hsu YC, Lee TY, Ren W, Qin C, Jun DW, Toyoda H, Wong VW, Cheung R, Zhu Q, Nguyen MH. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 538] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 9. | Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 683] [Cited by in F6Publishing: 710] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 10. | Yang JD, Kim B, Sanderson SO, St Sauver JL, Yawn BP, Pedersen RA, Larson JJ, Therneau TM, Roberts LR, Kim WR. Hepatocellular carcinoma in olmsted county, Minnesota, 1976-2008. Mayo Clin Proc. 2012;87:9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, Argo CK. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48:696-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 12. | Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183-2191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, Sumpter K, Stewart S, Rose J, Hudson M, Manas D, Reeves HL. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 382] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 14. | Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, Romero-Gomez M, George J, Ahmed A, Wong R, Younossi I, Ziayee M, Afendy A; Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17:748-755.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 478] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 15. | Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O, Ratziu V. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther. 2017;46:856-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Hester D, Golabi P, Paik J, Younossi I, Mishra A, Younossi ZM. Among Medicare Patients With Hepatocellular Carcinoma, Non-alcoholic Fatty Liver Disease is the Most Common Etiology and Cause of Mortality. J Clin Gastroenterol. 2020;54:459-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 18. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Dyal HK, Aguilar M, Bartos G, Holt EW, Bhuket T, Liu B, Cheung R, Wong RJ. Diabetes Mellitus Increases Risk of Hepatocellular Carcinoma in Chronic Hepatitis C Virus Patients: A Systematic Review. Dig Dis Sci. 2016;61:636-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 355] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 23. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-31.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 415] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 24. | Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 25. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T, Kawata S, Uto H, Takami S, Sumida Y, Takamura T, Kawanaka M, Okanoue T; Japan NASH Study Group, Ministry of Health, Labour, and Welfare of Japan. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-33; quiz e50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 26. | Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, Orsini L, Kim WR, Roberts LR. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617-23.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Schütte K, Schulz C, Poranzke J, Antweiler K, Bornschein J, Bretschneider T, Arend J, Ricke J, Malfertheiner P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Chun YS, Huang M, Rink L, Von Mehren M. Expression levels of insulin-like growth factors and receptors in hepatocellular carcinoma: a retrospective study. World J Surg Oncol. 2014;12:231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Edenvik P, Davidsdottir L, Oksanen A, Isaksson B, Hultcrantz R, Stål P. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int. 2015;35:1862-1871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 532] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 31. | Weinmann A, Alt Y, Koch S, Nelles C, Düber C, Lang H, Otto G, Zimmermann T, Marquardt JU, Galle PR, Wörns MA, Schattenberg JM. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15:210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Wong CR, Njei B, Nguyen MH, Nguyen A, Lim JK. Survival after treatment with curative intent for hepatocellular carcinoma among patients with vs without non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;46:1061-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Huang Y, Wallace MC, Adams LA, MacQuillan G, Garas G, Ferguson J, Samuelson S, Tibballs J, Jeffrey GP. Rate of Nonsurveillance and Advanced Hepatocellular Carcinoma at Diagnosis in Chronic Liver Disease. J Clin Gastroenterol. 2018;52:551-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Koh YX, Tan HJ, Liew YX, Syn N, Teo JY, Lee SY, Goh BKP, Goh GBB, Chan CY. Liver Resection for Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma. J Am Coll Surg. 2019;229:467-478.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Hassan I, Gane E. Improving survival in patients with hepatocellular carcinoma related to chronic hepatitis C and B but not in those related to non-alcoholic steatohepatitis or alcoholic liver disease: a 20-year experience from a national programme. Intern Med J. 2019;49:1405-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Gawrieh S, Dakhoul L, Miller E, Scanga A, deLemos A, Kettler C, Burney H, Liu H, Abu-Sbeih H, Chalasani N, Wattacheril J. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: a United States multicentre study. Aliment Pharmacol Ther. 2019;50:809-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 37. | Hong TP, Gow PJ, Fink M, Dev A, Roberts SK, Nicoll A, Lubel JS, Kronborg I, Arachchi N, Ryan M, Kemp WW, Knight V, Sundararajan V, Desmond P, Thompson AJ, Bell SJ. Surveillance improves survival of patients with hepatocellular carcinoma: a prospective population-based study. Med J Aust. 2018;209:348-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Jamwal R, Krishnan V, Kushwaha DS, Khurana R. Hepatocellular carcinoma in non-cirrhotic versus cirrhotic liver: a clinico-radiological comparative analysis. Abdom Radiol (NY). 2020;45:2378-2387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Phipps M, Livanos A, Guo A, Pomenti S, Yeh J, Dakhoul L, Burney H, Kettler C, Liu H, Miller E, Gawrieh S, deLemos A, Scanga A, Chalasani N, Wattacheril J. Gender Matters: Characteristics of Hepatocellular Carcinoma in Women From a Large, Multicenter Study in the United States. Am J Gastroenterol. 2020;115:1486-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Bengtsson B, Stål P, Wahlin S, Björkström NK, Hagström H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019;39:1098-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 41. | Tokushige K, Hashimoto E, Kodama K. Hepatocarcinogenesis in non-alcoholic fatty liver disease in Japan. J Gastroenterol Hepatol. 2013;28 Suppl 4:88-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, Humar A, Marsh JW, Geller DA, Tsung A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809-1819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 43. | Phan J, Ng V, Sheinbaum A, French S, Choi G, El Kabany M, Durazo F, Saab S, Tong M, Busuttil R, Han SH. Hyperlipidemia and Nonalcoholic Steatohepatitis Predispose to Hepatocellular Carcinoma Development Without Cirrhosis. J Clin Gastroenterol. 2019;53:309-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | van Meer S, van Erpecum KJ, Sprengers D, Coenraad MJ, Klümpen HJ, Jansen PL, IJzermans JN, Verheij J, van Nieuwkerk CM, Siersema PD, de Man RA. Hepatocellular carcinoma in cirrhotic versus noncirrhotic livers: results from a large cohort in the Netherlands. Eur J Gastroenterol Hepatol. 2016;28:352-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Yang JD, Ahmed Mohammed H, Harmsen WS, Enders F, Gores GJ, Roberts LR. Recent Trends in the Epidemiology of Hepatocellular Carcinoma in Olmsted County, Minnesota: A US Population-based Study. J Clin Gastroenterol. 2017;51:742-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Leung C, Yeoh SW, Patrick D, Ket S, Marion K, Gow P, Angus PW. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol. 2015;21:1189-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 84] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 47. | Kodama K, Kawaguchi T, Hyogo H, Nakajima T, Ono M, Seike M, Takahashi H, Nozaki Y, Kawanaka M, Tanaka S, Imajo K, Sumida Y, Kamada Y, Fujii H, Seko Y, Takehara T, Itoh Y, Nakajima A, Masaki N, Torimura T, Saibara T, Karino Y, Chayama K, Tokushige K. Clinical features of hepatocellular carcinoma in nonalcoholic fatty liver disease patients without advanced fibrosis. J Gastroenterol Hepatol. 2019;34:1626-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, Hanouneh IA, Alhaddad R, Alkhouri N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int. 2016;10:632-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Tobari M, Hashimoto E, Taniai M, Kodama K, Kogiso T, Tokushige K, Yamamoto M, Takayoshi N, Satoshi K, Tatsuo A. The characteristics and risk factors of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis. J Gastroenterol Hepatol. 2020;35:862-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Thompson SM, Garg I, Ehman EC, Sheedy SP, Bookwalter CA, Carter RE, Roberts LR, Venkatesh SK. Non-alcoholic fatty liver disease-associated hepatocellular carcinoma: effect of hepatic steatosis on major hepatocellular carcinoma features at MRI. Br J Radiol. 2018;91:20180345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Cotrim HP, Oliveira CP, Coelho HS, Alvares-da-Silva MR, Nabuco L, Parise ER, Ivantes C, Martinelli AL, Galizzi-Filho J, Carrilho FJ. Nonalcoholic steatohepatitis and hepatocellular carcinoma: Brazilian survey. Clinics (Sao Paulo). 2016;71:281-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Iannaccone R, Piacentini F, Murakami T, Paradis V, Belghiti J, Hori M, Kim T, Durand F, Wakasa K, Monden M, Nakamura H, Passariello R, Vilgrain V. Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: helical CT and MR imaging findings with clinical-pathologic comparison. Radiology. 2007;243:422-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 431] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 54. | De Minicis S, Agostinelli L, Rychlicki C, Sorice GP, Saccomanno S, Candelaresi C, Giaccari A, Trozzi L, Pierantonelli I, Mingarelli E, Marzioni M, Muscogiuri G, Gaggini M, Benedetti A, Gastaldelli A, Guido M, Svegliati-Baroni G. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS One. 2014;9:e97136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Janku F, Kaseb AO, Tsimberidou AM, Wolff RA, Kurzrock R. Identification of novel therapeutic targets in the PI3K/AKT/mTOR pathway in hepatocellular carcinoma using targeted next generation sequencing. Oncotarget. 2014;5:3012-3022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Kudo Y, Tanaka Y, Tateishi K, Yamamoto K, Yamamoto S, Mohri D, Isomura Y, Seto M, Nakagawa H, Asaoka Y, Tada M, Ohta M, Ijichi H, Hirata Y, Otsuka M, Ikenoue T, Maeda S, Shiina S, Yoshida H, Nakajima O, Kanai F, Omata M, Koike K. Altered composition of fatty acids exacerbates hepatotumorigenesis during activation of the phosphatidylinositol 3-kinase pathway. J Hepatol. 2011;55:1400-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Chettouh H, Lequoy M, Fartoux L, Vigouroux C, Desbois-Mouthon C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver Int. 2015;35:2203-2217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 58. | Hirsova P, Ibrabim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 59. | Afonso MB, Rodrigues PM, Carvalho T, Caridade M, Borralho P, Cortez-Pinto H, Castro RE, Rodrigues CM. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin Sci (Lond). 2015;129:721-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 60. | Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT, Bartneck M, Neumann UP, Canbay A, Reeves HL, Luedde M, Tacke F, Trautwein C, Heikenwalder M, Luedde T. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 61. | Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 702] [Cited by in F6Publishing: 754] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 62. | Bozaykut P, Sahin A, Karademir B, Ozer NK. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic steatohepatitis. Mech Ageing Dev. 2016;157:17-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Novo E, Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008;1:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 235] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 64. | Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1169] [Cited by in F6Publishing: 1283] [Article Influence: 91.6] [Reference Citation Analysis (1)] |
| 65. | Min HK, Mirshahi F, Verdianelli A, Pacana T, Patel V, Park CG, Choi A, Lee JH, Park CB, Ren S, Sanyal AJ. Activation of the GP130-STAT3 axis and its potential implications in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2015;308:G794-G803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Jiang N, Sun R, Sun Q. Leptin signaling molecular actions and drug target in hepatocellular carcinoma. Drug Des Devel Ther. 2014;8:2295-2302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Xing SQ, Zhang CG, Yuan JF, Yang HM, Zhao SD, Zhang H. Adiponectin induces apoptosis in hepatocellular carcinoma through differential modulation of thioredoxin proteins. Biochem Pharmacol. 2015;93:221-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Shen J, Yeh CC, Wang Q, Gurvich I, Siegel AB, Santella RM. Plasma Adiponectin and Hepatocellular Carcinoma Survival Among Patients Without Liver Transplantation. Anticancer Res. 2016;36:5307-5314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 500] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 70. | Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, Lorentzen A, Einer C, Schulz S, Clavel T, Protzer U, Thiele C, Zischka H, Moch H, Tschöp M, Tumanov AV, Haller D, Unger K, Karin M, Kopf M, Knolle P, Weber A, Heikenwalder M. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 475] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 71. | Lanthier N. Targeting Kupffer cells in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Why and how? World J Hepatol. 2015;7:2184-2188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 72. | Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012;72:3977-3986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 73. | Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 910] [Cited by in F6Publishing: 906] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 74. | Zheng X, Zeng W, Gai X, Xu Q, Li C, Liang Z, Tuo H, Liu Q. Role of the Hedgehog pathway in hepatocellular carcinoma (review). Oncol Rep. 2013;30:2020-2026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Della Corte CM, Viscardi G, Papaccio F, Esposito G, Martini G, Ciardiello D, Martinelli E, Ciardiello F, Morgillo F. Implication of the Hedgehog pathway in hepatocellular carcinoma. World J Gastroenterol. 2017;23:4330-4340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019;11:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 154] [Article Influence: 30.8] [Reference Citation Analysis (7)] |
| 77. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 441] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 78. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1343] [Cited by in F6Publishing: 2393] [Article Influence: 797.7] [Reference Citation Analysis (1)] |
| 79. | Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 80. | Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 213] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 81. | Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ohmoto Y, Amakawa K, Tsuji H, Kumada H. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 82. | Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, Roberts LR. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:907-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 83. | Turati F, Talamini R, Pelucchi C, Polesel J, Franceschi S, Crispo A, Izzo F, La Vecchia C, Boffetta P, Montella M. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer. 2013;108:222-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 84. | Degasperi E, Colombo M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2016;1:156-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 85. | Abdel-Rahman O, Helbling D, Schöb O, Eltobgy M, Mohamed H, Schmidt J, Giryes A, Mehrabi A, Iype S, John H, Tekbas A, Zidan A, Oweira H. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10:245-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 86. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 883] [Article Influence: 63.1] [Reference Citation Analysis (1)] |
| 87. | Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 820] [Cited by in F6Publishing: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 88. | Park H, Shin SK, Joo I, Song DS, Jang JW, Park JW. Systematic Review with Meta-Analysis: Low-Level Alcohol Consumption and the Risk of Liver Cancer. Gut Liver. 2020;14:792-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Sookoian S, Castaño GO, Pirola CJ. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: a meta-analysis of 43 175 individuals. Gut. 2014;63:530-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 90. | Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, Chen CJ. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 91. | Kanda T, Goto T, Hirotsu Y, Masuzaki R, Moriyama M, Omata M. Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 92. | Donati B, Dongiovanni P, Romeo S, Meroni M, McCain M, Miele L, Petta S, Maier S, Rosso C, De Luca L, Vanni E, Grimaudo S, Romagnoli R, Colli F, Ferri F, Mancina RM, Iruzubieta P, Craxi A, Fracanzani AL, Grieco A, Corradini SG, Aghemo A, Colombo M, Soardo G, Bugianesi E, Reeves H, Anstee QM, Fargion S, Valenti L. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7:4492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 93. | Wang P, Wu CX, Li Y, Shen N. HSD17B13 rs72613567 protects against liver diseases and histological progression of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:8997-9007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 94. | Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore). 2017;96:e5904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 95. | Jung YB, Yoo JE, Han DH, Kim KS, Choi JS, Kim DY, Park YN, Choi GH. Clinical and survival outcomes after hepatectomy in patients with non-alcoholic fatty liver and hepatitis B-related hepatocellular carcinoma. HPB (Oxford). 2021;23:1113-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Hester CA, Rich NE, Singal AG, Yopp AC. Comparative Analysis of Nonalcoholic Steatohepatitis- Versus Viral Hepatitis- and Alcohol-Related Liver Disease-Related Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2019;17:322-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 97. | Giannini EG, Marabotto E, Savarino V, Trevisani F, di Nolfo MA, Del Poggio P, Benvegnù L, Farinati F, Zoli M, Borzio F, Caturelli E, Chiaramonte M; Italian Liver Cancer (ITALICA) Group. Hepatocellular carcinoma in patients with cryptogenic cirrhosis. Clin Gastroenterol Hepatol. 2009;7:580-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Benhammou JN, Aby ES, Shirvanian G, Manansala K, Hussain SK, Tong MJ. Improved survival after treatments of patients with nonalcoholic fatty liver disease associated hepatocellular carcinoma. Sci Rep. 2020;10:9902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Viganò L, Conci S, Cescon M, Fava C, Capelli P, D'Errico A, Torzilli G, Di Tommaso L, Giuliante F, Vecchio FM, Salizzoni M, David E, Pinna AD, Guglielmi A, Capussotti L. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: A multicenter matched analysis with HCV-related HCC. J Hepatol. 2015;63:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 100. | Than NN, Ghazanfar A, Hodson J, Tehami N, Coldham C, Mergental H, Manas D, Shah T, Newsome PN, Reeves H, Shetty S. Comparing clinical presentations, treatments and outcomes of hepatocellular carcinoma due to hepatitis C and non-alcoholic fatty liver disease. QJM. 2017;110:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15:1450-1458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Tokushige K, Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Shiratori K. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J Gastroenterol. 2010;45:960-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 103. | Hernandez-Alejandro R, Croome KP, Drage M, Sela N, Parfitt J, Chandok N, Marotta P, Dale C, Wall W, Quan D. A comparison of survival and pathologic features of non-alcoholic steatohepatitis and hepatitis C virus patients with hepatocellular carcinoma. World J Gastroenterol. 2012;18:4145-4149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1211] [Cited by in F6Publishing: 1259] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 105. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 106. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3934] [Cited by in F6Publishing: 4885] [Article Influence: 814.2] [Reference Citation Analysis (0)] |
| 107. | Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020;158:1822-1830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 183] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 108. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2664] [Article Influence: 444.0] [Reference Citation Analysis (1)] |
| 109. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 986] [Cited by in F6Publishing: 1335] [Article Influence: 190.7] [Reference Citation Analysis (0)] |
| 110. | Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71:523-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 111. | Pennisi G, Celsa C, Giammanco A, Spatola F, Petta S. The Burden of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: Screening Issue and Future Perspectives. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 112. | Younes R, Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol. 2018;68:326-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 113. | Gori M, Arciello M, Balsano C. MicroRNAs in nonalcoholic fatty liver disease: novel biomarkers and prognostic tools during the transition from steatosis to hepatocarcinoma. Biomed Res Int. 2014;2014:741465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 114. | Peng L, Yuan XQ, Zhang CY, Peng JY, Zhang YQ, Pan X, Li GC. The emergence of long non-coding RNAs in hepatocellular carcinoma: an update. J Cancer. 2018;9:2549-2558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 115. | Zhang L, Wang K, Deng Q, Li W, Zhang X, Liu X. Identification of Key Hydroxymethylated Genes and Transcription Factors Associated with Alpha-Fetoprotein-Negative Hepatocellular Carcinoma. DNA Cell Biol. 2019;38:1346-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 116. | Hwang A, Shi C, Zhu E, Naaz F, Zhou P, Rasheed Z, Liu M, Jung LS, Duan B, Li J, Jiang K, Paka L, Gadhiya SV, Dana D, Ali Q, Yamin MA, Goldberg ID, Narayan P. Supervised learning reveals circulating biomarker levels diagnostic of hepatocellular carcinoma in a clinically relevant model of non-alcoholic steatohepatitis; An OAD to NASH. PLoS One. 2018;13:e0198937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 770] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 118. | Baumeister SE, Schlesinger S, Aleksandrova K, Jochem C, Jenab M, Gunter MJ, Overvad K, Tjønneland A, Boutron-Ruault MC, Carbonnel F, Fournier A, Kühn T, Kaaks R, Pischon T, Boeing H, Trichopoulou A, Bamia C, La Vecchia C, Masala G, Panico S, Fasanelli F, Tumino R, Grioni S, Bueno de Mesquita B, Vermeulen R, May AM, Borch KB, Oyeyemi SO, Ardanaz E, Rodríguez-Barranco M, Dolores Chirlaque López M, Felez-Nobrega M, Sonestedt E, Ohlsson B, Hemmingsson O, Werner M, Perez-Cornago A, Ferrari P, Stepien M, Freisling H, Tsilidis KK, Ward H, Riboli E, Weiderpass E, Leitzmann MF. Association between physical activity and risk of hepatobiliary cancers: A multinational cohort study. J Hepatol. 2019;70:885-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 119. | El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 553] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 120. | George ES, Sood S, Broughton A, Cogan G, Hickey M, Chan WS, Sudan S, Nicoll AJ. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients. 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 121. | Wijarnpreecha K, Thongprayoon C, Ungprasert P. Coffee consumption and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29:e8-e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 122. | Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open. 2017;7:e013739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 123. | Kimura T, Tanaka N, Fujimori N, Sugiura A, Yamazaki T, Joshita S, Komatsu M, Umemura T, Matsumoto A, Tanaka E. Mild drinking habit is a risk factor for hepatocarcinogenesis in non-alcoholic fatty liver disease with advanced fibrosis. World J Gastroenterol. 2018;24:1440-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 124. | Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT, Corey KE. Daily Aspirin Use Associated With Reduced Risk For Fibrosis Progression In Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17:2776-2784.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 125. | Bertot LC, Adams LA. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:179-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 126. | Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 127. | Zhou YY, Zhu GQ, Wang Y, Zheng JN, Ruan LY, Cheng Z, Hu B, Fu SW, Zheng MH. Systematic review with network meta-analysis: statins and risk of hepatocellular carcinoma. Oncotarget. 2016;7:21753-21762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 128. | Yokohama K, Fukunishi S, Ii M, Nakamura K, Ohama H, Tsuchimoto Y, Asai A, Tsuda Y, Higuchi K. Rosuvastatin as a potential preventive drug for the development of hepatocellular carcinoma associated with non-alcoholic fatty liver disease in mice. Int J Mol Med. 2016;38:1499-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 129. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881-91; quiz 892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 130. | Galle PR, Abou-Alfa GK. Decision making in systemic therapy of hepatocellular carcinoma: Should we pay attention to disease aetiology? J Hepatol. 2021;75:763-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 131. | Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. 2021;75:960-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 191] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 132. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2542] [Cited by in F6Publishing: 3317] [Article Influence: 829.3] [Reference Citation Analysis (1)] |
| 133. | au T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Begic D, Chen G, Neely J, Anderson J, Sangro B. LBA38_PR - CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874-v875. [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 433] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 134. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 1082] [Article Influence: 216.4] [Reference Citation Analysis (0)] |
| 135. | Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, Müller F, Sinha A, Friebel E, Engleitner T, Lenggenhager D, Moncsek A, Heide D, Stirm K, Kosla J, Kotsiliti E, Leone V, Dudek M, Yousuf S, Inverso D, Singh I, Teijeiro A, Castet F, Montironi C, Haber PK, Tiniakos D, Bedossa P, Cockell S, Younes R, Vacca M, Marra F, Schattenberg JM, Allison M, Bugianesi E, Ratziu V, Pressiani T, D'Alessio A, Personeni N, Rimassa L, Daly AK, Scheiner B, Pomej K, Kirstein MM, Vogel A, Peck-Radosavljevic M, Hucke F, Finkelmeier F, Waidmann O, Trojan J, Schulze K, Wege H, Koch S, Weinmann A, Bueter M, Rössler F, Siebenhüner A, De Dosso S, Mallm JP, Umansky V, Jugold M, Luedde T, Schietinger A, Schirmacher P, Emu B, Augustin HG, Billeter A, Müller-Stich B, Kikuchi H, Duda DG, Kütting F, Waldschmidt DT, Ebert MP, Rahbari N, Mei HE, Schulz AR, Ringelhan M, Malek N, Spahn S, Bitzer M, Ruiz de Galarreta M, Lujambio A, Dufour JF, Marron TU, Kaseb A, Kudo M, Huang YH, Djouder N, Wolter K, Zender L, Marche PN, Decaens T, Pinato DJ, Rad R, Mertens JC, Weber A, Unger K, Meissner F, Roth S, Jilkova ZM, Claassen M, Anstee QM, Amit I, Knolle P, Becher B, Llovet JM, Heikenwalder M. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 578] [Article Influence: 192.7] [Reference Citation Analysis (0)] |