Published online Nov 21, 2021. doi: 10.3748/wjg.v27.i43.7546
Peer-review started: May 23, 2021
First decision: June 12, 2021
Revised: July 19, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: November 21, 2021
Circulating tumor cells (CTCs) and survivin are indicators for tumor stage and metastasis, as well as epitheliomesenchymal transition, in various cancers, including hepatocellular cancer (HCC).
To explore the potential of survivin-positive CTCs, specifically, as a marker for tumor progression in HCC patients.
We examined the survivin expression pattern in CTCs obtained from 179 HCC patients, and investigated the in vitro effects of survivin silencing and overexpression on the proliferation and invasion of HCC cells. CTC count and survivin expression in patient samples were examined using RNA in situ hybridization.
All 179 patients were positive for CTC markers, and 94.41% of the CTCs were positive for survivin. The CTC and survivin-positive CTC counts were signi
Survivin shows upregulated expression (indicative of anti-apoptotic effects) in HCC. Thus, survivin-positive CTCs are promising as a predictor of HCC prognosis and metastasis, and their accurate measurement may be useful for the management of this cancer.
Core Tip: This study first analyzed surviving-positive circulating tumor cells (CTCs) in patients with hepatocellular carcinoma (HCC). The levels of survivin expression in different CTCs were significantly different. The rates of moderate and high expression in the mesenchymal and hybrid CTCs were significantly higher than those in the epithelial CTCs. Survivin overexpression induced HCC cell proliferation, promoted their invasive ability, and reduced apoptosis. The expression of survivin in mesen
- Citation: Yu J, Wang Z, Zhang H, Wang Y, Li DQ. Survivin-positive circulating tumor cells as a marker for metastasis of hepatocellular carcinoma. World J Gastroenterol 2021; 27(43): 7546-7562
- URL: https://www.wjgnet.com/1007-9327/full/v27/i43/7546.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i43.7546
According to 2020 statistics, hepatic cancer ranked sixth among malignant cancers in terms of incidence, and it ranked fourth globally as the main reason for cancer-related death[1]. Further, the 5-year overall survival of this cancer is quite abysmal, as it is less than 12%[1]. The prognosis of this disease continues to remain poor, even though there have been advances in the diagnostic and therapeutic strategies[2]. Hence, understanding the mechanisms underlying the metastasis of this cancer and assessing the status of disease progression are necessary.
The number of circulating tumor cells (CTCs) is an effective marker for solid tumors associated with metastasis[2]. CTC analysis is considered as real-time “liquid biopsy” for cancer patients, as it provides real-time monitoring of tumor progression and recurrence[3]. As a non-invasive biomarker, the CTC level can be used for comprehensive surveillance of cancer progression, in the case of both hepatocellular carcinoma (HCC) and cholangiocarcinoma[4]; in particular, it has been shown to be a good prognostic marker for HCC[5-7]. Currently, the most commonly used technique for CTC isolation is the cell search system, which is the only FDA-approved detection method; this method is based on positive immunoselection of epithelial cell adhesion molecule (EpCAM) and negative immunoselection of leukocytes (for which the general target is CD45). By using this technique, CTCs are divided into epithelial CTCs, mesenchymal CTCs (mCTCs), and hybrid CTCs[8]. According to this classification, CTCs have been reported to be useful as a marker for epithelial-mesenchymal transition (EMT) in HCC[9], and EMT is a known marker for the diagnosis and prognosis of cancer progression. Accordingly, previous studies have revealed that CTCs undergoing EMT are useful as indicators for diagnosing HCC and for predicting its prognosis[10]. The prognostic value of CTCs has also been demonstrated after surgery or during chemotherapy and recurrence[11,12].
Survivin is an anti-apoptotic protein (molecular weight, 16.5 kDa)[13] that plays an important role in inhibiting apoptosis in multicellular organisms and is overexpressed in many tumors, including HCC[14]. Studies have also shown that overexpression of survivin is associated with protection against apoptosis and propensity for metastasis in tumor cells, but this effect was not observed in normal cells[15]. Wurmbach et al[16] and Roessler et al[17] showed gene overexpression of survivin in HCC samples compared to normal liver samples, and the difference between the two groups was significant, as shown in Figure 1. Additionally, recent research has shown that insulin-like growth factor-1 induced EMT via activation of survivin in HCC cells[18]. Accordingly, overexpression of survivin mRNA and protein has been shown to stimulate EMT in HCC cells; this has been shown to increase their ability for invasion and migration and their cell proliferation rate, and decrease their apoptosis rate[15]. Thus, there is evidence for the potential of survivin as a marker for cancer cell proliferation and metastasis.
As discussed above, the research so far has indicated that both CTCs and survivin are markers for EMT and, therefore, cancer progression in HCC. However, the serum survivin levels of HCC patients are not different from those of healthy controls and patients with nonmalignant chronic liver diseases[19]. And no study so far has analyzed the prognostic value of survivin-expressing CTCs in HCC. Therefore, in the present study, we explored whether survivin-positive CTC levels are associated with cancer stage and clinicopathological characteristics in HCC, and whether survivin expression in CTCs has potential as a predictor of metastasis in HCC patients.
Cancer genome atlas data were retrieved from GEO database (http://www.ncbi.nlm.nih.gov/geo) and TCGA database (http://www.ualcan.path.uab.edu). The GSE6467 and GSE14520 datasets were downloaded for this study. Data was analyzed using software packages including ClusterProfiler, GSEquery, and pheatmap.
A study cohort (n = 179) included HCC patients who were enrolled at Hubei Cancer Hospital, China between May 2018 and December 2018. Only patients for whom HCC was confirmed based on pathological evidence, according to the World Health Organization criteria, and patients who had not undergone prior anticancer treatment were included. The tumors were staged as 0-A or B-C according to the Barcelona Clinic Liver Cancer (BCLC) staging system. Further, based on TNM classification, the tumors were staged as I, II, III, or IV. A control group comprised 70 healthy persons and 54 patients who tested positive for hepatitis B/C virus. The study was approved by the ethics committee of Hubei Cancer Hospital. (Ethical approval number: LLHBCH2019LW-002)
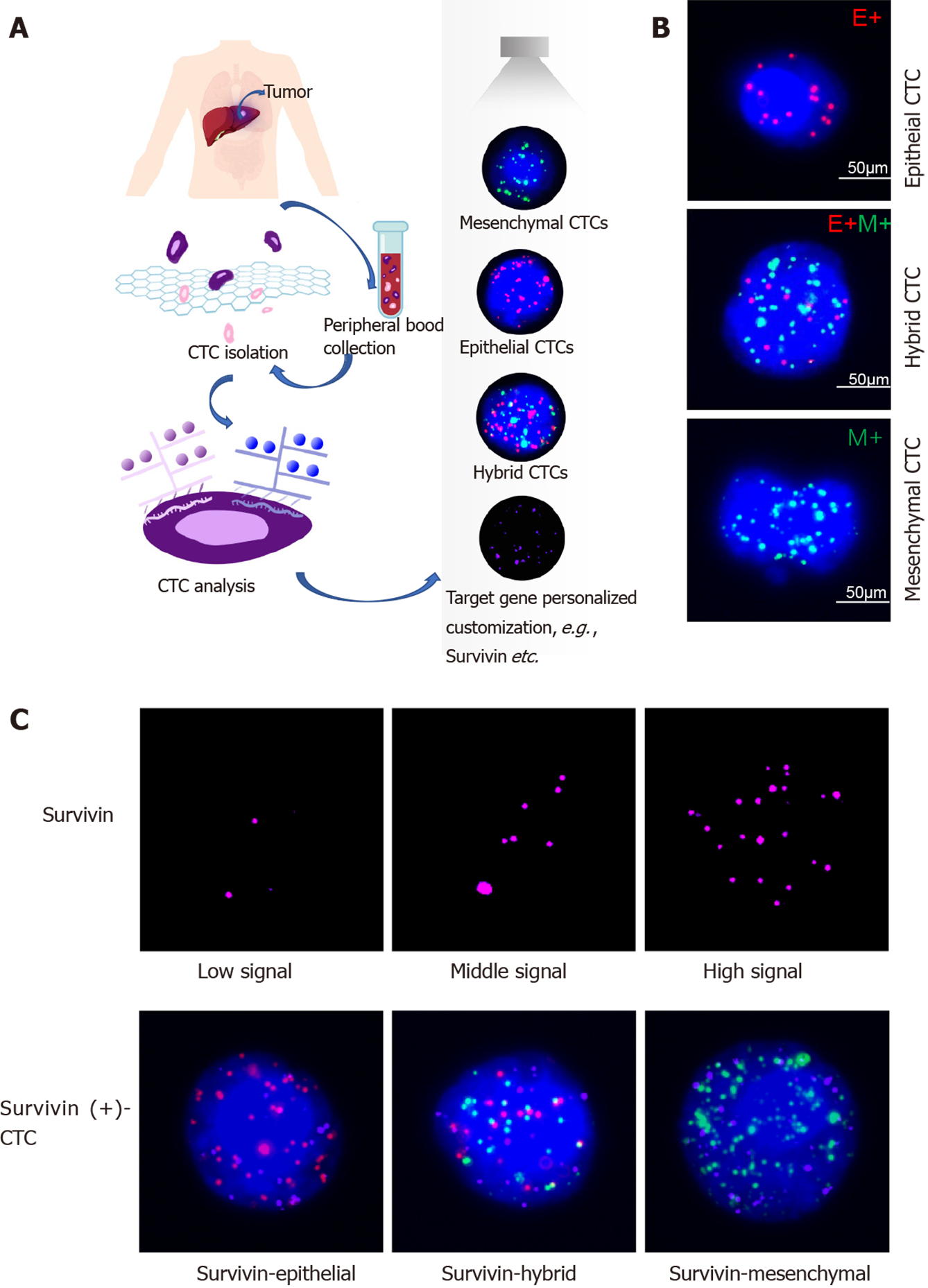
Samples of peripheral blood (a volume of 5 mL mixed with EDTA as an anticoagulant) were obtained via venipuncture. The CanPatrol CTC enrichment technique (SurExam, Guangzhou, China) was used to isolate CTCs from the blood samples and classify them. The samples were filtered through a membrane (pore diameter, 8 μm; Millipore, Billerica, United States), a vacuum plate that provided various valve settings (SurExam, Guangzhou, China), an E-Z 96 vacuum that also had various settings (Omega, Norcross, United States), and a vacuum pump (Auto Science, Tianjin, China). A cell lysis buffer that comprised 154 mmol/L NH4Cl, 10 mmol/L KHCO3, and 0.1 mmol/L EDTA (all the reagents were from Sigma, St. Louis, United States) was used to induce lysis of erythrocytes. The cell suspension was then resuspended for 5 min in phosphate-buffered saline (Sigma, St. Louis, United States) that contained 4% formaldehyde (Sigma, St. Louis, United States), and transferred to the specialized filtration system described earlier. The vacuum pump was turned on and set at a pressure of 0.08 MPa. The CTCs were separated using the membrane as mentioned above.
CTC phenotypes were detected by RNA in situ hybridization (ISH) with CD45 (a leukocyte marker), EpCAM and CK8/18/19 (epithelial cell markers), and vimentin and Twist (mesenchymal cell markers) as markers (Figure 1). A protease (Qiagen, Hilden, Germany) was used to pretreat the blood cells on the membrane. Following this, the cells were hybridized with a capture probe that targeted the genes for the markers mentioned above. Next, the cells were stained by 5-min incubation with 4,6-diamidino-2-phenylindole (Sigma, St. Louis, United States), and viewed under an automated imaging fluorescence microscope (Keyence, United States). Red fluorescence signals were considered to indicate epithelial cell markers, while green fluorescence signals were considered to indicate mesenchymal cell markers. Further, bright white fluorescence signals were considered to indicate CD45 expression.
Survivin expression in CTCs was assessed using the RNA-ISH method (Figure 1C). Survivin expression is indicated by purple fluorescence emitted by the CTCs (Alexa Fluor 647). According to the signal intensity score, survivin expression was classified as absent (0), low (1-2), moderate (3-9), and high (signal > 9), as shown in Figure 1C.
The HCC cell line HepG2 was obtained from the cell bank of the Chinese Academy of Science and cultured in Dulbecco’s modified Eagle medium with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator.
Short hairpin RNAs against the survivin sequence (5'-CTTACCAGGTGAGAA
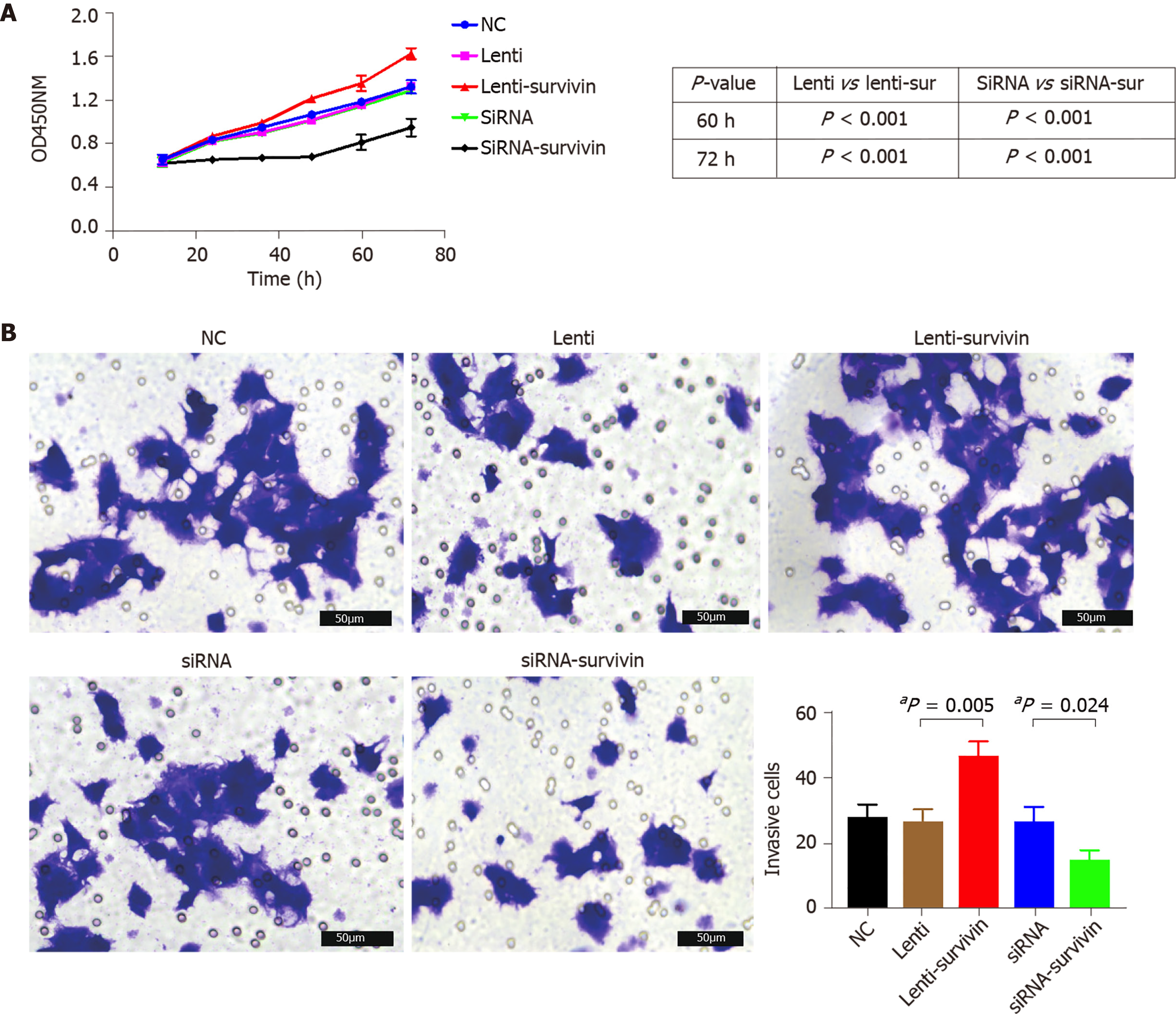
The CCK-8 kit (Bioswamp Company, China) was used to analyze cell proliferation according to the manufacturer’s protocol. The staining reagent used was CCK-8 (at a volume of 10 mL); the cells were incubated with the reagent for 2 h at 37 °C for 12 h, 24 h, 36 h, 48 h, 60 h, and 72 h after transfection (with the survivin siRNA or lentivirus). Following this, the absorbance of the cells was detected at 450 nm. Flow cytometry (Beckman, United States) was used to analyze cell apoptosis with the Annexin V-PE and 7AAD assay kit (Becton, Dickinson Company, United States). Apoptotic cells were defined as PE-positive and 7AAD-negative, while necrotic or dead cells were defined as 7AAD-positive and PE-negative.
For the invasion experiment, cells were seeded at a density of 5 × 104 cells/well in Transwell inserts (pore diameter, 8 µm) coated with Matrigel and containing cold serum-free medium. After an incubation period of 48 h, non-invasive cells that were present in the upper compartment were removed with a cotton swab, and the cells that had successfully adhered to the lower compartment were subjected to fixation with 10% paraformaldehyde and staining with 0.1% hexamethylpararosaniline for 30 min. The number of cells was counted under a microscope (Olympus BX53) in five selected fields. The average number of adherent cells was calculated.
Formalin-fixed paraffin-embedded specimens of HCC tissues from Hubei Cancer Hospital were prepared for immunohistochemical staining. The tissue slides were washed in phosphate-buffered saline (Bioswamp, China) after dewaxing and dehydration. The slides were placed in plastic tubes and incubated with boiling citric acid for antigen retrieval. In the next step, endogenous peroxidase activity was inhibited via 10-min incubation in methanol containing 3% H2O2. Next, the slides were blocked by 15-min incubation in 1% goat serum at 37 °C. Following this, they underwent incubation with anti-survivin rabbit polyclonal antibody (1:100 dilution; Abcam, United Kingdom) at 4 °C overnight in a moist chamber. After treatment with the secondary antibodies for 30 min, the slides were colored with diaminobenzidine and counterstained with hematoxylin. All the images were captured using an Olympus BX53 microscope. The protocol for Western blot assay of survivin protein expression in the tissue specimens was the same as that described for Western blot analysis of the HepG2 cells.
All analyses were conducted using SPSS version 20.0 (IBM Corp., Armonk, NY, United States). The Kolmogorov-Smirnov method was used to determine the normality of the CTC and survivin-positive CTC counts, which were found to be non-normally distributed. The phenotypic CTC counts and survivin expression in different groups were examined by the Fisher exact probability test. Correlations between variables were evaluated with the Spearman rank correlation test. P < 0.05 was considered to indicate statistical significance.
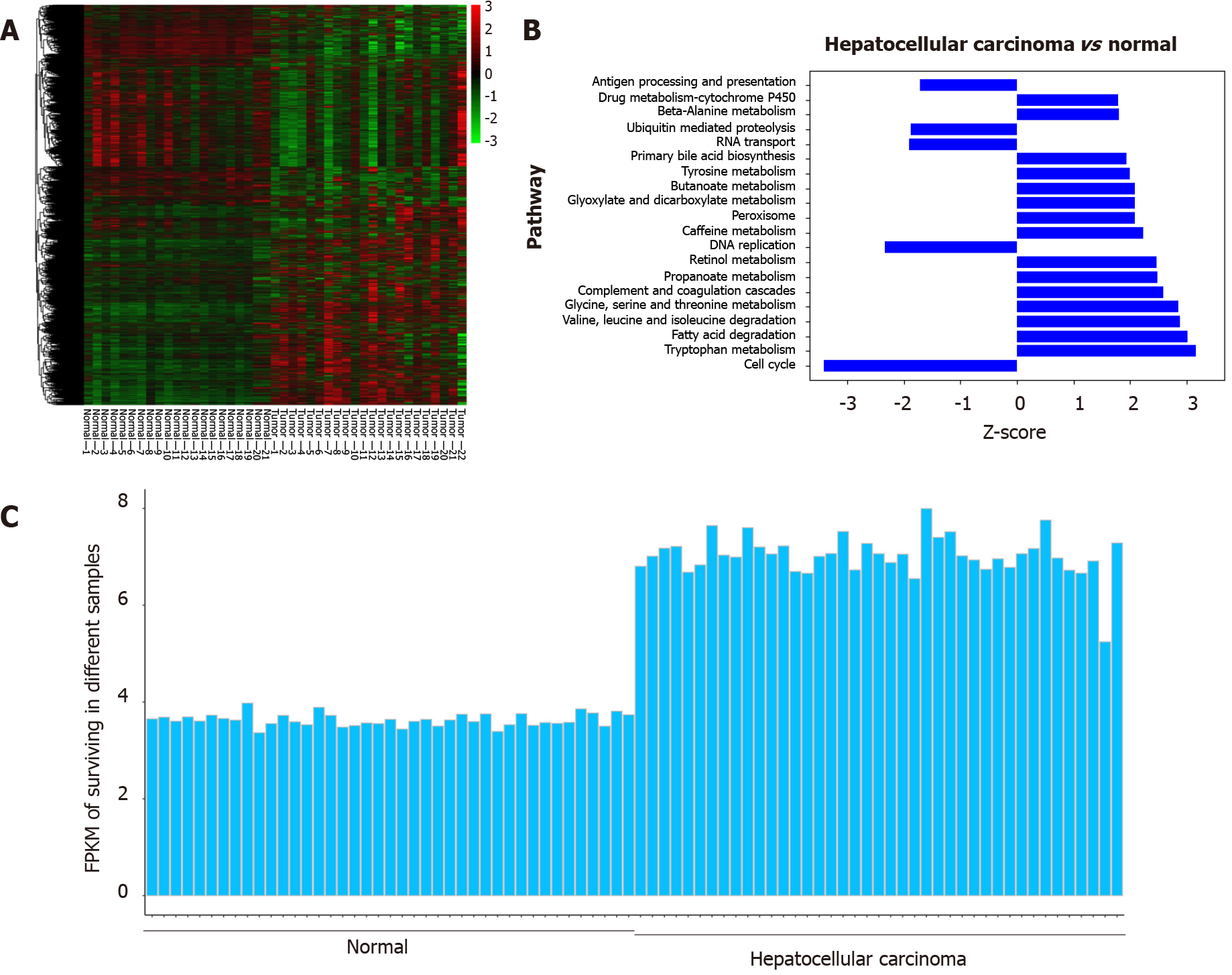
Based on the GSE6467 and GSE14520 datasets, gene expression was found to differ between tumor tissue and normal tissue, as evident from the survivin gene expression data shown in Figure 2A and 2C. Next, we found that DNA replication and cell cycle pathways were downregulated in HCC samples, based on the Ingenuity pathway analysis (Z-score < 0; Figure 2B).
Based on the TCGA data analysis, the expression of survivin in HCC tissue was significantly associated with stage and tumor grade. Survivin expression (fragments per kilobase of transcript per million fragments mapped) in patients with stages III and IV HCC was significantly higher than that in patients with stages I and II HCC (P < 0.05). And survivin expression (fragments per kilobase of transcript per million fragments mapped) in patients with grades 3 and 4 HCC was significantly higher than that in patients with grades 1 and 2 HCC (P < 0.05) (Supplementary Figure 1).
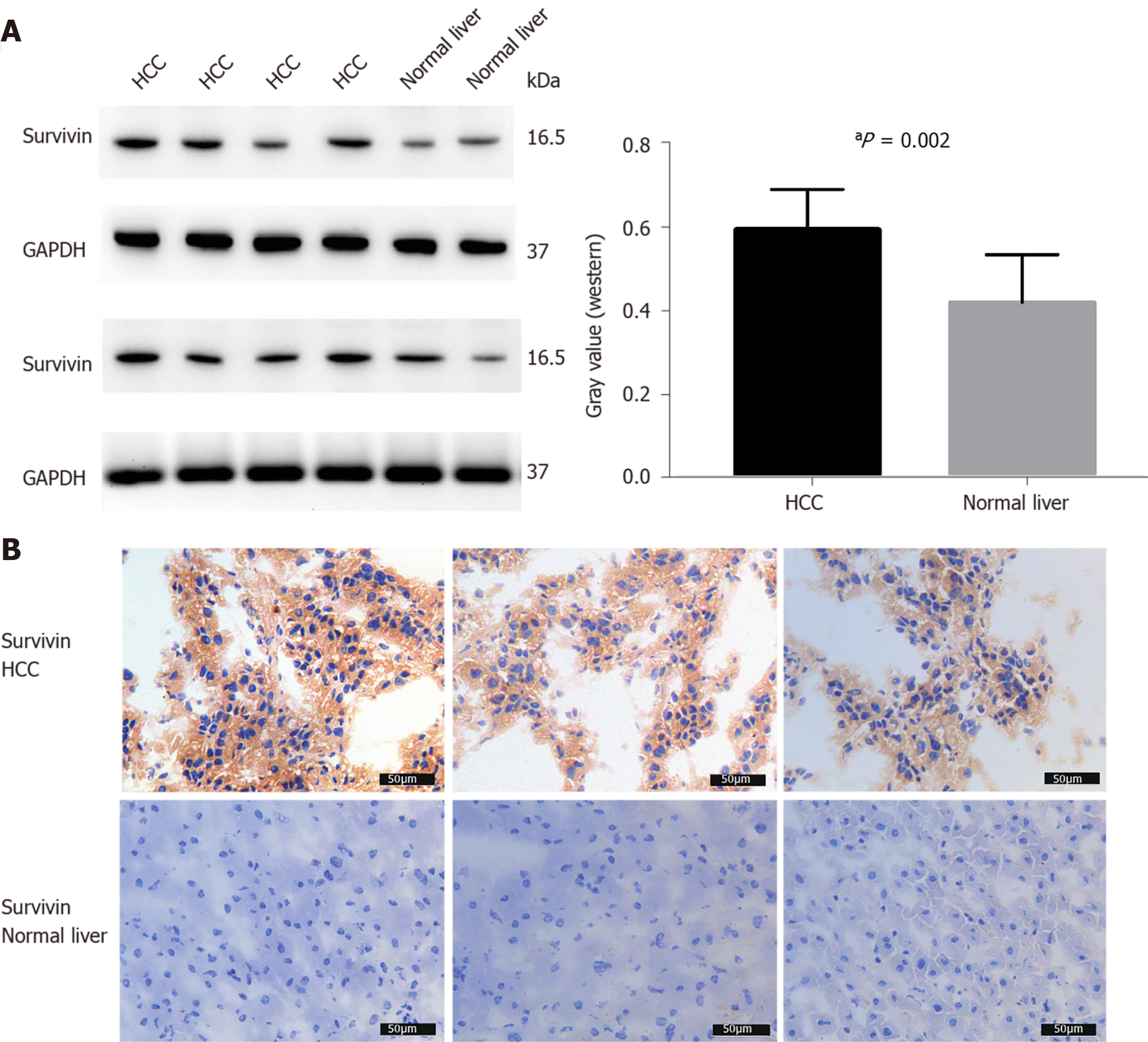
The survivin expression level in tissue samples from HCC patients (n = 8) was significantly higher than that in normal hepatic tissues (n = 4), as indicated in the Western blot in Figure 3A and the immunohistochemical staining images in Figure 3B.
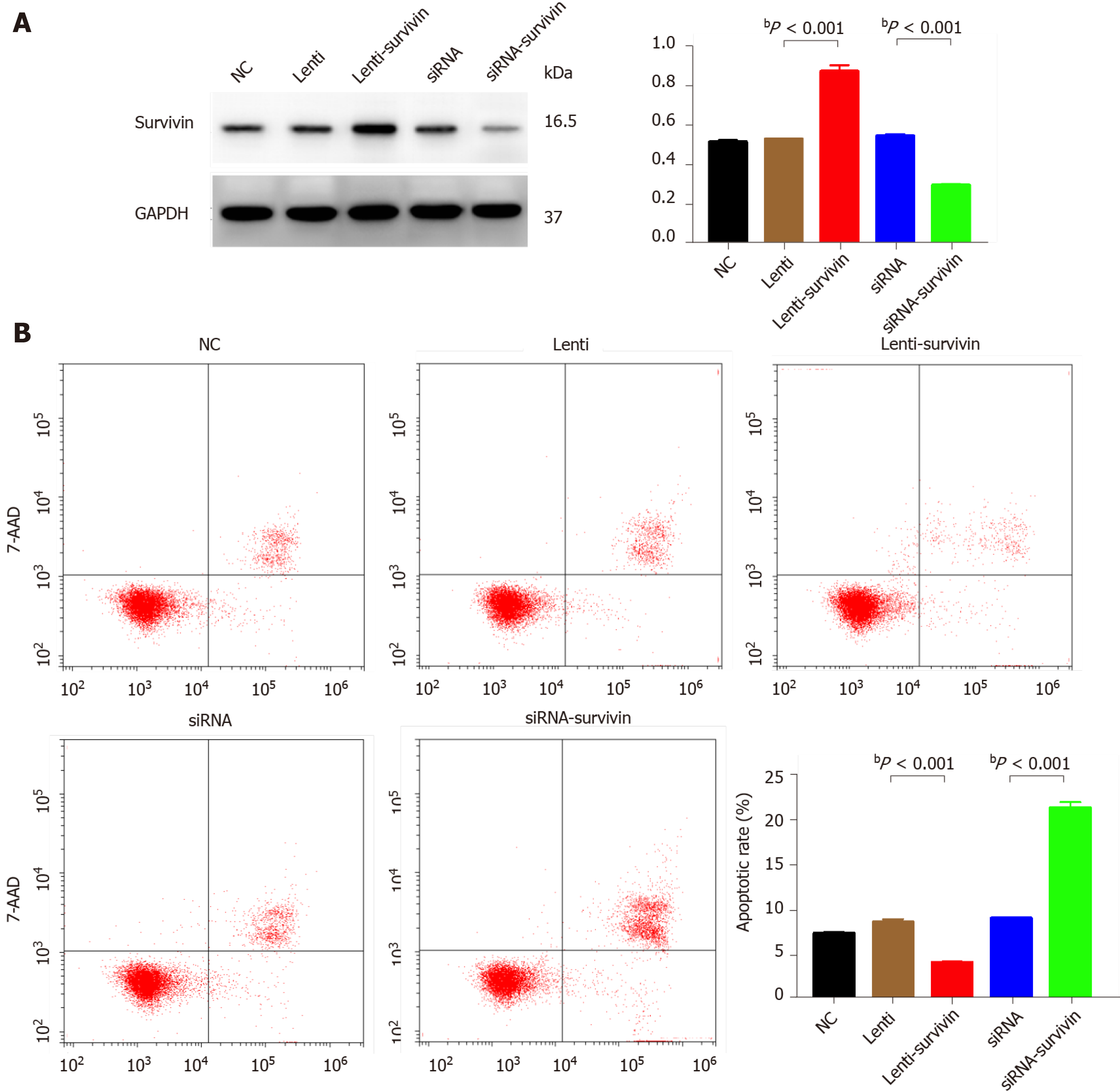
Silencing of survivin expression and overexpression of survivin were successfully induced by siRNA and lentivirus transfection, respectively (Figure 4A). At 12 h, 24 h, 48 h, 60 h, and 72 h post-transfection, the cell proliferation rate was significantly higher in the survivin-overexpression cells than in the control cells and survivin-silenced cells, while it was significantly lower in the survivin-silenced cells than in the control cells and survivin-overexpressing cells (Figure 5A). Accordingly, the apoptosis rate of survivin-silenced HepG2 cells was significantly higher than that of survivin-overexpressing and normal control cells (Figure 4B). Finally, the results for cell invasion rates obtained from the Transwell migration assays indicated that the survivin-overexpressing HepG2 cells had significantly higher invasive ability than the survivin-silenced and normal control cells (Figure 5B).
The observations of RNA-ISH analysis of CTCs based on EMT markers are shown in Figure 1B. The expression of survivin in these subtypes of CTCs is also indicated. The proportion of mCTCs is shown in Figure 6C. The number of mCTCs seemed to be higher in advanced-stage HCC (stage B-C) than in metastatic HCC.
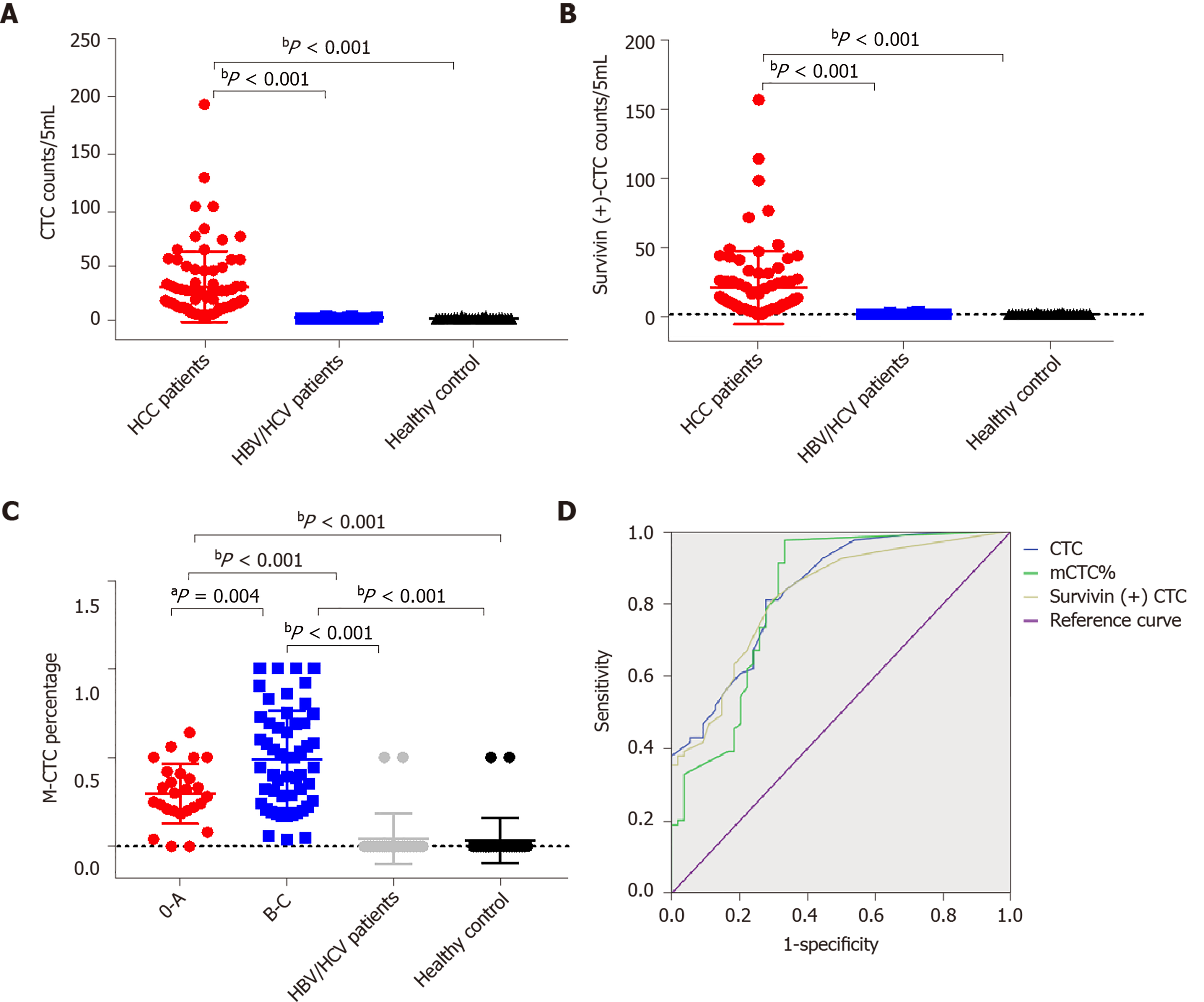
Table 1 shows the clinicopathological characteristics of the study cohort. A CTC count of > 5/5 mL was detected in 168 out of 179 blood samples (93.85%), and the median CTC count was 16/5 mL (range, 1-193 CTCs/5 mL). Further, survivin-positive CTCs were detected in 94.41% (169 of 179) of the patients. As shown in Figure 6, the median CTC count (Figure 6A) and survivin-positive CTC count (Figure 6B) in HCC patients were significantly higher than those in the HBV/HCV patients and healthy controls (P < 0.05). Patients with stage B/C HCC (or advanced HCC) had a significantly greater median CTC count than those with stage 0/A HCC (or early-stage HCC) (P = 0.024) (Table 1), and the median CTC count in patients with poorly differentiated tumors was significantly higher than that in patients with well-differentiated tumors (P = 0.002) (Table 1).
| Clinical factor | n | 1CTC count/5 mL | 2mCTC count/5 mL | 3Survivin (+)CTC count/5 mL | P value |
| Median, SE | Median, SE | Median, SE | |||
| Sex | |||||
| Male | 119 | 17, 3.57 | 6, 1.11 | 9, 3.05 | 0.2511, 0.8192, 0.9093 |
| Female | 60 | 12, 9.61 | 6, 3.19 | 9.5, 7.88 | |
| Age | |||||
| < 55 yeara | 89 | 19, 4.28 | 9, 1.50 | 11, 3.54 | 0.7001, 0.1412, 0.4673 |
| ≥ 55 years | 90 | 14, 5.75 | 4, 1.71 | 8, 4.88 | |
| HBV/HCV | |||||
| Positive | 130 | 17, 3.57 | 7, 1.16 | 10, 3.03 | 0.4021, 0.5392, 0.4363 |
| Negative | 49 | 13, 9.90 | 4, 3.12 | 6.5, 8.21 | |
| AFP levels | |||||
| < 7.0 | 80 | 14, 5.85 | 5, 1.85 | 7, 4.66 | 0.2851, 0.812, 0.2543 |
| ≥ 7.0 | 99 | 22.5, 4.42 | 7.5, 1.45 | 11, 3.92 | |
| Cirrhosis | |||||
| Yes | 119 | 16, 3.43 | 6, 1.19 | 9.0, 2.78 | 0.4981, 0.6712, 0.8293 |
| No | 60 | 19.5, 10.03 | 7.5, 2.94 | 10, 8.67 | |
| TNM stage | |||||
| I-II | 79 | 14, 2.93 | 6, 1.04 | 6, 1.49 | 0.1111, 0.4332, 0.0023 |
| III-IV | 100 | 24, 6.29 | 8.50, 2.00 | 18, 5.95 | |
| BCLC stage | |||||
| 0-A | 58 | 12.5, 3.04 | 4, 0.75 | 4, 1.25 | 0.0241, 0.0002, 0.0003 |
| B-C | 121 | 25, 4.94 | 9, 1.56 | 20, 4.64 | |
| Differentiation | |||||
| Well | 29 | 13, 2.04 | 5, 2.46 | 5, 1.70 | 0.0021 (well vs poor) |
| Moderate | 92 | 14.5, 5.35 | 6, 1.77 | 8.5, 5.15 | 0.9702 (well vs poor) |
| Poor | 58 | 37, 6.18 | 7.5, 1.55 | 24, 5.44 | 0.0053 (well vs poor) |
Out of the 179 patients, 138 had an mCTC count of > 2/5 mL. The mCTC count was not significantly associated with AFP level, tumor stage, or degree of differentiation (Table 1). However, the proportion and count of mCTC were associated with BCLC stage and metastasis (Figure 6C).
The area under the ROC curve was 0.84 [95% confidence interval (CI) = 0.77-0.91] for CTCs, 0.82 (95%CI = 0.75-0.89) for survivin-positive CTCs, and 0.82 (95%CI = 0.75-0.90) for the proportion of mCTCs (Figure 6D). The rational cut-off value for diagnosis was 12 for the CTC count (sensitivity = 60.89%, specificity = 91.13%), 4 for the survivin-positive CTC count (sensitivity = 81.01%, specificity = 78.23%), and 1.85% for the proportion of mCTCs (sensitivity = 84.92%, specificity = 72.58%).
The survivin-positive CTC counts were not significantly associated with AFP levels. However, the median survivin-positive CTC count in patients with stages III and IV HCC was significantly higher than that in patients with stages I and Ⅱ HCC (Table 1). Further, patients with stage B/C HCC (or advanced HCC) had a significantly greater median survivin-positive CTC count than those with stage 0/A HCC (or early-stage HCC) (P < 0.001) (Table 1). Finally, patients with poorly differentiated HCC had a significantly greater median survivin-positive CTC count than those with well-differentiated tumors (P = 0.005) (Table 1).
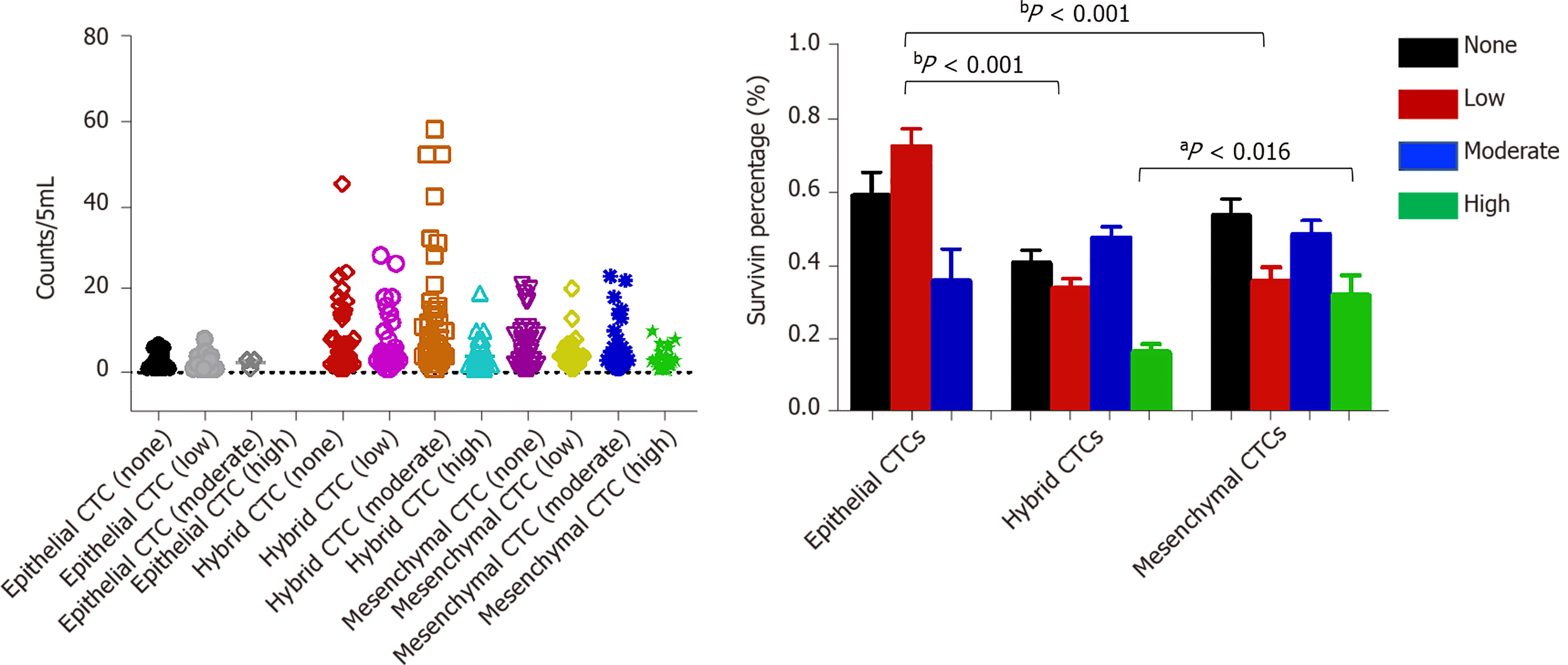
Survivin expression was detected in all CTC-positive patients (Figure 7), but the level of expression (based on the intensity of the purple fluorescent signal) differed between epithelial, mesenchymal, and hybrid CTCs, although not significantly: Survivin expression was observed in 62.7% of mCTCs, 74.61% of hybrid CTCs, and 73.53% of epithelial CTCs. However, the signal intensities were significantly different: Moderate- and high-intensity signals were observed in 48.53% and 31.91% of mCTCs, respectively, and in 47.65% and 16.5% of hybrid CTCs, respectively. The proportion of mesenchymal and hybrid CTCs with moderate- and high-intensity signals was significantly higher than that of epithelial CTCs (P < 0.001) (Figure 7A and B). In contrast, a significantly greater proportion of epithelial CTCs tended to have low signal intensity for survivin expression: 72.47% of epithelial CTCs vs 33.85% and 35.83% of hybrid and mCTCs, respectively (Figure 7A and B).
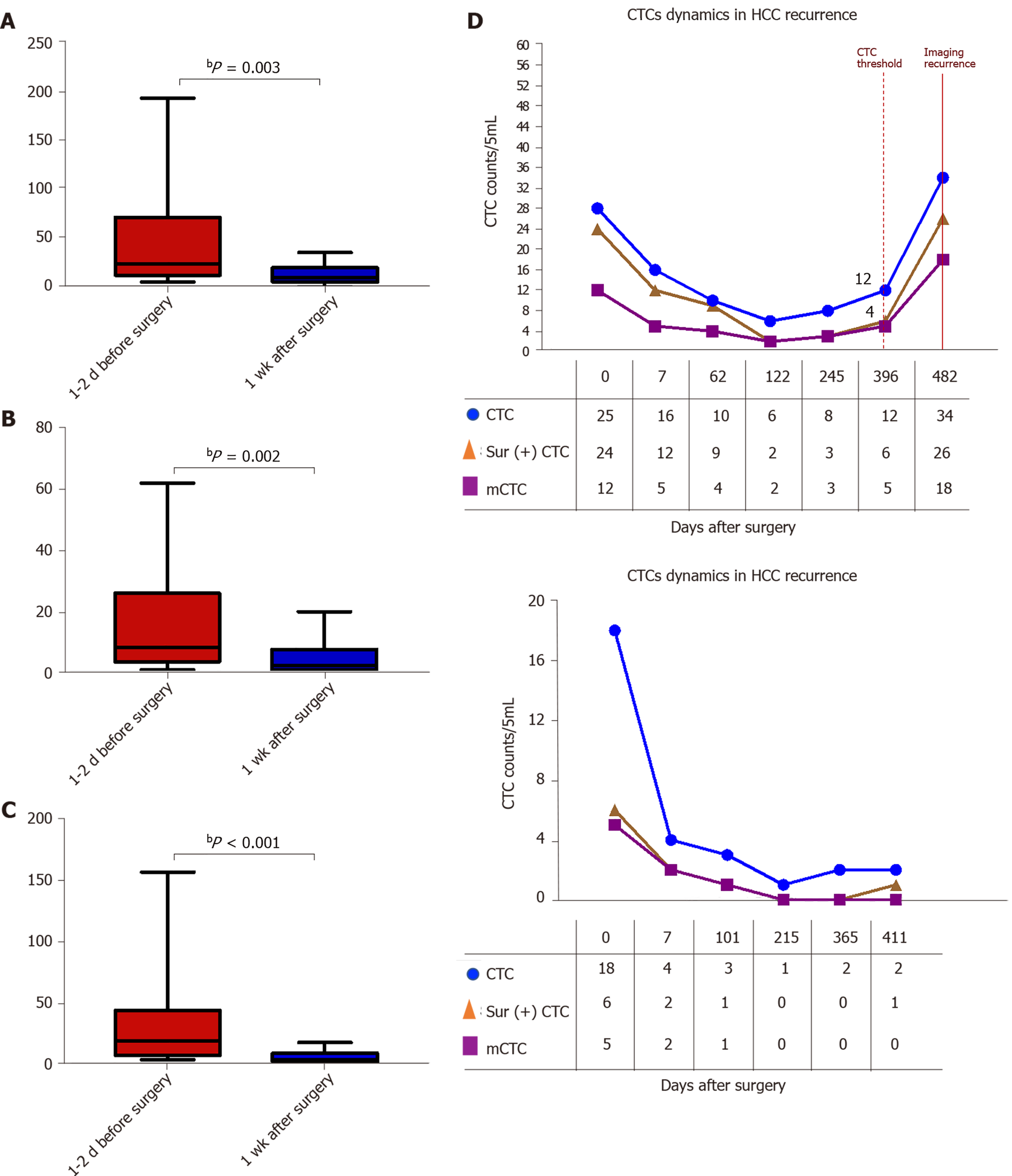
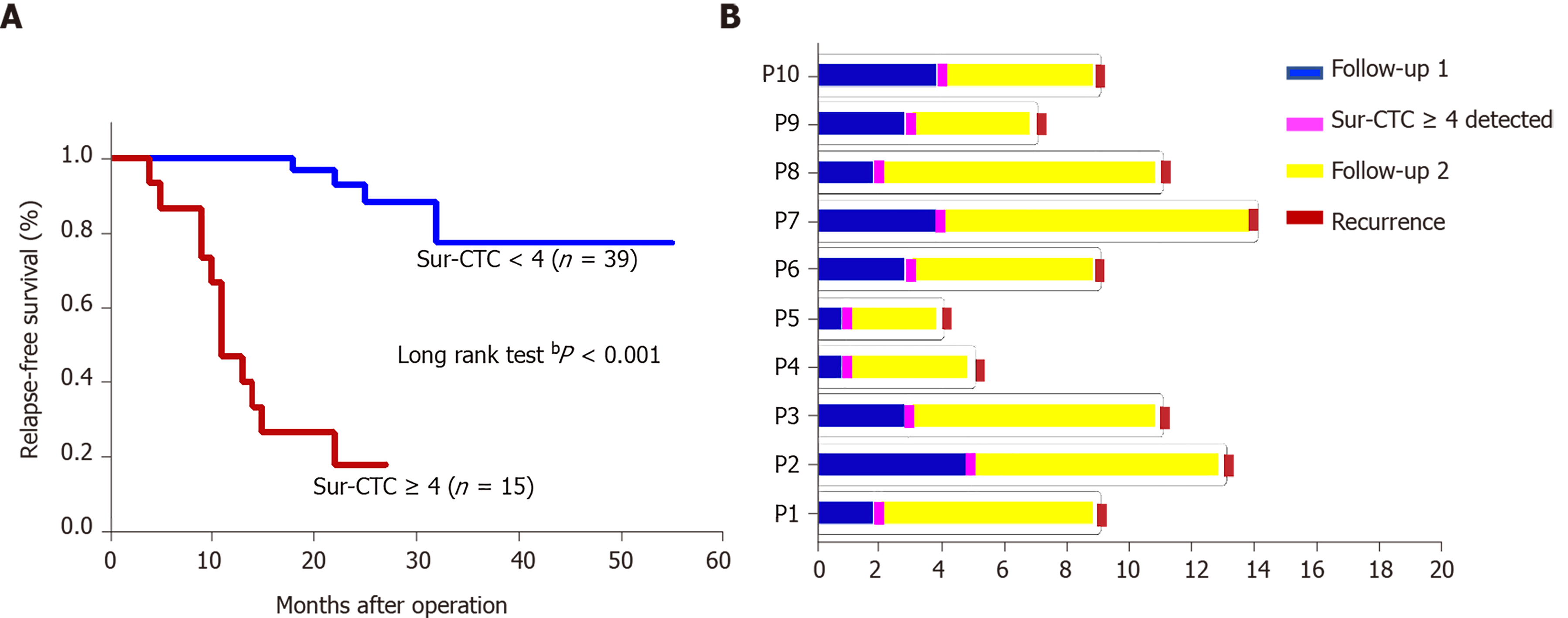
The survivin-positive CTC counts were detected in 22 patients at 1 wk following surgical resection. The total CTC count exhibited a considerable decrease after resection, as did the survivin-positive CTC and mCTC counts (Figure 8A-C). During the postoperative period, in 10 out of 22 HCC patients, the total CTC, mCTC, and survivin-positive CTC counts had increased over the thresholds (12 for CTCs, 4 for mCTCs and sur-CTCs) at 86 d before imaging recurrence or metastatic lesions were detected, while no increase was noted in the other 12 patients for more than 1 year (Figure 8D). Additionally, log-rank test revealed that the recurrence free survival rate was significantly associated with survivin-positive CTC count (Figure 9A). In all 10 cases, over 4 survivin-positive CTCs were detected before recurrence (Figure 9B).
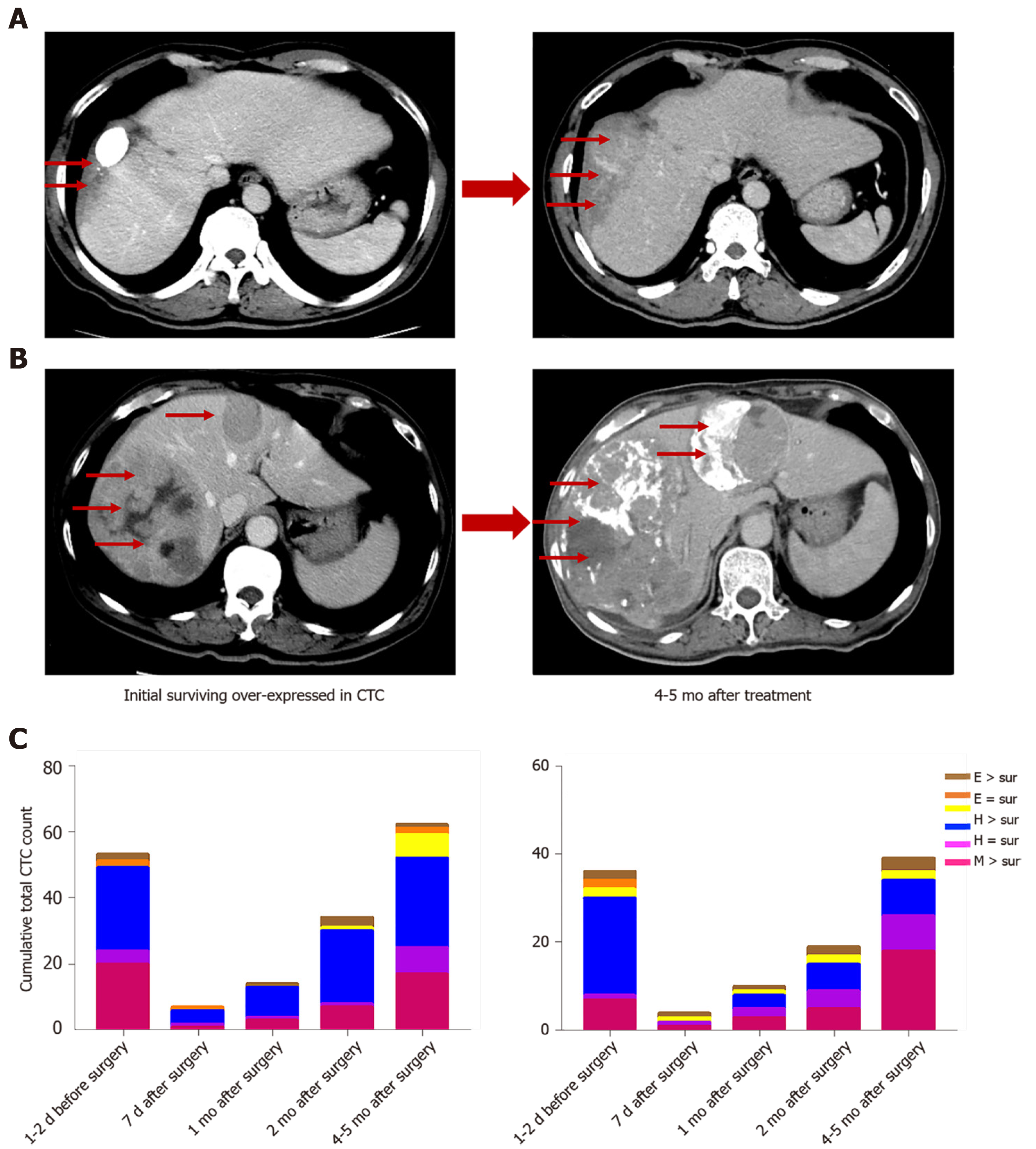
In two HCC patients, initial overexpression of survivin was observed in CTCs (counts/5 mL), while recurrence or metastasis (Figure 10A and B) was observed 4-5 mo after therapy in patients who were in the same BCLC stage before treatment. And increased surviving-positive CTC count is showed in Figure 10C.
In the present study, we analyzed the correlation between survivin-positive CTC expression patterns and the prognosis of HCC, in order to explore the potential of survivin-positive CTCs as markers for HCC stage and predictors of HCC metastasis. The prognostic value of survivin-positive CTCs in patients after surgical resection was also demonstrated.
Our in vitro experiments with HepG2 cells showed that siRNA-induced silencing of survivin expression resulted in a significant decrease in the proliferation and invasive ability of these cells, and a significant increase in their apoptosis rate; opposite results were obtained with overexpression of survivin. In agreement with this finding, previous studies have confirmed that survivin deficiency induces apoptosis in HepG2 cells[20,21]. These findings together indicate that survivin promotes the metastasis of HCC, and they are in agreement with a previous study which showed that knockdown of survivin expression suppressed HCC metastasis[22].
In our study, 94.41% of CTCs obtained from the HCC patients were positive for survivin expression. Further, we examined the survivin expression pattern in different phenotypes of CTCs, including mCTCs, hybrid CTCs, and epithelial CTCs. The findings showed that survivin expression was much higher in mesenchymal and hybrid CTCs than in epithelial CTCs, and a major proportion (62.7%) of mCTCs were positive for survivin expression. These findings indicate that survivin plays an important role in promoting EMT in HCC cells. During the process of carcinogenesis, tumor cells gain invasive ability by undergoing EMT, which occurs via downregulation of the epithelial cell marker E-cadherin (among others) and upregulation of the mesenchymal cell markers vimentin and N-cadherin (among others)[23]. Accordingly, it has also been reported that survivin mediates EMT in HCC cells via modulation of EMT marker expression[24]. Thus, based on these findings, it appears that survivin promotes metastasis of HCC through mediation of EMT events.
Our findings showed that survivin was expressed at significantly greater levels in HCC tissue than in normal liver tissue, and the expression levels were significantly associated with the degree of tumor differentiation, that is, poorly differentiated tumors had significantly higher survivin expression. We also found that survivin expression level was significantly greater in HCC tissue than in normal tissue. The high frequency of cytoplasmic survivin expression in HCC observed in our study is in agreement with that reported in previous studies[24,25]. Many studies have demonstrated that survivin plays a crucial role in the early and late stage of carcinogenesis[26]. In agreement with our findings, other in vitro studies have reported increased expression of survivin in tumor cells[27,28]. Based on these findings, we assumed that survivin plays an anti-apoptotic role and aids in the progression of HCC. Hence, HCC patients who are positive for survivin should be monitored carefully, and enhanced adjuvant therapy should be considered to help improve their prognosis[29,30]. It is not easy to obtain HCC tissue samples at every follow-up appointment; therefore, detection of survivin in CTCs may be a useful supplementary method.
Previous studies have mostly investigated the expression of survivin in cancer cells or tissue specimens from patients[25,31,32], but the strength of our study lies in its investigation of survivin expression in different subtypes of CTCs. This is the first study to have pursued this line of investigation. We found that the survivin-positive CTC counts are significantly associated with the TNM tumor stage, BCLC stage, and degree of differentiation. Thus, the survivin-positive CTC count may particularly be important as an indicator of tumor stage and cancer progression. Additionally, the proportion of mCTCs also increases with tumor progression, but the mCTC counts are not significantly different between the different tumor grades and AFP. This variable needs to be explored further in future studies for its potential as a tumor stage marker.
One of the limitations of this study is the small sample size. Future investigations on survivin-positive CTCs should use a larger sample. Additionally, because of the limited follow-up time, the relationship between survivin-positive CTC counts and overall survival could not be explored. Therefore, an analysis with a longer follow-up duration would be highly useful.
Thus, the present findings indicate that the survivin-positive CTC count might have potential as an indicator for disease stage and a predictor of recurrence in HCC patients. Thus, an efficient and reliable technique to measure survivin expression in CTCs from liver cancer patients could prove useful for predicting the prognosis and recurrence of this cancer, and this could help clinicians select an optimal and customized management strategy for the treatment of this malignancy.
Circulating tumor cells (CTCs), which are also known as liquid biopsy, are mainly used as a predictor to monitor the recurrence and metastasis of tumors including hepatocellular carcinoma (HCC). Survivin is an anti-apoptotic protein that plays an important role in inhibiting apoptosis and is overexpressed in many tumors, including HCC. No study has analyzed the value of survivin-expressing CTCs in HCC.
We aimed to study the expression of survivin in CTCs of HCC and indicate the role of survivin-expressing CTCs as a marker for EMT and cancer progression in HCC.
To explore the prognostic value of survivin-expressing CTCs in HCC, and estimate survivin-positive CTCs as a potential predictor of metastasis in HCC patients.
We examined the survivin expression patterns in CTCs of HCC patients, and investigated the in vitro effects of survivin silencing and overexpression on the proliferation and invasion of HCC cells. We also analysed the survivin protein expression in HCC tissue. And we observed the dynamic changes in survivin-positive CTC count following surgery and its prognostic significance.
The CTC and survivin-positive CTC counts were significantly higher in the HCC patients than in the normal controls. Further, survivin overexpression was found to induce HepG2 cell proliferation, reduce apoptosis, and improve invasive ability. Additionally, log-rank test revealed that the recurrence free survival rate was significantly associated with survivin-positive CTC count.
Survivin-positive CTCs are promising as a predictor of HCC prognosis and metastasis.
The dynamic monitoring of survivin-positive CTC count would help clinical therapy and improve the prognosis of HCC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Suzuki R S-Editor: Liu M L-Editor: Wang TQ P-Editor: Liu M
| 1. | Cao MD, Wang H, Shi JF, Bai FZ, Cao MM, Wang YT, Yan XX, Wang L, Huang Z, Ren JS, Zhao JJ, Dai M, Qu CF, Chen WQ. [Disease burden of liver cancer in China: an updated and integrated analysis on multi-data source evidence]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:1848-1858. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga JY, Bidard FC. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol. 2017;22:421-430. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Ignatiadis M, Lee M, Jeffrey SS. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin Cancer Res. 2015;21:4786-4800. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Yang JD, Campion MB, Liu MC, Chaiteerakij R, Giama NH, Ahmed Mohammed H, Zhang X, Hu C, Campion VL, Jen J, Venkatesh SK, Halling KC, Kipp BR, Roberts LR. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology. 2016;63:148-158. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Yu JJ, Xiao W, Dong SL, Liang HF, Zhang ZW, Zhang BX, Huang ZY, Chen YF, Zhang WG, Luo HP, Chen Q, Chen XP. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma. BMC Cancer. 2018;18:835. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Yin LC, Luo ZC, Gao YX, Li Y, Peng Q, Gao Y. Twist Expression in Circulating Hepatocellular Carcinoma Cells Predicts Metastasis and Prognoses. Biomed Res Int. 2018;2018:3789613. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Arrazubi V, Mata E, Antelo ML, Tarifa A, Herrera J, Zazpe C, Teijeira L, Viudez A, Suárez J, Hernández I, Vera R. Circulating Tumor Cells in Patients Undergoing Resection of Colorectal Cancer Liver Metastases. Clinical Utility for Long-Term Outcome: A Prospective Trial. Ann Surg Oncol. 2019;26:2805-2811. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Alix-Panabières C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016;6:479-491. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Qi LN, Xiang BD, Wu FX, Ye JZ, Zhong JH, Wang YY, Chen YY, Chen ZS, Ma L, Chen J, Gong WF, Han ZG, Lu Y, Shang JJ, Li LQ. Circulating Tumor Cells Undergoing EMT Provide a Metric for Diagnosis and Prognosis of Patients with Hepatocellular Carcinoma. Cancer Res. 2018;78:4731-4744. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Chen Y, Li S, Li W, Yang R, Zhang X, Ye Y, Yu J, Ye L, Tang W. Circulating tumor cells undergoing EMT are poorly correlated with clinical stages or predictive of recurrence in hepatocellular carcinoma. Sci Rep. 2019;9:7084. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Ou H, Huang Y, Xiang L, Chen Z, Fang Y, Lin Y, Cui Z, Yu S, Li X, Yang D. Circulating Tumor Cell Phenotype Indicates Poor Survival and Recurrence After Surgery for Hepatocellular Carcinoma. Dig Dis Sci. 2018;63:2373-2380. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Ye X, Li G, Han C, Han Q, Shang L, Su H, Han B, Gong Y, Lu G, Peng T. Circulating tumor cells as a potential biomarker for postoperative clinical outcome in HBV-related hepatocellular carcinoma. Cancer Manag Res. 2018;10:5639-5647. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Liu J, Cui Y, Yu S, Huang Y, Liu P, Song L, Sun J, Zhang Q, He J. Survivin expression and localization in different organs of yaks (Bos grunniens). Gen Comp Endocrinol. 2018;268:80-87. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Peroukides S, Bravou V, Alexopoulos A, Varakis J, Kalofonos H, Papadaki H. Survivin overexpression in HCC and liver cirrhosis differentially correlates with p-STAT3 and E-cadherin. Histol Histopathol. 2010;25:299-307. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Dai D, Liang Y, Xie Z, Fu J, Zhang Y, Zhang Z. Survivin deficiency induces apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Oncol Rep. 2012;27:621-627. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, Bottinger E, Friedman S, Waxman S, Llovet JM. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938-947. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Roessler S, Jia HL, Budhu A, Forgues M, Ye QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX, Wang XW. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70:10202-10212. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Liu F, Sun Y, Liu B, Lu J, Li H, Zhu H, Gao H, Zhou X, Chang H. Insulin-like growth factor-1 induces epithelial-mesenchymal transition in hepatocellular carcinoma by activating survivin. Oncol Rep. 2018;40:952-958. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Jia X, Gao Y, Zhai D, Liu J, Wang Y, Jing LI, DU Z. Survivin is not a promising serological maker for the diagnosis of hepatocellular carcinoma. Oncol Lett. 2015;9:2347-2352. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Ma J, Feng Y, Liu Y, Li X. PUMA and survivin are involved in the apoptosis of HepG2 cells induced by microcystin-LR via mitochondria-mediated pathway. Chemosphere. 2016;157:241-249. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Yenkejeh RA, Sam MR, Esmaeillou M. Targeting survivin with prodigiosin isolated from cell wall of Serratia marcescens induces apoptosis in hepatocellular carcinoma cells. Hum Exp Toxicol. 2017;36:402-411. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Liu W, Zhu F, Jiang Y, Sun D, Yang B, Yan H. siRNA targeting survivin inhibits the growth and enhances the chemosensitivity of hepatocellular carcinoma cells. Oncol Rep. 2013;29:1183-1188. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Rai K H, Ahmed J. A Correlative Study of N-Cadherin Expression with Different Grades of Oral Squamous Cell Carcinoma Projecting as a Marker of Epithelial to Mesenchymal Transition in Tumor Progression. Asian Pac J Cancer Prev. 2019;20:2327-2332. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Tang B, Liang X, Tang F, Zhang J, Zeng S, Jin S, Zhou L, Kudo Y, Qi G. Expression of USP22 and Survivin is an indicator of malignant behavior in hepatocellular carcinoma. Int J Oncol. 2015;47:2208-2216. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Li J, Ma R, Cao Z, Zhang D. Correlations of MRI manifestations with survivin gene expression in primary hepatic carcinoma. Cancer Biomark. 2018;23:45-51. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Poomsawat S, Punyasingh J, Vejchapipat P. Overexpression of survivin and caspase 3 in oral carcinogenesis. Appl Immunohistochem Mol Morphol. 2014;22:65-71. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Lebelt A, Rutkowski R, Och W, Jaczun K, Dziemiańczyk-Pakieła D, Milewski R, Mariak Z, Reszeć J. Survivin, caspase-3 and MIB-1 expression in astrocytic tumors of various grades. Adv Med Sci. 2016;61:237-243. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Wang JX, Zheng S. Caspase-3 and survivin expression in pediatric neuroblastoma and their roles in apoptosis. Chin Med J (Engl). 2004;117:1821-1824. [PubMed] [Cited in This Article: ] |
| 29. | Xia H, Chen J, Shi M, Deivasigamani A, Ooi LL, Hui KM. The over-expression of survivin enhances the chemotherapeutic efficacy of YM155 in human hepatocellular carcinoma. Oncotarget. 2015;6:5990-6000. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Denel-Bobrowska M, Marczak A. The role of survivin in the diagnosis and therapy of gynaecological cancers. Postepy Hig Med Dosw (Online). 2016;70:1182-1189. [PubMed] [Cited in This Article: ] |
| 31. | Fan Y, Chen J. Clinicopathological significance of survivin expression in patients with cervical cancer: A systematic meta-analysis. Bioengineered. 2017;8:511-523. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Marioni G, Ottaviano G, Marchese-Ragona R, Fasanaro E, Tealdo G, Zanotti C, Randon B, Giacomelli L, Stellini E, Blandamura S. Nuclear survivin expression correlates with endoglin-assessed microvascularisation in laryngeal carcinoma. J Clin Pathol. 2017;70:1033-1037. [PubMed] [DOI] [Cited in This Article: ] |