Published online Sep 28, 2021. doi: 10.3748/wjg.v27.i36.6025
Peer-review started: February 28, 2021
First decision: May 1, 2021
Revised: May 10, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: September 28, 2021
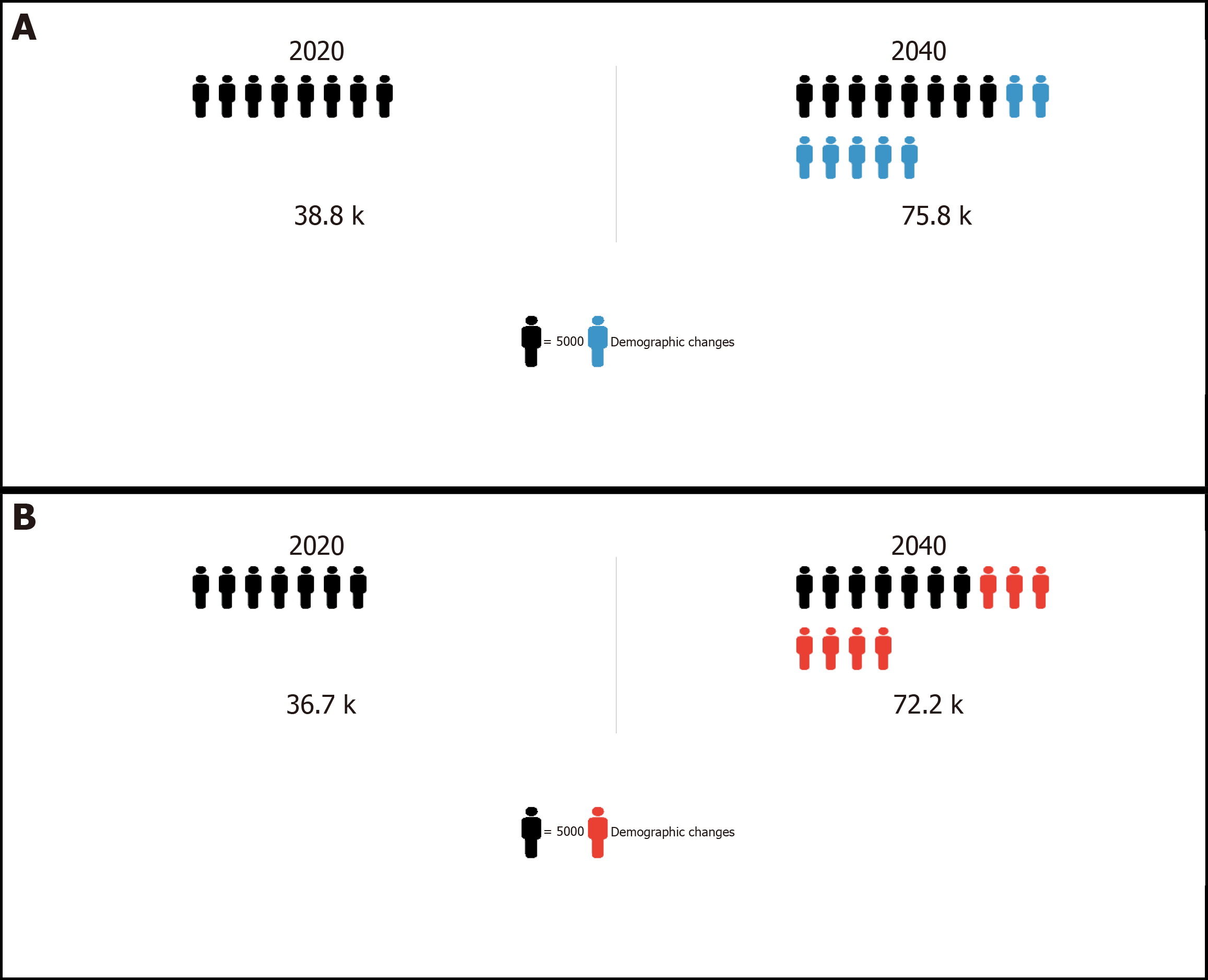
Elimination of viral hepatitis in sub-Saharan Africa by 2030 is an ambitious feat. However, as stated by the World Health Organization, there are unprecedented opportunities to act and make significant contributions to the elimination target. With 60 million people chronically infected with hepatitis B virus (HBV) of whom 38800 are at risk of developing highly fatal hepatocellular carcinoma (HCC) every year, sub-Saharan Africa faces one of the greatest battles towards elimination of viral hepatitis. There is a need to examine progress in controlling the disproportionate burden of HBV-associated HCC in sub-Saharan Africa within the context of this elimination target. By scaling-up coverage of hepatitis B birth dose and early childhood vaccination, we can significantly reduce new cases of HCC by as much as 50% within the next three to five decades. Given the substantial reservoir of chronic HBV carriers however, projections show that HCC incidence and mortality rates in sub-Saharan Africa will double by 2040. This warrants urgent public health attention. The trends in the burden of HCC over the next two decades, will be determined to a large extent by progress in achieving early diagnosis and appropriate linkage to care for high-risk chronic HBV infected persons.
Core Tip: Chronic hepatitis B virus (HBV) infection is the primary risk factor for hepatocellular carcinoma (HCC) in sub-Saharan Africa. In 2020, HBV-associated HCC accounted for approximately 36700 deaths. By 2040, it is projected that approximately 72200 people will die each year from this disease without an intensive public health response. The high mortality-to-incidence ratio associated with HCC in sub-Saharan Africa suggests significant inequities in access to appropriate health care. This review examines the evidence on the extent of the disease burden in sub-Saharan Africa and advocates for prioritizing HCC control as part of ongoing viral hepatitis elimination strategies within this region.
- Citation: Amponsah-Dacosta E. Hepatitis B virus infection and hepatocellular carcinoma in sub-Saharan Africa: Implications for elimination of viral hepatitis by 2030? World J Gastroenterol 2021; 27(36): 6025-6038
- URL: https://www.wjgnet.com/1007-9327/full/v27/i36/6025.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i36.6025
Despite the availability of a safe and effective prophylactic vaccine since 1982, chronic hepatitis B which is a serious liver disease caused by hepatitis B virus (HBV), remains a major global public health threat. Estimates from the World Health Organization’s (WHO) current Global Hepatitis Report suggest that in 2015, 3.5% (257 million persons) of the world’s population were living with chronic hepatitis B, with the Western Pacific and sub-Saharan African regions bearing the brunt (68%) of the disease burden (WHO, 2017). In addition to this, 887000 deaths due to HBV-associated hepatic sequelae such as acute hepatitis, liver cirrhosis and liver cancer or hepatocellular carcinoma (HCC), were recorded worldwide[1].
With an estimated 830180 associated deaths recorded in 2020, liver cancer remains a leading cause of cancer-related death worldwide, third only to lung (1.8 million deaths) and colorectal (935153 deaths) cancers[2]. Globally, incidence rates of liver cancer have remained high, with 905677 newly diagnosed cases in 2020 [compared to 748000 (5.9% of all cancers) new cases in 2008, for example[3]], representing 4.7% of all cancer cases recorded in that year alone[2]. The most common type of malignant transformation in the liver is HCC (75%-85%), followed by intrahepatic cholangiocarcinoma (10%-15%), with other rare types accounting for the remainder of all primary liver cancers. The geographic distribution of the incidence of HCC tends to mirror that of its major risk factors, chronic hepatitis B and hepatitis C virus (HCV) infection, which account for approximately 56% and 20% of all HCC cases, respectively[2,4-6]. This implies that the highest incidence rates [age-standardized incidence rate (ASIR) > 20 cases per 100000 persons per year] of HCC are recorded in hepatitis B endemic countries including those in sub-Saharan Africa, while non-endemic regions like Europe and North America report relatively lower incidence rates (ASIR < 10 cases per 100000 persons per year)[2,7-9]. A further cause for concern in most resource limited countries within regions like sub-Saharan Africa where HCC screening and diagnostic services, and medical interventions are often inadequate, is the poor survival and extremely high mortality rates associated with HCC. Approximately 93% of patients die within a year of the onset of symptoms[7,9-11]. Evidently, elimination of viral hepatitis caused by HBV and HCV presents the best opportunity to reduce the incidence of HCC, especially in regions like sub-Saharan Africa, where the disease burden and need for intervention are oftentimes the greatest.
Recognizing the devastating impact of viral hepatitis on global health, the WHO in May 2016 adopted a Global Health Sector Strategy on Viral Hepatitis aimed at achieving a 90% reduction in new cases and a 65% reduction in mortality due to HBV and HCV infection, towards an ambitious target of eliminating viral hepatitis by 2030[12]. To eliminate chronic hepatitis B, the health service targets to be attained by 2030 include; 90% coverage of routine childhood hepatitis B vaccination, a reduction in mother-to-child transmission (MTCT) of HBV such as through > 90% coverage of hepatitis B birth dose vaccination, 100% of all blood donations screened for HBV, 90% of all HBV infections diagnosed, and 80% of eligible persons with chronic hepatitis B linked to appropriate treatment and care[12]. While largely in the planning phase of executing this global strategy, a recent WHO report suggests that overall, member states are making progress in developing national viral hepatitis management guidelines and strategic plans towards attaining the elimination targets, although availability of dedicated funding to support implementation appears to be an important challenge in some countries[13].
To contribute to the knowledgebase on the scope of the burden of chronic hepatitis B in sub-Saharan Africa, the status and public health response to HBV-associated HCC in sub-Saharan Africa are reviewed. Opportunities and challenges towards achieving the 2030 viral hepatitis elimination target – at least where chronic hepatitis B and HCC are concerned – are also identified, with the intent of arguing for continued financial and technical investments to support ongoing health sector strategies and interventions within sub-Saharan Africa.
The seroprevalence of chronic hepatitis B which is based on the detection of the hepatitis B surface antigen (HBsAg) within the general population, is highly variable worldwide. This variability is demonstrated by substantial regional and inter-country disparities in the burden of the disease. Available estimates[14] suggest that HBsAg prevalence rates in the Americas, for example, range from < 2% in countries like the United States of America, Mexico, and Guatemala, to 13.55% (95%CI: 9.00-19.89) in Haiti. In the South East Asian region, HBsAg prevalence rates range from 0.82% (95%CI: 0.80–0.84) in Nepal to as high as 6.42% (95%CI: 6.37–6.47) in Thailand. Overall, countries within the Eastern Mediterranean and European regions mostly have lower-intermediate endemicity levels (HBsAg prevalence ranging from 2% to 4.99%) while the Western Pacific can be classified as a high-intermediate endemic region with most countries recording HBsAg prevalence rates > 5%. Within the African region, the lowest HBsAg prevalence rates are reported in countries like Seychelles [0.48% (95%CI: 0.12-1.90)], Eritrea [2.49% (95%CI: 2.32-2.67)], and Algeria [2.89% (95%CI: 2.50-3.33)], while Mauritania [16.16% (95%CI: 14.92-17.49)], Liberia [17.55% (95%CI: 15.70-19.55)], Swaziland [19% (95%CI: 17.65-20.43)], and South Sudan [22.38% (95%CI: 20.10-24.83)] have recorded some of the highest prevalence estimates[14].
Historically, sub-Saharan Africa has been classified as hyper-endemic for chronic hepatitis B based on the detection of HBsAg among ≥ 8% of the general population. Table 1 shows the variable prevalence of HBsAg among populations in some sub-Saharan African countries prior to introduction of universal hepatitis B vaccination and how this has changed over time post-vaccine introduction[15-47]. At the peak of the hepatitis B epidemic in sub-Saharan Africa, the disease burden was characterized by a preponderance of horizontal transmission of HBV among young children (between 1-4 years of age), 30%-50% of whom would go on to develop chronic hepatitis B, and later progress to potentially fatal sequelae (mainly liver cirrhosis and HCC) within 30-50 years after infection[15,29,48]. This contrasts with HBV infection acquired during adulthood which carries a considerably lower risk (< 5%) of progression to chronic disease as observed in non-endemic regions of the world.
| Country | Year of hepatitis B vaccine introduction1 | Prevalence of HBsAg (%) | ||
| Pre-vaccine introduction | Post-vaccine introduction | |||
| < 15-yr-olds | ≥ 15-yr-olds | |||
| Burundi | 2004 | 11.0[15] | 2.6[16] | 1.0-4.6[17] |
| Democratic Republic of Congo | 2007 | > 20.7[18] | 2.2[19] | 3.7[19] |
| Ethiopia | 2007 | 11.0[20] | 4.4[21] | 7.4[22] |
| Gambia | 1990 | 20.0[23] | 0.4[24] | 10.0[25] |
| Kenya | 2002 | 11.4[26] | 0.9[16] | 3.4[27] |
| Mali | 2003 | > 8.7[28] | 4.9[16] | 8.5[16] |
| Mozambique | 2001 | 14.6[29] | 3.7[16] | 4.5[30] |
| Namibia | 2009 | 14.0[31] | 2.7[32] | 1.8[33] |
| Nigeria | 2004 | 13.3[34] | 11.5[35] | 8.2[36] |
| Rwanda | 2002 | Approximately 5.0[37] | 1.7[16] | 2.2[38] |
| Senegal | 2004 | 11.8[39] | 1.6[40] | > 11.0[41] |
| South Africa | 1995 | 9.6[15] | 0.4[42] | 4.0[43] |
| Uganda | 2002 | 10.3[44] | 0.6[45] | 4.1[45] |
| Zimbabwe | 2000 | 15.4[46] | 4.4[16] | 3.3[47] |
Currently, the prevalence of HBsAg in sub-Saharan Africa is reported to be 6.1%, equating to 60 million people living with chronic hepatitis B, of which approximately 4.8 million are children < 5 years of age[1]. The decline in the prevalence of HBsAg within the population can be largely attributed to the success of universal childhood hepatitis B vaccination programmes implemented in sub-Saharan Africa since the early 1990s[49]. In most sub-Saharan African countries, the first dose of the hepatitis B vaccine is administered at 6 weeks of age, with the remainder of the regimen completed within the 1st year of life in an effort to interrupt transmission and prevent incident HBV infection in early childhood. Coverage of the third dose of the hepatitis B vaccine in the region is currently estimated at 73%[50]. Countries like The Gambia and South Africa with longstanding hepatitis B vaccination programmes have achieved marked declines in incident infections over time, especially among children < 5 years of age (Table 1).
Despite this success, we are still decades away from realizing the full benefits of universal childhood hepatitis B vaccination programmes in sub-Saharan Africa, given the protracted natural history of chronic hepatitis B, and the long interval between early childhood infection and development of chronic sequelae[51,52]. As such, high prevalence rates of HBsAg persist among a reservoir of adult populations, including women of childbearing age, most of whom were born before the introduction of the hepatitis B vaccine or were not fully vaccinated in infancy (Table 1). This continues to feed the epidemic in sub-Saharan Africa, contributing to the 87890 HBV-associated deaths (approximately 10% of the global total) recorded each year[1]. What further compounds the situation in sub-Saharan Africa is the disproportionate burden of human immunodeficiency virus (HIV) co-infection (69% of all HBV-HIV co-infected persons reside in sub-Saharan Africa) which is associated with a more severe prognosis than that observed in HBV mono-infected individuals[53-55]. Emerging evidence also suggests that perinatal transmission or MTCT of HBV, which was previously presumed to be insignificant in the epidemiology of chronic hepatitis B in sub-Saharan Africa, actually contributes to 367250 incident HBV infections (twice the number of paediatric HIV infections) among neonates annually[56]. In fact, the risk of HBV MTCT increases by up to 2.5-fold among the substantial population of HBV-HIV co-infected pregnant women in sub-Saharan Africa, compared to their HBV mono-infected counterparts[57-60]. This is concerning, given that perinatally acquired HBV infections carry a 90% risk of progression to chronic hepatitis B. This implies that hepatitis B vaccination from 6 weeks of age may be inadequate in preventing incident HBV infections among neonates, especially where the burden of maternal HBV-HIV co-infection is high and prevention of HBV MTCT (PMTCT) strategies are sub-optimal, as is the case in most sub-Saharan African countries. This is a stark contrast to HIV MTCT in sub-Saharan Africa which is on course for elimination due to rapid expansion of antenatal screening and access to timely HIV antiretroviral therapy[61]. The marked decline in HIV MTCT has led to a growing population of HIV-exposed uninfected children in sub-Saharan Africa. Of the 14.8 million HIV-exposed uninfected children in the world, 90% live in sub-Saharan Africa[62]. Although inconclusive, there is some evidence to suggest that HIV-exposed uninfected children may have a modified immune response to hepatitis B vaccination and may also be at increased risk for HBV infection, presenting an additional complexity to elimination strategies in sub-Saharan Africa[58,63-66].
Of the 60 million people currently living with chronic hepatitis B in sub-Saharan Africa, 38800 are at risk of developing HCC every year, characterized by an aggressive clinical course[2,67,68]. In addition, approximately 93% (36700) of persons with HCC will die within a year of their diagnosis without appropriate and timely medical intervention[2,11,67]. Most of these HCC cases (and deaths) will occur among a predominantly male population (sex ratio of 2:1) with age at diagnosis ranging between 38-67 years (compared to 50-70 years in resource rich countries), who are in their prime reproductive and working years, draining productive capacity, and placing a further burden on already strained economic, societal, and health, systems in sub-Saharan Africa[9,11,69-71]. While cases of paediatric HCC are diagnosed more frequently in some sub-Saharan African countries than those in Europe and North America for example, they remain uncommon when compared to the HCC incidence among adult populations[72-75]. Within the next two decades, HCC incidence and mortality rates in sub-Saharan Africa are predicted to double (Figure 1[67,76,77]) unless addressed through reforms in regional and national health policy and practice, including intensifying prevention, diagnostic and treatment strategies, in a whole system approach.
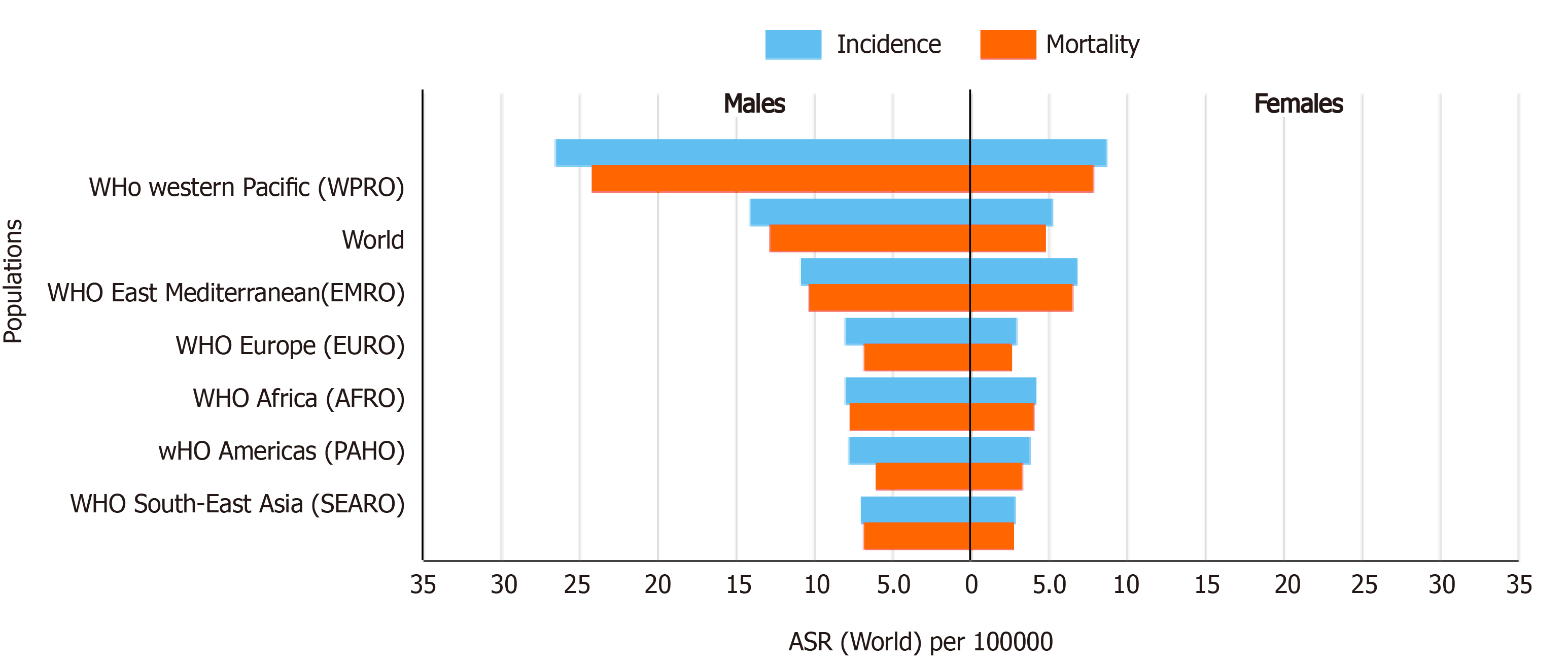
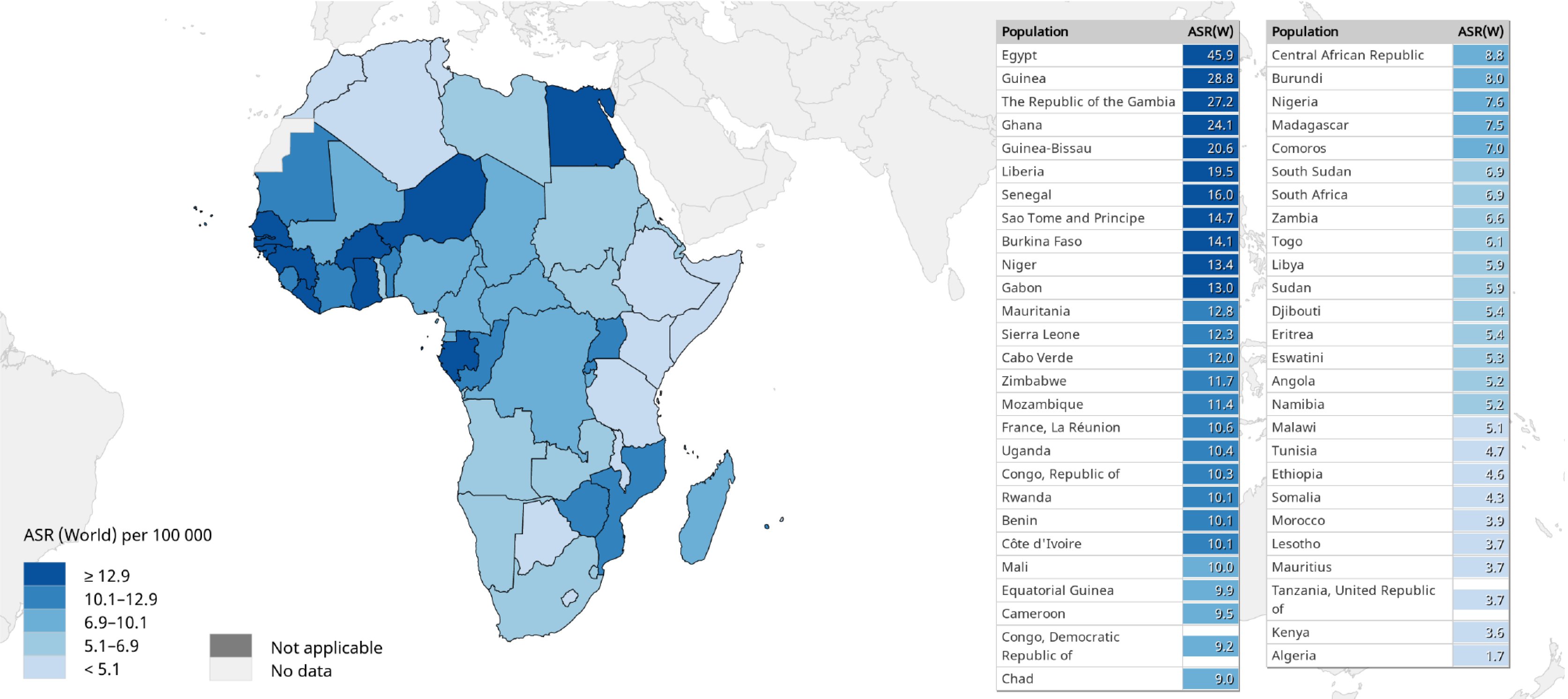
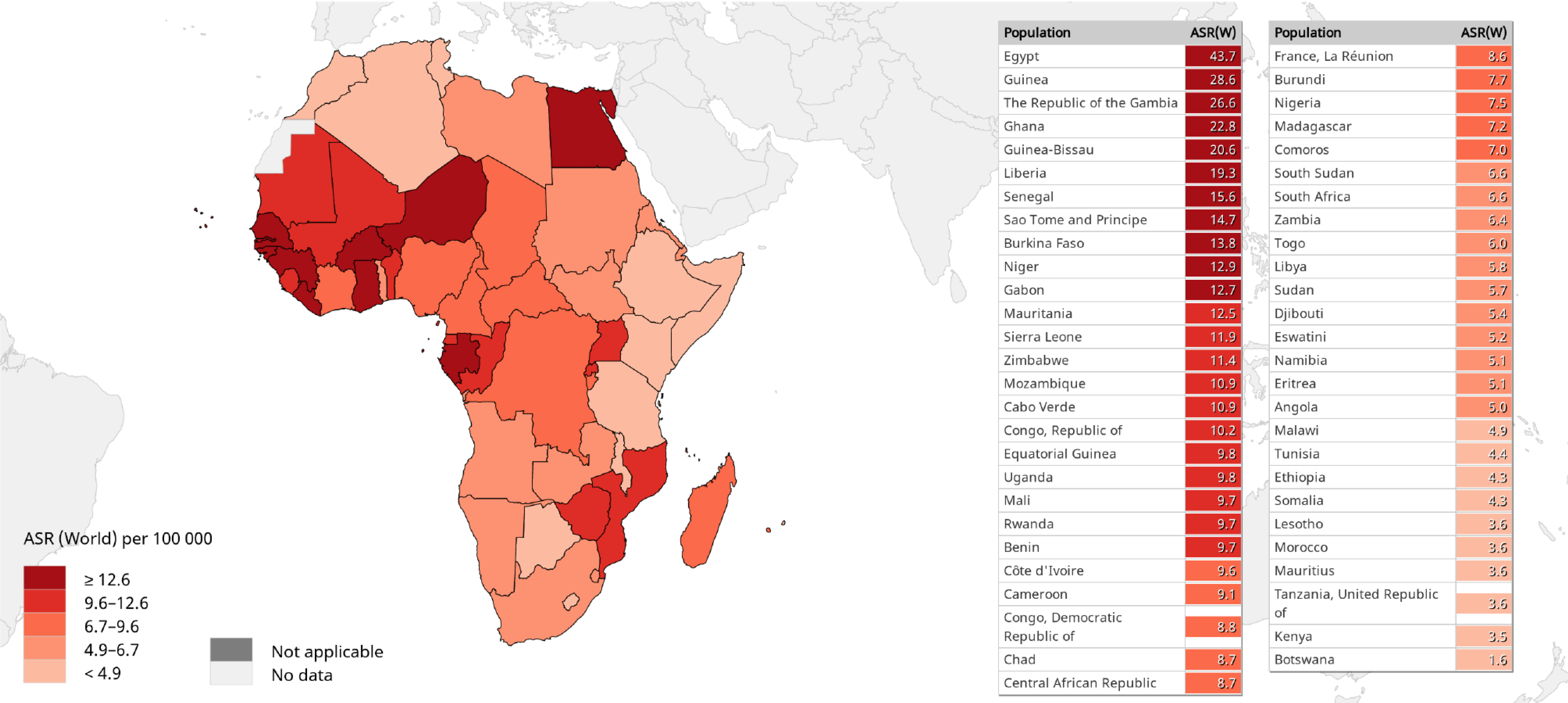
A comparison of the 2020 estimates of age-standardized liver cancer (mainly HCC) incidence and mortality (ASMR) rates in sub-Saharan Africa vs other regions of the world is shown in Figure 2[2,67,78]. With ASIRs of 8.1 and 4.2, and ASMRs of 7.8 and 4.0 per 100000 persons per year among men and women, respectively, HCC is a common cause of cancer-related morbidity and mortality in sub-Saharan Africa. There are substantial regional variations in HCC incidence and mortality rates across sub-Saharan Africa. Among males for example, the highest HCC ASIRs (> 12.9 per 100000 persons per year) and ASMRs (> 12.6 per 100000 persons per year) are recorded in West African countries, while East African countries generally appear to experience a lower burden (ASIRs < 5.1 and ASMRs < 4.9 per 100000 persons per year) of the disease (Figures 3 and 4)[2,67,78]. It should be noted however, that the true burden of HCC in sub-Saharan Africa is grossly underestimated by as much as 40%, given that cases are often underreported due to challenges in effectively diagnosing the disease, while the quality and coverage of data in population-based cancer registries are suboptimal[9,11,79].
The overall HCC mortality-to-incidence ratio (MIR = 0.95) in sub-Saharan Africa is comparable to that in the Western Pacific and South-East Asian regions (MIR = 0.95) but higher than that in Northern America (MIR = 0.82)[80]. This high MIR reflects disparities in outcomes of HCC in sub-Saharan Africa compared to resource-rich regions, owing to limited availability and accessibility of diagnostic and treatment services[81,82]. These disparities go beyond the clinical context and are rooted in socio-economic inequities. A recent South African study[82] investigating trends in liver cancer-associated mortality found key socio-economic and sex disparities. The average MIR for black South African men and women was 4.0 and 3.3 respectively, compared to 2.2 and 1.8 among their white counterparts. This underscores the inequities in HCC prognosis experienced by socio-economically disadvantaged populations[82]. Evidently, addressing the disproportionate burden of HCC in sub-Saharan Africa will require careful consideration of socio-economic and demographic inequities prevalent within its population.
The association of chronic hepatitis B with the development of most cases of HCC [attributable fraction, AF = 50% (95%CI: 39-60)] occurring among sub-Saharan African populations is well established in the literature[5,6,83,84]. The remainder of HCC cases are typically associated with HCV infection [AF = 21% (95%CI: 13-32)] and exposure to the dietary carcinogen, aflatoxin B1, although metabolic syndrome is emerging as an important risk factor in sub-Saharan Africa[6,85-88]. In comparison, the major risk factors for HCC development in low incidence regions of the world (North America, and Western, Central, and Eastern Europe) include host and environmental factors such as genetic predisposition to primary liver cancer, chronic alcohol intake, obesity, hemochromatosis, and exposure to nitrosamines, followed by HCV infection[71,89-91]. It is worth noting however, that the potential for interaction among these different risk factors, leading to synergistic or additive effects in the development of HCC in both endemic and non-endemic regions of the world cannot be undervalued.
The mechanism underlying HBV-associated hepatocarcinogenesis is multifactorial, involving various direct and indirect viral mechanisms required to stimulate the host oncogenic pathway and achieve hepatocyte transformation[92,93]. These mechanisms, which may act synergistically, include integration of the viral DNA into host genome, persistent and enhanced HBV replication, as well as infection with specific HBV genotypes and HBV genetic variants. Important HBV-specific risk factors involved in the development of HCC have been identified in previous studies conducted in sub-Saharan Africa. In a recent case control study, Atsama Amougou et al[94] demonstr
Management of chronic hepatitis B involves suppressing HBV viremia and minimizing the risk of progression to liver cirrhosis, chronic liver failure and HCC, using oral nucleotide/nucleoside analogues like tenofovir disoproxil fumarate (TDF) and entecavir. Treatment with nucleotide/nucleoside analogues is indicated in chronic carriers with elevated HBV viremia, elevated liver enzymes, and evidence of liver fibrosis and cirrhosis. Appropriate linkage to care requires the identification of chronically infected individuals who are eligible for treatment through HBV screening programmes[99]. It is estimated that < 1% of chronic HBV infected individuals in sub-Saharan Africa are currently being diagnosed[100]. In addition, there are major gaps in determining treatment eligibility. Significant limitations have been identified when applying internationally recommended treatment eligibility criteria in the sub-Saharan African context. Only 10%–15% of persons with liver cirrhosis are detected for linkage to appropriate treatment[99-102].
Low uptake of HBV screening and treatment services in sub-Saharan Africa has been attributed to the lack of publicly funded, national HBV screening and treatment programmes. This leaves the responsibility for seeking an HBV test to the patient, which is highly detrimental when considered in the context of limited public awareness of the virus, and the asymptomatic nature of the infection until onset of late-stage sequelae[103]. As currently available nucleotide/nucleoside analogues cannot eradicate intrahepatic HBV DNA, treatment is typically lifelong, and this amounts to a significant cost[100,102]. A recent WHO report indicates that by 2019, < 8 of the 47 member states within the WHO African region (WHO AFRO) had established subsidized HBV treatment programmes[16]. This suggests that a substantial proportion of chronic HBV carriers continue to incur undue financial burden as a consequence of paying out-of-pocket for the treatment they need, while others may be unable to afford it altogether. In Ghana, the annual cost of TDF is estimated at $670 which, when considered against an average annual income of $1778, is a major constraint to accessing lifesaving treatment[104]. It should not come as a surprise then that most patients in sub-Saharan Africa present to health care facilities with established liver cirrhosis or symptomatic advanced-stage HCC, at which point the prognosis is grim and therapeutic options are limited to palliative care.
Future trends in the burden of HCC in sub-Saharan Africa will be determined by our progress in preventing new HBV infections, screening and treating existing chronic hepatitis B cases, as well as detecting and appropriately managing HCC. As part of a scorecard to monitor progress towards elimination of viral hepatitis within WHO AFRO in 2019, six core indicators were listed; (1) development of national hepatitis policies in the form of a national strategic plan for viral hepatitis; (2) implementation of hepatitis B birth dose vaccination; (3) achieving > 90% national coverage of the third dose of the hepatitis B vaccine; (4) being on track for the HBV and HCV 2020 testing target; (5) implementation of a national hepatitis treatment programme; and (6) commemoration of World Hepatitis Day in 2018. Overall, most sub-Saharan African countries were found lagging in almost all indicators[16].
Within the next decade, there is a need to scale-up primary prevention of incident HBV infections in sub-Saharan Africa. Despite adopting resolutions to improve hepatitis B birth dose and routine childhood vaccination in WHO AFRO by 2020, implementation and coverage of the birth dose remains unacceptably low, while coverage of routine childhood vaccination remains well below the global average of 84%[49,105,106]. Recognizing the significant threat of HBV MTCT to public health in sub-Saharan Africa, there have been renewed calls to expand access to the hepatitis B birth dose and leverage HIV PMTCT infrastructure in screening pregnant women and providing timely prophylaxis[56,98,107]. The future research agenda in sub-Saharan Africa should include investigating the need for tailored hepatitis B vaccination strategies for unique populations like HIV-exposed uninfected children.
As national governments grapple with the feasibility of implementing subsidized hepatitis surveillance and treatment programmes, the success of the HIV test and treat model in sub-Saharan Africa has been recognized as an opportunity to expand screening and antiviral treatment for chronic hepatitis B in the interim. To ensure a comprehensive package of care, there is a need to prioritize integration of cost-effective point-of-care screening tests with good diagnostic accuracy, guided by appropriate treatment eligibility criteria[49,108].
Within the past decade, there have been significant advances in the development of new diagnostic and therapeutic approaches for HCC, with prospects for further innovation in the field[109,110]. These advances present unique opportunities to improve surveillance and management of HCC. Biannual surveillance among high-risk chronic carriers of HBV using both liver ultrasonography and serum α-fetoprotein concentrations, is commonly used for early detection of progression to HCC. Curative treatment approaches for early-stage HCC include surgical resection, tumour ablation, or liver transplantation. For advanced-stage HCC, combination treatment using atezolizumab and bevacizumab over sorafenib alone, have shown promising long-term outcomes in phase III clinical trials[110]. Other immunotherapeutic agents for the treatment of late-stage HCC have also been recently explored[109]. While all these advancements present much needed opportunities to improve the quality of life of patients with HCC, the cost and feasibility of implementation within the sub-Saharan African public health context are always important considerations.
Ultimately, sub-Saharan Africa faces one of the toughest battles against chronic hepatitis B and HCC. Despite this, there are opportunities to achieve significant reductions in incident HBV infections and alter future trends in the burden of HCC. This calls for strong political will, regional coordination, and effective partnerships with donor agencies and non-governmental organizations in order to mobilize financial investments and technical support. Finally, the role of advocacy and awareness campaigns like World Hepatitis Day in enabling public ownership and demand for accessible, equitable, and quality health services cannot be undervalued.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: World Society for Virology; Health Systems Global, No. 56106488.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Africa
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng S S-Editor: Wang JL L-Editor: Webster JR P-Editor: Li JH
| 1. | World Health Organization. Global hepatitis report, 2017. Geneva: World Health Organization, 2017. [Cited in This Article: ] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 43243] [Article Influence: 14414.3] [Reference Citation Analysis (47)] |
| 3. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 4. | Herbst DA, Reddy KR. Risk factors for hepatocellular carcinoma. Clin Liver Dis (Hoboken). 2012;1:180-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 341] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 6. | Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471-2477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 7. | Ozakyol A. Global Epidemiology of Hepatocellular Carcinoma (HCC Epidemiology). J Gastrointest Cancer. 2017;48:238-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY). 2018;43:13-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 9. | Zakharia K, Luther CA, Alsabbak H, Roberts LR. Hepatocellular carcinoma: Epidemiology, pathogenesis and surveillance - implications for sub-Saharan Africa. S Afr Med J. 2018;108:35-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 10. | Kew MC. Hepatocellular carcinoma in developing countries: Prevention, diagnosis and treatment. World J Hepatol. 2012;4:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Kew MC. Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Ann Hepatol. 2013;12:173-182. [PubMed] [Cited in This Article: ] |
| 12. | World Health Organization. Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis. Geneva: World Health Organization, 2016. [Cited in This Article: ] |
| 13. | Smith S, Harmanci H, Hutin Y, Hess S, Bulterys M, Peck R, Rewari B, Mozalevskis A, Shibeshi M, Mumba M, Le LV, Ishikawa N, Nolna D, Sereno L, Gore C, Goldberg DJ, Hutchinson S. Global progress on the elimination of viral hepatitis as a major public health threat: An analysis of WHO Member State responses 2017. JHEP Rep. 2019;1:81-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1806] [Cited by in F6Publishing: 1853] [Article Influence: 205.9] [Reference Citation Analysis (1)] |
| 15. | Kiire CF. The epidemiology and prophylaxis of hepatitis B in sub-Saharan Africa: a view from tropical and subtropical Africa. Gut. 1996;38 Suppl 2:S5-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | World Health Organization Regional Office for Africa. Hepatitis scorecard for the WHO Africa Region implementing the hepatitis elimination strategy. 2019. Available from: https://www.afro.who.int/publications/hepatitis-scorecard-who-africa-region-implementing-hepatitis-elimination-strategy. [Cited in This Article: ] |
| 17. | Kwizera R, Moibéni A, Shenawy F, Youssif M. The prevalence of hepatitis B and C among blood donors at the National Blood Transfusion Center (CNTS) in Burundi. Pan Afr Med J. 2018;31:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 18. | Werner GT, Frösner GG, Fresenius K. Prevalence of serological hepatitis A and B markers in a rural area of northern Zaire. Am J Trop Med Hyg. 1985;34:620-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Thompson P, Parr JB, Holzmayer V, Carrel M, Tshefu A, Mwandagalirwa K, Muwonga J, Welo PO, Fwamba F, Kuhns M, Jhaveri R, Meshnick SR, Cloherty G. Seroepidemiology of Hepatitis B in the Democratic Republic of the Congo. Am J Trop Med Hyg. 2019;101:226-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Tsega E, Mengesha B, Nordenfelt E, Hansson BG, Lindberg J. Prevalence of hepatitis B virus markers among Ethiopian blood donors: is HBsAg screening necessary? Trop Geogr Med. 1987;39:336-340. [PubMed] [Cited in This Article: ] |
| 21. | Argaw B, Mihret A, Aseffa A, Tarekegne A, Hussen S, Wachamo D, Shimelis T, Howe R. Sero-prevalence of hepatitis B virus markers and associated factors among children in Hawassa City, southern Ethiopia. BMC Infect Dis. 2020;20:528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Fortuin M, Chotard J, Jack AD, Maine NP, Mendy M, Hall AJ, Inskip HM, George MO, Whittle HC. Efficacy of hepatitis B vaccine in the Gambian expanded programme on immunisation. Lancet. 1993;341:1129-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Peto TJ, Mendy ME, Lowe Y, Webb EL, Whittle HC, Hall AJ. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986-90) and in the nationwide immunisation program. BMC Infect Dis. 2014;14:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Bittaye M, Idoko P, Ekele BA, Obed SA, Nyan O. Hepatitis B virus sero-prevalence amongst pregnant women in the Gambia. BMC Infect Dis. 2019;19:259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Hyams KC, Okoth FA, Tukei PM, Mugambi M, Johnson B, Morrill JC, Gray GC, Woody JN. Epidemiology of hepatitis B in eastern Kenya. J Med Virol. 1989;28:106-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Awili HO, Gitao GC, Muchemi GM. Seroprevalence and Risk Factors for Hepatitis B Virus Infection in Adolescent Blood Donors within Selected Counties of Western Kenya. Biomed Res Int. 2020;2020:8578172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Maupas P, Chiron JP, Goudeau A, Coursaget P, Perrin J, Barin F, Yvonnet B, Dubois F, Duflo B, Duflo-Moreau B, Sidibe S, Diallo AN. [Epidemiology and pathologic results of chronic carriers state of hepatitis B in Mali]. Bull Soc Pathol Exot Filiales. 1981;74:722-732. [PubMed] [Cited in This Article: ] |
| 29. | Kiire CF. Hepatitis B infection in sub-Saharan Africa. The African Regional Study Group. Vaccine. 1990;8 Suppl:S107-12; discussion S134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Mabunda N, Zicai AF, Ismael N, Vubil A, Mello F, Blackard JT, Lago B, Duarte V, Moraes M, Lewis L, Jani I. Molecular and serological characterization of occult hepatitis B among blood donors in Maputo, Mozambique. Mem Inst Oswaldo Cruz. 2020;115:e200006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Botha JF, Ritchie MJ, Dusheiko GM, Mouton HW, Kew MC. Hepatitis B virus carrier state in black children in Ovamboland: role of perinatal and horizontal infection. Lancet. 1984;1:1210-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 127] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Mhata P, Rennie TW, Small LF, Nyarang'o PM, Chagla Z, Hunter CJ. Distribution of hepatitis B virus infection in Namibia. S Afr Med J. 2017;107:882-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Mavenyengwa RT, Mukesi M, Chipare I, Shoombe E. Prevalence of human immunodeficiency virus, syphilis, hepatitis B and C in blood donations in Namibia. BMC Public Health. 2014;14:424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Nasidi A, Harry TO, Vyazov SO, Munube GM, Azzan BB, Ananiev VA. Prevalence of hepatitis B infection markers in representative areas of Nigeria. Int J Epidemiol. 1986;15:274-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Ezeilo MC, Engwa GA, Iroha RI, Odimegwu DC. Seroprevalence and Associated Risk Factors of Hepatitis B Virus Infection Among Children in Enugu Metropolis. Virology (Auckl). 2018;9:1178122X18792859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Federal Ministry of Health, Nigeria. Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) 2018: Technical Report. Abuja, Nigeria; 2019. Available from: https://www.naiis.ng/resource/NAIIS-Report-2018.pdf. [Cited in This Article: ] |
| 37. | Twagirumugabe T, Swaibu G, Walker TD, Lindh M, Gahutu JB, Bergström T, Norder H. Hepatitis B virus strains from Rwandan blood donors are genetically similar and form one clade within subgenotype A1. BMC Infect Dis. 2017;17:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Makuza JD, Nisingizwe MP, Rwema JOT, Dushimiyimana D, Habimana DS, Umuraza S, Serumondo J, Ngwije A, Semakula M, Gupta N, Nsanzimana S, Janjua NZ. Role of unsafe medical practices and sexual behaviours in the hepatitis B and C syndemic and HIV co-infection in Rwanda: a cross-sectional study. BMJ Open. 2020;10:e036711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Barin F, Perrin J, Chotard J, Denis F, N'Doye R, Diop Mar I, Chiron JP, Coursaget P, Goudeau A, Maupas P. Cross-sectional and longitudinal epidemiology of hepatitis B in Senegal. Prog Med Virol. 1981;27:148-162. [PubMed] [Cited in This Article: ] |
| 40. | Lô G, Sow-Sall A, Diop-Ndiaye H, Babacar N, Diouf NN, Daffé SM, Ndao B, Thiam M, Mbow M, Soumboundou MB, Lemoine M, Sylla-Niang M, Ndiaye O, Boye CS, Mboup S, Touré-Kane NC. Hepatitis B virus (HBV) infection amongst children in Senegal: current prevalence and seroprotection level. Pan Afr Med J. 2019;32:140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Coste M, De Sèze M, Diallo A, Carrieri MP, Marcellin F, Boyer S; ANRS 12356 AmBASS Study Group. Burden and impacts of chronic hepatitis B infection in rural Senegal: study protocol of a cross-sectional survey in the area of Niakhar (AmBASS ANRS 12356). BMJ Open. 2019;9:e030211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Prabdial-Sing N, Makhathini L, Smit SB, Manamela MJ, Motaze NV, Cohen C, Suchard MS. Hepatitis B sero-prevalence in children under 15 years of age in South Africa using residual samples from community-based febrile rash surveillance. PLoS One. 2019;14:e0217415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Samsunder N, Ngcapu S, Lewis L, Baxter C, Cawood C, Khanyile D, Kharsany ABM. Seroprevalence of hepatitis B virus: Findings from a population-based household survey in KwaZulu-Natal, South Africa. Int J Infect Dis. 2019;85:150-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Bwogi J, Braka F, Makumbi I, Mishra V, Bakamutumaho B, Nanyunja M, Opio A, Downing R, Biryahwaho B, Lewis RF. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 2009;9:98-108. [PubMed] [Cited in This Article: ] |
| 45. | Ministry of Health, Uganda. Uganda Population-based HIV Impact Assessment (UPHIA) 2016–2017: Final Report: Kampala, Ministry of Health; 2019. Available from: https://phia.icap.columbia.edu/wp-content/uploads/2019/07/UPHIA_Final_Report_Revise_07.11.2019_Final_for-web.pdf. [Cited in This Article: ] |
| 46. | Tswana S, Chetsanga C, Nyström L, Moyo S, Nzara M, Chieza L. A sero-epidemiological cross-sectional study of hepatitis B virus in Zimbabwe. S Afr Med J. 1996;86:72-75. [PubMed] [Cited in This Article: ] |
| 47. | Mavenyengwa RT, Moyo SR, Nordbø SA. Streptococcus agalactiae colonization and correlation with HIV-1 and HBV seroprevalence in pregnant women from Zimbabwe. Eur J Obstet Gynecol Reprod Biol. 2010;150:34-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris). 2010;58:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 49. | Spearman CW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, Dusheiko G, Gogela N, Kassianides C, Kew M, Lam P, Lesi O, Lohouès-Kouacou MJ, Mbaye PS, Musabeyezu E, Musau B, Ojo O, Rwegasha J, Scholz B, Shewaye AB, Tzeuton C, Sonderup MW; Gastroenterology and Hepatology Association of sub-Saharan Africa (GHASSA). Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol. 2017;2:900-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 50. | World Health Organization. World Health Organization/UNICEF joint reporting process. Geneva: World Health Organization/UNICEF, 2020. [Cited in This Article: ] |
| 51. | Hadziyannis SJ. Natural history of chronic hepatitis B in Euro-Mediterranean and African countries. J Hepatol. 2011;55:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 126] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 52. | Croagh CM, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol. 2014;20:10395-10404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 68] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 53. | Kew MC. Hepatitis B virus / human immunodeficiency virus co-infection and its hepatocarcinogenic potential in sub-saharan black africans. Hepat Mon. 2012;12:e7876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Matthews PC, Geretti AM, Goulder PJ, Klenerman P. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in Sub-Saharan Africa. J Clin Virol. 2014;61:20-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Platt L, French CE, McGowan CR, Sabin K, Gower E, Trickey A, McDonald B, Ong J, Stone J, Easterbrook P, Vickerman P. Prevalence and burden of HBV co-infection among people living with HIV: A global systematic review and meta-analysis. J Viral Hepat. 2020;27:294-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 56. | Keane E, Funk AL, Shimakawa Y. Systematic review with meta-analysis: the risk of mother-to-child transmission of hepatitis B virus infection in sub-Saharan Africa. Aliment Pharmacol Ther. 2016;44:1005-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 57. | Andersson MI, Maponga TG, Ijaz S, Barnes J, Theron GB, Meredith SA, Preiser W, Tedder RS. The epidemiology of hepatitis B virus infection in HIV-infected and HIV-uninfected pregnant women in the Western Cape, South Africa. Vaccine. 2013;31:5579-5584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Chasela CS, Kourtis AP, Wall P, Drobeniuc J, King CC, Thai H, Teshale EH, Hosseinipour M, Ellington S, Codd MB, Jamieson DJ, Knight R, Fitzpatrick P, Kamili S, Hoffman I, Kayira D, Mumba N, Kamwendo DD, Martinson F, Powderly W, Teo CG, van der Horst C; BAN Study Team. Hepatitis B virus infection among HIV-infected pregnant women in Malawi and transmission to infants. J Hepatol. 2014;60:508-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Thumbiran NV, Moodley D, Parboosing R, Moodley P. Hepatitis B and HIV co-infection in pregnant women: indication for routine antenatal hepatitis B virus screening in a high HIV prevalence setting. S Afr Med J. 2014;104:307-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Kafeero HM, Ndagire D, Ocama P, Walusansa A, Sendagire H. Sero-prevalence of human immunodeficiency virus-hepatitis B virus (HIV-HBV) co-infection among pregnant women attending antenatal care (ANC) in sub-Saharan Africa (SSA) and the associated risk factors: a systematic review and meta-analysis. Virol J. 2020;17:170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | UNAIDS. UNAIDS data 2020. 2nd edition, February 2021. Available from: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf. [Cited in This Article: ] |
| 62. | Slogrove AL, Powis KM, Johnson LF, Stover J, Mahy M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000-18: a modelling study. Lancet Glob Health. 2020;8:e67-e75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 63. | Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305:576-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 64. | Njom Nlend AE, Nguwoh PS, Ngounouh CT, Tchidjou HK, Pieme CA, Otélé JM, Penlap V, Colizzi V, Moyou RS, Fokam J. HIV-Infected or -Exposed Children Exhibit Lower Immunogenicity to Hepatitis B Vaccine in Yaoundé, Cameroon: An Appeal for Revised Policies in Tropical Settings? PLoS One. 2016;11:e0161714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Baruti K, Lentz K, Anderson M, Ajibola G, Phinius BB, Choga WT, Mbangiwa T, Powis KM, Sebunya T, Blackard JT, Lockman S, Moyo S, Shapiro R, Gaseitsiwe S. Hepatitis B virus prevalence and vaccine antibody titers in children HIV exposed but uninfected in Botswana. PLoS One. 2020;15:e0237252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Tamandjou Tchuem C, Cotton MF, Nel E, Tedder R, Preiser W, Violari A, Bobat R, Hovind L, Aaron L, Montepiedra G, Mitchell C, Andersson MI. Viral hepatitis B and C in HIV-exposed South African infants. BMC Pediatr. 2020;20:563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3585] [Cited by in F6Publishing: 4282] [Article Influence: 713.7] [Reference Citation Analysis (1)] |
| 68. | Okeke E, Davwar PM, Roberts L, Sartorius K, Spearman W, Malu A, Duguru M. Epidemiology of Liver Cancer in Africa: Current and Future Trends. Semin Liver Dis. 2020;40:111-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Howell J, Ladepa NG, Lemoinea M, Thursza MR. , Taylor-Robinsona SD. Hepatitis B in sub-Saharan Africa. South Sudan Med J. 2014;7:59-61. [Cited in This Article: ] |
| 70. | Shimakawa Y, Lemoine M, Njai HF, Bottomley C, Ndow G, Goldin RD, Jatta A, Jeng-Barry A, Wegmuller R, Moore SE, Baldeh I, Taal M, D'Alessandro U, Whittle H, Njie R, Thursz M, Mendy M. Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from The Gambia. Gut. 2016;65:2007-2016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 71. | Yang JD, Mohamed EA, Aziz AO, Shousha HI, Hashem MB, Nabeel MM, Abdelmaksoud AH, Elbaz TM, Afihene MY, Duduyemi BM, Ayawin JP, Gyedu A, Lohouès-Kouacou MJ, Ndam AW, Moustafa EF, Hassany SM, Moussa AM, Ugiagbe RA, Omuemu CE, Anthony R, Palmer D, Nyanga AF, Malu AO, Obekpa S, Abdo AE, Siddig AI, Mudawi HM, Okonkwo U, Kooffreh-Ada M, Awuku YA, Nartey YA, Abbew ET, Awuku NA, Otegbayo JA, Akande KO, Desalegn HM, Omonisi AE, Ajayi AO, Okeke EN, Duguru MJ, Davwar PM, Okorie MC, Mustapha S, Debes JD, Ocama P, Lesi OA, Odeghe E, Bello R, Onyekwere C, Ekere F, Igetei R, Mah'moud MA, Addissie B, Ali HM, Gores GJ, Topazian MD, Roberts LR; Africa Network for Gastrointestinal and Liver Diseases. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol. 2017;2:103-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 72. | Moore SW, Millar AJ, Hadley GP, Ionescu G, Kruger M, Poole J, Stones D, Wainwright L, Chitnis M, Wessels G. Hepatocellular carcinoma and liver tumors in South African children: a case for increased prevalence. Cancer. 2004;101:642-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Seleye-Fubara D, Jebbin NJ. Hepatocellular carcinoma in Port Harcourt, Nigeria: clinicopathologic study of 75 cases. Ann Afr Med. 2007;6:54-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Carreira H, Lorenzoni C, Carrilho C, Ferro J, Sultane T, Garcia C, Amod F, Augusto O, Silva-Matos C, La Vecchia C, Lunet N. Spectrum of pediatric cancers in Mozambique: an analysis of hospital and population-based data. Pediatr Hematol Oncol. 2014;31:498-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Stefan C, Bray F, Ferlay J, Liu B, Maxwell Parkin D. Cancer of childhood in sub-Saharan Africa. Ecancermedicalscience. 2017;11:755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 76. | Bray F, Møller B. Predicting the future burden of cancer. Nat Rev Cancer. 2006;6:63-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 77. | Ferlay J, Laversanne M, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Tomorrow. Lyon: International Agency for Research on Cancer, 2020. [Cited in This Article: ] |
| 78. | Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2020. [Cited in This Article: ] |
| 79. | Sartorius K, Sartorius B, Aldous C, Govender PS, Madiba TE. Global and country underestimation of hepatocellular carcinoma (HCC) in 2012 and its implications. Cancer Epidemiol. 2015;39:284-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 80. | Wang CC, Tsai MC, Peng CM, Lee HL, Chen HY, Yang TW, Sung WW, Lin CC. Favorable liver cancer mortality-to-incidence ratios of countries with high health expenditure. Eur J Gastroenterol Hepatol. 2017;29:1397-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Spearman CW, Sonderup MW. Health disparities in liver disease in sub-Saharan Africa. Liver Int. 2015;35:2063-2071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Mak D, Sengayi M, Chen WC, Babb de Villiers C, Singh E, Kramvis A. Liver cancer mortality trends in South Africa: 1999-2015. BMC Cancer. 2018;18:798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, Hainaut P, Hall AJ, Whittle H, Montesano R. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004;39:211-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 84. | Otedo A, Simbiri KO, Were V, Ongati O, Estambale BA. Risk factors for liver Cancer in HIV endemic areas of Western Kenya. Infect Agent Cancer. 2018;13:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 85. | Kirk GD, Lesi OA, Mendy M, Szymañska K, Whittle H, Goedert JJ, Hainaut P, Montesano R. 249(ser) TP53 mutation in plasma DNA, hepatitis B viral infection, and risk of hepatocellular carcinoma. Oncogene. 2005;24:5858-5867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 86. | Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22:305-310. [PubMed] [Cited in This Article: ] |
| 87. | Kew MC. Hepatitis C virus-induced hepatocellular carcinoma in sub-Saharan Africa. J Afr Cancer. 2013;5:169-174. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 88. | Amponsah-Dacosta E, Tamandjou Tchuem C, Anderson M. Chronic hepatitis B-associated liver disease in the context of human immunodeficiency virus co-infection and underlying metabolic syndrome. World J Virol. 2020;9:54-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72-S78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 90. | Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 91. | Petruzziello A. Epidemiology of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) Related Hepatocellular Carcinoma. Open Virol J. 2018;12:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 92. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 571] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 93. | Torresi J, Tran BM, Christiansen D, Earnest-Silveira L, Schwab RHM, Vincan E. HBV-related hepatocarcinogenesis: the role of signalling pathways and innovative ex vivo research models. BMC Cancer. 2019;19:707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 94. | Atsama Amougou M, Marchio A, Bivigou-Mboumba B, Noah Noah D, Banai R, Atangana PJA, Fewou Moundipa P, Pineau P, Njouom R. Enrichment in selected genotypes, basal core and precore mutations of hepatitis B virus in patients with hepatocellular carcinoma in Cameroon. J Viral Hepat. 2019;26:1086-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Mak D, Babb de Villiers C, Chasela C, Urban MI, Kramvis A. Analysis of risk factors associated with hepatocellular carcinoma in black South Africans: 2000-2012. PLoS One. 2018;13:e0196057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Mendy ME, Welzel T, Lesi OA, Hainaut P, Hall AJ, Kuniholm MH, McConkey S, Goedert JJ, Kaye S, Rowland-Jones S, Whittle H, Kirk GD. Hepatitis B viral load and risk for liver cirrhosis and hepatocellular carcinoma in The Gambia, West Africa. J Viral Hepat. 2010;17:115-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 97. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 961] [Cited by in F6Publishing: 1060] [Article Influence: 176.7] [Reference Citation Analysis (1)] |
| 98. | Wilson P, Parr JB, Jhaveri R, Meshnick SR. Call to Action: Prevention of Mother-to-Child Transmission of Hepatitis B in Africa. J Infect Dis. 2018;217:1180-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Sonderup MW, Dusheiko G, Desalegn H, Lemoine M, Tzeuton C, Taylor-Robinson SD, Spearman CW. Hepatitis B in sub-Saharan Africa-How many patients need therapy? J Viral Hepat. 2020;27:560-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Béguelin C, Fall F, Seydi M, Wandeler G. The current situation and challenges of screening for and treating hepatitis B in sub-Saharan Africa. Expert Rev Gastroenterol Hepatol. 2018;12:537-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Desalegn H, Aberra H, Berhe N, Gundersen SG, Johannessen A. Are non-invasive fibrosis markers for chronic hepatitis B reliable in sub-Saharan Africa? Liver Int. 2017;37:1461-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 102. | Lemoine M, Thursz MR. Battlefield against hepatitis B infection and HCC in Africa. J Hepatol. 2017;66:645-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 103. | Boye S, Shimakawa Y, Vray M, Giles-Vernick T. Limited Awareness of Hepatitis B but Widespread Recognition of Its Sequelae in Rural Senegal: A Qualitative Study. Am J Trop Med Hyg. 2020;102:637-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Adjei CA, Stutterheim SE, Naab F, Ruiter RAC. Barriers to chronic Hepatitis B treatment and care in Ghana: A qualitative study with people with Hepatitis B and healthcare providers. PLoS One. 2019;14:e0225830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | World Health Organization Regional Office for Africa. Sixty-fourth session of the WHO Regional Committee for Africa. 2014. Available from: https://www.afro.who.int/about-us/governance/sessions/sixty-fourth-session-who-regional-committee-africa. [Cited in This Article: ] |
| 106. | World Health Organization Regional Office for Africa. Regional strategic plan for immunization 2014 – 2020. 2015. Available from: https://www.afro.who.int/sites/default/files/2017-06/oms-ivb-rvap-afro-en-20150408_final_sent140317.pdf. [Cited in This Article: ] |
| 107. | Andersson MI, Rajbhandari R, Kew MC, Vento S, Preiser W, Hoepelman AI, Theron G, Cotton M, Cohn J, Glebe D, Lesi O, Thursz M, Peters M, Chung R, Wiysonge C. Mother-to-child transmission of hepatitis B virus in sub-Saharan Africa: time to act. Lancet Glob Health. 2015;3:e358-e359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 108. | Stockdale AJ, Geretti AM. Chronic hepatitis B infection in sub-Saharan Africa: a grave challenge and a great hope. Trans R Soc Trop Med Hyg. 2015;109:421-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Jia L, Gao Y, He Y, Hooper JD, Yang P. HBV induced hepatocellular carcinoma and related potential immunotherapy. Pharmacol Res. 2020;159:104992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 110. | Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |