Published online May 14, 2022. doi: 10.3748/wjg.v28.i18.1875
Peer-review started: January 19, 2022
First decision: March 8, 2022
Revised: March 8, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 14, 2022
Gut microbiota has a significant role in gut development, maturation, and immune system differentiation. It exerts considerable effects on the child's physical and mental development. The gut microbiota composition and structure depend on many host and microbial factors. The host factors include age, genetic pool, general health, dietary factors, medication use, the intestine's pH, peristalsis, and transit time, mucus secretions, mucous immunoglobulin, and tissue oxidation-reduction potentials. The microbial factors include nutrient availability, bacterial cooperation or antagonism, and bacterial adhesion. Each part of the gut has its microbiota due to its specific characteristics. The gut microbiota interacts with different body parts, affecting the pathogenesis of many local and systemic diseases. Dysbiosis is a common finding in many childhood disorders such as autism, failure to thrive, nutritional disorders, coeliac disease, Necrotizing Enterocolitis, helicobacter pylori infection, functional gastrointestinal disorders of childhood, inflammatory bowel diseases, and many other gastrointestinal disorders. Dysbiosis is also observed in allergic conditions like atopic dermatitis, allergic rhinitis, and asthma. Dysbiosis can also impact the development and the progression of immune disorders and cardiac disorders, including heart failure. Probiotic supplements could provide some help in managing these disorders. However, we are still in need of more studies. In this narrative review, we will shed some light on the role of microbiota in the development and management of common childhood disorders.
Core Tip: Gut microbiota has an intimate relationship with the various health conditions of the human body. It interacts with different body parts, affecting the pathogenesis of many local and systemic diseases. Gut dysbiosis is observed in many childhood disorders, inside and outside the gastrointestinal tract. Probiotic supplements could provide some help in managing these disorders. However, we are still in need of more studies. In this narrative review, we will shed some light on the role of microbiota in the development and management of common childhood disorders.
- Citation: Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O. Gut microbiota in various childhood disorders: Implication and indications. World J Gastroenterol 2022; 28(18): 1875-1901
- URL: https://www.wjgnet.com/1007-9327/full/v28/i18/1875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i18.1875
The human has an intimate symbiotic relationship with microbes. The human body harbors about 10-100 trillion microbial cells. Most of these microbes are present mainly in the gut as it provides a warm, stable, and eutrophic environment. There is significant variability in microbial composition at different body sites, with a vast difference between health and disease. Although the term microbiota is sometimes interchangeably used with the term microbiome, microbiota refers to the organisms living in a specific environment, and microbiome refers to the microorganisms and their genome in a particular environment[1]. The microbial microbiome has a set of genes of approximately 3.3 million active genes compared to 22000 human genes. The gut microbiota is the organisms that inhabit the gut, forming about 60% of the dry faces; 99% are anaerobic bacteria. Though bacteria form the main bulk of the microbiome, viruses, archaea, and eukaryotes are present in fewer numbers, but we should not ignore their presence[2].
Microbial colonization with more than 1000 species plays an essential role in gut development and maturation. There is evidence that gut colonization started in utero, and bacteria were detected from the amniotic fluid meconium and placenta in healthy term babies[3]. After delivery, the microbiota of the vaginally delivered neonates resembles those of their mother's vagina, while those delivered by cesarean section resemble those of the mother's skin. Then the infant microbiota changes gradually with every change in the infant diet from the simple neonatal microbiota with a predominance of facultative anaerobic bacteria, such as Enterobacteria, Enterococci, and Streptococci, to the more complex adult-type by the first few years of life with greater diversity and ability to biosynthesize vitamins and digest polysaccharides[4]. However, the child's microbiota continues to develop throughout childhood and adolescence. Despite being like the adult regarding the number of the detected species, the gut microbiota of children and adolescents may differ in genera's relative abundances[5]. Their gut microbiota has more abundances of Bifidobacterium spp., Faecalibacterium spp., and members of the Lachnospiraceae than the adults' gut microbiota with more abundances of Bacteroides spp. The microbiome also is different in children with more genes involved in amino acid degradation, vitamin synthesis, triggering mucosal inflammation, and oxidative phosphorylation compared with that observed in the adults with more genes associated with inflammation and obesity. So, as expected, the gut microbiota and microbiome go through a continuous and persistent development throughout life[6].
Gut microbiota exerts some essential functions in the human body's immunological, metabolic, structural, and neurological landscapes. Gut microbiota also significantly influences an individual's physical and mental health[7]. Gut microbiota significantly impacts normal and physiological gut development and helps gut mucosa maturation and differentiation and its immune system. It restricts the growth of the pathogenic and the potential pathogenic microbes, competes with them, and inhibits their ability to invade and implement the ecosystem. Some microbiota strains can secrete bacteriocins antimicrobial substances to inhibit other bacterial proliferation.
Other microbiota strains can ferment and digest nondigestible carbohydrates, fibers, and endogenous intestinal mucus, producing gases and short-chain fatty acids (SCFAs) such as acetate (the most abundant), propionate, and butyrate. These SCFAs can modulate the various activities in the gastrointestinal tract, including cell proliferation and differentiation, water and electrolytes absorption, hormonal secretion, and immune system activation[8,9]. SCFAs can serve as a food substrate for colonocytes (butyrate) and regulate leukocyte function and immune system activation by producing different eicosanoids, cytokines (IL-2, IL-6, IL-10, and TNF-α), and chemokines production with inducing balance among pro-inflammatory and anti-inflammatory mechanisms. SCFAs may also affect leucocyte chemotaxis, affecting their ability to migrate to the focus of infection or inflammation to destroy the target microbes[10].
Lack of SCFA is one of the causes of leaky gut and local gut inflammation that enhance microbial invasion. Butyrate can also induce colon cancer cells apoptosis and activate intestinal gluconeogenesis to enhance energy balance. It is crucial for glucose homeostasis by regulating hepatic gluconeogenesis and stimulating satiety signaling. The metabolic effects of SCFAs are not limited to the intestine but have extra-intestinal effects. Acetate SCFAs play a crucial role in regulating cholesterol metabolism and lipogenesis[11]. Microbiota also has an essential metabolic function in the biosynthesis of vitamins (vitamin K, biotin, folic acid, vitamin B12, and pantothenic acid) and amino acids from urea or ammonia. It also plays a role in xenobiotics and drug metabolism[12].
Gut microbiota can affect the host's energy balance through different mechanisms. It extracts energy from nondigestible dietary components and impacts gut transit, energy intake, and energy expenditure[13]. It also can modify the available pool of bile acids, affecting their composition and abundance. Gut microbiota-derived enzymes can metabolize the bile acids produced by the liver, a critically crucial process to maintain a healthy gut microbiota, enhance lipid and carbohydrate metabolism, increase insulin sensitivity, and enhance innate immunity[14]. The gut microbiota connects with the brain through several various mechanisms. These mechanisms include neurotransmitters production or modulation of their catabolism, vagus nerve signaling, and the hypothalamus-pituitary axis activation[15]. Gut microbiota produces hundreds of neurochemical substances used by the brain to regulate its basic physiological processes and mental functions such as learning, memory, and mood[16].
The type and the quantities of the gut microbiota show wide individual variability. Many host and bacterial-related factors affect bacterial colonization in the different parts of the human gut. The host factors include the host's age, genetic pool, general health, dietary factors, using medication, pH, peristalsis, and the transit time of the part of the intestine, mucus secretions containing immunoglobulin, and the tissue oxidation-reduction potentials. The microbial factors include nutrient availability, bacterial cooperation or antagonism, and bacterial adhesion[17,18]. Each part of the gut has its microbiota due to its specific characteristics. Table 1 shows the microbiota in the different parts of the gut.
| Site | pH | Predominant microbiota | Bacterial load (CFU/gram content)
| Other factors |
| Mouth | 6.5-7 | Bacteria (esp Fusobacterium nucleatum), fungi, viruses and protozoa | 700 species | Ideal warm environment |
| Stomach | Strong acidic | Lactobacilli, streptococci, Lactobacillus, Peptostreptococcus, Helicobacter pylori, and yeasts | Low (102) | Gastric acidity, Acid suppressive therapy, H. pylori colonization, the reflux of bile, mucus thickness and gastric peristalsis |
| Duodenum | 4-5 | Lactobacilli and Streptococci | More than (102-104) | Age, diet, antibiotic, and proton pump inhibitor use |
| Jejunum-ileum | 6-7.4 | Firmicutes and Proteobacteria | More than duodenum (106-108) | Nutrient reach environment faster transit time, bile acids, and antimicrobial peptide exposure |
| Colon | Left colon 6.1-7.5; Cecum 5.7; Rectum 6.7 | Bacteriodetes (especially the genera Bacteroides and Prevotella) and Firmicutes (especially members of the genus Clostridium). Methanogenic archaea and fungi; Cecum: Aerobic bacteria; Rectum: Bacteroides and Prevotella. | 1010-1012 | High diversity and density, no digestive secretions, nutrient-poor environment, & slow transit time (30 h) |
The ability of the host genetics pool to modify the gut microbiome structure is still controversial. There is a strong association between the Lactase gene and the relative abundance of Bifidobacterium. However, this association could be related to lactose consumption[19]. The vitamin D receptor gene is associated with some variation in gut microbiota[20]. Other studies proved the association of some host genetic variations with the abundance of certain microbiota species. However, the origin of association is still uncertain[21]. The host diet is crucial in developing gut microbiota as carbohydrate fermentation is one of its core functions. The microbiota of the small intestine adapts quickly to varying nutrient availability in the lumen and can rapidly metabolize the simple carbohydrates. On the other hand, the colon microbiota can degrade complex carbohydrates. A high-fat diet stimulates the proliferation of Clostridium and suppresses the proliferation of Bifidobacterium and Bacteroides[22]. Dietary modification produces rapid alteration of the colonic microbiota within two days and long-term changes[23].
The type of delivery can early-life microbiome. However, this effect may differ upon intrapartum antibiotic exposure. The gut microbiota of vaginally delivered infants shows enrichment of Bifidobacterium spp. and reduction of Enterococcus and Klebsiella spp. Over the first year of life, the gut microbiota in infants born with caesarean section appears less stable with a predominance of pathogenic bacteria such as Klebsiella and Enterococcus and delayed acquisition of the beneficial Bifidobacterium[24]. Breast or bottle feeding also significantly impacts the gut microbiota. Exclusively breastfed infants have lower microbial diversity with a predominance of infant-type Bifidobacteria than formula-fed babies whose gut microbiota is more diverse and like older children. The predominance of infant-type Bifidobacteria significantly impacts the immune system's maturation and development, which may help decrease the incidence of childhood infections[25].
The gut microbiota develops throughout human life in predictable patterns, with fast change from the neonatal pattern to the age of three, reaching the adult pattern. Then the microbiota goes into a stable phase until middle age, and then it goes into accelerated changes in late adulthood. These changes could be related to aging itself, underlying diseases, and the use of medications. At the same time, changes in the microbiota pattern can predict decreased longevity[26]. The use of the proton pump inhibitors is associated with decreased bacterial richness and predominance of an unhealthy gut microbiome which predisposes to Clostridium difficile enteric infections[27]. Antibiotics negatively impact the gut microbiota by reducing the species diversity, altering the metabolic activity, and favoring the predominance of antibiotic-resistant microbial strains, which sequentially can cause antibiotic-associated diarrhea and recurrent Clostridium difficile infections[28].
Microbial cooperation is a characteristic feature of microbial communities. An example of bacterial cooperation appears clearly in Bacteroidales, the predominant Gram-negative bacteria in the human gut. Bacteroides ovatus and Bacteroides vulgatus showed a mutual relation where Bacteroides ovatus can digest the dietary complex polysaccharide inulin producing energy and food source for other Bacteroides, including Bacteroides vulgatus. In return, Bacteroides vulgatus benefits Bacteroides ovatus by detoxifying inhibitory substances and the secretion of a depleted or growth-promoting factor. This bacterial cooperation is vital to stabilize the gut ecosystem[29]. Bacterial antagonism is common in microbial communities and contributes to specific bacterial strains' different compositions and relative abundance. It also helps for the long-term stability of the microbial community. This antagonism can occur by interference competition with the secretion of specific molecules such as antibacterial peptides and proteins that inhibit other strains. These antimicrobial toxins perform a significant role in microbiota-mediated colonization resistance by inhibiting the invasive pathogens[30]. Bacterial adhesion to gut epithelial surfaces affects their retention time and, therefore, considerably impacts interactions between the microbiota and their hosts. This adhesion ability of some bacteria could help their transient colonization in the gut and help to boost their immunomodulatory effects and enhance the gut barrier and metabolic functions[31].
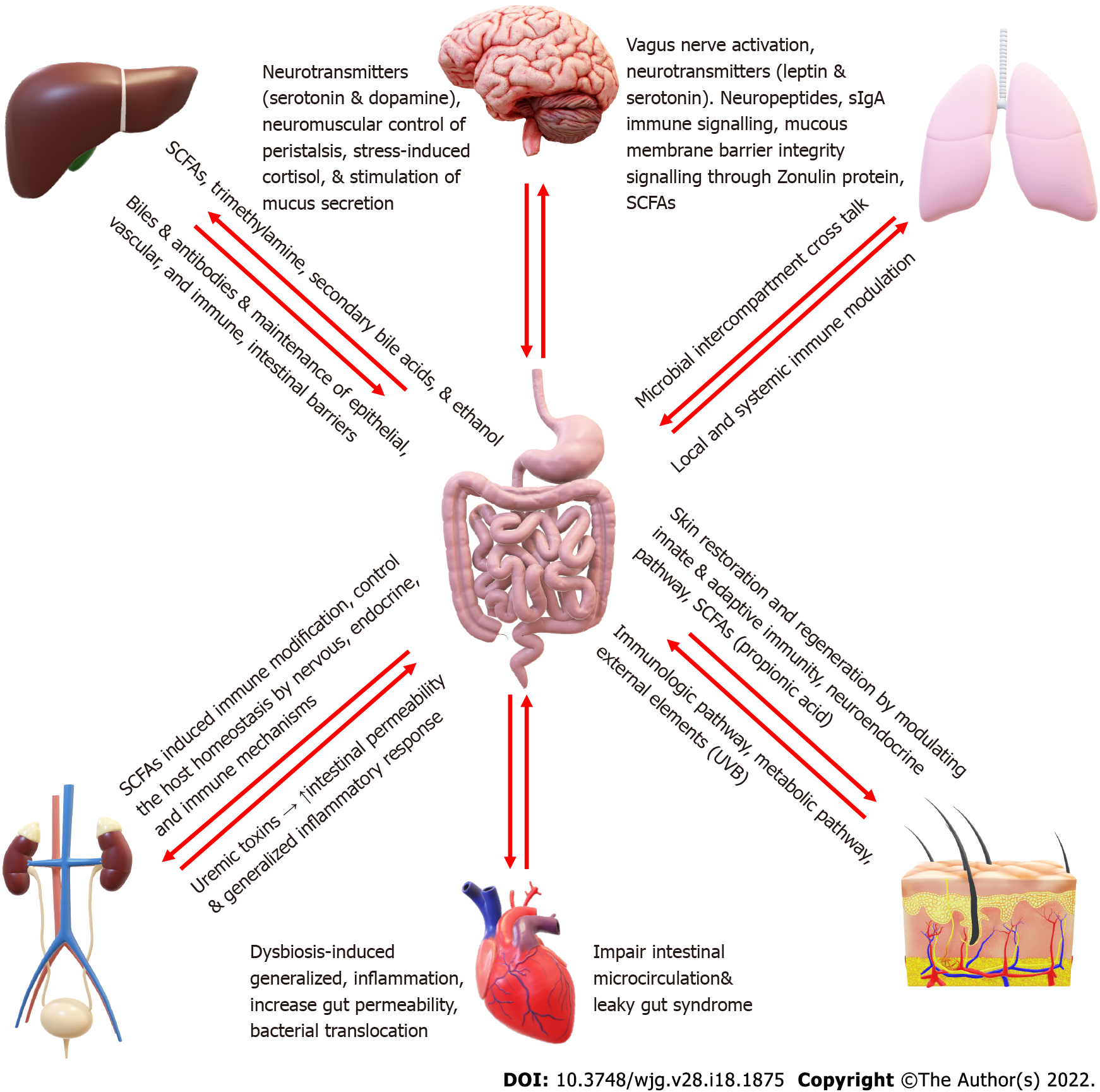
The intestinal microbiota is considered an organ of the human body, with its features making it crucial in various body functioning. There is a mutual bidirectional relation of the gut, microbiota, and the other body systems, forming different systemic axes, e.g., Brain-gut-microbiota axis, liver- gut-microbiota axis, skin- gut-microbiota axis, kidney- gut-microbiota axis, lung- gut-microbiota axis (Figure 1).
The brain and the gut interact together through the central and the enteric nervous system. The brain interacts with the gut through several mechanisms, including neurocrine and endocrine pathways, which may be involved in gut microbiota-to-brain signaling, and the brain can, in turn, alter gut microbiota composition. The brain controls the gut and gut microbiota through neurotransmitters such as serotonin and dopamine, neuromuscular control of peristalsis, stress-induced cortisol, and stimulation of mucus secretion[32]. On the other hand, the gut affects the brain through vagus nerve activation, neuropeptides, and neurotransmitters such as leptin and serotonin, immune signaling through secretory IgA, mucous membrane barrier integrity signaling through Zonulin protein, and SCFAs such as butyrate[15,33]. Alternatively, the microbiota affects the brain through different mechanisms. Some strains of Lactobacillus and Bifidobacterium can produce gamma-aminobutyric acid (GABA), which is the dominant brain inhibitory neurotransmitter. Other bacterial species such as Enterococcus and Escherichia and some candida strains can produce serotonin. Some bacillus species can produce dopamine neurotransmitters. Bacteria can also affect the brain by making SCFAs (such as butyric acid, propionic acid, and acetic acid), stimulating the sympathetic nervous system, and inducing mucosal serotonin release sequentially, impacting the memory and learning process in the brain[34].
The liver-gut-microbiota axis is a bidirectional relationship between the liver and the gut and its microbiota on the other side. The gut-derived products are transported directly to the liver through the portal veins, and the liver manufactures the bile and antibodies to be transported back to the intestine. The gut microbiota is essential for preserving the immune homeostasis of the liver-gut-microbiota axis. Microbe-derived metabolites, such as SCFAs, trimethylamine, secondary bile acids, and ethanol, may play a role in non-alcoholic fatty liver disease pathogenesis. On the other hand, liver cirrhosis induces intense changes in gut microbiota and impairment of the intestinal epithelial, vascular, and immune barriers[35]. A change in gut microbiota structure can activate the mucosal immune response triggering homeostasis imbalance. This imbalance results in bacterial transport and immune cells migrating to the liver, inducing inflammation-mediated liver injury and tumor progression[36,37].
The heart-gut axis is relatively newly described based on intestinal microbiota's ability to affect the cardiovascular status and vice versa. Gut dysbiosis is linked to the state of generalized inflammation associated with increased risk of obesity and type II diabetes mellitus, which are important cardiovascular risk factors, especially for atherosclerosis and heart failure. At the same time, the diet that can cause dysbiosis, e.g., a high fatty diet, can also cause metabolic syndrome. On the other side, Most cardiovascular disease (CVD) risk factors, such as aging, dietary patterns, obesity, and a sedentary lifestyle, can induce gut dysbiosis. Dysbiosis can also increase gut permeability, leaky gut syndrome, and bacterial translocation and are considered risk factors for CVD. Meanwhile, congestive heart failure will impair intestinal microcirculation aggravating the leaky gut syndrome and causing more bacterial translocation worsening the heart failure with a vicious cycle[38-40].
The gut microbiota has critical roles in various diseases involving hypertension and chronic kidney disease. The gut microbiota connects with the nervous, endocrine, and immune systems to control the host homeostasis, involving blood pressure and renal functions. The gut–kidney axis is conducted through metabolism-dependent mechanisms and immune pathways[41]. SCFAs produced by commensal gut microbiota can affect the kidneys through a wide range of mechanisms, including immune system modification and interactions with the renal cognate receptors and transporters[42]. On the other side, kidney injury causes uremic toxins accumulation in the intestine with increased intestinal permeability and generalized inflammatory response[43]. Uraemia increases bacterial translocation and impairs immunity by decreasing T and B cell responses from vaccination and decreasing the memory of T and B cells. Increased nitrogen waste products in uremia promote the overgrowth of proteolytic bacteria[44].
Despite the clear anatomical distinction between the gut and the lung, recent evidence showed that the gut and lung microbiota affect each other, significantly impacting respiratory diseases[45]. The lung microbiome is much lower than the gut microbiota. Its composition depends on the oropharynx and upper respiratory tract microbial colonization through salivary micro-inhalations, the host abilities for microbial elimination, primarily through cough and mucociliary clearance, the interactions with the host immune system, and on local conditions that control the microbial proliferation, such as oxygen concentration and local pH[46]. The lung microbiota composition is also strongly correlated with the gut microbiota composition. The gut microbiota enriches the lung bacteria, impacting the gut microbiota composition. For example, inhalation of gastroesophageal content (through gastroesophageal reflux) and sputum swallowing may explain this inter-organ connection. The lung-gut-microbiota axis may also involve indirect communications through the host immune modulation either by gut microbiota's local or systemic immune impact, especially on the pulmonary immune system[47].
Skin and gut play crucial immune and neuro-endocrine roles and are distinctively related in function. The gut microbiota affects the skin microbiome through the skin-gut-microbiota axis. SCFAs produced from fiber fermentation by gut microbiota have a significant role in skin microbiota composition and immune defense mechanisms[44]. Propionic acid has a powerful antimicrobial effect against the community-acquired methicillin-resistant Staphylococcus aureus[49]. Gut microbiota also helps skin restoration and regeneration by modulating innate and adaptive immunity. It enhances the skin barrier through modulation of T cell differentiation in response to different immune stimuli[50]. Several environmental factors, e.g., diet and psychological stress, can impact the gut microbiome, directly or indirectly influencing skin health.
Table 2 summarizes the disease-associated dysbiosis and the proposed probiotics.
| The disease | Encountered dysbiosis | The proposed probiotics |
| Autism[57,58] | Mother have abundance of Alphaproteobacteria, Proteobacteria, Acinetobacter, & Moraxellaceae. Children have more clostridial species, non-spore-forming anaerobes, and microaerophilic bacteria | No suggested type yet |
| Malnutrition[60] | Less Bifidobacteria. More pathogenic microbes (Escherichia coli, Fusobacterium mortiferum, & Streptococcus spp.) | The lack of strong evidence for specific types of probiotics |
| Obesity[75-78] | Less bifidobacteria. More Bacteroides & Staphylococcus spp. | Bifidobacterium lactis and Lactobacillus GG |
| Infant colic[85-87] | More abundance of Proteobacteria. Less abundance of the genera Lactobacillus & Bifidobacterium. Reduced gut bacterial diversity | Lactobacillus reuteri DSM17938 in breastfeeding infants |
| Functional abdominal pain[90,91] | More Prevotella, Lactobacillus, Veillonella, & Parasporo bacterium. Less Verrucomicrobium & Bifidobacterium | Sporobacter & Subdoligranulum |
| Functional constipation[94,95] | More Prevotella. More butyrate-producing bacteria as Roseburia, Coprococcus, & Faecalibacterium | Still investigational |
| Necrotizing enterocolitis[98,99] | More Citrobacter koseri and/or Klebsiella pneumoniae. Reduced diversity. Less Lactobacillus abundance | Bifidobacteria and Lactobacillus |
| Helicobacter pylori infection[102,106,107] | Prevotella, Clostridium, Proteobacteria, and Firmicutes. Less Bacteroides | Saccharomyces boulardii, L. acidophilus, L. casei DN-114001, L. gasseri, and Bifidobacterium infantis 2036 and Lactobacillus reuteri Gastrus |
| Coeliac disease[109,114-116] | Reduced Gram-positive/Gram-negative bacteria ratio. Less Bifidobacterium, Clostridium histolyticum, Clostridium. lituseburense and Faecalibacterium prausnitzii. More Bacteroides-Prevotella group. Less IgA coating the Bacteroides-Prevotella group | Lactobacillus rhamnosus, Bifidobactera breve & Longum, and Lactobacilli strains (L. ruminis, L. Johndoni, L. amylovorus, L. salivaris) |
| Inflammatory bowel diseases[122,126-128] | Less abundance of the healthy commensal (such as Clostridium IXa and IV groups, Bacteroides, Bifidobacteria). More abundance of the pathogenic bacteria as sulphate-reducing Escherichia coli | Still controversial. Saccharomyces boulardi. Escherichia coli Nissle1917, Bifidobacterium breve, Bifidobacterium bifidum, Lactobacillus acidophilus |
| Cystic fibrosis[135-137] | Aberrant colonization of gut and respiratory microbiota due to altered intestinal & airway microenvironment | Lactobacillus rhamnosus GG & Lactobacillus reuteri |
| Allergic rhinitis[140,142-144] | Decrease gut bacterial diversity | Lactobacillus paracasei. Bifidobacteria mixture |
| Bronchial asthma[147] | Relative abundance of the bacterial genera Rothia, Veillonella, Lachnospira, & Faecalibacterium. Low total & gut microbial diversity | Still controversial |
| Atopic dermatitis[154-157] | Reduced microbial diversity. More abundance of pathogenic Staphylococcus aureus and Malassezia. Presence of Clostridioides difficile. More Bifidobacteria abundance. Lower lactobacilli abundance | Topical Roseomonas mucosa |
| Psoriasis[160,161,163,164] | More bacterial diversity & heterogeneity. More Staphylococcus aureus. Less Staphylococcus epidermidis & Propionibacterium acnes. Reduced microbiota stability. Variable topographic dysbiosis | Sill controversial. Oral Lactobacillus, one sachet thrice daily with biotin |
| Systemic lupus erythematosus[166,168] | Less microbiota abundance and diversity | Animal studies showed Lactobacillus fermentum CECT5716 (LC40) |
| Juvenile idiopathic arthritis[172,174] | Less Faecalibacterium Prausnitzii abundance. More Bifidobacterium abundance, mostly B. adolescentis | Not conclusive. Trial with Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus rhamnosus, Bifidobacterium breve, Streptococcus thermophile & Bifidobacterium longum |
| Dental caries[176,178,179] | More abundance of Prevotella melaninogenica, Leptotrichia shahii, Leptotrichia HOT 498, Veillonella dispar, and Streptococcus mutans | Insufficient evidence. Lactobacillus rhamnosus may help |
| Chronic congestive heart failure[180,184,187,189] | Decreased gut microbiota diversity. More pathogenic Microbes as Campylobacter, Yersinia enterocolitica, Salmonella, Shigella & candida. Low Coriobacteriaceae, Erysipelotrichaceae and Ruminococcaceae | Bifidobacteria, yeasts, and lactic acid-producing bacteria such as Lactobacillus rhamnosus GR-1. Saccharomyces boulardii |
Gut microbiota exerts a considerable effect on the child's physical and mental development. The human brain has a rapid growth rate throughout the perinatal period, matching the remarkable maternal and infant microbiota changes[51]. The microbiota plays an essential role during brain development through its effects on gamma-aminobutyric acid and serotonin synthesis from tryptophan and altered neurotransmitters such as noradrenaline and dopamine. Serotonin is crucial to brain development. Decreased brain serotonin impair synaptogenesis and the brain wiring, causing long-term neurodevelopmental impairment[52]. About 95% of the body's serotonin is formed by the gut microbiota, affecting mood and gastrointestinal activity. However, scientists found that serotonin cannot cross the blood-brain barrier. So, it works mainly on the peripheral enteric nervous system and works as a hormone affecting different tissues, including those regulating metabolic homeostasis[53]. However, the beneficial role of probiotics in alleviating the manifestation of many psychiatric disorders such as depression and anxiety could be related to their ability to secrete serotonin, a significant player in many psychiatric disorders[54]. Meanwhile, animal studies showed that probiotic use might cause rising plasma tryptophan levels, decreased serotonin concentrations in the frontal cortex, and decreased cortical dopamine metabolites, thus improving depressive symptoms[55].
SCFAs, a product from the fermenting effects of the colonic bacteria, regulate microglial homeostasis. The effects of SCFAs are markedly observed during the early phases of brain development during the early postnatal stage, while brain plasticity is still preserved[56]. Two interesting studies showed that gut microbiota is crucial to maintain healthy microglia functions, vital to preventing neurodevelopmental and neurodegenerative disorders[57,58]. Tamana et al[59] showed that boys who have a higher Bacteroidetes ratio in the gut microbiota at one year have higher cognitive functions and advanced linguistic skills after one year of follow-up. They also observed that girls have cognitive and linguistic scores than boys at the same age. They also noted a higher Bacteroidetes ratio in girls than boys. They related this increase in cognitive function due to the sphingolipid production by Bacteroidetes, which is an essential substrate for brain structures and functions. Factors that deplete Bacteroidetes, e.g., caesarean section or flourish Bacteroidetes such as normal vaginal delivery, breastfeeding, high-fiber diet, exposure to pets, and outdoor nature with green spaces can negatively or positively impact child cognitive functions[60].
Investigating the underlying mechanisms of neural development and neuropsychiatric disorders proved that the intestinal microbiota could affect brain physiology and behavior through the humoral and neural pathways of gut-brain communication, suggesting that the gut microbiota has a vital role in many neuropsychiatric disorders[61]. Autism is a multifactorial disease in which the gut microbiota plays an important role. The gut microbiota in children with autism showed plenty of Bacteroidetes and a lesser amount of Firmicutes than controls with characteristic mucosal microbiota signatures. This dysbiosis observed in children with autism correlates with cytokine quantities and tryptophan homeostasis. However, we do not know whether the observed dysbiosis is a cause or a result of the associated behavior problem observed in children with autism[62,63]. The effect of the gut microbiota is not limited to the child's gut but is also related to the maternal gut microbiota. A study by Li et al[64] found significant differences in the gut microbiota composition between the mothers and children with autism spectrum disorder (ASD) compared to healthy children and their mothers. They found that mothers of children with ASD had more Alphaproteobacteria, Proteobacteria, Acinetobacter, and Moraxellaceae than mothers of healthy children. Children with late-onset (regressive) autism have more colony numbers of fecal clostridial species and non-spore-forming anaerobes and microaerophilic bacteria, which are absent in the typically developed children, which could be related to the frequent use of antibiotics, disrupting the microbiota with more colonization by these types of autism-promoting microbiota species[65]. According to this hypothesis, the use of minimally absorbed oral vancomycin can induce temporary improvement in autistic symptoms[66]. However, a metanalysis that included 28 studies done on children with autism by Bezawada et al[67] showed the inconsistency of the data due to heterogeneity of the included populations and the used methods. They suggested that despite several reasons to consider the role of gut microbiota and their product in the pathogenesis of autism, we need more studies to understand better and confirm their effects. The developing hypothesis of a microbiota-gut-brain axis proposes that gut microbiota modulation may be an amenable strategy to developing a new therapeutic approach for complex central nervous system disorders[68].
Epilepsy is a common childhood disorder. There is a close relation between epilepsy and autoimmune diseases and between gut microbiota and autoimmune disease; a suggested association arises between epilepsy and gut microbiota. An exciting study by Huang et al[69] presented forty children who developed benign infantile convulsions after mild gastroenteritis, linking changing the gut microbiota and the epileptogenesis. Şafak et al[70] tried to elaborate on the relationship between gut microbiota and epileptogenesis. They studied the intestinal microbiota composition in patients with idiopathic focal epilepsy and compared them to a healthy volunteer group. They found an increased prevalence of Fusobacteria species in patients with epilepsy (10.6%) but not in the healthy volunteer group. This significant taxonomic drift and variations in the intestinal microbiota and the resulting gut dysbiosis may be associated with certain forms of epilepsy.
Meanwhile, some anticonvulsants can be metabolized by the gut microbiota, affecting their efficacy. For example, the intestinal microbiota can metabolize Zonisamide to 2-sulfamoyl-acetyl-phenol, which is pharmacologically not active[71]. In addition, the anti-epileptic effects of the ketogenic diet used in drug-resistance epilepsy (although its exact mechanism of action is unclear) could be related to the ketogenic diet-induced changes in the gut microbiome composition and function of patients with epilepsy. The gut microbes modify the seizure vulnerability through mechanisms different from just alterations of beta-hydroxybutyrate levels (a measure of ketosis). The anti-seizure protective effects of diet and microbiota are associated with elevating hippocampal GABA relative to glutamate content[72]. The probiotics supplement could provide additional benefits to the anti-epileptics, especially in drug-resistant epilepsy. Gómez-Eguílaz et al[73] supplied patients with drug-resistant epilepsy with a probiotic mixture for four months. The patients showed a significant reduction in seizures frequency and improved quality of their life. Consequently, reformation of the gut microbiota through fecal microbiota transplantation, probiotic supplement, and the ketogenic diet has potential favorable impacts on drug-resistant epilepsy[74].
The gut microbiota typically develops hand in hand with the child's growth. The prenatal microbial communities affect fetal and postnatal development. Maternal microbiota is a crucial element for intrauterine growth. An exciting study by Sato et al[75] showed that the maternal gut microbiota correlates with the neonatal anthropometrics measures. In male neonates, the head circumference and weight are negatively correlated with genus Eggerthella and Parabacteroides. In female neonates, a high ratio of Streptococcus correlates with low anthropometric measures. Neonates with very low birth weight and restricted extrauterine growth had a predominance of Proteobacteria of their intestinal microbiota[76]. The gut microbiota affects growth by affecting growth hormone and insulin-like growth factor 1 production and regulation through its effects on the hypothalamic-pituitary–somatotropic axis. Delayed maturation and colonization of the gut microbiota may result from underlying food insecurity, malnutrition, and infections and could negatively impact the child's nutritional status[77]. The malnutrition-associated dysbiosis of the gut microbiota starts with depletion of the Bifidobacteria followed by the establishment of potentially pathogenic microbes (Escherichia coli, Fusobacterium mortiferum, and Streptococcus spp.), causing diarrhea and essential nutrients malabsorption[78]. Dysbiosis may result in a generalized inflammatory state and enteropathy that may precipitate growth faltering. The effects of these microbiota changes are significant in the first 1000 d. It provides a window of opportunity for modifying the gut microbiota through different interventions such as diet, antibiotic use, supplementary probiotics, prebiotics, symbiotics, postbiotics, or fecal microbiota transplantation to restore the proper growth and development[79].
Dysbiosis may explain why malnourished children may miss up the desired weight compared to their well-fed counterparts, despite gaining some weight and growing better with nutrient-rich supplements. Subramanian et al[80] showed significant differences in the proportions and species of gut microbiota in children up to two years of age with and without malnutrition. Children with malnutrition showed the immaturity of their gut microbiota, resembling their healthy counterparts but at a younger age. This malnutrition-induced microbiota imbalance fails to recover even after correcting the malnutrition. Oral probiotic supplements with beneficial gut bacteria and fecal transplantation from healthy children can restore the malnutrition-induced dysbiosis, and the malnourished children thrive. Probiotic supplements can improve a child's growth by preventing infections and micronutrient deficiency. They have been shown to improve the absorption of specific nutrients (vitamin B12, calcium, and zinc) and decrease the possibility of anemia[81]. However, there is no clear evidence to use them in the treatment of malnutrition. This lack of evidence is also augmented by the difficulty of modifying the gut microbiota. It resists long-term change and is affected by other factors such as diet and sleep pattern[82].
The cause of malnutrition also impacts the composition of gut microbiota. For example, moderate-to-severe diarrhea in children reduces bacterial diversity and changes gut microbiota composition[83]. Diarrhea can also affect weight, height, and the child's mental development, especially for diarrhea occurring below the age of 2 years[84]. The diarrhea-induced changes in gut microbiota increase the risk of persistent diarrhea, which causes stunted growth and decreases the affected children's mental abilities. Interventions to restore the gut microbiota as prebiotic and probiotic supplementation could help to combat the risk of diarrhea and the resulting malnutrition[85].
Meanwhile, subclinical changes in the gut microbiota may result in stunting even in the absence of clinically evident infections such as diarrhea. For example, poor hygiene may cause persistent exposure to environmental pathogens inducing subclinical alteration in the gut microbiota structure and function and consequently cause stunting[86]. For instance, poor sanitary conditions with chronic exposure to environmental pathogens resulting in subclinical alteration in the gut microbiota structure and function initiate a condition known as environmental enteric dysfunction. This environmental enteric dysfunction induces a cell-mediated inflammation that ends in stunting[87].
The link between gut microbiota and obesity was evident in the adult population but not yet well documented in childhood. Obesity correlates with altered gut microbiota distinguished by raised Firmicutes and reduced Bacteroidetes abundance. This correlation is believed to be through the powerful effects of the gut microbiota on the human metabolic and immune status. Higher levels of SCFAs, a fermentation product by the gut microbiota, were found in children with obesity which tightened the relationship between the gut microbiota and the development of obesity[88]. The maturation patterns of gut microbiota in infancy can impact the relative chance of developing overweight and obesity in later childhood. Pregnant women with a high body mass index (BMI) have a higher load of Bacteroides than those with normal BMI[89].
Consequently, the maternal microbiota during pregnancy and breastfeeding significantly affects the newborn microbiota. The presence of Bacteroides spp. or their relatives and the relative lower abundances of Bifidobacteria in early infancy are related to developing childhood overweight and obesity. Staphylococcus spices may also serve as a predictive but inconsistent tool for childhood BMI[90]. Children with an average BMI at seven years have more Bifidobacterium spp. in their gut during their first year of life than children with high BMI[91]. Gut microbiota could modify obesity through its role in metabolic regulation, food availability, and digestion. Gut microbiota has extra-intestinal effects involving the brain, liver, and adipose tissue, possibly linked to obesity, insulin resistance, diabetes mellitus type-II, and related cardiovascular disorders. Gut microbiota might also impact food intake and satiation through gut peptide signaling[92].
Consequently, gut microbiota can modify energy regulation and systemic inflammation, two crucial pillars for obesity development. Early modification and restoration of gut microbiota may be an encouraging tool to counteract the increasing childhood metabolic disorders, including overweight and obesity, providing the specific anti-obesity microbiota[93]. Despite the evidence of the beneficial effects of probiotics on glucose tolerance, insulin sensitivity, and inflammatory markers, there is no substantial evidence to recommend the use of probiotics in obesity[94]. Antenatal supplement with Bifidobacterium lactis and Lactobacillus GG decreases the risk of gestational diabetes mellitus and consequently reduces the risk of macrosomia and large baby size at birth, an effect that could last up to six months after birth[95,96].
Gut microbiota can modulate various types of chronic pain through direct modulation of neuronal excitability dorsal root ganglia and neuroinflammation regulation in the central and peripheral nervous systems[97]. Numerous studies reported a strong association between the human gut microbiota and the development of functional gastrointestinal disorders, especially for infant colic, functional constipation, and irritable bowel disease. Randomized controlled trials showed that probiotics could be helpful in a variety of functional gastrointestinal disorders, including infant colic and irritable bowel syndrome. Probiotics may induce gut microbiota diversity with strain-specific effects on colonization resistance, the integrity of the epithelial barrier, signal transduction modulation, with a significant impact on both innate and adaptive immune responses, and notable effects on visceral hyperalgesia[98,99].
About 20% of infants developed infant colic, with prolonged crying without apparent cause. The exact etiology of infant colic is unknown, but many factors are proposed to have a role, such as gastrointestinal, psychosocial, and neurodevelopmental factors[100]. Several studies addressed the role of the gut microbiota in developing infant colic. Gut dysbiosis was described in infant colic in the form of more abundance of Proteobacteria and less abundance of the genera Lactobacillus and Bifidobacterium with reduced gut bacterial diversity[101]. A metanalysis done by Sung et al[102] showed that Lactobacillus reuteri DSM17938 was an effective treatment for infant colic in breastfed infants. Still, they cannot generalize this recommendation to formula-fed infants with colic, which needs further research. The unique effect of Lactobacillus reuteri DSM17938 in the breastfed infant may be related to the distinctive structure of breast milk or probably the direct effects of Lactobacillus reuteri or human milk oligosaccharides in breast milk[103]. How probiotics improve infant colic is not yet determined but may be mediated via modifying the activity of the colonic intrinsic sensory neurons with improving the gut motility. In addition, they have positive impacts on function and visceral pain[104].
About one-third of the school-aged children suffer from abdominal pain weekly, which causes school absenteeism and limitation of their social activities in about 20% of them. In children, functional abdominal pain is defined as when it persists for two or more months without an evident organic cause[105]. Function abdominal pain is further subclassified into four conditions: irritable bowel syndrome (IBS), functional dyspepsia, abdominal migraine, and functional abdominal pain not otherwise specified[106]. Despite the high prevalence of dysfunctional abdominal pain, the exact pathogenesis is not well-defined. However, many risk factors increase the rate of dysfunctional abdominal pain, including the winter season, sleep, school stress, and diet. Many studies relate dysbiosis of the gut microbiota to dysfunctional abdominal pain such as irritable bowel syndrome. Rigsbee et al[107] showed that children with irritable bowel syndrome had more abundance of Prevotella, Lactobacillus, Veillonella, and Parasporo bacterium and less quantity of Verrucomicrobium and Bifidobacterium.
On the other hand, a low fermentable substrate diet decreased the abdominal pain frequency in children with irritable bowel syndrome by increasing the abundance of bacterial taxa belonging to the genera Sporobacter and Subdoligranulum and reduced the abundance of taxa belonging to Bacteroides[108]. Probiotic use might change the gut microbiota composition and decrease inflammation. It could also promote the physiology of the gut and improve functional symptoms. Some probiotics may impact colonic motility by increasing stool fluidity through modifying water and electrolytes secretion and absorption, smooth muscle cell contractions modification, increasing the production of lactate and SCFAs, and reducing intraluminal pH[109].
Functional constipation is a common childhood disorder characterized by reduced gut movements and/or hard stools without organic causes. Functional constipation affects about 18% of infants and 3% of children and adolescents worldwide, with considerable influence on the child's and family's quality of life[110]. The pathophysiology of functional constipation is multifactorial, with a complex interaction between gastrointestinal dysmotility, psychological factors, and gut microbiota. Disturbances in gut microbiota may promote development and affect the outcome of functional constipation in children[111]. Zhu et al[112] showed that obese children suffering from constipation had a low-fiber diet and lower prevalence of gut Prevotella with an increased ratio of butyrate-producing bacteria such as Roseburia, Coprococcus, and Faecalibacterium than the control, which could be related to the low fiber intake. Probiotics can enhance intestinal transit time, stool frequency, and consistency[113]. Data about the efficacy of probiotic use to treat functional constipation are conflicting. A metanalysis by Gomes et al[114] showed that "despite the probiotics' positive effects on certain characteristics of the intestinal habitat, there is still no evidence to recommend it in the treatment of constipation in pediatrics". So, the use of probiotics to treat functional constipation is still investigational.
Necrotizing enterocolitis (NEC) is a significant danger to neonatal life, especially preterm neonates. Prematurity is associated with many risk factors that alter the infant microbiome. These factors include mode of delivery, maternal microbiome diversity, feeding pattern, antibiotic use, environmental exposure to pathogenic and commensal bacteria in the neonatal intensive care unit[115]. Dysbiosis, alterations in the gut microbiome, and low microbial diversity of the preterm neonate significantly correlate with a higher risk and raised rate of complication of necrotizing enterocolitis and, consequently, the development of late-onset sepsis. Low microbiota diversity may provoke pathogenic bacteria overgrowth, a significant risk factor that promotes NEC development[116]. Dobbler et al[117] found powerful domination of Citrobacter koseri and/or Klebsiella pneumoniae, reduced diversity, less Lactobacillus abundance, and an altered microbial-network structure during the first days of life, correlate with NEC risk in preterm infants.
Oral administration of probiotics shows a significant reduction of NEC incidence. However, their safety still needs to be proven as preterm babies have immature immune systems with possible vulnerability even to the commensal bacteria[115]. Probiotic supplementation allows restoration of the normal commensal bacteria with the transition to the beneficial bacteria through enhancement of mucosal barrier function competitive inhibition of the pathogenic bacteria. It induces an anti-inflammatory effect on mucosal signaling[118]. Probiotics upregulate the cytoprotective genes of the gut and down-regulate the pro-inflammatory gene expression. They also enhance butyrate and other SCFAs productions to nourish colonocytes. Some probiotics decrease the pH and lower the oxygen tension in the intestinal lumen, thus inhibiting the growth of pathogenic bacteria, especially Enterobacteriaceae. Other probiotics can support the maturation intestinal barrier and functions and regulate cellular immunity through balancing the Th1:Th2 ratio[119].
Helicobacter pylori (H. pylori) is a flagellated, spiral-shaped, Gram-negative bacillus that colonizes the human gastric mucosa causing gastric mucosa inflammatory response, gastric and/or duodenal ulcers, intestinal metaplasia, or even gastric cancer[120]. H. pylori infection in children differs from the adults in the prevalence, rate of complications, difficulties in diagnosis, and the higher rate of antibiotic resistance. The prevalence of H. pylori in children is higher in developing countries (20%) than in developed countries (0.5%)[121]. H. pylori infection in children increases the risk of gut colonization with Prevotella, Clostridium, Proteobacteria, and Firmicutes compared to children without infection who have more Bacteroides. These changes in the gut microbiota associated with H. pylori infection could be related to the development of chronic gastrointestinal diseases and drug resistance[122]. Eradication of H. pylori has a positive and negative impact on the host. It may restore the gut microbiota with decreased abundance of Bacteroidetes and increase Firmicutes, producing plenty of SCFAs with positive and negative effects[123]. While supplements with probiotic strain can improve infection conditions, it is not enough to eradicate H. pylori infection[124]. Adding probiotics to the traditional triple therapy to eliminate H. pylori increases the chance of successful treatment and decreases the therapy-related side effects compared to treatment without probiotics[125]. The addition of Saccharomyces boulardii to the standard treatment of H. pylori increases the eradication rate and reduces the therapy-related side effects[126]. Addition of Lactobacillus- and Bifidobacterium-containing probiotics such as L. acidophilus, L. casei DN-114001, L. gasseri, Bifidobacterium infantis 2036, and Lactobacillus reuteri Gastrus had the same beneficial effects during H. pylori therapy[127]. Various studies reported that certain probiotic strains could demonstrate an inhibitory activity against H. pylori bacteria. In contrast, other strains can ease the side effects of antibiotic therapy and subsequently improve the H. pylori eradication rate[128].
Coeliac disease (CD) is a life-long chronic autoimmune inflammatory systemic disorder but mainly affects the small intestine due to a deviated immune response to dietary gluten proteins (glutenins and gliadins) in genetically susceptible individuals. Several risk factors increase the risk of CD, including a family history of CD or dermatitis herpetiformis, delivery with caesarean section, type-1 diabetes mellitus, chromosomal abnormalities (Down syndrome or Turner syndrome), Addison's disease, and presence of other autoimmune disorders as autoimmune thyroiditis, or microscopic colitis[129]. As mucosal immune response via IgA secretion is among the first defense lines accountable for neutralizing harmful antigens and pathogens, patients with CD have significantly lower levels of IgA-coated fecal bacteria than in healthy controls. De Palma et al[130] found a significant reduction of the Gram-positive/Gram-negative bacteria ratio in patients with CD than in healthy controls. They also found less predominance of Bifidobacterium, Clostridium histolyticum, Clostridium lituseburense and Faecalibacterium prausnitzii, more abundance of Bacteroides-Prevotella group, and reduced IgA coating the Bacteroides-Prevotella group. Dysbiosis and predominance of the bacteria associated with the development of CD can be a risk factor for CD, either by its direct effects on the mucosal immune responses or by increasing the inflammatory reactions to gluten[131]. Fasano et al[132] found increased Zonulin expression in the intestinal tissues during flaring of celiac disease. Zonulin is a human protein like a toxin derived from Vibrio cholera called Zonula occludens toxin. Both Zonulin and Zonula occludens toxin increase intestinal permeability by decreasing the mucosal epithelium's tight intercellular junction.
Meanwhile, dysbiosis may result in a complication of the strict gluten-free diet, which reduces the beneficial bacteria, especially Bifidobacterium and Lactobacillus, and abundance of gram-negative bacteria such as Bacteroides and Escherichia coli[133]. The gluten-free diet-induced dysbiosis results from excluding crucial dietary carbohydrate resources, the primary resources for the energy required by the beneficial bacteria[134]. Despite being the only available treatment for CD, compliance with the gluten-free diet is complex. Consequently, there is a strong need for alternative therapy. Probiotics could supplement a gluten-free diet in patients refractory to the gluten-free diet. Probiotics can help to support the gluten-free diet through different mechanisms: improving the intestinal barrier function by Lactobacillus rhamnosus containing probiotics[135], anti-inflammatory modulation by Bifidobacterium breve and Bifidobacterium longum[136], and gluten degradation, lysing the proline/glutamine-rich gluten peptides, and reduction of the gluten concentration and toxicity by Lactobacilli strains (L. ruminis, L. Johndoni, L. amylovorus, L. salivaris)[137]. The use of probiotics enriched with Lactobacilli species may relieve the effects of accidental or contaminant gluten exposure by chopping up gluten proteins into smaller portions, not triggering an immune reaction or damaging the patients[138]. However, we need more studies and effort to evaluate probiotics in CD management.
Inflammatory bowel diseases (IBD, Crohn's disease, ulcerative colitis, and unclassified) are a group of chronic, relapsing, and remitting inflammatory diseases of the gastrointestinal tract in a genetically predisposed person due to an aberrant immune response against gut microbiota, causing intestinal damage[139]. About 25% of the patients develop the disease before the age of 20 years, 18% before the age of 10 years, and 4% before the age of 5 years, and still on the rise[140]. Crohn's disease and ulcerative colitis affect the terminal ileum and colon, where there is heavy bacterial colonization. The presence of IgG antibodies and hyperactive presentation of T-lymphocytes in the intestinal mucosa indicates a decrease in the local tolerance mechanisms[141]. In normal situations, the commensal bacteria cannot invade the intestinal mucosal barrier. Even when succussed to pass through it, it is rapidly phagocytosed and eliminated by the mucosal macrophage. Under unusual conditions, these commensal bacteria can cross the mucosal barrier and induce the inflammatory cascade[142]. Fava et al[143] found that patients with IBDs have different microbiota composition than that observed in the healthy controls, with decreased abundance of the healthy commensal (such as Clostridium IXa and IV groups, Bacteroides, Bifidobacteria) and increased the pathogenic bacteria as sulfate-reducing Escherichia coli reaching up to 40% of the dominant bacteria and consequently decreasing the microbiota diversity. The observed dysbiosis coupled with defective innate immunity and reduced bacterial killing ability due to impaired phagocytosis, decreased mucosal IgA and defensins, and over destructive adaptive immunity with ineffective regulatory T cells and antigen-presenting cells initiate the process of the pathogenesis of IBDs[144].
Treatment of pediatric IBDs is one of the fundamental challenges to pediatricians with frequent treatment failure and numerous therapy-associated side effects. Gut microbiota modification is one of the promising therapies for IBDs but is still controversial. Probiotics supplementation can restore the metabolic activity of the intestinal microbiota and modify their relative components by inhibiting the pathogenic bacterial overgrowth, decomposing their antigen, secreting antimicrobial substances, and increasing mucosal IgA. Probiotics also help to improve mucosal barrier function and preserve their integrity by tightening the epithelial junction and stabilizing the intestinal permeability. They also modulate intestinal epithelial and mucosal cells' immune response and induce T-cell apoptosis. Consequently, probiotics regulate the immune response and decrease the production of pro-inflammatory factors[145,146].
However, probiotic treatment for IBDs is still controversial. Vilela et al[147] showed that administering Saccharomyces boulardii helped maintain remission, improve intestinal permeability, and bowel sealing in patients with Crohn's disease. Kato et al[148] and Kruis et al[149] showed that Escherichia coli Nissle1917, Bifidobacterium breve, Bifidobacterium bifidum, and Lactobacillus acidophilus showed a promising effect on in maintaining the remission phase in patients with ulcerative colitis as effective as the standard mesalazine therapy but with high safety and tolerability profiles. However, Bousvaros et al[150] showed that the addition of probiotic Lactobacillus rhamnosus strain GG (LGG) to the standard therapy showed no significant differences compared to placebo in prolonging remission in children with Crohn's disease. Moreover, we need more randomized controlled studies to evaluate the effectiveness of Lactobacillus GG and other probiotic strains in children with IBDs.
The gastrointestinal microbiota plays a crucial role in future lung development and future health status. Perinatal antibiotic use induces highly selective alterations on the resident gut microbiota, leading to precise modifications in susceptibility to TH2- or TH1-/TH17- determined lung inflammatory disorders[151].
The coronavirus disease 2019 (COVID-19) is a real worldwide threat for all individuals of different ages, including children. Despite being mainly a respiratory disease, the gastrointestinal tract is a significant target, especially children. The virus can actively infect the gastrointestinal tract cells and replicate in the epithelium of the small and large intestine, stimulating an excessive immunological reaction in the host[152]. Angiotensin-converting enzyme-2 (ACE2) receptors are highly expressed in the upper esophagus and absorptive enterocytes from the ileum till the colon. The ACE2 receptor is an essential receptor for the virus entry to the cell membrane of host cells, with the interaction between the S protein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This interaction induces a state of an inflammatory cascade that ends with dysbiosis and leaky gut syndrome. The degree of expression of ACE2 throughout the gut is an essential factor that aggravates or alleviates the resulting gut dysbiosis and gastrointestinal leakage[153]. Meanwhile, SARS-CoV-2 infection causes many plasma cells and lymphocytes infiltration. It possibly provokes interstitial edema and the deterioration of the intestinal-blood barrier, causing the spread of endotoxins, viruses, bacteria, and microbial metabolites into the systemic circulation, impairing the host's response to SARS-CoV-2 infection and causing multisystem dysfunction and even septic shock[154].
The resulting dysbiosis lasts for a long time after clearance of SARS-CoV-2 virus from the body, indicating the presence of a more long-long-term harmful effect on the gut microbiome, in the form of reduced beneficial bacteria such as Lactobacillus, Bifidobacterium, and Faecalibacterium prausnitzii and more abundance of Clostridium hathewayi, Clostridium ramosum, Coprobacillus, Candida and Aspergillus. The presence of comorbidities such as diabetes mellitus, hypertension, and old age, and antibiotics, antivirals, antifungal, and steroid use increase dysbiosis severity[155]. Diversity of the gut microbiota and the gut predominance of beneficial bacteria share in determining the course of COVID-19 infection. Restoring the gut microbiota diversity could help improve the severity of the disease. Dietary supplements with specialized pre/probiotics such as fructooligosaccharide, galactooligosaccharide could improve gut dysbiosis, especially in patients presenting with gastrointestinal manifestations such as diarrhea and thus improving the overall immune response in these patients[156]. Probiotics can produce bioactive peptides capable of inhibiting the ACE receptors by blocking the active sites, preventing the entry of SARS-CoV-2 from attacking the enterocytes. We can use prebiotics, probiotics, or symbiotics to protect the high-risk groups, such as healthy contacts with a suspected case or the front-line caregivers[157]. However, microbiota modulation as a treatment method of patients with COVID-19 disease is based on indirect evidence and needs further studies.
Cystic fibrosis is an inherited systemic disease that produces severe injury to the lungs, digestive system, and other body organs and might lead to death. The relation between the microbiota and cystic fibrosis is bidirectional. Loss of the function of cystic fibrosis transmembrane conductance regulator results in aberrant colonization of gut and respiratory microbiota due to altered intestinal and airway microenvironment even in the absence of antibiotic use[158,159]. Homozygous cystic fibrosis is associated with more significant changes in the gut microbiome and the severity of the disease. The resulting changes in the gut microbiota associated with cystic fibrosis induce changes in the airway microbiota due to the dynamic interaction between gut and airway microbiota[160].
On the other hand, the gut microbiota could impact the disease severity and progression. Restoration of the gut microbiota in cystic fibrosis either by adding oral probiotics, prebiotics, or even postbiotics by adding certain bacterial strains, indigestible fibers, or SCFAs; namely, butyrate improves the gut and the systemic inflammation, energy intake, nutritional status, and the respiratory function of the patients[161]. Oral probiotic intake, especially with LGG and Lactobacillus reuteri, can reduce inflammation, improve body weight, reduce pulmonary exacerbations, and upper respiratory infections and improve the pulmonary functions in children with cystic fibrosis with mild-to-moderate lung disease. These effects are related to the probiotics' anti-inflammatory and immunomodulatory properties and their impact on the intestinal barrier[162-164]. However, a recent multicentre study by Bruzzese et al[165] showed that LGG supplementation had no significant effect on the respiratory and nutritional outcomes in a large group of children with cystic fibrosis. This study's failure to show a beneficial effect for LGG supplementation could be related to the lower dose of probiotics. They used 109 colony-forming units instead of 1010 in the previous two studies. Meanwhile, we remain in need of more studies to confirm these effects.
Allergic rhinitis in children has a significant impact on the child's health with many comorbidities, impaired quality of life, and poor educational performance. It may progress to asthma or complicate the control of existing asthma[166]. Many factors that cause decreased microbial diversity (e.g., delivery by caesarean section) are associated with an increased risk of allergic rhinitis and other atopic diseases such as atopic dermatitis and asthma. Bisgaard et al[167] found that the bacterial diversity in the early gut microbiota at one and twelve months after birth was negatively associated with the increased risk of allergic sensitization, peripheral blood eosinophilia, and allergic rhinitis. As the gut microbiome shows significant development during the first year of life, it is highly susceptible to disruption during that time. Early antibiotic use has a significant adverse effect on the gut microbiota by modifying the relative abundance of the bacterial composition and initiating dysbiosis, with increasing the risk for childhood allergic diseases[168].
Oral Probiotic supplementation can alter the gut microbiota in children with notable positive immunomodulatory effects help prevention of allergic diseases, including allergic rhinitis. Lin et al[169] examined the effects of Lactobacillus paracasei supplementation on the treatment of perennial allergic rhinitis in children between 6-13 years. They found significant improvement in individual parameters in the rhino-conjunctivitis quality of life questionnaires, including sneezing, nasal itching, and swollen puffy eyes in the supplemented group, but without significant effects on total symptom score and the nasal total symptoms score. Miraglia Del Giudice et al[170] found that children supplemented with probiotic Bifidobacteria mixture for four weeks achieved a significant improvement of allergic rhinitis symptoms than the control without probiotic supplementation. A metanalysis by Güvenç et al[171] showed the evident beneficial immunologic and clinical effects of probiotics, especially for Lactobacillus paracasei-33 strains in managing patients with allergic rhinitis despite the high heterogeneity among the included studies. However, despite the beneficial effects of probiotics in improving allergic rhinitis symptoms and the patient quality of life, there is limited evidence for the primary preventive role of probiotics supplementations in children with a high risk of allergic rhinitis[172,173].
Asthma is a prevalent childhood disease. More than 300 million children and adults are affected by asthma worldwide. The development of asthma is multifactorial and is affected by environmental and other exogenous factors and genetic predisposition. Shaping the lung microbiota, especially during birth and very early life, plays a crucial role in asthma development. Arrieta et al[174] found a significant decrease in the relative abundance of the bacterial genera Rothia, Veillonella, Lachnospira, and Faecalibacterium, in children at risk of asthma. The noticed abundance of these bacteria decreases the fecal acetate levels and consequently induces dysregulation of enterohepatic metabolites. In the same context, Abrahamsson et al[175] showed that children who developed asthma at the age of 7 years had a reduced total and gut microbial diversity in the first month of life than healthy children.
On the other hand, more abundance of the good bacteria as Bifidobacterium longum and less quantity of bacteroid fragilis in the gut microbiota early in life reduces the risk of asthma[176]. The recent decrease in pediatric asthma incidence, noted in some parts of Europe and North America, could be related to judicious antibiotic use during early infancy and childhood that preserve the gut microbiota community[177]. The use of oral probiotics, prebiotics, or synbiotics (combination of pro and prebiotics) could modify the airway microbiota directly through microaspiration of the probiotic strain from the gastrointestinal tract to the airway or indirectly through their metabolic products[178]. Probiotics might generate local effects, such as reducing mucosal permeability and thus decreasing systemic antigens penetration, enhancing local IgA production, and tolerance induction. Their systemic anti-inflammatory effects are mediated through Toll-like receptors, stimulating Th1 response to allergens, enhancing tolerogenic dendritic cells, and the production of Treg[179]. Probiotic supplementation could restore the airway microbiota dysbiosis, promoting the healthy microbiota, which could modify the course of the pulmonary disorders. However, there are not enough studies concerned with the effects of probiotic supplementation on childhood asthma. A systemic review by Lin et al[180] failed to confirm the beneficial role of probiotic supplementation on the disease course in children with bronchial asthma.
Atopic dermatitis (AD) is a common chronic, recurrent inflammatory skin disease in children, affecting about 20% worldwide and on the rise, especially in developed countries. Skin microbiota can reflect general human health. The quantitative and qualitative skin and gut microbiota composition alteration can trigger various diseases, including allergic dermatoses[181]. The skin microbiota of children with AD shows significant dysbiosis, with reduced microbial diversity and more abundance of pathogenic Staphylococcus aureus and Malassezia[182]. Melli et al[183] showed an association between the presence of Clostridioides difficile, more quantity of Bifidobacteria, and a lower abundance of lactobacilli in the gut microbiota of children with atopic dermatitis. Bacterial strains such as Staphylococcus epidermidis, Staphylococcus cohnii, Gram-negative Roseomonas mucosa, and Cutibacterium strains that inhibit Staphylococcus aureus can serve as potential probiotics in children with atopic dermatitis[184]. Myles et al[185] showed that the local application of Roseomonas mucosa to the skin of 10 adults and five children with atopic dermatitis was associated with significant improvement of atopic dermatitis severity, a decrease in topical steroid requirement, and Staphylococcus aureus burden with no adverse events or treatment complications. Probiotics can decrease the severity and progression of atopic dermatitis by reducing inflammation through modulating T-cell immune response and improving the Th1/Th2 ratio; inhibiting Th2 cell response, and decreasing cytokines production such as IL-4, IL-5, IL-6, IL-13, and INF, enhance phagocytosis, increase serum IgA is increased[186]. Probiotics also inhibit the differentiation of mature dendritic cells and naive T cells' transformation into Th2[158]. Probiotics also can regulate brain function involving stress response on the gut-brain axis[187].
Psoriasis is a chronic, complex, immune-mediated, inflammatory disease characterized by keratinocytes hyperproliferation. Unlike atopic dermatitis, patients with psoriasis have more bacterial diversity and heterogeneity with increased Staphylococcus aureus and decreased Staphylococcus epidermidis and Propionibacterium acnes and reduced microbiota stability than in healthy controls. Staphylococcus aureus colonization of the skin triggers Th17-induced inflammation with impaired community stability and accumulation of pathogenic bacteria[188]. The bacterial dysbiosis in psoriasis shows topographic changes. An exciting study by Fahlén et al[189] showed a significant decrease in the ratio of Staphylococci and Propionibacteria in psoriasis limb skin and enriched Proteobacteria in the trunk skin in patients with psoriasis than in controls. Gut dysbiosis also plays a significant role in psoriasis. There is a decrease in gut Bifidobacterium and Firmicutes and an increase in Bacteroidetes in patients with psoriasis than in healthy children. This gut dysbiosis also correlates with the severity of the disease[190]. Probiotics use in the treatment of psoriasis is promising via immune modifying response through restoring the gut microbiome. Vijayashankar et al[191] described the successful use of oral Lactobacillus strain with biotin to treat pustular psoriasis. However, a systematic review and meta-analysis by Zeng et al[192] showed that prebiotics might positively impact relieving the clinical symptoms of psoriasis with a low incidence of side effects. The probiotics exert their effects through their immunomodulatory effect on the skin and repair the skin barrier by decreasing the bacterial load and restoring the skin microbiota.
The relation between adaptive immunity and gut microbiota is well documented. Systemic lupus erythematosus (SLE) is a chronic systemic severe autoimmune disease that affects connective tissues. Pathogenesis of SLE results from the interaction between genetic and environmental factors[193]. Gut microbiota dysbiosis with disturbed composition and activity plays a role in many autoimmune diseases, including SLE and rheumatoid arthritis. A study done by Ma et al[194] found that the fecal microbiota of SLE mice had lesser community abundance and diversity than healthy mice. They can also induce anti-double-stranded DNA (anti-dsDNA) antibodies production in germ-free mice, promote the inflammatory response resembling SLE inflammation, and modify the SLE susceptibility genes expression in these mice by fecal microbiota transplantation. Another interesting experimental study by Ma et al[195] performed fecal microbiota transplantation from healthy controls and patients with active untreated SLE to germ-free mice. The Germ-free mice developed a series of lupus-like phenotypic and laboratory features that confirm the contributing role of abnormal gut microbiota in promoting SLE development.
On the other hand, Toral et al[196] found that Lactobacillus fermentum CECT5716 (LC40) ameliorates disease activity and cardiovascular complications in female mice models by improving gut barrier integrity. At the same time, de la Visitación et al[197] showed that Lactobacillus fermentum CECT5716 (LC40) prevented renal damage in a female mouse model of SLE. Long-standing use of probiotics is supposed to counteract the imbalance in the gut microbiota that causes reduced antibody production and attenuated inflammatory response, resulting in reduced severity and improving the signs and the manifestation of SLE[198]. However, we need more human-based studies on patients with SLE, as most animal-based studies confirmed the potential beneficial role for oral probiotics intake, which can alter the gut microbiome's composition and prevent SLE progression.
Gut dysbiosis is also a potential pathogenic factor for developing juvenile idiopathic arthritis (JIA). Wu et al[199] were able to induce autoimmune arthritis in mice using segmented filamentous bacteria, which could elaborate the potential role of the microbiota and development of autoimmune arthritis. On the other hand, some degree of intestinal inflammation is observed in about two-thirds of children with spondyloarthritis arthritis which may indicate that gut microbiota in children with spondyloarthritis is both modified and unusually affected by the deviated immune system[200]. Meanwhile, Stoll et al[201] found less abundance of Faecalibacterium Prausnitzii and a more abundance of Bifidobacterium, mostly B. adolescentis, in children with enthesitis-related arthritis than in healthy control. However, Öman et al[202] found no significant variations in microbiota α-diversity or composition between children with JIA, their healthy siblings, or unrelated healthy controls. Trials to modify gut microbiota using probiotics, exclusive enteral nutrition, or other modalities have variable success. Esmaeili et al[203] found no significant differences in the improvement criteria of rheumatoid arthritis in patients supplemented with synbiotic (500 mg capsule containing a prebiotic (fructooligosaccharides) and probiotics including 109 CFU/mL of Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus rhamnosus, Bifidobacterium breve, Streptococcus thermophile, and Bifidobacterium longum,) for three months and placebo groups. They suggested that lack of response is probably related to the short duration of the treatment, but we think that the dose also was suboptimal. However, we need more studies with different probiotic strains and concentrations.
Dental caries is a common pediatric disorder, especially in children with special needs. Understanding the association between specific bacterial strains in dental biofilms and different health conditions is crucial to preventing and combating dental caries. Richard et al[204] found that the microbiomes of supragingival dental plaque vary considerably between tooth surfaces and in children with different caries activities. Qudeimat et al[205] found that children with active caries have a significantly higher abundance of Prevotella melaninogenica, Leptotrichia shahii, Leptotrichia HOT 498, Veillonella dispar, and Streptococcus mutans. In comparison, children without active caries had a significantly higher abundance of Lautropia mirabilis, Corynebacterium durum, Corynebacterium matruchotii, and Neisseria elongata. Kanasi et al[206] also confirmed the presence of diverse microbiota that varied in children with severe caries from caries-free children. Probiotics might be helpful to inhibit or treat dental caries, periodontitis, or gingivitis. Certain probiotic bacterial strains have variable effects on the gut microbiome. Each probiotic bacterial strain has specific abilities to inhibit the growth of particular strains, particularly cariogenic bacterial strains and yeast[207].
Probiotic dairy products have a naturally occurring buffer to acid. When combined with calcium and calcium lactate effects, it produces anti-cariogenic properties that benefit the oral cavity. In the short term, probiotic products can hamper the development of harmful strains, but the long-term effects have not been thoroughly studied. Lee et al[208] showed that Lactobacillus species strongly inhibited the growth of oral streptococci. They also showed that Lactobacillus rhamnosus might inhibit oral biofilm formation by decreasing the glucan production of Streptococcus mutans. Systematic review and meta-analysis by Gruner et al[209] showed insufficient current evidence to recommend probiotics in managing dental caries but support using probiotics to manage gingivitis or periodontitis. Future studies are needed to confirm the role of probiotics in the management of dental caries.
Recent evidence revealed that modifications of the gut microbiota composition and function could accelerate the progression of CVDs. The gut microbiota has a crucial effect in inducing inflammatory and immune responses that could link the gut microflora to heart failure. The gut microbiota of patients with chronic heart failure has more pathogenic bacteria such as Campylobacter and Shigella and more candida than the healthy controls. The ratios of these pathogenic bacteria and candida positively correlated with the severity of heart failure[210]. Increased intestinal permeability is observed in a significant portion of patients with congestive or right-sided heart failure, which correlates with right atrial pressure[211]. This observation could explain the increased serum endotoxin levels in patients with chronic heart failure. Reduced cardiac output and systemic congestion observed in heart failure cause intestinal mucosal ischemia and/or edema, which increases the bacterial translocation and the circulating endotoxins that can promote the underlying inflammation observed in patients with heart failure[212]. These mucosal changes also cause enhanced bacterial growth, which is reflected by the increase in serum levels of immunoglobulin A-antilipopolysaccharide[213]. In heart failure, there is a decreased gut microbiota diversity with an increased ratio of the pathogenic bacteria such as Shigella, Campylobacter, Yersinia enterocolitica, Salmonella, and Candida species[214]. Luedde et al[215] also observed significant downregulation of key intestinal bacterial groups such as Coriobacteriaceae, Erysipelotrichaceae, and Ruminococcaceae.
Probiotics may have significant beneficial effects on cardiovascular health. The effects are strain-specific and target specific cardiovascular risk factors. For example, Lactobacillus rhamosus can significantly reduce body weight while Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium bifidum can dramatically lower blood glucose levels by 38% in patients with DM type II. At the same time, Lactobacillus acidophilus and Bifidobacterium lactis Bb12 significantly lower fasting blood glucose, hemoglobin A1c, and malondialdehyde and raise erythrocyte glutathione peroxidase and superoxide dismutase activities and improve total antioxidant states. Meanwhile, Lactobacillus acidophilus, Lactobacillus reuteri, and Bifidobacterium longum improve dyslipidemia, increase high-density lipoprotein (HDL) cholesterol level, and reduce low-density lipoprotein (LDL)/HDL cholesterol ratio. Lactobacillus curvatus and Lactobacillus Plantarum increase apolipoprotein AV and LDL particles size. Streptococcus thermophiles can significantly decrease systolic blood pressure[92,216-219].
Probiotics may have a role in managing heart failure, primarily those containing Bifidobacteria, yeasts, and lactic acid-producing bacteria, as they can reduce inflammation, repair, protect the intestinal mucosal barrier, and improve its function[220]. Gan et al[221] showed that six weeks of supplementation with Lactobacillus rhamnosus GR-1-containing probiotic could significantly improve left ventricular hypertrophy and increase its ejection fraction in rates with induced myocardial infarction due to coronary artery occlusion. Animal studies also showed that probiotics could decrease myocardial cell apoptosis and alleviate ventricular remodeling in rat models of spontaneous hypertension[222]. There are very few studies of the effects of probiotics in human patients with heart failure. Costanza et al[223] studied the impact of three months of supplementation with S. boulardii (1000 mg per day) on outpatients with heart failure with NYHA class II or III and left ventricular ejection fraction (LVEF) < 50%. The supplemented patients showed a significant reduction of left atrium diameter and improvement of LVEF compared to the patients supplemented with placebo. Children with heart failure may have underlying cardiac conditions that increase the risk of infective endocarditis. Probiotics in such patients are not entirely safe, and there is a risk of bacterial translocation with a possible occurrence of sepsis and infective endocarditis. Their safety in such vulnerable patients requires additional studies.
Despite probiotics being part of the body's good microbiota and are safe in most cases, there are some limitations to their use. The side effects of probiotics lie in four categories: excessive immune stimulation, adverse metabolic activities, generalized infections, and gene transfer[224]. They may occasionally trigger allergic reactions, especially with Saccharomyces boulardii in those with a history of yeast allergy, and should be used judiciously. Abdominal discomfort could happen in the first few days of therapy, occasionally with diarrhea and bloating. Probiotics are safe to be used by children, especially those containing Bifidobacteria or Lactobacillus, which can be used for up to one year without any safety issues[225]. Bacteriemia and infective endocarditis have been recorded in a few patients taking probiotics containing Bifidobacteria or Lactobacillus probiotics, especially among patients with central lines or impaired immunity, e.g., who are suffering from tuberculosis or acquired immune deficiency syndrome[226]. Probiotics containing Lactobacilli can cause mural and valvular infective endocarditis. Although this is extremely rare, patients with valvular heart disease may be more prone to this complication. Before dental or surgical procedures, patients with valvular or congenital heart disease with high-pressure shunt should discontinue probiotic use[227]. Some probiotics may transmit antibiotic resistance genes, such as enterococci. Other probiotic strains such as the Bacillus cereus group can produce enterotoxins and emetic toxins[228]. Another limitation is the lack of international regulatory measures that control probiotics production and prescription. Another limitation is the need to elucidate the mechanism of action of each probiotic, the ideal strain and its effect for each medical condition, and which health benefits can be gained[229]. We need to do more clinical and mechanistic studies to understand better the interaction between the microbes and host cells, including the mucus and immune defenses, and produce effective interventions.
There is an intimate relationship between the human and his body microbes. The gut is the primary residence for this microbiota, as it provides the bacteria with a convenient environment for thriving. The microbiota plays a significant role in gut development, maturation, and immune system differentiation. It exerts a considerable effect on the child's physical and mental development. Gut dysbiosis is also a potential pathogenic factor for developing various childhood disorders inside and outside the gastrointestinal tract. Probiotics may have a role in managing these disorders with variable degrees. Even though probiotics could help address these disorders, we need more studies to prove the efficacy, select the proper probiotic for each disease, the appropriate dose, and ensure its safety.
We thank the anonymous referees for their valuable suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liao JX, China; Liu Z, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823-1836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1710] [Cited by in F6Publishing: 1525] [Article Influence: 217.9] [Reference Citation Analysis (2)] |
| 2. | Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70 Suppl 1:S38-S44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 524] [Cited by in F6Publishing: 575] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 3. | Collado MC, Segata N. Initial exploration of in utero microbial colonization. Nat Med. 2020;26:469-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Silverstein RB, Mysorekar IU. Group therapy on in utero colonization: seeking common truths and a way forward. Microbiome. 2021;9:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Ihekweazu FD, Versalovic J. Development of the Pediatric Gut Microbiome: Impact on Health and Disease. Am J Med Sci. 2018;356:413-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, Paliy O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol. 2011;77:404-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 8. | Engevik MA, Versalovic J. Biochemical Features of Beneficial Microbes: Foundations for Therapeutic Microbiology. Microbiol Spectr. 2017;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325-2340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2408] [Cited by in F6Publishing: 2684] [Article Influence: 244.0] [Reference Citation Analysis (2)] |
| 10. | Russo E, Giudici F, Fiorindi C, Ficari F, Scaringi S, Amedei A. Immunomodulating Activity and Therapeutic Effects of Short Chain Fatty Acids and Tryptophan Post-biotics in Inflammatory Bowel Disease. Front Immunol. 2019;10:2754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 11. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1566] [Cited by in F6Publishing: 1805] [Article Influence: 225.6] [Reference Citation Analysis (0)] |
| 12. | Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 993] [Cited by in F6Publishing: 1253] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 13. | Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 839] [Cited by in F6Publishing: 949] [Article Influence: 158.2] [Reference Citation Analysis (0)] |
| 14. | Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 945] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 15. | Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 16. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104 Suppl 2:S1-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1349] [Cited by in F6Publishing: 1226] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 18. | Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska H, Vinderola G. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18:649-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 544] [Article Influence: 181.3] [Reference Citation Analysis (1)] |
| 19. | Kato K, Ishida S, Tanaka M, Mitsuyama E, Xiao JZ, Odamaki T. Association between functional lactase variants and a high abundance of Bifidobacterium in the gut of healthy Japanese people. PLoS One. 2018;13:e0206189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen FA, Rühlemann MC, Szymczak S, Holm K, Esko T, Sun J, Pricop-Jeckstadt M, Al-Dury S, Bohov P, Bethune J, Sommer F, Ellinghaus D, Berge RK, Hübenthal M, Koch M, Schwarz K, Rimbach G, Hübbe P, Pan WH, Sheibani-Tezerji R, Häsler R, Rosenstiel P, D'Amato M, Cloppenborg-Schmidt K, Künzel S, Laudes M, Marschall HU, Lieb W, Nöthlings U, Karlsen TH, Baines JF, Franke A. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48:1396-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 432] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 21. | Hughes DA, Bacigalupe R, Wang J, Rühlemann MC, Tito RY, Falony G, Joossens M, Vieira-Silva S, Henckaerts L, Rymenans L, Verspecht C, Ring S, Franke A, Wade KH, Timpson NJ, Raes J. Genome-wide associations of human gut microbiome variation and implications for causal inference analyses. Nat Microbiol. 2020;5:1079-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 22. | Martinez-Guryn K, Hubert N, Frazier K, Urlass S, Musch MW, Ojeda P, Pierre JF, Miyoshi J, Sontag TJ, Cham CM, Reardon CA, Leone V, Chang EB. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe. 2018;23:458-469.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 334] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 23. | Kastl AJ Jr, Terry NA, Wu GD, Albenberg LG. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell Mol Gastroenterol Hepatol. 2020;9:33-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 24. | Reyman M, van Houten MA, van Baarle D, Bosch AATM, Man WH, Chu MLJN, Arp K, Watson RL, Sanders EAM, Fuentes S, Bogaert D. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10:4997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 25. | Ma J, Li Z, Zhang W, Zhang C, Zhang Y, Mei H, Zhuo N, Wang H, Wang L, Wu D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci Rep. 2020;10:15792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 26. | Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, Earls JC, Zimmer A, Glusman G, Robinson M, Yurkovich JT, Kado DM, Cauley JA, Zmuda J, Lane NE, Magis AT, Lovejoy JC, Hood L, Gibbons SM, Orwoll ES, Price ND. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3:274-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 225] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 27. | Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 780] [Cited by in F6Publishing: 756] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 28. | Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major Disruptors of Gut Microbiota. Front Cell Infect Microbiol. 2020;10:572912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 278] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 29. | Rakoff-Nahoum S, Foster KR, Comstock LE. The evolution of cooperation within the gut microbiota. Nature. 2016;533:255-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 350] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 30. | García-Bayona L, Comstock LE. Bacterial antagonism in host-associated microbial communities. Science. 2018;361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 31. | de Wouters T, Jans C, Niederberger T, Fischer P, Rühs PA. Adhesion Potential of Intestinal Microbes Predicted by Physico-Chemical Characterization Methods. PLoS One. 2015;10:e0136437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018;6:133-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 603] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 33. | Osadchiy V, Martin CR, Mayer EA. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol. 2019;17:322-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 34. | Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 774] [Cited by in F6Publishing: 831] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 35. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 812] [Article Influence: 203.0] [Reference Citation Analysis (0)] |
| 36. | Yang X, Lu D, Zhuo J, Lin Z, Yang M, Xu X. The Gut-liver Axis in Immune Remodeling: New insight into Liver Diseases. Int J Biol Sci. 2020;16:2357-2366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Wu X, Tian Z. Gut-liver axis: gut microbiota in shaping hepatic innate immunity. Sci China Life Sci. 2017;60:1191-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Forkosh E, Ilan Y. The heart-gut axis: new target for atherosclerosis and congestive heart failure therapy. Open Heart. 2019;6:e000993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 39. | Sata Y, Marques FZ, Kaye DM. The Emerging Role of Gut Dysbiosis in Cardio-metabolic Risk Factors for Heart Failure. Curr Hypertens Rep. 2020;22:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Ahmad AF, Ward NC, Dwivedi G. The gut microbiome and heart failure. Curr Opin Cardiol. 2019;34:225-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14:442-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 364] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 42. | Foresto-Neto O, Ghirotto B, Câmara NOS. Renal sensing of bacterial metabolites in the gut-kidney axis. Kidney360. 2021;2:1501-1509. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant. 2015;30:924-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 44. | Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 45. | Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, Delhaes L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front Cell Infect Microbiol. 2020;10:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 343] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 46. | Wilson MT, Hamilos DL. The nasal and sinus microbiome in health and disease. Curr Allergy Asthma Rep. 2014;14:485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Chiu L, Bazin T, Truchetet ME, Schaeverbeke T, Delhaes L, Pradeu T. Protective Microbiota: From Localized to Long-Reaching Co-Immunity. Front Immunol. 2017;8:1678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 48. | Salem I, Ramser A, Isham N, Ghannoum MA. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front Microbiol. 2018;9:1459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 49. | Jones CJ, Brinton LA, Hamman RF, Stolley PD, Lehman HF, Levine RS, Mallin K. Risk factors for in situ cervical cancer: results from a case-control study. Cancer Res. 1990;50:3657-3662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 50. | Hacini-Rachinel F, Gheit H, Le Luduec JB, Dif F, Nancey S, Kaiserlian D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS One. 2009;4:e4903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Harkness S. A cultural model for the acquisition of language: implications for the innateness debate. Dev Psychobiol. 1990;23:727-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1438] [Cited by in F6Publishing: 1242] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 52. | Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2013;18:1106-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 53. | Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv Nutr. 2020;11:709-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 278] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 54. | Evrensel A, Ceylan ME. The Gut-Brain Axis: The Missing Link in Depression. Clin Psychopharmacol Neurosci. 2015;13:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 55. | Huang R, Wang K, Hu J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2016;8:483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 267] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 56. | Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1623] [Cited by in F6Publishing: 1967] [Article Influence: 218.6] [Reference Citation Analysis (0)] |
| 57. | Majnik AV, Lane RH. The relationship between early-life environment, the epigenome and the microbiota. Epigenomics. 2015;7:1173-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Harry GJ. Microglia during development and aging. Pharmacol Ther. 2013;139:313-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 324] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 59. | Tamana SK, Tun HM, Konya T, Chari RS, Field CJ, Guttman DS, Becker AB, Moraes TJ, Turvey SE, Subbarao P, Sears MR, Pei J, Scott JA, Mandhane PJ, Kozyrskyj AL. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes. 2021;13:1-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 60. | Zhang S, Qian Y, Li Q, Xu X, Li X, Wang C, Cai H, Zhu J, Yu Y. Metabolic and Neural Mechanisms Underlying the Associations Between Gut Bacteroides and Cognition: A Large-Scale Functional Network Connectivity Study. Front Neurosci. 2021;15:750704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Cryan JF, Dinan TG. More than a gut feeling: the microbiota regulates neurodevelopment and behavior. Neuropsychopharmacology. 2015;40:241-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 71] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 62. | Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA 3rd. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 637] [Cited by in F6Publishing: 643] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 63. | Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, Williams KC. Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cell Mol Gastroenterol Hepatol. 2017;3:218-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 64. | Li N, Yang J, Zhang J, Liang C, Wang Y, Chen B, Zhao C, Wang J, Zhang G, Zhao D, Liu Y, Zhang L, Li G, Gai Z, Zhao G. Correlation of Gut Microbiome Between ASD Children and Mothers and Potential Biomarkers for Risk Assessment. Genomics Proteomics Bioinformatics. 2019;17:26-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 65. | Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM, Collins MD, Lawson PA, Summanen P, Baysallar M, Tomzynski TJ, Read E, Johnson E, Rolfe R, Nasir P, Shah H, Haake DA, Manning P, Kaul A. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6-S16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 493] [Cited by in F6Publishing: 457] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 66. | Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML, Nelson MN, Wexler HM. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 375] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 67. | Bezawada N, Phang TH, Hold GL, Hansen R. Autism Spectrum Disorder and the Gut Microbiota in Children: A Systematic Review. Ann Nutr Metab. 2020;76:16-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Al-Beltagi M. Autism medical comorbidities. World J Clin Pediatr. 2021;10:15-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 59] [Cited by in F6Publishing: 63] [Article Influence: 21.0] [Reference Citation Analysis (9)] |
| 69. | Huang TS, Lu XG, Li B, Chen Y, Wen JL, Hu Y, Chen L, Xiao YH, Zhang J, Liao JX. [Benign infantile convulsions with mild gastroenteritis: clinical analysis of 40 cases]. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:533-535. [PubMed] [Cited in This Article: ] |
| 70. | Şafak B, Altunan B, Topçu B, Eren Topkaya A. The gut microbiome in epilepsy. Microb Pathog. 2020;139:103853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 71. | Kitamura S, Sugihara K, Kuwasako M, Tatsumi K. The role of mammalian intestinal bacteria in the reductive metabolism of zonisamide. J Pharm Pharmacol. 1997;49:253-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Dahlin M, Prast-Nielsen S. The gut microbiome and epilepsy. EBioMedicine. 2019;44:741-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 73. | Gómez-Eguílaz M, Ramón-Trapero JL, Pérez-Martínez L, Blanco JR. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef Microbes. 2018;9:875-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 74. | Yue Q, Cai M, Xiao B, Zhan Q, Zeng C. The Microbiota-Gut-Brain Axis and Epilepsy. Cell Mol Neurobiol. 2022;42:439-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 75. | Sato Y, Sakurai K, Tanabe H, Kato T, Nakanishi Y, Ohno H, Mori C. Maternal gut microbiota is associated with newborn anthropometrics in a sex-specific manner. J Dev Orig Health Dis. 2019;10:659-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 76. | Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The Human Microbiome and Child Growth - First 1000 Days and Beyond. Trends Microbiol. 2019;27:131-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 77. | Li H, He Z, Gao D, Lv Y, Zhou Q, Xiao B, Huang W. Characteristics of the Intestinal Microbiota in Very Low Birth Weight Infants With Extrauterine Growth Restriction. Front Pediatr. 2019;7:99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Becker-Dreps S, Allali I, Monteagudo A, Vilchez S, Hudgens MG, Rogawski ET, Carroll IM, Zambrana LE, Espinoza F, Azcarate-Peril MA. Gut Microbiome Composition in Young Nicaraguan Children During Diarrhea Episodes and Recovery. Am J Trop Med Hyg. 2015;93:1187-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Salvucci E. The human-microbiome superorganism and its modulation to restore health. Int J Food Sci Nutr. 2019;70:781-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 80. | Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA Jr, Ahmed T, Gordon JI. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 807] [Cited by in F6Publishing: 803] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 81. | Onubi OJ, Poobalan AS, Dineen B, Marais D, McNeill G. Effects of probiotics on child growth: a systematic review. J Health Popul Nutr. 2015;34:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Tidjani Alou M, Million M, Traore SI, Mouelhi D, Khelaifia S, Bachar D, Caputo A, Delerce J, Brah S, Alhousseini D, Sokhna C, Robert C, Diallo BA, Diallo A, Parola P, Golden M, Lagier JC, Raoult D. Gut Bacteria Missing in Severe Acute Malnutrition, Can We Identify Potential Probiotics by Culturomics? Front Microbiol. 2017;8:899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 83. | Pop M, Walker AW, Paulson J, Lindsay B, Antonio M, Hossain MA, Oundo J, Tamboura B, Mai V, Astrovskaya I, Corrada Bravo H, Rance R, Stares M, Levine MM, Panchalingam S, Kotloff K, Ikumapayi UN, Ebruke C, Adeyemi M, Ahmed D, Ahmed F, Alam MT, Amin R, Siddiqui S, Ochieng JB, Ouma E, Juma J, Mailu E, Omore R, Morris JG, Breiman RF, Saha D, Parkhill J, Nataro JP, Stine OC. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol. 2014;15:R76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 84. | Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut--a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 85. | Bandsma RHJ, Sadiq K, Bhutta ZA. Persistent diarrhoea: current knowledge and novel concepts. Paediatr Int Child Health. 2019;39:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Korpe PS, Petri WA Jr. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 319] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 87. | Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004;39:153-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, Berry D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017;19:95-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 89. | Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 547] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 90. | Jian C, Carpén N, Helve O, de Vos WM, Korpela K, Salonen A. Early-life gut microbiota and its connection to metabolic health in children: Perspective on ecological drivers and need for quantitative approach. EBioMedicine. 2021;69:103475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 91. | Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7:91-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 92. | Sanchez M, Panahi S, Tremblay A. Childhood obesity: a role for gut microbiota? Int J Environ Res Public Health. 2014;12:162-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 93. | Korpela K, Renko M, Vänni P, Paalanne N, Salo J, Tejesvi MV, Koivusaari P, Ojaniemi M, Pokka T, Kaukola T, Pirttilä AM, Tapiainen T. Microbiome of the first stool and overweight at age 3 years: A prospective cohort study. Pediatr Obes. 2020;15:e12680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33:2277-2284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 422] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 95. | Luoto R, Kalliomäki M, Laitinen K, Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int J Obes (Lond). 2010;34:1531-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 96. | Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103:1792-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 97. | Guo R, Chen LH, Xing C, Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth. 2019;123:637-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 98. | Abrahamsson TR, Wu RY, Sherman PM. Microbiota in Functional Gastrointestinal Disorders in Infancy: Implications for Management. Nestle Nutr Inst Workshop Ser. 2017;88:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 99. | Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6:39-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 533] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 100. | Mai T, Fatheree NY, Gleason W, Liu Y, Rhoads JM. Infantile Colic: New Insights into an Old Problem. Gastroenterol Clin North Am. 2018;47:829-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 101. | Dubois NE, Gregory KE. Characterizing the Intestinal Microbiome in Infantile Colic: Findings Based on an Integrative Review of the Literature. Biol Res Nurs. 2016;18:307-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Sung V, D'Amico F, Cabana MD, Chau K, Koren G, Savino F, Szajewska H, Deshpande G, Dupont C, Indrio F, Mentula S, Partty A, Tancredi D. Lactobacillus reuteri to Treat Infant Colic: A Meta-analysis. Pediatrics. 2018;141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 103. | Chichlowski M, German JB, Lebrilla CB, Mills DA. The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annu Rev Food Sci Technol. 2011;2:331-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 104. | Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1150] [Cited by in F6Publishing: 1046] [Article Influence: 58.1] [Reference Citation Analysis (6)] |
| 105. | Choung RS, Locke GR 3rd. Epidemiology of IBS. Gastroenterol Clin North Am. 2011;40:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 106. | Thapar N, Benninga MA, Crowell MD, Di Lorenzo C, Mack I, Nurko S, Saps M, Shulman RJ, Szajewska H, van Tilburg MAL, Enck P. Paediatric functional abdominal pain disorders. Nat Rev Dis Primers. 2020;6:89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 107. | Rigsbee L, Agans R, Shankar V, Kenche H, Khamis HJ, Michail S, Paliy O. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2012;107:1740-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 108. | Chumpitazi BP, Hollister EB, Oezguen N, Tsai CM, McMeans AR, Luna RA, Savidge TC, Versalovic J, Shulman RJ. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014;5:165-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 109. | van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006;101:2401-2409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 390] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 110. | Waller PA, Gopal PK, Leyer GJ, Ouwehand AC, Reifer C, Stewart ME, Miller LE. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46:1057-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 111. | Avelar Rodriguez D, Popov J, Ratcliffe EM, Toro Monjaraz EM. Functional Constipation and the Gut Microbiome in Children: Preclinical and Clinical Evidence. Front Pediatr. 2020;8:595531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 112. | Zhu L, Liu W, Alkhouri R, Baker RD, Bard JE, Quigley EM, Baker SS. Structural changes in the gut microbiome of constipated patients. Physiol Genomics. 2014;46:679-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 113. | Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100:1075-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 114. | Gomes DOVS, Morais MB. Gut microbiota and the use of probiotics in constipation in children and adolescents: systematic review. Rev Paul Pediatr. 2020;38:e2018123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | Baranowski JR, Claud EC. Necrotizing Enterocolitis and the Preterm Infant Microbiome. Adv Exp Med Biol. 2019;1125:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 116. | Zhuang L, Chen H, Zhang S, Zhuang J, Li Q, Feng Z. Intestinal Microbiota in Early Life and Its Implications on Childhood Health. Genomics Proteomics Bioinformatics. 2019;17:13-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 117. | Dobbler PT, Procianoy RS, Mai V, Silveira RC, Corso AL, Rojas BS, Roesch LFW. Low Microbial Diversity and Abnormal Microbial Succession Is Associated with Necrotizing Enterocolitis in Preterm Infants. Front Microbiol. 2017;8:2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 118. | Patel RM, Denning PW. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: what is the current evidence? Clin Perinatol. 2013;40:11-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 119. | Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380:1158-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 120. | Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Semin Pediatr Surg. 2018;27:39-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 121. | Kalach N, Bontems P, Raymond J. Helicobacter pylori infection in children. Helicobacter. 2017;22 Suppl 1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Benavides-Ward A, Vasquez-Achaya F, Silva-Caso W, Aguilar-Luis MA, Mazulis F, Urteaga N, Del Valle-Mendoza J. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res Notes. 2018;11:468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 123. | Yap TW, Gan HM, Lee YP, Leow AH, Azmi AN, Francois F, Perez-Perez GI, Loke MF, Goh KL, Vadivelu J. Helicobacter pylori Eradication Causes Perturbation of the Human Gut Microbiome in Young Adults. PLoS One. 2016;11:e0151893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 124. | Mourad-Baars P, Hussey S, Jones NL. Helicobacter pylori infection and childhood. Helicobacter. 2010;15 Suppl 1:53-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | Losurdo G, Cubisino R, Barone M, Principi M, Leandro G, Ierardi E, Di Leo A. Probiotic monotherapy and Helicobacter pylori eradication: A systematic review with pooled-data analysis. World J Gastroenterol. 2018;24:139-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 65] [Cited by in F6Publishing: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 126. | Szajewska H, Horvath A, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2015;41:1237-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 127. | Emara MH, Mohamed SY, Abdel-Aziz HR. Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Therap Adv Gastroenterol. 2014;7:4-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 128. | Sharma N, Bhatia S, Chunduri V, Kaur S, Sharma S, Kapoor P, Kumari A, Garg M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front Nutr. 2020;7:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 129. | Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol. 2015;21:10644-10653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 71] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (3)] |
| 130. | De Palma G, Nadal I, Medina M, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10:63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 131. | Sjöberg V, Sandström O, Hedberg M, Hammarström S, Hernell O, Hammarström ML. Intestinal T-cell responses in celiac disease - impact of celiac disease associated bacteria. PLoS One. 2013;8:e53414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 132. | Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 392] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 133. | Nadal I, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol. 2007;56:1669-1674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 134. | Sánchez E, De Palma G, Capilla A, Nova E, Pozo T, Castillejo G, Varea V, Marcos A, Garrote JA, Polanco I, López A, Ribes-Koninckx C, García-Novo MD, Calvo C, Ortigosa L, Palau F, Sanz Y. Influence of environmental and genetic factors linked to celiac disease risk on infant gut colonization by Bacteroides species. Appl Environ Microbiol. 2011;77:5316-5323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 135. | De Angelis M, Rizzello CG, Fasano A, Clemente MG, De Simone C, Silano M, De Vincenzi M, Losito I, Gobbetti M. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue. Biochim Biophys Acta. 2006;1762:80-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 136. | Primec M, Mičetić-Turk D, Langerholc T. Analysis of short-chain fatty acids in human feces: A scoping review. Anal Biochem. 2017;526:9-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 137. | Duar RM, Clark KJ, Patil PB, Hernández C, Brüning S, Burkey TE, Madayiputhiya N, Taylor SL, Walter J. Identification and characterization of intestinal lactobacilli strains capable of degrading immunotoxic peptides present in gluten. J Appl Microbiol. 2015;118:515-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 138. | Greco L, Gobbetti M, Auricchio R, Di Mase R, Landolfo F, Paparo F, Di Cagno R, De Angelis M, Rizzello CG, Cassone A, Terrone G, Timpone L, D'Aniello M, Maglio M, Troncone R, Auricchio S. Safety for patients with celiac disease of baked goods made of wheat flour hydrolyzed during food processing. Clin Gastroenterol Hepatol. 2011;9:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 139. | Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120:622-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 140. | Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 383] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 141. | Kelsall BL. Innate and adaptive mechanisms to control [corrected] pathological intestinal inflammation. J Pathol. 2008;214:242-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 142. | Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222-2226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 838] [Cited by in F6Publishing: 779] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 143. | Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol. 2011;17:557-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 203] [Cited by in F6Publishing: 190] [Article Influence: 14.6] [Reference Citation Analysis (2)] |
| 144. | Niess JH. Role of mucosal dendritic cells in inflammatory bowel disease. World J Gastroenterol. 2008;14:5138-5148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 64] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 145. | Salminen S, Benno Y, de Vos W. Intestinal colonisation, microbiota and future probiotics? Asia Pac J Clin Nutr. 2006;15:558-562. [PubMed] [Cited in This Article: ] |
| 146. | Morais MB, Jacob CM. The role of probiotics and prebiotics in pediatric practice. J Pediatr (Rio J). 2006;82:S189-S197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 147. | Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, Guerra Pinto A, Carolina Carneiro Aguirre A, Paiva Martins F, Marcos Andrade Goulart E, Sales Da Cunha A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol. 2008;43:842-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 148. | Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A, Arakawa Y. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20:1133-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 149. | Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617-1623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 851] [Cited by in F6Publishing: 761] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 150. | Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, Goldin B, Hartigan L, Kugathasan S, Levy J, Murray KF, Oliva-Hemker M, Rosh JR, Tolia V, Zholudev A, Vanderhoof JA, Hibberd PL. A randomized, double-blind trial of Lactobacillus GG vs placebo in addition to standard maintenance therapy for children with Crohn's disease. Inflamm Bowel Dis. 2005;11:833-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 151. | Russell SL, Gold MJ, Reynolds LA, Willing BP, Dimitriu P, Thorson L, Redpath SA, Perona-Wright G, Blanchet MR, Mohn WW, Finlay BB, McNagny KM. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol. 2015;135:100-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 152. | McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 153. | Ojetti V, Saviano A, Covino M, Acampora N, Troiani E, Franceschi F; GEMELLI AGAINST COVID‐19 group. COVID-19 and intestinal inflammation: Role of fecal calprotectin. Dig Liver Dis. 2020;52:1231-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 154. | Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 Links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 895] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 155. | Kim S, Rigatto K, Gazzana MB, Knorst MM, Richards EM, Pepine CJ, Raizada MK. Altered Gut Microbiome Profile in Patients With Pulmonary Arterial Hypertension. Hypertension. 2020;75:1063-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 156. | Al-Beltagi M, Saeed NK, Bediwy AS, El-Sawaf Y. Paediatric gastrointestinal disorders in SARS-CoV-2 infection: Epidemiological and clinical implications. World J Gastroenterol. 2021;27:1716-1727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 157. | Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 364] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 158. | Olaimat AN, Aolymat I, Al-Holy M, Ayyash M, Abu Ghoush M, Al-Nabulsi AA, Osaili T, Apostolopoulos V, Liu SQ, Shah NP. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci Food. 2020;4:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 159. | Cox MJ, Allgaier M, Taylor B, Baek MS, Huang YJ, Daly RA, Karaoz U, Andersen GL, Brown R, Fujimura KE, Wu B, Tran D, Koff J, Kleinhenz ME, Nielson D, Brodie EL, Lynch SV. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One. 2010;5:e11044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 327] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 160. | Rogers GB, Carroll MP, Hoffman LR, Walker AW, Fine DA, Bruce KD. Comparing the microbiota of the cystic fibrosis lung and human gut. Gut Microbes. 2010;1:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 161. | Li L, Somerset S. The clinical significance of the gut microbiota in cystic fibrosis and the potential for dietary therapies. Clin Nutr. 2014;33:571-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 162. | Bruzzese E, Raia V, Spagnuolo MI, Volpicelli M, De Marco G, Maiuri L, Guarino A. Effect of Lactobacillus GG supplementation on pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Clin Nutr. 2007;26:322-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 163. | Weiss B, Bujanover Y, Yahav Y, Vilozni D, Fireman E, Efrati O. Probiotic supplementation affects pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Pediatr Pulmonol. 2010;45:536-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 164. | Di Nardo G, Oliva S, Menichella A, Pistelli R, De Biase RV, Patriarchi F, Cucchiara S, Stronati L. Lactobacillus reuteri ATCC55730 in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2014;58:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 165. | Bruzzese E, Raia V, Ruberto E, Scotto R, Giannattasio A, Bruzzese D, Cavicchi MC, Francalanci M, Colombo C, Faelli N, Daccò V, Magazzù G, Costa S, Lucidi V, Majo F, Guarino A. Lack of efficacy of Lactobacillus GG in reducing pulmonary exacerbations and hospital admissions in children with cystic fibrosis: A randomised placebo controlled trial. J Cyst Fibros. 2018;17:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 166. | Schuler Iv CF, Montejo JM. Allergic Rhinitis in Children and Adolescents. Pediatr Clin North Am. 2019;66:981-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 167. | Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, Stokholm J, Smith B, Krogfelt KA. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646-52.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 168. | Ni J, Friedman H, Boyd BC, McGurn A, Babinski P, Markossian T, Dugas LR. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr. 2019;19:225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 169. | Lin WY, Fu LS, Lin HK, Shen CY, Chen YJ. Evaluation of the effect of Lactobacillus paracasei (HF.A00232) in children (6-13 years old) with perennial allergic rhinitis: a 12-week, double-blind, randomized, placebo-controlled study. Pediatr Neonatol. 2014;55:181-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 170. | Miraglia Del Giudice M, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr. 2017;43:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 171. | Güvenç IA, Muluk NB, Mutlu FŞ, Eşki E, Altıntoprak N, Oktemer T, Cingi C. Do probiotics have a role in the treatment of allergic rhinitis? Am J Rhinol Allergy. 2016;30:157-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 172. | Zajac AE, Adams AS, Turner JH. A systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis. Int Forum Allergy Rhinol. 2015;5:524-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 173. | Peng Y, Li A, Yu L, Qin G. The role of probiotics in prevention and treatment for patients with allergic rhinitis: A systematic review. Am J Rhinol Allergy. 2015;29:292-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 174. | Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T; CHILD Study Investigators, Mohn WW, Turvey SE, Finlay BB. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 956] [Cited by in F6Publishing: 1036] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 175. | Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 176. | Vael C, Nelen V, Verhulst SL, Goossens H, Desager KN. Early intestinal Bacteroides fragilis colonisation and development of asthma. BMC Pulm Med. 2008;8:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 177. | Patrick DM, Sbihi H, Dai DLY, Al Mamun A, Rasali D, Rose C, Marra F, Boutin RCT, Petersen C, Stiemsma LT, Winsor GL, Brinkman FSL, Kozyrskyj AL, Azad MB, Becker AB, Mandhane PJ, Moraes TJ, Sears MR, Subbarao P, Finlay BB, Turvey SE. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med. 2020;8:1094-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 178. | Forsythe P. Probiotics and lung diseases. Chest. 2011;139:901-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 179. | Sestito S, D'Auria E, Baldassarre ME, Salvatore S, Tallarico V, Stefanelli E, Tarsitano F, Concolino D, Pensabene L. The Role of Prebiotics and Probiotics in Prevention of Allergic Diseases in Infants. Front Pediatr. 2020;8:583946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 180. | Lin J, Zhang Y, He C, Dai J. Probiotics supplementation in children with asthma: A systematic review and meta-analysis. J Paediatr Child Health. 2018;54:953-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 181. | Murzina E, Kaliuzhna L, Bardova K, Yurchyk Y, Barynova M. Human Skin Microbiota in Various Phases of Atopic Dermatitis. Acta Dermatovenerol Croat. 2019;27:245-249. [PubMed] [Cited in This Article: ] |
| 182. | Koh LF, Ong RY, Common JE. Skin microbiome of atopic dermatitis. Allergol Int. 2022;71:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 183. | Melli LCFL, Carmo-Rodrigues MSD, Araújo-Filho HB, Mello CS, Tahan S, Pignatari ACC, Solé D, Morais MB. Gut microbiota of children with atopic dermatitis: Controlled study in the metropolitan region of São Paulo, Brazil. Allergol Immunopathol (Madr). 2020;48:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 184. | Rusu E, Enache G, Cursaru R, Alexescu A, Radu R, Onila O, Cavallioti T, Rusu F, Posea M, Jinga M, Radulian G. Prebiotics and probiotics in atopic dermatitis. Exp Ther Med. 2019;18:926-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 185. | Myles IA, Earland NJ, Anderson ED, Moore IN, Kieh MD, Williams KW, Saleem A, Fontecilla NM, Welch PA, Darnell DA, Barnhart LA, Sun AA, Uzel G, Datta SK. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 186. | Kim HJ, Kim HY, Lee SY, Seo JH, Lee E, Hong SJ. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 187. | Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 832] [Cited by in F6Publishing: 858] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 188. | Chang HW, Yan D, Singh R, Liu J, Lu X, Ucmak D, Lee K, Afifi L, Fadrosh D, Leech J, Vasquez KS, Lowe MM, Rosenblum MD, Scharschmidt TC, Lynch SV, Liao W. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018;6:154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 189. | Fahlén A, Engstrand L, Baker BS, Powles A, Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 190. | Huang L, Gao R, Yu N, Zhu Y, Ding Y, Qin H. Dysbiosis of gut microbiota was closely associated with psoriasis. Sci China Life Sci. 2019;62:807-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 191. | Vijayashankar M, Raghunath N. Pustular psoriasis responding to Probiotics – a new insight. Our Dermatology Online. 2012;3:326-328. [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 192. | Zeng L, Yu G, Wu Y, Hao W, Chen H. The Effectiveness and Safety of Probiotic Supplements for Psoriasis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Preclinical Trials. J Immunol Res. 2021;2021:7552546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 193. | Picascia A, Grimaldi V, Pignalosa O, De Pascale MR, Schiano C, Napoli C. Epigenetic control of autoimmune diseases: from bench to bedside. Clin Immunol. 2015;157:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 194. | Ma Y, Xu X, Li M, Cai J, Wei Q, Niu H. Gut microbiota promote the inflammatory response in the pathogenesis of systemic lupus erythematosus. Mol Med. 2019;25:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 195. | Ma Y, Guo R, Sun Y, Li X, He L, Li Z, Silverman GJ, Chen G, Gao F, Yuan J, Wei Q, Li M, Lu L, Niu H. Lupus gut microbiota transplants cause autoimmunity and inflammation. Clin Immunol. 2021;233:108892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 196. | Toral M, Robles-Vera I, Romero M, de la Visitación N, Sánchez M, O'Valle F, Rodriguez-Nogales A, Gálvez J, Duarte J, Jiménez R. Lactobacillus fermentum CECT5716: a novel alternative for the prevention of vascular disorders in a mouse model of systemic lupus erythematosus. FASEB J. 2019;33:10005-10018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 197. | de la Visitación N, Robles-Vera I, Toral M, O'Valle F, Moleon J, Gómez-Guzmán M, Romero M, Duarte M, Sánchez M, Jiménez R, Duarte J. Lactobacillus fermentum CECT5716 prevents renal damage in the NZBWF1 mouse model of systemic lupus erythematosus. Food Funct. 2020;11:5266-5274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 198. | Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1157] [Cited by in F6Publishing: 1159] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 199. | Esmaeili SA, Mahmoudi M, Momtazi AA, Sahebkar A, Doulabi H, Rastin M. Tolerogenic probiotics: potential immunoregulators in Systemic Lupus Erythematosus. J Cell Physiol. 2017;232:1994-2007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 200. | Stoll ML. Gut microbes, immunity, and spondyloarthritis. Clin Immunol. 2015;159:134-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 201. | Stoll ML, Kumar R, Morrow CD, Lefkowitz EJ, Cui X, Genin A, Cron RQ, Elson CO. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther. 2014;16:486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 202. | Öman A, Dicksved J, Engstrand L, Berntson L. Fecal Microbiota in Untreated Children With Juvenile Idiopathic Arthritis: A Comparison With Healthy Children and Healthy Siblings. J Rheumatol. 2021;48:1589-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 203. | Esmaeili F, Salesi M, Askari G, Esmaeilisharif A, Maracy M, Karimzadeh H, Shojaie B. Efficacy of synbiotic supplementation in improving rheumatoid arthritis. Res Pharm Sci. 2020;15:263-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 204. | Richards VP, Alvarez AJ, Luce AR, Bedenbaugh M, Mitchell ML, Burne RA, Nascimento MM. Microbiomes of Site-Specific Dental Plaques from Children with Different Caries Status. Infect Immun. 2017;85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 205. | Qudeimat MA, Alyahya A, Karched M, Behbehani J, Salako NO. Dental plaque microbiota profiles of children with caries-free and caries-active dentition. J Dent. 2021;104:103539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 206. | Kanasi E, Dewhirst FE, Chalmers NI, Kent R Jr, Moore A, Hughes CV, Pradhan N, Loo CY, Tanner AC. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44:485-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 207. | Lee SH, Kim YJ. A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch Microbiol. 2014;196:601-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 208. | Jindal G, Pandey RK, Singh RK, Pandey N. Can early exposure to probiotics in children prevent dental caries? J Oral Biol Craniofac Res. 2012;2:110-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 209. | Gruner D, Paris S, Schwendicke F. Probiotics for managing caries and periodontitis: Systematic review and meta-analysis. J Dent. 2016;48:16-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 210. | Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL, Lefer DJ. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ Heart Fail. 2016;9:e002314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 211. | Polsinelli VB, Sinha A, Shah SJ. Visceral Congestion in Heart Failure: Right Ventricular Dysfunction, Splanchnic Hemodynamics, and the Intestinal Microenvironment. Curr Heart Fail Rep. 2017;14:519-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 212. | Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, Kalra PR, Buhner S, Herrmann R, Springer J, Doehner W, von Haehling S, Anker SD, Rauchhaus M. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 213. | Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse-Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64:1092-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 214. | Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, Dioguardi F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016;4:220-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 215. | Luedde M, Winkler T, Heinsen FA, Rühlemann MC, Spehlmann ME, Bajrovic A, Lieb W, Franke A, Ott SJ, Frey N. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017;4:282-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 216. | Jia Q, Li H, Zhou H, Zhang X, Zhang A, Xie Y, Li Y, Lv S, Zhang J. Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure. Cardiovasc Ther. 2019;2019:5164298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 217. | Moroti C, Souza Magri LF, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 218. | Kiessling G, Schneider J, Jahreis G. Long-term consumption of fermented dairy products over 6 mo increases HDL cholesterol. Eur J Clin Nutr. 2002;56:843-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 219. | Ahn HY, Kim M, Chae JS, Ahn YT, Sim JH, Choi ID, Lee SH, Lee JH. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduces fasting triglycerides and enhances apolipoprotein A-V levels in non-diabetic subjects with hypertriglyceridemia. Atherosclerosis. 2015;241:649-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 220. | Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci. 2000;83:255-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 221. | Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR, Reid G, Karmazyn M. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 222. | Lin PP, Hsieh YM, Kuo WW, Lin YM, Yeh YL, Lin CC, Tsai FJ, Tsai CH, Huang CY, Tsai CC. Probiotic-fermented purple sweet potato yogurt activates compensatory IGFIR/PI3K/Akt survival pathways and attenuates cardiac apoptosis in the hearts of spontaneously hypertensive rats. Int J Mol Med. 2013;32:1319-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 223. | Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015;179:348-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 224. | Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 225. | Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol. 2012;5:111-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 226. | Boumis E, Capone A, Galati V, Venditti C, Petrosillo N. Probiotics and infective endocarditis in patients with hereditary hemorrhagic telangiectasia: a clinical case and a review of the literature. BMC Infect Dis. 2018;18:65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 227. | Banerjee S. Recommendation and limitation of probiotics supplements. Curr Trends Pharm Pharm Chem. 2021;3:19-22. [DOI] [Cited in This Article: ] |
| 228. | Anadón A, Martínez-Larrañaga MR, Aranzazu Martínez M. Probiotics for animal nutrition in the European Union. Regulation and safety assessment. Regul Toxicol Pharmacol. 2006;45:91-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 229. | Ayichew T, Belete A, Alebachew T, Tsehaye H, Berhanu H and Minwuyelet A. Bacterial Probiotics their Importances and Limitations: A Review. J Nutr Health Sci. 2017;4:1-8. [Cited in This Article: ] |