Published online Feb 14, 2021. doi: 10.3748/wjg.v27.i6.501
Peer-review started: December 12, 2020
First decision: December 17, 2020
Revised: December 30, 2020
Accepted: January 12, 2021
Article in press: January 12, 2021
Published online: February 14, 2021
Early detection of advanced cystic mucinous neoplasms [(A-cMNs), defined as high-grade dysplasia or malignancy] of the pancreas is of great significance. As a simple and feasible detection method, serum tumor markers (STMs) may be used to predict advanced intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs). However, there are few studies on the usefulness of STMs other than carbohydrate antigen (CA) 19-9 for early detection of A-cMNs.
To study the ability of five STMs-CA19-9, carcinoembryonic antigen (CEA), CA125, CA724, and CA242 to predict A-cMNs and distinguish IPMNs and MCNs.
We mainly measured the levels of each STM in patients pathologically diagnosed with cMNs. The mean levels of STMs and the number of A-cMN subjects with a higher STM level than the cutoff were compared respectively to identify the ability of STMs to predict A-cMNs and distinguish MCNs from IPMNs. A receiver operating characteristic curve with the area under curve (AUC) was also created to identify the performance of the five STMs.
A total of 187 patients with cMNs were identified and 72 of them showed A-cMNs. We found that CA19-9 exhibited the highest sensitivity (SE) (54.2%) and accuracy (76.5%) and a moderate ability (AUC = 0.766) to predict A-cMNs. In predicting high-grade dysplasia IPMNs, the SE of CA19-9 decreased to 38.5%. The ability of CEA, CA125, and CA724 to predict A-cMNs was low (AUC = 0.651, 0.583, and 0.618, respectively). The predictive ability of CA242 was not identified. The combination of STMs improved the SE to 62.5%. CA125 may be specific to the diagnosis of advanced MCNs.
CA19-9 has a moderate ability, and CEA, CA125, and CA724 have a low ability to predict A-cMNs. The combination of STM testing could improve SE in predicting A-cMNs.
Core Tip: The value of serum tumor markers (STMs) in predicting advanced cystic mucinous neoplasms (A-cMNs) has not been well studied except for carbohydrate antigen (CA) 19-9. Our study illustrates for the first time the potential value of multiple STMs in the diagnosis of A-cMNs. CA19-9 was the most accurate STM in predicting A-cMNs with a moderate ability. CEA, CA125, and CA724 showed a low ability to predict A-cMNs. CA125 may be specific to the diagnosis of advanced mucinous cystic neoplasms. CA242 was not identified as a useful STM in predicting A-cMNs in our study. The combination of STMs could improve sensitivity in predicting A-cMNs.
- Citation: Sun LQ, Peng LS, Guo JF, Jiang F, Cui F, Huang HJ, Jin ZD. Validation of serum tumor biomarkers in predicting advanced cystic mucinous neoplasm of the pancreas. World J Gastroenterol 2021; 27(6): 501-512
- URL: https://www.wjgnet.com/1007-9327/full/v27/i6/501.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i6.501
Pancreatic cystic lesions (PCLs) are being detected more frequently due to advancements in radiological technologies. Their prevalence ranges from 1.9% to 49.1% in different races[1-3]. The primary management goal of PCLs is to correctly identify malignant and invasive PCLs. Serum tumor markers (STMs) may play an adjunct role to imaging features in the evaluation of PCLs.
Cystic mucinous neoplasms (cMNs) of the pancreas, including mucinous cystic neoplasms (MCNs) and intraductal papillary mucinous neoplasms (IPMNs), have the potential to undergo malignant transformation, which is a cause for concern. Early detection of cMNs with high-grade dysplasia or invasive cancer [advanced cMNs (A-cMNs)] is important for improving prognoses.
MCN and IPMN have different biological behaviors and originate from different embryonic layers[4]. Although they may have similar imaging presentations, especially branch duct IPMN and MCN, they are different tumor types. Theoretically, they may also show different specific STMs. However, there is little available data demonstrating the difference.
STM testing is accessible in routine health checkups. It is more suitable as a screening modality than cross-imaging technologies. Several STMs, including carbohydrate antigen (CA) 19-9, carcinoembryonic antigen (CEA), CA125, CA724, and CA242, have been reported to be associated with the presence, metastasis, and recurrence of pancreatic adenocarcinoma[5-9]. However, the value of these STMs for PCLs is not well studied except for CA19-9 and CEA. CA19-9 is considered an independent predictor of malignancy in PCLs by many guidelines[10-12]. The value of CEA is mostly based on evidence of cyst fluid analyses, and it has the ability to distinguish mucinous from non-mucinous PCLs[13]. The sensitivity (SE) of CEA in serum is considered relatively low in predicting advanced IPMNs[14-16]. No previous studies have reported the value of CA125, CA724, or CA242 in predicting A-cMNs.
To evaluate the ability of CA19-9, CEA, CA125, CA724, and CA242 in predicting A-cMNs and whether the STM levels vary in MCNs and IPMNs, we conducted a retrospective cohort study of 187 patients diagnosed with PCLs and assessed their STM levels. Although we have obtained some promising findings, as a retrospective study with incomplete data, these findings need to be further confirmed by multi-center prospective studies.
We conducted a retrospective study at Changhai Hospital affiliated to Second Military Medical University, one of the largest pancreatic disease research centers in Shanghai, China. Patients who underwent surgical resection for an identified pancreatic cyst and whose final pathological outcomes were identified with cMNs were included in our study. A-cMNs were defined as MCNs and IPMNs with high-grade dysplasia or malignancy identified by pathological outcomes. Patient demographics, pathologic data, and STMs were extracted. The Institutional Review Board of Changhai Hospital approved the study.
The decision regarding surgical treatment was made by our multidisciplinary hepato-pancreatobiliary team for the vast majority of patients according to the FG guidelines of 2006 and 2012[17,18]; the decisions for the remaining cases were made according to the patients’ own wishes.
Serum CA19-9, CA125, CA724, CA242, and CEA were measured by using electrochemiluminescence immunoassays on a Roche Cobas E601 immunoassay analyzer (Roche Diagnostics, Mannheim, Germany) equipped with dedicated reagents, according to the manufacturer’s instructions. All of the assays were carried out at the Department of Laboratory Medicine, Changhai Hospital.
In our center, the cutoff values for STMs were: CA19-9, 37.0 U/mL; CEA, 5.0 ng/mL; CA125, 35.0 U/mL; CA724, 9.8 U/mL; and CA242, 20.0 U/mL. The mean levels of STMs and the number of A-cMN subjects with a higher STM level than the cutoff were compared to identify the ability of STMs to predict A-cMNs. STMs with a significant difference between A-cMNs and non-A-cMNs for both the mean level and number of subjects were considered to have the ability to predict A-cMNs. A receiver operating characteristic curve was created to identify the true ability of these STMs in predicting A-cMNs. STMs with areas under curve (AUCs) higher than 0.9, 0.7-0.9, and lower than 0.7 were considered to have strong, moderate, and low abilities in predicting A-cMNs, respectively. The SE, specificity (SP), and accuracy (AC) were also calculated to measure performance of the five STMs. For combination analysis, if any of these STM levels were elevated (higher than the cutoff level) in one subject, the combination was considered positive.
Categorical data were reported as frequencies or percentages. Continuous data are reported as the mean ± SD. Two-tailed Student’s t tests for continuous variables and chi-square tests for categorical variables were used to identify predictors of A-cMNs. The Z test was calculated to determine whether the AUCs are significantly different between STMs. P < 0.05 was considered statistically significant. Data were analyzed with IBM Statistic Package for Social Science Statistics version 22.0 (IBM Corp., Armonk, NY, United States).
From January 2013 to June 2019, 187 patients were identified with cMNs by surgical resection specimens in our center and were included in our study. Of the 187 patients, 151 (80.7%) had IPMNs, and 36 (19.3%) had MCNs. Of the 151 IPMN subjects, 46 IPMNs with invasive cancer and 13 IPMNs with high-grade dysplasia were identified. Of the 36 MCN subjects, 12 MCNs with invasive cancer and one MCN with high-grade dysplasia (HGD) were identified. In total, 72 advanced-cMNs were included in our study. The mean age of the overall study cohort was 60.2 ± 11.9 years, and males (109, 58.3%) were slightly more predominant than females (78, 41.7%). The median cyst size (interquartile range) of the study cohort was 28.5 mm (19-42 mm). The number of patients with cysts located in the pancreatic head/body/tail/whole pancreas was 111, 31, 30, and 15, respectively (Table 1). Male sex (P = 0.001), aging (P = 0.014), and large cyst size (P < 0.0001) were associated with the malignant transformation of MNs.
| Overall | Benign | Advanced | P value1 | |
| Patients characteristics (n = 187) | ||||
| Male (n, %) | 109 (58.3) | 63 (54.8) | 46 (63.9) | 0.007 |
| Age (Median, IQR) | 62 (54-68) | 61 (51-68) | 63.5 (56-70) | 0.014 |
| Cyst size (Median, IQR, mm) | 28.5 (19-42) | 24.5 (15-37) | 38.5 (28-59.5) | < 0.0001 |
| Location (H/B/T/D) | 111/31/30/15 | 63/20/20/10 | 48/11/10/5 | |
| CA19-9 (n = 187) | ||||
| Median, IQR (U/mL) | 10.11(4.4-52.9) | 7.1(3.4-16.3) | 55.5 (9.3-319.3) | < 0.0001 |
| ≥ 37/< 37 U/mL | 50/137 | 11/104 | 39/33 | < 0.0001 |
| CEA (n = 187) | ||||
| Median, IQR (ng/mL) | 2.37 (1.54-3.98) | 2.23 (1.5-3.3) | 3.1 (2.02-5.2) | < 0.0001 |
| ≥ 5/< 5 ng/mL | 27/160 | 9/106 | 18/54 | 0.001 |
| CA125 (n = 135) | ||||
| Median, IQR (U/mL) | 12.2 (8.2-18.8) | 12.1 (7.0-16.8) | 12.75 (8.9-23.4) | 0.002 |
| ≥ 35 /< 35 U/mL | 10/125 | 1/82 | 9/43 | 0.002 |
| CA724 (n = 116) | ||||
| Median, IQR (U/mL) | 1.6 (1.025-3.15) | 1.44 (1.0-2.66) | 2.4 (1.07-6.8) | 0.014 |
| ≥ 9.8/< 9.8 U /mL | 10/106 | 3/70 | 7/36 | 0.05 |
| CA242 (n = 31) | ||||
| Median, IQR (U/mL) | 7.13 (3.6-12.7) | 5.35 (2.7-7.9) | 11.1 (6.95-31.7) | 0.136 |
| ≥ 20/< 20 U/mL | 6/25 | 1/15 | 5/10 | 0.08 |
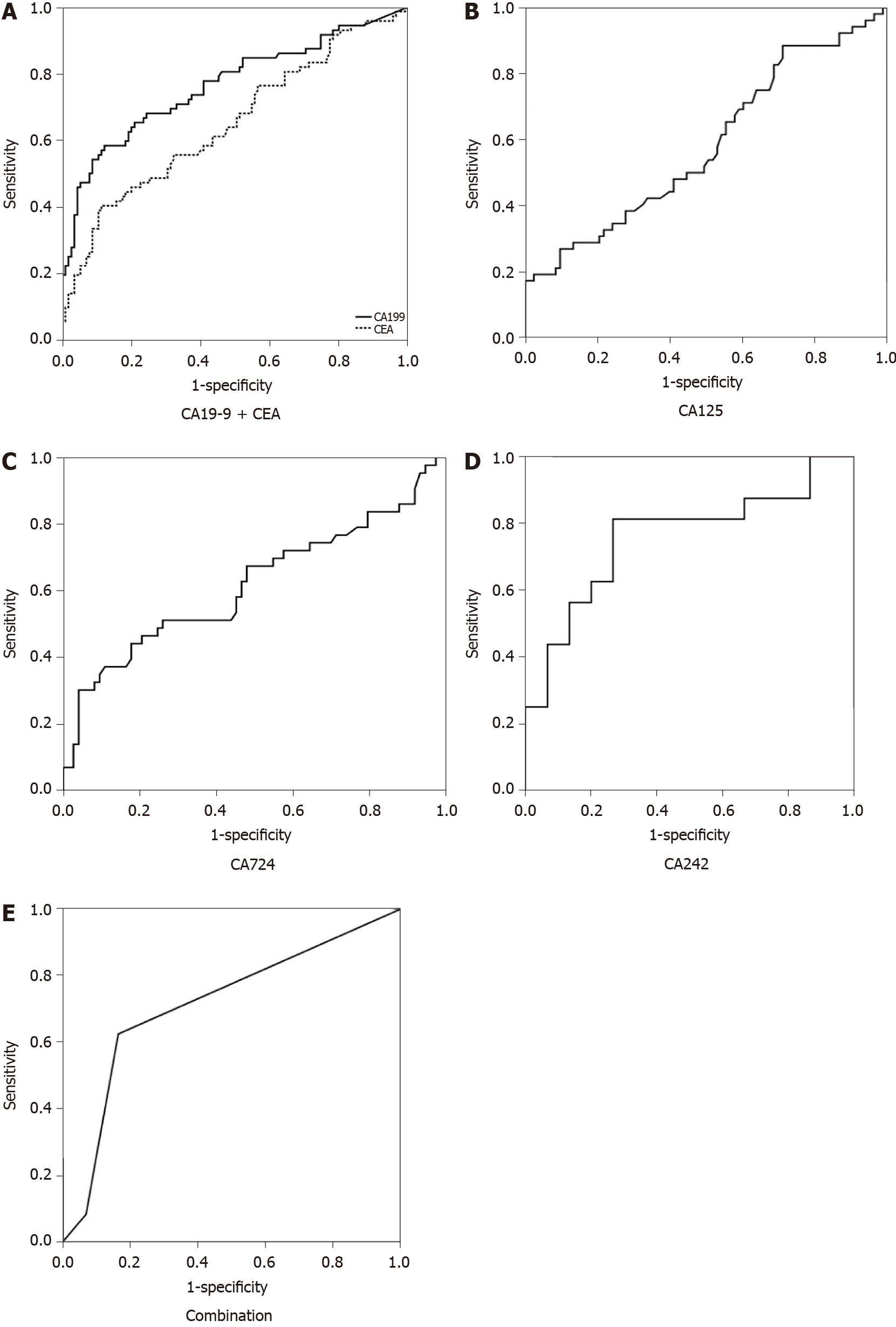
Receiver operating characteristic curves are demonstrated in Figure 1. The baseline characteristics and performance of each STM, including the overall characteristics and the classification of benign vs malignant by surgical pathology, are shown in Table 1. The SE, SP, positive predictive value, negative predictive value, AC, and AUC of each STM and their combination in predicting A-cMNs are presented in Table 2.
| SE (95%CI) | SP (95%CI) | PPV (95%CI) | NPV (95%CI) | AC | AUC | P value | |
| CA19-9 (%) | 54.2 (42.1-65.8) | 90.4 (83.2-94.5) | 78 (63.6.7-88) | 75.9 (67.7-82.6) | 76.5 | 0.766 | |
| CEA (%) | 25 (15.9-45.9) | 92.17 (85.26-96.13) | 66.67 (46-82.8) | 66.3 (58.3-73.4) | 66.31 | 0.651 | 0.04 |
| CA125 (%) | 17.3 (8.7-30.8) | 98.8 (92.54-99.9) | 90 (54.1-99.5) | 65.5 (56.5-73.7) | 67.4 | 0.583 | 0.004 |
| CA724 (%) | 16.3 (7.3-31.3) | 95.9 (87.7-98.9) | 70 (35.4-91.9) | 66 (56.1-74.8) | 66.4 | 0.618 | 0.03 |
| CA242 (%) | 31.25 (12.1-58.5) | 93.3 (66-99.7) | 83.3 (36.5-99.1) | 56 (35.3-75) | 61.3 | 0.758 | 0.81 |
| CA199 ± any one of other STMs | 62.5 (50.3-73.4) | 83.5 (75.1-89.5) | 70.3 (57.4-80.8) | 78 (69.5-84.8) | 75.4 | 0.715 | 0.32 |
Serum CA19-9 tests were available for all 187 subjects. The mean CA19-9 level was 121.3 ± 10.11 U/mL, and CA19-9 ≥ 37 U/mL was observed in 50 subjects, including 39 advanced cases. Serum CEA tests were also available for all 187 subjects. The mean CEA level was 3.46 ± 3.75 ng/mL, and CEA ≥ 5 ng/mL was observed in 27 subjects, including 18 advanced cases. Serum CA125 tests were available for 135 (72.2%) subjects, and the mean level was 17.65 ± 21.56 U/mL. An elevated CA125 level ≥ 35 U/mL was observed in ten subjects, including nine advanced cases. Serum CA724 tests were available for 116 (62%) subjects, and the mean level was 5.46 ± 17.56 U/mL. An elevated CA724 level ≥ 9.8 U/mL was observed in ten subjects, including seven advanced cases. Serum CA242 tests were available for 31 (16.6%) subjects, and the mean level was 32.12 ± 92.2 U/mL. An elevated CA242 Level ≥ 20 U/mL was observed in six subjects, including five advanced cases (Table 1).
CA19-9 (both P < 0.0001), CEA (P < 0.0001 and 0.001), CA125 (both P = 0.002), and CA724 (P = 0.014 and 0.05) all had the ability to predict A-cMNs because they showed significant differences in mean levels between the benign and advanced groups and in the number of advanced cases with a higher STM level than the cutoff (Table 1). These STMs had relatively high SP (90.4%, 92.17%, 98.8%, and 95.9%). However, their SE was suboptimal (54.2%, 25%, 17.3%, and 16.3%) (Table 2). Comparing their AUCs, CA19-9 had a significantly better performance (0.766) than CEA (0.651, P = 0.04), CA125 (0.583, P = 0.004), and CA724 (0.618, P = 0.03). CEA, CA125, and CA724 had comparable performances in predicting A-cMNs (P > 0.05 for all) (Table 3).
| ROC curve | ΔAUC1 | Standard error | Z statistic | P value |
| CA19-9 vs CEA | 0.115 | 0.038 and 0.042 | 2.03 | 0.04 |
| CA19-9 vs CA125 | 0.183 | 0.038 and 0.051 | 2.88 | 0.004 |
| CA19-9 vs CA724 | 0.148 | 0.038 and 0.058 | 2.14 | 0.03 |
| CA19-9 vs CA242 | 0.008 | 0.038 and 0.090 | 0.08 | 0.81 |
| CEA vs CA125 | 0.068 | 0.042 and 0.051 | 1.03 | 0.30 |
| CEA vs CA724 | 0.033 | 0.042 and 0.058 | 0.46 | 0.62 |
| CA125 vs CA724 | 0.035 | 0.051 and 0.058 | 0.45 | 0.63 |
The ability of CA242 to predict A-cMNs was not identified by our study. We found no significantly higher mean level nor any significantly greater number of subjects with a higher STM level than the cutoff in the advanced groups (P = 0.136 and 0.08) despite the comparable AUC to CA19-9 (0.758, P = 0.81). The combination of STMs increased the SE to 62.5%, but the SP decreased to 83.5%. However, the diagnostic AC remained high (75.4%) (Table 2). The AUC was comparable to that of CA19-9 alone (P = 0.32).
In all 151 IPMN subjects, both CA19-9 and CEA were available. CA125, CA724, and CA242 were available in 109, 96, and 28 subjects, respectively. CA19-9, CEA, and CA724 were identified as having definite abilities in predicting advanced IPMNs (Table 4).
| Overall | Benign | Advanced | P value1 | |
| CA19-9 (n = 151) | ||||
| Median, IQR (U/mL) | 9.7 (3.75-46.3) | 6.05 (3.1-15.2) | 46.3 (9.0-166.4) | < 0.0001 |
| ≥ 37/< 37 U/mL | 40/111 | 9/83 | 31/8 | < 0.0001 |
| CEA (n = 151) | ||||
| Median, IQR (ng/mL) | 2.7 (1.85-4.16) | 2.46 (1.67-3.44) | 3.1 (2.05-5.3) | 0.002 |
| ≥ 5/< 5 ng/mL | 24/127 | 9/83 | 15/44 | 0.01 |
| CA125 (n = 109) | ||||
| Median, IQR (U/mL) | 11.9 (8.0-17.35) | 11.65 (6.8-16.6) | 12.1 (8.7-18.8) | 0.051 |
| ≥ 35 /< 35 U/mL | 5/104 | 1/63 | 4/41 | 0.182 |
| CA724 (n = 96) | ||||
| Median, IQR (U/mL) | 1.64 (1.04-3.17) | 1.44 (1.02-2.7) | 2.5 (1.16-6.8) | 0.022 |
| ≥ 9.8/< 9.8 U /mL | 9/87 | 2/56 | 7/31 | 0.035 |
| CA242 (n = 28) | ||||
| Median, IQR (U/mL) | 7.44 (4.1-12.7) | 5.7 (3.6-8.23) | 9.8 (6.9-20.7) | 0.174 |
| ≥ 20/< 20 U/mL | 5/23 | 1/12 | 4/11 | 0.416 |
In all 36 MCN subjects, both CA19-9 and CEA were available. CA125, CA724, and CA242 were available in 26, 20, and 3 subjects, respectively. CA19-9, CEA, and CA125 were identified as having definite abilities in predicting advanced MCNs. Moreover, the subjects with advanced MCN were much older than those with non-advanced MCN (P = 0.005) (Table 5).
| Overall | Benign | Advanced | P value1 | |
| CA19-9 (n = 31) | ||||
| Median, IQR (U/mL) | 14.7 (7.3-108.1) | 7.73 (5.63-24.2) | 157 (15.7-1100) | 0.001 |
| ≥ 37/< 37 U/mL | 10/26 | 2/21 | 8/5 | 0.003 |
| CEA (n = 31) | ||||
| Median, IQR (ng/mL) | 1.5 (1.05-2.58) | 1.5 (1.0-1.87) | 2.56 (1.31-5.57) | 0.004 |
| ≥ 5/< 5 ng/mL | 3/33 | 0/23 | 3/10 | 0.04 |
| CA125 (n = 26) | ||||
| Median, IQR (U/mL) | 14.5 (10.0-25.7) | 14.4 (9.2-20.1) | 68.6 (11.9-125) | < 0.0001 |
| ≥ 35 /< 35 U/mL | 5/21 | 0/19 | 5/2 | < 0.0001 |
| CA724 (n = 20) | ||||
| Median, IQR (U/mL) | 1.5 (0.96-2.7) | 1.52 (0.99-2.24) | 1.53 (0.8-6.8) | 0.538 |
| ≥ 9.8/< 9.8 U /mL | 1/19 | 1/14 | 0/5 | 1.00 |
| CA242 (n = 3) | ||||
| Median, IQR (U/mL) | 2.39 (0.1) | 1.25 (0.1) | 35.35 | Not applicable |
| ≥ 20/< 20 U/mL | 1/2 | 0/2 | 1/0 | 0.333 |
The mean level and elevated number of subjects for CA19-9, CEA, CA724, and CA242 were not significantly different between advanced MCNs and IPMNs. However, the mean CA125 Level in advanced MCNs was 74.4 ± 60.3 U/mL, which was much higher than that in advanced IPMNs (17.3 ± 16.4 U/mL, P < 0.0001). CA125 tests were available in 7 of 13 advanced MCNs, and five (71.4%) subjects had elevated CA125 Levels. Meanwhile, CA125 tests were available in 45 of 59 advanced IPMNs, and four (8.9%) subjects had elevated CA125 levels. The number of subjects with elevated CA125 levels in advanced MCNs was also higher than that in IPMNs (P < 0.0001) (Table 6). The SE, SP, AC, and AUC of the standard serum CA125 cutoff level (35 U/L) in differentiating advanced MCNs and advanced IPMNs were 71.4%, 91.1%, 88.5%, and 0.779, respectively. Therefore, in addition to the ability to predict A-cMNs, serum CA125 testing may be useful to differentiate advanced MCNs and advanced IPMNs.
| Overall | Benign | Advanced | |
| mean ± SD (U/mL) (IPMN) | 14.6 ± 12.2 | 12.7 ± 7.56 | 17.3 ± 16.4 |
| mean ± SD (U/mL) (MCN) | 30.5 ± 40.5 | 14.3 ± 6.4 | 74.4 ± 60.3 |
| Comparison P value | 0.001 | 0.69 | < 0.0001 |
| ≥ 35/< 35 U/mL (IPMN) | 5/104 | 1/63 | 4/41 |
| ≥ 35/< 35 U/mL (MCN) | 5/21 | 0/19 | 5/2 |
| Comparison P value | 0.03 | 1.00 | < 0.0001 |
Thirteen cases of IPMN and one case of MCN with high-grade dysplasia were identified in our study. Due to the small number of MCNs with HGD, we only assessed the performance of STMs in predicting IPMN with HGD. CA19-9 and CEA were both available in all 13 HGD IPMNs. The mean levels of CA19-9 and CEA were 182.5 ± 356.5 U/mL and 3.77 ± 2.17 ng/mL for HGD IPMNs, respectively. There were five cases with CA19-9 levels > 37 U/mL and three cases with CEA levels > 5 ng/mL. In addition, seven patients with HGD IPMNs were tested for CA125 (mean level 10.9 ± 2.17 U/mL), five for CA724 (mean level 3.09 ± 2.4 U/mL), and three for CA242 (mean level 172.7 ± 283.5 U/mL). Comparison of levels of the five STMs between benign and HGD IPMN patients showed that only CA19-9 had the ability to predict HGD IPMNs. The SE, SP, AC, and AUC values of CA199 for differentiating HGD IPMNs and benign IPMNs were 38.5%, 90.2%, 83.8%, and 0.643, respectively.
Due to the absence of reliable standards for distinguishing advanced PCLs from non-advanced PCLs, the clinical management of PCLs remains difficult. PCLs, especially cMNs, have the potential to undergo carcinogenesis, and malignant transformation may take a relatively long time[3]. Therefore, early detection of A-cMNs is key to the management of PCLs. Current guidelines recommend long-term, routine yearly surveillance by magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP), and/or endoscopic ultrasound (EUS)[10-12]. The benefit of yearly surveillance in reducing pancreatic cancer-related mortality remains to be established. Further, routine surveillance may impose psychological and economic burdens[19]. STMs are much more accessible than MRI/MRCP/EUS, which can be obtained during health checkups. The value of STMs in predicting advanced PCLs remains to be fully elucidated.
The role of STMs in the early detection of pancreatic ductal adenocarcinoma (PDA) is doubtful due to their low SE. However, as a tumor progresses, the level of STMs increases[14]. Moreover, the 5-year survival rate after surgical resection of A-MNS was much higher than that of PDA[20-23]. In other words, although STMs may not be able to detect early A-MNs, they can contribute to finding late A-MNs that are more indolent than PDA, and the prognosis is still satisfactory[24]. Thus, unlike the role of STMs in PDA, STM testing may play a more active role in the management of PCLs.
CA19-9 was widely acknowledged as the most accurate STM for predicting A-cMNs, and the value of CA19-9 was also identified in our study. Although false negative results and false positive results (caused by biliary tract obstruction and inflammation, pancreatitis, and other digestive cancers)[9] limit its clinical application, it is still widely used in the management of PCLs. The pooled SE for CA19-9 in predicting advanced PCLs was approximately 40% by a meta-analysis[14]. As our study showed, although the best performances were observed in all analysis groups, the SE was suboptimal, and the AUC for CA19-9 was 0.766, which indicated that the ability of CA19-9 to predict A-cMNs was moderate. Moreover, if merely based on the commonly used cutoff (37 U/mL), up to 33/72 (45.8%) malignant subjects would be considered negative, and 11/115 (9.6%) benign subjects would be considered positive. Furthermore, we for the first time report that CA19-9 was still a useful diagnostic tool for predicting HGD IPMNs, but the SE and AUC decreased as expected.
The reported SE and SP of CEA for IPMNs were 18% and 93%, respectively[14]. In our study, serum CEA detection had the ability to distinguish A-cMNs from non-A-cMNs. However, the AUC for CEA was 0.651, and the SE was 25%. Both indicators performed worse than CA19-9. The results indicated that the predictive value of CEA for A-cMNs was low. Therefore, it is essential to explore other biomarkers to supplement CA19-9 and CEA to improve the diagnosis of A-cMNs.
Our study identified CA125 and CA724 as useful STMs in predicting A-cMNs with a relatively low SE (17.3% and 16.3%) and low AUC (0.583 and 0.618), which indicated that both STMs were weak predictors of A-cMNs. Therefore, CA125 and CA724 could not be identified as valuable screening markers for A-cMNs alone despite their relatively high SP (98.8% and 95.9%). Nevertheless, we identified that serum CA125 was more likely a predictor of advanced MCN than advanced IPMN. The results were similar to those of two recently published studies[25,26]. The possible reason may be the tissue histogenesis of MCN. Elevated CA125 levels are common in serous ovarian tumors. However, for mucinous ovarian tumors, an increase in serum CA125 levels is not uncommon[27]. MCNs and mucinous ovarian tumors both originate from primordial germ cells[4], and ovarian-like stroma can be obtained from the tissue of advanced MCNs[28]; thus, advanced MCNs may theoretically have an increased CA125 level as well as ovarian tumors. If a patient's serum CA125 level is elevated, it may be necessary to examine the pancreas to exclude advanced MCNs after an ovarian examination. However, the result was concluded from a small sample size, and the true value of CA125 in the management of A-cMNs needs to be further studied.
We did not find that CA242 showed predictive value. This may be due to our study’s small sample size. The recognition of CA242 has not been established until recent years in our hospital. As a result, there were fewer patients who underwent CA242 testing in our hospital than those who were tested for other STMs. However, CA242 had a comparable AUC to CA19-9 (0.758 vs 0.766, P = 0.81). The value of CA242 in PCLs may be verified by enlarging the sample size.
The combination of multiple STMs can improve their diagnostic AC in pancreatic cancer[5]. However, no one has explored this field in PCLs. In our study, the combination of STMs slightly increased the SE of CA19-9 to 62.5%. However, the SP decreased to 83.5%, and the AUC decreased to 0.715. The bias may exist due to the incomplete data for CA125, CA724, and CA242. Overall, the more data from these incomplete STMs that were included in our study, the more positive subjects were associated with combined results, and the SE was improved. Since STMs are the modalities for screening, the high SE of the combination will lead to more subjects undergoing further imaging evaluation to avoid missed diagnosis of advanced cases, which we deem justified. In addition, the reported AC of CT for identifying benign from malignant cysts was 71%–80%, and the AC of MRI or MRCP for differentiating a benign from a malignant cyst ranged from 55% to 76%[12]. The diagnostic accuracies were very similar to the AC of STMs that we reported in this research. Although imaging modalities can provide more information, we still believe that as a screening tool, serum STM detection may play a role in the management of PCLs.
Our study has limitations that merit discussion. First, this work was designed to be a retrospective, single-center study; hence, selection bias could not be completely avoided. Thus, the results of this study might not be applicable to other medical settings. Second, the CA125, CA724, and CA242 data were incomplete, which might influence the statistical results and decrease the AC of the combination analysis. Third, our analysis focused on cMNs. The application of our study might be limited in clinical practice due to the difficulty of making a definitive diagnosis preoperatively. Fourth, due to the lack of data, other STMs associated with pancreatic cancer, such as CA50, were not included.
In conclusion, this is the first study to illustrate the potential value of multiple STMs for the diagnosis of A-cMNs. CA19-9 is the most accurate STM for predicting A-cMNs, showing a moderate ability. CEA, CA125, and CA724 present a low ability to predict A-cMNs. CA125 may be specific to the diagnosis of advanced-MCNs. CA242 is not identified as a useful STM for predicting A-cMNs in our study; however, the AUC for CA242 is comparable to that for CA19-9. Thus, a greater sample size will be necessary to identify the true value of CA242. A combination of the five STMs could improve SE in predicting A-cMNs.
Early detection of advanced cystic mucinous neoplasms (A-cMNs) of the pancreas is key to the management of pancreatic cystic lesions (PCLs) in relation to the carcinogenic potential of cMNs. However, the long-term, routine yearly surveillance by imaging methods recommended by the current guidelines will impose heavy psychological and economic burdens on patients.
As an economical and feasible detection method, serum tumor markers (STMs) can be obtained during ordinary health checkups. However, the role of STMs in predicting advanced PCLs remains elusive. In view of the consistency between the increasing level of STMs and tumor progression, STM detection may play an important role in the early diagnosis of advanced PCLs.
This study aimed to evaluate the ability of five common serum tumor markers to predict A-cMNs separately and in combination. The relevant research results may be beneficial to the management of PCLs and the optimization of guidance or consensus.
This retrospective cohort study mainly measured the levels of serum carbohydrate antigen (CA) 19-9, carcinoembryonic antigen (CEA), CA125, CA724, and CA242 in patients pathologically diagnosed with cMNs to identify the ability of these STMs to predict A-cMNs and distinguish mucinous cystic neoplasms (MCNs) from intraductal papillary mucinous neoplasms. A receiver operating characteristic curve with an area under curve and the sensitivity (SE), specificity (SP), and accuracy were also created to identify the performance of the five STMs.
CA19-9 showed the highest SE and accuracy and a moderate ability to predict A-cMNs. The ability of CEA, CA125, and CA724 to predict A-cMNs was low. The predictive ability of CA242 was not identified. A combination of STMs could improve SE. CA125 was more likely a predictor of advanced MCNs than advanced intraductal papillary mucinous neoplasms.
CA19-9 showed a moderate ability, and CEA, CA125, and CA724 showed a low ability to predict A-cMNs. Detection of multiple STMs could improve SE in predicting A-cMNs, which has great potential to improve the early diagnosis rate of advanced PCLs in clinical practice. CA125 may be specific to the diagnosis of advanced MCNs and can be used as a reminder for physicians not to ignore pancreatic examination in patients with elevated CA125.
A multicenter prospective study that monitors PCL patients with detailed data on serum tumor markers should be performed.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Chinese Society of Digestive Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Viswanath YK S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Sun L, Wang Y, Jiang F, Qian W, Shao C, Jin Z. Prevalence of pancreatic cystic lesions detected by magnetic resonance imaging in the Chinese population. J Gastroenterol Hepatol. 2019;34:1656-1662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Zerboni G, Signoretti M, Crippa S, Falconi M, Arcidiacono PG, Capurso G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology. 2019;19:2-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 3. | Kromrey ML, Bülow R, Hübner J, Paperlein C, Lerch MM, Ittermann T, Völzke H, Mayerle J, Kühn JP. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 4. | Elias KM, Tsantoulis P, Tille JC, Vitonis A, Doyle LA, Hornick JL, Kaya G, Barnes L, Cramer DW, Puppa G, Stuckelberger S, Hooda J, Dietrich PY, Goggins M, Kerr CL, Birrer M, Hirsch MS, Drapkin R, Labidi-Galy SI. Primordial germ cells as a potential shared cell of origin for mucinous cystic neoplasms of the pancreas and mucinous ovarian tumors. J Pathol. 2018;246:459-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, Yang J, Li H, Wu Y, Zhang H, Chen W. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med. 2015;8:11683-11691. [PubMed] [Cited in This Article: ] |
| 6. | Dong D, Jia L, Zhang L, Ma N, Zhang A, Zhou Y, Ren L. Periostin and CA242 as potential diagnostic serum biomarkers complementing CA19.9 in detecting pancreatic cancer. Cancer Sci. 2018;109:2841-2851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Liu P, Zhu Y, Liu L. CA724 is a novel factor for predicting the unresectability in pancreatic adenocarcinoma. Int J Clin Exp Pathol. 2015;8:15112-15117. [PubMed] [Cited in This Article: ] |
| 8. | Liu L, Xu HX, Wang WQ, Wu CT, Xiang JF, Liu C, Long J, Xu J, Fu de L, Ni QX, Houchen CW, Postier RG, Li M, Yu XJ. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden. Oncotarget. 2016;7:5943-5956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Luo G, Liu C, Guo M, Cheng H, Lu Y, Jin K, Liu L, Long J, Xu J, Lu R, Ni Q, Yu X. Potential Biomarkers in Lewis Negative Patients With Pancreatic Cancer. Ann Surg. 2017;265:800-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in F6Publishing: 971] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 11. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 793] [Cited by in F6Publishing: 704] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 12. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 329] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 13. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [PubMed] [Cited in This Article: ] |
| 14. | Wang W, Zhang L, Chen L, Wei J, Sun Q, Xie Q, Zhou X, Zhou D, Huang P, Yang Q, Xie H, Zhou L, Zheng S. Serum carcinoembryonic antigen and carbohydrate antigen 19-9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Biomed Rep. 2015;3:43-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Jang JY, Park T, Lee S, Kim Y, Lee SY, Kim SW, Kim SC, Song KB, Yamamoto M, Hatori T, Hirono S, Satoi S, Fujii T, Hirano S, Hashimoto Y, Shimizu Y, Choi DW, Choi SH, Heo JS, Motoi F, Matsumoto I, Lee WJ, Kang CM, Han HS, Yoon YS, Sho M, Nagano H, Honda G, Kim SG, Yu HC, Chung JC, Nagakawa Y, Seo HI, Yamaue H. Proposed Nomogram Predicting the Individual Risk of Malignancy in the Patients With Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann Surg. 2017;266:1062-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Kim JR, Jang JY, Kang MJ, Park T, Lee SY, Jung W, Chang J, Shin Y, Han Y, Kim SW. Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19-9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas. J Hepatobiliary Pancreat Sci. 2015;22:699-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1539] [Cited by in F6Publishing: 1391] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 18. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1714] [Cited by in F6Publishing: 1540] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 19. | Andermann A, Blancquaert I, Beauchamp S, Déry V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ. 2008;86:317-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 507] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 20. | Hirono S, Shimizu Y, Ohtsuka T, Kin T, Hara K, Kanno A, Koshita S, Hanada K, Kitano M, Inoue H, Itoi T, Ueki T, Shimokawa T, Hijioka S, Yanagisawa A, Nakamura M, Okazaki K, Yamaue H. Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J Gastroenterol. 2020;55:86-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Antoñanzas J, Cienfuegos JA, Hurtado-Pardo L, Panadero P, Benito A, Pardo F, Rotellar F, Martí-Cruchaga P, Zozaya G, Valentí V, Hernández Lizoain JL. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: clinicopathological features and long-term outcomes following a pancreatectomy. Rev Esp Enferm Dig. 2018;110:768-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Al Efishat M, Attiyeh MA, Eaton AA, Gönen M, Basturk O, Klimstra D, D'Angelica MI, DeMatteo RP, Kingham TP, Balachandran V, Jarnagin WR, Allen PJ. Progression Patterns in the Remnant Pancreas after Resection of Non-Invasive or Micro-Invasive Intraductal Papillary Mucinous Neoplasms (IPMN). Ann Surg Oncol. 2018;25:1752-1759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Marchegiani G, Mino-Kenudson M, Ferrone CR, Morales-Oyarvide V, Warshaw AL, Lillemoe KD, Castillo CF. Patterns of Recurrence After Resection of IPMN: Who, When, and How? Ann Surg. 2015;262:1108-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Li Y, Zhu Z, Peng L, Jin Z, Sun L, Song B. The pathological features and prognoses of intraductal papillary mucinous neoplasm and mucinous cystic neoplasm after surgical resection: a single institution series. World J Surg Oncol. 2020;18:287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Deng S, Fan Z, Gong Y, Cheng H, Jin K, Qian Y, Xiao Z, Liu Y, Wang R, Zheng Y, Ni Q, Yu X, Liu C, Luo G. Clinical implication of serum CA125 for the prediction of malignancy in mucinous cystic neoplasms of the pancreas. Exp Ther Med. 2020;20:158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Zhao ZM, Jiang N, Gao YX, Yin ZZ, Zhao GD, Tan XL, Xu Y, Liu R. Clinical diagnosis and management of pancreatic mucinous cystadenoma and cystadenocarcinoma: Single-center experience with 82 patients. World J Gastrointest Oncol. 2020;12:642-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 27. | Moss EL, Hollingworth J, Reynolds TM. The role of CA125 in clinical practice. J Clin Pathol. 2005;58:308-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 28. | Crinò SF, Bernardoni L, Gabbrielli A, Capelli P, Salvia R, Rusev BC, Scarpa A, Manfrin E. Beyond Pancreatic Cyst Epithelium: Evidence of Ovarian-Like Stroma in EUS-Guided Through-the-Needle Micro-Forceps Biopsy Specimens. Am J Gastroenterol. 2018;113:1059-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |