Published online Dec 28, 2021. doi: 10.3748/wjg.v27.i48.8242
Peer-review started: March 2, 2021
First decision: April 17, 2021
Revised: June 4, 2021
Accepted: November 30, 2021
Article in press: November 30, 2021
Published online: December 28, 2021
Inflammatory bowel disease (IBD) is a chronic disease that requires chronic treatment throughout the evolution of the disease, with a complex physiopathology that entails great challenges for the development of new and specific treatments for ulcerative colitis and Crohn´s disease. The anti-tumor necrosis factor alpha therapy has impacted the clinical course of IBD in those patients who do not respond to conventional treatment, so there is a need to develop new therapies and markers of treatment response. Various pathways involved in the development of the disease are known and the new therapies have focused on blocking the inflammatory process at the gastrointestinal level by oral, intravenous, subcutaneous, and topical route. All these new therapies can lead to more personalized treatments with higher success rates and fewer relapses. These treatments have not only focused on clinical remission, but also on achieving macroscopic changes at the endoscopic level and microscopic changes by achieving mucosal healing. These treatments are mainly based on modifying signaling pathways, by blocking receptors or ligands, reducing cell migration and maintaining the integrity of the epithelial barrier. Therefore, this review presents the efficacy and safety of the new treatments that are currently under study and the advances that have been made in this area in recent years.
Core Tip: This review is to present the efficacy and safety of novel treatments for inflammatory bowel disease. The new treatments that may be available in the future are new anti-tumor necrosis factor alpha, anti-integrines, anti-interleukines, modulation of sphingosine-1-phosphate, janus kinase inhibitors, toll like receptor agonist, therapy on the integrity of the epithelial barrier, phosphodiesterase-4 inhibitors and antisense oligonucleotide therapy, currently in clinical studies. Many of them with encouraging results in clinical studies, while others have not been able to maintain significant results in the final phases.
- Citation: Yamamoto-Furusho JK, Parra-Holguín NN. Emerging therapeutic options in inflammatory bowel disease. World J Gastroenterol 2021; 27(48): 8242-8261
- URL: https://www.wjgnet.com/1007-9327/full/v27/i48/8242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i48.8242
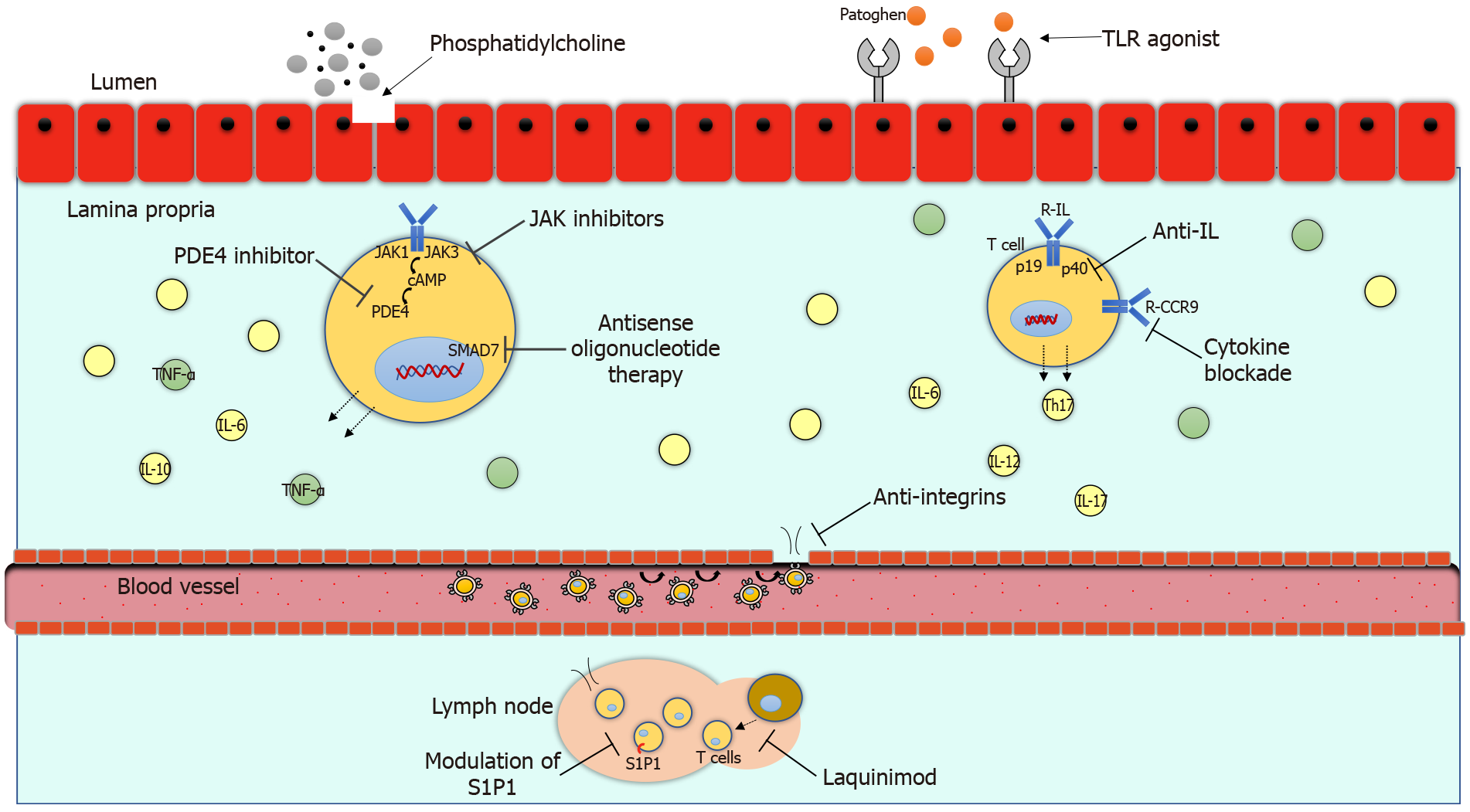
The pathogenesis of inflammatory bowel disease (IBD) is multifactorial and involves a series of factors specific to the patient and the environment. The chronic inflammatory process in ulcerative colitis (UC) and Crohn´s disease (CD) is causing damage to the intestinal mucosa with gastrointestinal and systemic symptoms. The anti-tumor necrosis factor alpha (TNF-α) therapy has impacted in the clinical course of IBD in those patients who do not respond to conventional treatment. Up to 30.0% of patients may not respond to initial anti-TNF alfa therapy and up to 46.0% may lose response during disease evolution[1]. Therefore, there is a need to innovate with the development of new treatments to be able to modify the clinical course of IBD including fewer clinical relapses, hospitalizations, surgeries and better quality of life. Currently, the approved biological treatments have great limitations such as their route of administration and adverse events. In recent years, new therapies have been developed to reduce the inflammatory process through different signaling pathways. There are several new mechanisms of action available such as anti-integrines, anti-interleukines, modulation of sphingosine-1-phosphate (S1P1), janus kinase (JAK) inhibitors, toll like receptor (TLR) agonist, phosphatidylcholin, phosphodiesterase-4 (PDE4) inhibitors and antisense oligonucleotide therapy, which are promising therapies currently in clinical studies. The mechanisms of action of the new biological treatments are illustrated in Figure 1. The purpose of this review is to present the efficacy and safety of novel treatments for IBD.
IBD is now recognized as an immune-mediated disease that occurs in genetically susceptible hosts and can be described as chronic perturbations in homeostasis between the host and the external environment. The interface of these interactions can be divided into three critical elements: intestinal epithelium, immune cells, and commensal microbiota.
One consensus hypothesis is that each of these three major host compartments that functions as an integrated supraorganism is affected by specific environmental (enteropathogens, antibiotics, smoking etc.) and genetic factors that come together in a susceptible host and lead to chronic dysregulation and development of inflammation[2]. Thus, in both UC and CD, an inflammatory pathway likely emerges from the genetic predisposition that is associated with inappropriate innate immune and epithelial sensing and reactivity to commensal microbiota that secrete inflammatory mediators, together with inadequate regulatory pathways that lead to activated CD4+ T cells within the intestinal epithelium and lamina propria, secreting excessive quantities of inflammatory cytokines relative to anti-inflammatory cytokines. Some activate other inflammatory cells (macrophages and B cells) and others act indirectly to recruit other lymphocytes, inflammatory leukocytes, and mononuclear cells from the vasculature into the gut, through interactions between homing receptors on leukocytes (e.g., α4β7 integrin) and addressins on the vascular endothelium (e.g., MadCAM1). Neutralization of TNF or α4β7 integrin is consistent with an effective treatment of IBD. There are three major types of CD4+ T cells that promote inflammation in the gut, all of which are possibly associated with colitis in animal models and humans: TH1 cells (secrete interferon, TNF), TH2 cells [secrete interleukin (IL)-4, IL-5, IL-13] and TH17 cells (secrete IL-17, IL-21). Each of these subsets of T cells cross-regulate each other. The TH1 cytokine pathway is initiated by IL-12, a key cytokine in the pathogenesis of experimental models of mucosal inflammation. IL-4 and IL-23, together with Il-6 and transforming growth factor beta (TGF-β), induce TH2 and TH17 cells, respectively. IL-23 also inhibits the suppressive functions of regulatory T cells[3]. Activated macrophages secrete TNF and IL-6.
Understanding inflammatory pathways has led to the development of new therapies, such as monoclonal antibodies that block pro-inflammatory cytokines or the signaling by their receptors (e.g., anti-TNF-α anti-IL-12, anti-IL-23, anti-IL-6 or JAK inhibitors); molecules associated with leukocyte recruitment (e.g., anti-α4β7); and the use of cytokines that inhibit inflammation (e.g., IL-10) or promote intestinal barrier function (e.g., epidermal growth factor), which may be beneficial to humans with intestinal inflammation.
We performed an exhaustive search, encompassing the last 10 years, in the Medline/PubMed, the Cochrane Database, EMBASE (Ovid), and LILACS databases, using the following MeSH terms: ulcerative colitis, Crohn’s disease, inflammatory bowel disease, pathogenesis, biologic therapy, new anti-TNF-α agents, anti-integrin therapy, vedolizumab, etrolizumab, abrilumab, ontamalimab, cytokine blockade, anti-interleukin therapy, vercirnon, anti-interlukin 23, eldelumab, rizankizumab, mirikizumab, brazikumab, guselkumab, briakinumab, anti-interleukin 17, secukinumab, brodalumab, anti-interleukin 6, interleukin 22, JAK inhibitors, upadacitinib, filgotinib, peficitinib, modulation of SIP1, ozanimod, etrasimod, amiselimod, laquinimod, toll like receptor agonist, cobitolimob, phosphatidylcholine, PDE4 inhibitor, apremilast, antisense oligonucleotide therapy, mongersen, GATA3 DNAzyme, alicaforsen. The search was limited to randomized controlled trials (RCTs) conducted on human subjects. Language: English. We also searched for any relevant RCTs included in the IBD Group Specialized Trials Register, the World Health Organization International Clinical Trials Registry, the European Union Clinical Trials Register, and the ClinicalTrials.gov to ensure identification of all eligible studies; and recent conference proceedings (European Crohn’s and Colitis Organisation, United European Gastroenterology Week, and Digestive Disease Week). Finally, we conducted supplemental searches of the regulatory authorities’ websites (European Medicines Agency: www.ema.europa.eu; United States Food and Drug Administration: www.fda.gov) to obtain details on study characteristics or outcomes.
This is a polyclonal anti-TNF antibody, currently in development and it has been tested in patients with moderate to severe disease UC activity. There is few information about its mechanism of action, it has been proposed to act locally in the gastrointestinal tract named AVX-470 has shown to inhibit gut inflammation in mice[4]. It is considered a large weight molecule of 160–900 kDa, with an oral administration which can avoid systemic adverse events. In phase 1 clinical trial, patients receive AVX-470 at doses of 0.2, 1.6 or 3.5 g a day, clinical response was an achievement in 7 (25.9%) with AVX-470 groups vs 1 (11.1%) in the placebo group and a significant reduction in serum C reactive protein (CRP) and IL-6. Low levels of anti-TNF antibodies were observed in patients who received this treatment, the antibody levels were lower compared to other anti-TNF therapies, having less immunogenicity avoiding future loss of response to this treatment, with a good safety profile, there were no serious adverse events in this human trial[5]. The phases of clinical trials of these new treatments are listed in Table 1.
| Treatment | UC | CD | Treatment | UC | CD | Treatment | UC | CD | Treatment | UC | CD |
| Anti-IL | Anti-integrin | JAK inhibitors | Other therapies | ||||||||
| Rizankinumab | III | III | Ontamalimab | III | II | Upadacitinib | III | III | AVX-470 | I | |
| Mirikizumab | III | II | Etrolizumab | III | III | Filgotinib | III | III | Laquinimod | - | II |
| Brazikumab | II | III | Abrilumab | II | II | Peficitinib | II | - | Cobitolimod | III | - |
| Guselkumab | II | III | AJM300 | III | - | TD-1473 | II | II | BL-7040 | II | - |
| Briakinumab | - | II | Cytokine blockade | Modulation of SIP1 | Phosphatidylcholine | III | - | ||||
| PTG‑200 | - | II | Vercirnon | - | III | Ozanimod | III | III | Apremilast | II | - |
| Secukinumab | - | II | Eldelumab | II | II | Etrasimod | III | - | Mongersen | - | II |
| Brodalumab | - | II | GSK3050002 | I | - | Amiselimod | - | II | GATA3 DNAzyme | II | - |
| PF-04236921 | - | II | KRP-203 | II | - | STNM01 | II | ||||
Integrins are receptors found on the cell surface for cell proliferation, signaling, and migration, its subunits binds to cell adhesion molecules (CAMs). The α4β1 integrin heterodimer binds VCAM-1 or fibronectin, α4β7 integrin heterodimer binds mucosal vascular addressin (MAd) CAM-1 and the αEβ7 integrin heterodimer binds E-cadherin[6]. Inhibiting these molecules have a therapeutic effect since it decreases the cell migration of pro-inflammatory cells in the gastrointestinal tract[7].
This is a fully human anti-MAdCAM-1 antibody, reducing lymphocyte migration. In a phase 2 study (TURANDOT trial) in patients with moderate to severe UC who failed conventional treatment, were randomized to receive ontamalimab subcutaneously (SC) at a dose of 7.5 mg, 22.5 mg, 75 mg, 225 mg or placebo every 4 wk, clinical remission was presented in 8 (11.3%), 12 (16.7%), 11 (15.5%) and 4 (5.7%) in the groups respectively and in the placebo group only in 2.7% of patients[8]. In the open label study for UC patients (TURANDOT II trial) mucosal healing increased from 20.3% from baseline to 28.5% at week 16 and was maintained until week 144 of follow-up[9]. The phase 3 study for patients with UC is currently recruiting patients[10]. In the phase 2 study (OPERA) in patients with CD, the results did not show significant differences compared to the placebo group[11], therefore, the phase 3 study in CD was suspended by the sponsor[12].
This is a humanized IgG1 monoclonal antibody (mAb) for the β7 integrin subunit and blocks the interactions of α4β7 with MAdCAM-1 and αEβ7 with E-cadherin[13]. This therapy suppresses the trafficking of lymphocytes in the intestine and the retention of lymphocytes in the intraepithelial compartment. In a phase 2 study, its efficacy for induction of remission in patients with UC was demonstrated previously with subcutaneous administration[11]. Currently, the phase 3 study is underway for patients with UC and CD with moderate to severe activity, it is composed of multiple randomized control trials HIBISCUS I and II, GARDENIA, LAUREL, HICKORY, ERGAMOT and open-label extension trials COTTONWOOD and JUNIPER. Also the purpose of these studies is not only to verify its efficacy and safety, but to compare with other biological treatments such as adalimumab and infliximab[14].
This is a fully humanized IgG2 mAb, with the same mechanism of action like vedolizumab, against the integrin α4β7[15]. A phase 2 study was conducted in patients with moderate to severe UC refractory to anti-TNF alpha and immunomodulatory therapy, were randomized to receive abrilumab SC at doses of 7, 21 or 70 mg on day 1, week 2 and 4, then every 4 wk, abrilumab 210 mg on day 1 or placebo. The clinical remission rates were 98 (13.3%), 79 (12.7%) and 116 (4.3%) (P ≤ 0.05) for abrilumab 70 mg, 210 mg and for placebo respectively at week 8. No serious adverse events occurred during the study. The most frequent adverse events reported for both groups was the reaction at the injection site, nasopharyngitis, headache, and arthralgias[16] . For patients with CD, a phase 2 study was conducted and were randomized to receive placebo or abrilumab at doses of 21 mg or 70 mg SC on day 1, weeks 2 and 4, and every 4 wk for 24 wk or only one dose of 210 mg SC on day 1, the primary endpoint was not reached and there were no significant differences in clinical remission compared to the placebo group[17].
AJM300 is an oral small molecule antagonist of α4 and target α4β7 and α4β1 integrin. Previous studies have demonstrated, a significant decrease in the number of T lymphocytes in the lamina propria in mice[18]. The therapeutic efficacy and safety of AJM300 were tested in a phase 2a study with 102 UC patients and were administered 960 mg orally for 8 wk, 3 times a day or placebo, to evaluate the induction to clinical remission. Clinical response rates were 32 (62.7%) and 13 (25.5%) (P = 0.0002), clinical remission in 12 (23.5%) and 2 (3.9%) (P = 0.0099), mucosal healing in 30 (58.8%) and 15 (29.4%) (P = 0.0014) at week 8 in the AJM300 and placebo group, respectively. This study demonstrated a significant improvement in clinical response, endoscopic remission, and histological response. No serious adverse effects were documented and only the most common adverse event was nasopharyngitis[19]. A phase 3 study with the same doses is currently being conducted to evaluate the efficacy and safety in patients with UC[20].
This is an antagonist against the receptor CCR9, inhibiting leukocyte traffic to the small intestine[21]. In a study phase 2 in patients with CD, subjects received 250 mg once daily, 250 mg twice daily, or 500 mg once daily of vercirnon or placebo for 12 wk as induction therapy and then they receive 250 mg of vercirnon through week 16 if they response were randomly assigned to receive 250 mg of vercirnon twice a day or placebo for 36 wk. Response rates for the induction therapy at week 12 was about 55 (56.0%, P = 0.168), 47 (49.0%, P = 0.792), 59 (61.0%, P = 0.039) in vercinon groups and 68 (47%) in the placebo group. In the maintenance period, 68 (47%) of subjects on vercirnon were in remission vs 29 (31%) in the placebo group (P = 0.012)[22] During the phase 3 study, patients were randomized into three groups to receive vercirnon 500 mg once a day, 500 twice a day, or placebo, clinical response at week 12 was in 56 (27.6%, P = 0.546), 55 (27.2%, P = 0.648) and 51 (25.1%), respectively. The most frequent adverse events were headache, worsening of CD and abdominal pain. This treatment failed to show the effectiveness of previous studies and no significant differences between the all study groups[23], so subsequent studies were canceled.
Eldelumab is a fully human mAb against the chemokine CXCL10, this chemokine is also involved in the traffic of leukocytes to the colon, its receptor CXCR3 is expressed on most T cells. In a phase 2 study in patients with UC, they receive 10 mg/kg of eldelumab or placebo intravenously (IV) every other week. The primary and secondary endpoints of clinical response, clinical remission and mucosal healing at day 57 were not met, but the clinical response and clinical remission rates were associated with higher drug exposure[24]. A phase 2 trial in patients with CD receives eldelumab 10 mg, 20 mg or placebo at days 1 and 8 and alternate weeks. Clinical remission was 29.3%, 22.5% and 20.0% in the 20 mg/kg, 10 mg/kg and placebo groups at week 11, but they were not significantly superior to the placebo group[25]. Despite the encouraging results of the clinical response related to drug exposure and a good safety profile, the response rates were lower, so further studies were not continued in IBD.
This is a mAb IgG1 with affinity to chemokine CCL20, binds to its receptor CCR6 expressed mainly in dendritic cells and B cells. The chemokine CCL20 is up-regulated in active IBD[26]. Currently, there are only phase 1 studies focused on patients with UC. In a study with healthy volunteers, they were administered, dose escalation of IV GSK3050002. With a half-life time of 2 wk, with a dose dependent decrease in CCR6, and a good safety profile at doses from 0.1 to 20 mg/kg[27].
In genome association studies, a strong association with the production of IL-17 and IL-23 has been shown, especially in patients with CD[28,29], as well as an increase in the expression of messenger RNA of these molecules and their intracellular proteins in the lamina propria of the gastrointestinal tract of patients with IBD[30,31].
This is a mAb that targets the p19 subunit, specific for IL-23. In the phase 2 studies for the induction of clinical remission in patients with moderate to severe CD, risankizumab was administered at doses of 200 and 600 mg IV where clinical remission was obtained in 12 (31%) vs 6 (15%) patients in the placebo group at week 12[32]. The maintenance of clinical remission with risankizumab in patients with CD, it was maintained in 44 (71%) of patients, 50 (81%) patients had a clinical response, 22 (35%) obtained endoscopic remission, 15 (24%) mucosal healing and 18 (29%) achieved clinical and endoscopic (deep) remission at week 52[33]. A phase 2 and 3 studies are currently recruiting patients with moderate to severe UC activity, with IV induction doses and subcutaneous maintenance SC doses[34], a phase 3 study of maintenance of remission is planned for patients who achieved clinical response and remission in the induction study[35]. A phase 3 study for induction of remission in CD and its maintenance until week 52[36].
This is a mAb that blocks selectively the p19 subunit of IL-23. In the phase 2 study in patients with moderate to severe activity of UC were randomized into four groups to receive doses at 50 mg, 200 mg, 600 mg and placebo SC at 4 and 8 wk. Clinical remission was obtained in 10 (15.9%), 14 (22.6%) and in 7 (11.5%) patients, respectively, compared with only 3 (4.8%) patients in the placebo group at week 12. The maintenance of clinical remission at doses of 200 mg every 4 wk, 200 mg every 12 wk and placebo, with 22 (46.8%), 17 (37.0%) and 1 (7.7%) of patients at week 52 in the maintenance of clinical remission[37]. The most frequently reported adverse effects were nasopharyngitis, nausea and worsening of UC. A phase 3 study (LUCENT 1) for induction of remission in 12 wk for UC patients with moderate to severe activity is currently under recruitment[38], as well as maintenance of remission (LUCENT 3)[39]. A phase 2 study for patients with CD (SERENITY) and a phase 3 study with an active arm for ustekinumab[40].
This is a mAb selectively directed to the p19 subunit of IL-23. Efficacy was evaluated in patients with CD and moderate to severe activity, who had a failure to anti-TNF-α, they were randomized with a dose of brazikumab of 700 mg IV or placebo at weeks 0 and 4. Followed by maintenance doses of 210 mg SC every 4 wk from weeks 12 to 112. Clinical response was measured in 29 (49.2%) vs 16 (26.7%) response from the placebo group at week 8. At week 24, the clinical response of 28 (53.8%) in the brazikumab group vs 30 (57.7%) in patients in the placebo group. A secondary outcome was to measure the expression of IL-22, a pro-inflammatory cytokine induced by the action of IL-23. Patients with a higher expression of IL-22 at the start of treatment was associated with a higher probability of response to brazikumab compared to the placebo group. The most frequently adverse effects were headache, nasopharyngitis, abdominal pain, arthralgia and proctalgia[41]. In patients with UC with moderate to severe activity named the EXPEDITION, which is a long-term phase 2 study of brazikumab in patients with UC with moderate to severe activity, is underway with IV brazikumab on days 1, 15 and 43, followed by brazikumab SC starting on day 71 every 4 wk[42]. It is also being evaluated in CD patients in a phase 3 study with severe activity, with IV brazikumab on days 1, 29, and 57, followed by SC brazikumab. For CD, a phase 3 study with an active arm is being recruited to compare adalimumab in which IL-22 was also included as a prognostic factor of response to treatment[43].
This is a mAb against the p19 subunit, whose efficacy has been proven and was approved for psoriasis treatment[44]. There are no data available so far on its efficacy and safety in patients with IBD, data are only available in patients with psoriasis and psoriatic arthritis where it has shown successful results with few adverse effects. There is an ongoing phase 2 study with combined therapy with guselkumab and golimumab in patients with moderate to severe UC activity. Participants will receive guselkumab at first dose as an IV infusion and the second one as a SC injection in addition to golimumab two doses as an SC injection and placebo[45]. For CD, a phase 2 study (GALAXI 1) is underway, participants will be assigned to five treatment groups, where groups 1 to 3will receive two doses of guselkumab IV and SC; group 4 will receive ustekinumab IV infusion followed by SC dosing, and group 5 will receive IV placebo at week 12. Those patients who do not respond will receive two doses of ustekinumab IV and SC. In GALAXI 2 and 3 studies, participants will be randomized to guselkumab, ustekinumab, or placebo[46]. A phase 3 study, is ongoing in patients with moderate to severe CD activity with IV guselkumab (3 doses) followed by SC guselkumab[47].
This is a mAb antibody against the p40 subunit of IL-12 and 23. Early studies, showed significant decreased in Th1 Lymphocytes in the gastrointestinal tract[48]. Currently it is only being evaluated for the treatment of psoriasis. In a phase 2 study, patients with CD were included in four treatment groups, they received briakinumab doses of 200 mg, 400 mg, 700 mg and placebo at weeks 0, 4 and 8. Patients who responded with doses of 400 mg and 700 mg were included in the maintenance phase at doses of 200 mg, 400 mg, 700 mg and placebo at weeks 12, 16 and 20. At week 24, 21 (43%), 21 (48%), 21 (57%) and 14 (29%) patients were in remission in the respectively groups. The most frequent adverse effect reported were respiratory infections in 20.7%, nausea in 17.3%, abdominal pain and headache 14.3%[49]. No current studies are undergoing in patients with CD and briakinumab.
This is a selective inhibitor blocks the IL-23 receptor, it has the main advantage of oral administration. In vivo studies, have demonstrated that a high concentration of this molecule at the gastrointestinal level and a minimum concentration at the systemic level. Phase 1 trials in healthy volunteers showed few adverse effects, none of them serious, with a half-life of approximately 1.5 h[50]. A phase 2 study is currently underway in patients with CD with moderate to severe activity to evaluate the efficacy and safety for 12 wk, with daily oral administration of PTG-200[51].
The IL-23 is involved in the signaling pathway of Th17 cells, these lymphocytes are producers of cytokines that enhance or regulate immune responses by interacting with other inflammatory cells such as macrophages, neutrophils, eosinophils, and basophils. These cells participate in the expression of subsets regulatory T cells and Th1, Th2, and Th17 lymphocytes[52]. Stimulation of neutrophil activation and IL-23-mediated induction of IL-17 and IL-22 production by neutrophils. All IL-17 producing cells predominate in patients with UC, mainly in the lamina propria, and CD transmurally[30].
Is a mAb of the IgG type which binds selectively to IL-17, preventing its union with its receptor, with this action the inflammatory process caused by this cytokine. In a phase 2 study carried out in patients with CD with moderate to severe active disease in which 59 patients were included who received IV secukinumab or placebo, 31% of patients in the secukinumab group discontinued the study prematurely due to lack of response to treatment. Higher rates of adverse effects were observed compared to the placebo group, 29 (74.4%) vs 10 (50%) patients. The most frequent adverse event were infections, worsening of CD, abdominal pain and arthralgias[53]. Secukinumab was approved for the treatment of psoriasis, but have been reported cases of IBD after the application of these biological in this group of patients[54,55], therefore, its use in patients with known IBD is not recommended and no new studies are undergoing.
Is a mAb that acts directed against the IL-17 receptor, inhibiting the inflammatory activity of this interleukin with high affinity[56]. Its availability is limited to psoriasis patients with moderate to severe disease. In the phase 2 study, patients with moderate to severe CD were enrolled to receive different doses of brodalumab 210, 350 and 700 mg at weeks 0 and 4 compared to a placebo group. This study was interrupted for aggravation of CD activity. Only 130 patients were randomized to receive treatment groups with clinical response in 1 (3.1%), 5 (15.2%), 3 (9.1%) and 1 (3.1%) in the brodalumab at 210 mg, 350 mg, 700 mg and placebo respectively at week 6. The most frequent adverse effect was worsening activity of CD[57]. There are no ongoing studies for Brodalumab in IBD.
This cytokine has inflammatory effects and inhibits apoptosis of T lymphocytes in the gastrointestinal mucosa[58]. Serum IL-6 concentrations are elevated in patients, with CD and correlates with CRP levels[59].
The PF-04236921 molecule is a IgG2 mAb that inhibits the action of IL-6, it has an approximate half-life of 36 to 51 d. The induction of clinical remission was evaluated with doses of 10 mg, 50 mg, 200 mg and placebo. The response rate at dose of 50 mg was 49.3% vs 30.6% (P ≤ 0.05) in the placebo group at week 8 and 27.4% and 10.9% (P ≤ 0.05) respectively at week 12. Common adverse effects were headache, abdominal pain and nasopharyngitis while serious adverse effects were presented in 3 (4.5%), in 7 (9.9%), in 8 (20%) patients in the 10 mg, 50 mg and 200 mg groups respectively, which include perforation and abscess formation[60].
Unlike the previous interleukins, IL-22 has an anti-inflammatory mechanism, it is elevated during inflammatory processes, with multiple functions such as regulation of the interaction between bacteria-host, protection and healing of the mucosa[59]. In patients with CD, it is higher compared to patients with UC, since previous studies have shown greater expression in the small intestine[61,62] and patients with active UC[63].
In a phase 1 stage in healthy volunteers, ascending doses of this molecule were used by IV and SC routes where they showed adequate pharmacokinetics with a good level of safety[64]. A phase 2 study is currently being recruited in patients with moderate to severe active UC, which will also include active arms with vedolizumab for the induction of clinical remission at week 8 as well as a maintenance phase will be evaluated as the primary objective until week 30[65].
This is a selective oral inhibitor of JAK1 compared to JAK2, JAK3 and TYK-2[66,67]. Upadacitinib down-regulates multiple pro-inflammatory cytokines, including the following interleukins: IL-2, 4, 6, 7, 9, 15, 21, and interferon gamma that are relevant to the pathogenesis of IBD[68]. A total of 220 patients were included to evaluate the induction of clinical remission in patients with CD who received upadacitinib orally twice a day, the clinical remission was reach in 39 (13%) with 3 mg, in 37 (27%, P < 0.1) with 6 mg, in 36 (11%) with 12 mg, 35 (14%) with 24 mg and 37 (11%) in the placebo group once a day at week 16. Endoscopic remission was greater the higher the dose, but not the clinical remission[66]. These results are similar for UC at doses of 7.5 mg, 15 mg, 30 mg or 45 mg once a day, with clinical remission in 4 (8.5%, P = 0.052), in (14.3%, P = 0.013), in 7 (13.5%, P = 0.011), in 11 (19.6%, P = 0.002) respectively and 0% in the placebo group at week 8[69]. Currently are conducting phase 3 studies for both diseases[70,71].
This is an inhibitor with higher selectivity for JAK1 over JAK2 and JAK3[72] in order to assess the induction of remission in patients with moderate to severe CD, 200 mg orally was administered once daily against placebo over a period of 10 wk, in 60 patients (47%) who received filgotinib achieved clinical remission at week 10 vs 10 (23%, P = 0.0077) patients in the placebo group, the most frequent adverse effects were: nasopharyngitis and urinary tract infections[73]. It is currently in recruitment in phase 3 study for patients with CD[74] and UC[75] with moderate to severe activity naïve to biological therapy or who had failure or intolerance to any other biological treatment.
Peficitinib inhibits selectively for JAK3 over JAK1, JAK2, and TYK2[76]. In phase 2 with UC patients, it was evaluated the efficacy at doses of 25 mg, 75 mg, 150 mg once a day, 75 mg twice a day and placebo orally. The primary endpoint of dose-response was not reached at week 8, but the clinical response, clinical remission and mucosal healing were higher at doses of ≥ 75 mg. Biochemical markers like fecal calprotectin and CRP were not significantly reduced with peficitinib. The most frequent adverse events were worsening of UC, increased blood creatine phosphokinase and anemia[77].
TD-1473 is a gut-selective pan-JAK inhibitor, administered orally, inhibits cytokine signaling directly in the gastrointestinal tract avoiding systemic effects. Phase 1 in mice and healthy volunteers show high intestinal drug exposure compared with plasma. The Phase 1 study was done in UC with moderate to severe active disease, and evaluate 3 doses 20 mg, 80 mg and 270 mg orally once a day after an overnight fast for 28 d, no efficacy analysis was carried out but tendencies to decrease UC activity were found[78]. A phase 2 study is currently being carried out in patients with CD (DIONE)[79] and a phase 2 and 3 for patients with UC (RHEA)[80].
Small molecule drugs have intrinsic properties that distinguish them from biological therapies: they are administered orally, have a short half-life and a low risk of immunogenicity[81].
This is an oral agonist of the S1P1 and 5 receptors, decreasing the number of activated lymphocytes circulating to the gastrointestinal tract. The clinical remission occurred in 11 (16%, P = 0.048) who received 1 mg ozanimod and in 9 (14%, P = 0.14) who received 0.5 mg ozanimod, compared with 4 (6%) patients who received placebo at week 8. In the maintenance period, the clinical remission was in 14 (21%, P = 0.01) in the ozanimod 1 mg group, 17 (26%, P = 0.002) in the 0.5 mg group, and 6% in the placebo group at week 32. The main adverse effects presented were anemia and headache[82]. Preliminary results in CD receiving ozanimod 1 mg orally daily showed improvement in mucosal healing in patients with moderate to severe CD treated for 12 wk[83]. A phase 3 study, is currently being carried out to evaluate the induction and maintenance of clinical remission for CD and a phase 3 for UC is completed pending publication of official results[84,85].
This is a selective modulator of the S1P1, S1P4 and S1P5 sphingosine receptors, decreasing the production of several cytokines[86]. After treatment with etrasimod 2 mg once daily, an approximately 53% decreased in mean lymphocyte count was observed in healthy volunteer patients on day 3, with a continuous decrease in 69% of patients by day 21. In a phase 2 study in UC, were randomized in 3 groups: 1 mg, 2 mg and placebo for 12 wk orally once a day, the primary endpoint was an improvement in the modified Mayo index that evaluates the frequency of stools, rectal bleeding and endoscopic findings. Clinical remission was observed in 33.0% (P ≤ 0.001) of the etrasimod 2 mg group compared with 8.1% of the placebo group. Endoscopic improvement occurred in 41.8% (P = 0.003) in the 2 mg group. No significant differences were found concerning adverse effects compared with the placebo group[87]. A phase 3 study is recruiting patients, with UC for the administration of etrasimod 2 mg orally for 52 wk[88].
This is a S1P1 receptor modulator, with more favorable cardiac safety profile than other S1P1 receptor modulators[89]. It was evaluated in patients with CD, with clinically active disease and elevated biomarkers, in patients who were previously treated with steroids, immunomodulators and/or anti-TNF-α treatment. The dose evaluated was 0.4 mg orally once a day for 14 wk. The primary endpoint of CDAI100 was achieved in 19 (48.7%) in the amiselimod group vs 20 (54.1%) patients in the placebo group. Adverse effects were observed in both groups, infections occurred in 26% vs 13% of the placebo group. Cardiac disorders such as ventricular tachycardia, bradycardia, ventricular extrasystoles were observed[90].
This is a S1P1, 4, 5 receptor agonist and partial agonist of S1P3 receptor. In a phase 2 with moderate UC activity and 5-aminosalicylate refractory patients. They received 1.2 mg of KRP203 or placebo daily for 8 wk. No statistically significant differences were found between both groups, but the frequency of clinical remission was 14% and 0% in the placebo group. No adverse cardiac events were reported during the study, the most frequent adverse events were gastrointestinal disorders and headache[91].
This an oral small-molecule with a direct inhibitory effect on T cells and causes a decreased pro-inflammatory cytokines in the gastrointestinal tract[92]. In a phase 2 study in patients with active CD, they receive 0.5 mg, 1.5 mg, or 2 mg a day of laquinimod or a placebo, for 8 wk. The primary endpoint was a clinical response of 70 or 100 points of CDAI reduction from baseline or remission and no treatment failure. A dose of 0.5 mg showed improvement on remission rates in 14 patients (48.3%) vs 10 patients (15.9%), a response of 100 CDAI of 55.2% vs 31.7% and response CDAI 70 in 62.1% vs 34.9% in the placebo group. The most frequents adverse events were headache and abdominal pain[93].
The TLR-9 is mainly expressed on dendritic cells and macrophages, the TLR recognize pathogenic molecules to release anti-inflammatory mechanisms. TLR-9 expression is upregulated in the mucosa of the rectum in UC patients with active disease compared with healthy controls and patients with UC in remission. Activation of the TLR-9 receptor has been proposed to stimulate intestinal mucosal healing[94].
This is a TLR-9 agonist which is a synthetic oligonucleotide that induced the production of IL-10 and other anti-inflammatory cytokines[95]. Furthermore, it has been seen in cell studies to increase steroid sensitivity in patients with steroid-resistant UC patients[96]. In UC patients refractory to conventional treatment and anti-TNF-α therapy, were included to receive rectally DIMS0150 30 mg or placebo. No statistical differences between 30 mg and placebo were found, with the induction of clinical remission at week 12 in 44.4% and 46.5% respectively. With symptomatic remission in 32.1% vs 14.0% in the 30 mg and placebo group (P = 0.020) at week 4, and 44.4% vs 27.9% at week 8 (P = 0.061). Mucosal healing at week 4 in 21.0% vs 4.7% (P = 0.01), there were no major safety events during study development[97]. A phase 2 trial (CONDUCT study) patients were randomized to receive rectal enemas at doses of 31 mg, 125 mg or 250 mg at weeks 0 and 3, and cobitolimod at doses of 125 mg or placebo at week 0, 1, 2 and 3. There were statistically significant differences for clinical remission at week 6 in the 250 mg group in the 21.0% vs 7% in placebo (P = 0.0025)[98].
This is a TLR-9 modulator, in phase 2, in UC with moderate clinical activity, received BL-7040 orally, 12 mg for 19–21 d followed by 40 mg for an additional 14 d, clinical remission was achieved in 12.5%, mucosal healing was achieved in 50%, and was well tolerated with one serious adverse event not related to the study[99].
Is usually found in the intestinal barrier, maintaining its integrity, it is decreased in patients with UC and cause epithelial permeability[100], these changes have developed in mice models and a probable role in the pathogenesis of IBD development has been demonstrated[101]. In a phase 2 study in UC patients, the treatment was administered orally with pellets, four times daily at doses of 0, 0.8, 1.6, or 3.2 g. Clinical remission was achieved in the 31.4% of 3.2 g vs 15.0% in the placebo group (P = 0.089). Mucosal healing was achieved in 47.4% vs 32.5% (P = 0.098), histologic remission in 47 (40.5%) vs 8 (20.0%) respectively (P = 0.016)[102]. A phase 3 study was recently conducted (PROTECT-2) compared with mesalamine and placebo for the maintenance of remission in patients with UC, but the results have not been published so far[103]. The study for induction of remission (PROTECT-3) in UC was terminated because it did not show any efficacy for achieving induction of remission[104].
Apremilast: This an oral small molecule that specifically inhibits PDE4[105] ,with activation of intracellular cAMP levels and an increase the production of anti-inflammatory cytokines with effects on innate inmmunity[106] and is currently approved for the use in psoriasis. In the phase 2 study in patients with UC, patients were randomized to receive apremilast 30 mg, 40 mg or placebo twice daily for 12 wk and subsequently randomized to receive 30 or 40 mg for 40 wk. Clinical remission was achieved in 31.6% and in 12.1%, (P = 0.01) in the groups of 30 mg and placebo, respectively at week 12, without significant differences for the group of 40 mg. During the maintenance period, clinical remission was achieved in 40.4% in the 30 mg group vs 32.7% in the 40 mg group[107].
Mongersen GED0301: TGF-β is an important cytokine with an anti-inflammatory functions, with a regulatory function of T cells[108]. The activation of this factor causes a phosphorylation of the SMAD2/3complex complex, in this pathway SMAD7 acts, which is responsible for downregulating TGF-Β, blocking the activation of the SMAD2/3complex complex. TGF-Β is normally produced in patients with IBD but it did not achieve its anti-inflammatory effect due to the high production of SMAD7 in these patients[109]. Mongersen is an anti-SMAD7 oligonucleotide, against SMAD7 mRNA, decreasing the production of this inhibitor[110]. Mongersen is for oral use and binds to the TGF-β receptor inhibiting the signal of SMAD2 and 3[111], and reduce pro inflammatory cytokines[112]. A phase 2 study of Mongersen was conducted in CD patients with doses of 10, 40, 160 and placebo, clinical remission at 2 wk was archived in 55% and 65% in the groups of 40 and 160 mg respectively (P ≤ 0.001), with no significant differences in the 10 mg group[113]. A subsequent study was performed, with a dose of 160 mg in three groups 4, 8 and 12 wk of follow-up with clinical remission in 32%, 35% and 48% respectively[114]. In the phase 3 study was cancelled for findings of non-effectiveness in this group of CD patients[115].
The inflammatory process is regulated by lymphocytes Th2 and the production of IL-4, 5 and 13 in UC. In CD the response is characterized by Th1 and release of interferon gamma and TNF. This treatment was first studied in patients with asthma and the evidence was shown a decrease in IL production[116]. GATA3 is a transcription factor for the transcription of cytokines of Th2 response[117], and GATA3 RNA transcripts are higher in colonic UC biopsies[116]. Animal models treated with a DNAzyme anti-GATA3 with intrarectal administration showed a reduction in the production of pro-inflammatory cytokines[118]. Phase 2 was conducted to evaluate the efficacy and safety of a topical formulation by enema in patients with moderate to severe active UC, but results have not been published yet[119].
In patients with CD, the development of fibrotic stenoses is common due to the chronic inflammation that causes a remodeling process. The treatment of this issue is endoscopic or surgical resection. In recent years, the enzyme carbohydrate sulfotransferase 15 (CHST15) was discovered, is responsible for regulating the production of glycosaminoglycans that cause the fibrotic process in patients with CD[109]. STNM01 is an RNA oligonucleotide against CHST15, inhibits the expression of mRNA with less production of glycosaminoglycans in the colon. The first studies in mice were carried out using direct submucosal injections into the colon[120]. The study in CD patients with ulcerative lesions was randomized to receive a single submucosal injection by endoscopic route or placebo, in the largest ulcerated lesion that was visualized by colonoscopy. A decrease in the extent of fibrosis was documented by histology, and no adverse effects were documented during the study[121]. A phase 2a study was conducted in patients with refractory and left-sided UC in 24 patients. They were randomized into 3 groups to receive a single dose of 25 nM, 250 nM or placebo by submucosal injection. The primary endpoint was mucosal healing on days 14 and 25, which was achieved in 62.5% vs 28.6% in the 250 nM and placebo group, respectively. Clinical response was shown by 62.5% in the STNM01 250 nM group (P = 0.3200) vs 28.6% in the placebo group and clinical remission in 50.0% in the 250 nM vs 14.3% in the placebo group (P = 0.04), with a good safety profile[122].
This a 20-base ICAM-1 human antisense oligonucleotide that targets the mRNA of ICAM-1 and causes its inactivation[123]. Initially, it was used in patients with CD, IV and SC with few results, in recent years alicaforsen was reformulate to its use in enemas for patients with UC and pouchitis. A randomized phase 2 study was carried out in patients with UC with mild to moderate distal disease, they received a 60 mL enema with 0.1, 0.5, 2 or 4 mg/mL or placebo once daily for 28 d. Alicaforsen improves the disease activity index in 70% vs 28% patients in the placebo group (P = 0.004) at day 29. The most frequent adverse events were asthenia, infections, and nausea. No serious adverse events related to the medical treatment[124]. In another phase 2 clinical trial, no significant difference was observed between treatment arms and placebo in the primary endpoint[125]. In a case series in patients with refractory pouchitis, clinical improvement was achieved in 84.6%, but 81.8% patients had a relapse after a median of 16 wk[126]. A phase 3 study was performed in patients with pouchitis who failed at least one course of antibiotics and received alicaforsen 240 mg or placebo once daily for 6 wk. Preliminary results showed reduction in the stool frequency in 33.8% and 26.2% in the treatment group vs placebo, respectively[127].
The clinical course of the disease in IBD may change in the coming years with the evolution of the new therapies that are being studied at this time. Most of these new therapies are in advanced phases of study with promising results, with similar response rates to currently approved therapies. The purpose of these new therapeutic targets will allow us to personalize medicine to treat IBD, according to the characteristic pathogenesis of each patient. More studies are needed to verify their efficacy and safety, as well as studies comparing these therapies with emerging or approved therapies to have accurate results.
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ji G S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol. 2016;7:e135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 432] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2894] [Cited by in F6Publishing: 3117] [Article Influence: 183.4] [Reference Citation Analysis (8)] |
| 3. | Nascimento Santos L, Carvalho Pacheco LG, Silva Pinheiro C, Alcantara-Neves NM. Recombinant proteins of helminths with immunoregulatory properties and their possible therapeutic use. Acta Trop. 2017;166:202-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Burton RE, Kim S, Patel R, Hartman DS, Tracey DE, Fox BS. Structural features of bovine colostral immunoglobulin that confer proteolytic stability in a simulated intestinal fluid. J Biol Chem. 2020;295:12317-12327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Harris MS, Hartman D, Lemos BR, Erlich EC, Spence S, Kennedy S, Ptak T, Pruitt R, Vermeire S, Fox BS. AVX-470, an Orally Delivered Anti-Tumour Necrosis Factor Antibody for Treatment of Active Ulcerative Colitis: Results of a First-in-Human Trial. J Crohns Colitis. 2016;10:631-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Dotan I, Allez M, Danese S, Keir M, Tole S, McBride J. The role of integrins in the pathogenesis of inflammatory bowel disease: Approved and investigational anti-integrin therapies. Med Res Rev. 2020;40:245-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Slack RJ, Macdonald SJF, Roper JA, Jenkins RG, Hatley RJD. Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 152] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 8. | Vermeire S, Sandborn WJ, Danese S, Hébuterne X, Salzberg BA, Klopocka M, Tarabar D, Vanasek T, Greguš M, Hellstern PA, Kim JS, Sparrow MP, Gorelick KJ, Hinz M, Ahmad A, Pradhan V, Hassan-Zahraee M, Clare R, Cataldi F, Reinisch W. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 9. | Shire. Long-Term Safety of PF-00547659 In Ulcerative Colitis (TURANDOT II). [accessed 2020 Dec 27]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01771809 ClinicalTrials.gov Identifier: NCT01771809. [Cited in This Article: ] |
| 10. | Takeda. A Safety Extension Study of Ontamalimab in Participants With Moderate to Severe Ulcerative Colitis or Crohn’s Disease (AIDA). [accessed 2020 Dec 27]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03283085 ClinicalTrials.gov Identifier: NCT03283085. [Cited in This Article: ] |
| 11. | Saruta M, Park DI, Kim YH, Yang SK, Jang BI, Cheon JH, Im JP, Kanai T, Katsuno T, Ishiguro Y, Nagaoka M, Isogawa N, Li Y, Banerjee A, Ahmad A, Hassan-Zahraee M, Clare R, Gorelick KJ, Cataldi F, Watanabe M, Hibi T. Anti-MAdCAM-1 antibody (PF-00547659) for active refractory Crohn's disease in Japanese and Korean patients: the OPERA study. Intest Res. 2020;18:45-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Takeda. Efficacy and Safety Study of Ontamalimab as Induction Therapy in Participants With Moderate to Severe Crohn’s Disease (CARMEN CD 306) (CARMEN CD 306). [accessed 2020 Dec 27]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03566823 ClinicalTrials.gov Identifier: NCT03566823. [Cited in This Article: ] |
| 13. | Vermeire S, O'Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, Lamb CA, Feagan BG, Panes J, Salas A, Baumgart DC, Schreiber S, Dotan I, Sandborn WJ, Tew GW, Luca D, Tang MT, Diehl L, Eastham-Anderson J, De Hertogh G, Perrier C, Egen JG, Kirby JA, van Assche G, Rutgeerts P. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 14. | Sandborn WJ, Vermeire S, Tyrrell H, Hassanali A, Lacey S, Tole S, Tatro AR; Etrolizumab Global Steering Committee. Etrolizumab for the Treatment of Ulcerative Colitis and Crohn's Disease: An Overview of the Phase 3 Clinical Program. Adv Ther. 2020;37:3417-3431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 15. | Pan WJ, Köck K, Rees WA, Sullivan BA, Evangelista CM, Yen M, Andrews JM, Radford-Smith GL, Prince PJ, Reynhardt KO, Doherty DR, Patel SK, Krill CD, Zhou K, Shen J, Smith LE, Gow JM, Lee J, Treacy AM, Yu Z, Platt VM, Borie DC. Clinical pharmacology of AMG 181, a gut-specific human anti-α4β7 monoclonal antibody, for treating inflammatory bowel diseases. Br J Clin Pharmacol. 2014;78:1315-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Hibi T, Motoya S, Ashida T, Sai S, Sameshima Y, Nakamura S, Maemoto A, Nii M, Sullivan BA, Gasser RA Jr, Suzuki Y. Efficacy and safety of abrilumab, an α4β7 integrin inhibitor, in Japanese patients with moderate-to-severe ulcerative colitis: a phase II study. Intest Res. 2019;17:375-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Amgen. Abrilumab (AMG 181) in Adults With Moderate to Severe Crohn´s Disease. [accessed 2020 Dec 27]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01696396 ClinicalTrials.gov Identifier: NCT01696396. [Cited in This Article: ] |
| 18. | Sugiura T, Kageyama S, Andou A, Miyazawa T, Ejima C, Nakayama A, Dohi T, Eda H. Oral treatment with a novel small molecule alpha 4 integrin antagonist, AJM300, prevents the development of experimental colitis in mice. J Crohns Colitis. 2013;7: e533-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Yoshimura N, Watanabe M, Motoya S, Tominaga K, Matsuoka K, Iwakiri R, Watanabe K, Hibi T; AJM300 Study Group. Safety and Efficacy of AJM300, an Oral Antagonist of α4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology. 2015;149:1775-1783.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Eisai Inc. A Study to Evaluate the Safety and Efficacy of AJM300 in Participants with Active Ulcerative Colitis. [accessed 2020 Dec 27]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03531892 ClinicalTrials.gov Identifier: NCT03531892. [Cited in This Article: ] |
| 21. | Misselwitz B, Juillerat P, Sulz MC, Siegmund B, Brand S; Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Emerging Treatment Options in Inflammatory Bowel Disease: Janus Kinases, Stem Cells, and More. Digestion. 2020;101 Suppl 1:69-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Keshav S, Vaňásek T, Niv Y, Petryka R, Howaldt S, Bafutto M, Rácz I, Hetzel D, Nielsen OH, Vermeire S, Reinisch W, Karlén P, Schreiber S, Schall TJ, Bekker P; Prospective Randomized Oral-Therapy Evaluation in Crohn’s Disease Trial-1 PROTECT-1 Study Group. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn's disease. PLoS One. 2013;8:e60094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Feagan BG, Sandborn WJ, D'Haens G, Lee SD, Allez M, Fedorak RN, Seidler U, Vermeire S, Lawrance IC, Maroney AC, Jurgensen CH, Heath A, Chang DJ. Randomised clinical trial: vercirnon, an oral CCR9 antagonist, vs. placebo as induction therapy in active Crohn's disease. Aliment Pharmacol Ther. 2015;42:1170-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Ragusa F. Th1 chemokines in ulcerative colitis. Clin Ter. 2015;166:e126-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 25. | Sandborn WJ, Rutgeerts P, Colombel JF, Ghosh S, Petryka R, Sands BE, Mitra P, Luo A. Eldelumab [anti-interferon-γ-inducible protein-10 antibody] Induction Therapy for Active Crohn's Disease: a Randomised, Double-blind, Placebo-controlled Phase IIa Study. J Crohns Colitis. 2017;11:811-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Skovdahl HK, Damås JK, Granlund AVB, Østvik AE, Doseth B, Bruland T, Mollnes TE, Sandvik AK. C-C Motif Ligand 20 (CCL20) and C-C Motif Chemokine Receptor 6 (CCR6) in Human Peripheral Blood Mononuclear Cells: Dysregulated in Ulcerative Colitis and a Potential Role for CCL20 in IL-1β Release. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Bouma G, Zamuner S, Hicks K, Want A, Oliveira J, Choudhury A, Brett S, Robertson D, Felton L, Norris V, Fernando D, Herdman M, Tarzi R. CCL20 neutralization by a monoclonal antibody in healthy subjects selectively inhibits recruitment of CCR6+ cells in an experimental suction blister. Br J Clin Pharmacol. 2017;83:1976-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ; NIDDK IBD Genetics Consortium, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E; Belgian-French IBD Consortium; Wellcome Trust Case Control Consortium, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2041] [Cited by in F6Publishing: 1984] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 29. | Sedda S, Bevivino G, Monteleone G. Targeting IL-23 in Crohn's disease. Expert Rev Clin Immunol. 2018;14:907-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Fonseca-Camarillo G, Mendivil EJ, Furuzawa-Carballeda J, Yamamoto-Furusho JK. Interleukin 17 gene and protein expression are increased in patients with ulcerative colitis. Inflamm Bowel Dis. 2011;17:E135-E136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Siakavellas SI, Bamias G. Role of the IL-23/IL-17 axis in Crohn's disease. Discov Med. 2012;14:253-262. [PubMed] [Cited in This Article: ] |
| 32. | Feagan BG, Sandborn WJ, D'Haens G, Panés J, Kaser A, Ferrante M, Louis E, Franchimont D, Dewit O, Seidler U, Kim KJ, Neurath MF, Schreiber S, Scholl P, Pamulapati C, Lalovic B, Visvanathan S, Padula SJ, Herichova I, Soaita A, Hall DB, Böcher WO. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn's disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 317] [Article Influence: 45.3] [Reference Citation Analysis (1)] |
| 33. | Feagan BG, Panés J, Ferrante M, Kaser A, D'Haens GR, Sandborn WJ, Louis E, Neurath MF, Franchimont D, Dewit O, Seidler U, Kim KJ, Selinger C, Padula SJ, Herichova I, Robinson AM, Wallace K, Zhao J, Minocha M, Othman AA, Soaita A, Visvanathan S, Hall DB, Böcher WO. Risankizumab in patients with moderate to severe Crohn's disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018;3:671-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | AbbVie. A Multicenter, Randomized, Double-Blind, Placebo Controlled Induction Study to Evaluate the Efficacy and Safety of Risankizumab in Participants With Moderately to Severely Active Ulcerative Colitis. [accessed 2020 Dec 27]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03398148 ClinicalTrials.gov Identifier: NCT03398148. [Cited in This Article: ] |
| 35. | AbbVie. A Study to Assess the Efficacy and Safety of Risankizumab in Participants With Ulcerative Colitis. [accessed 2020 Dec 27]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03398135 ClinicalTrials.gov Identifier: NCT03398135. [Cited in This Article: ] |
| 36. | AbbVie. A Study of the Efficacy and Safety of Risankizumab in Participants With Crohn’s Disease. [accessed 2020 Dec 27]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03105128 ClinicalTrials.gov Identifier: NCT03105128. [Cited in This Article: ] |
| 37. | Sandborn WJ, Ferrante M, Bhandari BR, Berliba E, Hibi T, D'Haens GR, Tuttle JL, Krueger K, Friedrich S, Durante M, Arora V, Naegeli AN, Schmitz J, Feagan BG. Efficacy and Safety of Continued Treatment With Mirikizumab in a Phase 2 Trial of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 38. | Eli Lilly and Company. An Induction Study of Mirikizumab in Participants With Moderately to Severely Active Ulcerative Colitis (LUCENT 1). [accessed 2020 Dec 28]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03518086 ClinicalTrials.gov Identifier: NCT03518086. [Cited in This Article: ] |
| 39. | Eli Lilly and Company. A Study to Evaluate the Long-Term Efficacy and Safety of Mirikizumab in Participants With Moderately to Severely Active Ulcerative Colitis (LUCENT 3). [accessed 2020 Dec 28]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03519945 ClinicalTrials.gov Identifier: NCT03519945. [Cited in This Article: ] |
| 40. | Eli Lilly and Company. A Study of Mirikizumab (LY3074828) in Participants With Crohn’s Disease (VIVID-1). [accessed 2020 Dec 28]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03926130 ClinicalTrials.gov Identifier: NCT03926130. [Cited in This Article: ] |
| 41. | Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, Higgins PDR, Newbold P, Faggioni R, Patra K, Li J, Klekotka P, Morehouse C, Pulkstenis E, Drappa J, van der Merwe R, Gasser RA Jr. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn's Disease: A Phase 2a Study. Gastroenterology. 2017;153:77-86.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 42. | Allergan. An Active and Placebo-Controlled Study of Brakizumab in Participants with Moderately to Severely Active Ulcerative Colitis [EXPEDITION]. [accessed 2020 Dec 28]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03616821 ClinicalTrials.gov Identifier: NCT0361682. [Cited in This Article: ] |
| 43. | Allergan. An Active and Placebo-Controlled Study of Brakizumab in Participant With Moderately to Severely Active Crohn´s Disease. [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03759288 ClinicalTrials.gov Identifier: NCT03759288. [Cited in This Article: ] |
| 44. | MacDonald JK, Nguyen TM, Khanna R, Timmer A. Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2016;11:CD007572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Janssen Research & Development, LLC. A Study of Efficacy and Safety of Combination Therapy With Guselkumab and Golimumab in Participants With Moderately to Severely Active Ulcerative Colitis (VEGA). [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03662542 ClinicalTrials.gov Identifier: NCT03662542. [Cited in This Article: ] |
| 46. | Janssen Research & Development, LLC. A Study of the Efficacy and Safety of Guselkumab in Participants With Moderately to Severely Active Crohn’s Disease (GALAXI). [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT0346641 ClinicalTrials.gov Identifier: NCT0346641. [Cited in This Article: ] |
| 47. | Janssen Pharmaceutical K. K. A Study of Guselkumab in Participants With Moderately to Severely Active Crohn’s Disease. [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: clinicaltrials.gov/ct2/show/NCT04397263 ClinicalTrials.gov Identifier: NCT04397263. [Cited in This Article: ] |
| 48. | Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, Quezado M, Yang Z, Neurath MF, Salfeld J, Veldman GM, Schwertschlag U, Strober W; Anti-IL-12 Crohn's Disease Study Group. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069-2079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 690] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 49. | Panaccione R, Sandborn WJ, Gordon GL, Lee SD, Safdi A, Sedghi S, Feagan BG, Hanauer S, Reinisch W, Valentine JF, Huang B, Carcereri R. Briakinumab for treatment of Crohn's disease: results of a randomized trial. Inflamm Bowel Dis. 2015;21:1329-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Potagonist Therapeutics, Inc. Pharmacokinetics and Pharmacodynamics of Differente PTG-300 Regimen in Healthy Volunteers. [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04516382 ClinicalTrials.gov Identifier: NCT04516382. [Cited in This Article: ] |
| 51. | Janssen Research & Development, LLC. A Study Evaluating Participants With Moderately to Severely Active Crohn’s Disease (PRISM). [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04102111 ClinicalTrials.gov Identifier: NCT04102111. [Cited in This Article: ] |
| 52. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 805] [Cited by in F6Publishing: 935] [Article Influence: 116.9] [Reference Citation Analysis (0)] |
| 53. | Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, Karczewski J, Pezous N, Bek S, Bruin G, Mellgard B, Berger C, Londei M, Bertolino AP, Tougas G, Travis SP; Secukinumab in Crohn's Disease Study Group. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1055] [Cited by in F6Publishing: 1099] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 54. | Ojeda Gómez A, Madero Velázquez L, Buendía Sanchez L, Pascual Sánchez I, Pérez Rabasco E, García Monsalve A, González Ferrández JA, García Sepulcre MF. Inflammatory bowel disease new-onset during secukinumab therapy: real-world data from a tertiary center. Rev Esp Enferm Dig. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Wang J, Bhatia A, Krugliak Cleveland N, Gupta N, Dalal S, Rubin DT, Sakuraba A. Rapid Onset of Inflammatory Bowel Disease after Receiving Secukinumab Infusion. ACG Case Rep J. 2018;5:e56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 56. | Papp KA, Reid C, Foley P, Sinclair R, Salinger DH, Williams G, Dong H, Krueger JG, Russell CB, Martin DA. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. J Invest Dermatol. 2012;132:2466-2469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 57. | Targan SR, Feagan B, Vermeire S, Panaccione R, Melmed GY, Landers C, Li D, Russell C, Newmark R, Zhang N, Chon Y, Hsu YH, Lin SL, Klekotka P. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients With Moderate-to-Severe Crohn's Disease. Am J Gastroenterol. 2016;111:1599-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 58. | Allocca M, Jovani M, Fiorino G, Schreiber S, Danese S. Anti-IL-6 treatment for inflammatory bowel diseases: next cytokine, next target. Curr Drug Targets. 2013;14:1508-1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Mitsuyama K, Tomiyasu N, Suzuki A, Takaki K, Takedatsu H, Masuda J, Yamasaki H, Matsumoto S, Tsuruta O, Toyonaga A, Sata M. A form of circulating interleukin-6 receptor component soluble gp130 as a potential interleukin-6 inhibitor in inflammatory bowel disease. Clin Exp Immunol. 2006;143:125-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Danese S, Vermeire S, Hellstern P, Panaccione R, Rogler G, Fraser G, Kohn A, Desreumaux P, Leong RW, Comer GM, Cataldi F, Banerjee A, Maguire MK, Li C, Rath N, Beebe J, Schreiber S. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn's disease (ANDANTE I and II). Gut. 2019;68:40-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 61. | Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 62. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 639] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 63. | Yamamoto-Furusho JK, Miranda-Pérez E, Fonseca-Camarillo G, Sánchez-Muñoz F, Dominguez-Lopez A, Barreto-Zuñiga R. Colonic epithelial upregulation of interleukin 22 (IL-22) in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16:1823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Rothenberg ME, Wang Y, Lekkerkerker A, Danilenko DM, Maciuca R, Erickson R, Herman A, Stefanich E, Lu TT. Randomized Phase I Healthy Volunteer Study of UTTR1147A (IL-22Fc): A Potential Therapy for Epithelial Injury. Clin Pharmacol Ther. 2019;105:177-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of UTTR1147A Compared With Placebo and With Vedolizumab in Participants With Moderate to Severe Ulcerative Colitis (UC). [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03558152 ClinicalTrials.gov Identifier: NCT03558152. [Cited in This Article: ] |
| 66. | Lucaciu LA, Seicean R, Seicean A. Small molecule drugs in the treatment of inflammatory bowel diseases: which one, when and why? Eur J Gastroenterol Hepatol. 2020;32:669-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, Camp HS, Padley RJ, George JS, Hyland D, Rosebraugh M, Wishart N, Olson L, Long AJ. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 68. | McInnes IB, Byers NL, Higgs RE, Lee J, Macias WL, Na S, Ortmann RA, Rocha G, Rooney TP, Wehrman T, Zhang X, Zuckerman SH, Taylor PC. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21:183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 69. | Sandborn WJ, Ghosh S, Panes J, Schreiber S, D'Haens G, Tanida S, Siffledeen J, Enejosa J, Zhou W, Othman AA, Huang B, Higgins PDR. Efficacy of Upadacitinib in a Randomized Trial of Patients With Active Ulcerative Colitis. Gastroenterology. 2020;158:2139-2149.e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 70. | AbbVie. A Study of the Efficacy and Safety of Upadacitnib (ABT-494) in Participants With Moderately to Severely Active Crohn’s Disease Who Have Inadequately Responded to or Are Intolerant to Conventional and/or Biologic. [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03345849 ClinicalTrials.gov Identifier: NCT03345849. [Cited in This Article: ] |
| 71. | AbbVie. A Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Participants With Moderately to Severely Active Ulcerative Colitis (U-Accomplish). [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03653026 ClinicalTrials.gov Identifier: NCT03653026. [Cited in This Article: ] |
| 72. | Van Rompaey L, Galien R, van der Aar EM, Clement-Lacroix P, Nelles L, Smets B, Lepescheux L, Christophe T, Conrath K, Vandeghinste N, Vayssiere B, De Vos S, Fletcher S, Brys R, van 't Klooster G, Feyen JH, Menet C. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol. 2013;191:3568-3577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 73. | Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A, Sike R, Stoyanova K, Tasset C, Van der Aa A, Harrison P. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 74. | Sciences Gilead. Filgotinib in the Induction and Maintenance of Remission in Adultos With Moderately to Severely Acrive Crohn´s Disease (Diversity 1). [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02914561 ClinicalTrials.gov Identifier: NCT02914561. [Cited in This Article: ] |
| 75. | Sciences Gilead. Filgotinib in the Induction and Maintenance of Remission in Adultos With Moderately to Severely Active Ulcerative Colitis (SELECTION1). [accessed 2020 Dec 29]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02914522 ClinicalTrials.gov Identifier: NCT02914522. [Cited in This Article: ] |
| 76. | Hamaguchi H, Amano Y, Moritomo A, Shirakami S, Nakajima Y, Nakai K, Nomura N, Ito M, Higashi Y, Inoue T. Discovery and structural characterization of peficitinib (ASP015K) as a novel and potent JAK inhibitor. Bioorg Med Chem. 2018;26:4971-4983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 77. | D'Amico F, Fiorino G, Furfaro F, Allocca M, Danese S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin Investig Drugs. 2018;27:595-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | Davies SC, Hussein IM, Nguyen TM, Parker CE, Khanna R, Jairath V. Oral Janus kinase inhibitors for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;1:CD012381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Biopharma Theravance. Efficacy and Safety of TD-1473 in Crohn’s Disease (DIONE). [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03635112 ClinicalTrials.gov Identifier: NCT03635112. [Cited in This Article: ] |
| 80. | Biopharma Theravance. Efficacy & Safety of TD-1473 in Ulcerative Colitis (RHEA). [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03758443 ClinicalTrials.gov Identifier: NCT03758443. [Cited in This Article: ] |
| 81. | Ma C, Battat R, Dulai PS, Parker CE, Sandborn WJ, Feagan BG, Jairath V. Innovations in Oral Therapies for Inflammatory Bowel Disease. Drugs. 2019;79:1321-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Sandborn WJ, Feagan BG, Wolf DC, D'Haens G, Vermeire S, Hanauer SB, Ghosh S, Smith H, Cravets M, Frohna PA, Aranda R, Gujrathi S, Olson A; TOUCHSTONE Study Group. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N Engl J Med. 2016;374:1754-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 83. | Celgene. Efficacy and Safety Trial of RPC1063 for Moderate to Severe Croh´s Diseaase. [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02531113 ClinicalTrials.gov Identifier: NCT02531113. [Cited in This Article: ] |
| 84. | Celgene. Safety and Efficacy Trial of RPC1063 for Moderate to Severe Ulcerative Colitis. [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02435992 ClinicalTrials.gov Identifier: NCT02435992. [Cited in This Article: ] |
| 85. | Celgene. Induction Study #1 of Oral Oznimod as Induction Therapy for Moderately to Severely Active Crohn's Disease. [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03440372 ClinicalTrials.gov Identifier: NCT03440372. [Cited in This Article: ] |
| 86. | Buzard DJ, Kim SH, Lopez L, Kawasaki A, Zhu X, Moody J, Thoresen L, Calderon I, Ullman B, Han S, Lehmann J, Gharbaoui T, Sengupta D, Calvano L, Montalban AG, Ma YA, Sage C, Gao Y, Semple G, Edwards J, Barden J, Morgan M, Chen W, Usmani K, Chen C, Sadeque A, Christopher RJ, Thatte J, Fu L, Solomon M, Mills D, Whelan K, Al-Shamma H, Gatlin J, Le M, Gaidarov I, Anthony T, Unett DJ, Blackburn A, Rueter J, Stirn S, Behan DP, Jones RM. Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor. ACS Med Chem Lett. 2014;5: 1313-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 87. | Sandborn WJ, Peyrin-Biroulet L, Zhang J, Chiorean M, Vermeire S, Lee SD, Kühbacher T, Yacyshyn B, Cabell CH, Naik SU, Klassen P, Panés J. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology. 2020;158:550-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 88. | Pharmaceuticals Arena. Etrasimod Versus Placebo for the Treatment of Moderately to Severely Active Ulcerative Colitis (ELEVATE UC 52). [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03945188 ClinicalTrials.gov Identifier: NCT03945188. [Cited in This Article: ] |
| 89. | Shimano K, Maeda Y, Kataoka H, Murase M, Mochizuki S, Utsumi H, Oshita K, Sugahara K. Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 functional antagonist, inhibits progress of chronic colitis induced by transfer of CD4+CD45RBhigh T cells. PLoS One. 2019;14:e0226154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Mitsubishi Tanabe Pharma Corporation. Safety and Efficacy of MT-1303 in Subjects With Moderate to Severe Active Crohn´s Disease. [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02378688 ClinicalTrials.gov Identifier: NCT02378688. [Cited in This Article: ] |
| 91. | Radeke HH, Stein J, Van Assche G, Rogler G, Lakatos PL, Muellershausen F, Moulin P, Jarvis P, Colin L, Gergely P, Kruis W. A Multicentre, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy, Safety, and Tolerability of the S1P Receptor Agonist KRP203 in Patients with Moderately Active Refractory Ulcerative Colitis. Inflamm Intest Dis. 2020;5:180-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 92. | Brunmark C, Runström A, Ohlsson L, Sparre B, Brodin T, Aström M, Hedlund G. The new orally active immunoregulator laquinimod (ABR-215062) effectively inhibits development and relapses of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;130:163-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | D'Haens G, Sandborn WJ, Colombel JF, Rutgeerts P, Brown K, Barkay H, Sakov A, Haviv A, Feagan BG; Laquinimod for Crohn's Disease Investigators. A phase II study of laquinimod in Crohn's disease. Gut. 2015;64:1227-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 94. | Sánchez-Muñoz F, Fonseca-Camarillo G, Villeda-Ramírez MA, Miranda-Pérez E, Mendivil EJ, Barreto-Zúñiga R, Uribe M, Bojalil R, Domínguez-López A, Yamamoto-Furusho JK. Transcript levels of Toll-Like Receptors 5, 8 and 9 correlate with inflammatory activity in Ulcerative Colitis. BMC Gastroenterol. 2011;11:138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Obermeier F, Hofmann C, Falk W. Inflammatory bowel diseases: when natural friends turn into enemies-the importance of CpG motifs of bacterial DNA in intestinal homeostasis and chronic intestinal inflammation. Int J Inflam. 2010;2010:641910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Kuznetsov NV, Zargari A, Gielen AW, von Stein OD, Musch E, Befrits R, Lofberg R, von Stein P. Biomarkers can predict potential clinical responders to DIMS0150 a toll-like receptor 9 agonist in ulcerative colitis patients. BMC Gastroenterol. 2014;14:79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Atreya R, Reinisch W, Peyrin-Biroulet L, Scaldaferri F, Admyre C, Knittel T, Kowalski J, Neurath MF, Hawkey C. Clinical efficacy of the Toll-like receptor 9 agonist cobitolimod using patient-reported-outcomes defined clinical endpoints in patients with ulcerative colitis. Dig Liver Dis. 2018;50:1019-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Atreya R, Peyrin-Biroulet L, Klymenko A, Augustyn M, Bakulin I, Slankamenac D, Miheller P, Gasbarrini A, Hébuterne X, Arnesson K, Knittel T, Kowalski J, Neurath MF, Sandborn WJ, Reinisch W; CONDUCT study group. Cobitolimod for moderate-to-severe, left-sided ulcerative colitis (CONDUCT): a phase 2b randomised, double-blind, placebo-controlled, dose-ranging induction trial. Lancet Gastroenterol Hepatol. 2020;5:1063-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Dotan I, Levy-Nissenbaum E, Chowers Y, Fich A, Israeli E, Adar T, Shteingart S, Soreq H, Goldin E. Ameliorating Active Ulcerative Colitis via an Orally Available Toll-Like Receptor-9 Modifier: A Prospective Open-Label, Multicenter Phase II Trial. Dig Dis Sci. 2016;61:3246-3254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Stremmel W, Hanemann A, Braun A, Stoffels S, Karner M, Fazeli S, Ehehalt R. Delayed release phosphatidylcholine as new therapeutic drug for ulcerative colitis--a review of three clinical trials. Expert Opin Investig Drugs. 2010;19:1623-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Stremmel W, Hanemann A, Ehehalt R, Karner M, Braun A. Phosphatidylcholine (lecithin) and the mucus layer: Evidence of therapeutic efficacy in ulcerative colitis? Dig Dis. 2010;28:490-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Karner M, Kocjan A, Stein J, Schreiber S, von Boyen G, Uebel P, Schmidt C, Kupcinskas L, Dina I, Zuelch F, Keilhauer G, Stremmel W. First multicenter study of modified release phosphatidylcholine "LT-02" in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses. Am J Gastroenterol. 2014;109:1041-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 103. | Dr. Falk Pharma GmBh. Phosphatidylcholine (LT-02) vs. Placebo vs. Mesalamine for Maintenance of Remission in Ulcerative Colitis (PROTECT-2) (PROTECT-2). [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02280629 ClinicalTrials.gov Identifier: NCT02280629. [Cited in This Article: ] |
| 104. | Prometheus Labratories. A Study to Investigate the Safety and Efficacy of LT-02 in Patients With Mesalamine Refractory Ulcerative Colitis (UC) (PROTECT-3). [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02849951 ClinicalTrials.gov Identifier: NCT02849951. [Cited in This Article: ] |
| 105. | Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 106. | Schafer PH, Parton A, Capone L, Cedzik D, Brady H, Evans JF, Man HW, Muller GW, Stirling DI, Chopra R. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016-2029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 107. | Danese S, Neurath MF, Kopoń A, Zakko SF, Simmons TC, Fogel R, Siegel CA, Panaccione R, Zhan X, Usiskin K, Chitkara D. Effects of Apremilast, an Oral Inhibitor of Phosphodiesterase 4, in a Randomized Trial of Patients With Active Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:2526-2534.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 108. | Monteleone G, Boirivant M, Pallone F, MacDonald TT. TGF-beta1 and Smad7 in the regulation of IBD. Mucosal Immunol. 2008;1 Suppl 1:S50-S53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Coskun M, Vermeire S, Nielsen OH. Novel Targeted Therapies for Inflammatory Bowel Disease. Trends Pharmacol Sci. 2017;38:127-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 110. | Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 441] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 111. | Scarozza P, Schmitt H, Monteleone G, Neurath MF, Atreya R. Oligonucleotides-A Novel Promising Therapeutic Option for IBD. Front Pharmacol. 2019;10:314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Monteleone G, Fantini MC, Onali S, Zorzi F, Sancesario G, Bernardini S, Calabrese E, Viti F, Monteleone I, Biancone L, Pallone F. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn's disease. Mol Ther. 2012;20:870-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 113. | Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, Scribano ML, Armuzzi A, Caprioli F, Sturniolo GC, Rogai F, Vecchi M, Atreya R, Bossa F, Onali S, Fichera M, Corazza GR, Biancone L, Savarino V, Pica R, Orlando A, Pallone F. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn's disease. N Engl J Med. 2015;372:1104-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 304] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 114. | Ardizzone S, Bevivino G, Monteleone G. Mongersen, an oral Smad7 antisense oligonucleotide, in patients with active Crohn's disease. Therap Adv Gastroenterol. 2016;9:527-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 115. | Bevivino G, Sedda S, Marafini I, Monteleone G. Oligonucleotide-Based Therapies for Inflammatory Bowel Disease. BioDrugs. 2018;32:331-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Corren J. New Targeted Therapies for Uncontrolled Asthma. J Allergy Clin Immunol Pract. 2019;7:1394-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 117. | Tindemans I, Serafini N, Di Santo JP, Hendriks RW. GATA-3 function in innate and adaptive immunity. Immunity. 2014;41:191-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 118. | Ray K. IBD: A role for GATA3 in ulcerative colitis. Nat Rev Gastroenterol Hepatol. 2016;13:624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 119. | Sterna Biologicals GmbH & Co. KG. Efficacy, Pharmacokinetics, Tolerability, Safety of SB012 Intrarectally Applied in Active Ulcerative Colitis Patients (SECURE). [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02129439 ClinicalTrials.gov Identifier: NCT02129439. [Cited in This Article: ] |
| 120. | Suzuki K, Arumugam S, Yokoyama J, Kawauchi Y, Honda Y, Sato H, Aoyagi Y, Terai S, Okazaki K, Suzuki Y, Mizumoto S, Sugahara K, Atreya R, Neurath MF, Watanabe K, Hashiguchi T, Yoneyama H, Asakura H. Pivotal Role of Carbohydrate Sulfotransferase 15 in Fibrosis and Mucosal Healing in Mouse Colitis. PLoS One. 2016;11:e0158967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 121. | Suzuki K, Yokoyama J, Kawauchi Y, Honda Y, Sato H, Aoyagi Y, Terai S, Okazaki K, Suzuki Y, Sameshima Y, Fukushima T, Sugahara K, Atreya R, Neurath MF, Watanabe K, Yoneyama H, Asakura H. Phase 1 Clinical Study of siRNA Targeting Carbohydrate Sulphotransferase 15 in Crohn's Disease Patients with Active Mucosal Lesions. J Crohns Colitis. 2017;11:221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 122. | Atreya R, Kühbacher T, Waldner MJ, Hirschmann S, Drvarov O, Abu Hashem R, Maaser C, Kucharzik T, Dinter J, Schramm C, Mertens J, Holler B, Mössner J, Suzuki K, Yokoyama J, Terai S, Yoneyama H, Asakura H, Hibi T, Neurath MF. DOP073 Submucosal injection of the oligonucleotide STNM01 is able to induce clinical remission, mucosal healing and histological response in left-sided ulcerative colitis patients with moderate-to-severe disease. J Crohn´s Colitis. 2017;11:S69. [DOI] [Cited in This Article: ] |
| 123. | Jairath V, Khanna R, Feagan BG. Alicaforsen for the treatment of inflammatory bowel disease. Expert Opin Investig Drugs. 2017;26:991-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 124. | Greuter T, Vavricka SR, Biedermann L, Pilz J, Borovicka J, Seibold F, Sauter B, Rogler G. Alicaforsen, an Antisense Inhibitor of Intercellular Adhesion Molecule-1, in the Treatment for Left-Sided Ulcerative Colitis and Ulcerative Proctitis. Dig Dis. 2018;36:123-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 125. | van Deventer SJ, Wedel MK, Baker BF, Xia S, Chuang E, Miner PB Jr. A phase II dose ranging, double-blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left-sided ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1415-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 126. | Greuter T, Biedermann L, Rogler G, Sauter B, Seibold F. Alicaforsen, an antisense inhibitor of ICAM-1, as treatment for chronic refractory pouchitis after proctocolectomy: A case series. United European Gastroenterol J. 2016;4:97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 127. | Atlantic Pharmaceuticals Ltd. Efficacy of Alicaforsen in Pouchitis Patients Who Have Failed to Respond to at Least One Course of Antibiotics. [accessed 2020 Dec 30]. In: ClinicalTrials.gov [Internet]. Bethseda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02525523 ClinicalTrials.gov Identifier: NCT02525523. [Cited in This Article: ] |