Published online Sep 14, 2021. doi: 10.3748/wjg.v27.i34.5700
Peer-review started: February 28, 2021
First decision: April 18, 2021
Revised: May 14, 2021
Accepted: August 20, 2021
Article in press: August 20, 2021
Published online: September 14, 2021
Incidental pancreatic cysts are commonly encountered with some cysts having malignant potential. The most common pancreatic cystic neoplasms include serous cystadenoma, mucinous cystic neoplasm and intraductal papillary mucinous neoplasm. Risk stratifying pancreatic cysts is important in deciding whether patients may benefit from endoscopic ultrasound (EUS) or surgical resection. Surgery should be reserved for patients with malignant cysts or cysts at high risk for developing malignancy as suggested by various risk features including solid mass, nodule and dilated main pancreatic duct. EUS may supplement magnetic resonance imaging findings for cysts that remain indeterminate or have concerning features on imaging. Various cyst fluid markers including carcinoembryonic antigen, glucose, amylase, cytology, and DNA markers help distinguish mucinous from nonmucinous cysts. This review will guide the practicing gastroenterologist in how to evaluate incidental pancreatic cysts and when to consider referral for EUS or surgery. For presumed low risk cysts, surveillance strategies will be discussed. Managing pancreatic cysts requires an individualized approach that is directed by the various guidelines.
Core Tip: Incidental pancreatic cysts are common, and some have malignant potential. magnetic resonance imaging of the pancreas should be used to risk stratify pancreatic cysts and decide whether patients may benefit from endoscopic ultrasound or surgical resection. Presumed low risk cysts should undergo surveillance unless the patient is not a surgical candidate or has a pseudocyst or serous cystadenoma. We discuss the approach to diagnosis and management of incidental pancreatic cysts.
- Citation: Lee LS. Updates in diagnosis and management of pancreatic cysts. World J Gastroenterol 2021; 27(34): 5700-5714
- URL: https://www.wjgnet.com/1007-9327/full/v27/i34/5700.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i34.5700
Pancreatic cysts are typically discovered incidentally with the increased use and quality of abdominal imaging. They are identified in up to 20% of magnetic resonance imaging (MRI) and 3% of computed tomography (CT) scans with older patients more likely to have pancreatic cysts[1-3]. The malignant potential of some pancreatic cysts creates consternation for patients and providers alike. A retrospective study of Veterans Administration patients with pancreatic cysts noted a hazard ratio of 19.64 for pancreatic cancer[4]. However, other studies of incidental pancreatic cysts demonstrated lower risk of malignancy with a hazard ratio of 3.0 for pancreatic adenocarcinoma[5]. The American Gastroenterological Association (AGA) technical review of incidental pancreatic cysts estimated incident risk of malignancy at 0.24% per year with a prevalent malignant risk of 0.25% at the time the cyst is identified[6].
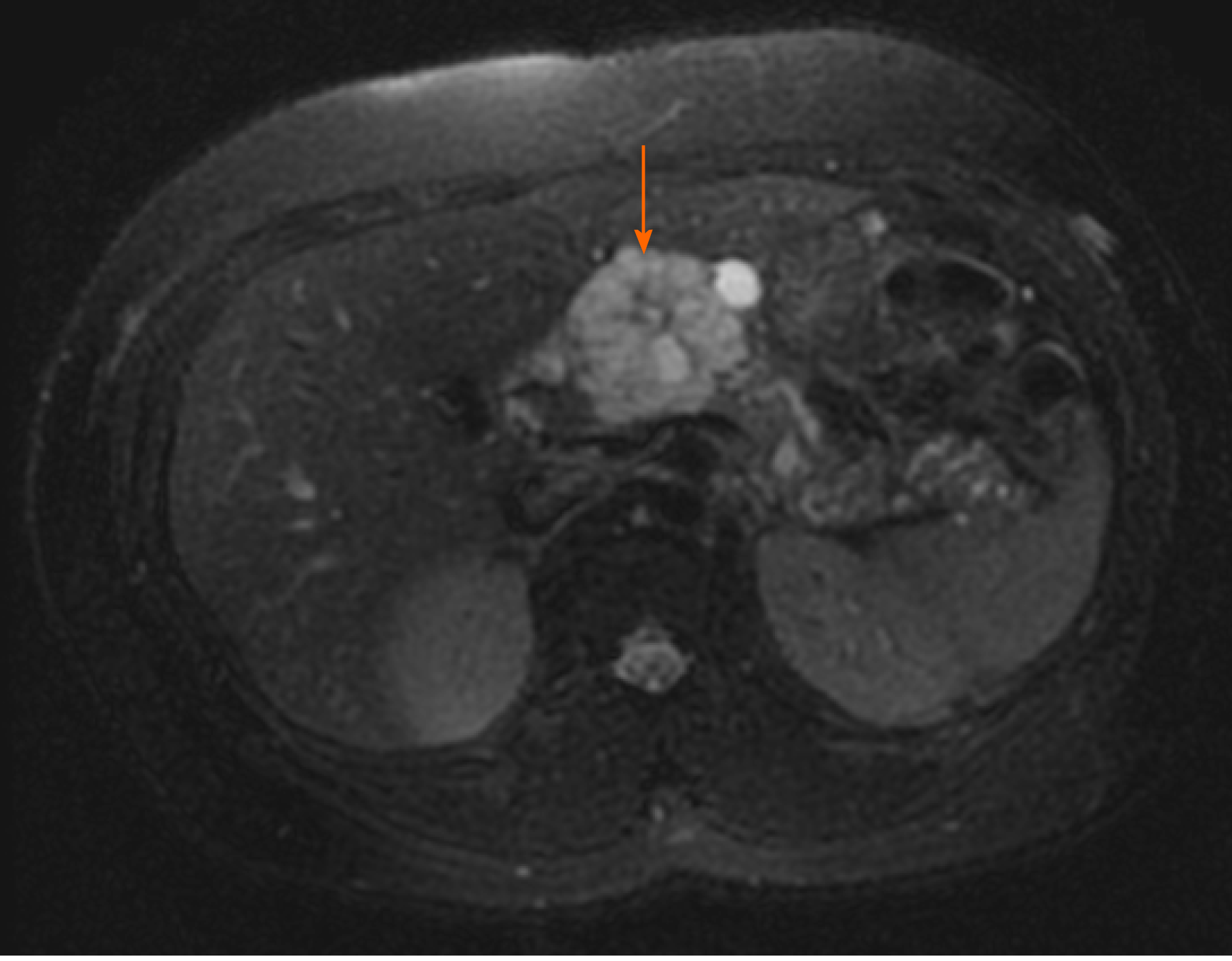
The most commonly encountered benign pancreatic cysts include serous cystadenoma (SCA), mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasm (IPMN). MCN and IPMN have varying malignant potential. SCAs classically appear lobular with multiple septations and microcysts (Figure 1). If densely septated, the cyst can appear mass-like on imaging and endoscopic ultrasound (EUS). A central scar which may be calcified is pathognomonic for SCA but occurs in only 15-20% of all SCA. A surgical series reported 51% preoperative diagnostic accuracy mainly based on radiologic imaging studies for SCA[7]. On pathology, SCA are defined by glycogen-containing cuboidal epithelial cells. The natural history of SCAs may involve slow gradual growth, which occurs in less than half of all SCAs with cysts larger than 4 cm potentially growing faster[8,9].
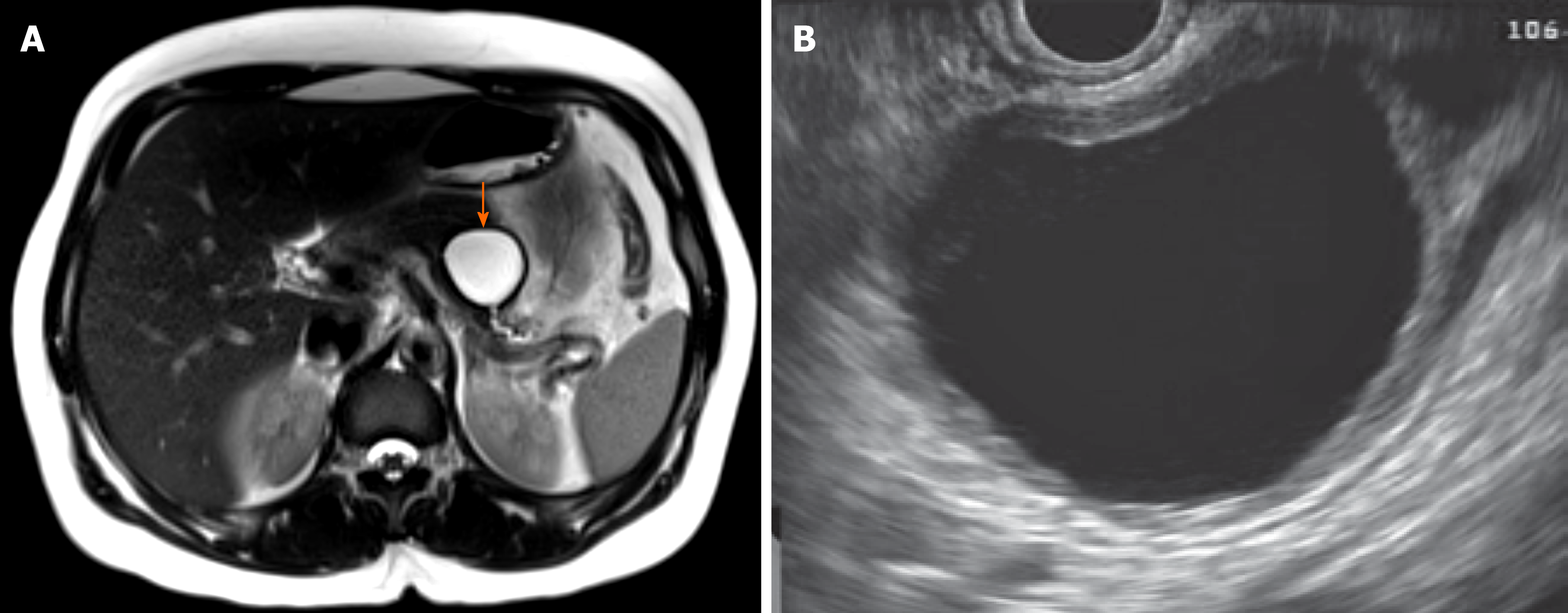
MCNs are defined by the presence of ovarian-like stroma, which explains why they occur almost exclusively in women. On imaging, MCNs are usually characterized by unilocular cysts in the body and/or tail (Figure 2). Peripheral calcifications of the wall or septa can occur in about 25% of MCNs. Approximately 15% of resected MCNs contain invasive cancer, and risk factors for malignancy include size > 6 cm, enhancing nodule, thick irregular wall, and peripheral calcifications[6,10]. While MCNs have malignant potential, less than 0.4% of MCNs smaller than 3 cm without a nodule harbor high-grade dysplasia or invasive cancer[11]. This has led to the suggestion by some that not all MCNs must be resected.
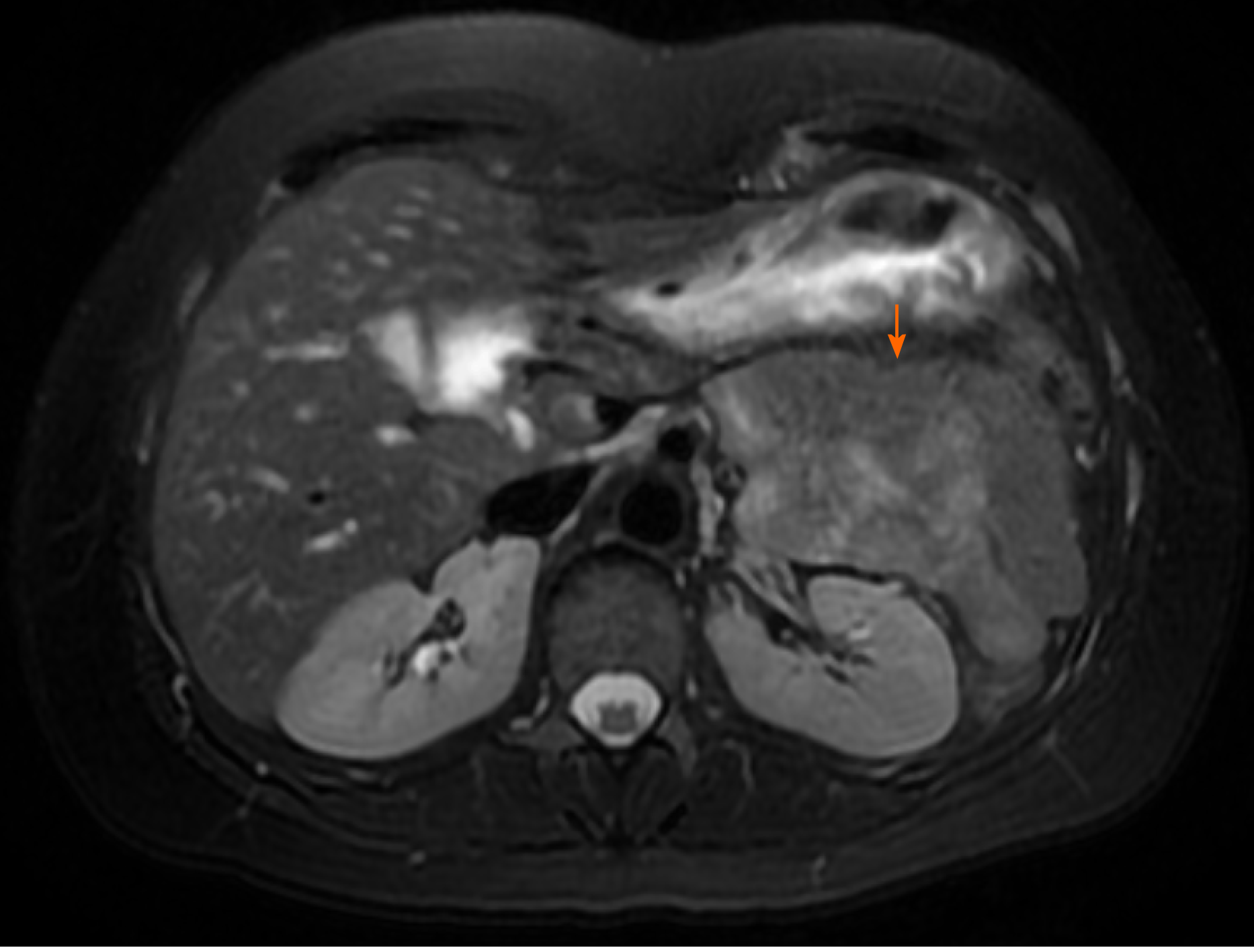
IPMNs are more common with radiologic and histologic subtypes that confer different risks of malignancy. Main duct IPMNs (MD-IPMN) have the highest malignant potential (approximately 60%-80%) and are characterized by diffuse or segmental dilatation of the main pancreatic duct to > 5 mm from a cystic tumor producing mucus within the duct (Figure 3)[12]. Branch duct IPMNs (BD-IPMN) arise within the branches of a nondilated main pancreatic duct (Figure 4) with malignant risk ranging from 3% to 26%[6,12]. Mixed type IPMNs have features of both MD-IPMN and BD-IPMN with approximately 20% to 30% of BD-IPMN ultimately proven to be mixed type IPMN following surgical resection[13]. The malignant potential of mixed type IPMN is comparable to MD-IPMN although mixed type IPMN with only microscopic involvement of the main pancreatic duct may carry less malignant risk[14]. High risk features for malignant IPMNs include solid component, enhancing nodule, main pancreatic duct dilation > 1 cm, jaundice and cytology with high-grade dysplasia or cancer, which were associated with lower 5-year survival compared with size > 3 cm, main pancreatic duct 5-9 mm, pancreatitis and non-enhancing nodules15. Other findings potentially associated with development of risk features include rapid rate of growth > 2.5 mm per year, body mass index > 26.4, smoking, and history of extrapancreatic malignancy[15-17]. Gastric histology is associated with a more benign course and more commonly found with BD-IPMN and microscopic mixed type IPMN while intestinal histology tends to occur with MD-IPMNs[18]. The less common pancreatobiliary and oncocytic histologic subtypes are mainly associated with high-grade dysplasia[19].
Solid pseudopapillary neoplasms (SPEN) also have malignant potential with characteristic pseudopapillae and cystic spaces containing hemorrhage and cholesterol clefts in myxoid stroma alternating with solid tissue. Therefore, these lesions appear solid and cystic, and typically occur in young women (Figure 5).
Evaluating a patient with an incidental pancreatic cyst should begin with a targeted history focused on acute or chronic pancreatitis and family history of pancreatic cancer or hereditary cancer syndromes associated with increased risk of pancreatic cancer. Patients at increased risk of pancreatic cancer due to family history should be managed differently and these patients are not the focus of this review. For those with incidental cysts, MRI pancreas with magnetic resonance cholangiopancreatography in 1.5 or 3 tesla should be performed. MRI has 55% to 76% accuracy for differentiating benign from malignant cysts while it is only 40% to 50% accurate for diagnosing the specific type of cyst[20]. MRI is superior for identifying communication between the main pancreatic duct and cyst, and thus better at diagnosing BD-IPMNs. If MRI cannot be performed, pancreatic protocol CT with contrast-enhanced images during the pancreatic and portal venous phases should be obtained. The imaging findings will help determine whether EUS is needed for further diagnostic evaluation, the patient should undergo surgical resection, or begin a surveillance program. The overarching questions when reviewing imaging are: (1) Is the cyst malignant; (2) If not, is it a mucinous cyst; and (3) If it is a mucinous cyst, what is the malignant potential[21-23]?
Before embarking on further evaluation for incidental pancreatic cysts, discussion should occur with select patients about whether ongoing management fits with the patients’ overall clinical status. All pancreatic cyst guidelines agree that patients who are not surgically fit do not need ongoing surveillance. A study of 725 patients with IPMNs noted that patients with higher Charlson comorbidity index ≥ 7 were 11 times more likely to die from non-IPMN related causes within 3 years of cyst diagnosis and had significantly shorter median survival (43 mo vs 180 mo)[24]. Another study used the Adult Comorbidity Evaluation 27 scoring system in 793 patients with IPMNs followed for over a year with similar conclusions that patients with higher scores were more likely to die from non-IPMN related causes[25]. These may help risk stratify patients with multiple comorbidities who likely will not need ongoing attention to their cysts. The other group of patients who do not need ongoing evaluation include those with pseudocysts or SCAs although the European cyst guideline suggests a one-time surveillance MRI one year after identification of SCAs[26].
The multiple guidelines that have been proposed to assist clinicians with managing pancreatic cystic lesions share many commonalities although the AGA guideline is the significant outlier (Table 1)[12,20,26-28]. The controversial AGA guideline increased the threshold for sending a patient to EUS-FNA as well as surgery from one to at least two risk features. While this may be expected to decrease the unnecessary resection of benign cysts, the impact on the negative predictive value has not been proven[29,30]. In our experience, we found similar rates of missing malignant cysts when comparing the International Association of Pancreatology Fukuoka and AGA guidelines (19% by Fukuoka, 25% by AGA) and preventing unnecessary surgeries (69% by Fukuoka, 74% by AGA)[29]. Therefore, the guidelines all serve as guides but cannot dictate management of patients with pancreatic cysts.
| Specifics of Guidelines | 2015 AGA | 2017 Fukuoka | 2017 ACG | 2017 ACR | 2018 European Study Group |
| Patient population | Incidental pancreatic cysts | Suspected MCN and IPMN | All pancreatic cysts | Incidental pancreatic cysts | All pancreatic cysts |
| Threshold for EUS and/or surgery | At least 2 risk factors | 1 risk factor | 1 risk factor | 1 risk factor | 1 risk factor |
| Surveillance recommendations in unresected cysts | MRI in 1 year, then every 2 yr | Surveillance based on cyst size | Surveillance based on cyst size | Surveillance based on cyst size and age | Surveillance based on cyst size and diagnosis |
| Stopping surveillance | (1) After 5 yr of stability without development of high-risk features; (2) Surgically unfit; and (3) Select resected cysts including BD-IPMN with no, low or moderate-grade dysplasia | (1) Surgically unfit; and (2) Following resection of serous cystadenoma and MCN without invasive cancer | (1) Surgically unfit; (2) Following resection of serous cystadenoma and MCN without invasive cancer; and (3) Individualize approach to patients > 75 | (1) 9-10 yr and stop at age 80; and (2) For cysts discovered > age 80, limited surveillance for 4 yr only if stable | Surgically unfit |
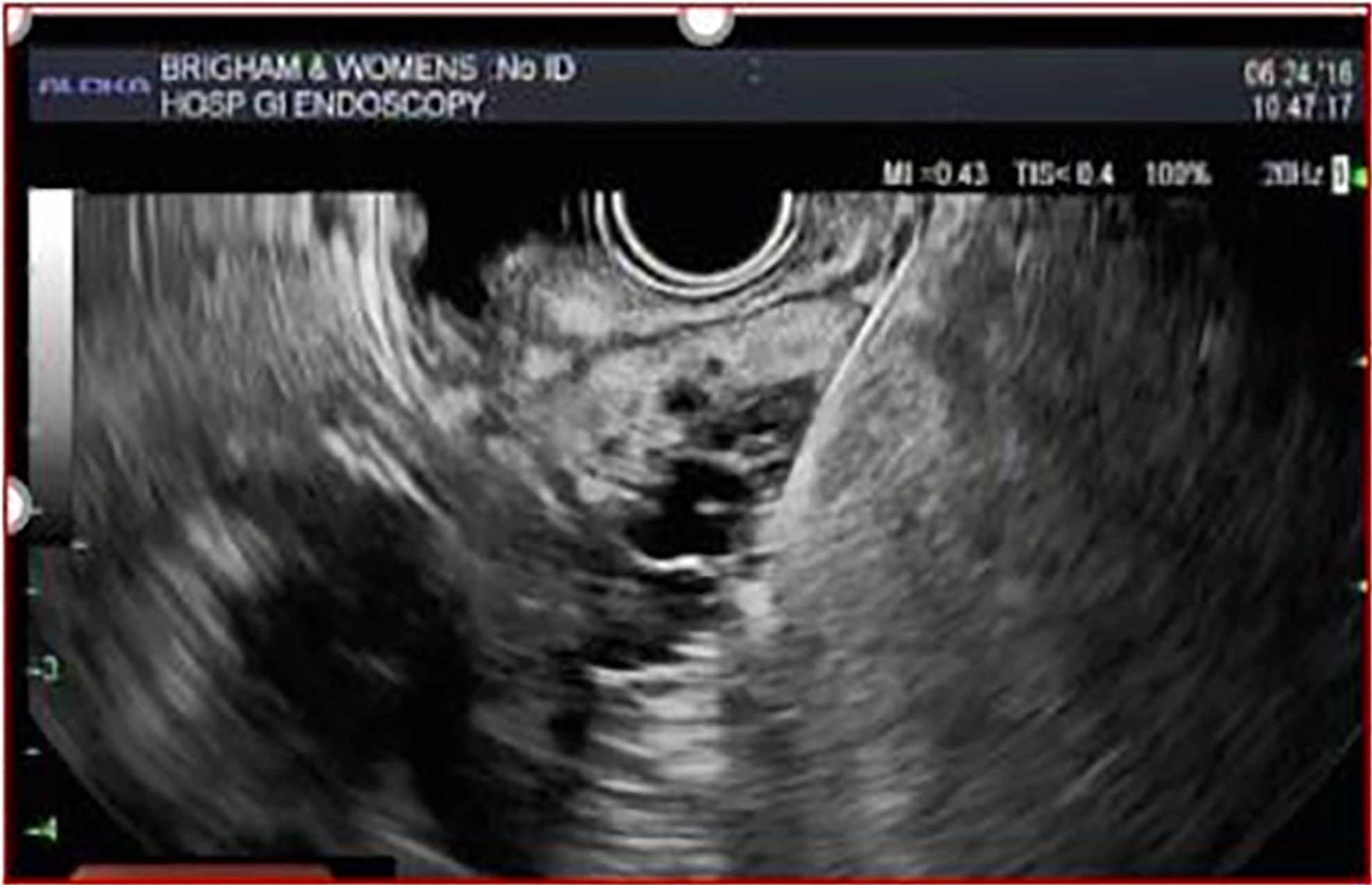
Following radiologic imaging, the role of EUS in the diagnostic work-up of pancreatic cysts includes evaluating cysts that are indeterminate or have risk features, or if EUS may change management. EUS-FNA is helpful in differentiating mucinous from nonmucinous cysts when imaging is indeterminate and in diagnosing suspected cystic neuroendocrine tumors and solid pseudopapillary neoplasms[31]. The Fukuoka and AGA guidelines offer guidance on whom to select for EUS-FNA based on presence of specific risk features. The Fukuoka guideline for suspected MCN and IPMN recommends EUS for patients with any one of these clinical or radiologic worrisome features (pancreatitis, size ≥ 3 cm, enhancing nodule < 5 mm, main pancreatic duct 5-9 mm, thick cyst wall, abrupt change in main pancreatic duct diameter with upstream parenchymal atrophy, lymphadenopathy, elevated serum CA 19-9, cyst growth ≥ 5 mm/2 years)[12]. On the other hand, the AGA guideline suggests EUS-FNA only for cysts with two high-risk imaging features (size ≥ 3 cm, solid component, or dilated main pancreatic duct) or if significant changes develop in the cyst during surveillance[27]. However, it is certainly reasonable to perform EUS-FNA in certain situations even with a single risk feature such as a solid component or significantly dilated main pancreatic duct given the relatively high risk of malignancy associated with these features.
EUS imaging is limited in diagnostic accuracy for identifying mucinous cysts with only 51% accuracy[32]. Moreover, among expert endosonographers there remains wide variation in interobserver agreement of neoplastic features[33,34]. Agreement is reportedly best for nodules, moderate for solid component and cystic communication with pancreatic duct, and fair for suspicion of malignancy[34].
Endosonographers can distinguish nodules from mucus by comparing the echogenicity relative to adjacent tissue and assessing the mobility of the structure with patient repositioning and probing with the needle. Nodules appear iso- or hypoechoic without a smooth edge or hyperechoic rim compared with mucus which have a smooth-edged hyperechoic rim surrounding a hypoechoic center[35]. Contrast-enhanced harmonic EUS, which involves intravenous injection of special contrast agents that highlight microvasculature differences between normal and abnormal tissue, appears helpful in not only diagnosing nodules, but also differentiating high-grade dysplasia and cancerous nodules from nodules with low-grade dysplasia[36]. Nodules taller than 5 mm have greater association with malignancy.
Because of the inadequate diagnostic capabilities of EUS imaging alone, intense interest has focused on searching for the ideal cyst fluid marker whether protein, DNA, RNA, metabolite that would diagnose the cyst, distinguish a mucinous from nonmucinous cyst, and predict malignant progression of the cyst. Cyst fluid for cytology typically has low diagnostic yield with less than 50% sensitivity for mucinous lesions, however, it is helpful when positive for a specific diagnosis. Similarly, cytology is highly specific for malignancy with at best 60% sensitivity for malignancy[27,37]. Fluid cytology appears more useful for SPEN with 70-81% accuracy and cystic neuroendocrine tumors with 70%-89% diagnostic yield[38-41]. Cyst fluid from pancreatic lymphangioma has a characteristic chylous appearance, elevated triglyceride levels, and numerous benign lymphocytes[42]. Improved diagnostic yield for mucinous or malignant cysts by 29% has been reported with cyst wall cytology, obtained by repeatedly passing the needle back and forth through the collapsed cyst wall[43]. Therefore, cyst wall cytology may be preferred over fluid alone, unless copious fluid is available for cytology. A small study suggests greater diagnostic yield with EUS-fine needle biopsy using a core biopsy needle[44].
If there is enough fluid, before sending it for analysis it should be evaluated for string sign, which is highly specific (95%) for a mucinous cyst[45]. This is defined as cyst fluid extending for at least 1 cm and 1 s from the tip of the EUS needle or between two fingers that are separated after placing a drop of fluid on them. The remaining fluid should be sent for carcinoembryonic antigen (CEA), amylase, glucose and potentially DNA analyses (Table 2).
| Cyst fluid test | Test characteristics | Diagnosis |
| String sign ≥ 1 cm, ≥ 1 s | 95% specificity, 94% positive predictive value | Mucinous |
| Cyst fluid cytology | 63% sensitivity | - |
| Cyst wall cytology | 29% increased diagnostic yield | - |
| CEA > 192 ng/mL | 75% sensitivity, 84% specificity | Mucinous |
| CEA < 5 ng/mL | 50% sensitivity, 95% specificity | Serous cystadenoma, pseudocyst, cystic neuroendocrine tumor |
| Amylase < 250 U/L | 44% sensitivity, 98% specificity | Excludes pseudocyst |
| Glucose < 50 mg/dL | 89% sensitivity, 78% specificity | Mucinous |
| KRAS/GNAS mutation | 89% sensitivity, 100% specificity | Mucinous |
While CEA is not predictive of malignancy, it remains the most widely used and accurate tumor marker for differentiating mucinous from non-mucinous pancreatic cysts[46]. However, it does not distinguish IPMN from MCN. Other limitations with CEA assays are they were validated for serum but not for cyst fluid, and significant CEA variation exists among different assays[47]. The appropriate CEA threshold is debated with CEA greater than 192 ng/mL having 73% sensitivity and 84% specificity for mucinous cysts[32]. Cyst fluid glucose may be more accurate than CEA for mucinous cysts with a recent meta-analysis reporting 91% sensitivity and 75% specificity for glucose compared with 67% sensitivity and 80% specificity for CEA[48]. A common cutoff used for cyst fluid glucose in diagnosing mucinous cysts is < 50 mg/dL. Very low CEA levels < 5 ng/mL are specific for SCA, pseudocyst, or cystic neuroendocrine tumor[49]. Amylase is helpful in excluding pseudocysts if less than 250 U/L[49].
DNA analysis of cyst aspirates may be helpful especially when less than 0.5cc of fluid is available as this precludes many chemistry and tumor marker analyses. A recent meta-analysis noted that KRAS and GNAS mutations were more accurate for diagnosing mucinous cysts and IPMNs than CEA alone[50]. Other studies have noted high specificity > 90% for mucinous cysts and IPMNs but lower sensitivity (65%) with KRAS and GNAS[51]. Using both CEA and KRAS may be increase sensitivity to 83% for mucinous cysts but at the expense of specificity (85%)[52]. How useful DNA mutations are for identifying cysts with high-grade dysplasia or cancer remains to be determined with some preliminary studies of various DNA mutation panels suggesting high sensitivity and specificity[53,54]. Our study found the addition of DNA analysis consisting of KRAS, loss of heterozygosity, DNA quantity and quality did not improve upon the Sendai guideline in detecting malignant cysts[55].
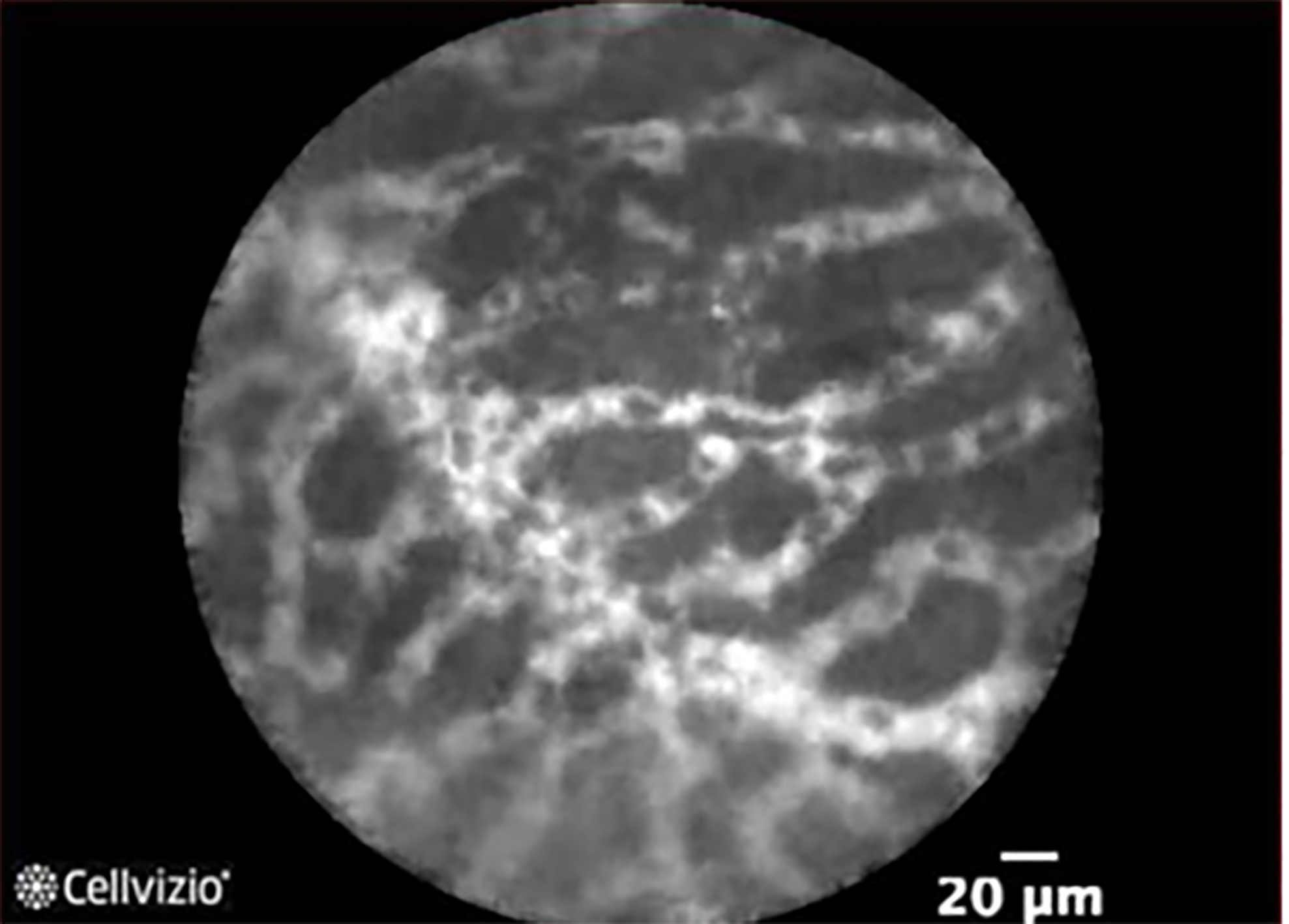
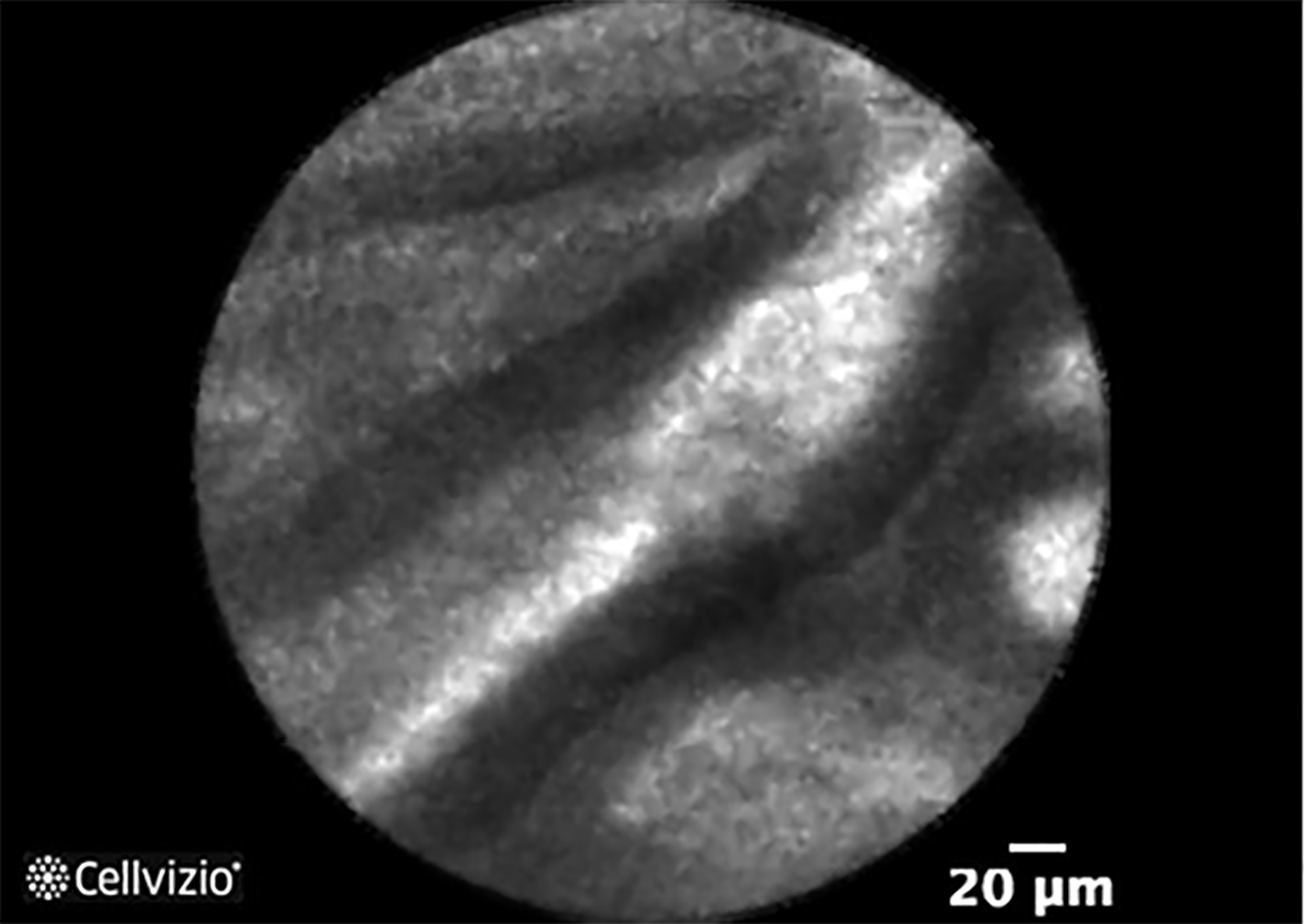
Newer tools during EUS that may improve diagnosis of pancreatic cysts include needle-based confocal laser endomicroscopy (nCLE) (Mauna Kea Technologies, Paris, France) and microbiopsy forceps (Steris, Mentor, OH, Figure 6). Both require passing the nCLE probe or microbiopsy forceps through a 19-gauge needle, which limits the size and location of cysts eligible for these techniques. EUS-nCLE allows real-time microscopic imaging of the cyst wall. Visualizing a superficial vascular network pattern is consistent with SCA with 56% to over 90% sensitivity and 100% specificity (Figure 7)[56,57]. Sensitivity for mucinous cysts ranges from 66% to over 90% with finger-like papilla consistent with IPMN (Figure 8) and epithelial bands seen in MCN[56,58]. The most common complication with nCLE is pancreatitis which ranges from 1.2% to 12%. Recommended techniques to minimize pancreatitis include preloading the nCLE probe in the FNA needle, nCLE duration less than 10 minutes and avoiding brushing the tip of the nCLE probe along the cyst wall.
A systematic review of 9 studies with 463 patients undergoing EUS with microbiopsy forceps reported 68.6% diagnostic accuracy with near 10% complications[59]. A death has been reported in Europe following this procedure as well as up to 4% severe complications[60]. Therefore, while nCLE and microbiopsy forceps are exciting tools that may improve diagnosis of pancreatic cysts, further studies are necessary to understand their safety profile as well as the optimal place these tools have amidst the currently available cyst fluid markers.
Surgery should be considered for patients with cysts that are symptomatic, malignant, or at high-risk for malignancy. Stratifying the malignant risk of a pancreatic cyst is simplest when the diagnosis of the cyst is known, but also possible for indeterminate cysts by assessing for risk features. Surgical resection is recommended for symptomatic SCAs only[20,26]. On the other hand, resection of MCNs should be considered for all patients or select higher risk patients with symptoms, nodule or size ≥ 4 cm[12,26]. Similarly, due to higher malignant risk of MD-IPMN and mixed type IPMNs, surgical resection is suggested for these lesions[12,26]. Resection is recommended for select BD-IPMN with a risk feature. Absolute indications for resection include main pancreatic duct ≥ 1 cm, enhancing nodule ≥ 5 mm, solid mass, jaundice, or cytology suspicious or positive for malignancy. Relative indications for resection of BD-IPMN include main pancreatic duct 5-9 mm, cyst ≥ 4 cm, enhancing nodule < 5 mm, growth rate ≥ 5 mm/year, serum CA 19-9 ≥ 37 U/mL, acute pancreatitis, or new onset diabetes. SPENs are considered premalignant with up to 15% incidence of local invasion or metastatic disease[39]. Given their malignant potential, favorable post-resection outcomes, and occurrence in mainly young women, referral for surgical resection is most appropriate[20,26].
The AGA guideline is the outlier by requiring the presence of at least 2 risk features before sending a patient to surgery. In the AGA technical review, 15% of all resected pancreatic cyst specimens had invasive malignancy while the rate of high-grade dysplasia was not assessed[6]. Of surgically resected IPMNs, 25% had invasive malignancy and 42% had either high-grade dysplasia and/or invasive malignancy[6]. Whether resecting IPMN with low-grade dysplasia in 58% of cases is acceptable can be debated, but as a result, the AGA guideline increased the threshold for surgery to requiring 2 risk features (solid component, dilated main pancreatic duct, and/or concerning features on EUS-FNA) in the hopes of improving the positive predictive value for resecting pancreatic cysts[27]. However, this has not proven true from multiple studies including our experience[29]. A recent meta-analysis comparing the Fukuoka and AGA guidelines reported they both had similarly modest sensitivity (67% and 59%, respectively) and specificity (64% and 77%, respectively) for detecting high-grade dysplasia and invasive cancer[61].
The AGA technical review identified the following as the greatest risk factors for malignancy in incidental pancreatic cysts: solid component [odds ratio (OR) 7.7], cyst size > 3 cm (OR 3), and dilated main pancreatic duct (MPD) (OR 2.4)[6]. One caveat is that the included studies used varying definitions for dilated main pancreatic duct ranging from ≥ 3mm to > 6 mm. Studies have suggested that the degree of main pancreatic duct dilation correlates with varying malignant potential with higher risk associated with duct > 7-8 mm[62,63]. Regarding cyst size, a study of 563 resected and radiologically diagnosed BD-IPMN noted that 18% of cysts > 3 cm had high-grade dysplasia or invasive cancer, while no malignancy was detected in cysts < 2 cm and no high-grade dysplasia was noted in lesions < 1 cm[64]. Larger cysts seem associated with development of high-risk features including nodule and main pancreatic duct dilation[16]. Assessment of cyst size and nodules may vary depending on the imaging modality. EUS may be more accurate than CT or MRI for cyst size measurements and for detecting nodules[35,65].
Careful surgical planning is important with MD-IPMN, and partial pancreatectomy is reasonable in cases with segmental dilation or diffuse dilation with focal lesions[12]. Resection should continue until the margins are negative for high-grade dysplasia and cancer. However, determining how much pancreas to resect in MD-IPMN with diffuse dilation without focal lesions can be challenging. ERCP with pancreatoscopy and/or intraductal ultrasound (IDUS) and EUS may help with surgical planning. Case series have suggested the utility of pancreatoscopy with or without IDUS in mapping IPMNs preoperatively leading to more or less extensive surgeries than previously planned in about 60% of patients[26,66,67]. Therefore, these tools may be used selectively to guide surgical planning. Regarding total pancreatectomy, some suggest this should be reserved for younger healthy patients who will tolerate brittle diabetes or exocrine insufficiency postoperatively while others advocate for this in all patients with diffuse main pancreatic duct dilation or those with family history of pancreatic cancer[12,26].
Patients with cysts at low risk for malignancy and following resection of certain cysts should undergo surveillance. MRI is preferred over CT for surveillance due to reduced radiation exposure and potentially improved ability to detect communication with the main pancreatic duct and nodules[20,26,28]. Recent reports of gadolinium deposition in the brain has raised concern over repeated injections of gadolinium during surveillance MRIs[68]. A few studies have suggested that MRI performed with vs without gadolinium did not change management decisions[69,70]. Non-contrast MRI scans have demonstrated similar efficacy to contrast-enhanced MRI in discerning benign from malignant disease[69]. Therefore, surveillance MRIs may be performed without gadolinium unless concerning findings are noted on the non-contrast MRI.
Surveillance is recommended at various intervals for unresected pancreatic cystic neoplasms depending on size and other criteria for different guidelines (Table 3)[12,20,26-28]. The surveillance interval may be lengthened if there are no concerning features or changes found on repeated testing[26,27]. If a cyst had been followed with imaging every 6 mo, this may be extended to every year while annual surveillance may be lengthened to biennial follow-up. Similarly, if new onset or worsening diabetes or concerning changes (risk features) develop on imaging, surveillance interval should be shortened and/or EUS performed and patient referred to a multidisciplinary pancreas center.
| 2015 AGA | 2017 Fukuoka | 2017 ACG | 2017 ACR | 2018 European Study Group |
| Repeat 1 yr then q2 years until 5 yr | < 1 cm: q2-3 years | < 1 cm: q2 years x4 years | < 1.5 cm + < 65-year-old: q1 year x5, q2 years x2 | < 1.5 cm: q1 year x3 years then q2 years |
| 1-2 cm: q1 year x2 years | 1-2 cm: q1 year x3 years then q2 years x4 years | < 1.5 cm + ≥ 65-year-old: q2 years x5 | ≥ 1.5 cm: q6 mo x2 then q1 year | |
| 2-3 cm: EUS in 3-6 mo then increase interval and alternate with MRI | 2-3 cm: MRI/EUS q6-12 mo x3 years then q1 year x4 years | 1.5-1.9 cm connected to MPD: q1 year x5 then q2 years x2 | IPMN or MCN: q6 mo x2 then q1 year | |
| > 3 cm: MRI alternate with EUS q3-6 mo | > 3 cm: Consider referral to multidisciplinary pancreas group; MRI/EUS q6 mo x3 years, then MRI/EUS q1 year x4 years | 2-2.5 cm connected to MPD or 1.5-2.5 cm without MPD connection or > 2.5 cm: q6 mo x4 then q1 year x2 then q2 years x3 |
While the other gastroenterology guidelines suggest surveillance intervals based on cyst size and other factors without an explicit recommendation to stop surveillance except in surgically unfit patients, the AGA guideline endorses a simplified surveillance protocol of MRI in 1 year and then every 2 years for 5 years followed by stopping if the cyst remains stable without developing high risk features[27]. This is perhaps the most controversial aspect of the AGA guideline. Numerous long-term surveillance studies have been published following the release of the AGA guidelines, most of which noted low risk of malignancies developing after 5 years of surveillance. A Japanese study of 1404 patients with BD-IPMN excluding cysts with nodules or main pancreatic duct dilation > 1 cm noted gradually increasing incidence rates of cancer over time (3.3% at 5 years, 6.6% at 10 years and 15% at 15 years)[71]. Long-term surveillance of 1036 BD-IPMNs without worrisome or high-risk features in Italy found 1.1% developed cancer after median 62 mo follow-up[72]. Development of worrisome features or cyst size growth > 2.5 mm/year were associated with risk for cancer. Another study of 363 patients with BD-IPMN followed for over 5 years noted that cyst size > 1.5 cm was associated with higher risk of developing cancer (7.5% vs 0.9%)[73]. Therefore, surveillance should not be stopped for all patients after 5 years, and in fact, should continue for most patients except those who are no longer surgical candidates. As discussed earlier, various comorbidity scoring systems including the Charlson comorbidity index and Adult Comorbidity Evaluation 27 scoring system may serve as aides to identify patients at higher risk from their comorbidities than their pancreatic cyst[24,25]. These patients likely would not benefit from ongoing surveillance. The American College of Gastroenterology guideline also suggests individualizing surveillance in patients over 75 years old analogous to the United States Preventive Services Taskforce recommendations for colon cancer screening.
Following surgical resection of SCA or MCN without invasive features, surveillance is not necessary as resection is considered curative. This is because no recurrence of MCN without invasive cancer was noted in patients after nearly 5 years[74]. For higher risk IPMNs including high-grade dysplasia at the surgical margins, non-intestinal subtype, or family history of pancreatic cancer, the Fukuoka guideline recommends repeat imaging at least every 6 mo[12]. For other IPMNs, surveillance every 6-12 mo is suggested. Those with resected invasive cancer should continue surveillance as per patients with pancreatic ductal adenocarcinoma. The AGA guideline supports postoperative surveillance only following resection of high-grade dysplasia or invasive cancer with MRI every 2 years[27]. A concern with this recommendation is that early recurrences, especially in patients with invasive cancer, may be missed.
Unresolved questions remain regarding surveillance including the optimal surveillance interval and duration in unresected cysts.
While the various guidelines provide a foundation for managing pancreatic cysts, the approach to each patient with a pancreatic cyst should be individualized based on clinical status and comorbidities, risk of malignancy, and personal preferences. A good quality MRI of the pancreas may help identify malignant and mucinous cysts. If the diagnosis of the cyst remains uncertain or there are concerning features on imaging, EUS should be pursued although the ideal diagnostic markers and tools remain elusive. Surgical resection should be reserved for patients with high risk features including solid mass, nodule or dilated main pancreatic duct. The ongoing challenge with surgical referral remains accurately identifying signs predictive of malignancy that allow early surgery to improve long-term survival while sparing patients with low-risk cysts the morbidity and mortality of pancreatic surgery. High quality studies are necessary to discover better diagnostic markers and tools, to improve risk stratification of patients, and to understand the optimal interval and duration of surveillance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corrales FJ, Fiori E S-Editor: Wang JL L-Editor: A P-Editor: Xing YX
| 1. | de Oliveira PB, Puchnick A, Szejnfeld J, Goldman SM. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS One. 2015;10:e0121317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Megibow AJ, Baker ME, Gore RM, Taylor A. The incidental pancreatic cyst. Radiol Clin North Am. 2011;49:349-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Chu LC, Singhi AD, Haroun RR, Hruban RH, Fishman EK. The many faces of pancreatic serous cystadenoma: Radiologic and pathologic correlation. Diagn Interv Imaging. 2017;98:191-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest Endosc. 2016;84:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Chernyak V, Flusberg M, Haramati LB, Rozenblit AM, Bellin E. Incidental pancreatic cystic lesions: is there a relationship with the development of pancreatic adenocarcinoma and all-cause mortality? Radiology. 2015;274:161-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824-48.e22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 255] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 7. | Kang JS, Kim HJ, Choi YJ, Byun Y, Lee JM, Han Y, Kim H, Kwon W, Jang JY. Clinicoradiological features of resected serous cystic neoplasms according to morphological subtype and preoperative tentative diagnosis: can radiological characteristics distinguish serous cystic neoplasms from other lesions? Ann Surg Treat Res. 2020;98:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM, Zaheer A, Wolfgang C, Hruban R, Marchegiani G, Fernández Del Castillo C, Brugge W, Ha Y, Kim MH, Oh D, Hirai I, Kimura W, Jang JY, Kim SW, Jung W, Kang H, Song SY, Kang CM, Lee WJ, Crippa S, Falconi M, Gomatos I, Neoptolemos J, Milanetto AC, Sperti C, Ricci C, Casadei R, Bissolati M, Balzano G, Frigerio I, Girelli R, Delhaye M, Bernier B, Wang H, Jang KT, Song DH, Huggett MT, Oppong KW, Pererva L, Kopchak KV, Del Chiaro M, Segersvard R, Lee LS, Conwell D, Osvaldt A, Campos V, Aguero Garcete G, Napoleon B, Matsumoto I, Shinzeki M, Bolado F, Fernandez JM, Keane MG, Pereira SP, Acuna IA, Vaquero EC, Angiolini MR, Zerbi A, Tang J, Leong RW, Faccinetto A, Morana G, Petrone MC, Arcidiacono PG, Moon JH, Choi HJ, Gill RS, Pavey D, Ouaïssi M, Sastre B, Spandre M, De Angelis CG, Rios-Vives MA, Concepcion-Martin M, Ikeura T, Okazaki K, Frulloni L, Messina O, Lévy P. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016;65:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413-9; discussion 419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 305] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 10. | Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited: part II. Mucinous cystic neoplasms. Surg Oncol. 2011;20:e93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Nguyen D, Dawson DW, Hines OJ, Reber HA, Donahue TR. Mucinous cystic neoplasms of the pancreas: are we overestimating malignant potential? Am Surg. 2014;80:915-919. [PubMed] [Cited in This Article: ] |
| 12. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in F6Publishing: 971] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 13. | Fritz S, Werner J, Büchler MW. Reply to Letter: "Sendai Consensus Guidelines for Branch-duct IPMN: Guidelines Are Just Guidelines". Ann Surg. 2015;262:e66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Sahora K, Fernández-del Castillo C, Dong F, Marchegiani G, Thayer SP, Ferrone CR, Sahani DV, Brugge WR, Warshaw AL, Lillemoe KD, Mino-Kenudson M. Not all mixed-type intraductal papillary mucinous neoplasms behave like main-duct lesions: implications of minimal involvement of the main pancreatic duct. Surgery. 2014;156:611-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Capurso G, Crippa S, Vanella G, Traini M, Zerboni G, Zaccari P, Belfiori G, Gentiluomo M, Pessarelli T, Petrone MC, Campa D, Falconi M, Arcidiacono PG. Factors Associated With the Risk of Progression of Low-Risk Branch-Duct Intraductal Papillary Mucinous Neoplasms. JAMA Netw Open. 2020;3:e2022933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Han Y, Lee H, Kang JS, Kim JR, Kim HS, Lee JM, Lee KB, Kwon W, Kim SW, Jang JY. Progression of Pancreatic Branch Duct Intraductal Papillary Mucinous Neoplasm Associates With Cyst Size. Gastroenterology. 2018;154:576-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Overbeek KA, Alblas M, Gausman V, Kandel P, Schweber AB, Brooks C, Van Riet PA, Wallace MB, Gonda TA, Cahen DL, Bruno MJ. Development of a stratification tool to identify pancreatic intraductal papillary mucinous neoplasms at lowest risk of progression. Aliment Pharmacol Ther. 2019;50:789-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Yamada S, Fujii T, Shimoyama Y, Kanda M, Nakayama G, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Nakao A, Kodera Y. Clinical implication of morphological subtypes in management of intraductal papillary mucinous neoplasm. Ann Surg Oncol. 2014;21:2444-2452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Patra KC, Bardeesy N, Mizukami Y. Diversity of Precursor Lesions For Pancreatic Cancer: The Genetics and Biology of Intraductal Papillary Mucinous Neoplasm. Clin Transl Gastroenterol. 2017;8:e86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 329] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 21. | Lee LS. Diagnostic approach to pancreatic cysts. Curr Opin Gastroenterol. 2014;30:511-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Lee LS. Incidental Cystic Lesions in the Pancreas: Resect? Curr Treat Options Gastroenterol. 2014;12:333-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Chiang AL, Lee LS. Clinical approach to incidental pancreatic cysts. World J Gastroenterol. 2016;22:1236-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Sahora K, Ferrone CR, Brugge WR, Morales-Oyarvide V, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Effects of Comorbidities on Outcomes of Patients With Intraductal Papillary Mucinous Neoplasms. Clin Gastroenterol Hepatol. 2015;13:1816-1823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Kawakubo K, Tada M, Isayama H, Sasahira N, Nakai Y, Takahara N, Miyabayashi K, Yamamoto K, Mizuno S, Mohri D, Kogure H, Sasaki T, Yamamoto N, Tateishi R, Hirano K, Ijichi H, Tateishi K, Koike K. Risk for mortality from causes other than pancreatic cancer in patients with intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2013;42:687-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 793] [Cited by in F6Publishing: 704] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 27. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 663] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 28. | Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14:911-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 29. | Lee A, Kadiyala V, Lee LS. Evaluation of AGA and Fukuoka Guidelines for EUS and surgical resection of incidental pancreatic cysts. Endosc Int Open. 2017;5:E116-E122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Singhi AD, Zeh HJ, Brand RE, Nikiforova MN, Chennat JS, Fasanella KE, Khalid A, Papachristou GI, Slivka A, Hogg M, Lee KK, Tsung A, Zureikat AH, McGrath K. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: a clinicopathologic study of 225 patients with supporting molecular data. Gastrointest Endosc. 2016;83:1107-1117.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Kadiyala V, Lee LS. Endosonography in the diagnosis and management of pancreatic cysts. World J Gastrointest Endosc. 2015;7:213-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 32. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1016] [Cited by in F6Publishing: 857] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 33. | de Jong K, Verlaan T, Dijkgraaf MG, Poley JW, van Dullemen H, Bruno MJ, Fockens P. Interobserver agreement for endosonography in the diagnosis of pancreatic cysts. Endoscopy. 2011;43:579-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Ahmad NA, Kochman ML, Brensinger C, Brugge WR, Faigel DO, Gress FG, Kimmey MB, Nickl NJ, Savides TJ, Wallace MB, Wiersema MJ, Ginsberg GG. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 35. | Zhong N, Zhang L, Takahashi N, Shalmiyev V, Canto MI, Clain JE, Deutsch JC, DeWitt J, Eloubeidi MA, Gleeson FC, Levy MJ, Mallery S, Raimondo M, Rajan E, Stevens T, Topazian M. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol. 2012;10:192-198, 198.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 36. | Kamata K, Kitano M, Omoto S, Kadosaka K, Miyata T, Yamao K, Imai H, Sakamoto H, Harwani Y, Chikugo T, Chiba Y, Matsumoto I, Takeyama Y, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48:35-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. 2008;15:3187-3192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Karsenti D, Caillol F, Chaput U, Perrot B, Koch S, Vuitton L, Jacques J, Valats JC, Poincloux L, Subtil C, Chabrun E, Williet N, Vanbiervliet G, Belkhodja H, Charachon A, Wangermez M, Coron E, Cholet F, Privat J, Le Baleur Y, Bichard P, Ah Soune P, Lecleire S, Palazzo M; from the GRAPHE. Safety of Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Pancreatic Solid Pseudopapillary Neoplasm Before Surgical Resection: A European Multicenter Registry-Based Study on 149 Patients. Pancreas. 2020;49:34-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, Dal Molin M, Moran RA, Khashab MA, Ahuja N, Goggins M, Hruban RH, Wolfgang CL, Lennon AM. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 40. | Ridtitid W, Halawi H, DeWitt JM, Sherman S, LeBlanc J, McHenry L, Coté GA, Al-Haddad MA. Cystic pancreatic neuroendocrine tumors: outcomes of preoperative endosonography-guided fine needle aspiration, and recurrence during long-term follow-up. Endoscopy. 2015;47:617-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Lee LS. Diagnosis of pancreatic neuroendocrine tumors and the role of endoscopic ultrasound. Gastroenterol Hepatol (N Y). 2010;6:520-522. [PubMed] [Cited in This Article: ] |
| 42. | Barnes EL, Lee LS. Got milk? Gastroenterology. 2015;148:e1-e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Hong SK, Loren DE, Rogart JN, Siddiqui AA, Sendecki JA, Bibbo M, Coben RM, Meckes DP, Kowalski TE. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc. 2012;75:775-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Phan J, Dawson D, Sedarat A, Fejleh MP, Marya N, Thaker AM, Rogers M, Kim S, Muthusamy VR. Clinical Utility of Obtaining Endoscopic Ultrasound-Guided Fine-Needle Biopsies for Histologic Analyses of Pancreatic Cystic Lesions. Gastroenterology. 2020;158:475-477.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Bick BL, Enders FT, Levy MJ, Zhang L, Henry MR, Abu Dayyeh BK, Chari ST, Clain JE, Farnell MB, Gleeson FC, Kendrick ML, Pearson RK, Petersen BT, Rajan E, Vege SS, Topazian M. The string sign for diagnosis of mucinous pancreatic cysts. Endoscopy. 2015;47:626-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Ngamruengphong S, Bartel MJ, Raimondo M. Cyst carcinoembryonic antigen in differentiating pancreatic cysts: a meta-analysis. Dig Liver Dis. 2013;45:920-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Boot C. A review of pancreatic cyst fluid analysis in the differential diagnosis of pancreatic cyst lesions. Ann Clin Biochem. 2014;51:151-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Faias S, Cravo M M, Chaves P, Pereira L. A comparative analysis of glucose and carcinoembryonic antigen in diagnosis of pancreatic mucinous cysts: a systematic review and meta-analysis. Gastrointest Endosc. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 365] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 50. | McCarty TR, Paleti S, Rustagi T. Molecular analysis of EUS-acquired pancreatic cyst fluid for KRAS and GNAS mutations for diagnosis of intraductal papillary mucinous neoplasia and mucinous cystic lesions: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:1019-1033.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Singhi AD, Nikiforova MN, Fasanella KE, McGrath KM, Pai RK, Ohori NP, Bartholow TL, Brand RE, Chennat JS, Lu X, Papachristou GI, Slivka A, Zeh HJ, Zureikat AH, Lee KK, Tsung A, Mantha GS, Khalid A. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res. 2014;20:4381-4389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 52. | Al-Haddad M, DeWitt J, Sherman S, Schmidt CM, LeBlanc JK, McHenry L, Coté G, El Chafic AH, Luz L, Stuart JS, Johnson CS, Klochan C, Imperiale TF. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 53. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131-2141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 54. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA Jr, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 55. | Li ZH, Zhong LQ, Mu WN, Wu YH. Toxicity of Tributyltin in Juvenile Common Carp (Cyprinus Carpio): Physiological Responses, Hepatic Gene Expression, and Stress Protein Profiling. J Biochem Mol Toxicol. 2016;30:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Napoleon B, Palazzo M, Lemaistre AI, Caillol F, Palazzo L, Aubert A, Buscail L, Maire F, Morellon BM, Pujol B, Giovannini M. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: a prospective multicenter validation study in patients with definite diagnosis. Endoscopy. 2019;51:825-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 57. | Keane MG, Wehnert N, Perez-Machado M, Fusai GK, Thorburn D, Oppong KW, Carroll N, Metz AJ, Pereira SP. A prospective trial of CONfocal endomicroscopy in CYSTic lesions of the pancreas: CONCYST-01. Endosc Int Open. 2019;7:E1117-E1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Kadayifci A, Atar M, Basar O, Forcione DG, Brugge WR. Needle-Based Confocal Laser Endomicroscopy for Evaluation of Cystic Neoplasms of the Pancreas. Dig Dis Sci. 2017;62:1346-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Balaban VD, Cazacu IM, Pinte L, Jinga M, Bhutani MS, Saftoiu A. EUS-through-the-needle microbiopsy forceps in pancreatic cystic lesions: A systematic review. Endosc Ultrasound. 2021;10:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Kovacevic B, Klausen P, Rift CV, Toxværd A, Grossjohann H, Karstensen JG, Brink L, Hassan H, Kalaitzakis E, Storkholm J, Hansen CP, Hasselby JP, Vilmann P. Clinical impact of endoscopic ultrasound-guided through-the-needle microbiopsy in patients with pancreatic cysts. Endoscopy. 2021;53:44-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Wu J, Wang Y, Li Z, Miao H. Accuracy of Fukuoka and American Gastroenterological Association Guidelines for Predicting Advanced Neoplasia in Pancreatic Cyst Neoplasm: A Meta-Analysis. Ann Surg Oncol. 2019;26:4522-4536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Abdeljawad K, Vemulapalli KC, Schmidt CM, Dewitt J, Sherman S, Imperiale TF, Al-Haddad M. Prevalence of malignancy in patients with pure main duct intraductal papillary mucinous neoplasms. Gastrointest Endosc. 2014;79:623-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Sugimoto M, Elliott IA, Nguyen AH, Kim S, Muthusamy VR, Watson R, Hines OJ, Dawson DW, Reber HA, Donahue TR. Assessment of a Revised Management Strategy for Patients With Intraductal Papillary Mucinous Neoplasms Involving the Main Pancreatic Duct. JAMA Surg. 2017;152:e163349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, Pitman MB, Warshaw AL, Lillemoe KD, Fernandez-del Castillo CF. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? Ann Surg. 2013;258:466-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 65. | Du C, Chai NL, Linghu EQ, Li HK, Sun LH, Jiang L, Wang XD, Tang P, Yang J. Comparison of endoscopic ultrasound, computed tomography and magnetic resonance imaging in assessment of detailed structures of pancreatic cystic neoplasms. World J Gastroenterol. 2017;23:3184-3192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Cheon YK, Cho YD, Jeon SR, Moon JH, Jeong SW, Hur KY, Jin SY, Lee JS. Pancreatic resection guided by preoperative intraductal ultrasonography for intraductal papillary mucinous neoplasm. Am J Gastroenterol. 2010;105:1963-1969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Tyberg A, Raijman I, Siddiqui A, Arnelo U, Adler DG, Xu MM, Nassani N, Sejpal DV, Kedia P, Nah Lee Y, Gress FG, Ho S, Gaidhane M, Kahaleh M. Digital Pancreaticocholangioscopy for Mapping of Pancreaticobiliary Neoplasia: Can We Alter the Surgical Resection Margin? J Clin Gastroenterol. 2019;53:71-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Choi JW, Moon WJ. Gadolinium Deposition in the Brain: Current Updates. Korean J Radiol. 2019;20:134-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 69. | Nougaret S, Reinhold C, Chong J, Escal L, Mercier G, Fabre JM, Guiu B, Molinari N. Incidental pancreatic cysts: natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. Eur Radiol. 2014;24:1020-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Macari M, Lee T, Kim S, Jacobs S, Megibow AJ, Hajdu C, Babb J. Is gadolinium necessary for MRI follow-up evaluation of cystic lesions in the pancreas? AJR Am J Roentgenol. 2009;192:159-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Oyama H, Tada M, Takagi K, Tateishi K, Hamada T, Nakai Y, Hakuta R, Ijichi H, Ishigaki K, Kanai S, Kogure H, Mizuno S, Saito K, Saito T, Sato T, Suzuki T, Takahara N, Morishita Y, Arita J, Hasegawa K, Tanaka M, Fukayama M, Koike K. Long-term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology. 2020;158:226-237.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 72. | Marchegiani G, Andrianello S, Pollini T, Caravati A, Biancotto M, Secchettin E, Bonamini D, Malleo G, Bassi C, Salvia R. "Trivial" Cysts Redefine the Risk of Cancer in Presumed Branch-Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Potential Target for Follow-Up Discontinuation? Am J Gastroenterol. 2019;114:1678-1684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 73. | Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, Brugge WR, Mino-Kenudson M, Patino M, Sahani DV, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology. 2017;153:1284-1294.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 74. | Gil E, Choi SH, Choi DW, Heo JS, Kim MJ. Mucinous cystic neoplasms of the pancreas with ovarian stroma. ANZ J Surg. 2013;83:985-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |