Published online Feb 28, 2021. doi: 10.3748/wjg.v27.i8.708
Peer-review started: October 24, 2020
First decision: November 23, 2020
Revised: December 17, 2020
Accepted: January 13, 2021
Article in press: January 13, 2021
Published online: February 28, 2021
A recent investigation showed that the prevalence of type 2 diabetes mellitus (T2DM) is 12.8% among individuals of Han ethnicity. Gut microbiota has been reported to play a central role in T2DM. Goto-Kakizaki (GK) rats show differences in gut microbiota compared to non-diabetic rats. Previous studies have indicated that berberine could be successfully used to manage T2DM. We sought to understand its hypoglycaemic effect and role in the regulation of the gut microbiota.
To determine whether berberine can regulate glucose metabolism in GK rats via the gut microbiota.
GK rats were acclimatized for 1 wk. The GK rats were randomly divided into three groups and administered saline (Mo), metformin (Me), or berberine (Be). The observation time was 8 wk, and weight, fasting blood glucose (FBG), insulin, and glucagon-like peptide-1 (GLP-1) were measured. Pancreatic tissue was observed for pathological changes. Additionally, we sequenced the 16S rRNA V3-V4 region of the gut microbiota and analysed the structure.
Compared with the Mo group, the Me and Be groups displayed significant differences in FBG (P < 0.01) and GLP-1 (P < 0.05). A significant decrease in weight and homeostatic model assessment-insulin resistance was noted in the Be group compared with those in the Me group (P < 0.01). The pancreatic islets of the Me- and Be-treated rats showed improvement in number, shape, and necrosis compared with those of Mo-treated rats. A total of 580 operational taxonomic units were obtained in the three groups. Compared to the Mo group, the Me and Be groups showed a shift in the structure of the gut microbiota. Correlation analysis indicated that FBG was strongly positively correlated with Clostridia_UCG-014 (P < 0.01) and negatively correlated with Allobaculum (P < 0.01). Body weight showed a positive correlation with Desulfovibrionaceae (P < 0.01) and a negative correlation with Akkermansia (P < 0.01). Importantly, our results demonstrated that Me and Be could significantly decrease Bacteroidetes (P < 0.01) and the Bacteroidetes/Firmicutes ratio (P < 0.01). Furthermore, Muribaculaceae (P < 0.01; P < 0.05) was significantly decreased in the Me and Be groups, and Allobaculum (P < 0.01) was significantly increased.
Berberine has a substantial effect in improving metabolic parameters and modulating the gut microbiota composition in T2DM rats.
Core Tip: Recent studies suggested that the gut microbiota has an essential role in type 2 diabetes mellitus. Our study verifies the effects of berberine in regulating glucose metabolism and reducing pancreas injury in Goto-Kakizaki rats. Berberine also provides an alternative means of modulating the gut microbiota. The results revealed evidence of a decrease in Desulfobacterota, Desulfovibrionaceae, and Clostridia_UCG-014 and an increase in Verrucomicrobiota, Akkermansia, and Allobaculum. Correlation analysis indicated that fasting blood glucose was strongly positively correlated with Clostridia_UCG-014 and negatively correlated with Allobaculum.
- Citation: Zhao JD, Li Y, Sun M, Yu CJ, Li JY, Wang SH, Yang D, Guo CL, Du X, Zhang WJ, Cheng RD, Diao XC, Fang ZH. Effect of berberine on hyperglycaemia and gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. World J Gastroenterol 2021; 27(8): 708-724
- URL: https://www.wjgnet.com/1007-9327/full/v27/i8/708.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i8.708
Type 2 diabetes mellitus (T2DM) has become a critical and urgent human health concern worldwide. A recent epidemiological investigation shows that the prevalence of T2DM is 12.8% among individuals of Han ethnicity[1]. T2DM is a metabolic disorder and affects patients’ quality of life. Its physiological hallmarks are insulin resistance and β-cell failure[2]. Accumulating evidence has suggested that diabetes is also associated with gut microbiota composition and homeostasis[3].
Goto-Kakizaki (GK) rats, which provide an elevated blood glucose and non-obese T2DM experimental model, have been widely used to study T2DM[4,5]. When GK rats are 28 d of age, basal hyperglycaemia, impaired insulin secretion by pancreatic β-cells, and increased hepatic glucose production are observed[6,7]. Typical T2DM characteristics are more obvious in 8-week-old rats[8].
T2DM patients and animals show significant differences in gut microbiota composition compared to their non-diabetic counterparts[9-11]. Hence, regulators of gut microbiota may become potential alternative targets for diabetes treatment. Metformin is a first-line oral agent used to treat T2DM. Metformin therapy for T2DM has been extensively used for 70 years[12]. Its main effect involves decreasing hepatic glucose production and improving glucose uptake and insulin sensitivity[13,14]. Some studies also suggest that metformin treatment modulates the gut microbiota, thus contributing to the anti-diabetic effect[15-17].
For many years, studies have shown that tradition Chinese medicine (TCM) exhibits an effect against the development of T2DM and treats T2DM by decreasing fasting blood glucose (FBG) and glycated haemoglobin A1c, improving insulin resistance, and protecting β-cells and pancreatic islet function[18-20]. Berberine has been used as a TCM or an alternative treatment for T2DM. Berberine is derived from Rhizoma Coptidis, Cortex Phellodendri Amurensis, Radix Scutellariae Baicalensis, and others. Recently, many scholars have demonstrated that berberine is an effective, potent anti-diabetic agent[21,22]. At present, there are many reports indicating that berberine can regulate blood glucose, improve blood lipids, and reduce insulin resistance[19,23,24]. Some studies have reported that berberine repairs the gut barrier structure and alters the diversity of the gut microbiota[25,26], suggesting that its effects are exerted via modulation of the gut bacteria.
More information regarding the influence of berberine on T2DM hyperglycaemia and gut microbiota composition is needed. In this study, we evaluated the effects of berberine in regulating glucose metabolism and its influence on imbalanced gut microbiota in T2DM GK rats to elucidate the hypoglycaemic mechanism of berberine.
Berberine chloride hydrate was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Metformin was purchased from Sino-American Shanghai Squibb Pharmaceuticals Ltd., (Shanghai, China). Plasma insulin and stimulate glucagon-like peptide-1 (GLP-1) concentrations were measured with a Mercodia ELISA kit (Uppsala, Sweden). The assay kits for FBG, total cholesterol (TC), and triglycerides (TG) were provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Thirty male GK rats (6 wk old) were obtained from the Shanghai Laboratory Animal Center (Shanghai, China; certificate No. 2015000535026). The animals were acclimated in a specific pathogen-free animal laboratory at 22 ± 2 °C with 50%-70% relative humidity under a 12/12 h light/dark cycle. They were fed standard laboratory chow and provided with water ad libitum.
Following acclimatization for 1 wk, GK rats with high FBG levels exceeding 11.1 mmol/L were randomly divided into three groups (n = 10): A model group (Mo), which received an equal volume of saline intragastrically once daily; a metformin group (Me), which received 100 mg/kg intragastrically once daily; and a berberine group (Be), which received 200 mg/kg intragastrically once daily. The course of treatment was 8 wk, and body weight was measured every 2 wk. One rat from each of the three groups (3 total) died during the experiment; we speculate that the cause may be related to high blood glucose, infection, or other conditions.
At the end of the observation period, 27 rats were weighed and deeply anaesthetized with intraperitoneal pentobarbital sodium (30 mg/kg, Merck, United States). Blood samples were collected in non-heparinized tubes, incubated on ice for 30-60 min, and centrifuged at 4000 rpm for 10 min at 4 °C, and the entire contents of the colon were carefully removed and placed in Eppendorf tubes. The serum, pancreas, and colon contents were then collected and stored at -80 °C. The FBG and lipid parameters were analysed using an automatic biochemistry analyser (AU 7600, HITACHI, Japan). Serum insulin and GLP-1 were measured using a Multi-Mode Microplate Reader (Synergy Neo2, Biotek, United States) according to the manufacturer’s instructions. Isolated pancreas tissues were fixed in a tube containing 4% paraformaldehyde solution for 24 h.
Pancreatic tissues were washed with phosphate-buffered saline, embedded in paraffin wax, sliced into 5-μm sections, and stained with haematoxylin and eosin (H&E). The sections were observed using a Zeiss Axioskop 2 plus upright light microscope and camera (Zeiss, Oberkochen, Germany; × 100 magnification).
Total bacterial DNA was extracted using a TIANamp Stool DNA kit (Tiangen Biotech Co., Ltd., Beijing, China) following the manufacturers’ instructions and stored at -20 °C prior to further analysis. The 16S rRNA gene from DNA samples was amplified using the conventional barcoded universal bacterial primers F338/R806 targeting the V3-V4 region, which were selected to analyse the taxonomic composition of the gut microbiota. Sequencing of polymerase chain reaction products was performed using the Illumina HiSeq platform (Illumina, San Diego, CA, United States) for pair-end reads with an average length of 451 bp. The trimmed sequences were de-multiplexed and quality-filtered to the Quantitative Insights Into Microbial Ecology (QIIME, v1.9.1) and R software (v3.3.1)[10,27]. The acquired data were analysed by the Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China)[28].
The results of the shared and unique classification of operational taxonomic units (OTUs) were determined using the Greengenes Database (http://greengenes.secondgenome.com/) following the 97% homology cut-off value[29]. Taxonomic annotation of OTUs was performed using the RDP Classifier (https://sourceforge.net/projects/rdp-classifier/) against the SILVA 16S rRNA database (https://www.arb-silva.de/), which was performed using R software. Venn diagrams, community bar charts, unweighted UniFrac-based principal coordinates analysis (PCoA), and correlation heat-maps were generated using R software.
All data are expressed as the mean ± SD for each group. The data were analysed by one-way analysis of variance (ANOVA) followed by the least significant differences test or Tamhane's T2 and Spearman’s correlation with SPSS 23.0 software (NY, United States). P values < 0.05 were considered indicative of statistical significance. GraphPad Prism 5.0 software and Multi Experiment Viewer software (v4.9.0) were used to perform the statistical analyses.
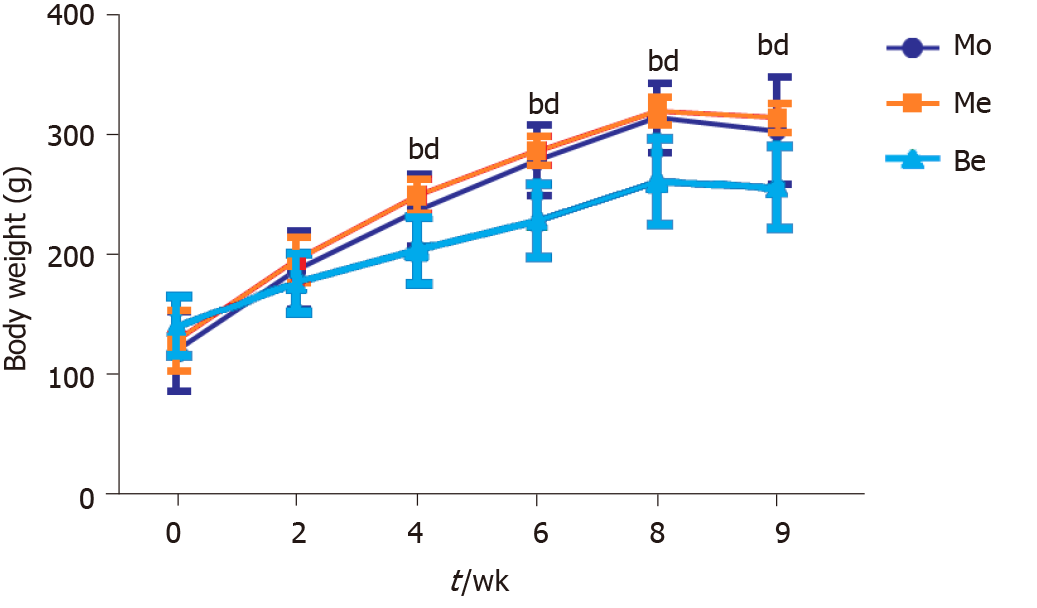
Before treatment, body weight was not significantly different (P > 0.05) among the three groups. Compared with the Mo and Me groups, the Be group had a significantly lower weight from 4 to 9 wk (P < 0.01). However, the body weight of Me rats was not significantly different from that of the Mo group (Figure 1).
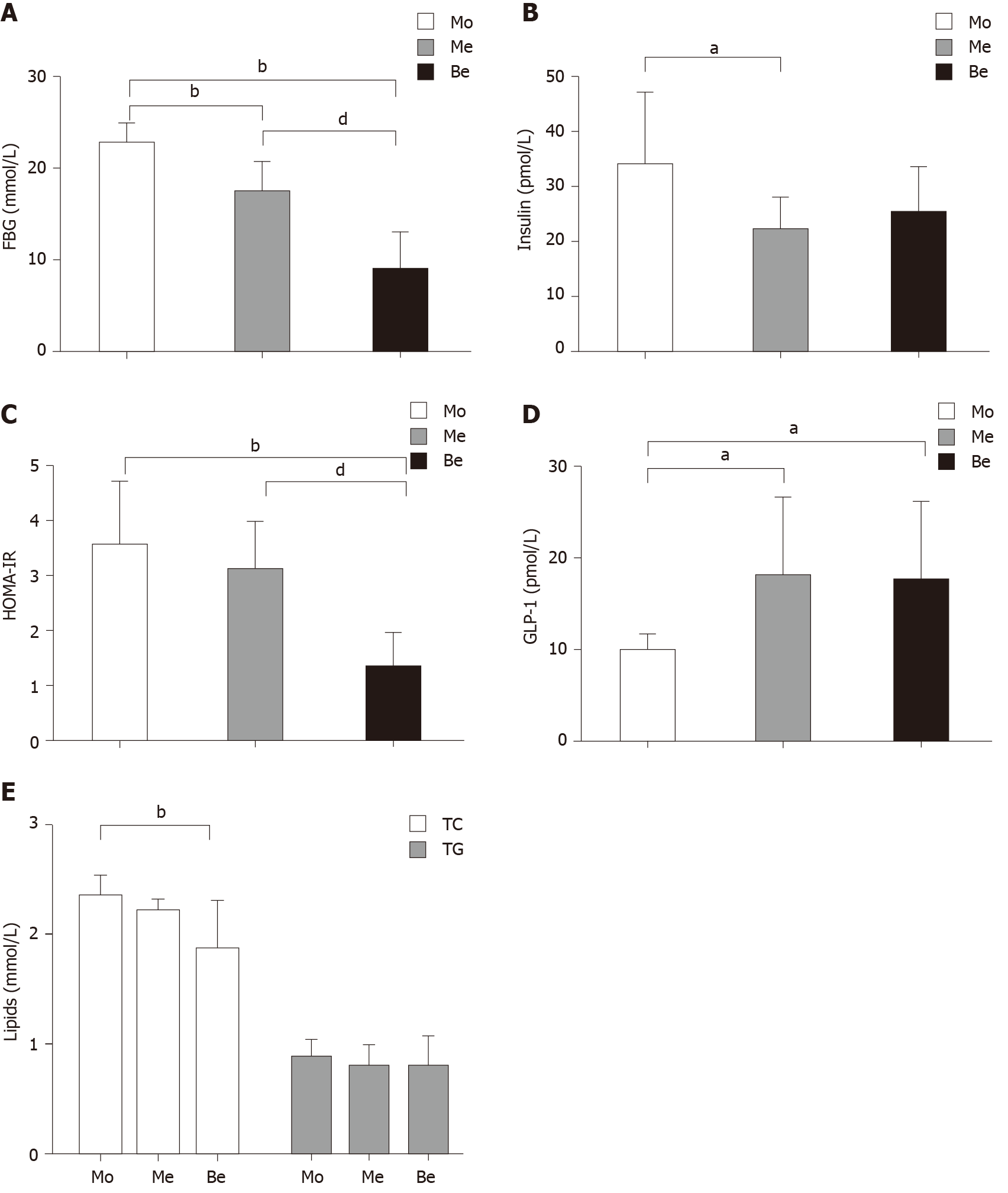
The FBG levels were also not significantly different (P > 0.05) before the experiment. Compared with those of the Mo group, the FBG levels of the Me and Be groups were notably lower. Moreover, the Be group exhibited a marked reduction compared with the Me group (P < 0.01) (Figure 2A). The fasting insulin level was significantly different between the Mo and Me groups (P < 0.05), and it was close to being significantly different in the Be group (P = 0.058) (Figure 2B). In addition, homeostatic model assessment-insulin resistance (HOMA-IR) showed a greater reduction in insulin resistance after treatment in the Be group than in the Mo or Me groups (P < 0.01) (Figure 2C). Compared with that in the Mo group, the GLP-1 levels of the Me and Be groups were notably increased (P < 0.05). There were no significant differences in fasting insulin or GLP-1 between the Me and Be groups (P > 0.05) (Figure 2B and D).
Interestingly, the Be group showed lower TC values than the Mo group (P < 0.01). There was no significant difference between the Be and Me groups (P > 0.05); however, both showed a similar trend in blood lipid regulation. In addition, the TG levels were not significantly different after treatment (Figure 2E).
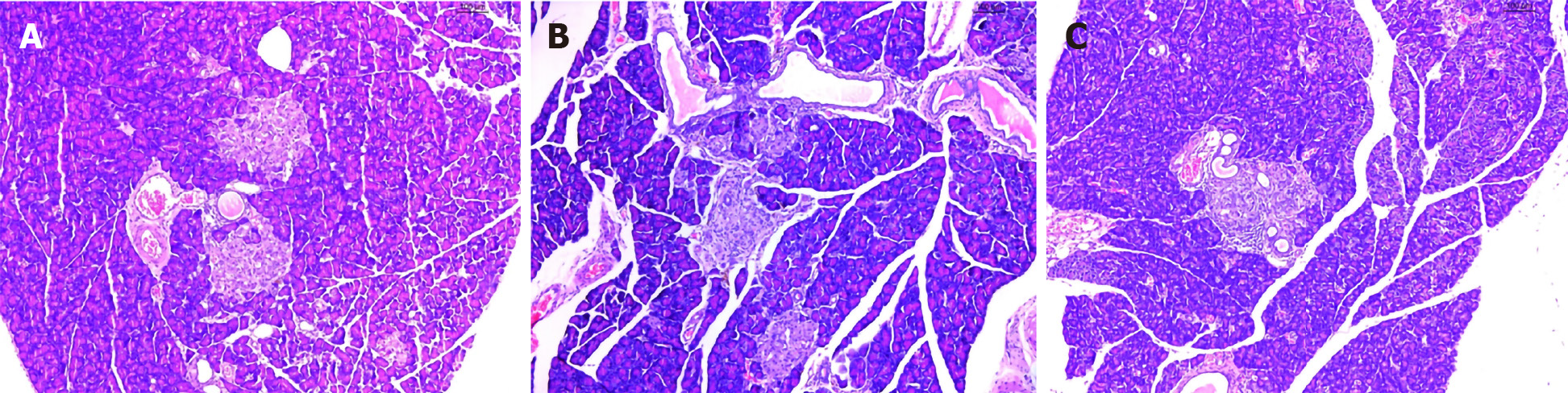
As expected, H&E staining revealed that the pancreatic islet β cells in the Mo group were reduced, and the pancreatic islets had mild amyloidosis. The size of the β cells was decreased, the nuclei showed pyknosis, endocrine granules were significantly reduced, and lipids were deposited in the cell cytoplasm (Figure 3A). In the Me and Be groups, the shape of the pancreatic islets was still regular, the number of pancreatic islet β cells was slightly increased, the nucleus was enlarged and rounded, the H&E staining was lighter, and the capillaries in the pancreatic islets were proliferated (Figure 3B and C).
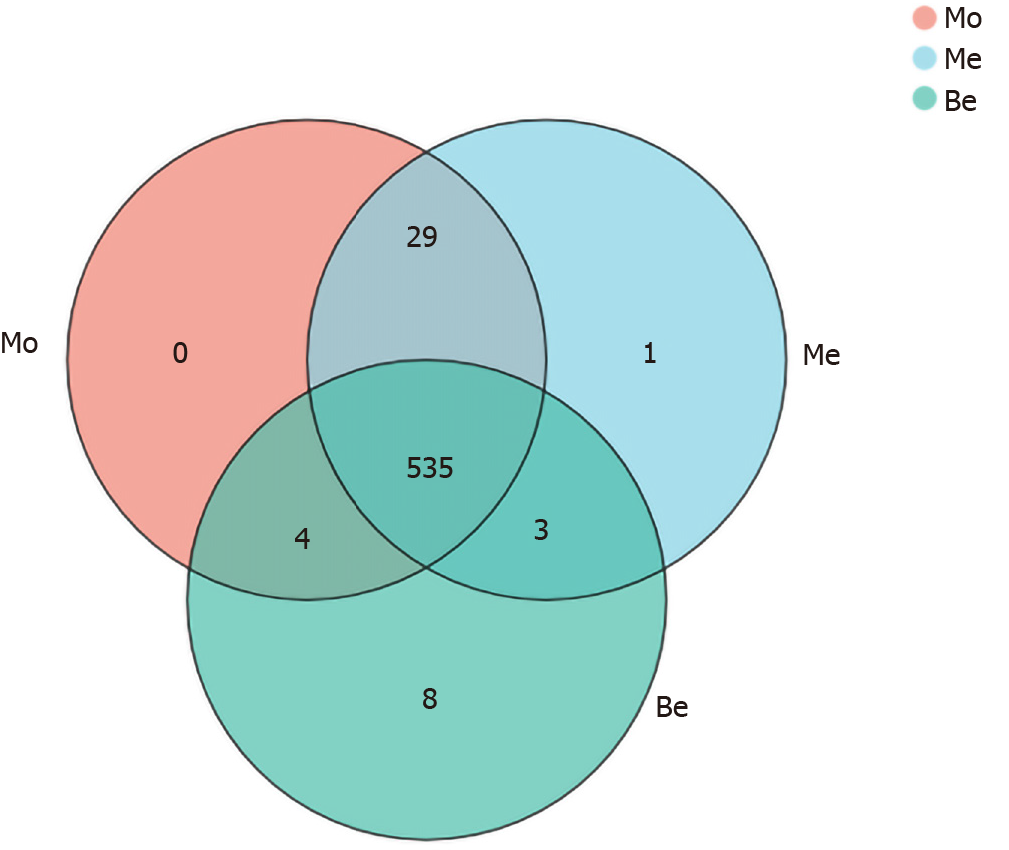
To characterize the composition of the gut microbiota, a total of 1505750 clean reads were generated from 27 samples, providing a total of 580 OTUs. Notably, eight unique OTUs were found in the Be group, one was found in the Me group, and there were no unique OTUs in the Mo group (Figure 4).
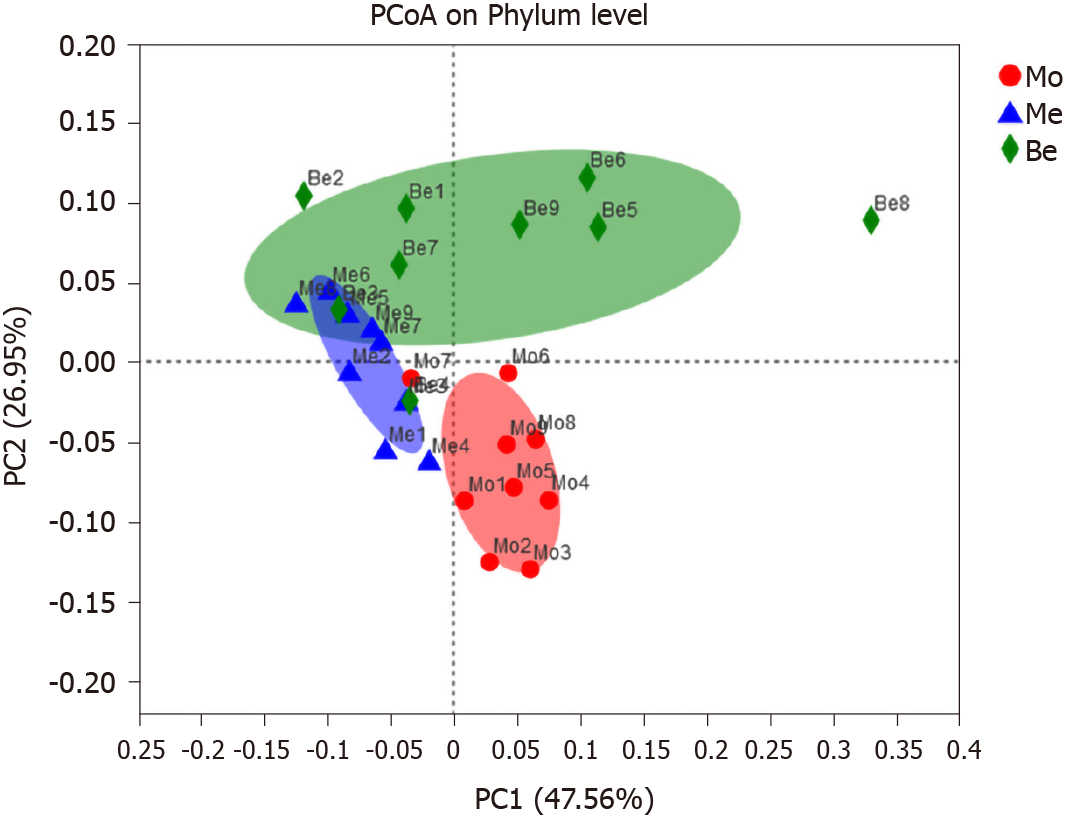
PCoA score plots based on phylogenetic statistical analysis methods indicated clustering of the gut microbiota within groups. PCoA of both unweighted UniFrac distances showed a clear separation in the three groups [the first principal component 47.56%, the second principal component (PC2) 26.95%]. The gut microbiota structure of the Me and Be groups showed the same trend, particularly an obvious shift along PC2 with Be treatment (Figure 5).
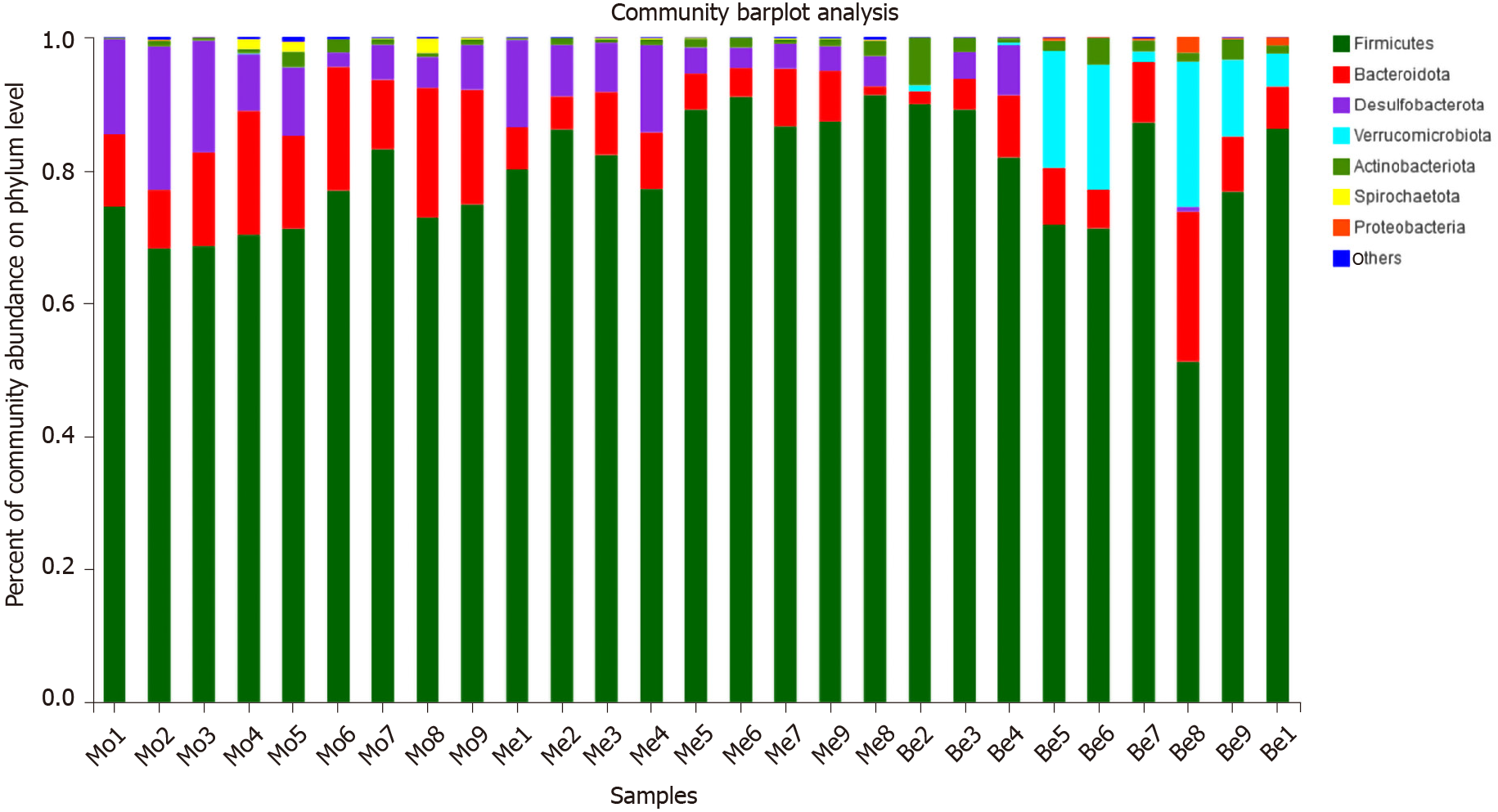
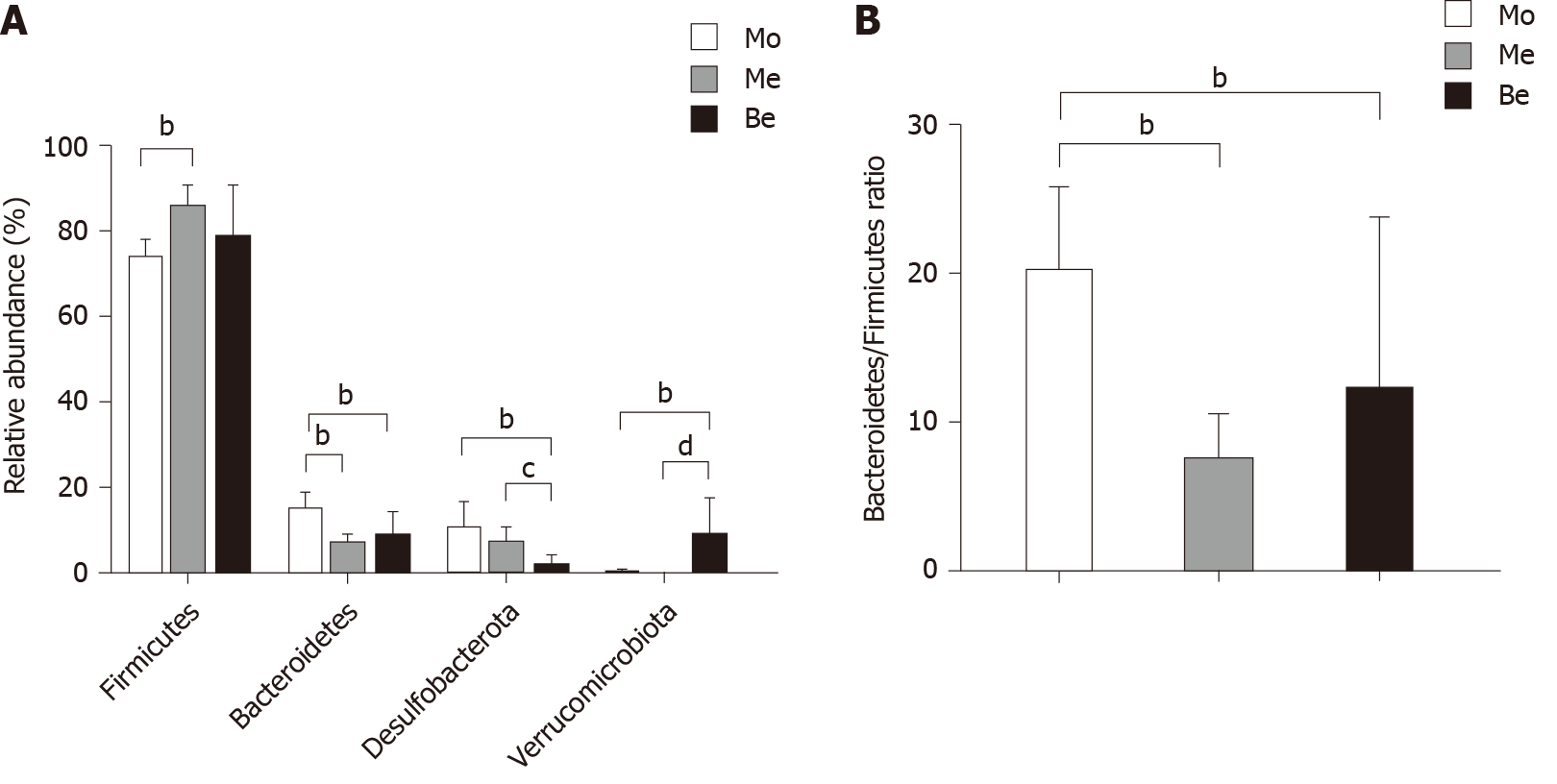
The dominant gut bacteria were of the phylum Firmicutes and Bacteroidetes. The composition of each sample is listed in detail (Figure 6). The abundance of Firmicutes in the Me group (85.62% ± 4.85%) was significantly higher than that in the Mo group (73.21% ± 4.63%) (P < 0.01). The proportion of Firmicutes showed an increasing trend in the Be group (78.30% ± 12.39%), but there was no significant difference compared with that in the Mo and Me groups (P > 0.05). In contrast, the composition of Bacteroidetes was significantly lower in the Me and Be groups (6.30% ± 2.56%, 8.38% ± 5.51%) than in the Mo group (14.65% ± 4.01%) (P < 0.01) (Figure 7). The gut microbiota of the Mo group was characterized by an increased Bacteroidetes/Firmicutes ratio (20.10 ± 5.66), and this ratio showed significant differences with lower proportions in the Me and Be groups (7.48 ± 3.24, 12.15 ± 11.66) (P < 0.01) (Figure 7). There were no significant differences in Firmicutes, Bacteroidetes, or the Bacteroidetes/Firmicutes ratio between the Me and Be groups (P > 0.05) (Figure 7).
Desulfobacterota abundance in the Be group (1.36% ± 2.63%) was significantly lower than that in the Mo and Me groups (9.93% ± 6.37%, 6.64% ± 4.00%) (P < 0.01 or P < 0.05). The proportion of Desulfobacterota showed a decreasing trend in the Me group, but there was no significant difference compared with that in the Mo group (P > 0.05) (Figure 7). Furthermore, Verrucomicrobiota in the Be group (8.59% ± 8.87%) was significantly higher than that in the Mo and Me groups (P < 0.01), but these changes were not significantly different between the Mo and Me groups. The mean did not exceed 0.05% in the Me and Be groups (Figure 7).
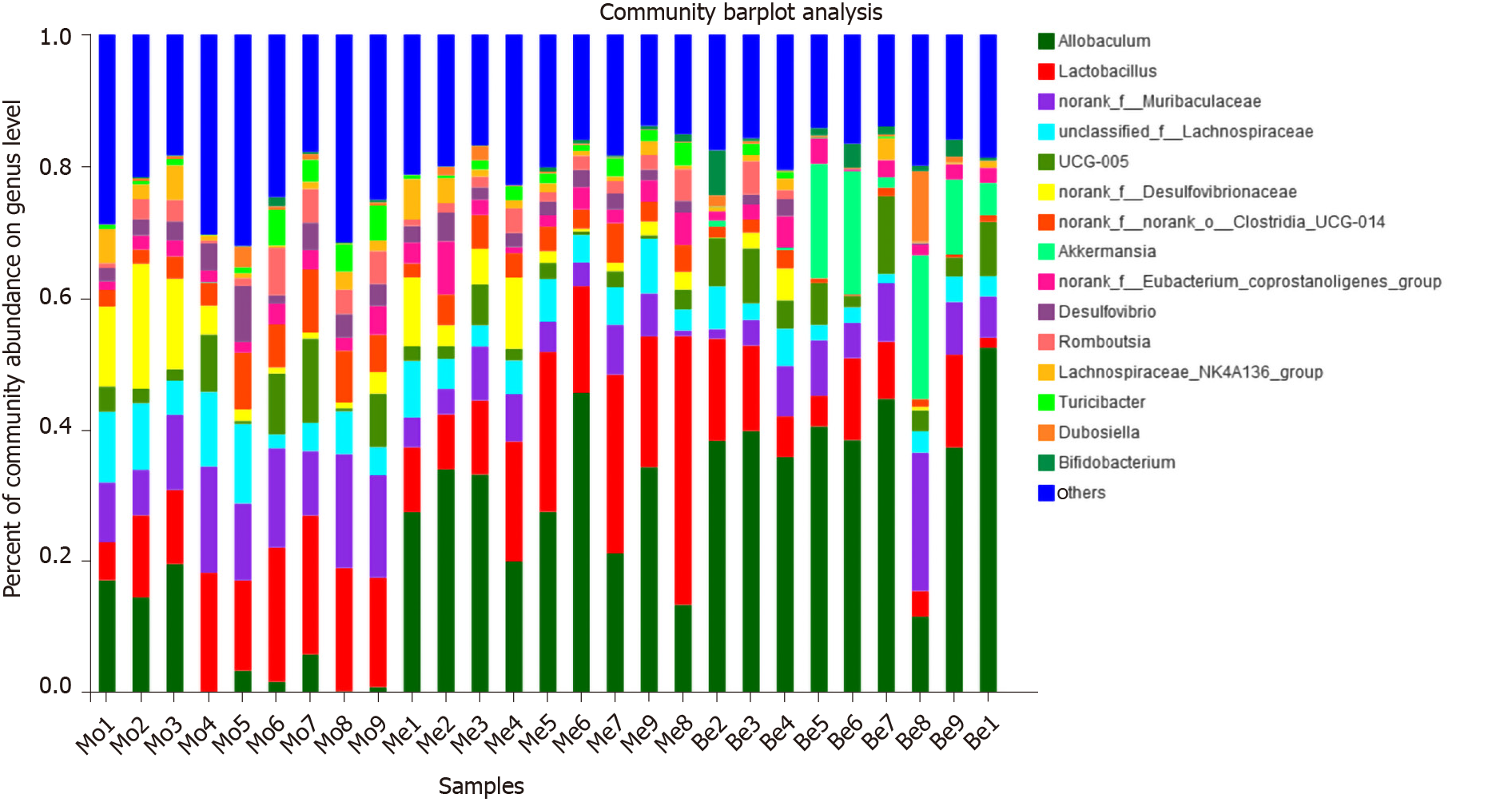
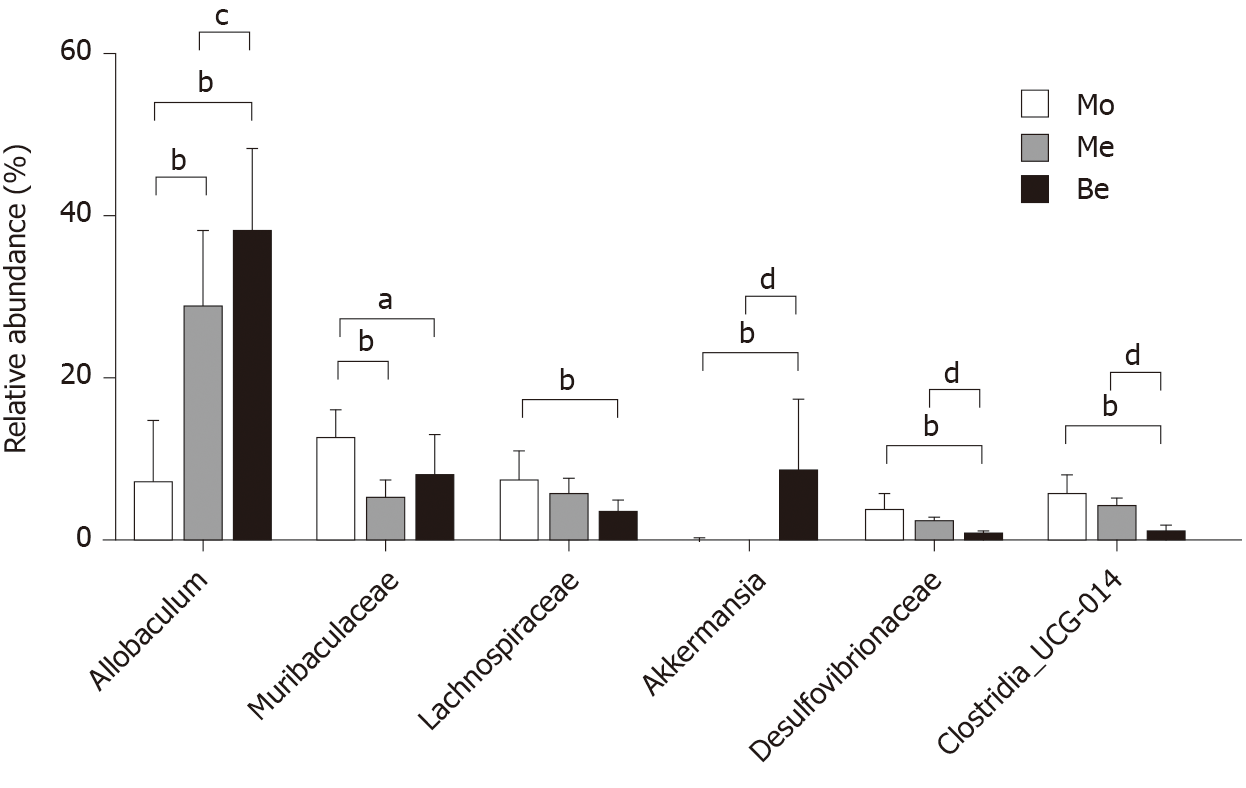
At the genus level, the microbiota composition varied greatly among the three groups (Figure 8). The relative abundance of Allobaculum contributed to 24.36% of the total faecal microbiota population. A higher proportion of Allobaculum was observed in the Be and Me groups (37.76% ± 10.35%, 28.49% ± 9.55%) than in the Mo group (7.07% ± 7.81%) (P < 0.01). Moreover, the Be group exhibited a remarkable increment compared with the Me group (P < 0.05) (Figure 9). Reductions in Muribaculaceae, Lachnospiraceae, Desulfovibrionaceae, and Clostridia_UCG-014 were detected in the Be group compared with those in the Mo group (P < 0.01, P < 0.05). Furthermore, the advantages of the Be group were more prominent than those in the Me group at Desulfovibrionaceae and Clostridia_UCG-014 (P < 0.01) (Figure 9). The Be group exhibited a significant increase in the relative abundance of Akkermansia compared with that in the Mo and Me groups (P < 0.01) (Figure 9).
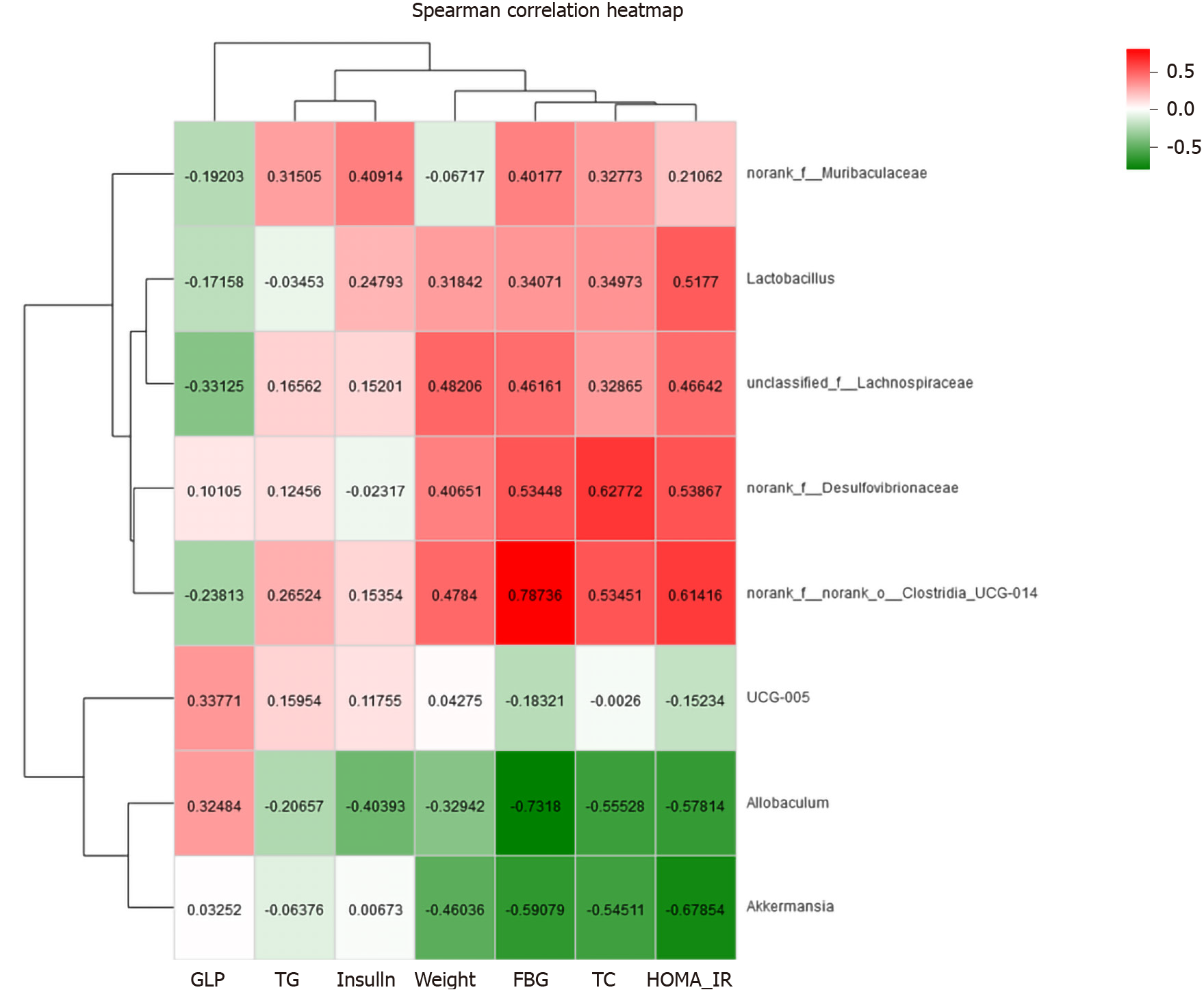
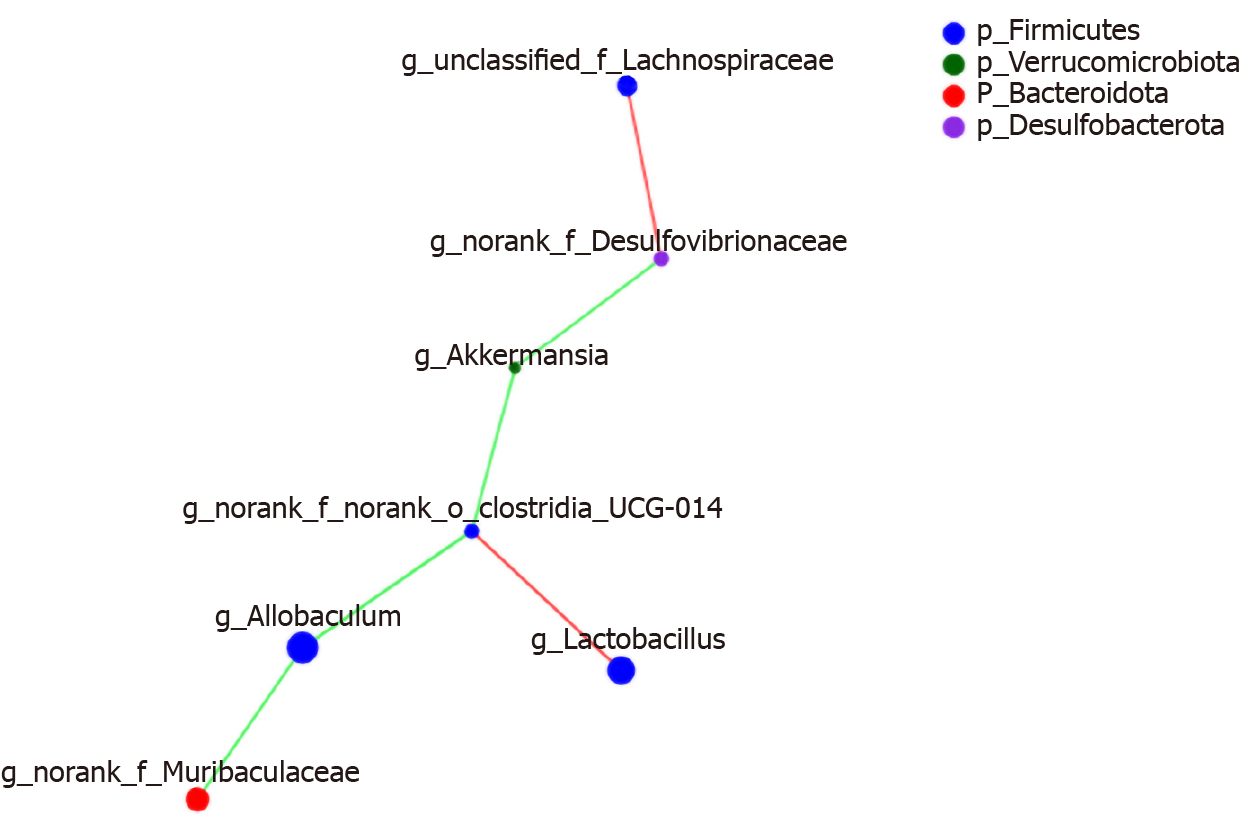
Correlation heat-map analysis was applied to investigate T2DM-related factors that significantly affected gut microbiota at the genus level (Figure 10). Based on the heat map, we found that Desulfovibrionaceae, Lachnospiraceae, and Clostridia_UCG-014 were positively correlated with the FBG levels, while Allobaculum and Akkermansia were negatively associated with the FBG concentration (P < 0.05, P < 0.01). Among them, a strong correlation with Clostridia_UCG-014 and Allobaculum was observed. HOMA-IR and Clostridia_UCG-014 were significantly positively correlated, and HOMA-IR was negatively correlated with Akkermansia (P < 0.05). In addition, both FBG and TC levels showed positive correlations with Desulfovibrionaceae and Clostridia_UCG-014 and negative correlations with Allobaculum and Akkermansia (P < 0.05). Body weight showed a positive correlation with Desulfovibrionaceae, Lachnospiraceae, and Clostridia_UCG-014 and a negative correlation with Akkermansia (P < 0.05) (Figure 11).
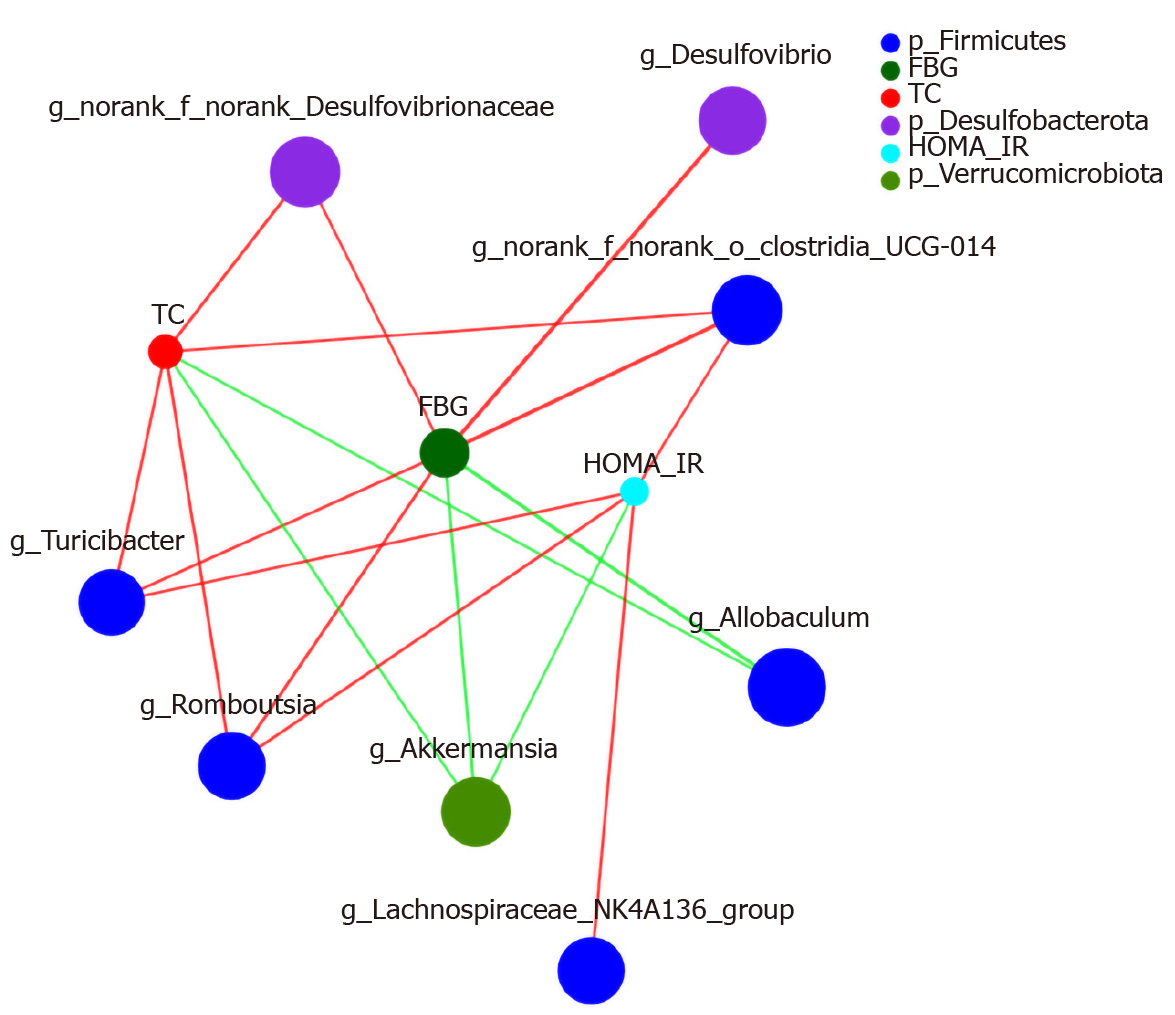
Networkx analysis was performed to build a species correlation network and between-species and T2DM-related factors correlation network (Figures 11 and 12). In the single-factor correlation network comparisons, we found that Allobaculum had a direct negative correlation with Clostridia_UCG-014 and Muribaculaceae. Clostridia_UCG-014 had a direct negative correlation with Akkermansia but not with Allobaculum. Meanwhile, a positive correlation with Lactobacillus was shown. In addition, there were certain associations among seven of the eight most abundant bacterial species (Figure 13). Based on the 16 most abundant bacteria and seven indicators related to T2DM, a two-factor correlation network diagram was formed and involved eight bacterial species and three indicators (Figure 12). FBG was positively correlated with five bacterial species and negatively correlated with two bacterial species. HOMA-IR was positively correlated with four bacterial species and negatively correlated with one bacterial species. TC was positively correlated with four bacterial species and negatively correlated with two bacterial species.
In the present study, we found that administration of metformin for 8 wk did not effectively lower body weight compared with saline. In previous research, the results indicated that metformin treatment improved markers of metabolic disorders and that body weight of T2DM patients or animals remained constant[10,30]. The main reasons may be as follows: GK rats are not obese and do not show significant weight gain. Furthermore, standard rodent feed was used in this study. In addition, although metformin can reduce weight, the effect is not obvious in non-obese subjects. It has been demonstrated that metformin could modulate FBG and insulin in our study, and the same results were obtained in previous studies[31,32]. We did not observe a significant improvement in HOMA-IR with metformin treatment, which may be because of the short-term treatment or because normal feed was used in the present study unlike that in other research. Therefore, we need to conduct long-term observation in further studies.
Although metformin is a powerful drug in T2DM, berberine showed the best hypoglycaemic and body weight-lowering effect in this research. Body weight has positive and negative correlations with gut microbiota; adjustment of the gut microbiota has an effect on body weight. Moreover, Chinese medicine theory believes that berberine has the characteristics of bitterness and coldness; thus, it may reduce food intake and energy intake, which leads to a significant reduction in body weight and FBG. In addition, berberine improves HOMA-IR, thus exerting a strong anti-glycaemic effect. These findings are similar to those of a previous systematic review[22]. In addition, our current results show that berberine treatment provided a beneficial effect on elevated levels of TC compared with saline. This may be due to the means by which berberine promotes lipid metabolism, improves antioxidant capacity, and inhibits preadipocyte differentiation[33].
Short chain fatty acids (SCFAs) as fermentation products of gut microbiota are involved in stimulating the secretion of a number of gut peptide hormones, including PYY, GLP-1, and GLP-2[34]. GLP-1 has an effect on insulin secretion following meal consumption. It is also involved in the regulation of β-cell proliferation and protection of β-cells in the pancreatic islets[35]. It is well known that the insulin-producing β-cells are important in maintaining balance in glucose metabolism. The pancreatic islets of rats in the Mo group displayed severe pathology, including fewer cells, smaller size of cells, and necrosis. The pancreatic islets of rats in the Me and Be groups showed improvement compared with those in the Mo group. These results agree with the findings of Cui et al[36].
A previous study showed that the secretion of GLP-1 was lower in T2DM mice[37]. In the present study, we found that the administration of metformin and berberine for 8 wk was effectively anti-diabetic, and we also observed an increase in GLP-1 secretion when rats were supplemented with metformin or berberine because these compounds can increase the proliferation of L cells and enhance glucagon and prohormone invertase synthesis[38].
Gut microbiota plays an important role in the regulation of multiple host metabolic pathways (e.g., glucose and lipid homeostasis). Berberine can notably change the composition of the gut microbiota in T2DM rats and can particularly influence certain bacteria correlated with SCFAs production and anti-inflammatory activity, further contributing to its biological effects through the modulation of gut farnesoid X receptor and/or the G-protein-coupled receptor activity[39,40]. Firmicutes and Bacteroidetes were the two main bacterial phylum in the gut microbiota. The present study showed that Firmicutes and Verrucomicrobiota distinctly decreased and that Bacteroidetes, the Bacteroidetes/Firmicutes ratio, and Desulfobacterota significantly increased in T2DM. At the phylum level, current studies identified that the Firmicutes, Bacteroidetes, and Bacteroidetes/Firmicutes ratio were modified after oral administration of berberine. Similar results[41] were observed in studies by Zhao et al[40] and Tian et al[42]. Berberine led to changes in proteins involved in microbial defense and stress responses and enrichment of proteins from the Verrucomicrobiota phylum[43]. For instance, Desulfovibrionaceae was enriched in patients with T2DM. Our results are supported by research that indicated a negative correlation between the abundance of Desulfovibrionaceae and mRNA expression of interleukin-6 and tumor necrosis factor α[44].
The data suggested that berberine mainly reversed the decrease in the proportions of Muribaculaceae, Lachnospiraceae, Desulfovibrionaceae, and Clostridia_UCG-014 and the increase in the relative abundance of Allobaculum and Akkermansia in T2DM rats. Correlation analysis indicated that these bacteria were significantly associated with the levels of FBG, TC, HOMA-IR, and body weight. These results suggest that OTUs of the gut microbiota are associated with specific metabolic biomarkers. The control of blood glucose is the most important observation indicator for the treatment of diabetes, especially FBG. Therefore, we need to up-regulate Akkermansia and Allobaculum and down-regulate Romboutsia, Turicibacter, Desulfovibrio, Desulfovibrionaceae, and Clostridia_UCG-014 to balance the microbiota, allowing FBG to return to normal levels as these bacteria are closely related to FBG.
We did not find a relationship between Muribaculaceae and berberine in previous T2DM studies. N. sativa is an annual herb, and it significantly increases the abundance of Muribaculaceae[45], which is inconsistent with our research results. However, further studies are needed to probe the controversy regarding this genus[46]. Berberine reduced the populations of Lachnospiraceae and Desulfovibrionaceae in our study. These results agree with the research by Cui et al[36]. The abundances of certain SCFAs-producing Lachnospiraceae were significantly increased in T2DM patients[10]. Desulfovibrionaceae induced abnormal proliferation and metabolism of epithelial cells, which impaired the gut barrier function[27,47]. Although our data illustrated that Clostridia_UCG-014 might play a significantly positive role in improvements and has a strong positive correlation with FBG levels, the role of this genus in T2DM has not yet been explored. However, its appearance in the results of our analyses suggests that it might be a future target of interest. The Clostridia_UCG-014 and Clostridium spp belong to the Clostridiaceae family. A study suggested that berberine reduced Clostridium spp by altering bile acid metabolism and activating FXR signalling[48].
In this study, Allobaculum is the most abundant bacterial species. Because Allobaculum was strongly negatively associated with FBG, the relative abundance of Allobaculum was reduced. Allobaculum belonging to putative SCFAs-producing bacteria was markedly increased by berberine. In agreement with previous studies, we showed that the selective modulations of specific gut microbial phylotypes, particularly the enrichment of SCFAs-producing Allobaculum, may participate in the actions of berberine in alleviating the T2DM[25,49].
These may participate in the actions of berberine in alleviating hyperglycaemia. Some other studies also demonstrated that berberine increased the abundance of probiotic Akkermansia in T2DM mice[48,50]. Akkermansia can reduce systemic inflammation and improve insulin resistance[17,51], which may contribute to the metabolic protective effects of berberine. We have observed that berberine regulates T2DM associated with the gut microbiota by increasing the number of SCFAs-producing bacteria and probiotics, and reducing opportunistic pathogens. Berberine mixture was more effective than metformin in altering features of the gut microbiota.
In summary, the gut microbiota plays crucial roles in fighting T2DM. GK rats have been shown to have altered gut microbiota composition. Disturbance of the gut microbiota can directly or indirectly lead to insulin resistance or T2DM. As expected, our results showed that berberine could effectively promote insulin secretion, gut hormone secretion, and pancreatic islet β cells and improve the symptoms of body weight, hyperglycaemia, and insulin resistance; intestinal barrier; inflammation; and lipid metabolism disorders resulting from T2DM. Thus, it can promote the achievement of diabetes treatment and reduce the various complications of diabetes. We demonstrated that gut microbiota is a pharmacological target to exert a hypoglycaemic effect after berberine treatment of T2DM rats, but the mechanism is not yet entirely clear. Gut microbiota may also have a potential signalling function that needs to be studied for wider clinical and experimental usage[52].
Type 2 diabetes mellitus (T2DM) has become a critical and urgent human health concern worldwide. Accumulating evidence has suggested that diabetes is also associated with gut microbiota composition and homeostasis. Hence, regulators of gut microbiota may become potential alternative targets for diabetes treatment. For many years, studies have shown that traditional Chinese medicine (TCM) exhibits an effect against the development of T2DM and can be used to treat T2DM. Thus, exploring a TCM monomer that can effectively regulate gut microbiota could benefit the prognosis of T2DM.
Goto-Kakizaki (GK) rats have been widely used to study T2DM, and berberine has been used for T2DM. At present, there are many reports indicating that berberine can regulate blood glucose, improve blood lipids, and reduce insulin resistance. Some studies have reported that berberine repairs the gut barrier structure and alters the diversity of the gut microbiota. Therefore, this study aimed to verify the effect of berberine treatment in GK rats and explore the underlying mechanism about gut microbiota.
To determine whether berberine can regulate the glucose metabolism in GK rats via regulating the gut microbiota.
Male GK rats were randomly divided into a saline (Mo), metformin (Me), or berberine (Be) group, with ten rats in each group. The observation time was 8 wk, and weight, fasting blood glucose (FBG), insulin, and glucagon-like peptide-1 (GLP-1) were measured. The pathology of the pancreatic tissue was observed. We also sequenced the 16S rRNA V3-V4 region of the gut microbiota and analysed the relationship to metabolic parameters.
The FBG, GLP-1, and homeostatic model assessment-insulin resistance (HOMA-IR) levels and pancreatic islets in the Me and Be groups were significantly different from those of the Mo group. Correlation analysis indicated that FBG was strongly positively correlated with Clostridia_UCG-014 and negatively correlated with Allobaculum. Importantly, our results demonstrated that Me and Mo could significantly decrease Bacteroidetes and the Bacteroidetes/Firmicutes ratio. Furthermore, Muribaculaceae (P < 0.01; P < 0.05) was significantly decreased in the Me and Be groups, and Allobaculum (P < 0.01) was significantly increased.
Berberine has a substantial effect in regulating the gut microbiota to improve metabolic parameters in GK rats.
We observed that berberine might decrease FBG, reduce HOMA-IR, and increase GLP-1 in GK rats. Further investigation of the underlying molecular mechanisms of berberine in regulating the gut farnesoid X receptor and/or the G-protein-coupled receptor is required to provide experimental evidence for wider clinical and experimental usage.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li H, Olt S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, Shi B, Sun H, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Ning G, Mu Y, Zhao J, Teng W, Shan Z. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 759] [Cited by in F6Publishing: 711] [Article Influence: 177.8] [Reference Citation Analysis (1)] |
| 2. | Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1276] [Cited by in F6Publishing: 1292] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 3. | Burcelin R, Serino M, Chabo C, Blasco-Baque V, Amar J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol. 2011;48:257-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Movassat J, Calderari S, Fernández E, Martín MA, Escrivá F, Plachot C, Gangnerau MN, Serradas P, Alvarez C, Portha B. Type 2 diabetes - a matter of failing beta-cell neogenesis? Diabetes Obes Metab. 2007;9 Suppl 2:187-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Portha B, Lacraz G, Kergoat M, Homo-Delarche F, Giroix MH, Bailbé D, Gangnerau MN, Dolz M, Tourrel-Cuzin C, Movassat J. The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol Cell Endocrinol. 2009;297:73-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Goto Y, Suzuki K, Ono T, Sasaki M, Toyota T. Development of diabetes in the non-obese NIDDM rat (GK rat). Adv Exp Med Biol. 1988;246:29-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Portha B, Giroix MH, Serradas P, Gangnerau MN, Movassat J, Rajas F, Bailbe D, Plachot C, Mithieux G, Marie JC. beta-cell function and viability in the spontaneously diabetic GK rat: information from the GK/Par colony. Diabetes. 2001;50 Suppl 1:S89-S93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci USA. 2009;106:13998-14003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 9. | Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1783] [Cited by in F6Publishing: 1887] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 10. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3971] [Cited by in F6Publishing: 4291] [Article Influence: 357.6] [Reference Citation Analysis (0)] |
| 11. | Zhang C, Wu W, Xin X, Li X, Liu D. Extract of ice plant (Mesembryanthemum crystallinum) ameliorates hyperglycemia and modulates the gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. Food Funct. 2019;10:3252-3261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Miller RA, Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. J Clin Invest. 2010;120:2267-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 711] [Cited by in F6Publishing: 753] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 14. | Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahrén B, Byiers S, Shao Q, Dejager S. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. 2014;80:5935-5943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 260] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 16. | de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care. 2017;40:54-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 438] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 17. | Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1031] [Cited by in F6Publishing: 1050] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 18. | Tong XL, Wu ST, Lian FM, Zhao M, Zhou SP, Chen XY, Yu B, Zhen Z, Qi LW, Li P, Wang CZ, Sun H, Yuan CS. The safety and effectiveness of TM81, a Chinese herbal medicine, in the treatment of type 2 diabetes: a randomized double-blind placebo-controlled trial. Diabetes Obes Metab. 2013;15:448-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Dai P, Wang J, Lin L, Zhang Y, Wang Z. Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus: Evaluation via biochemical markers and color Doppler ultrasonography. Exp Ther Med. 2015;10:869-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Jung CH, Seog HM, Choi IW, Choi HD, Cho HY. Effects of wild ginseng (Panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J Ethnopharmacol. 2005;98:245-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan J, Sun G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Yan A, Li S, Liu B, Li H, Yan Y. Efficacy and safety of berberine in the treatment of type 2 diabetes with insulin resistance: Protocol for a systematic review. Medicine (Baltimore). 2019;98:e16947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH, Li J, Ye JM. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 398] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 24. | Martínez N, White V, Kurtz M, Higa R, Capobianco E, Jawerbaum A. Activation of the nuclear receptor PPARα regulates lipid metabolism in foetal liver from diabetic rats: implications in diabetes-induced foetal overgrowth. Diabetes Metab Res Rev. 2011;27:35-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, Li M, Zhang C, Zhang Z, Zhang Y, Li X, Ning G, Zhao L. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 26. | Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS One. 2011;6:e24520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1766] [Cited by in F6Publishing: 1823] [Article Influence: 165.7] [Reference Citation Analysis (0)] |
| 28. |
Liu DQ, Tong C.
Bacterial community diversity of traditional fermented vegetables in China, |
| 29. | Amato KR, Yeoman CJ, Kent A, Righini N, Carbonero F, Estrada A, Gaskins HR, Stumpf RM, Yildirim S, Torralba M, Gillis M, Wilson BA, Nelson KE, White BA, Leigh SR. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013;7:1344-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 905] [Cited by in F6Publishing: 725] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 30. | Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 824] [Cited by in F6Publishing: 846] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 31. | Gao K, Yang R, Zhang J, Wang Z, Jia C, Zhang F, Li S, Wang J, Murtaza G, Xie H, Zhao H, Wang W, Chen J. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol Res. 2018;130:93-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Shin NR, Bose S, Wang JH, Ansari A, Lim SK, Chin YW, Choi HS, Kim H. Flos Lonicera Combined with Metformin Ameliorates Hepatosteatosis and Glucose Intolerance in Association with Gut Microbiota Modulation. Front Microbiol. 2017;8:2271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Li JW, Yuan K, Shang SC, Guo Y. A safer hypoglycemic agent for type 2 diabetes–Berberine organic acid salt. J Functional Foods. 2017;38:399-408. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Lü F, Jin T, Drucker DJ. Proglucagon gene expression is induced by gastrin-releasing peptide in a mouse enteroendocrine cell line. Endocrinology. 1996;137:3710-3716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Tanaka T, Yano T, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:515-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Cui HX, Hu YN, Li JW, Yuan K. Hypoglycemic Mechanism of the Berberine Organic Acid Salt under the Synergistic Effect of Intestinal Flora and Oxidative Stress. Oxid Med Cell Longev. 2018;2018:8930374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Sogin EM, Anderson P, Williams P, Chen CS, Gates RD. Application of 1H-NMR metabolomic profiling for reef-building corals. PLoS One. 2014;9:e111274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Wang ZK, Yang YS, Chen Y, Yuan J, Sun G, Peng LH. Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J Gastroenterol. 2014;20:14805-14820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 76] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Wang LL, Guo HH, Huang S, Feng CL, Han YX, Jiang JD. Comprehensive evaluation of SCFA production in the intestinal bacteria regulated by berberine using gas-chromatography combined with polymerase chain reaction. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1057:70-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Zhao L, Zhang Q, Ma W, Tian F, Shen H, Zhou M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017;8:4644-4656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 352] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 41. | Li T, Chiang JY. Nuclear receptors in bile acid metabolism. Drug Metab Rev. 2013;45:145-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Tian Y, Cai J, Gui W, Nichols RG, Koo I, Zhang J, Anitha M, Patterson AD. Berberine Directly Affects the Gut Microbiota to Promote Intestinal Farnesoid X Receptor Activation. Drug Metab Dispos. 2019;47:86-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 43. | Li L, Chang L, Zhang X, Ning Z, Mayne J, Ye Y, Stintzi A, Liu J, Figeys D. Berberine and its structural analogs have differing effects on functional profiles of individual gut microbiomes. Gut Microbes. 2020;11:1348-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Liu G, Bei J, Liang L, Yu G, Li L, Li Q. Stachyose Improves Inflammation through Modulating Gut Microbiota of High-Fat Diet/Streptozotocin-Induced Type 2 Diabetes in Rats. Mol Nutr Food Res. 2018;62:e1700954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 45. | Dong J, Liang Q, Niu Y, Jiang S, Zhou L, Wang J, Ma C, Kang W. Effects of Nigella sativa seed polysaccharides on type 2 diabetic mice and gut microbiota. Int J Biol Macromol. 2020;159:725-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 46. | Zhao J, Li Y, Sun M, Xin L, Wang T, Wei L, Yu C, Liu M, Ni Y, Lu R, Bao T, Zhang L, Wu Y, Fang Z. The Chinese Herbal Formula Shenzhu Tiaopi Granule Results in Metabolic Improvement in Type 2 Diabetic Rats by Modulating the Gut Microbiota. Evid Based Complement Alternat Med. 2019;2019:6976394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42:1571-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Zhang W, Xu JH, Yu T, Chen QK. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed Pharmacother. 2019;118:109131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 49. | Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, Zhang X, Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 427] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 50. | Zhu L, Zhang D, Zhu H, Zhu J, Weng S, Dong L, Liu T, Hu Y, Shen X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe-/- mice. Atherosclerosis. 2018;268:117-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 51. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066-9071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2639] [Cited by in F6Publishing: 2896] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 52. | Zhao J, Li Y, Xin L, Sun M, Yu C, Shi G, Bao T, Liu J, Ni Y, Lu R, Wu Y, Fang Z. Clinical Features and Rules of Chinese Herbal Medicine in Diabetic Peripheral Neuropathy Patients. Evid Based Complement Alternat Med. 2020;2020:5795264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |