Published online Aug 7, 2021. doi: 10.3748/wjg.v27.i29.4763
Peer-review started: February 18, 2021
First decision: May 1, 2021
Revised: May 10, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: August 7, 2021
The emergence and rapid spread of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 180 million confirmed cases resulting in over 4 million deaths worldwide with no clear end in sight for the coronavirus disease 19 (COVID-19) pandemic. Most SARS-CoV-2 exposed individuals experience mild to moderate symptoms, including fever, cough, fatigue, and loss of smell and taste. However, many individuals develop pneumonia, acute respiratory distress syndrome, septic shock, and multiorgan dysfunction. In addition to these primarily respiratory symptoms, SARS-CoV-2 can also infiltrate the central nervous system, which may damage the blood-brain barrier and the neuron's synapses. Resultant inflammation and neurodegeneration in the brain stem can further prevent efferent signaling to cranial nerves, leading to the loss of anti-inflammatory signaling and normal respiratory and gastrointestinal functions. Additionally, SARS-CoV-2 can infect enterocytes resulting in gut damage followed by microbial dysbiosis and translocation of bacteria and their byproducts across the damaged epithelial barrier. As a result, this exacerbates pro-inflammatory responses both locally and systemically, resulting in impaired clinical outcomes. Recent evidence has highlighted the complex interactions that mutually modulate respiratory, neurological, and gastrointestinal function. In this review, we discuss the ways SARS-CoV-2 potentially disrupts the gut-brain-lung axis. We further highlight targeting specific responses to SARS-CoV-2 for the development of novel, urgently needed therapeutic interventions. Finally, we propose a prospective related to the individuals from Low- and Middle-Income countries. Here, the underlying propensity for heightened gut damage/microbial translocation is likely to result in worse clinical outcomes during this COVID-19 pandemic.
Core Tip: Severe acute respiratory syndrome coronavirus 2 has spread rapidly, infecting and killing millions worldwide. In addition to respiratory symptoms, coronavirus disease 19 (COVID-19) is associated with enterocyte infection leading to intestinal inflammation, gut barrier damage, and microbial dysbiosis exacerbating the systemic inflammatory response. Viral infiltration to the central nervous system from cranial nerve innervation of the lungs and gut can also cause neuroinflammation and degeneration, which further dysregulates gut and lungs. This review summarizes recent findings on COVID-19 pathogenesis in the gut-brain-lung axis and offers therapeutic interventions to improve clinical outcomes.
- Citation: Johnson SD, Olwenyi OA, Bhyravbhatla N, Thurman M, Pandey K, Klug EA, Johnston M, Dyavar SR, Acharya A, Podany AT, Fletcher CV, Mohan M, Singh K, Byrareddy SN. Therapeutic implications of SARS-CoV-2 dysregulation of the gut-brain-lung axis. World J Gastroenterol 2021; 27(29): 4763-4783
- URL: https://www.wjgnet.com/1007-9327/full/v27/i29/4763.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i29.4763
Starting from late 2019 and through 2020, a novel coronavirus, the causative agent of coronavirus disease 19 (COVID-19), initiated one of the deadliest global pandemics warranting extensive panic for the underprepared global health system[1-4]. The pandemic has claimed countless lives (approximately 4 million deaths worldwide of 188 million confirmed cases as of July 15, 2021) and presented unprecedented socio-economic losses[5-8]. The primary mode of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission is airborne, with infectious droplets gaining access into the host respiratory tract. Subsequently, standard clinical indicators comprise fever, cough, fatigue, chills, nausea, headache, dyspnea. Additionally, the loss of taste or smell often ensues[9,10]. Coronavirus infections primarily target the upper respiratory tract, but they also manifest in other regions such as the gas
SARS-CoV-2 has been shown to infect GIT cells such as human gut enterocytes[13]. SARS-CoV-2 RNA has also been detected in gastrointestinal glandular epithelial cells[14-17]. During infection, compromised gastrointestinal symptom involvement has worse clinical outcomes and takes longer to recover than those with only respiratory symptoms[18-20]. Subsequently, patients who test positive for viral RNA in feces have poor viral clearance and prolonged delays for infection resolution[21]. SARS-CoV-2 tropism in the lungs and GIT is supported by abundant cellular viral entry receptors such as the angiotensin-converting enzyme-2 (ACE2). ACE2 exists in abundance on the epithelia of the lungs and small intestine (ileum)[22,23]. ACE2 catalyzes the conversion of angiotensin II into angiotensin 1-7; it is crucial for maintaining gut balance through regulation of amino acid homeostasis, innate immunity, the balance of the intestinal microbiome, and by limiting diarrhea[24,25].
The gut is the largest immune organ and is a crucial interconnecting hub, which synchronously orchestrates multiple physiological, cellular, and organ functions[26]. The gut maintains an anti-inflammatory state that supports the growth of trillions of commensal bacteria, which modulate the function of other organs like the brain, heart, kidney, lungs, and liver while favoring its core functions like digestion, absorption of nutrients, and excretion of waste matter[27-30]. This inter-compartmentalized crosstalk infers the need to develop effective gut-specific therapeutics that could limit systemic pathology during diverse viral infections such as human immunodeficiency virus (HIV), SARS-CoV-2, and other related respiratory viruses.
The gut is one of the most complex immune organs containing a wide variety of immune cells, including myeloid cells, conventional T cells, innate lymphoid cells (e.g., natural killer cells and specialized intraepithelial lymphocyte populations), and epithelial cells[31]. Homeostasis in a healthy gut is mainly fostered through the sustenance of an anti-inflammatory environment crucial for maintaining tolerance to oral antigens, commensal microflora, and bridging an intact mucosal barrier[32,33]. Commensal microflora competes with pathobionts (potentially pathogenic bacteria which are benign during homeostasis) for nutrients within the gut microenvironment, secrete toxins that favor their predominance, and release metabolites that shape gut immunity by providing appropriate anti-inflammatory signals to other immune cells such as macrophages[31,34].
Critical genera of the microbiome also ferment dietary fiber into short-chain fatty acids that support gut immune responses by sustaining the balance of T regulatory (T reg) cells and activated T cells[35]. The released short-chain fatty acids such as butyrate facilitate CD103+ dendritic cell production of retinoic acid, which in turn aid in the generation of FoxP3+ T reg cells and sampling of an antigen across the gut lumen. Increased frequencies of CD4+ regulatory T cells maintain a basal anti-inflammatory state of the gut. Released acetate also improves the integrity of gut epithelial cells[36,37]. Additionally, lamina propria macrophages are highly phagocytic and engulf any foreign microorganisms that may have breached the intact gut mucosal barrier[38]. The gut epithelial cells secrete anti-inflammatory cytokines such as interleukin 10 (IL-10) and transforming growth factor-β (TGF-β), which promote increased frequencies of T regs that are crucial for immunological suppression and induction of mucosal tolerance[39-41].
During gut infection, SARS-CoV-2 uses its spike (S) proteins on the virus's surface to directly bind to the ACE2 receptor that is highly expressed on target intestinal epithelial cells (enterocytes and goblet cells). A transmembrane serine protease (TMPRSS2) acts synergistically and cleaves the S protein into S1 and S2 subunits, facilitating the host cell's viral entry[15]. Like other viruses such as rotavirus, norovirus, and enteroviruses, the interferon-stimulated genes are activated, resulting in the reinstatement of an antiviral state and restricted SARS-CoV-2 replication[42-44]. Simultaneously, pro-inflammatory cytokines like IL-6, IL-1, and tumor necrosis factor-α (TNF-α) are also released[44]. Severe enterocyte apoptosis follows as soon as the virus overcomes the interferon-mediated antiviral state[16]. The tremendous loss of intestinal epithelial cells diminishes the gut barrier’s integrity and gravely impacts the absorption of nutrients, secretion of mucus, and production of antimicrobial peptides[45]. The elevated secretion of pro-inflammatory cytokines also simultaneously activates macrophages towards pro-inflammatory states, which involves the increased surface expression and later shedding of markers such as CD14 and CD163 that are critical for detecting microorganisms and limiting inflammation[16].
A recent unpublished study that comprehensively investigated changes in gut dynamics during SARS-CoV-2 infection shows that there were increases in permeability of gut tight junctions as estimated using zonulin, microbial translocation, lipid-binding protein, and myeloid activation using soluble CD14[46]. Gut barrier damage, microbial translocation, and myeloid cell activation worsened as the disease transitioned into more severe states. All this was heralded when a two-fold increment in tight junction permeability was observed in individuals who died vs COVID-19 survivors. Unsurprisingly, alterations in gut homeostasis positively correlated with elevated IL-6, thus confirming increased inflammation as a conduit for loss of gut barrier integrity[46]. Lastly, progressive increments of disrupted citrulline metabolism accompanying the severity of SARS-CoV-2 indicate ongoing microbial dysbiosis and impaired intestinal function[47].
An optimal balance between T regs and inflammatory T helper 17 (Th17) cells is maintained within a healthy gut. As inflammation occurs, this balance is tipped as pro-inflammatory bacteria are favored over commensals[37]. These pro-inflammatory bacteria induce the secretion of cytokines like IL-6 and IL-17 that select for increased frequencies of Th17 pro-inflammatory CD4+ T cells and susceptibility to inflammatory conditions such as inflammatory bowel disease (IBD)[37].
An increased expression of SARS-CoV-2 receptors during other inflammatory conditions has been suggested to affect disease pathogenesis. During IBD and Crohn's disease (CD), epithelial ACE2 expression is elevated in the colon and rectum while diminished in the ileum[48]. As anticipated, gut dysbiosis occurs as pathobionts outcompete commensal bacteria, and macrophage populations transition towards inflammatory states where they secrete increased amounts of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-12[49,50]. The resultant inflammatory damage leads to the destruction of tight junctions within the gut barrier. This later leads to leakage of microbial contents into the systemic circulation. Microbial translocation then ensues and later fuels chronic immune activation[49].
Several studies have investigated the co-occurrence of IBD and COVID-19-related illnesses[51-53]. Here, we discuss the possibility of leveraging IBD therapeutics for treating COVID-19-related symptoms. Current IBD treatments can be divided into two broad categories: (1) Non-biologic; and (2) Biologic therapeutics. Examples of non-biologic therapeutics include small molecules corticosteroids, thiopurines, and 5-aminosalicylates. Each of these therapeutics non-specifically targets multiple inflammatory processes.
On the other hand, biologic therapies target specific inflammatory mediators in the gut to modulate their ability to induce signal transduction[41]. Current biologics used to treat IBD include the monoclonal antibodies infliximab, ustekinumab, and vedolizumab. Infliximab and ustekinumab target pro-inflammatory cytokines TNF-α and IL-12/IL-23, respectively. Since these cytokines are not specific to intestinal inflammation, antibodies that inhibit their action can act throughout the periphery. More specific therapies target IBD like vedolizumab function by blocking the gut homing receptor α4β7 integrin, thereby blocking inflammatory lymphocyte trafficking resulting in reduced inflammation[41,54,55]. Similarly, the biologic natalizumab, an anti-α4 integrin antibody, is also used to treat Crohn's disease, albeit less frequently due to an increased risk of progressive multifocal leukoencephalopathy (Table 1)[56].
| Therapy | Target | Impact | Clinical outcome |
| Infliximab[57,58] | TNF-α | Reduced pro-inflammatory response induction and leukocyte migration | Reduced infection rate, symptoms, hospital rate, and mortality |
| Ustekinumab[57] | IL-12/IL-23 | Blocks T cell activation | Possible improved outcome (pooled with other biologics) |
| Vedolizumab[54,57] | α4β7 integrin | Reduced leukocyte trafficking to gut and associated inflammation | Possible improved outcome (pooled with other biologics) |
| Corticosteroids[57,58,63] | Glucocorticoid and mineralocorticoid receptors | Reduced inflammatory response | Better or worse clinical outcomes, depending on the timing |
| Microbiome[176] | Butyrate production | Improved gut barrier integrity and decreased microbial translocation | Reduced inflammatory response |
| Vitamin D[70,72,74] | Th17 cells | Reduced gut and systemic inflammation | Reduced infection risk and enhanced clinical outcomes |
| Nicotine and related agonists[142,152,153] | α7nAChR | Reduced Inflammatory Response | Reduced infection rate |
| Vagus nerve stimulation[151] | α7nAChR | Increased acetylcholine release | Reduced inflammation |
Several studies have suggested that IBD non-biologic therapies may worsen clinical outcomes during COVID-19[57-59]. For example, 5-aminosalicylate and its prodrug form sulfasalazine have increased the risk of SARS-CoV-2 infection and increased the risk of hospitalization and mortality[57-59]. Other immunomodulators include thiopurines, calcineurin inhibitors, and methotrexate. When grouped in meta-analyses, these immunomodulators were also associated with an increased risk of infection, hospitalization, and mortality during SARS-CoV-2 infection[57-59]. The data for corticosteroids is more complicated[57-59]. Owing to their nature as non-selective hormone analogs, corticosteroids target gut inflammation non-specifically, resulting in a dampening of the inflammatory response beyond the intestines. This dampened immune response can therefore inhibit the clearance of replicating virus and prolong the duration of infection. Corticosteroids may further negatively impact clinical outcomes by increasing the pro-thrombotic response to SARS-CoV-2, exacerbating already frequent clotting in intrinsically vascular disease. Several case studies have linked this hypercoagulable state in COVID-19 to worsening in the gut, including ischemic colitis, potentially aggravating an already disrupted gut homeostasis[60,61]. Therefore, it is unsurprising that studies to determine the impact of IBD therapy on COVID-19 have not been pursued. Nonetheless, corticosteroids for IBD are typically administered long-term and precede SARS-CoV-2 infection. Corticosteroids, particularly dexamethasone, reduce mortality in hospitalized SARS-CoV-2 patients receiving oxygen and reduce intensive care unit hospitalization length when given at moderate doses over a short period (6 mg for up to 10 d)[20,62]. In this context, dexamethasone is typically given after the body has begun clearing the virus but before the worst symptoms of COVID-19 due to cytokine storm arise[63]. The difference in infection rate and clinical outcomes for patients receiving treatment before infection suggests that timing is critical for optimizing corticosteroid use.
In addition to modulating immune responses directly, non-biologic medications may influence COVID-19 pathogenesis in other ways. For instance, people receiving non-biologic medications such as corticosteroids, thiopurines, and 5-aminosalicylates have reduced ACE2 and TMPRRS2 expression on enterocytes in the inflamed colon rectum, but not the ileum[48]. This suggests that despite the adverse clinical outcomes with these medications, they may reduce productive gut epithelial infections in people living with IBD. However, these drugs did not cause changes to receptor expression in the uninflamed gut[48]. In contrast, some biologics used to treat IBD have been mostly, though not exclusively, linked to improved clinical outcomes during SARS-CoV-2 infection, offering novel therapeutic interventions. Early studies have shown that the patients administered biologics did not have an increased risk of COVID-19, despite being considered immunocompromised[64,65]. Individual case studies demonstrate that targeting TNF-α with infliximab promises to improve pulmonary symptoms related to COVID-19[66]. Further, an early study grouping multiple biologic therapies showed that clinical outcomes for individuals taking these biologics were five times less likely to be diagnosed with a SARS-CoV-2 infection[67]. However, this analysis was limited due to the small sample size and inability to separate the specific therapies.
A more recent meta-analysis, again grouping different biologics (anti-TNF-α, ustekinumab, and vedolizumab), demonstrated that their use was associated with a risk ratio of hospitalization 0.34 [95% confidence interval (CI) 0.19-0.61], need for intensive care unit 0.49 (95%CI 0.33-0.72), and mortality 0.22 (95%CI 0.13-0.38), suggesting a substantial improvement in clinical outcome[57]. However, one concern is that because people treated with biologics have such muted symptomology, they may serve as silent carriers of SARS-CoV-2[68]. In addition to reducing symptoms, infliximab has also been shown to reduce the expression of ACE2 in the colon, potentially limiting the productive infection of the gut[48]. Because of these findings, a continued study of these monoclonal antibodies is warranted. Luckily, several clinical trials are currently underway, including anti-TNF- α therapies infliximab and adalimumab.
In addition to prescription therapeutics, other recommendations for IBD can also be applied to SARS-CoV-2 infection. COVID-19 pathogenesis has been characterized primarily by dysregulation of the systemic pro-inflammatory response of Th17 cells, particularly during the cytokine storm. This includes decreases in CD4+ T cells expressing Th17 markers CCR6 and CD161, increasing senescence and exhaustion markers CD57 and PD-1[69]. The total number of IL-17A-producing CD4+ and CD8+ T cells was significantly increased despite these findings. These studies suggest that targeting IL-17 may improve clinical outcomes for COVID-19 patients[69].
Further, early during the pandemic, low circulating vitamin D became a known risk factor for SARS-CoV-2 infection, and severe pathogenesis, including associations with significantly higher pro-inflammatory cytokines, including IL-6[70,71] was noted. For this reason, vitamin D supplementation has been recommended for high-risk individuals[70]. Oral vitamin D supplements are absorbed by the small intestine, where they may modulate local Th17 cells, including reduced production of IL-17[72]. In clinical practice, vitamin D is frequently recommended for patients prescribed corticosteroids to manage their IBD symptoms to prevent bone density loss[73]. It is also recommended to reduce or protect against IBD onset of symptoms, in part by suppressing IL-17 production[72]. Further, vitamin D has been linked to increased expression of tight epithelial junctions and blocking apoptosis in the intestine, strengthening gut integrity and preventing bacterial translocation[74,75]. However, whether dampened COVID-19 pathogenesis is linked to inhibition of pro-inflammatory Th17 cells or by a different mechanism remains unknown.
One final concern regarding SARS-CoV-2 during IBD has been the safety and efficacy of vaccination with current therapeutics, many of which are immunosuppressive because so far, phase III clinical trials have excluded patients with IBD[76,77]. Previous studies have found IBD patients treated with anti-TNF-α therapies have a muted response when administered other vaccines (pneumococcal, influenza), leading to further questioning of efficacy[76,78,79]. Despite these concerns, the benefits of vaccination outweigh many of the risks of SARS-CoV-2 infection, and vaccination is likely beneficial for most patients with IBD, even if receiving immunosuppressive therapies[76,80,81]. However, future studies are needed to determine efficacy for this population.
SARS-CoV-2-related microbial dysbiosis manifests the enrichment of pathobionts such as Ruminococcus gnavus, Ruminococcus torques, and Bacteroides dorei (B. dorei) Bacteroides vulgatus. These bacteria have also been implicated in IBD and ulcerative colitis pathogenesis, further strengthening their role in gut dysfunction[82,83]. SARS-CoV-2 dysbiosis was also accompanied by loss of crucial bacteria that have been previously implicated in the lowering of inflammation, such as Bifidobacterium adolescentis, Faecalibacterium prausnitzii (F. prausnitzii), Collinsella aerofaciens, and Eubacterium rectale (E. rectale)[84] (Figure 1). Another study highlighted that Clostridium hathewayi (C. hathewayi), Clostridium ramosum (C. ramosum), and Coprobacillus were linked to COVID-19 severity[85]. Unsurprisingly, these microbial signatures (C. hathewayi, C. ramosum, and Coprobacillus) have also been associated with pathogenic/inflammatory gut conditions such as diarrhea, colitis, IBD, and overall inflammation[86-88]. This situation's gravitas is further supported by observations made in a separate study that showed that the inflammatory species, Proteobacteria, was reported in the guts of COVID-19 patients[89].
Alternatively, several anti-inflammatory bacteria, including F. prausnitzii, B. dorei, Bacteroides thetaiotaomicron, Bacteroides massiliensis, and Bacteroides ovatus were linked to improved clinical outcomes in SARS-CoV-2 patients. Remarkably, Bacteroides were reported to down-regulate ACE2 expression in the gut[85]. The fecal microbiome has also been determined to be dysbiotic for periods as long as 30 d after clearance of SAR-CoV-2 infection, further contributing to the extensive list of health complications reported in COVID long haulers who never wholly recover and recuperate to a healthy state[84,90]. To this effect, it was noted that microbiomes of recovered patients were enriched in Bifidobacterium dentium and Lactobacillus ruminis species and reduced E. rectale, Ruminococcuss bromii, F. prausnitzii, and Bifidobacterium longum[84]. Therapeutics involving antibiotics to stabilize the gut microbiome by eliminating SARS-CoV-2 associated pathobionts revealed that these regimens did not affect the recovery of the microbiome. Therefore, these findings negate this approach as a possible future gut microbial rejuvenation strategy[84]. In contrast, previous studies have shown that probiotics improve immune responses to other respiratory tract viral infections such as Influenza and Rhinoviruses[91,92]. Also, in patients with severe COVID-19 disease who cannot ingest probiotics, the use of butyrate enemas could act as adjunctive therapy for repairing intestinal epithelial cell wall damage, lowering chronic immune activation promoting gut-brain axis function[93].
Analysis of the fungal mycobiome using deep shotgun metagenomic sequencing revealed significant proportions of opportunistic fungal pathogens such as Candida albicans, Candida auris, and Aspergillus flavus were enriched in the gut microbiota of COVID-19 hospitalized patients[94]. Similarly, separate studies have detected Aspergillus in respiratory tract secretions and tracheal aspirates of patients with COVID-19[95,96]. Unsurprisingly, in patients with Aspergillus in their fecal mic
The gut-lung axis has been proposed to be a bi-directional conduit by which each organ can modulate the function and inflammatory state. This includes the production of metabolites such as short-chain fatty acids (SCFAs) by the gut microbiome, lymphocyte trafficking from the lungs or the gut into the periphery where they redirect systemic immune responses, and indirect neuronal innervation via the central nervous system (CNS) and cranial nerves bridging each organ[98,99]. The bi-directionality of the gut-lung axis, while capable of promoting beneficial co
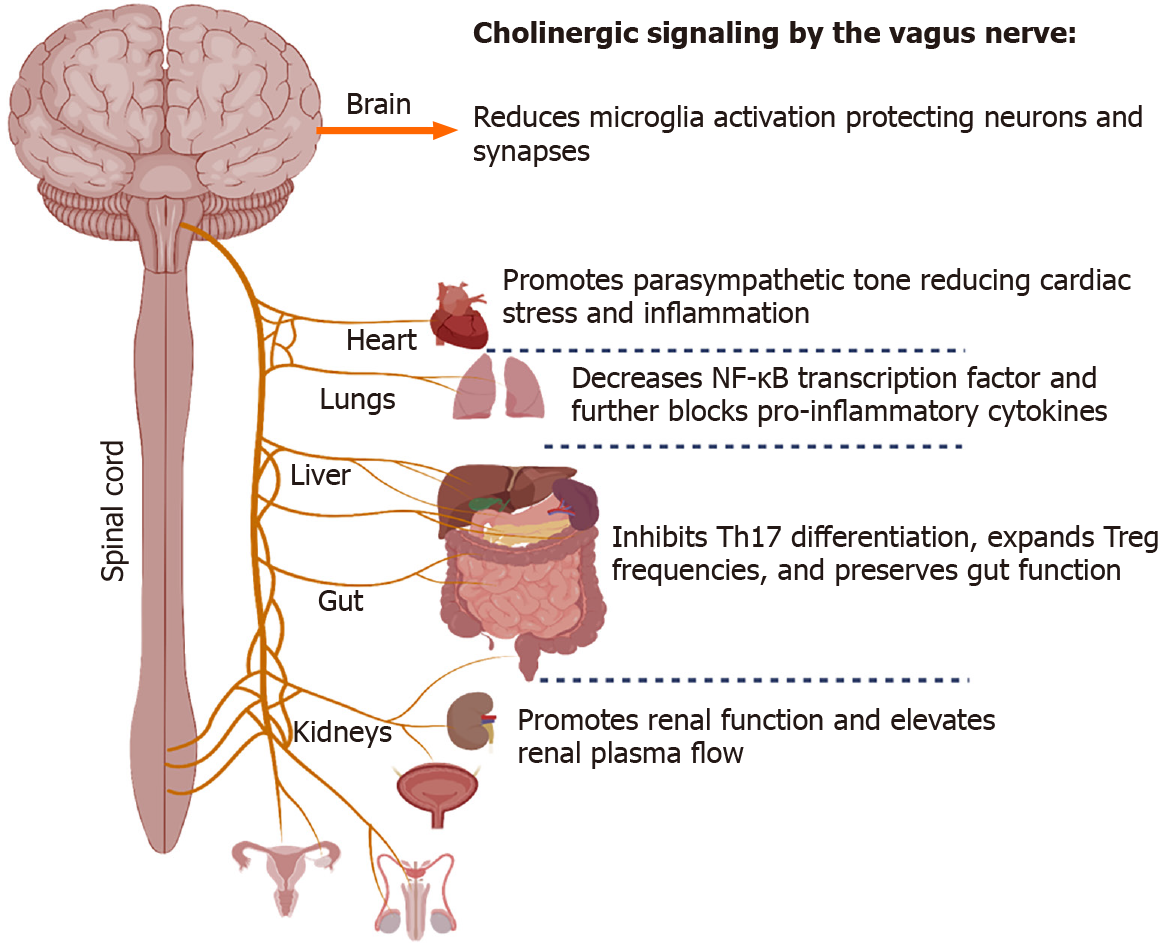
Another branch that extends from the lung-gut axis includes the lung-brain conduit, which proposes to connect severe neurological dysfunction and injury with associated lung injury. In general, CoV-induced inflammation and oxidative stress may represent a crucial mechanism for the onset of neurological symptoms. SARS-CoV-2, in particular, has been shown to have the neuroinvasive potential[104]. The vagus nerve innervates the lung's distal airway, and upon stimulation, nerve endings release acetylcholine (ACh), which functions to regulate lung infection[105] (Figure 2). ACh secreted from neuronal and non-neuronal sources acts to decrease inflammation, and using an Influenza A model, a previous study showed that viral infection caused an influx of cholinergic lymphocytes while inhibiting ACh synthesis resulted in extended pulmonary inflammation, increased inflammatory macrophage activation, and delayed tissue repair (Reviewed in[106]).
Similarly, it was demonstrated that a deficiency to the α7 nicotinic ACh receptor (nAChR) acts to worsen lung infection, inflammation, and injury while increasing circulatory levels of pro-inflammatory cytokines. Additionally, α7 nAChR activation by GTS-21, which enhances the phagocytic ability of hyperoxic macrophages, is a prominent method for improving bacterial clearance and reducing lung injury[107], suggesting an involvement of lung-gut-brain connection. During infection, viruses may breach the CNS by various routes, including the vasculature, olfactory and trigeminal nerves, or the lymphatic system. However, the direct mechanism that SARS-CoV-2 utilizes is unknown[108]. One hypothesis is that SARS-CoV-2 enters the CNS by breaching the olfactory route, thereby circumventing the blood-brain barrier (BBB). More than 80% of COVID-19 patients presented with gustatory and olfactory impairments lending support to this access point[104].
Furthermore, in a cohort of 214 COVID-19 patients, 36.4% developed neurologic symptoms ranging from acute cerebrovascular diseases, consciousness impairment, and skeletal muscle symptoms[109]. As reviewed by Nuzzo and Picone, another study used a cellular model to demonstrate that respiratory syncytial virus infection propagates reactive oxygen species (ROS) production transitively, creating oxidative stress that supports the idea that lung inflammation may be a determinant of systemic oxidative stress[104]. The brain, a primary tissue for metabolizing oxygen, is particularly vulnerable to ROS, and SARS-CoV-2 could generate enough ROS to cause brain injury.
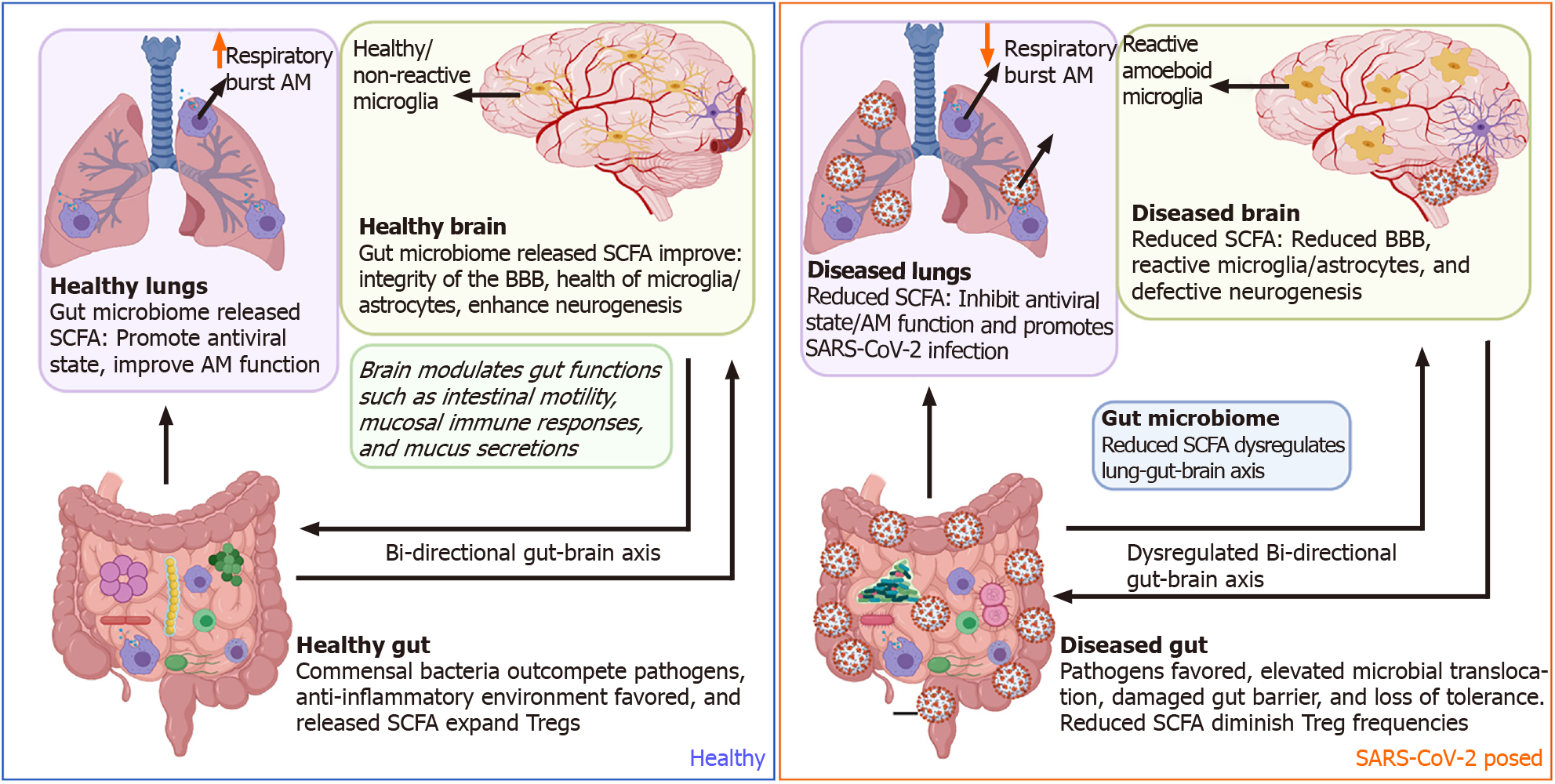
In addition to the host immune system's direct mutual interaction in the gut-brain-lung axis, commensal and pathogenic bacteria in the gut microbiome can also influence COVID-19 pathogenesis. SCFAs such as acetate, butyrate, and propionate, being the most prominent immunomodulatory metabolites, maintain and reinforce intestinal epithelial integrity to decrease inflammation in the gastrointestinal tract and respiratory tract by increasing differentiation and fortifying against tight junction permeability[110]. During SARS-CoV-2 infection, increased permeability was associated with clinical worsening, including multiorgan failure[111]. SCFAs produced by the microbiota influence the inflammatory state of the host's epithelial cells, innate and adaptive immune cells directly or indirectly, and utilize the mesenteric lymphatic system for translocation of intact bacteria, their fragments or metabolites through the intestinal barrier, thereby modulating the lung immune response upon entry to the systemic circulation[112,113]. Additionally, SFCA butyrate suppresses intestinal inflammation by inhibiting histone deacetylase (HDAC) activity. Inhibition of HDAC enhances the mTOR-S6K pathway required for T cell differentiation and cytokine expression and enhances histone 3 acetylation at Foxp3 Locus to allow transcription to occur and Treg cell differentiation[114,115] Further, GPR43 activation by butyrate can inhibit NF-κB activity and stimulate anti-inflammatory signaling, including IL-8 and IL-10[116].
Previous work on other respiratory viruses also documents disruptions to the gut microbiota. Interestingly, SARS-CoV-2 has been reported to deplete the levels of short-chain fatty acid-producing bacteria such as Parabacteriodes merdae, Bacteriodes stercoris, and Lachnospiraceae bacterium[117]. Thus, respiratory influenza can indirectly induce intestinal immune injury and alter gut microbiota, resulting in the outgrowth of Enterobacteriaceae and reduction of Lactobacilli and Lactococci[113]. In the influenza-related lung response in mice, intestinal TLR activation is required for NF-κB-dependent pathways for the innate immune response. In this case, disruptions of the microbiota were unrelated to the influenza virus and mediated by Th17 cells[118].
Similarly, other respiratory infections, such as a respiratory syncytial virus (RSV), distort the gut microbiome's balance by depleting Lachnospiraceae and Lactobacillaceae families while enriching Bacteroidaceae[119]. Experimental studies designed to administer high fiber diets favor the enrichment of short-chain fatty acid butyrate-producing bacteria, thus promoting the interferon antiviral state within the lungs of RSV-infected individuals[120]. In turn, depletion of the gut microbiome has been shown to hinder optimal alveolar macrophages' optimal functionality by reducing oxidative respiratory burst capacity and later diminishing their bactericidal effects[121].
The gut microbiome has been shown to directly modulate multiple brain functions such as immune modalities like inflammation and neuroendocrine states like mood, stress, anxiety, and memory functions[122-125]. Within the gut, two plexuses comprise the enteric nervous system (ENS): one at the submucosa and the other associated with the muscularis propria. Together, these two plexuses sense the inflammatory and local microbial milieu. The ENS can regulate many aspects of CNS health and behavior through the production of inflammatory mediators, hormones, and direct neuronal innervation of the vagus nerve from the medulla oblongata[126,127]. The release of short-chain fatty acids by gut commensal bacteria improves the BBB's integrity, reduces inflammation signaling in microglia/astrocytes, and enhances neurogenesis within neurons. Collectively, this promotes reduced aging, better memory, and improved cognitive development[128].
During gut inflammation, as exemplified with IBD, distorted microbiota populations drive mucosal myeloid cells' activation and subsequent release of pro-inflammatory cytokines that sustain epithelial gut damage. In turn, this activates a cascade of gut pain sensory pathways, dysregulates the enteric nervous system, and simultaneous gut-brain dysfunctions[129-132]. Besides distortion of the lung-gut axis, SARS-CoV-2 dysbiosis of the microbiome could also negatively impact other organs such as the brain. SARS-CoV-2 diminishes crosstalk with the brain by depleting crucial short-chain fatty acid-producing bacteria within the gut microbiome[117] (Figure 3). Furthermore, SARS-CoV-2 has been shown to gain access to the brain by crossing the neural–mucosal interface in olfactory mucosa, where the olfactory mucosal, endothelial, and nervous tissue are closely intertwined[133]. After this, SARS-CoV-2 localizes within the medulla, where respiratory and cardiovascular activities are controlled[133-135]. Thus, this could account for the neurological symptoms such as loss of smell and taste, persistent headaches, confusion, cerebrovascular disease, muscle pain, ataxia, seizures, and dizziness observed in COVID-19 patients[136]. The CNS also supports gut function making the gut-brain axis bi-directional[137,138]. However, the impact of SARS-CoV-2 pathology within the CNS on the direction of gut functions such as peristalsis, modulation of gut immune responses, and directing digestion is yet to be fully comprehended[139].
Several epidemiological studies from the United States, Europe, and south and east Asia have concluded that tobacco smokers have a lower risk of SARS-CoV-2 infection[140-144]. Additionally, smokers may also have a reduced risk of significant symptoms once infected with SARS-CoV-2, despite typically experiencing increased morbidity and mortality during other respiratory infections[142]. Nicotine is the receptor agonist for nAChRs that contribute to tobacco smoke's addictive and psychoactive properties. In contrast to exogenous nicotine, the endogenous agonist for nAChRs, ACh is produced by neurons and lymphocytes, an essential neuro-immune modulator. The α7nAChR, in particular, regulates the cholinergic anti-inflammatory response (CAIP)[145]. During acute inflammation, efferent signals from the CNS induce the release of ACh at the enteric nervous system, where they modulate T-cell dynamics, including the induction of Tregs and inhibition of Th17 cells and suppression of local inflammation, including the releases of TNF-α, IL-1β, and HMGB1[146,147]. One hypothesis for nicotine's inhibitory effects is that exogenous α7nAChR agonists recapitulate the effects of ACh release by the vagus nerve, thereby reducing cytokine storm risk both locally at the inhalation site (the lungs) and systemically, including in the gut. This mechanism may be a likely reason nicotine improves ulcerative colitis symptoms[146,148]. The argument for cholinergic modulation is made even more compelling when considering that neither smoking nor nicotine consumption decreases ACE2 or TMPRSS2 and may increase their expression in the lower airways[149,150]. COVID-19 patients should not be encouraged to smoke or consume nicotine. However, alternative methods of modulating the CAIP have been suggested. Vagus nerve stimulation (VNS) delivers electrical impulses to the left vagus nerve via a small device implanted below the collar bone currently used to treat treatment-resistant epilepsy and major depressive disorder. However, less-invasive transcutaneous auricular VNS is being developed. Although more extensive clinical studies are ongoing, case studies have indicated possible benefits in reducing plasma IL-6 levels when using these devices[151]. Alternatively, α7nAChR agonists or acetylcholinesterase inhibitors have been proposed to reduce systemic inflammation[152,153].
A recent report describing ACE2 and TMPRSS2 expression during chronic vagus nerve stimulation showed no changes in either protein or enterocytes[154], although each gene was strongly correlated with CHRNA7 gene expression RNA-seq[154]. Another report separately described how the spike glycoprotein's receptor-binding domain on SARS-CoV-2 possesses significant structural homology with α-bun
It should be noted that in addition to a disruption to its anti-inflammatory functions, the vagus nerve may also contribute to the COVID-19 pathology in other ways. During Parkinson's disease, α-synuclein aggregates travel from the gut to the CNS in the vagus nerve via retrograde axonal transport[158]. The innervation of both the lungs and gut, where significant viral replication occurs, may allow a similar direct transport to the CNS[159]. This hypothesis is corroborated by the recent reports, which showed that the brain stem has an increased inflammatory response during COVID-19 compared with other brain regions. Where cranial nerves IX and X (glossopharyngeal and vagus nerves) meet, the medulla oblongata, in particular, has a higher viral load than the rest of the CNS[160]. The axonal and synaptic loss at the dorsal motor nucleus and nucleus solitarius near this region may be contributing to the loss of autonomic control of respiratory function, cardiac arrhythmias, and gastrointestinal symptoms like diarrhea and vomiting[161,162]. Complicating the role of nicotine in COVID-19 pathogenesis, its administration can inhibit Th17-driven neuroinflammation, thereby possibly protecting these regions in smokers[163]. A better understanding of this process, if it exists, could contribute significantly to minimizing neuropathogenesis during SARS-CoV-2 infection.
SARS-CoV-2 infection in the gut leads to alterations in gut microbiota with depletion of bacterial genera that produce crucial metabolites such as short-chain fatty acids that sustain optimal brain function. Future studies focusing on using probiotics to replenish commensals' dwindling populations as a strategy to reverse gut dysbiosis while maintaining the gut-brain axis during COVID-19 are needed[164]. Furthermore, additional efforts should be applied to the testing strategies currently used to manage inflammatory conditions affecting the gut for their potential in the direction of COVID-19 associated dysbiosis, gut damage, and microbial translocation. Targeting vagal stimulation and diverse nicotinic receptors could offer lower organ inflammation and confer systemic protection across the gut-brain and lung organs.
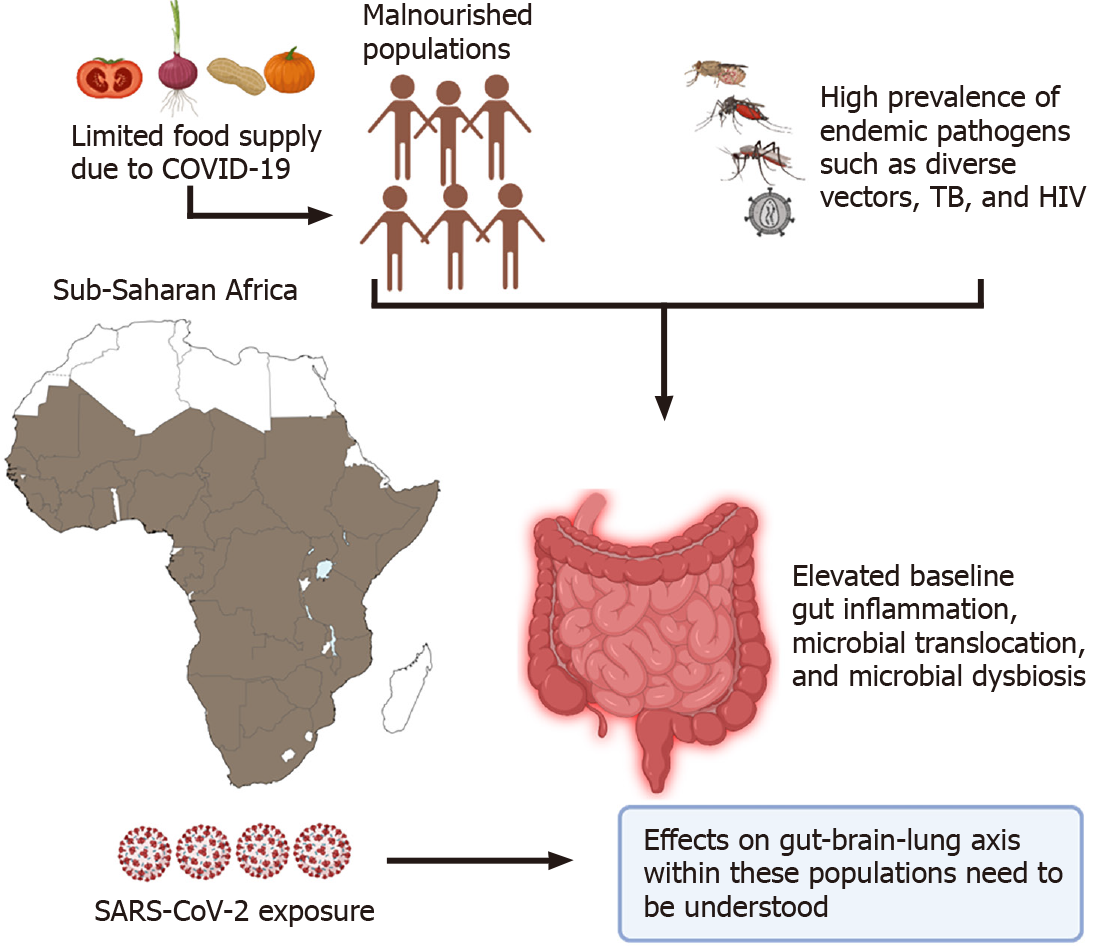
Lastly, special consideration should be given to populations worldwide where there is a high incidence of coinfections such as HIV and tuberculosis (TB) that target the gut early on in infection, cause dysbiosis and lead to microbial translocation[165-167]. In the world's poorest regions like Sub-Saharan Africa, most individuals are continuously exposed to endemic pathogens like helminths and TB that could lead to elevated baseline levels of gut damage, immune activation, and possible microbial translocation[168-171]. Recently, Bhaskaran et al[172] analyzed data from 27480 individuals co-infected with HIV within the United Kingdom. Their data revealed that people living with HIV had a higher risk of COVID-19 death than those without HIV, after adjusting for the age and sex with a hazard ratio (HR) of 290 (95%CI 0.196-0.430; P < 0.0001). However, the absence of testing, limited case reporting, and poor health care limits our understanding of the SARS-CoV-2 pandemic on this continent[172].
The sudden lifestyle changes imparted by the pandemic within these populations have tremendously strained their chances of survival. In countries where restrictions or lockdowns are enforced, most individuals from these regions rely on the government to supply free food rations[173]. However, the low quality of this freely distributed food fuels the already highly prevalent risk of malnutrition within these populations[174]. As we have illustrated, limited dietary access within these co
In these trying times of the COVID-19 pandemic, the connotation of a healthy gut-healthy mind could translate into one's ability to orchestrate a meaningful immune response that could limit SARS-CoV-2 infection and maintain systemic homeostasis and diminish side effects from virus exposure. Leveraging therapies currently being used to improve gut health could be exploited for better disease outcomes during severe disease. Additional studies on the gut-brain axis, particularly from diverse populations across the world, are warranted.
Manuscript source: Invited manuscript
Specialty type: Virology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yuan J S-Editor: Wu YXJ L-Editor: A P-Editor: Li X
| 1. | Unwin HJT, Mishra S, Bradley VC, Gandy A, Mellan TA, Coupland H, Ish-Horowicz J, Vollmer MAC, Whittaker C, Filippi SL, Xi X, Monod M, Ratmann O, Hutchinson M, Valka F, Zhu H, Hawryluk I, Milton P, Ainslie KEC, Baguelin M, Boonyasiri A, Brazeau NF, Cattarino L, Cucunuba Z, Cuomo-Dannenburg G, Dorigatti I, Eales OD, Eaton JW, van Elsland SL, FitzJohn RG, Gaythorpe KAM, Green W, Hinsley W, Jeffrey B, Knock E, Laydon DJ, Lees J, Nedjati-Gilani G, Nouvellet P, Okell L, Parag KV, Siveroni I, Thompson HA, Walker P, Walters CE, Watson OJ, Whittles LK, Ghani AC, Ferguson NM, Riley S, Donnelly CA, Bhatt S, Flaxman S. State-level tracking of COVID-19 in the United States. Nat Commun. 2020;11:6189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Carvalho VM, Hansen S, Ortiz A, Garcia JR, Rodrigo T, Rodriguez Mora S, Ruiz de Aguirre P. Tracking the COVID-19 crisis with high-resolution transaction data. 2020 April 16 [cited Feb 1, 2021]. In: BBVA Research [Internet]. Available from: https://www.bbvaresearch.com/en/publicaciones/tracking-the-covid-19-crisis-with-high-resolution-transaction-data/. [Cited in This Article: ] |
| 3. | Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, Ganesh S, Varsavsky T, Cardoso MJ, El-Sayed Moustafa JS, Visconti A, Hysi P, Bowyer RCE, Mangino M, Falchi M, Wolf J, Ourselin S, Chan AT, Steves CJ, Spector TD. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1095] [Cited by in F6Publishing: 829] [Article Influence: 207.3] [Reference Citation Analysis (0)] |
| 4. | Chong YY, Cheng HY, Chan HYL, Chien WT, Wong SYS. COVID-19 pandemic, infodemic and the role of eHealth literacy. Int J Nurs Stud. 2020;108:103644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | COVID Data Tracker. [cited July 15, 2021]. In: Centers for Disease Control and Prevention (CDC) [Internet]. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home. [Cited in This Article: ] |
| 6. | 2020 Global Nutrition Report. 2020 [cited Feb 1, 2021]. In: United Nations International Children’s Emergency Fund (UNICEF) [Internet]. Available from: https://globalnutritionreport.org/reports/2020-global-nutrition-report/. [Cited in This Article: ] |
| 7. | Belanger MJ, Hill MA, Angelidi AM, Dalamaga M, Sowers JR, Mantzoros CS. Covid-19 and Disparities in Nutrition and Obesity. N Engl J Med. 2020;383:e69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 8. | WHO Coronavirus (COVID-19) Dashboard 2021. [cited July 15, 2021]. In: World Health Organization [Internet]. Available from: https://covid19.who.int/. [Cited in This Article: ] |
| 9. | Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nature Rev Microbiol. 2020;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2083] [Cited by in F6Publishing: 2519] [Article Influence: 839.7] [Reference Citation Analysis (0)] |
| 10. | Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2713] [Cited by in F6Publishing: 2658] [Article Influence: 664.5] [Reference Citation Analysis (0)] |
| 11. | Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 429] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 12. | Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 617] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 13. | Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB, Diamond MS, Ciorba MA, Whelan SPJ, Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 768] [Cited by in F6Publishing: 701] [Article Influence: 175.3] [Reference Citation Analysis (0)] |
| 14. | Segal JP, Mak JWY, Mullish BH, Alexander JL, Ng SC, Marchesi JR. The gut microbiome: an under-recognised contributor to the COVID-19 pandemic? Therap Adv Gastroenterol. 2020;13:1756284820974914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1985] [Cited by in F6Publishing: 1903] [Article Influence: 475.8] [Reference Citation Analysis (1)] |
| 16. | Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1245] [Cited by in F6Publishing: 1186] [Article Influence: 296.5] [Reference Citation Analysis (0)] |
| 17. | Lee JJ, Kopetz S, Vilar E, Shen JP, Chen K, Maitra A. Relative Abundance of SARS-CoV-2 Entry Genes in the Enterocytes of the Lower Gastrointestinal Tract. Genes (Basel). 2020;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7635] [Cited by in F6Publishing: 5811] [Article Influence: 1452.8] [Reference Citation Analysis (0)] |
| 19. | Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Translation Res. 2020;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 186] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 20. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1160] [Cited by in F6Publishing: 1137] [Article Influence: 284.3] [Reference Citation Analysis (0)] |
| 21. | Hillman ET, Kozik AJ, Hooker CA, Burnett JL, Heo Y, Kiesel VA, Nevins CJ, Oshiro JMKI, Robins MM, Thakkar RD, Wu ST, Lindemann SR. Comparative genomics of the genus Roseburia reveals divergent biosynthetic pathways that may influence colonic competition among species. Microb Genom. 2020;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3643] [Cited by in F6Publishing: 3934] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 23. | Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, Spadaccini M, Colombo M, Gabbiadini R, Artifon ELA, Repici A, Sharma P. Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2011335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 269] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 24. | Perlot T, Penninger JM. ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 25. | Zhang YB, Liu SJ, Hu ZD, Zhou JX, Wang YZ, Fang B, Wong KW, Xia F. Increased Th17 activation and gut microbiota diversity are associated with pembrolizumab-triggered tuberculosis. Cancer Immunol Immunother. 2020;69:2665-2671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | News and Highlights. Mucosal Immunol. 2008;1:246-247. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Ochoa-Repáraz J, Kasper LH. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr Obes Rep. 2016;5:51-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Johnson A. Gut feelings: the world of the second brain. Lancet Gastroenterol Hepatol. 2018;3:536. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Onal EM, Afsar B, Covic A, Vaziri ND, Kanbay M. Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens Res. 2019;42:123-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Nagatomo Y, Tang WH. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J Card Fail. 2015;21:973-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 31. | Van Kaer L, Olivares-Villagómez D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J Immunol. 2018;200:2235-2244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Commins SP. Mechanisms of Oral Tolerance. Pediatr Clin North Am. 2015;62:1523-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, Klimešová K, Přibylová J, Bártová J, Sanchez D, Fundová P, Borovská D, Srůtková D, Zídek Z, Schwarzer M, Drastich P, Funda DP. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 473] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 34. | Isaacs-Ten A, Echeandia M, Moreno-Gonzalez M, Brion A, Goldson A, Philo M, Patterson AM, Parker A, Galduroz M, Baker D, Rushbrook SM, Hildebrand F, Beraza N. Intestinal Microbiome-Macrophage Crosstalk Contributes to Cholestatic Liver Disease by Promoting Intestinal Permeability in Mice. Hepatology. 2020;72:2090-2108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Ma H, Tao W, Zhu S. T lymphocytes in the intestinal mucosa: defense and tolerance. Cell Mol Immunol. 2019;16:216-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 36. | Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, Mackay CR. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016;15:2809-2824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 402] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 37. | Omenetti S, Pizarro TT. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front Immunol. 2015;6:639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 304] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 38. | Wang S, Ye Q, Zeng X, Qiao S. Functions of Macrophages in the Maintenance of Intestinal Homeostasis. J Immunol Res. 2019;2019:1512969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 39. | Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 765] [Cited by in F6Publishing: 774] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 40. | Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 41. | Ansari AA, Byrareddy SN. The Role of Integrin Expressing Cells in Modulating Disease Susceptibility and Progression (January 2016). Int Trends Immun. 2016;4:11-27. [PubMed] [Cited in This Article: ] |
| 42. | Good C, Wells AI, Coyne CB. Type III interferon signaling restricts enterovirus 71 infection of goblet cells. Sci Adv. 2019;5:eaau4255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Drummond CG, Bolock AM, Ma C, Luke CJ, Good M, Coyne CB. Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. Proc Natl Acad Sci USA. 2017;114:1672-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 44. | Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036-1045.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3305] [Cited by in F6Publishing: 2882] [Article Influence: 720.5] [Reference Citation Analysis (0)] |
| 45. | Patankar JV, Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol. 2020;17:543-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 46. | Giron LB, Dweep H, Yin X, Wang H, Damra M, Goldman AR, Gorman N, Palmer CS, Tang HY, Shaikh MW, Forsyth CB, Balk RA, Zilberstein NF, Liu Q, Kossenkov A, Keshavarzian A, Landay A, Abdel-Mohsen M. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front Immunol. 2021;12:686240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 47. | Kao CC, Cope JL, Hsu JW, Dwarkanath P, Karnes JM, Luna RA, Hollister EB, Thame MM, Kurpad AV, Jahoor F. The Microbiome, Intestinal Function, and Arginine Metabolism of Healthy Indian Women Are Different from Those of American and Jamaican Women. J Nutr. 2015;146:706-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Suárez-Fariñas M, Tokuyama M, Wei G, Huang R, Livanos A, Jha D, Levescot A, Irizar H, Kosoy R, Cording S, Wang W, Losic B, Ungaro RC, Di'Narzo A, Martinez-Delgado G, Suprun M, Corley MJ, Stojmirovic A, Houten SM, Peters L, Curran M, Brodmerkel C, Perrigoue J, Friedman JR, Hao K, Schadt EE, Zhu J, Ko HM, Cho J, Dubinsky MC, Sands BE, Ndhlovu L, Cerf-Bensusan N, Kasarskis A, Colombel JF, Harpaz N, Argmann C, Mehandru S. Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps With the Pathogenesis of SARS-CoV-2-related Disease. Gastroenterology. 2021;160:287-301.e20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 49. | Vrakas S, Mountzouris KC, Michalopoulos G, Karamanolis G, Papatheodoridis G, Tzathas C, Gazouli M. Intestinal Bacteria Composition and Translocation of Bacteria in Inflammatory Bowel Disease. PLoS One. 2017;12:e0170034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 50. | Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16:531-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 51. | Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 52. | Gajendran M, Perisetti A, Aziz M, Raghavapuram S, Bansal P, Tharian B, Goyal H. Inflammatory bowel disease amid the COVID-19 pandemic: impact, management strategies, and lessons learned. Ann Gastroenterol. 2020;33:591-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Jablaoui A, Kriaa A, Mkaouar H, Akermi N, Soussou S, Wysocka M, Wołoszyn D, Amouri A, Gargouri A, Maguin E, Lesner A, Rhimi M. Fecal Serine Protease Profiling in Inflammatory Bowel Diseases. Front Cell Infect Microbiol. 2020;10:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 54. | Arseneau KO, Cominelli F. Targeting leukocyte trafficking for the treatment of inflammatory bowel disease. Clin Pharmacol Ther. 2015;97:22-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Arthos J, Cicala C, Nawaz F, Byrareddy SN, Villinger F, Santangelo PJ, Ansari AA, Fauci AS. The Role of Integrin α4β7 in HIV Pathogenesis and Treatment. Curr HIV/AIDS Rep. 2018;15:127-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Armuzzi A, Felice C. Natalizumab in Crohn's disease: past and future areas of applicability. Ann Gastroenterol. 2013;26:189-190. [PubMed] [Cited in This Article: ] |
| 57. | Singh AK, Jena A, Kumar-M P, Sharma V, Sebastian S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. United European Gastroenterol J. 2021;9:159-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 58. | Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier J-F, Reinisch W, Ruemmele FM, Steinwurz F, Underwood FE, Zhang X, Colombel J-F, Kappelman MD. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159:481-491.e483. [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 535] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 59. | Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70:725-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 212] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 60. | Chan KH, Lim SL, Damati A, Maruboyina SP, Bondili L, Abu Hanoud A, Slim J. Coronavirus disease 2019 (COVID-19) and ischemic colitis: An under-recognized complication. Am J Emerg Med. 2020;38:2758.e1-2758.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | González Lázaro P, Lomas Meneses A, Del Val Zaballos F, Morandeira Rivas A. Ischemic colitis and short bowel disease due to choronavirus disease 2019 (COVID 19). Clin Nutr ESPEN. 2020;40:406-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | RECOVERY Collaborative Group. , Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6502] [Cited by in F6Publishing: 6616] [Article Influence: 2205.3] [Reference Citation Analysis (1)] |
| 63. | Matthay MA, Thompson BT. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020;8:1170-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 64. | Aziz M, Fatima R, Haghbin H, Lee-Smith W, Nawras A. The Incidence and Outcomes of COVID-19 in IBD Patients: A Rapid Review and Meta-analysis. Inflamm Bowel Dis. 2020;26:e132-e133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 65. | Marafini I, Salvatori S, Sena G, Calabrese E, Biancone L, Monteleone G. Low frequency of COVID-19 in inflammatory bowel diseases. Dig Liver Dis. 2020;52:1234-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Bezzio C, Manes G, Bini F, Pellegrini L, Saibeni S. Infliximab for severe ulcerative colitis and subsequent SARS-CoV-2 pneumonia: a stone for two birds. Gut. 2021;70:623-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 67. | Bezzio C, Pellegrini L, Manes G, Arena I, Picascia D, Della Corte C, Devani M, Schettino M, Saibeni S. Biologic Therapies May Reduce the Risk of COVID-19 in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:e107-e109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Baradaran Ghavami SH, Shahrokh SH, Hossein-Khannazer N, Shpichka A, Asadzadeh Aghdaei H, Timashev P, Vosough M. IBD Patients Could Be Silent Carriers for Novel Coronavirus and Less Prone to its Severe Adverse Events: True or False? Cell J. 2020;22:151-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 69. | De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, Gozzi L, Iannone A, Lo Tartaro D, Mattioli M, Paolini A, Menozzi M, Milić J, Franceschi G, Fantini R, Tonelli R, Sita M, Sarti M, Trenti T, Brugioni L, Cicchetti L, Facchinetti F, Pietrangelo A, Clini E, Girardis M, Guaraldi G, Mussini C, Cossarizza A. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11:3434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 540] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 70. | Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10:20191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 71. | Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1018] [Cited by in F6Publishing: 1039] [Article Influence: 259.8] [Reference Citation Analysis (0)] |
| 72. | Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285:38751-38755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 73. | Fletcher J, Cooper SC, Ghosh S, Hewison M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 74. | Lee C, Lau E, Chusilp S, Filler R, Li B, Zhu H, Yamoto M, Pierro A. Protective effects of vitamin D against injury in intestinal epithelium. Pediatr Surg Int. 2019;35:1395-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | He L, Liu T, Shi Y, Tian F, Hu H, Deb DK, Chen Y, Bissonnette M, Li YC. Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology. 2018;159:967-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | D'Amico F, Rabaud C, Peyrin-Biroulet L, Danese S. SARS-CoV-2 vaccination in IBD: more pros than cons. Nat Rev Gastroenterol Hepatol. 2021;18:211-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 77. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10556] [Cited by in F6Publishing: 9250] [Article Influence: 2312.5] [Reference Citation Analysis (1)] |
| 78. | Fiorino G, Peyrin-Biroulet L, Naccarato P, Szabò H, Sociale OR, Vetrano S, Fries W, Montanelli A, Repici A, Malesci A, Danese S. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2012;18:1042-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 79. | Mamula P, Markowitz JE, Piccoli DA, Klimov A, Cohen L, Baldassano RN. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:851-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 80. | Siegel CA, Melmed GY, McGovern DPB, Rai V, Krammer F, Rubin DT, Abreu MT, Dubinsky MC. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70:635-640. [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 132] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 81. | Alexander JL, Moran GW, Gaya DR, Raine T, Hart A, Kennedy NA, Lindsay JO, MacDonald J, Segal JP, Sebastian S, Selinger CP, Parkes M, Smith PJ, Dhar A, Subramanian S, Arasaradnam R, Lamb CA, Ahmad T, Lees CW, Dobson L, Wakeman R, Iqbal TH, Arnott I, Powell N; Inflammatory Bowel Disease section of the British Society of Gastroenterology and the the Inflammatory Bowel Disease Clinical Research Group. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol Hepatol. 2021;6:218-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 82. | Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 488] [Article Influence: 48.8] [Reference Citation Analysis (1)] |
| 83. | Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn's disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci USA. 2019;116:12672-12677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 84. | Yeoh YK, Zuo T, Lui GC-Y, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS-C, Chow KM, Ng SSS, Li TC-M, Ng RW, Yip TC, Wong GL-H, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698-706. [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 667] [Article Influence: 222.3] [Reference Citation Analysis (0)] |
| 85. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944-955.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 896] [Article Influence: 224.0] [Reference Citation Analysis (0)] |
| 86. | Manzoor SE, McNulty CAM, Nakiboneka-Ssenabulya D, Lecky DM, Hardy KJ, Hawkey PM. Investigation of community carriage rates of Clostridium difficile and Hungatella hathewayi in healthy volunteers from four regions of England. J Hosp Infect. 2017;97:153-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Du Z, Hudcovic T, Mrazek J, Kozakova H, Srutkova D, Schwarzer M, Tlaskalova-Hogenova H, Kostovcik M, Kverka M. Development of gut inflammation in mice colonized with mucosa-associated bacteria from patients with ulcerative colitis. Gut Pathog. 2015;7:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Nishino K, Imaeda H, Sakai S, Ohno M, Nishida A, Andoh A. The Abundance of Clostridium Hathewayi, a Potent Inducer of T Helper 17 (Th17) Cells, is Associated with the Disease Severity of Crohn's Disease. Gastroenterology. 2017;152:S993. [Cited in This Article: ] |
| 89. | Carvelli J, Demaria O, Vély F, Batista L, Chouaki Benmansour N, Fares J, Carpentier S, Thibult ML, Morel A, Remark R, André P, Represa A, Piperoglou C; Explore COVID-19 IPH group; Explore COVID-19 Marseille Immunopole group; Cordier PY, Le Dault E, Guervilly C, Simeone P, Gainnier M, Morel Y, Ebbo M, Schleinitz N, Vivier E. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020;588:146-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 331] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 90. | Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 91. | Zelaya H, Alvarez S, Kitazawa H, Villena J. Respiratory Antiviral Immunity and Immunobiotics: Beneficial Effects on Inflammation-Coagulation Interaction during Influenza Virus Infection. Front Immunol. 2016;7:633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 92. | Turner RB, Woodfolk JA, Borish L, Steinke JW, Patrie JT, Muehling LM, Lahtinen S, Lehtinen MJ. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection - a randomised controlled trial. Benef Microbes. 2017;8:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 93. | Archer DL, Kramer DC. The Use of Microbial Accessible and Fermentable Carbohydrates and/or Butyrate as Supportive Treatment for Patients With Coronavirus SARS-CoV-2 Infection. Front Med (Lausanne). 2020;7:292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Zuo T, Zhan H, Zhang F, Liu Q, Tso EYK, Lui GCY, Chen N, Li A, Lu W, Chan FKL, Chan PKS, Ng SC. Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge. Gastroenterology. 2020;159:1302-1310.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 95. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6231] [Cited by in F6Publishing: 6354] [Article Influence: 1588.5] [Reference Citation Analysis (0)] |
| 96. | Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati F, Le Hingrat Q, Enouf V, Houhou-Fidouh N, Valette M, Mailles A, Lucet JC, Mentre F, Duval X, Descamps D, Malvy D, Timsit JF, Lina B, van-der-Werf S, Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 783] [Cited by in F6Publishing: 750] [Article Influence: 187.5] [Reference Citation Analysis (0)] |
| 97. | Baba R, Takaoka H, Kamo T, Arai D, Takahashi H, Masaki K, Shinoda Y, Hagiwara S, Fukunaga K, Nakachi I. Clinical interpretations and therapeutic significance of isolating aspergillus species from respiratory specimens. Am J Respir Crit Care Med. 2020;201:A2117. [DOI] [Cited in This Article: ] |
| 98. | Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 622] [Cited by in F6Publishing: 798] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 99. | Magnotti LJ, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg. 1999;134:1333-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Ahlawat S, Asha, Sharma KK. Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res. 2020;286:198103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 101. | Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319:G245-G252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 102] [Article Influence: 25.5] [Reference Citation Analysis (2)] |
| 102. | Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2397-2410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 103. | Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 954] [Cited by in F6Publishing: 908] [Article Influence: 227.0] [Reference Citation Analysis (1)] |
| 104. | Nuzzo D, Picone P. Potential neurological effects of severe COVID-19 infection. Neurosci Res. 2020;158:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 105. | Huang Y, Zhao C, Su X. Neuroimmune regulation of lung infection and inflammation. QJM. 2019;112:483-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Horkowitz AP, Schwartz AV, Alvarez CA, Herrera EB, Thoman ML, Chatfield DA, Osborn KG, Feuer R, George UZ, Phillips JA. Acetylcholine Regulates Pulmonary Pathology During Viral Infection and Recovery. Immunotargets Ther. 2020;9:333-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 107. | Sitapara RA, Antoine DJ, Sharma L, Patel VS, Ashby CR Jr, Gorasiya S, Yang H, Zur M, Mantell LL. The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function. Mol Med. 2014;20:238-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses. 2019;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 590] [Cited by in F6Publishing: 650] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 109. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4761] [Cited by in F6Publishing: 4395] [Article Influence: 1098.8] [Reference Citation Analysis (0)] |
| 110. | Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. Fiber-utilizing capacity varies in Prevotella- vs Bacteroides-dominated gut microbiota. Sci Rep. 2017;7:2594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 111. | Uzzan M, Corcos O, Martin JC, Treton X, Bouhnik Y. Why is SARS-CoV-2 infection more severe in obese men? Med Hypotheses. 2020;144:110023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Bingula R, Filaire M, Radosevic-Robin N, Bey M, Berthon JY, Bernalier-Donadille A, Vasson MP, Filaire E. Desired Turbulence? J Oncol. 2017;2017:5035371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 113. | Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 456] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 114. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2937] [Cited by in F6Publishing: 3373] [Article Influence: 306.6] [Reference Citation Analysis (0)] |
| 115. | Husain A, Byrareddy SN. Rapamycin as a potential repurpose drug candidate for the treatment of COVID-19. Chem Biol Interact. 2020;331:109282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 116. | Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 248] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 117. | Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, Chen Z, Boon SS, Chan FK, Chan PK, Ng SC. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 238] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 118. | Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108:5354-5359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 967] [Cited by in F6Publishing: 1037] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 119. | Groves HT, Higham SL, Moffatt MF, Cox MJ, Tregoning JS. Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. mBio. 2020;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 120. | Antunes KH, Fachi JL, de Paula R, da Silva EF, Pral LP, Dos Santos AÁ, Dias GBM, Vargas JE, Puga R, Mayer FQ, Maito F, Zárate-Bladés CR, Ajami NJ, Sant'Ana MR, Candreva T, Rodrigues HG, Schmiele M, Silva Clerici MTP, Proença-Modena JL, Vieira AT, Mackay CR, Mansur D, Caballero MT, Marzec J, Li J, Wang X, Bell D, Polack FP, Kleeberger SR, Stein RT, Vinolo MAR, de Souza APD. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat Commun. 2019;10:3273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 121. | Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun. 2014;82:4596-4606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 122. | Liu L, Zhu G. Gut-Brain Axis and Mood Disorder. Front Psychiatry. 2018;9:223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 123. | Bioque M, González-Rodríguez A, Garcia-Rizo C, Cobo J, Monreal JA, Usall J, Soria V; PNECAT Group; Labad J. Targeting the microbiome-gut-brain axis for improving cognition in schizophrenia and major mood disorders: A narrative review. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 124. | Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, Li Z, Giovannoni F, Batterman KV, Scalisi G, Zandee SEJ, Heck ES, Alsuwailm M, Rosene DL, Becher B, Chiu IM, Prat A, Quintana FJ. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature. 2021;590:473-479. [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 159] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 125. | Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1548] [Cited by in F6Publishing: 1648] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 126. | Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17:338-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 250] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 127. | Sharkey KA, Beck PL, McKay DM. Neuroimmunophysiology of the gut: advances and emerging concepts focusing on the epithelium. Nat Rev Gastroenterol Hepatol. 2018;15:765-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 128. | Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 780] [Cited by in F6Publishing: 1005] [Article Influence: 251.3] [Reference Citation Analysis (0)] |
| 129. | Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 130. | Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39:1033-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 131. | Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20:14105-14125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 215] [Cited by in F6Publishing: 195] [Article Influence: 19.5] [Reference Citation Analysis (2)] |
| 132. | Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 133. | Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 799] [Cited by in F6Publishing: 796] [Article Influence: 265.3] [Reference Citation Analysis (0)] |
| 134. | Deigendesch N, Sironi L, Kutza M, Wischnewski S, Fuchs V, Hench J, Frank A, Nienhold R, Mertz KD, Cathomas G, Matter MS, Siegemund M, Tolnay M, Schirmer L, Pröbstel AK, Tzankov A, Frank S. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 2020;140:583-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 135. | Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH, de Bree GJ, Bulle EB, Aronica EM, Florquin S, Fronczek J, Heunks LMA, de Jong MD, Guo L, du Long R, Lutter R, Molenaar PCG, Neefjes-Borst EA, Niessen HWM, van Noesel CJM, Roelofs JJTH, Snijder EJ, Soer EC, Verheij J, Vlaar APJ, Vos W, van der Wel NN, van der Wal AC, van der Valk P, Bugiani M. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290-e299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 358] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 136. | Acharya A, Kevadiya BD, Gendelman HE, Byrareddy SN. SARS-CoV-2 Infection Leads to Neurological Dysfunction. J Neuroimmune Pharmacol. 2020;15:167-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 137. | Bercik P, Collins S, Verdu E. Microbes and the gut‐brain axis. Neurogastroenterol Motil. 2012;24:405-413. [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 138. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] [Cited in This Article: ] |
| 139. | JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [DOI] [Cited in This Article: ] [Cited by in Crossref: 856] [Cited by in F6Publishing: 922] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 140. | Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 216] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 141. | Saurabh S, Verma MK, Gautam V, Kumar N, Jain V, Goel AD, Gupta MK, Sharma PP, Bhardwaj P, Singh K, Nag VL, Garg MK, Misra S. Tobacco, alcohol use and other risk factors for developing symptomatic COVID-19 vs asymptomatic SARS-CoV-2 infection: a case-control study from western Rajasthan, India. Trans R Soc Trop Med Hyg. 2021;115:820-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 142. | Farsalinos K, Bagos PG, Giannouchos T, Niaura R, Barbouni A, Poulas K. Smoking prevalence among hospitalized COVID-19 patients and its association with disease severity and mortality: an expanded re-analysis of a recent publication. Harm Reduct J. 2021;18:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 143. | Meini S, Fortini A, Andreini R, Sechi LA, Tascini C. The Paradox of the Low Prevalence of Current Smokers Among Covid-19 Patients Hospitalized in Non-Intensive Care Wards: Results From an Italian Multicenter Case-Control Study. Nicotine Tob Res. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 144. | Gonzalez-Rubio J, Navarro-Lopez C, Lopez-Najera E, Lopez-Najera A, Jimenez-Diaz L, Navarro-Lopez JD, Najera A. Cytokine Release Syndrome (CRS) and Nicotine in COVID-19 Patients: Trying to Calm the Storm. Front Immunol. 2020;11:1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 145. | Brinkman DJ, Ten Hove AS, Vervoordeldonk MJ, Luyer MD, de Jonge WJ. Neuroimmune Interactions in the Gut and Their Significance for Intestinal Immunity. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 146. | Galitovskiy V, Qian J, Chernyavsky AI, Marchenko S, Gindi V, Edwards RA, Grando SA. Cytokine-induced alterations of α7 nicotinic receptor in colonic CD4 T cells mediate dichotomous response to nicotine in murine models of Th1/Th17- vs Th2-mediated colitis. J Immunol. 2011;187:2677-2687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 147. | Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 148. | Lunney PC, Leong RW. Review article: Ulcerative colitis, smoking and nicotine therapy. Aliment Pharmacol Ther. 2012;36:997-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 149. | Aliee H, Massip F, Qi C, Stella de Biase M, van Nijnatten JL, Kersten ETG, Kermani NZ, Khuder B, Vonk JM, Vermeulen RCH, Neighbors M, Tew GW, Grimbaldeston M, ten Hacken NHT, Hu S, Guo Y, Zhang X, Sun K, Hiemstra PS, Ponder BA, Makela MJ, Malmstrom K, Rintoul RC, Reyfman PA, Theis FJ, Brandsma C-A, Adcock I, Timens W, Xu CJ, van den Berge M, Schwarz RF, Koppelman GH, Nawijn MC, Faiz A. Determinants of SARS-CoV-2 receptor gene expression in upper and lower airways. [Cited February 1, 2021]. 2020 Preprint. Available from: medRxiv: 20169946. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 150. | Voinsky I, Gurwitz D. Smoking and COVID-19: Similar bronchial ACE2 and TMPRSS2 expression and higher TMPRSS4 expression in current vs never smokers. Drug Dev Res. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 151. | Boezaart AP, Botha DA. Treatment of Stage 3 COVID-19 With Transcutaneous Auricular Vagus Nerve Stimulation Drastically Reduces Interleukin-6 Blood Levels: A Report on Two Cases. Neuromodulation. 2021;24:166-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 152. | Ahmad F. COVID-19 induced ARDS, and the use of galantamine to activate the cholinergic anti-inflammatory pathway. Med Hypotheses. 2020;145:110331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 153. | Douaoui S, Djidjik R, Boubakeur M, Ghernaout M, Touil-Boukoffa C, Oumouna M, Derrar F, Amrani Y. GTS-21, an α7nAChR agonist, suppressed the production of key inflammatory mediators by PBMCs that are elevated in COPD patients and associated with impaired lung function. Immunobiology. 2020;225:151950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 154. | Ten Hove AS, Brinkman DJ, Li Yim AYF, Verseijden C, Hakvoort TBM, Admiraal I, Welting O, van Hamersveld PHP, Sinniger V, Bonaz B, Luyer MD, de Jonge WJ. The role of nicotinic receptors in SARS-CoV-2 receptor ACE2 expression in intestinal epithelia. Bioelectron Med. 2020;6:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 155. | Farsalinos K, Eliopoulos E, Leonidas DD, Papadopoulos GE, Tzartos S, Poulas K. Nicotinic Cholinergic System and COVID-19: In Silico Identification of an Interaction between SARS-CoV-2 and Nicotinic Receptors with Potential Therapeutic Targeting Implications. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 156. | Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 157. | Leung JM, Yang CX, Sin DD. COVID-19 and nicotine as a mediator of ACE-2. Eur Respir J. 2020;55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 158. | Uemura N, Yagi H, Uemura MT, Hatanaka Y, Yamakado H, Takahashi R. Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol Neurodegener. 2018;13:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 159. | Tassorelli C, Mojoli F, Baldanti F, Bruno R, Benazzo M. COVID-19: what if the brain had a role in causing the deaths? Eur J Neurol. 2020;27:e41-e42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 160. | Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, Dottermusch M, Heinemann A, Pfefferle S, Schwabenland M, Sumner Magruder D, Bonn S, Prinz M, Gerloff C, Püschel K, Krasemann S, Aepfelbacher M, Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 855] [Cited by in F6Publishing: 799] [Article Influence: 199.8] [Reference Citation Analysis (0)] |
| 161. | U R A, Verma K. Pulmonary Edema in COVID19-A Neural Hypothesis. ACS Chem Neurosci. 2020;11:2048-2050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 162. | Chigr F, Merzouki M, Najimi M. Autonomic Brain Centers and Pathophysiology of COVID-19. ACS Chem Neurosci. 2020;11:1520-1522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 163. | Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009;183:6681-6688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 164. | Baud D, Dimopoulou Agri V, Gibson GR, Reid G, Giannoni E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front Public Health. 2020;8:186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 165. | Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11:182-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 166. | Li W, Zhu Y, Liao Q, Wang Z, Wan C. Characterization of gut microbiota in children with pulmonary tuberculosis. BMC Pediatr. 2019;19:445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 167. | Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, Amara RR. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122:1712-1716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 168. | Olwenyi OA, Naluyima P, Cham F, Quinn TC, Serwadda D, Sewankambo NK, Gray RH, Sandberg JK, Michael NL, Wabwire-Mangen F. Differential associations of IL-6 and intestinal fatty acid-binding protein with progressive untreated HIV-1 infection in Rakai, Uganda. JAIDS. 2016;72:15-20. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 169. | Muñoz-Nevárez LA, Imp BM, Eller MA, Kiweewa F, Maswai J, Polyak C, Olwenyi OA, Allen IE, Rono E, Milanini B, Kibuuka H, Ake JA, Eller LA, Valcour VG. Monocyte activation, HIV, and cognitive performance in East Africa. J Neurovirol. 2020;26:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 170. | Onkoba NW, Chimbari MJ, Mukaratirwa S. Malaria endemicity and co-infection with tissue-dwelling parasites in Sub-Saharan Africa: a review. Infect Dis Poverty. 2015;4:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Degarege A, Veledar E, Degarege D, Erko B, Nacher M, Madhivanan P. Plasmodium falciparum and soil-transmitted helminth co-infections among children in sub-Saharan Africa: a systematic review and meta-analysis. Parasit Vectors. 2016;9:344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 172. | Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, Eggo RM, Morton CE, Bacon SCJ, Inglesby P, Douglas IJ, Walker AJ, McDonald HI, Cockburn J, Williamson EJ, Evans D, Forbes HJ, Curtis HJ, Hulme WJ, Parry J, Hester F, Harper S, Evans SJW, Smeeth L, Goldacre B. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24-e32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 273] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 173. | Ayanlade A, Radeny M. COVID-19 and food security in Sub-Saharan Africa: implications of lockdown during agricultural planting seasons. NPJ Sci Food. 2020;4:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 174. | Bain LE, Awah PK, Geraldine N, Kindong NP, Sigal Y, Bernard N, Tanjeko AT. Malnutrition in Sub-Saharan Africa: burden, causes and prospects. Pan Afr Med J. 2013;15:120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 175. | Alegbeleye BJ, Mohammed RK. Challenges of healthcare delivery in the context of COVID-19 pandemic in Sub-Saharan Africa. Iberoamerican J Med. 2020;2:100-109. [Cited in This Article: ] |
| 176. | Dillon SM, Kibbie J, Lee EJ, Guo K, Santiago ML, Austin GL, Gianella S, Landay AL, Donovan AM, Frank DN, McCARTER MD, Wilson CC. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS. 2017;31:511-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |