Published online Dec 7, 2021. doi: 10.3748/wjg.v27.i45.7859
Peer-review started: July 4, 2021
First decision: August 8, 2021
Revised: August 11, 2021
Accepted: November 24, 2021
Article in press: November 24, 2021
Published online: December 7, 2021
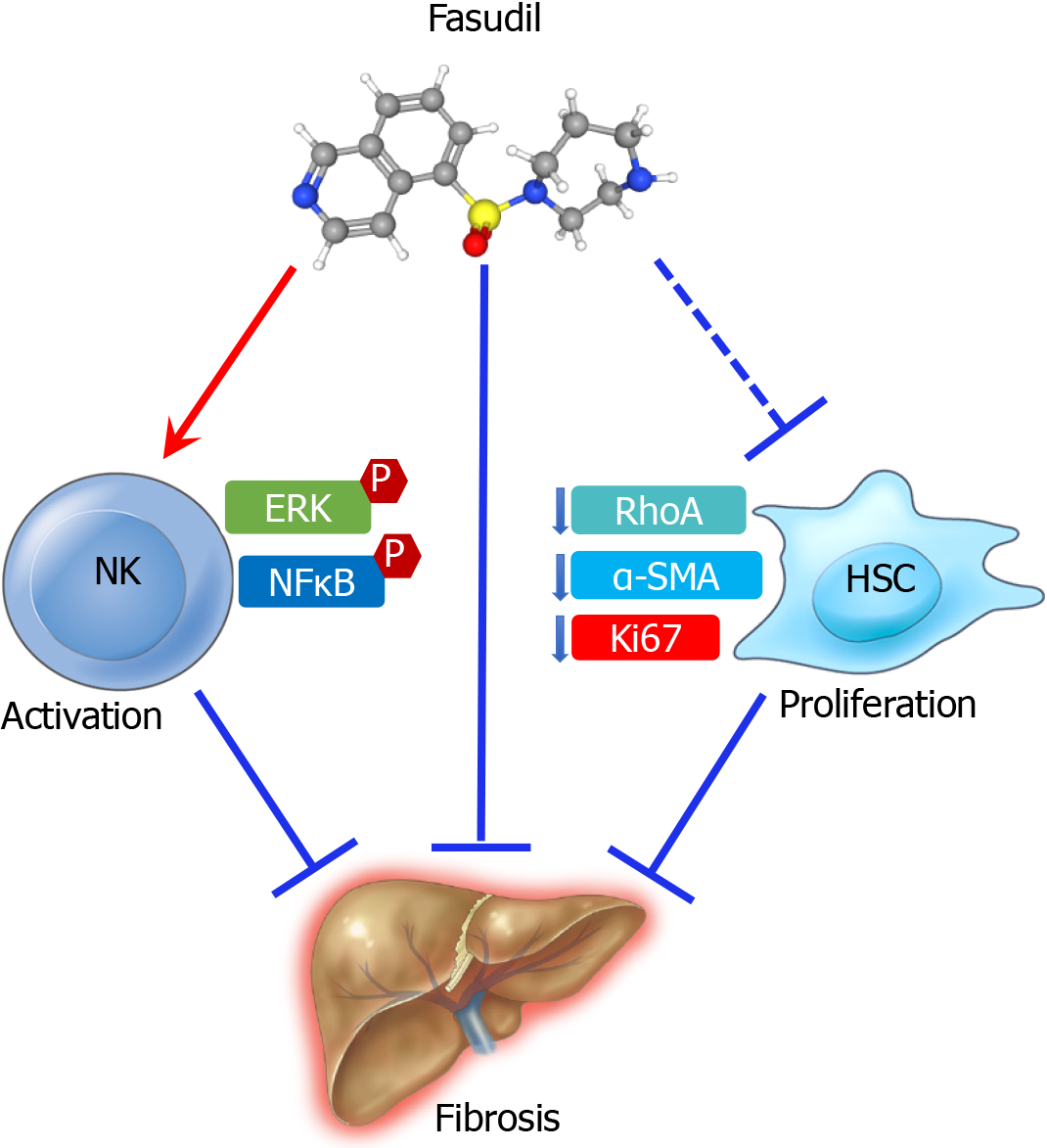
Fasudil has the potential to prevent liver fibrosis by activating natural killer cells and inhibiting the proliferation of hepatic stellate cells. Fasudil may be a promi
Core Tip: This letter to the editor is to supplement the ongoing discussion on the therapeutic potentials of Fasudil in the treatment of hepatic fibrosis. Fasudil is potential for the treatment of liver fibrosis through activating natural killer cells and inhibiting the proliferation of hepatic stellate cells. Fasudil, a vasodilator used in clinical treat
- Citation: Xi Y, Xu PF. Therapeutic potentials of fasudil in liver fibrosis. World J Gastroenterol 2021; 27(45): 7859-7861
- URL: https://www.wjgnet.com/1007-9327/full/v27/i45/7859.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i45.7859
We read with great interest the recent basic study by Han et al[1], that reported Fasudil, a potent RhoA/ROCK inhibitor and vasodilator, prevents and treats liver fibrosis and liver injury. They found Fasudil alleviates thioacetamide (TAA)-induced liver fibrosis in mice. The anti-fibrotic phenotypic exhibition of Fasudil is impressive. Hepatic fibrosis is the formation of scar tissue in response to chronic liver damage, such as chronic hepatitis and hepatic steatosis[2]. Currently, there is no pharmacotherapy available approved by Food and Drug Administration (FDA) in the treatment of liver fibrosis[3]. Fasudil has been approved in Japan and China for the prevention of artery tightening and ischemia caused by cerebral vasospasm and pulmonary hyper
Hepatic stellate cells (HSCs) and natural killer (NK) cells play key roles in the pathogenesis of liver fibrosis. They isolated NK cells from mice treated with vehicle, TAA, or TAA and Fasudil and treated the NK-92 cells with different concentrations of Fasudil. These results showed that Fasudil robustly promotes NK cell activation. When discussing the effect of Fasudil on HSCs, they used human stellate cell line LX2 cells and observed that Fasudil directly induces apoptosis and inhibits the proliferation of LX2 cells. LX2 cell is indeed a model for the study of HSC activation. But to investigate HSCs activation, the model of primary HSCs subjected to culture activation and LX2 cells subjected to the stimulation of the potent profibrogenic cytokine transforming growth factor-beta 1 (TGF-β1) and then treated with the drugs under study are more widely accepted. Here, the authors proposed that Fasudil inhibited liver fibrosis by blocking HSCs activation by directly using the LX2 cells treated with Fasudil, which is far-fetched and hard to interpret. As primary HSCs are activated by prolonged culture, HSCs isolated from human or mouse livers and treated with the studied drug may be a more comprehensive approach to evaluate HSC activation.
Other studies also showed that Fasudil has anti-fibrotic phenotypic exhibition in rat models of hepatic fibrosis, such as Fasudil alleviated hepatic fibrosis in type 1 and 2 diabetic rats and carbon tetrachloride (CCl4)-induced rat liver injury[5-7]. Combined with these studies, we proposed that Fasudil is potential for the treatment of liver fibrosis through multitargeted effects, as outlined in Figure 1. Taken together, Fasudi is a promising medication for the prevention and treatment of liver fibrosis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, 252088.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang J S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Han QJ, Mu YL, Zhao HJ, Zhao RR, Guo QJ, Su YH, Zhang J. Fasudil prevents liver fibrosis via activating natural killer cells and suppressing hepatic stellate cells. World J Gastroenterol. 2021;27:3581-3594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Tanwar S, Rhodes F, Srivastava A, Trembling PM, Rosenberg WM. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J Gastroenterol. 2020;26:109-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 78] [Cited by in F6Publishing: 84] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 3. | Alukal JJ, Thuluvath PJ. Reversal of NASH fibrosis with pharmacotherapy. Hepatol Int. 2019;13:534-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Zhao J, Zhou D, Guo J, Ren Z, Zhou L, Wang S, Xu B, Wang R. Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2006;46:421-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Xie Y, Song T, Huo M, Zhang Y, Zhang YY, Ma ZH, Wang N, Zhang JP, Chu L. Fasudil alleviates hepatic fibrosis in type 1 diabetic rats: involvement of the inflammation and RhoA/ROCK pathway. Eur Rev Med Pharmacol Sci. 2018;22:5665-5677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 6. | Zhou H, Fang C, Zhang L, Deng Y, Wang M, Meng F. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, ameliorates hepatic fibrosis in rats with type 2 diabetes. Chin Med J (Engl). 2014;127:225-231. [PubMed] [Cited in This Article: ] |
| 7. | Ikeda H, Kume Y, Tejima K, Tomiya T, Nishikawa T, Watanabe N, Ohtomo N, Arai M, Arai C, Omata M, Fujiwara K, Yatomi Y. Rho-kinase inhibitor prevents hepatocyte damage in acute liver injury induced by carbon tetrachloride in rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G911-G917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |