Published online Sep 28, 2021. doi: 10.3748/wjg.v27.i36.6142
Peer-review started: April 27, 2021
First decision: June 13, 2021
Revised: June 17, 2021
Accepted: August 19, 2021
Article in press: August 19, 2021
Published online: September 28, 2021
Patients with inflammatory bowel disease (IBD) are associated with increased cardiovascular risk and have increased overall cardiovascular burden. On the other hand, urotensin II (UII) is one of the most potent vascular constrictors with immunomodulatory effect that is connected with a number of different car
To determine serum UII levels in patients with IBD and to compare them to control subjects, as well as investigate possible associations with relevant clinical and biochemical parameters.
This cross sectional study consecutively enrolled 50 adult IBD patients (26 with Crohn’s disease and 24 with ulcerative colitis) and 50 age and gender matched controls. Clinical assessment was performed by the same experienced gastroenterologist according to the latest guidelines. Ulcerative Colitis Endoscopic Index of Severity and Simple Endoscopic Score for Crohn’s Disease were used for en
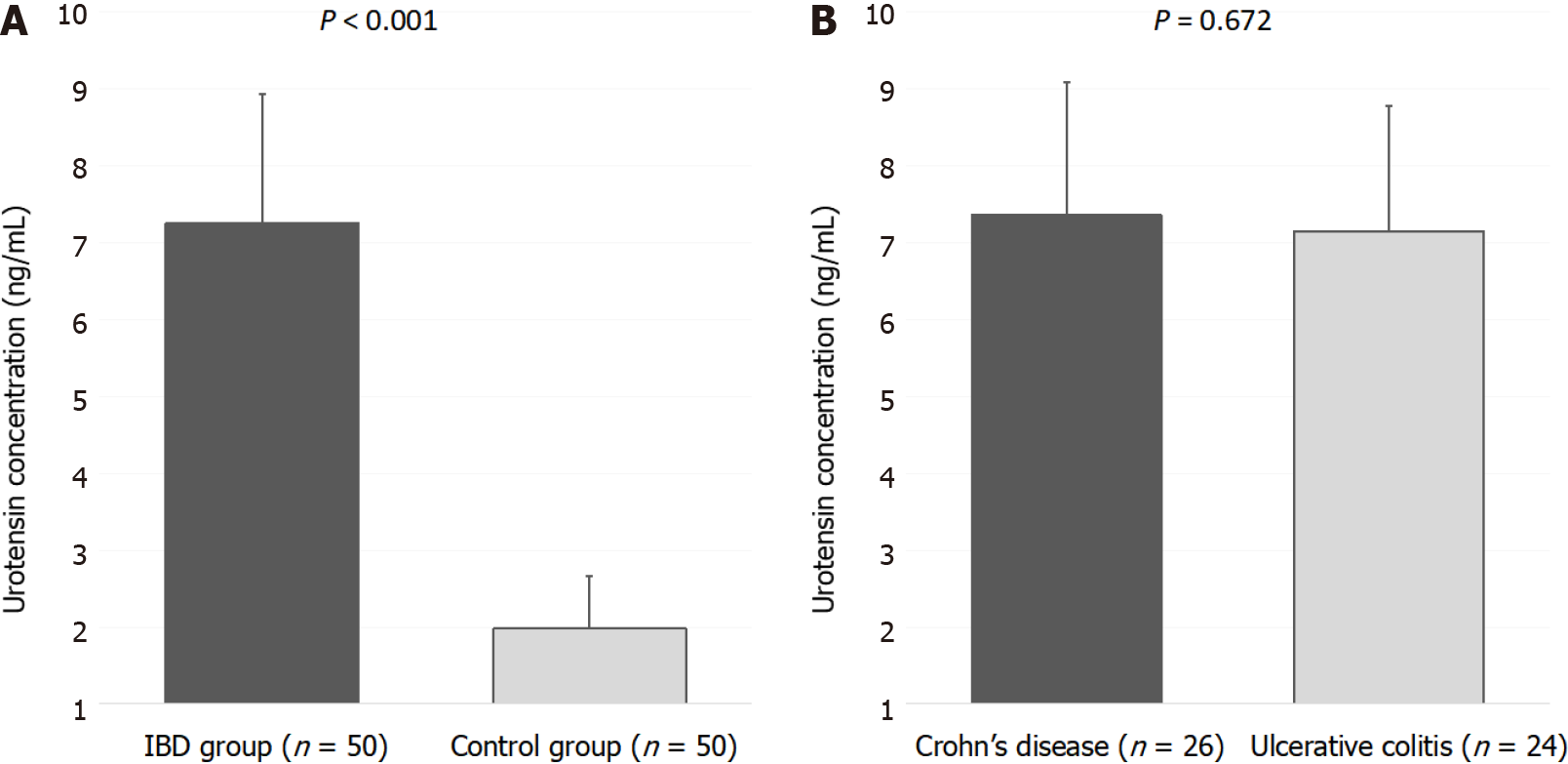
IBD patients have significantly higher concentrations of UII when compared to control subjects (7.57 ± 1.41 vs 1.98 ± 0.69 ng/mL, P < 0.001), while there were no significant differences between Crohn’s disease and ulcerative colitis patients (7.49 ± 1.42 vs 7.65 ± 1.41 ng/mL, P = 0.689). There was a significant positive correlation between serum UII levels and high sensitivity C reactive peptide levels (r = 0.491, P < 0.001) and a significant negative correlation between serum UII levels and total proteins (r = -0.306, P = 0.032). Additionally, there was a significant positive correlation between serum UII levels with both systolic (r = 0.387, P = 0.005) and diastolic (r = 0.352, P = 0.012) blood pressure. Moreover, serum UII levels had a significant positive correlation with Ulcerative Colitis Endoscopic Index of Severity (r = 0.425, P = 0.048) and Simple Endoscopic Score for Crohn’s Disease (r = 0.466, P = 0.028) scores. Multiple linear regression analysis showed that serum UII levels retained significant association with high sensitivity C reactive peptide (β ± standard error, 0.262 ± 0.076, P < 0.001) and systolic blood pressure (0.040 ± 0.017, P = 0.030).
It is possible that UII is involved in the complex pathophysiology of cardio
Core Tip: Urotensin II (UII) is a potent vasoconstrictor with an immunomodulatory effect that is connected to various cardiovascular disorders. On the other hand, patients with inflammatory bowel disease (IBD) have increased cardiovascular burden as well as increased expression of UII receptors. It is plausible that UII is involved in the complex pathophysiology of IBD complications. In the current study, we investigated UII levels in the IBD population and compared it to matched control subjects, as well as connection of UII with relevant clinical and biochemical parameters. The results of this study showed that serum UII levels are higher in IBD patients in comparison with the control group.
- Citation: Alicic D, Martinovic D, Rusic D, Zivkovic PM, Tadin Hadjina I, Vilovic M, Kumric M, Tokic D, Supe-Domic D, Lupi-Ferandin S, Bozic J. Urotensin II levels in patients with inflammatory bowel disease. World J Gastroenterol 2021; 27(36): 6142-6153
- URL: https://www.wjgnet.com/1007-9327/full/v27/i36/6142.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i36.6142
Inflammatory bowel disease (IBD) is a relapsing chronic inflammation of the gas
Urotensin II (UII) is a pleotropic peptide originally found 40 years ago in the neurosecretory system of the teleost fish, while in the meantime its activity was also found in humans. UII is considered to be the most potent vasoconstrictor discovered so far, with the effect 10-fold stronger than that of endothelin-1[7]. Furthermore, its expression was found distributed in most organs and tissues, including both the central nervous and cardiovascular systems, as well as the lungs, kidneys, spleen, hypophysis, adrenal glands, stomach, pancreas, ovaries and liver[8,9]. UII activity is regulated through the urotensin receptor (UTR), which after activation induces calcium mobilization in cellular plasma, smooth muscle cells proliferation and co
In the last few decades, the extraintestinal manifestations and complications of IBD are a major issue that is increasingly investigated for an improvement of both diagnostic and treatment criteria. It is well-established that patients with IBD are associated with a high cardiovascular risk, and it was shown that they have a higher prevalence cardiovascular diseases[16-18]. However, a Danish cohort study showed that patients with IBD have a lower prevalence of the traditional cardiovascular risk factors in comparison to the general population, while on the other hand they had a higher cardiovascular burden[19]. This ambiguity is well-established, yet it is unclear what are the factors that contribute to high cardiovascular risk in patients with IBD. Since UII is one of the most potent vasoconstrictors known, and it is well-known that it is associated with cardiovascular diseases, it is reasonable to presume that there is a possible connection between cardiovascular risk in IBD patients and UII[20,21]. Moreover, seeing that recent studies are pointing to UII immunomodulatory effect and since the hallmark of IBD is the chronic inflammation, this further suggests the need to investigate clinically the possible association between them[14,15]. Additionally, a recent study conducted on patients with UC showed that they have an increased expression of UTR compared to healthy controls[22]. Moreover, that expression was found to be increased in both disease lesions and healthy tissue biopsies.
Hence, the aim of this study was to evaluate serum UII levels in patients with IBD and to compare them with healthy, gender and age matched controls. Moreover, we further investigated the possible association between UII levels and the relevant clinical and biochemical parameters.
This cross-sectional study was conducted at the University Hospital of Split and the University of Split School of Medicine during the period from January 2018 to March 2019.
Before the start of the study, every participant was informed about the aim, course and procedures involved, and they all signed an informed written consent. The study was conducted in accordance with all ethical principles of the Seventh Revision of the Helsinki Declaration, and it was approved by the Ethics Committee of University Hospital of Split (date of approval: November 23, 2017).
This study included 50 adult patients with pre-diagnosed IBD (24 patients with UC and 26 patients with CD) and 50 healthy, age and gender matched controls. The diagnosis of UC and CD was established on clinical, radiological, endoscopic and histological traits in accordance with the European Consensus on Crohn’s Disease and Ulcerative Colitis[23]. Inclusion criteria were: Disease duration of at least 1 year, stable disease activity in the past 3 mo and age between 18 and 65 years. Exclusion criteria were: Diabetes, obesity, arterial hypertension, use of statins, cardiovascular disorders, therapy with corticosteroids during 3 mo prior to study onset, substance abuse and consumption of alcohol more than 40 g/d.
Additionally, we checked medical records of the control subjects regarding gas
Disease activity evaluation was performed using clinical and endoscopic indices. The assessment was conducted by the same experienced gastroenterologist according to the latest guidelines, and the colonoscopy needed for the evaluation of the disease activity was performed within 2 wk of blood sampling. We used endoscopic indices for the evaluation of the disease activity since they have an advantage before clinical indices according to the European Consensus on Crohn’s Disease and Ulcerative Colitis guidelines[25]. Moreover, all IBD patients had their high sensitivity C reactive peptide (hsCRP) and fecal calprotectin evaluated to assess further the activity of the disease.
Ulcerative Colitis Endoscopic Index of Severity (UCEIS) is a quantitative grading system used for the evaluation of UC activity. Depending on the score, there are four possible grades for disease activity: (0-1)–remission; (2-4)–mild; (5-6)–moderate and (7-8)–severe activity[26].
Simple Endoscopic Score for CD (SES-CD) is a quantitative grading system used for the evaluation of CD activity. According to the majority of studies, the threshold values for interpretation of the results are: (0-2)–remission; (3-6)–mild activity; (7-15)–moderate activity and (≥ 16)–severe disease[27].
All blood samples were taken from the cubital vein after 12-h fasting. After extraction, all samples were processed in the same day except for the UII samples, which were centrifuged and stored at -80 °C for further analysis. All the procedures were conducted according to the international standards, in the same laboratory and by the same experienced medical biochemist who was blinded to the subjects group in the study. Serum levels of UII were determined using the enzyme immunoassay kit for human UII (Phoenix Pharmaceuticals, Burlingame, CA, United States), according to the manufacturer’s instructions. Concentration of the analyzed quality control sample that arrived from the manufacturer was within predefined acceptable deviation. The linear range of the assay was 0.06-8.2 ng/mL, and sensitivity was 0.06 ng/mL. Coefficient of variation within the probe was less than 10% and between probes was less than 15%. Other biochemical parameters were analyzed according to standard laboratory procedures.
Stool samples were received by a trained laboratory technician in sterile containers within 3 d of sampling. Fecal extraction and analyses were performed by an experi
All participants were subjected to detailed anamnesis, physical examination and measurements of anthropometric characteristics - body weight, body height and body mass index (BMI). For measurement of body weight and height, a calibrated medical scale with built-in heights (Seca, Birmingham, United Kingdom) was used. BMI was calculated according to the formula = [body weight (kg)]/[height per square (m2)].
Collected data were analyzed with statistical software MedCalc (version 17.4.1; MedCalc Software, Ostend, Belgium,). Quantitative data were expressed as mean ± SD or median and interquartile range, while qualitative data were expressed as whole number and percentage. Kolmogorov-Smirnov test was used to estimate the normality of data distribution. Comparison of serum UII levels and other parameters between patients with IBD and control subjects was done by Student t-test for independent samples or Mann-Whitney U test. For comparison of qualitative variables, Chi-squared test was used. Pearson’s correlation or Spearman’s correlation was used to estimate the correlation between biochemical, anthropometric and clinical parameters with serum UII levels. Furthermore, multiple linear regression analysis was used to determine significant independent predictors of serum UII levels, with reporting corresponding P values with unstandardized β-coefficients, standard error and t-values. The level of statistical significance was set at P < 0.05.
There were no statistically significant differences regarding age, gender and anthropometric features between the IBD patients and healthy controls (Table 1). Laboratory analyses showed that the IBD group compared to the control group had significantly lower erythrocytes (4.7 ± 0.5 vs 5.0 ± 0.4 × 1012/L, P = 0.020), hemoglobin (140.4 ± 17.3 vs 148.1 ± 13.7 g/L, P = 0.015) and albumins (39.5 ± 5.1 vs 43.7 ± 2.4 g/L, P < 0.001), while they had significantly higher hsCRP levels (3.4 ± 2.6 vs 1.2 ± 1.1 mg/L, P < 0.001) (Table 2).
| Parameter | IBD group (n = 50) | Control group (n = 50) | P value1 |
| Male gender, n (%) | 31 (62) | 29 (58) | 0.838 |
| Age (yr) | 44.3 ± 14.8 | 40.6 ± 12.3 | 0.181 |
| Body weight (kg) | 78.4 ± 14.0 | 81.1 ± 15.0 | 0.356 |
| Body height (cm) | 176.9 ± 9.8 | 179.6 ± 7.9 | 0.130 |
| Body mass index (kg/m2) | 23.9 ± 3.7 | 24.9 ± 3.4 | 0.136 |
| SBP (mmHg) | 119.5 ± 11.2 | 116.6 ± 9.2 | 0.156 |
| DBP (mmHg) | 77.7 ± 8.3 | 75.0 ± 8.6 | 0.112 |
| Smoking, n (%) | 12 (24.0) | 9 (18.4) | 0.660 |
| Disease duration (yr)2 | 6.0 (3.0-11.0) | - | - |
| UCEIS (score) | 6.0 (5.0-7.0) | - | - |
| SES-CD (score) | 9.2 (6.6-12.0) | - | - |
| Aminosalycilates | 32 (64.0%) | - | - |
| DMARD | 15 (30.0%) | - | - |
| Monoclonal antibodies | 29 (58.0%) | - | - |
| Parameter | IBD group (n = 50) | Control group (n = 50) | P value1 |
| Erythrocytes (× 1012/L) | 4.7 ± 0.5 | 5.0 ± 0.4 | 0.020 |
| Hemoglobin (g/L) | 140.4 ± 17.3 | 148.1 ± 13.7 | 0.015 |
| Fasting glucose (mmol/L) | 5.1 ± 0.9 | 5.0 ± 0.6 | 0.653 |
| Urea (mmol/L) | 4.7 ± 1.5 | 5.6 ± 1.6 | 0.004 |
| Creatinine (μmol/L) | 71.4 ± 15.3 | 75.9 ± 14.7 | 0.134 |
| Total proteins (g/L) | 71.2 ± 6.6 | 72.1 ± 3.9 | 0.394 |
| Albumins (g/L) | 39.5 ± 5.1 | 43.7 ± 2.4 | < 0.001 |
| hsCRP (mg/L) | 3.4 ± 2.6 | 1.2 ± 1.1 | < 0.001 |
| Triglycerides (mmol/L) | 1.3 ± 0.9 | 1.1 ± 0.6 | 0.311 |
| Total cholesterol (mmol/L) | 4.8 ± 1.3 | 5.2 ± 1.2 | 0.091 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.4 | 1.4 ± 0.3 | 0.447 |
| LDL cholesterol (mmol/L) | 2.6 (2.1-3.4) | 3.2 (2.4-3.9) | 0.018 |
| FC (μg/g) | 231 (61.5-619.2) | - | - |
Serum UII levels were significantly higher in the IBD group compared to the control group (7.26 ± 1.67 vs 1.98 ± 0.68 ng/mL, P < 0.001) (Figure 1A). Furthermore, there were no statistically significant differences in serum UII levels between the patients with UC and patients with CD (7.15 ± 1.63 vs 7.36 ± 1.73 ng/mL, P = 0.672) (Figure 1B).
There was a significant positive correlation between serum UII levels and hsCRP levels (r = 0.491, P < 0.001) and a significant negative correlation between serum UII levels and total proteins (r = -0.306, P = 0.032). There were no significant correlations with other biochemical parameters (Table 3).
| Parameter | r1 | P value |
| hsCRP (mg/L) | 0.491 | < 0.001 |
| Total proteins (g/L) | -0.306 | 0.032 |
| Albumins (g/L) | -0.182 | 0.210 |
| Triglycerides (mmol/L) | 0.057 | 0.698 |
| Total cholesterol (mmol/L) | -0.114 | 0.439 |
| HDL (mmol/L) | -0.153 | 0.298 |
| LDL (mmol/L) | -0.1033 | 0.487 |
| Urea (mmol/L) | 0.013 | 0.928 |
| Creatinine (μmol/L) | 0.133 | 0.356 |
| Age (yr) | -0.072 | 0.614 |
| Body mass index (kg/m2) | -0.037 | 0.800 |
| SBP (mmHg) | 0.387 | 0.005 |
| DBP (mmHg) | 0.352 | 0.012 |
| IBD duration (yr)2 | 0.0453 | 0.751 |
| FC (μg/g) | 0.0483 | 0.812 |
| UCEIS (score) | 0.4253 | 0.048 |
| SES-CD (score) | 0.4663 | 0.028 |
There was a significant positive correlation between serum UII levels with both systolic (r = 0.387, P = 0.005) and diastolic (r = 0.352, P = 0.012) blood pressure. Moreover, serum UII levels had a significant positive correlation with UCEIS (r = 0.425, P = 0.048) and SES-CD (r = 0.466, P = 0.028) scores (Table 3).
Multiple linear regression analysis showed that serum UII levels retained significant association with hsCRP (β ± standard error, 0.262 ± 0.076, P < 0.001) and systolic blood pressure (0.040 ± 0.017, P = 0.030) after model adjustment for age, gender, BMI and diastolic blood pressure, with serum UII levels as a dependent variable (Table 4).
The results of this study showed that serum UII levels are higher in patients with IBD compared to the healthy controls, while there was no significant difference between patients with UC and patients with CD. To the best of our knowledge, this is the first clinical study to investigate serum UII levels in both UC and CD.
Association between UII and IBD was only explored in a recent experimental pilot study that investigated expression of the UII receptor UTR in patients with UC[22]. They measured UTR expression from biopsies of the UC lesions and healthy colon tissue, and their outcomes determined that UTR expression was significantly higher in both the UC lesions and healthy tissue of the UC patients compared to the control group biopsies. Furthermore, a Chinese animal study conducted on mice with dextran sulfate sodium induced colitis explored the mechanisms of UTR in colonic inflammation[28]. They administrated the mice with urantide, a special antagonist of UTR that consequently alleviated rectal bleeding, tissue injury and production of inter
According to these results, it could be hypothesized that in IBD, among other mechanisms, higher UII levels and consequently greater UTR activity can be asso
Moreover, it is important to highlight that the previous studies have shown that high TNF-α levels play a major role in the disruption of macro and microvascular circulation[33]. It induces the production of reactive oxygen species, which results in endothelial dysfunction, while several studies presented that the administration of anti-TNF-α therapy to patients with IBD results in a significant improvement of endothelial dysfunction[32,34]. It is possible that functional and structural changes of the vascular endothelium due to chronic inflammation consequently results in higher cardiovascular risk in IBD patients[35]. Furthermore, with more severe disease activity and consequent greater inflammation, the resulting endothelial dysfunction that accompanies these changes is more advanced. It was presented by two recent studies that heart dysfunction, as well as fibrosis and cell hypertrophy, were significantly decreased in experimental heart models treated with UTR antagonists[36,37]. More
In the last 2 decades, it has become clear that chronic systemic inflammation plays a major part in the initiation and progression of atherosclerosis[39,40]. Circulating UII was reported to promote increase of reactive oxygen species levels, which are important molecules in the initiation of atherosclerosis[41]. In a Chinese animal study, urantide administration reduced the proportion of the macrophage lesion area as well as improved the plaque characteristics in hyperlipidemic rabbits by increasing the collagen content[42]. Even though UTR antagonist downregulated proinflammatory cytokines, it did not significantly change the lipid profile. In summary, although the UTR antagonist did not change the progression of atherosclerosis, it significantly affected composition of atherosclerotic plaque. These results imply that UII is asso
Our results also determined a significant positive correlation between serum UII levels with systolic and diastolic blood pressure. This is in alignment with several other clinical and experimental studies that have found an association between UII and arterial pressure, probably present due to its established vasoconstrictive effect[43,44]. Moreover, studies have shown that IBD patients have a lower incidence of some of the traditional risk factors for cardiovascular diseases, including hypertension[19]. In a current scenario, it is still questionable why IBD patients have lower incidence of hypertension, although UII levels are elevated in comparison with healthy subjects. It is hard to hypothesize from our results what are the possible reasons for this ambiguity, and further studies are needed to elaborate this issue. However, it is possible that UII vasoconstriction effect is diminished by other factors that are present in IBD patients.
This study had several limitations. It was a single center study with a cross-sectional design. Moreover, our sample size was relatively low, and we were not able to eliminate completely all possible confounding effects.
In conclusion, this study showed that patients with IBD have a higher serum level of UII compared to the control group. This implied association with IBD was further supported with the positive correlation between UII and hsCRP, UCEIS and SES-CD. All of these results suggest that UII could be involved in the pathophysiology of IBD, especially in the inflammation severity and disease activity. However, future studies need to clarify these connections.
Patients with inflammatory bowel disease (IBD) are associated with increased car
While a recent study showed that patients with ulcerative colitis (UC) have increased expression of urotensin II receptor in comparison to healthy controls, a larger clinical study regarding UII serum levels in patients with IBD is still missing.
The aim of this study was to compare serum levels of UII between patients with IBD and healthy controls. The additional goal was to investigate the association of serum UII levels with the anthropometric, clinical and biochemical parameters.
This study included 50 adult patients with pre-diagnosed IBD (24 patients with UC and 26 patients with Crohn’s disease (CD) and 50 healthy, age and gender matched controls. Serum levels of UII were determined using the enzyme immunoassay kit for human UII, according to the manufacturer’s instructions. Other parameters were analyzed according to the standard laboratory procedures.
Analysis has shown that IBD patients have significantly higher concentrations of UII when compared to control subjects (7.57 ± 1.41 vs 1.98 ± 0.69 ng/mL, P < 0.001), while there were no significant differences between CD and UC patients (7.49 ± 1.42 vs 7.65 ± 1.41 ng/mL, P = 0.689). There was a significant positive correlation between serum UII levels and high sensitivity C reactive peptide levels (r = 0.491, P < 0.001), UC Endoscopic Index of Severity (r = 0.425, P = 0.048) and Simple Endoscopic Score for CD (r = 0.466, P = 0.028) scores.
Our clinical results suggest that UII could be involved in the pathophysiology of IBD, especially in the inflammation severity and disease activity.
Future larger scale multicenter studies need to clarify the connection between UII and IBD.
The authors would like to thank to Behmen D, MA for her careful language assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sassaki LY S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 751] [Cited by in F6Publishing: 799] [Article Influence: 79.9] [Reference Citation Analysis (12)] |
| 2. | Brnic D, Martinovic D, Zivkovic PM, Tokic D, Vilovic M, Rusic D, Tadin Hadjina I, Libers C, Glumac S, Supe-Domic D, Tonkic A, Bozic J. Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease. World J Gastroenterol. 2020;26:4866-4877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Brnić D, Martinovic D, Zivkovic PM, Tokic D, Tadin Hadjina I, Rusic D, Vilovic M, Supe-Domic D, Tonkic A, Bozic J. Serum adropin levels are reduced in patients with inflammatory bowel diseases. Sci Rep. 2020;10:9264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J Gastroenterol. 2019;25:2162-2176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 93] [Cited by in F6Publishing: 95] [Article Influence: 19.0] [Reference Citation Analysis (7)] |
| 5. | Filimon AM, Negreanu L, Doca M, Ciobanu A, Preda CM, Vinereanu D. Cardiovascular involvement in inflammatory bowel disease: Dangerous liaisons. World J Gastroenterol. 2015;21:9688-9692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Cheng K, Faye AS. Venous thromboembolism in inflammatory bowel disease. World J Gastroenterol. 2020;26:1231-1241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 52] [Cited by in F6Publishing: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Svistunov AA, Tarasov VV, Shakhmardanova SA, Sologova SS, Bagaturiya ET, Chubarev VN, Galenko-Yaroshevsky PA, Avila-Rodriguez MF, Barreto GE, Aliev G. Urotensin II: Molecular Mechanisms of Biological Activity. Curr Protein Pept Sci. 2018;19:924-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Federico A, Zappavigna S, Dallio M, Misso G, Merlino F, Loguercio C, Novellino E, Grieco P, Caraglia M. Urotensin-II Receptor: A Double Identity Receptor Involved in Vasoconstriction and in the Development of Digestive Tract Cancers and other Tumors. Curr Cancer Drug Targets. 2017;17:109-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Pereira-Castro J, Brás-Silva C, Fontes-Sousa AP. Novel insights into the role of urotensin II in cardiovascular disease. Drug Discov Today. 2019;24:2170-2180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Castel H, Desrues L, Joubert JE, Tonon MC, Prézeau L, Chabbert M, Morin F, Gandolfo P. The G Protein-Coupled Receptor UT of the Neuropeptide Urotensin II Displays Structural and Functional Chemokine Features. Front Endocrinol (Lausanne). 2017;8:76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Eyre HJ, Speight T, Glazier JD, Smith DM, Ashton N. Urotensin II in the development and progression of chronic kidney disease following ⅚ nephrectomy in the rat. Exp Physiol. 2019;104:421-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Khan K, Albanese I, Yu B, Shalal Y, Al-Kindi H, Alaws H, Tardif JC, Gourgas O, Cerutti M, Schwertani A. Urotensin II, urotensin-related peptide, and their receptor in aortic valve stenosis. J Thorac Cardiovasc Surg. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Chen X, Yin L, Jia WH, Wang NQ, Xu CY, Hou BY, Li N, Zhang L, Qiang GF, Yang XY, Du GH. Chronic Urotensin-II Administration Improves Whole-Body Glucose Tolerance in High-Fat Diet-Fed Mice. Front Endocrinol (Lausanne). 2019;10:453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Sun SL, Liu LM. Urotensin II: an inflammatory cytokine. J Endocrinol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Liang DY, Liu LM, Ye CG, Zhao L, Yu FP, Gao DY, Wang YY, Yang ZW. Inhibition of UII/UTR system relieves acute inflammation of liver through preventing activation of NF-κB pathway in ALF mice. PLoS One. 2014;8:e64895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Bigeh A, Sanchez A, Maestas C, Gulati M. Inflammatory bowel disease and the risk for cardiovascular disease: Does all inflammation lead to heart disease? Trends Cardiovasc Med. 2020;30:463-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Singh S, Kullo IJ, Pardi DS, Loftus EV Jr. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:26-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Zivkovic PM, Matetic A, Tadin Hadjina I, Rusic D, Vilovic M, Supe-Domic D, Borovac JA, Mudnic I, Tonkic A, Bozic J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death--a Danish nationwide cohort study. PLoS One. 2013;8:e56944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Ahmed AH, Maulood IM. Endothelin-1 and angiotensin-II modulate urotensin-II vasoconstriction in rat aorta exposed to mercury. Bratisl Lek Listy. 2018;119:444-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Jumaah S, Çelekli A, Sucu M. The role of human urotensin-II in patients with hypertrophic cardiomyopathy. J Immunoassay Immunochem. 2018;39:150-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Gravina AG, Dallio M, Tuccillo C, Martorano M, Abenavoli L, Luzza F, Stiuso P, Lama S, Grieco P, Merlino F, Caraglia M, Loguercio C, Federico A. Urotensin II receptor expression in patients with ulcerative colitis: a pilot study. Minerva Gastroenterol Dietol. 2020;66:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 23. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1024] [Cited by in F6Publishing: 1094] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 24. | Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil. 2017;23:151-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 25. | Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, Vavricka SR, Verstockt B, van Rheenen P, Tolan D, Taylor SA, Rimola J, Rieder F, Limdi JK, Laghi A, Krustiņš E, Kotze PG, Kopylov U, Katsanos K, Halligan S, Gordon H, González Lama Y, Ellul P, Eliakim R, Castiglione F, Burisch J, Borralho Nunes P, Bettenworth D, Baumgart DC, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 208] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 26. | Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lichtenstein GR, Marteau PR, Reinisch W, Sands BE, Yacyshyn BR, Schnell P, Bernhardt CA, Mary JY, Sandborn WJ. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145:987-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 27. | Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A, Mary JY, Colombel JF, Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 999] [Cited by in F6Publishing: 1104] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 28. | Yang Y, Zhang J, Chen X, Wu T, Xu X, Cao G, Li H, Li Y. UII/GPR14 is involved in NF-κB-mediated colonic inflammation in vivo and in vitro. Oncol Rep. 2016;36:2800-2806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Liu LM, Liang DY, Ye CG, Tu WJ, Zhu T. The UII/UT system mediates upregulation of proinflammatory cytokines through p38 MAPK and NF-κB pathways in LPS-stimulated Kupffer cells. PLoS One. 2015;10:e0121383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Lichtenstein GR. Comprehensive review: antitumor necrosis factor agents in inflammatory bowel disease and factors implicated in treatment response. Therap Adv Gastroenterol. 2013;6:269-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Friedrich M, Pohin M, Powrie F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity. 2019;50:992-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 32. | Schinzari F, Armuzzi A, De Pascalis B, Mores N, Tesauro M, Melina D, Cardillo C. Tumor necrosis factor-alpha antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin Pharmacol Ther. 2008;83:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Zhang H, Park Y, Wu J, Chen Xp, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond). 2009;116:219-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 34. | Zanoli L, Inserra G, Cappello M, Ozturk K, Castellino P. Aortic Stiffness in Patients With Inflammatory Bowel Disease Reduced After Anti-Tumor Necrosis Factor Therapy. J Am Coll Cardiol. 2019;73:981-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Cibor D, Domagala-Rodacka R, Rodacki T, Jurczyszyn A, Mach T, Owczarek D. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J Gastroenterol. 2016;22:1067-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 61] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Park CH, Lee JH, Lee MY, Lee BH, Oh KS. A novel role of G protein-coupled receptor kinase 5 in urotensin II-stimulated cellular hypertrophy in H9c2UT cells. Mol Cell Biochem. 2016;422:151-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Oh KS, Lee JH, Yi KY, Lim CJ, Park BK, Seo HW, Lee BH. A novel urotensin II receptor antagonist, KR-36996, improved cardiac function and attenuated cardiac hypertrophy in experimental heart failure. Eur J Pharmacol. 2017;799:94-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Demirpence M, Guler A, Yilmaz H, Sayin A, Pekcevik Y, Turkon H, Colak A, Ari EM, Aslanipour B, Kocabas GU, Calan M. Is elevated urotensin II level a predictor for increased cardiovascular risk in subjects with acromegaly? J Endocrinol Invest. 2019;42:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Chistiakov DA, Grechko AV, Myasoedova VA, Melnichenko AA, Orekhov AN. The role of monocytosis and neutrophilia in atherosclerosis. J Cell Mol Med. 2018;22:1366-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 41. | Mohammadi A, Najar AG, Khoshi A. Effect of urotensin II on apolipoprotein B100 and apolipoprotein A-I expression in HepG2 cell line. Adv Biomed Res. 2014;3:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Yu QQ, Cheng DX, Xu LR, Li YK, Zheng XY, Liu Y, Li YF, Liu HL, Bai L, Wang R, Fan JL, Liu EQ, Zhao SH. Urotensin II and urantide exert opposite effects on the cellular components of atherosclerotic plaque in hypercholesterolemic rabbits. Acta Pharmacol Sin. 2020;41:546-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Watanabe T, Kanome T, Miyazaki A, Katagiri T. Human urotensin II as a link between hypertension and coronary artery disease. Hypertens Res. 2006;29:375-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Debiec R, Christofidou P, Denniff M, Bloomer LD, Bogdanski P, Wojnar L, Musialik K, Charchar FJ, Thompson JR, Waterworth D, Song K, Vollenweider P, Waeber G, Zukowska-Szczechowska E, Samani NJ, Lambert D, Tomaszewski M. Urotensin-II system in genetic control of blood pressure and renal function. PLoS One. 2013;8:e83137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |