Published online Sep 21, 2021. doi: 10.3748/wjg.v27.i35.5967
Peer-review started: March 29, 2021
First decision: May 28, 2021
Revised: May 29, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: September 21, 2021
Perivascular epithelioid cell tumor (PEComa) is an uncommon tumor of mesenchymal origin. Cases of PEComa in the liver are extremely rare.
To analyze the clinicopathological features and treatment of hepatic PEComa and to evaluate the prognosis after different treatments.
Clinical and pathological data of 26 patients with hepatic PEComa were collected. All cases were analyzed by immunohistochemistry and clinical follow-up.
This study included 17 females and 9 males, with a median age of 50 years. Lesions were located in the left hepatic lobe in 13 cases, in the right lobe in 11, and in the caudate lobe in 2. The median tumor diameter was 6.5 cm. Light microscopy revealed that the tumor cells were mainly composed of epithelioid cells. The cytoplasm contained heterogeneous eosinophilic granules. There were thick-walled blood vessels, around which tumor cells were radially arranged. Immunohistochemical analysis of pigment-derived and myogenic markers in PEComas revealed that 25 cases were HMB45 (+), 23 were Melan-A (+), and 22 SMA (+). TFE3 and Desmin were negative in all cases. All the fluorescence in situ hybridization samples were negative for TFE3 gene break-apart probe. Tumor tissues were collected by extended hepatic lobe resection or simple hepatic tumor resection as the main treatments. Median follow-up was 62.5 mo. None of the patients had metastasis or recurrence, and there were no deaths due to the disease.
Hepatic PEComa highly expresses melanin and smooth muscle markers, and generally exhibits an inert biological behavior. The prognosis after extended hepatic lobe resection and simple hepatic tumor resection is semblable.
Core Tip: Hepatic perivascular epithelioid cell tumor (PEComa) exhibits an inert biological behavior, and its diagnosis, treatment, and follow-up are challenging. Our study revealed that there was no difference in the prognosis between simple resection of liver tumor and extended resection of liver lobe. Optimal surgical resection currently is the best treatment option, and radiotherapy, chemotherapy, and immunotherapy may become more effective in future. The number of cases in the current retrospective study was limited by the rarity of hepatic PEComa. Therefore, further multicenter, larger-cohort studies are warranted to investigate the clinicopathological features and biological behavior of hepatic PEComa.
- Citation: Zhang S, Yang PP, Huang YC, Chen HC, Chen DL, Yan WT, Yang NN, Li Y, Li N, Feng ZZ. Hepatic perivascular epithelioid cell tumor: Clinicopathological analysis of 26 cases with emphasis on disease management and prognosis. World J Gastroenterol 2021; 27(35): 5967-5977
- URL: https://www.wjgnet.com/1007-9327/full/v27/i35/5967.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i35.5967
Perivascular epithelioid cells were first described in 1992 by Bonetti et al[1]. In 2013, the World Health Organization[2] defined perivascular epithelioid cell tumor (PEComa) as “a mesenchymal tumor, which shows a local association with the vessel wall and usually expresses melanocyte markers and smooth muscle markers.” Bonetti et al[1] were the first to propose the concept of a PEComa family, which includes angiomyolipoma, clear cell sugar tumor of the lung, lymphangioleiomyomatosis, and a group of histologically and immunophenotypically similar tumors that include primary extrapulmonary sugar tumor, clear cell myomelanocytic tumor, and abdominopelvic sarcoma of perivascular epithelioid cells. PEComas are mainly composed of eosinophilic and clear epithelioid cells, which are usually arranged in nests of different sizes associated with blood vessels[3,4]. The diagnosis of PEComa relies on its pathological features, including epithelioid cellular shapes with ample clear to eosinophilic cytoplasm, and in some cases, arrangement around thick-walled blood vessels and immunohistochemical phenotypes, including melanocyte and smooth muscle markers[1,4,5]. Cases of PEComa in the liver are extremely rare[6], and surgical resection currently is the most effective therapeutic strategy to cure patients or prolong the survival period. In this study, the clinical and pathological features, immunohistochemical phenotypes, and information on treatment modalities of 26 cases of hepatic PEComa were collected, and the effects of different surgical methods on prognosis were evaluated to provide information for the guidance of clinical treatment.
The study included 17 women and 9 men who were diagnosed with hepatic PEComa for the first time. Tumor tissue samples were collected at the time of diagnosis between January 2010 and December 2018 at the First Affiliated Hospital of Bengbu Medical College (Anhui Province, China). None of the patients received preoperative radio- or chemo-therapy. Sixteen patients underwent extended hepatic lobe resection, eight underwent simple hepatic tumor resection, and two received the oral mTOR inhibitor sirolimus. None of the 26 patients had metastasis or recurrence, and there were no deaths due to the disease. Only two patients with extended liver lobectomy had a poor prognosis (one had postoperative pain in the liver area, and the other was diagnosed with liver cancer 2 years after surgery). Informed consent was obtained from all patients. The study protocol was approved by the ethics committees of the hospitals partaking in this study.
Imaging data of all patients were collected and reviewed by two experienced physicians who analyzed the imaging characteristics of the patients.
Two experienced pathologists reviewed hematoxylin and eosin-stained sections of each tissue sample, marked the representative regions of tissue blocks, and assessed the following histological features: Tumor boundary (infiltration), tumor cell structure (trabecular and nested), tumor cell type (epithelial and fusiform), cytological features (cytoplasm and nucleus), nuclear features (atypical and pleomorphic), presence of pleomorphic tumor cells, and tumor necrosis.
Immunohistochemical staining was conducted on 4-μm-thick serial PEComa tissue sections using the standard ElivisionTM Plus/HRP detection system (Fuzhou Maixin Biotechnology, Fuzhou, China) and DAB substrate, generating a brown color. The antibodies, clones, dilutions, and pretreatment conditions used, as well as the positively stained sites, are listed in Table 1. Serial sections were incubated in parallel with rabbit IgG instead of the primary antibody as a negative control. Immunoreactivity was graded according to the percentage of positive tumor cells (0, negative; 1+, 1%-5%; 2+, 6%-25%; 3+, 26%-50%; 4+, 51%-100%), and tumor cell immunoreactivity was also semi-qualitatively graded: Weak, heterogeneous, or strong[7,8]. For calculation of IHC totals, a score of 1+ with weak, heterogeneous, or strong staining was considered positive for all antibodies except TFE3. A minimum of 3+ was required for TFE3 immunopositivity[8].
| Antigen | Clone | Dilution | Antigen retrieval | Localization |
| HMB-45 | HMB-45 | 1:400 | None | Cytoplasm |
| Melan-A | A103 | 1:200 | Citrate buffer pressure cook | Cytoplasm |
| SMA | 1A4 | 1:20000 | None | Cytoplasm |
| Desmin | D33 | 1:500 | None | Cytoplasm |
| S100 protein | Polyclonal | 1:4000 | Citrate buffer pressure cook | Cytoplasm/nucleus |
| Hepatocyte | OCH1E5 | 1:1000 | Citrate buffer pressure cook | Cytoplasm |
| Vimentin | V9 | 1:200 | Citrate buffer pressure cook | Cytoplasm |
| CD34 | QBEnd/10 | 1:500 | Citrate buffer pressure cook | Cell membrane |
| TFE-3 | MRQ-0663 | 1:500 | ETDA buffer pressure cook | Nucleus |
| Ki-67 | MX006 | 1:200 | Citrate buffer pressure cook | Nucleus |
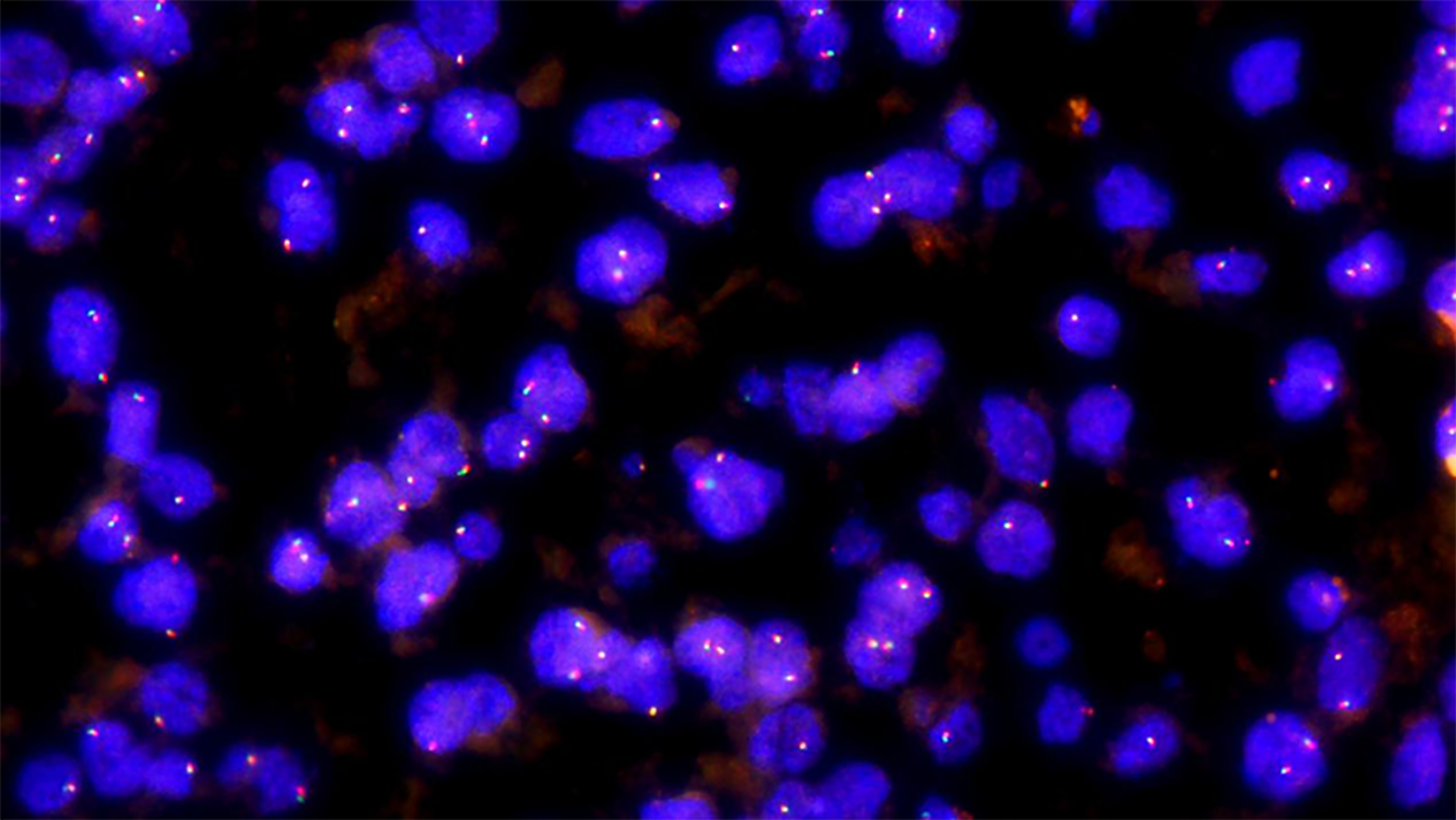
FISH was performed on paraffin-embedded tissue sections with a thickness of 4 μm and labeled with a TFE3 gene break-apart probe (Guangzhou Anbiping Medical, Guangzhou, Guangdong Province, China). For probe preparation, TFE3 gene was labeled with green fluorescence on the centromere side and red fluorescence on the telomere side. FISH interpretation criteria are as follows: The positive pattern for TFE3 translocation should be 1 red, 1 green, and 1 fusion (yellow) signal in females, and 1 red, 1 green, and 1 negative signal in males; the pattern for intact TFE3 alleles should be 2 fusion (yellow) signals in females and 1 fusion (yellow) signal in males. When the distance between the red and green signals exceeds 1 fusion signal size, it is interpreted as a red-green signal separation. A case was scored as positive if at least 10% of 100 scored nuclei showed a split signal pattern.
The clinical and pathological data for all 26 cases are summarized in Table 2. We enrolled 26 patients, including 17 females and 9 males. The median patient age was 50 years (range, 26–77 years). Of the 26 patients, 23 had liver-occupying lesions, 2 had hepatic hemangioma, and 1 had hepatic hamartoma. Six patients had a history of liver disease (cysts, hamartoma, or hemangioma). The most common site of tumors was the left hepatic lobe. Sixteen patients underwent extended hepatic lobe resection, eight underwent simple hepatic tumor resection, and two were treated only with the mTOR inhibitor sirolimus (both patients were treated for 8 mo). The clinical symptoms of hepatic PEComa were non-specific. Most patients were admitted to one of our hospitals because of space-occupying lesions in the liver during medical examination, nausea, vomiting, loss of appetite, or weight loss. During physical examination, the abdomen was soft, with no tenderness or rebound tenderness, occasional contact with the ribs at the liver margin, and no pain in the liver area. Some patients experienced compression pain under the ribs and xiphoid, or in the right abdomen when the tumor involved the caudate lobe, or in the right kidney.
| No. | Sex/age (yr) | Tumor location | Tumor size (cm) | First diagnosis | Treatment | Follow-up (mo) and prognosis |
| 1 | F/40 | Left lobe | 2.5 | Left lobe occupying lesion | Left hepatic tumor simple resection | 91, favorable prognosis |
| 2 | M/57 | Left lobe | 7.5 | Left lobe occupying lesion | Left hepatic tumor simple resection | 80, favorable prognosis |
| 3 | F/58 | Left lobe | 8.5 | Left lobe occupying lesion | Left hepatic tumor simple resection | 79, favorable prognosis |
| 4 | F/48 | Right lobe | 8.0 | Right lobe occupying lesion | Right hepatic tumor simple resection | 69, favorable prognosis |
| 5 | F/64 | Right lobe | 7.0 | Right lobe occupying lesion | Right hepatic tumor simple resection | 66, favorable prognosis |
| 6 | M/72 | Right lobe | 8.0 | Right lobe occupying lesion | Right hepatic tumor simple resection | 59, favorable prognosis |
| 7 | F/26 | Right lobe | 3.0 | Right hepatic hamartoma | Extended hepatic lobe resection | 55, favorable prognosis |
| 8 | M/47 | Right lobe | 6.5 | Right lobe occupying lesion | mTOR inhibitor-sirolimus | 51, favorable prognosis |
| 9 | F/47 | Left lobe | 5.5 | Left lobe occupying lesion | Extended hepatic lobe resection | 25, favorable prognosis |
| 10 | M/72 | Right lobe | 8.0 | Right lobe occupying lesion | Extended right hepatic lobe resection | 57, favorable prognosis |
| 11 | F/56 | Right lobe | 8.0 | Right lobe occupying lesion | mTOR inhibitor-sirolimus | 32, favorable prognosis |
| 12 | F/54 | Right lobe | 13.0 | Left lobe occupying lesion | Extended left hepatic lobe resection | 99, favorable prognosis |
| 13 | F/41 | Caudate lobe | 8.0 | Caudate lobe occupying lesion | Caudate hepatic tumor simple resection | 98, favorable prognosis |
| 14 | F/46 | Left lobe | 2.0 | Left lobe occupying lesion | Extended left hepatic lobe resection | 99, favorable prognosis |
| 15 | F/54 | Right lobe | 8.0 | Right hepatic hemangioma | Extended Rright hepatic lobe resection | 84, favorable prognosis |
| 16 | F/41 | Caudate lobe | 6.0 | Caudate lobe occupying lesion | Extended caudate hepatic lobe resection | 87, hepatic pain often occurs after discharge |
| 17 | M/45 | Right lobe | 0.5 | Right hepatic hemangioma | Extended hepatic lobe resection | 85, favorable prognosis |
| 18 | F/66 | Right lobe | 5.5 | Right lobe occupying lesion | Extended hepatic lobe resection | 59, favorable prognosis |
| 19 | F/43 | Right lobe | 2.8 | Right lobe occupying lesion | Extended hepatic lobe resection | 47, favorable prognosis |
| 20 | F/41 | Left lobe | 5.0 | Left lobe occupying lesion | Extended hepatic lobe resection | 49, reoperation for liver cancer in 2017 |
| 21 | M/52 | Left lobe | 7.5 | Left lobe occupying lesion | Left hepatic tumor simple resection | 48, favorable prognosis |
| 22 | F/48 | Right lobe | 9.5 | Right lobe occupying lesion | Extended right hepatic lobe resection | 71, favorable prognosis |
| 23 | M/58 | Left lobe | 4.0 | Left lobe occupying lesion | Left hepatic tumor simple resection | 70, favorable prognosis |
| 24 | M/77 | Left lobe | 4.0 | Left lobe occupying lesion | Extended left hepatic lobe resection | 47, favorable prognosis |
| 25 | M/62 | Left lobe | 6.5 | Left lobe occupying lesion | Extended left hepatic lobe resection | 36, favorable prognosis |
| 26 | F/45 | Left lobe | 3.0 | Left lobe occupying lesion | Extended left hepatic lobe resection | 35, favorable prognosis |
B-ultrasound usually revealed strong echoes in the liver, the boundary was clear, and the internal echo was uneven, suggesting that the liver had substantial space-occupying lesions (data not shown). Plain computed tomography (CT) scans commonly revealed an irregular soft tissue density (Figure 1A). Enhanced scanning in the arterial phase revealed obvious enhancement of the mass edge and of central heterogeneity (Figure 1B). Portal vein scanning revealed a low mass density (Figure 1C). Magnetic resonance imaging (MRI) revealed a solid cystic space in the liver, and tumors had clear boundaries and uneven internal signal (data not shown).
The median tumor diameter was 6.5 cm (range, 0.5-13.0 cm). PEComa tumors were located in the liver parenchyma and were round or oval. The surface was smooth and occasionally highlighted the surface of the liver. The boundary was clear and appeared to be enveloped. Tumors did not invade the surrounding tissue. The cut surface was solid and grayish yellow, had a slightly hard texture, and showed loose necrotic tissue in the center. The liver tissue surrounding the tumor was normal, and the lymph nodes in the hilar region were not swollen. Focal hemorrhage and necrosis were seen in two cases.
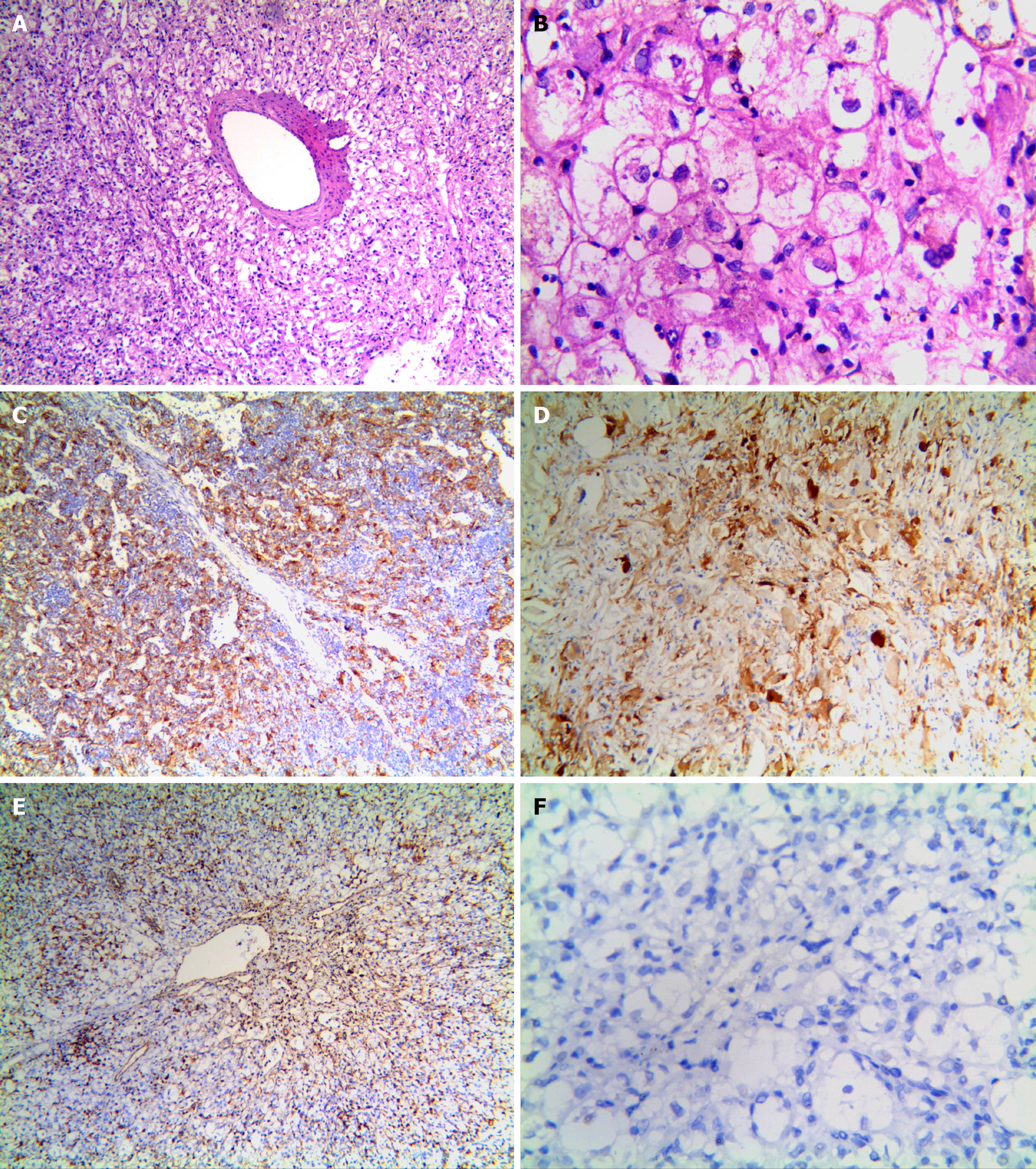
Microscopically, the tumor cells were clearly distinct from normal liver cells, and were largely composed of proliferating epithelioid cells and spindle cells, nested in trabeculae or lamellae. In most cases, the tumor cell nest was surrounded by capillaries. Tumor cells were arranged radially around the thick-walled blood vessels (Figure 2A). Tumor cells were polygonal and cytoplasm was translucent, with heterogeneous eosinophilic particles; tumor nuclei were round or oval, nucleoli were obvious, chromatin was sparse, part of the cells were heteromorphic, and mitotic figures were not common. Collagen fibers were observed in the interstitium and were generally feathery, and a few fibers were accompanied by hemorrhage and necrosis (Figure 2B).
Immunohistochemistry findings are summarized in Table 3. Of the 26 cases, 25 were HMB45 (+), usually with multifocal or diffuse distribution and occasionally, with scattered distribution (Figure 2C), 23 were Melan-A (+) (Figure 2D), 22 were SMA (+) (Figure 2E), 20 were VIM (+), and 12 were S-100 (+). Only three cases showed focal staining (1%-5%) for TFE3. All tumors were desmin (–) (Figure 2F). The positive rate for Ki-67 was < 10%. All cases expressed at least one smooth muscle or melanocyte marker. FISH showed that no abnormal TFE3 separation signal was found in 26 cases of hepatic PEComa (Figure 3).
| Target protein | Positive cases (n)/total | % Positive |
| HMB45 | 25/26 | 96.2 |
| Melan-A | 23/26 | 88.5 |
| SMA | 22/26 | 83.6 |
| Desmin | 1/26* | 3.8 |
| S100 | 14/26 | 53.8 |
| Hepatocyte | 9/26 | 34.6 |
| Vimentin | 20/26 | 76.9 |
| CD34 | 18/26 | 69.2 |
| TFE3 | 0/26 | 0 |
| Ki-67 (> 10%) | 1/26 | 3.8 |
Sixteen patients underwent extended hepatic lobe resection, eight underwent simple hepatic tumor resection, and two were treated with the mTOR inhibitor sirolimus. During a follow-up period of 25 mo to 99 mo, none of the 26 patients had metastasis or recurrence, and there were no deaths due to the disease. Only two patients with extended liver lobectomy had a poor prognosis (one had postoperative pain in the liver area, and the other was diagnosed with liver cancer 2 years after surgery). There was no difference in patient prognosis between the two surgical treatment methods, and long-term follow-up indicated that the patients went into remission.
Hepatic PEComa is a rare mesenchymal tumor derived from pericytes. Ultrasound, CT, and MRI are commonly used for preoperative diagnosis of PEComa. On contrast-enhanced CT, PEComa is characterized by vascular proliferation and arteriovenous connections[5,9,10]. MRI scans have revealed significant enhancement in PEComa in the arterial phase, but not in the portal venous and delayed phases[10]. Contrast-enhanced ultrasonography is another commonly used diagnostic method, in which the contrast agent characteristically reaches the tumor rapidly and drains the arterial blood rapidly to the vein[11]. However, due to the different proportions of smooth muscle cells, adipose tissue, blood vessels, and rare tumors, the accuracy of preoperative diagnosis is currently low. In our study, only one patient was diagnosed with hepatic PEComa before undergoing surgery.
Martignoni et al[12] defined PEComa as a tumor that is composed mainly of epithelioid cells and is closely associated with dilated blood vessels and contains eosinophils, but not fat cells or disordered blood vessels. The final diagnosis of PEComa currently depends on pathological features and immunohistochemical analysis. Hepatic PEComa is mainly composed of proliferating epithelioid cells and spindle cells. The tumor cells are polygonal, have translucent cytoplasm, and contain eosinophilic particles, and thick-walled blood vessels are visible in the tumors. Epithelioid cells are arranged radially around thick-walled blood vessels. Feather-like collagen fibers are visible. Nearly all PEComas have specific immunological characteristics, with melanocyte markers (e.g., HMB-45 and/or melan-A) and smooth muscle markers (e.g., SMA) being strongly expressed[11,13], whereas desmin, hepatocyte-specific antigen, and TFE3 are generally negative. In this study, 25 cases were HMB-45 (+), 17 were SMA (+), and only 3 showed focal staining (1%-5%) for TFE3.
TFE3 is a member of the MiTF family of transcription factors. A recent study[14] showed that TFE3 gene rearrangements occur in approximately 14% of PEComas. Similar to other TFE3 translocation-associated tumors, TFE3 (+) PEComa usually exhibits an acinar structure and epithelioid cell morphology, shows aggressive biological behavior, and has a poor prognosis. PSF-TFE3 gene fusion has been detected in gastrointestinal tract PEComa, but fusion partners in other cases remain unknown[15]. In this study, TFE3 expression was weak and detected in only three patients with small tumors and typical morphological PEComa images, and was associated with a low malignancy and good prognosis. Moreover, no break-apart of the TFE3 gene was detected by FISH method. Whether there is a TFE3 fusion gene still needs to be confirmed by subsequent studies. This suggests that liver PEComa may be less malignant than PEComas in other organs.
PEComas are mainly benign tumors[16] that usually do not recur after surgical resection; however, some are malignant, and their biological behavior has not been fully elucidated. In 2005, Folpe et al[17] reviewed 26 cases of PEComa of soft tissue and gynecological origin, and suggested to classify PEComa into benign, uncertain malignant potential, and malignant. Further, the authors proposed seven evaluation criteria for PEComa malignancies: (1) Tumor size > 5 cm; (2) Infiltration and growth into surrounding normal tissue; (3) High nuclear grade; (4) Excessive cells; (5) Mitotic figures in > 1/50 high-power fields; (6) Coagulative necrosis of tumor; and (7) Vascular invasion. PEComas with two or more of these features are considered to be malignant, and tumors with only nuclear polymorphism, multinucleated giant cells, or tumors > 5 cm in size are considered to have malignant potential[18].
Because of the rare disease types and the scarcity of cases, treatment plans for hepatic PEComa can only be developed based on statistical analysis of a small number of cases. Surgical resection currently is the main means of treating hepatic PEComa. In clinical practice, surgical methods are usually selected based on the tumor size and on whether the tumor is benign or malignant. Larger and malignant tumors are removed by extended hepatic lobe resection, whereas simple hepatic tumor resection is used for smaller or benign tumors. In this study, the 26 cases showed clinical and biological manifestations of inertness, and no morphological criteria for malignant PEComa. Sixteen patients underwent extended hepatic lobe resection, eight underwent simple hepatic tumor resection, and two received sirolimus. The survival rate of the patients treated with the three different modalities was good, and there was no significant difference among the treatments. Hepatic pain complications were reported only in a few cases with extended lobe resection. It has been reported that when the tumor diameter is less 5 cm, resection can be suspended or regular follow-up suffices[18].
Current data do not support that chemo- or radio-therapy improves the survival time in patients with PEComa[12]; however, sirolimus is expected to improve outcomes either when used alone or in combination with other treatments[4,10,19,20]. A 31-year-old woman with hepatic PEComa showed a significant reduction in tumor volume after 8 mo of treatment with sirolimus[19]. After subsequent surgical resection, there were no complications and the prognosis was favorable. This suggests that hepatic PEComa has a better prognosis when surgery is combined with chemotherapy[13,14]. In addition, Wagner et al[21] treated three patients with PEComa with sirolimus and found that the tumors responded to the drug, suggesting that sirolimus can be used alone or in combination to treat PEComa. Italiano et al[22] reported similar efficacy in a number of cases. However, large-scale clinical trials are needed. Numerous previous studies and this study showed that hepatic PEComa displays an inert biological behavior. However, due to the heterogeneous nature of PEComa, the existing diagnostic criteria cannot accurately determine the nature of this tumor, which has led to overtreatment in some cases. In addition, because the nature of hepatic PEComa is not entirely clear, there is no standard treatment, and it is difficult to develop an optimal treatment plan. Therefore, clinical observation and follow-up of more cases, and the establishment of a clinical online registration system for hepatic PEComa are needed to provide clinical data for future exploration of the differentiation and distribution of the disease and the development of more accurate diagnostic criteria.
Hepatic PEComa is a rare mesenchymal tumor that exhibits an inert biological behavior, and its diagnosis, treatment, and follow-up are challenging. Our study of 26 cases of hepatic PEComa revealed that there was no difference in the prognosis between simple resection of liver tumor and extended resection of liver lobe. Optimal surgical resection currently is the best treatment option, and radiotherapy, chemotherapy, and immunotherapy may become more effective in future[4,9]. The number of cases in the current retrospective study was limited by the rarity of hepatic PEComa. Therefore, further multicenter, larger-cohort studies are warranted to investigate the clinicopathological features and biological behavior of hepatic PEComa.
Perivascular epithelioid cell tumor (PEComa) is an uncommon tumor of mesenchymal origin. Cases of PEComa in the liver are extremely rare.
Cases of PEComa in the liver are extremely rare, and surgical resection currently is the most effective therapeutic strategy to cure patients or prolong the survival period. In this study, the clinical and pathological features, immunohistochemical phenotypes, and information on treatment modalities of 26 cases of hepatic PEComa were collected, and the effects of different surgical methods on prognosis were evaluated to provide information for the guidance of clinical treatment.
We aimed to analyze the clinicopathological features and treatment of hepatic PEComa and to evaluate the prognosis after different treatments.
Clinical and pathological data of 26 patients with hepatic PEComa were collected. All cases were analyzed by immunohistochemistry and clinical follow-up.
This study included 17 females and 9 males, with a median age of 50 years. Lesions were located in the left hepatic lobe in 13 cases, in the right lobe in 11, and in the caudate lobe in 2. The median tumor diameter was 6.5 cm. Light microscopy revealed that the tumor cells were mainly composed of epithelioid cells. The cytoplasm contained heterogeneous eosinophilic granules. There were thick-walled blood vessels, around which tumor cells were radially arranged. Immunohistochemical analysis of pigment-derived and myogenic markers in PEComa tumors revealed that 25 cases were HMB45 (+), 23 were Melan-A (+), and 22 SMA (+). TFE3 and Desmin were negative in all cases. All the FISH samples were negative for TFE3 gene break-apart probe. Tumor tissues were collected by extended hepatic lobe resection or simple hepatic tumor resection as the main treatments. Median follow-up was 62.5 mo. None of the patients had metastasis or recurrence, and there were no deaths due to the disease.
Hepatic PEComa is a rare mesenchymal tumor that exhibits an inert biological behavior, and its diagnosis, treatment, and follow-up are challenging. Our study of 26 cases of hepatic PEComa revealed that there was no difference in the prognosis between simple resection of liver tumor and extended resection of liver lobe. Optimal surgical resection currently is the best treatment option, and radiotherapy, chemotherapy, and immunotherapy may become more effective in future.
The number of cases in the current retrospective study was limited by the rarity of hepatic PEComa. Therefore, further multicenter, larger-cohort studies are warranted to investigate the clinicopathological features and biological behavior of hepatic PEComa.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yasukawa K S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 276] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Abhirup B, Kaushal K, Sanket M, Ganesh N. Malignant hepatic perivascular epithelioid cell tumor (PEComa) - Case report and a brief review. J Egypt Natl Canc Inst. 2015;27:239-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Parfitt JR, Bella AJ, Izawa JI, Wehrli BM. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch Pathol Lab Med. 2006;130:1219-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Fang SH, Zhou LN, Jin M, Hu JB. Perivascular epithelioid cell tumor of the liver: a report of two cases and review of the literature. World J Gastroenterol. 2007;13:5537-5539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Strzelczyk JM, Durczynski A, Szymanski D, Jablkowski M, Dworniak D, Sporny S. Primary perivascular epithelioid cell tumor (PEComa) of the liver: report of a case. Surg Today. 2009;39:916-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Schoolmeester JK, Dao LN, Sukov WR, Wang L, Park KJ, Murali R, Hameed MR, Soslow RA. TFE3 translocation-associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: morphology, immunophenotype, differential diagnosis. Am J Surg Pathol. 2015;39:394-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Schoolmeester JK, Howitt BE, Hirsch MS, Dal Cin P, Quade BJ, Nucci MR. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: clinicopathologic and immunohistochemical characterization of 16 cases. Am J Surg Pathol. 2014;38:176-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Högemann D, Flemming P, Kreipe H, Galanski M. Correlation of MRI and CT findings with histopathology in hepatic angiomyolipoma. Eur Radiol. 2001;11:1389-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Tan Y, Xiao EH. Hepatic perivascular epithelioid cell tumor (PEComa): dynamic CT, MRI, ultrasonography, and pathologic features--analysis of 7 cases and review of the literature. Abdom Imaging. 2012;37:781-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Akitake R, Kimura H, Sekoguchi S, Nakamura H, Seno H, Chiba T, Fujimoto S. Perivascular epithelioid cell tumor (PEComa) of the liver diagnosed by contrast-enhanced ultrasonography. Intern Med. 2009;48:2083-2086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Bergamo F, Maruzzo M, Basso U, Montesco MC, Zagonel V, Gringeri E, Cillo U. Neoadjuvant sirolimus for a large hepatic perivascular epithelioid cell tumor (PEComa). World J Surg Oncol. 2014;12:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Argani P, Aulmann S, Illei PB, Netto GJ, Ro J, Cho HY, Dogan S, Ladanyi M, Martignoni G, Goldblum JR, Weiss SW. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34:1395-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 276] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 15. | Tanaka M, Kato K, Gomi K, Matsumoto M, Kudo H, Shinkai M, Ohama Y, Kigasawa H, Tanaka Y. Perivascular epithelioid cell tumor with SFPQ/PSF-TFE3 gene fusion in a patient with advanced neuroblastoma. Am J Surg Pathol. 2009;33:1416-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Selvaggi F, Risio D, Claudi R, Cianci R, Angelucci D, Pulcini D, D'Aulerio A, Legnini M, Cotellese R, Innocenti P. Malignant PEComa: a case report with emphasis on clinical and morphological criteria. BMC Surg. 2011;11:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 581] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 18. | Tang D, Wang J, Tian Y, Li Q, Yan H, Wang B, Xiong L. Hepatic perivascular epithelioid cell tumor: Case report and brief literature review. Medicine (Baltimore). 2016;95:e5572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Khaja F, Carilli A, Baidas S, Sriharan A, Norford S. PEComa: A Perivascular Epithelioid Cell Tumor in the Liver-A Case Report and Review of the Literature. Case Rep Med. 2013;2013:904126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Bleeker JS, Quevedo JF, Folpe AL. "Malignant" perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma. 2012;2012:541626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, Ligon AH, Antonescu CR, Ramaiya NH, Demetri GD, Kwiatkowski DJ, Maki RG. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 22. | Italiano A, Delcambre C, Hostein I, Cazeau AL, Marty M, Avril A, Coindre JM, Bui B. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Ann Oncol. 2010;21:1135-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |