Published online Jul 21, 2021. doi: 10.3748/wjg.v27.i27.4453
Peer-review started: February 21, 2021
First decision: May 13, 2021
Revised: May 16, 2021
Accepted: July 7, 2021
Article in press: July 7, 2021
Published online: July 21, 2021
Most hepatocellular carcinomas (HCCs) are hypervascular, with characteristic features of hepatic arterial supply to the tumor. The factors involved in tumor angiogenesis include angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), and vascular endothelial growth factor (VEGF).
To investigate the profiles of plasma levels of angiogenesis markers in patients with HCC and evaluate their roles in predicting overall survival (OS) and progression-free survival (PFS).
Plasma samples from 240 prospectively enrolled HCC patients in the very early to advanced stages were used to measure the levels of Ang-1, Ang-2, and VEGF. Their associations with clinical characteristics, OS, and PFS were analyzed.
The median plasma levels of Ang-1, Ang-2, and VEGF were 3216 pg/mL, 1684 pg/mL, and 26.5 pg/mL, respectively. The plasma level of Ang-2 showed a significant increase from early stage [Barcelona clinic liver cancer (BCLC) A] to intermediate (BCLC B) and advanced stage HCC (BCLC C/D), whereas Ang-1, VEGF, and alpha-fetoprotein (AFP) levels in the plasma did not show any such changes. Multivariable analysis, propensity score-matched analysis, and time-dependent receiver operating curve analysis revealed that Ang-2 levels had the highest predictive power for OS and PFS. Neither Ang-1 nor VEGF was significantly associated with OS or PFS. The neutrophil-to-lymphocyte ratio was an independent factor for OS and PFS.
The plasma levels of Ang-2 correlated with liver function, tumor stage, and tumor invasiveness, showing better performance in predicting OS and PFS than AFP, Ang-1, or VEGF.
Core Tip: Most hepatocellular carcinomas (HCCs) are hypervascular tumor, thus angiogenesis markers can be a potential biomarker. This study explored the potential of each plasma level of angiopoietin (Ang)-1, Ang-2, and vascular endothelial growth factor as a prognostic biomarker for very early to advanced stages of HCC via detailed analysis in comparison with alpha-fetoprotein. The plasma level of Ang-2 correlated with liver function, tumor stage, and tumor invasiveness. Multivariable and propensity score-matched analyses revealed Ang-2 levels with the highest predictive power for overall survival in patients with HCC.
- Citation: Choi GH, Jang ES, Kim JW, Jeong SH. Prognostic role of plasma level of angiopoietin-1, angiopoietin-2, and vascular endothelial growth factor in hepatocellular carcinoma. World J Gastroenterol 2021; 27(27): 4453-4467
- URL: https://www.wjgnet.com/1007-9327/full/v27/i27/4453.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i27.4453
Hepatocellular carcinoma (HCC) is the third most common malignancy in men, and seventh in women, and the second leading cause of cancer-related deaths worldwide[1]. Most HCCs are hypervascular with characteristic features of hepatic arterial supply to the tumor, frequent portal vein invasion, and intrahepatic metastasis. Based on these characteristics, the diagnosis of HCC based on radiological criteria was established to minimize the bleeding and seeding complications of tumor biopsy in most cases.
Therapeutically, transarterial chemoembolization (TACE), a standard treatment modality for the intermediate stage of HCC, uses the characteristic tumor blood supply for the selective arterial delivery of chemotherapeutic agents and the blockade of tumor-supplying vessels with embolic material. In advanced-stage HCC, several targeted therapeutics, including sorafenib, regorafenib, lenvatinib, ramucirumab, and cabozantinib, inhibit the angiogenic pathway along with other signaling pathways[2-4]. Recently, the combination of an anti-angiogenic agent and an immune checkpoint inhibitor showed a superior effect to sorafenib in the treatment of advanced HCC[5]. Therefore, the development of biomarkers for angiogenesis to assess treatment response and prognosis for HCC is becoming important.
Angiogenesis, the formation of new blood vessels from pre-existing ones, is a fundamental process in HCC development, progression, and metastasis[6,7]. In contrast to normal vessels, tumor vessels have highly proliferative endothelial cells with a leaky vasculature, which is unable to provide oxygen and nutrient supply to the tumor, leading to aberrant tumor microenvironments. The factors involved in tumor angiogenesis include vascular endothelial growth factor (VEGF), angiopoietin (Ang), fibroblast growth factor, epidermal growth factor, insulin-like growth factor, and platelet-derived growth factor. This makes the tumor angiogenesis system highly complex[8]. Among these, VEGF and Ang-tyrosine kinase with Ig and EGF homology domains-2 (Tie2) pathways, including Ang-1 and Ang-2[7,9,10], are the two dominant therapeutic targets of anti-angiogenesis in HCC.
However, investigations on the prognostic value of blood angiogenesis biomarkers across all HCC stages till date are limited. Therefore, this study aimed to investigate the plasma levels of Ang-1, Ang-2, and VEGF, and to evaluate their roles in predicting the overall survival (OS) and progression-free survival (PFS) in patients with very early to advanced stages of HCC.
A total of 251 newly diagnosed HCC patients who agreed to the collection of their information and blood samples at the time of diagnosis were prospectively enrolled in the Hepatology unit of the Seoul National University Bundang Hospital (SNUBH) between March 2012 and April 2016 (Supplementary Figure 1). Patients with extrahepatic malignancies that might affect survival (n = 9) and those with combined hepatocellular cholangiocarcinoma (n = 2) were excluded accordingly. The final HCC group hence, included 240 patients. HCC diagnosis was based on histologic examination and/or typical features (nodules ≥ 1 cm with arterial hypervascularity and portal or delayed washout) on dynamic computed tomography and/or magnetic resonance imaging[11]. Written informed consent was obtained from each HCC patient after approval by the Institutional Review Board (IRB) of the SNUBH. (IRB No. B-1201/143-002)
Clinical and pathological data of the HCC group were collected from electronic medical records. Data included age, sex, height, weight, etiology of HCC, comorbi
The primary outcome was OS, and the secondary outcome was the PFS. The index date was defined as the date when patients were diagnosed with HCC on their first liver protocol computed tomography or magnetic resonance imaging. The HCC patients were followed up until December 2019 for a median duration of 3.6 years. The mortality of each patient was confirmed by the requested data from Statistics Korea, and the cause of death was checked with the help of their medical records. PFS was estimated as the interval between the date of diagnosis and the date of progression confirmed on imaging study, death, or the end of follow-up.
Blood samples were collected from patients with HCC before treatment. The collected samples were centrifuged at 4000 × g for 10 min to obtain plasma and stored at −70 °C until use. Plasma levels of Ang-1, Ang-2, and VEGF were measured using a commercial enzyme-linked immunoassay (Luminex® screening and performance assays, R&D systems, Minneapolis, MN, United States) according to the manufac
The baseline characteristics of the patients were compared using the chi-square test for categorical variables, and a t-test was used to compare continuous variables. Spearman rank correlation was used to calculate the correlation coefficient between the ranked variables. OS and PFS were estimated using the Kaplan–Meier method and compared using the log-rank test. The Cox proportional hazards model was employed to determine the hazard ratio (HR) of OS and PFS in the univariate and subsequent multivariate analyses.
The prognostic values of the plasma biomarkers were determined by time-dependent receiver operating characteristic (ROC) curve analysis from censored survival data using the nearest-neighbor estimation method. All P values were two-sided, and values < 0.05, were considered significant. SPSS (version 21; SPSS, Inc., Chicago, IL, United States) and R (version 3.6.3., http://cran.r-project.org/) software was used for the statistical analyses.
To confirm the prognostic value of Ang-2 by reducing the impact of potential confounding effects, significant differences in baseline characteristics between the high and low Ang-2 groups were adjusted by propensity score (PS) matching. We used nearest-neighbor matching with a caliper size of 0.1, and matched the patients in a 1:1 ratio.
Baseline characteristics of the subjects are presented in Table 1. The 240 HCC patients had a mean age of 60.9 years, and there was a male proportion of 80.4%. The etiologies of HCC were as follows: Hepatitis B virus (HBV) (69.6%), hepatitis C virus (10.0%), alcohol (11.7%), and others (7.9%). The HCC group included patients with Child-Pugh class B or C (14.9%), TNM stage I or II (65.5%), and BCLC stage 0 or A (55.5%) (Table 1). There were no missing values for the variables.
| Characteristic | HCC patients (n = 240) |
| Age, mean age ± SD, year | 60.9 ± 11.2 |
| Male sex, n (%) | 193 (80.4) |
| BMI, mean ± SD, kg/m2 | 24.1 ± 3.0 |
| Etiology, n (%) | |
| HBV/HCV/HBV + HCV | 167 (69.6)/24 (10)/2 (0.8) |
| Alcohol/Others | 28 (11.7)/19 (7.9) |
| Presence of cirrhosis, n (%) | 184 (76.7) |
| Child-Pugh score, n (%) | |
| A/B/C | 204 (85.1)/35 (14.5)/1 (0.4) |
| ECOG performance status, n (%) | |
| 0/1–2/3 | 156 (65)/80 (33.3)/4 (1.7) |
| Maximal tumor size, median (IQR), cm | 3.6 (2.0–7.0) |
| Multi-nodularity of tumor, n (%) | 105 (43.8) |
| Vascular invasion of HCC, n (%) | 45 (18.8) |
| Presence of distant metastasis, n (%) | 18 (7.5) |
| BCLC staging, n (%) | |
| 0/A | 52 (21.7)/81 (33.8) |
| B/C/D | 43 (17.9)/59 (24.6)/5 (2.1) |
| TNM staging, n (%) | |
| I/II | 115 (48.0)/42 (17.5) |
| III/IV | 65 (27.1)/18 (7.5) |
| Initial treatment, n (%) | |
| Resection | 34 (14.2) |
| RFA/TACE | 27 (11.3)/165 (69.2) |
| Sorafenib/BSC | 2 (0.8)/11 (4.6) |
| Laboratory results | |
| WBC, median (IQR), × 103/uL | 5.1 (4.0–6.6) |
| Hemoglobin, median (IQR), g/dL | 13.6 (12.0–14.8) |
| Platelet count, median (IQR), × 109/uL | 130 (88–184) |
| Prothrombin time, median (IQR), INR | 1.11 (1.04–1.18) |
| AST, median (IQR), IU/mL | 45 (32–73) |
| ALT, median (IQR), IU/mL | 38 (23–61) |
| Albumin; median (IQR), g/dL | 4.0 (3.5–4.2) |
| Total bilirubin, median (IQR), mg/dL | 0.8 (0.5–1.1) |
| Creatinine, median (IQR), mg/dL | 0.8 (0.7–1.0) |
| AFP, median (IQR), ng/mL | 17.9 (4.0–698) |
| Angiogenesis marker | |
| Ang-1, median (IQR), pg/mL | 3216 (1565–6266) |
| Ang-2, median (IQR), pg/mL | 1684 (1107–3064) |
| Ang-2/Ang-1 ratio, median (IQR) | 0.56 (0.25–1.39) |
| VEGF, median (IQR), pg/mL | 26.5 (13.8–51.3) |
| NLR, mean ± SD | 2.8 ± 3.5 |
| PLR, mean ± SD | 118.2 ± 119.7 |
The median plasma levels of Ang-1, Ang-2, VEGF, and AFP were 3216 pg/mL, 1684 pg/mL, 26.5 pg/mL, and 17.9 ng/mL, respectively. All biomarkers showed an increasing trend as the BCLC stage progressed (Figure 1). However, only the median plasma level of Ang-2 showed a significant increase from the early stage HCC (BCLC A: 1289 pg/mL) to intermediate (BCLC B; 1808 pg/mL) and advanced stage HCC (BCLC C/D; 3653 pg/mL), whereas Ang-1, VEGF, and AFP levels did not show any changes. Ang-2 and AFP levels showed an increasing trend as the TNM stage progressed, while Ang-1 and VEGF levels did not (Supplementary Figure 2). NLR and PLR showed an increasing trend as the BCLC stage progressed (Supplementary Figure 3).
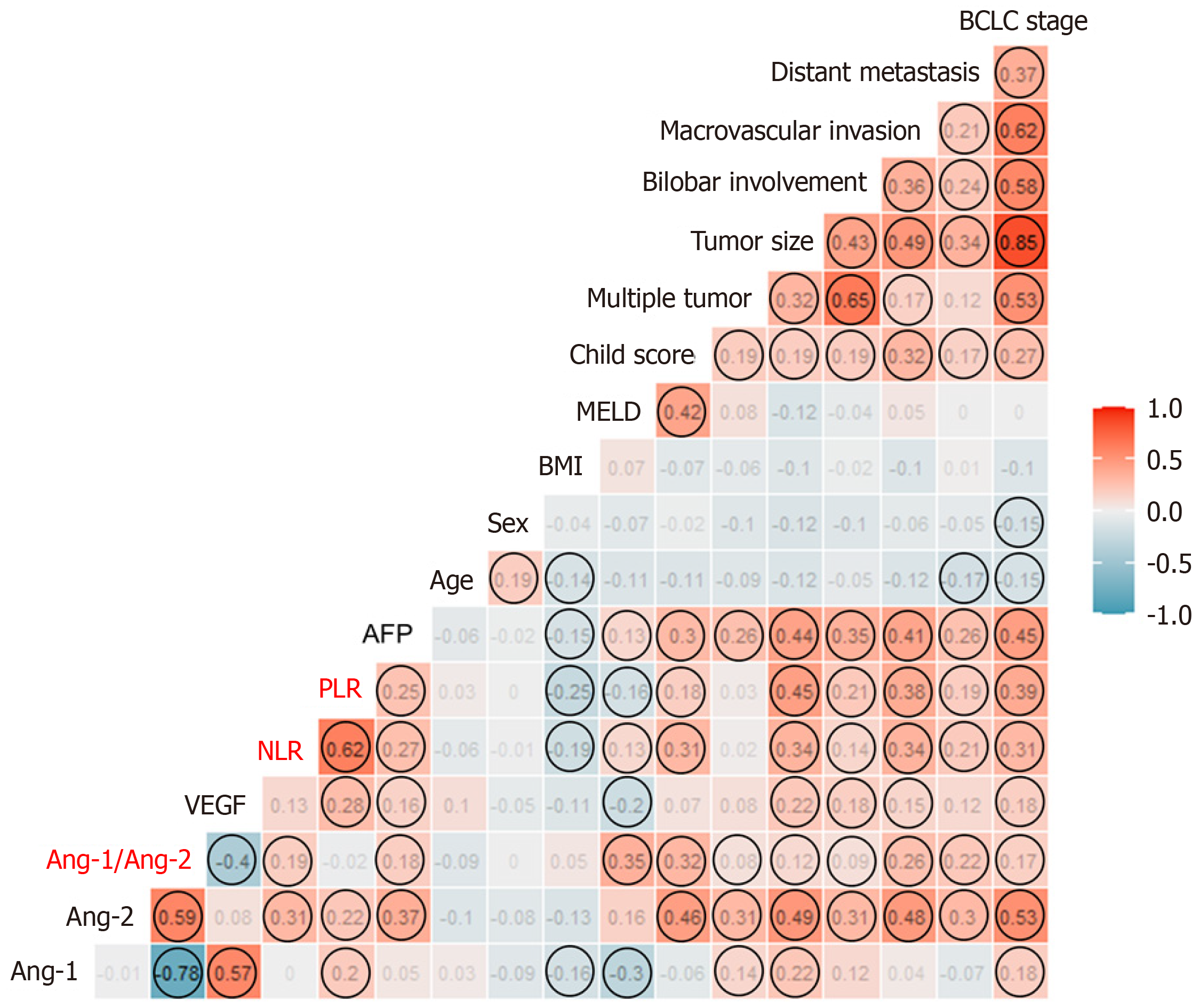
The correlation plot between each plasma level of the three biomarkers and clinical factors is shown in Figure 2. Ang-2 levels were significantly correlated with tumor extent and poor liver function, while VEGF levels were correlated with tumor extent. Meanwhile, the rho value between Ang-1 or VEGF levels and liver function or tumor extent was not significant. Ang-2 was significantly correlated with both NLR and PLR, while Ang-1 and VEGF were correlated with PLR only. Ang-2 levels were not correlated with VEGF, but negatively correlated with Ang-1 levels. Meanwhile, Ang-1 was positively correlated with VEGF levels in the HCC group (Figure 2).
The median plasma levels of Ang-2 and VEGF did not differ according to the etiology of HCC (Supplementary Figure 4A and B). The correlation plot between each plasma level of the biomarkers and HBV DNA is shown in Supplementary 4C and D among CHB patients (n = 156). HBV DNA levels were not significantly correlated with either Ang-2 or VEGF levels.
The comparison of the clinical factors between high- and low-level groups of Ang-2 or VEGF using the median level as a cutoff is shown in Supplementary Table 1. The high Ang-2 and high VEGF groups showed more advanced tumor stages than the low Ang-2 and low VEGF groups. The high Ang-2 group had a significantly higher proportion of Child-Pugh class B or C than the low Ang-2 group (26.1% vs 4.1%, P < 0.001). On the contrary, the high VEGF group showed a significantly lower MELD score than the low VEGF group (3.8 vs 5.6, P = 0.01). The NLR and PLR of the high Ang-2 group were significantly higher than those of the low Ang-2 group (3.5 vs 2.2, P = 0.007; 138.3 vs 98.5. P = 0.01). However, there was no difference in the NLR and PLR between the high and low VEGF groups.
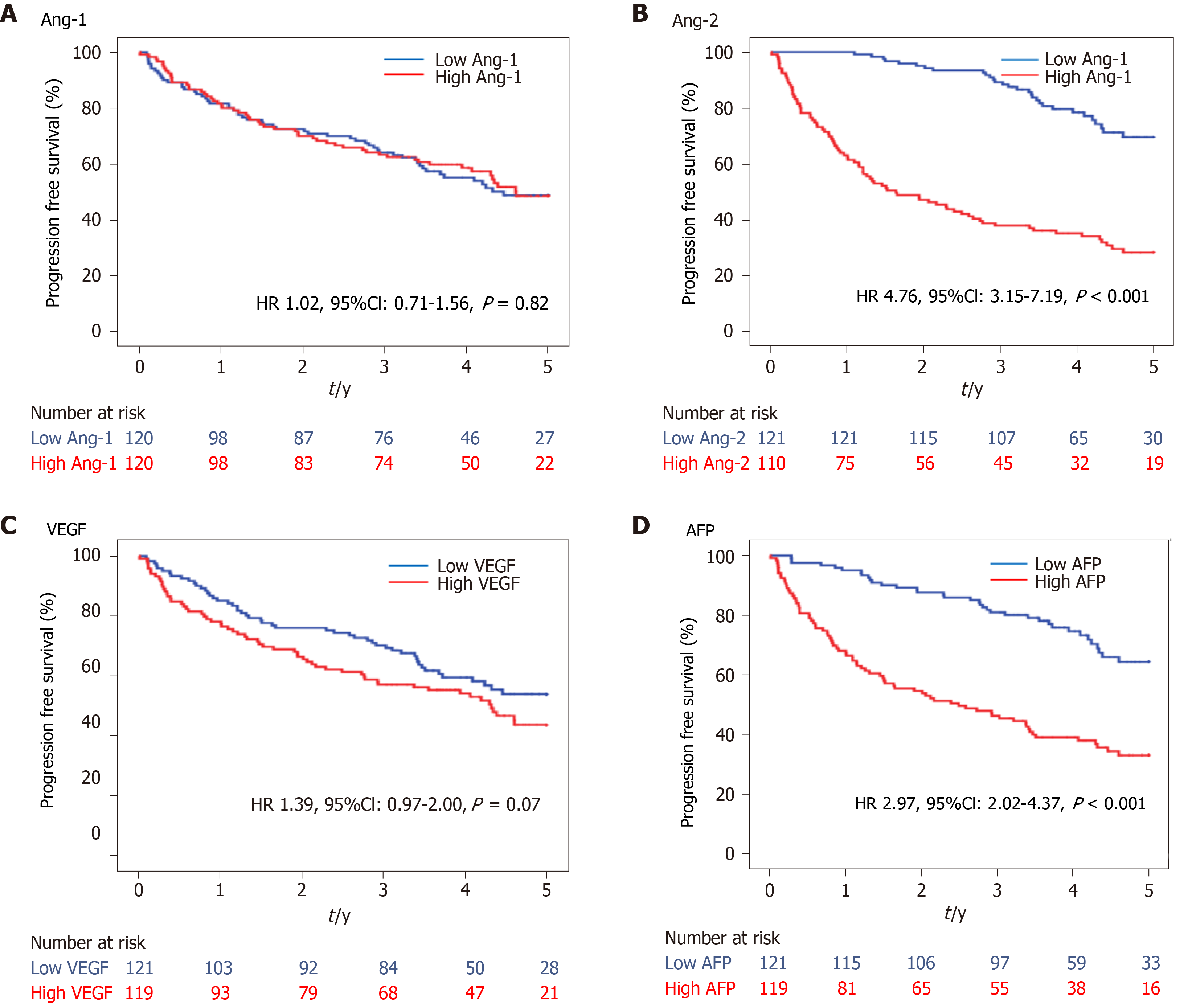
During a median follow-up of 3.6 years, 118 deaths were observed in the HCC group. The OS curves between the low and high levels of Ang-1, Ang-2, VEGF, and AFP using the median level as a cutoff are shown in Figure 3. The OS was significantly shorter in the high Ang-2 group [HR 4.76, 95% confidence interval (CI): 3.15–7.19, P < 0.001; Figure 3B] and high AFP group (≥20 ng/mL) (HR 2.97, 95%CI: 2.02–4.37, P < 0.001; Figure 3D) than in the low Ang-2 and low AFP groups, respectively. However, the OS rate was not significantly different between the high and low Ang-1 groups (HR 1.02, 95%CI: 0.71-1.56, P = 0.82; Figure 3A) and between the high and low VEGF groups (HR 1.39, 95%CI: 0.97–2.00, P = 0.07; Figure 3C).
The results of the univariate and multivariate analyses for the factors associated with OS are shown in Table 2. The high Ang-2 group was an independent factor (HR 5.96, 95%CI: 1.58–22.43, P < 0.001) associated with OS, along with NLR (HR 4.45, 95%CI: 1.77–11.23, P = 0.002), Child-Pugh class B or C, and TNM stage. Meanwhile, VEGF and AFP levels were not associated with OS (Table 2).
| Variable | Univariate analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Ang-2 group (cut-off: Median level) | ||||
| Low Ang-2 group | Reference | – | Reference | – |
| High Ang-2 group | 4.76 (3.15 – 7.20) | < 0.001 | 5.96 (1.58–22.43) | < 0.001 |
| VEGF group (cut-off: Median level) | ||||
| Low VEGF group | Reference | – | Reference | – |
| High VEGF group | 1.39 (0.97–2.00) | 0.07 | 0.80 (0.33–1.98) | 0.63 |
| Ang-2/Ang-1 ratio | 1.02 (0.94–1.06) | 0.26 | – | – |
| AFP (≥ 20 ng/mL) | 2.97 (2.02–4.37) | < 0.001 | 2.63 (0.85–8.09) | 0.09 |
| Age | 1.00 (0.98–1.02) | 0.93 | – | – |
| Male sex | 1.05 (0.68–1.64) | 0.82 | – | – |
| BMI > 25 kg/m2 | 0.70 (0.47–1.03) | 0.07 | – | – |
| Presence of cirrhosis | 1.02 (0.67–1.57) | 0.92 | – | – |
| Child-Pugh class B or C | 3.68 (2.42–5.58) | < 0.001 | 5.59 (2.44–12.81) | < 0.001 |
| NLR | 3.24 (1.49–7.06) | 0.003 | 4.45 (1.77–11.23) | 0.002 |
| PLR | 2.68 (1.26–5.70) | 0.01 | 0.56 (0.24–1.33) | 0.19 |
| TNM stage | ||||
| I | Reference | – | Reference | – |
| II | 6.30 (1.15–34.42) | 0.03 | 3.61 (0.62–21.08) | 0.15 |
| III | 26.43 (6.01–116.24) | < 0.001 | 14.20 (2.82–71.45) | 0.001 |
| IV | 70.42 (15.00–330.6) | < 0.001 | 41.39 (6.62–258.67) | < 0.001 |
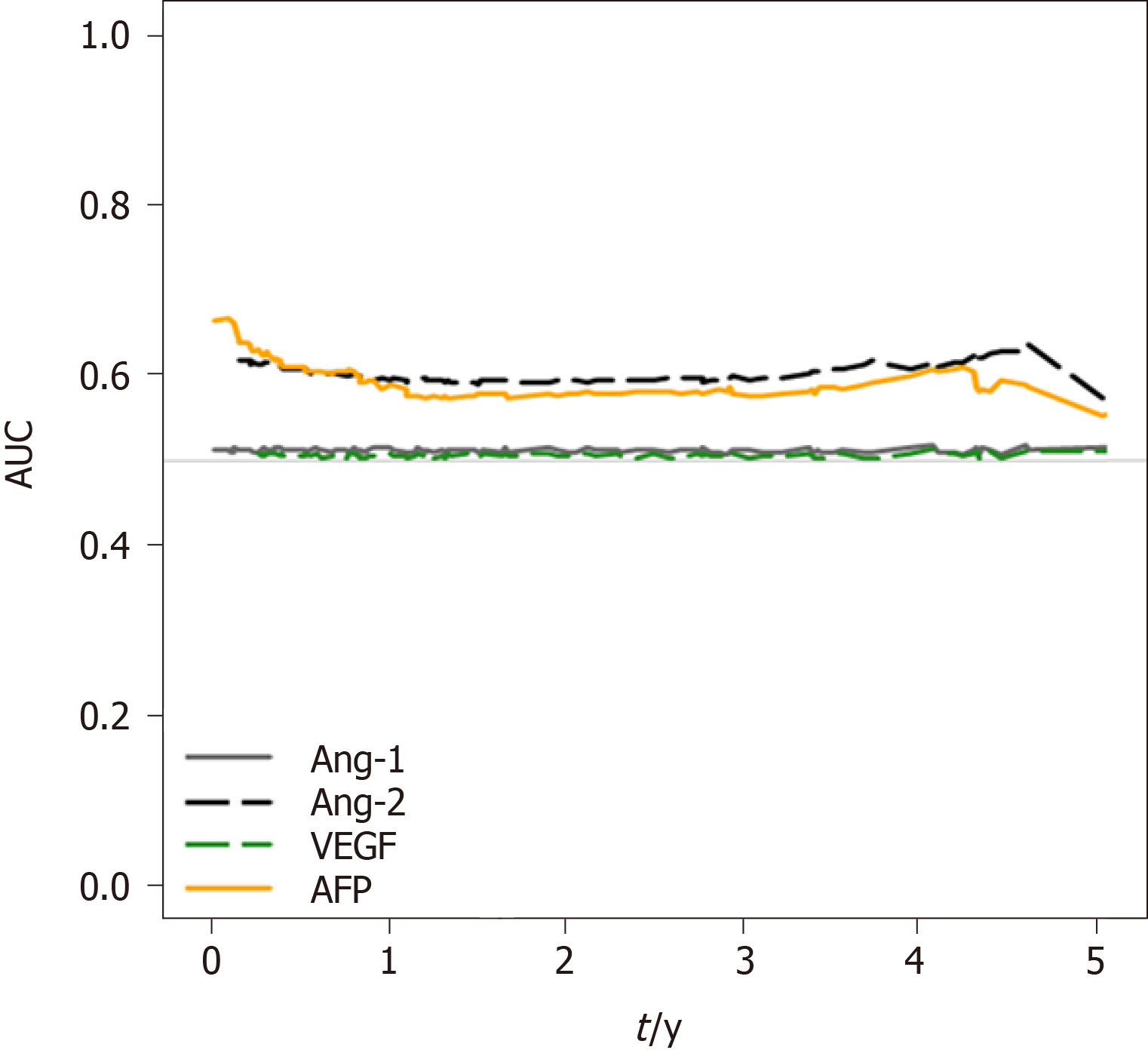
To analyze the survival prediction power of Ang-2 levels, time-dependent area under the ROC (AUROC) analysis for survival prediction showed that the AUROC of baseline Ang-2 levels (0.909) for the 1-year survival prediction was significantly higher than that of AFP (AUROC 0.817, P = 0.03), Ang-1 (AUROC 0.535, P < 0.001), and VEGF (AUROC 0.577, P < 0.001; Figure 4). The AUROC of Ang-2 (0.873) for the two-year survival prediction was also significantly higher than that of AFP (AUROC 0.767, P = 0.01), Ang-1 (AUROC 0.541, P < 0.001), and VEGF (AUROC 0.581, P < 0.001). Moreover, baseline Ang-2 levels showed better survival predictive power than the other plasma biomarkers throughout the 5 years (Figure 4).
PS-matching yielded 37 matched pairs of patients from the high Ang-2 and low Ang-2 groups (Supplementary Table 2). Within this matched cohort, there were no significant between-group differences in most baseline characteristics, except for the proportion of male patients (75.8% vs 83.8%). The high Ang-2 group showed a significantly higher risk of death (HR 2.29, 95%CI: 1.10–4.76, P = 0.03) than the low Ang-2 group (Supplementary Figure 5A).
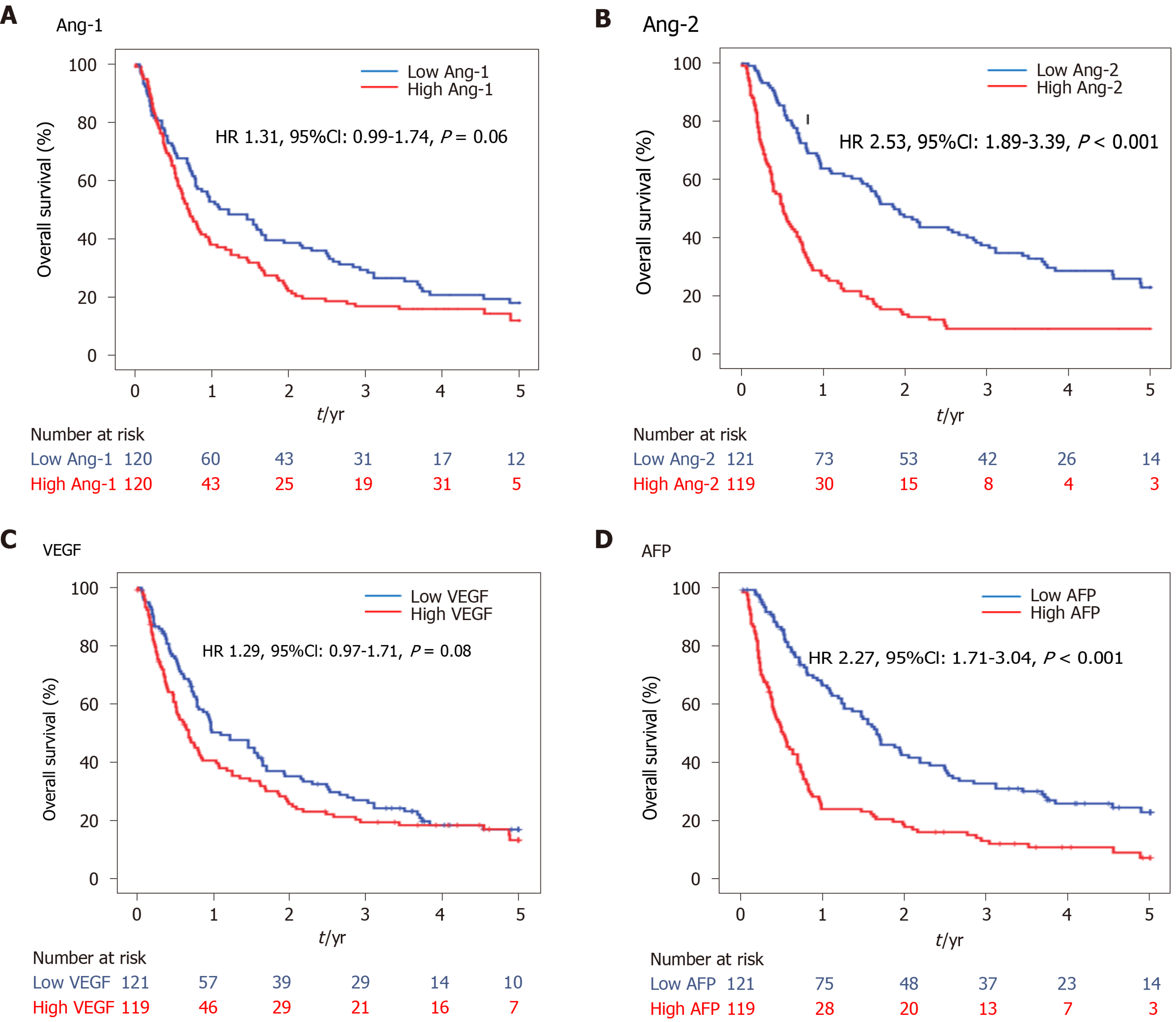
During the median follow-up period, 171 progressions and 18 deaths were observed in the HCC group. The PFS curve between low and high levels of Ang-1, Ang-2, VEGF, and AFP using the median level as a cutoff are shown in Figure 5. The PFS was significantly shorter in the high Ang-2 group (HR 2.53, 95%CI: 1.89–3.39, P < 0.001; Figure 5B) and high AFP group (≥ 20 ng/mL) (HR 2.27, 95%CI: 1.71–3.04, P < 0.001; Figure 5D) than in the low Ang-2 and low AFP groups, respectively. However, the PFS rate was not significantly different between the high and low Ang-1 groups (HR 1.31, 95%CI: 0.99–1.74, P = 0.06; Figure 5A) or between the high and low VEGF groups (HR 1.29, 95%CI: 0.97–1.71, P = 0.08; Figure 5C).
The results of univariate and multivariate analyses for the factors associated with PFS are shown in Table 3. The high Ang-2 group was an independent factor (HR 1.55, 95%CI: 1.10–2.20; P = 0.01) associated with PFS, along with NLR (HR 1.95, 95%CI: 1.23–3.08, P = 0.004), AFP (HR 1.54, 95%CI: 1.13–2.11, P = 0.007), Child-Pugh class B or C, BMI, and TNM stage. However, VEGF and PLR were not (Table 3).
| Variable | Univariate analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Ang-2 group (cut-off: Median level) | ||||
| Low Ang-2 group | Reference | – | Reference | – |
| High Ang-2 group | 2.53 (1.89–3.39) | < 0.001 | 1.55 (1.10–2.20) | 0.01 |
| VEGF group (cut-off: Median level) | ||||
| Low VEGF group | Reference | – | Reference | – |
| High VEGF group | 1.29 (0.97–1.71) | 0.08 | 1.08 (0.80–1.46) | 0.61 |
| Ang-2/Ang-1 ratio | 1.02 (0.98–1.05) | 0.32 | – | – |
| AFP (≥ 20 ng/mL) | 2.27 (1.713.04) | < 0.001 | 1.54 (1.13–2.11) | 0.007 |
| Age | 0.99 (0.98–1.01) | 0.30 | – | – |
| Male sex | 1.32 (0.91–1.92) | 0.15 | – | – |
| BMI > 25 kg/m2 | 0.67 (0.50–0.91) | 0.01 | 0.72 (0.53–0.97) | 0.03 |
| Presence of cirrhosis | 1.04 (0.74–1.46) | 0.81 | – | – |
| Child-Pugh class B or C | 2.17 (1.48–3.18) | < 0.001 | 1.54 (1.01–2.35) | 0.05 |
| NLR > 4 | 2.13 (1.45–3.12) | < 0.001 | 1.95 (1.23–3.08) | 0.004 |
| PLR > 150 | 2.05 (1.45–2.90) | < 0.001 | 1.02 (0.67–1.57) | 0.93 |
| TNM stage | ||||
| I | Reference | – | Reference | – |
| II | 1.94 (1.31–2.89) | 0.001 | 1.75 (1.16–2.63) | 0.008 |
| III | 5.47 (3.81–116.24) | < 0.001 | 4.35 (2.91–6.50) | < 0.001 |
| IV | 70.42 (15.00–330.6) | < 0.001 | 9.35 (5.00–17.46) | < 0.001 |
Within 37 pairs of PS-matched cohorts, PFS was not significantly different between the high and low Ang-2 groups (HR 1.19, 95%CI: 0.72–1.96, P = 0.51; Supplementary Figure 5B). This is probably due to the small sample size of PFS during follow-up.
This study explored the potential of each plasma level of Ang-1, Ang-2, and VEGF as a prognostic biomarker for very early to advanced stages of HCC via detailed analysis in comparison with AFP. Multivariable and PS-matched analyses revealed Ang-2 levels with the highest predictive power for OS in patients with HCC. Moreover, Ang-2 and AFP levels were independent factors for PFS. In contrast, neither Ang-1 nor VEGF was significantly associated with OS or PFS. NLR was an independent factor for both OS and PFS.
In this study, the median levels of Ang-1, Ang-2, and VEGF were lower than those in previous reports that included only patients with advanced-stage HCC, mostly treated with sorafenib. This may be due to the relatively low proportion of advanced-stage HCC cases included in this study (27%). Ang-2 levels were higher in patients with advanced HCC than in those with early HCC. Moreover, the majority of our patients underwent locoregional therapy (radiofrequency ablation, 11.3%; TACE, 69.2%). Ang-2 Levels showed a positive correlation with liver function indicators, such as MELD score and Child-Pugh score, and tumor extent or aggressiveness, which are the two major axes of HCC prognosis. Similarly, a recent study reported that Ang-2 levels were associated with 90-d mortality, acute kidney injury stage, and risk of renal replacement therapy in a cohort of patients with decompensated cirrhosis[13]. Meanwhile, Ang-1 and VEGF levels were generally associated with tumor extent rather than liver function. Therefore, this study suggests that Ang-2 levels are better prognostic biomarkers for HCC than Ang-1 or VEGF levels, especially after locoregional treatment.
The potential of Ang-2 as a prognostic marker was confirmed by survival analysis. Our multivariable analysis showed that the plasma level of Ang-2 (median 1684 pg/mL), but not of VEGF (median 26.5 pg/mL), was an independent predictor of OS. In addition, time-dependent ROC curve analysis also demonstrated that Ang-2 level is a better predictor of OS than AFP, Ang-1, and VEGF levels, which was supported by PS-matched analysis.
Several studies have investigated the prognostic role of Ang-2 in patients with advanced HCC. In 2013, Miyahara et al[14] investigated eight cytokine levels in 120 patients with advanced HCC who were treated with sorafenib. The results showed that the Ang-2 level (median, 721.3 pg/mL) was associated with OS and PFS, but VEGF (median 68.6 pg/mL) was not[14]. This result is consistent with that of our study. However, in our study, the median levels of Ang-2 (1684 pg/mL) and VEGF (26.5 pg/mL) were different. Llovet et al[15] reported that the plasma levels of Ang-2 (median, 6043.5 pg/mL) and VEGF (median, 1019 pg/mL) were independent predictors of OS in 602 advanced HCC enrolled in the phase 3, randomized controlled sorafenib HCC assessment randomized protocol (SHARP) trial, both of which were not significant response-prediction biomarkers in the sorafenib-treated cohort[15].
In a Chinese study of 173 HCC patients, Ang-2 level (mean, 18000 pg/mL) was an independent prognostic factor with a cutoff level of 6433 pg/mL[16]. Pestana et al[6] reported that a high plasma Ang-2 level (above mean value of 15300 pg/mL) was associated with lower OS, but a high Ang-1 level (above mean value of 16000 pg/mL) was associated with longer OS in 767 HCC patients treated at MD Anderson Cancer Center (77% in advanced stage)[6]. In this study, the Ang-1 and Ang-2 levels were markedly higher than that of ours and of previous studies because they showed mean rather than median values and used a different ELISA kit. A small-scale study in Spain (n = 33) showed that Ang-2 levels in peripheral blood and hepatic veins were well correlated (r = 0.95), and serum Ang-2 levels were significantly associated with tumor extent and aggressiveness. However, it was not related to treatment response.
VEGF is secreted by HCC cells and enhances endothelial cell survival, proliferation, invasion, and migration in response to tumor hypoxia[9]. Ang-1 and Ang-2 are both secreted proteins with 60% amino acid sequence homology, and they interact with Tie2, the endothelial cell-specific tyrosine kinase receptor[10]. While Ang-1 binds Tie2 to promote vessel stability and quiescence, Ang-2 binds to Tie2 to promote vessel permeability, instability, and eventual tissue hypoxia. This antagonistic relationship was consistent with the negative correlation between the plasma levels of Ang-1 and Ang-2 in our study. Angiogenic switch by Ang-2 promotes VEGF expression and promotes tumor angiogenesis.
On the other hand, Ang-2 acts as a chemoattractant by promoting recruitment of proangiogenic myeloid cells, especially Tie2 expressing macrophages (TEMs)[17,18]. TEMs become more proangiogenic and immunosuppressive in the tumor microenvironment. This is a potential mechanism for resistance to antiangiogenic therapy and poor survival in patients with high Ang-2 expression in HCC[19]. Interestingly, NLR and PLR, both inflammatory biomarkers, were significantly correlated with Ang-2 levels in this study. A recent meta-analysis showed that preoperative NLR was positively correlated with the risk of microvascular invasion in HCC[20]. Moreover, many studies have confirmed that a high baseline NLR is an independent factor for OS and PFS[21-23]. This is consistent with the results of our study.
Angiogenesis inhibitors have become an important therapeutic approach for the treatment of solid cancers. First-generation anti-angiogenic agents, including bevacizumab, sorafenib, pazopanib, vandeltanib, cabozantinib, axitinib, VEGF-trap ziv-aflibercept, and ramucirumab, target VEGF signaling[8]. Second-generation antiangiogenic agents, including trebananib, nesvacumab, rebastinib, and MEDI3617, target Ang/Tie2 signaling in clinical development[8,19,24]. However, resistance to these agents, lack of improvement of OS, and common adverse events, including hypertension and bleeding, are challenges faced by current anti-angiogenic therapies. Therefore, combination therapy of VEGF and Ang-2 targeted agents as well as a combination of anti-angiogenic and chemotherapeutic agents, radiation, or immune modulators are being developed accordingly. Recent studies suggested that predicting OS was made possible by periodically measuring serum angiogenic cytokines, especially Ang-2 or VEGF, in patients with HCC treated with sorafenib or lenvatinib[14,25,26]. Moreover, a single-nucleotide polymorphism of Ang-2 is an independent prognostic factor for sorafenib-treated advanced HCC[27]. A high increase in the serum VEGF level after TACE among patients with HCC was associated with distant metastasis and unfavorable outcomes[28,29]. Therefore, identifying tumors using circulating biomarkers that are sensitive to antiangiogenic therapy and predictive of OS and PFS could improve therapeutic approaches.
This study had several limitations. First, this was a single-center study in an HBV-endemic area[30]. Although there was no difference in Ang-2 and VEGF levels according to the etiology of HCC in this study, the results need external validation. Second, the angiogenic biomarkers were measured only once at the time of HCC diagnosis, so the longitudinal profile according to tumor progression or treatment response could not be analyzed. Third, this study did not evaluate the plasma levels of these three angiogenesis factors in normal control subjects or cirrhotic patients because we focused on the role of Ang-1, Ang-2, and VEGF as prognostic rather than diagnostic markers. Fourth, this study did not evaluate other valuable angiogenesis markers, such as microvessel density, PDGF/PDGFR, FGF/FGFR, and endoglin (CD105)[9,31]. Further comprehensive studies including these angiogenesis markers are hence needed. Lastly, the number of patients who underwent surgery and sorafenib treatment was small. Therefore, it is difficult to generalize that angiogenesis markers can predict the prognosis of patients receiving these treatments.
In conclusion, the plasma level of Ang-2 correlated with liver function, tumor stage, and tumor invasiveness. It also performed better than AFP, Ang-1, and VEGF in terms of predicting OS and PFS.
Most hepatocellular carcinomas (HCCs) are hypervascular, with characteristic features of hepatic arterial supply to the tumor. The factors involved in tumor angiogenesis include angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), and vascular endothelial growth factor (VEGF).
Angiogenesis markers can be a potential biomarker.
To investigate the profiles of plasma levels of angiogenesis markers in patients with HCC and evaluate their roles in predicting overall survival (OS) and progression-free survival (PFS).
Plasma samples from 240 prospectively enrolled HCC patients in the very early to advanced stages were used to measure the levels of Ang-1, Ang-2, and VEGF. Their associations with clinical characteristics, OS, and PFS were analyzed.
The plasma level of Ang-2 correlated with liver function, tumor stage, and tumor invasiveness. Multivariable and propensity score-matched analyses revealed Ang-2 Levels with the highest predictive power for OS in patients with HCC.
The plasma levels of Ang-2 can be a better biomarker than AFP in predicting OS or RFS.
Identifying HCCs using circulating biomarkers that are sensitive to antiangiogenic therapy and predictive of OS and PFS could improve therapeutic approaches.
We are grateful to our research coordinator (Da Woon Jeong) and the laboratory technician (Yun Suk Choi).
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang CY, Porrello G, Zeng YY S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1729] [Cited by in F6Publishing: 1954] [Article Influence: 244.3] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3934] [Cited by in F6Publishing: 4891] [Article Influence: 815.2] [Reference Citation Analysis (0)] |
| 3. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2107] [Cited by in F6Publishing: 2574] [Article Influence: 429.0] [Reference Citation Analysis (2)] |
| 4. | Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A, Hoshi SS, Mimura F, Haneda T, Fukuda Y, Kamata JI, Takahashi K, Matsukura M, Wakabayashi T, Asada M, Nomoto KI, Watanabe T, Dezso Z, Yoshimatsu K, Funahashi Y, Tsuruoka A. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 312] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 5. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2542] [Cited by in F6Publishing: 3319] [Article Influence: 829.8] [Reference Citation Analysis (1)] |
| 6. | Pestana RC, Hassan MM, Abdel-Wahab R, Abugabal YI, Girard LM, Li D, Chang P, Raghav K, Morris J, Wolff RA, Rashid A, Amin HM, Kaseb A. Clinical and prognostic significance of circulating levels of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. Oncotarget. 2018;9:37721-37732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, Nakamura T, Sakata R, Hashimoto O, Sakamoto M, Kumashiro R, Sata M, Nakashima O, Yano H, Kojiro M. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J Hepatol. 2004;40:799-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Biel NM, Siemann DW. Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Lett. 2016;380:525-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:912-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 300] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 10. | Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 11. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 12. | Di Nunno V, Santoni M, Cimadamore A, Battelli N, Massari F. Re: Gladell P. Paner, Walter M. Stadler, Donna E. Hansel, Rodolfo Montironi, Daniel W. Lin, Mahul B. Amin. Updates in the Eighth Edition of the Tumor-node-metastasis Staging Classification for Urologic Cancers. Eur Urol 2018;73:560-9: Tumour, Node, and Metastasis Staging System for Urological Malignancies: Are We Ready for the Next Step? Eur Urol. 2018;74:e118-e119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Allegretti AS, Vela Parada X, Ortiz GA, Long J, Krinsky S, Zhao S, Fuchs BC, Sojoodi M, Zhang D, Karumanchi SA, Kalim S, Nigwekar SU, Thadhani RI, Parikh SM, Chung RT. Serum Angiopoietin-2 Predicts Mortality and Kidney Outcomes in Decompensated Cirrhosis. Hepatology. 2019;69:729-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Miyahara K, Nouso K, Morimoto Y, Takeuchi Y, Hagihara H, Kuwaki K, Onishi H, Ikeda F, Miyake Y, Nakamura S, Shiraha H, Takaki A, Honda M, Kaneko S, Sato T, Sato S, Obi S, Iwadou S, Kobayashi Y, Takaguchi K, Kariyama K, Takuma Y, Takabatake H, Yamamoto K; Okayama Liver Cancer Group. Pro-angiogenic cytokines for prediction of outcomes in patients with advanced hepatocellular carcinoma. Br J Cancer. 2013;109:2072-2078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J; SHARP Investigators Study Group. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290-2300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 436] [Article Influence: 36.3] [Reference Citation Analysis (2)] |
| 16. | Chen Y, Wu Y, Zhang X, Zeng H, Liu Y, Wu Q, Chen Y, Zhu G, Pan Q, Jin L, Guo L, Sun F. Angiopoietin-2 (Ang-2) is a useful serum tumor marker for liver cancer in the Chinese population. Clin Chim Acta. 2018;478:18-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y, Lewis CE. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270-5280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 18. | Matsubara T, Kanto T, Kuroda S, Yoshio S, Higashitani K, Kakita N, Miyazaki M, Sakakibara M, Hiramatsu N, Kasahara A, Tomimaru Y, Tomokuni A, Nagano H, Hayashi N, Takehara T. TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis. Hepatology. 2013;57:1416-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Gillen J, Richardson D, Moore K. Angiopoietin-1 and Angiopoietin-2 Inhibitors: Clinical Development. Curr Oncol Rep. 2019;21:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Zeng F, Chen B, Zeng J, Wang Z, Xiao L, Deng G. Preoperative neutrophil-lymphocyte ratio predicts the risk of microvascular invasion in hepatocellular carcinoma: A meta-analysis. Int J Biol Markers. 2019;34:213-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Qi X, Li J, Deng H, Li H, Su C, Guo X. Neutrophil-to-lymphocyte ratio for the prognostic assessment of hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Oncotarget. 2016;7:45283-45301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Peng C, Cheng Z, Wang X, Wu L, Li J, Huang C, Guo Q, Cai H. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: A systematic review and meta-analysis. Int J Surg. 2018;55:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Zheng J, Cai J, Li H, Zeng K, He L, Fu H, Zhang J, Chen L, Yao J, Zhang Y, Yang Y. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: a Meta-Analysis and Systematic Review. Cell Physiol Biochem. 2017;44:967-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 24. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1126] [Cited by in F6Publishing: 1147] [Article Influence: 191.2] [Reference Citation Analysis (0)] |
| 25. | Chuma M, Uojima H, Numata K, Hidaka H, Toyoda H, Hiraoka A, Tada T, Hirose S, Atsukawa M, Itokawa N, Arai T, Kako M, Nakazawa T, Wada N, Iwasaki S, Miura Y, Hishiki S, Nishigori S, Morimoto M, Hattori N, Ogushi K, Nozaki A, Fukuda H, Kagawa T, Michitaka K, Kumada T, Maeda S. Early Changes in Circulating FGF19 and Ang-2 Levels as Possible Predictive Biomarkers of Clinical Response to Lenvatinib Therapy in Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Kim HY, Lee DH, Lee JH, Cho YY, Cho EJ, Yu SJ, Kim YJ, Yoon JH. Novel biomarker-based model for the prediction of sorafenib response and overall survival in advanced hepatocellular carcinoma: a prospective cohort study. BMC Cancer. 2018;18:307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Marisi G, Petracci E, Raimondi F, Faloppi L, Foschi FG, Lauletta G, Iavarone M, Canale M, Valgiusti M, Neri LM, Ulivi P, Orsi G, Rovesti G, Vukotic R, Conti F, Cucchetti A, Ercolani G, Andrikou K, Cascinu S, Scartozzi M, Casadei-Gardini A. ANGPT2 and NOS3 Polymorphisms and Clinical Outcome in Advanced Hepatocellular Carcinoma Patients Receiving Sorafenib. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Poon RT, Lau C, Yu WC, Fan ST, Wong J. High serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: a prospective study. Oncol Rep. 2004;11:1077-1084. [PubMed] [Cited in This Article: ] |
| 29. | Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037-2044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Korean Liver Cancer Study Group (KLCSG); National Cancer Center; Korea (NCC). 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015;9:267-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Zhang Q, Wu J, Bai X, Liang T. Evaluation of Intra-Tumoral Vascularization in Hepatocellular Carcinomas. Front Med (Lausanne). 2020;7:584250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |