Published online Jul 14, 2021. doi: 10.3748/wjg.v27.i26.4194
Peer-review started: February 26, 2021
First decision: April 18, 2021
Revised: April 28, 2021
Accepted: June 18, 2021
Article in press: June 18, 2021
Published online: July 14, 2021
Endoscopic ultrasound tissue acquisition, in the form of both fine needle aspiration (EUS-FNA) and fine needle biopsy (EUS-FNB), is utilized for pancreatic mass lesions, subepithelial lesions, and lymph node biopsy. Both procedures are safe and yield high diagnostic value. Despite its high diagnostic yield, EUS-FNA has potential limitations associated with cytological aspirations, including inability to determine histologic architecture, and a small quantitative sample for further immunohistochemical staining. EUS-FNB, with its larger core biopsy needle, was designed to overcome these potential limitations. However, it remains unclear which technique should be used and for which lesions. Comparative trials are plagued by heterogeneity at every stage of comparison; including variable needles used, and different definitions of endpoints, which therefore limit generalizability. Thus, we present a review of prospective trials, systematic reviews, and meta-analyses on studies examining EUS-FNA vs EUS-FNB. Prospective comparative trials of EUS-FNA vs EUS-FNB primarily focus on pancreatic mass lesions, and yield conflicting results in terms of demonstrating the superiority of one method. However, consistent among trials is the potential for diagnosis with fewer passes, and a larger quantity of sample achieved for next generation sequencing. With regard to subepithelial lesions and lymph node biopsy, fewer prospective trials exist, and larger prospective studies are necessary. Based on the available literature, we would recommend EUS-FNB for peri-hepatic lymph nodes.
Core Tip: Endoscopic ultrasound fine needle aspiration (EUS-FNA) and fine needle biopsy (EUS-FNB) provide two methods for endoscopic ultrasound tissue acquisition for pancreatic mass lesions, subepithelial lesions, and lymph node biopsy. Both methods are safe and provide high diagnostic yield. Prospective comparative trials of EUS-FNA vs EUS-FNB primarily focus on pancreatic lesions. EUS-FNB provides diagnostic accuracy with fewer needle passes, and may provide higher diagnostic yield for peri-hepatic lymph nodes.
- Citation: Levine I, Trindade AJ. Endoscopic ultrasound fine needle aspiration vs fine needle biopsy for pancreatic masses, subepithelial lesions, and lymph nodes. World J Gastroenterol 2021; 27(26): 4194-4207
- URL: https://www.wjgnet.com/1007-9327/full/v27/i26/4194.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i26.4194
As medical and surgical therapeutics continue to evolve, there is a renewed emphasis on timely diagnosis of various illnesses. This mantra certainly holds true in gastrointestinal (GI) diseases, including pancreatic malignancies and GI tumors, where early and specific diagnosis guides management and impacts morbidity and mortality[1]. While cross-sectional imaging can characterize lesions, a tissue diagnosis is often required for a definitive diagnosis prior to therapy[2-5]. Endoscopic ultrasound tissue acquisition (EUS-TA) has improved the ability for tissue diagnosis using a minimally invasive technique. The two modalities for EUS-TA, endoscopic ultrasound fine needle aspiration (EUS-FNA) and endoscopic ultrasound fine needle biopsy (EUS-FNB) vary in technique and utility. Common indications for EUS-TA include the diagnosis and staging of pancreaticobiliary and luminal GI malignancy, and assessing lymphadenopathy associated with luminal GI and lung cancers[6]. Additionally, EUS-TA aids in the evaluation of potentially neoplastic GI subepithelial lesions[6]. Comparative studies on the diagnostic ability of EUS-FNA and EUS-FNB have yielded conflicting results. Here we review prospective comparative data on EUS-FNA vs EUS-FNB for pancreatic masses, subepithelial lesions, and lymph node biopsy (Table 1-3).
| Ref. | Study design | Number of subjects | Needle size (FNA, FNB) | Diagnostic yield/specimen adequacy (EUS-FNA vs EUS-FNB) | Diagnostic accuracy (EUS-FNA vs EUS-FNB) | Number of passes needed (EUS-FNA vs EUS-FNB) | Comments |
| Bang et al[9], 2012 | RCT | 56 | 22 G, 22 G Procore | 66.7% vs 80% (NS) | N/A | 1.61 vs 1.28 (NS) | |
| Aadam et al[30], 2015 | RCT | 73 | Variable, variable | 78.4% vs 91.7% (NS) | 67.5% vs 83.3% (NS) | N/A | |
| Tian et al[31], 2018 | RCT | 36 | 22 G, 22 G ProCore | 83.3% vs 83.3% | N/A | 1.83 vs 1.11 (P = 0.049) | |
| Hedenstrom et al[33], 2018 | RCT, crossover | 68 | 25G, 22G reverse bevel Wilson Cook | N/A | 78% vs 69% (NS) | N/A | In a subset of non-pancreatic adenocarcinoma, combined modality (EUS-FNA + FNB) was significantly higher compared to EUS-FNA alone |
| Oppong et al[34], 2020 | RCT, crossover | 108 | Variable, variable Sharkcore | 71% vs 82% (OR 3.23, sig) | 64% vs 79% (OR 4.79, sig) | N/A | Shorter sampling time and pathology viewing time with EUS-FNB. Equivalent cost analysis. |
| Kandel et al[35], 2020 | RCT, crossover | 50 | 25 G, variable Sharkcore | 100% vs 86% (NS) | 100% vs 100% | N/A | Primary outcome of DNA concentration, significantly higher in EUS-FNB than in EUS-FNA |
| Wang et al[26], 2017 | Meta-analysis | 921 | Variable, variable | 81.4% vs 88.3% (OR 0.57, sig) | 84.0% vs 87.8% (NS) | Fewer in EUS-FNB | |
| Li et al[27], 2018 | Meta-analysis | 1382 | Variable, variable | 82.3% vs 89.4% (OR 1.83, sig) | 84.3% vs 89.6% (OR 1.62, sig) | Fewer in EUS-FNB | |
| Ref. | Study design | Number of subjects | Needle size (FNA, FNB) | Lesions sampled | Diagnostic yield/specimen adequacy (EUS-FNA vs EUS-FNB) | Diagnostic accuracy (EUS-FNA vs EUS-FNB) | Number of needle passes needed (EUS-FNA vs EUS-FNB) | Comments |
| Kim et al[47], 2014 | RCT | 22 | 22 G, 22 G Procore | All SELs | 20% vs 75% (P = 0.01) | N/A | 4 vs 2 (P = 0.025) | |
| Iwai et al[43], 2017 | RCT, crossover | 23 | Variable, variable Procore | Gastric SELs | 73.9% vs 91.3% (P = 0.12) | N/A | N/A | Histology positive significantly higher in EUS-FNB for 21 mm-30 mm lesions |
| Hedenstrom et al[48], 2018 | RCT, crossover | 70 | Variable, variable reverse-bevel Wilson-Cook | All SELs | N/A | 49% vs 83% (P < 0.001) | N/A | Extramural lesions lower sensitivity for EUS-FNA but not EUS-FNB) |
| Nagula et al[49], 2018 | RCT | 18 | Variable, variable Procore | All SELs | 83.3% vs 75% (NS) | N/A | 2 vs 2 (NS) |
| Ref. | Study design | Number of subjects | Needle size (FNA, FNB) | Lymph nodes sampled | Diagnostic yield/specimen adequacy (EUS-FNA vs EUS-FNB) | Diagnostic accuracy (EUS-FNA vs EUS-FNB) | Number of needle passes needed (EUS-FNA vs EUS-FNB) | Comments |
| Nagula et al[49], 2018) | RCT | 46 | Variable, variable Procore | All lymph nodes | 92.9% vs 94.4% (NS) | N/A | 2 vs 2 (NS) | |
| de Moura et al[52], 2020) | Retrospective study of prospectively collected data | 209 | Variable, variable | All lymph nodes | N/A | 78.8% vs 83.2% (NS) | N/A | For peri-hepatic lesions, EUS-FNB was significantly more accurate |
EUS-FNA was first introduced in 1992. It is often combined with rapid onsite evaluation (ROSE) to improve diagnostic ability[7,8]. EUS-FNA is now standard of care for sampling pancreatic solid masses, subepithelial lesions, and lymph nodes, among others. The European Society of Gastroenterology and American Society of Gastroenterology recommend EUS-FNA as first line for diagnosing pancreatic lesions[9-11].
Marked variability exists in EUS-FNA equipment and technique. Several different needle sizes are available including 19 G, 20 G, 22 G, and 25 G. Additionally, variability exists in aspiration technique, including the use of negative pressure suction (used with either a 5 mL or 10 mL syringe) or slow stylet pull. The aspirate from EUS-FNA is often sufficient for cytology and adequate for diagnosis, with diagnostic accuracy ranging from 77% to 95% for pancreatic masses[9,10]. Given its minimally invasive technique and small needle size, EUS-FNA has low rates of morbidity[12].
However, several limitations exist for EUS-FNA which obtains a cytological specimen. EUS-FNA is limited by an inability to obtain histological architecture, and the inability to perform immunohistochemical analysis and molecular profiling. This is of particular importance as certain neoplasms, such as stromal cell tumors and lymphomas, may be difficult to diagnose without histologic samples, as their tissue architecture and morphology are essential for accurate pathologic assessment and histochemical studies[9,13-17]. Furthermore, with the increased attention on personalized or precision medicine in oncology, a sufficient tissue sample to perform next generation sequencing is required. Current National Comprehensive Cancer Network guidelines recommend germline testing for any patient with confirmed pancreatic cancer using comprehensive gene panels for hereditary cancer syndromes, as well as tumor/somatic gene profiling for patients with locally advanced or metastatic disease to identify mutations that may benefit from anti-cancer therapy. Testing on tumor tissue is preferred; however, cell-free DNA testing can also be considered[18]. There is uncertainty whether EUS-FNA will be able to routinely provide adequate material for these studies[6].
In an attempt to overcome the limitations of EUS-FNA, EUS-FNB was first introduced in the early 2000s to obtain tissue specimens as opposed to aspiration-based cytology. With the goal of evaluating tissue core, EUS-FNB provided novel needles for improved diagnostic accuracy.
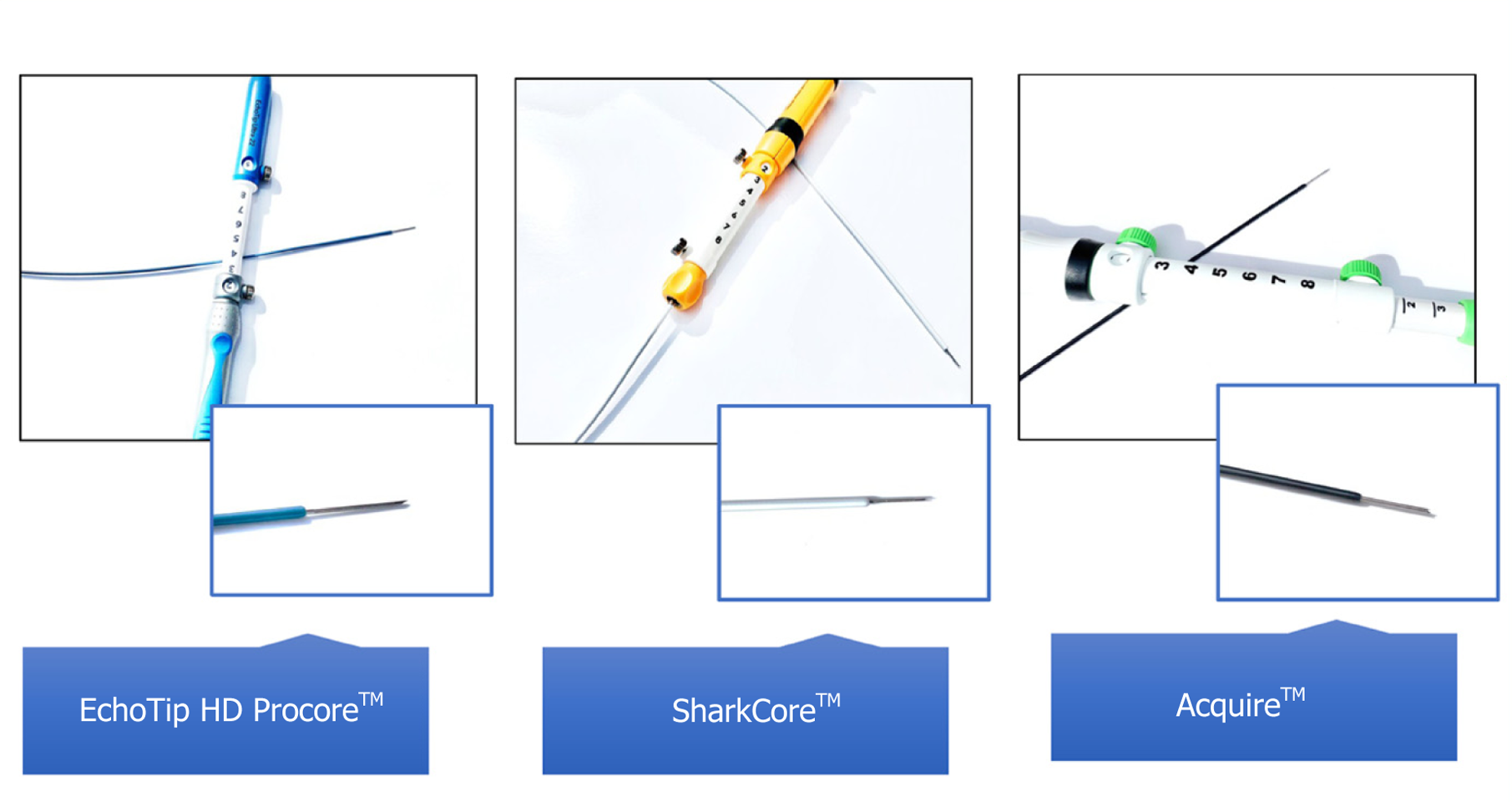
Early models of EUS-FNB utilized Trucut needle biopsy, with a tissue penetrating stylet within an outer cannula. The 19 G Trucut FNB proved more accurate than EUS-FNA for diagnosing lymphomas and stromal tumors, but was limited by mechanical failure when attempting to biopsy pancreatic head masses and duodenal lesions due to the torqued echoendoscope and mechanical friction[16,19]. Newer models, including EchoTip HD ProCoreTM (Wilson-Cook Medical Inc., Winston-Salem, NC, United States) is available in 19-25 G, and provides two cutting surfaces, a tip and reverse bevel, to further preserve histological architecture[1]. The reverse or opposing cutting bevel design of the EUS-FNB needle allows for the biopsy of core histopathologic tissue. This aspect has the potential advantage of improving diagnostic performance, but also allowing a wide range of follow-up testing[20]. Immunohistochemistry, which is required for the diagnosis of autoimmune pancreatitis, lymphoma, and metastasis, can be performed on the tissue core. Furthermore, molecular analysis, which is now standard of care for pancreatic malignancies, can also be performed. Other needles, including SharkCoreTM (Medtronic Inc., Sunnyvale, CA, United States) and AcquireTM (Boston Scientific, Malborough, MA, United States), (Figure 1) may achieve even higher diagnostic accuracy[21,22].
Studies have demonstrated high diagnostic yields of core specimens with EUS-FNB with fewer needle passes[23-25]. The potential concern for increased bleeding when using EUS-FNB is offset by the fewer passes required for diagnosis.
Several comparative trials have evaluated EUS-FNA vs EUS-FNB. Interpreting the conflicting data is challenging, as trials are plagued by heterogeneity in every stage of comparison. Reported outcomes as well as definition of those outcomes vary between studies. For example, inconsistent use of the term “diagnostic accuracy” and “diagnostic adequacy” creates confusion. Furthermore, heterogeneity exists within equipment use (needle size), and technique (suction vs slow pull; specified number of passes). Additionally, designing strong randomized trials is limited by the inability to blind endosonographers, and sometimes cytopathologists, to the type of needle used[26-57].
In compiling this review article, we performed a literature search utilizing PUBMED, EMBASE, and Google Scholar for comparative trials of EUS-FNA vs EUS-FNB for pancreatic mass lesions, subepithelial lesions, and lymph nodes. A total of 77 articles were identified. Trials were excluded if they were retrospective (n = 26), if they did not directly compare EUS-FNA and EUS-FNB (n = 18), or if they were incomplete manuscripts (n = 6). Any study performed on a variety of mass lesions without subcategories for the aforementioned groups was also excluded (n = 4).
Pancreatic adenocarcinoma is characterized by a poor prognosis, with a 5-year survival rate of 5%-6%[27]. Pancreatic adenocarcinoma may be difficult to differentiate from other pancreatic mass lesions based on cross-sectional imaging and abdominal ultrasound[2-5]. The reported sensitivity of EUS in the detection of pancreatic cancer is between 94% and 100%[28]. Compared to computed tomography (CT), EUS can detect up to 14% of pancreatic tumors that were not visualized on CT, especially tumors smaller than 20 mm[11]. As such, EUS is currently the standard method for tissue diagnosis of pancreatic masses[11].
Tissue sampling of pancreatic mass lesions by EUS is vital in diagnosis. Several sampling approaches are possible depending on the location of the pancreatic mass lesion. A trans-duodenal approach may be optimal for lesions in the pancreatic head, while the transgastric approach is more appropriate for lesions in the pancreatic body and tail. Bang et al[29] proposed an algorithm for needle selection based on anatomical site; a 25 G needle for the trans-duodenal approach and a 22 G or 25 G for all other punctures.
Comparative trials of EUS-FNA vs EUS-FNB for pancreatic mass lesions focus mostly on safety, diagnostic accuracy, sample adequacy for diagnosis and further testing (Table 1).
Bang et al[9] performed the earliest randomized controlled trial (RCT) comparing EUS-FNA and EUS-FNB. The study randomized 56 patients to receive either EUS-FNA 22 G or EUS-FNB 22 G ProCore for pancreatic mass lesions, with the primary outcome being the number of passes required to establish a diagnosis with ROSE. They found no significant difference in the median number of passes required to establish on-site diagnosis, and overall similar rates of diagnosis were achieved within 3 passes (100% EUS-FNA, 89% EUS-FNB). Incomplete diagnosis by EUS-FNB was due to diagnostic failure in two patients, and technical failure in 1 patient. Procedural complications among the two techniques were similar (one patient with post-procedural abdominal pain in the EUS-FNA cohort, and one patient with pancreatitis in the EUS-FNB cohort). With regard to secondary outcomes, EUS-FNA had a higher proportion of samples with histologic core tissue present (100% vs 88.3%, not-significant) but EUS-FNB had a higher percentage of histologic core tissue optimal for histochemical testing.
It is noteworthy that their technique varied from subsequent trials in several respects, and perhaps limited the study’s generalizability. First, ROSE was carried out for all specimens, thereby possibly preferentially inflating the diagnostic ability of EUS-FNA. Additionally, they utilized an earlier model of FNB, the 22 G Echotip ProCoreTM devise. Lastly, they utilized fewer needle movements for the EUS-FNB cohort (only 4 movements to and fro).
A subsequent larger RCT by Aadam et al[30] similarly showed no difference in diagnostic yield or specimen adequacy between EUS-FNA and EUS-FNB in patients with pancreatic lesions.
A 2018 RCT by Tian et al[31] of 36 patients similarly showed no superiority in diagnostic accuracy between EUS-FNA and EUS-FNB; although they did find a difference in the number of passes needed to make a diagnosis. Similar to Bang et al[9], patients were randomized to either EUS-FNA or EUS-FNB ProCore for solid pancreatic masses, although ROSE was not performed on any of the specimens. For the primary outcome of diagnostic yield, the authors found identical results (83%). However, among their secondary outcomes, EUS-FNB required fewer passes to make a diagnosis (1.11 vs 1.83, P < 0.05). It is noteworthy that a smaller percentage of their cohort were diagnosed with pancreatic adenocarcinoma (66.7%) compared to other trials. There were no complications in either cohort in their study.
Similar findings were also demonstrated in a larger, more recent RCT performed by Chen et al[32]. The authors randomized 235 patients with pancreatic mass lesions to EUS-FNA + ROSE (n = 120) vs EUS-FNB (22 G or 25 G Fork-tip needle, n = 115). For the primary outcome of diagnostic accuracy, the authors found no difference (92.2% vs 93.3%, respectively). However, among the secondary outcomes, EUS-FNB was associated with fewer needle passes to make a diagnosis compared to EUS-FNA + ROSE (2.3 vs 3.0) and decreased procedure time (19.3 min vs 22.7 min). There were no adverse events in the EUS-FNB cohort, and three adverse events in the EUS-FNA cohort (2 pancreatitis, 1 bleeding).
In contradistinction to the aforementioned articles, in several trials patients underwent both EUS-FNA and EUS-FNB in a crossover study design, thereby allowing direct comparison between specimen procurement in the same patients and providing an internal control. Hedenstrom et al[33] randomized 68 patients with a pancreatic mass to receive either EUS-FNA (25 G) followed by EUS-FNB (22 G), 1 pass each, or vice versa. A reverse bevel EUS-FNB 22 G needle was used (Wilson-Cook Medical) and further passes were performed by alternating the two needles. They utilized similar suction (10 cc) and fanning techniques for both EUS-FNA and EUS-FNB. ROSE was carried out for the majority of both EUS-FNA and EUS-FNB samples. The primary outcome of diagnostic accuracy was not significantly different between the two methods of tissue acquisition. No adverse events were recorded.
Utilizing a newer model of EUS-FNB Fork tip (SharkCoreTM FNB Needle), Oppong et al[34] randomized 108 patients with pancreatic mass lesions to EUS-FNA and then EUS-FNB, 3 passes each, or vice versa. The primary endpoint was diagnostic per
Other studies have utilized alternative endpoints to diagnostic accuracy or adequacy. As discussed previously, obtaining a diagnosis for pancreatic adenocarcinoma may still require further testing for personalized medicine, and therefore additional tissue may be required. Kandel et al[35] performed a RCT of 50 consecutive patients to assess adequacy for genomic profiling. In their study, they randomized patients to EUS-FNA followed by EUS-FNB (or vice versa) in a randomized order. They also utilized the SharkCoreTM FNB needle. The first pass with each needle was used for histology, and subsequent passes were used to collect DNA. They found that EUS-FNB yielded significantly higher mean DNA concentrations compared to EUS-FNA (5.930 μg/mL vs 3.365 μg/mL, P = 0.01).
These findings have unclear clinical significance, since despite the quantitative difference in DNA acquired, both acquisition techniques yielded sufficient DNA for next generation sequencing (approximately 10 ng/μL). Furthermore, it is noteworthy that the EUS-FNA utilized a smaller needle (25 G) compared to both EUS-FNB needles (19 G or 22 G). This was likely done to maximize diagnostic accuracy, which was similar in both cohorts (100% final diagnosis in both), but may come at the expense of the DNA quantity acquired.
Several systematic reviews and meta-analyses have attempted to summarize the conflicting data on pancreatic lesions. However, heterogeneity in the studies included and outcomes measured further perpetuate the confusion.
In 2017, Wang et al[26] performed a meta-analysis on 8 RCTs to determine diagnostic accuracy. Significant variability existed within needle size and suction technique between the trials. For diagnostic accuracy, they found no significant difference between EUS-FNA (84%) and EUS-FNB (88%, OR 0.72; 95%CI: 0.49-1.07). Among the 5 trials that reported specimen adequacy, and the four trials reporting the number of needle passes required, EUS-FNB demonstrated superiority (OR 0.57, 95%CI: 0.37-0.89; and OR 0.86, 95%CI: 0.45-1.26, respectively). Among the five studies that reported adverse events, the rates were low and not significantly different between the two groups (2/313 in the EUS-FNA group, and 4/311 in the EUS-FNB group), and specific complications were not mentioned.
One year later, in 2018, Li et al[27] performed a meta-analysis with the same 8 RCTs, and included an additional 3 RCTs, and yielded different results. They found that EUS-FNB had significantly better specimen adequacy (OR 1.83, 95%CI: 1.27-2.64), and higher diagnostic accuracy (OR 1.62, 95%CI: 1.17-2.26) than EUS-FNA, again with fewer needle passes (MD -0.69, 95%CI: -1.18 to -0.2). There was no difference in complications or technical success.
However, a larger 2019 meta-analysis by Facciorusso et al[20] of 27 RCTs found different results. They evaluated diagnostic accuracy, and found no significance difference between needle type (EUS-FNA or EUS-FNB) or needle size. The authors summarized the adverse events as rare among their studies; however, most studies did not itemize the etiology of the adverse events. The only studies that specifically reported bleeding episodes, all reported bleeding in the EUS-FNA cohort. Of note, the authors performed a network meta-analysis technique, thereby utilizing both direct RCT (EUS-FNA vs EUS-FNB) as well as indirect evidence (RCT of EUS-FNA vs EUS-FNA, or EUS-FNB vs EUS-FNB) and then extrapolated the data. Only 14 of the 27 trials included were actually EUS-FNA vs EUS-FNB. As such, their results should be interpreted with caution.
Conflicting data exist among prospective studies evaluating the superiority of different EUS-TA techniques. Taken together, both methods provide overall high, and comparable, diagnostic accuracy and specimen adequacy for diagnosis. Adverse events, including bleeding, are rare in both techniques, with pancreatitis being the most common adverse event. Multiple trials have demonstrated that fewer passes are required for EUS-FNB compared to EUS-FNA. The ramifications of this, with the resulting decreased procedural time and likely fewer adverse events, may prove beneficial when applied broadly, but larger trials are required for further elucidation. Additionally, clinical benefit from the increased quantity of tissue obtained remains unclear, if standardized testing and next generation sequencing can be performed on all samples.
Subepithelial lesions (SELs) of the GI tract are tumors that originate from the muscularis mucosa, submucosa, or muscularis propria[36]. Initial management of SELs focuses on proper diagnosis and determination of malignant potential, to guide further resection recommendations. EUS is the most accurate imaging method for evaluating SELs of the GI tract[37-39], because it can delineate the individual histologic layers and likely site of tumor origin. Certain SELs have a distinct endoscopic appearance, such as lipomas, duplication cysts, and ectopic pancreas, and endoscopic appearance may be considered diagnostic[36]. However, endoscopic appearance alone is not sufficient for diagnosis in many cases, such as hypoechoic and heterogeneous lesions from the submucosal and muscularis propria, and tissue acquisition is often required. Standard biopsy forceps and jumbo biopsy forceps (bite on bite technique) have low diagnostic yield[40,41].
EUS-FNA is the most widely used method for obtaining SEL tissue arising from the submucosal and muscularis propria layer[36]. However, the diagnostic accuracy of EUS-FNA is variable, ranging from 34% to 93%[39,42]. Additionally, the amount of cytological material obtained by EUS-FNA is often insufficient for the immunohistochemical staining required to differentiate different SELs[43].
There are few prospective comparison trials of EUS-FNA and EUS-FNB focused solely on SELs, although several larger prospective trials contained cohorts of SELs (Table 2). We excluded trials that did not perform subgroup analysis on this SEL subgroup in isolation[30,44-46].
The first RCT focused solely on SELs was performed by Kim et al[47] in 2014. The authors randomized 22 patients with GI SELs of all types to either EUS-FNA (n = 10) or EUS-FNB (n = 12, ProCore). The patients did not receive both methods of tissue acquisition. The cohort was comprised of mostly gastric SELs (17/22), and mainly arising from the muscularis propria (20/22). The needle size was dependent on tumor diameter at the time of EUS, with a 22 G needle used if the tumor was estimated to be < 30 mm, and 19 G used if the tumor was > 30 mm. The authors utilized the unique endpoint of the number of passes required to obtain macroscopically optimal core samples. Since ROSE was not carried out at all sites, the endoscopist immediately inspected the material for the presence of tissue core, defined as whitish pieces of tissue with apparent bulk. If present, no further passes were obtained. However, if absent, the endoscopist proceeded with an additional pass with a maximum of 3 passes. If the sample still did not contain macroscopic tissue core, the number of passes was recorded at 4, and the patient crossed over to the other cohort. The authors found that the median number of needle passes required to obtain macroscopically optimal core sampled by EUS-FNB was significantly lower than that by EUS-FNA (2 vs 4, P = 0.025). Despite being macroscopically defined as optimal core samples, the core samples were suboptimal for microscopic analysis in three cases. Overall, the rates of obtaining macroscopically and histologically optimal core samples with EUS-FNB (92% and 75%, respectively) were superior to EUS-FNA (30% and 20%, respectively). No technical difficulties were encountered, and one patient in the entire cohort developed post-procedural bleeding which was managed conservatively. A limitation of the study design was lack of blinding of the endoscopist who assessed the primary endpoint.
A follow-up study by Iwai et al[43] in 2017, focused solely on gastric SELs arising from the muscularis propria and randomized 24 patients to receive either EUS-FNA followed by EUS-FNB or vice versa. The two needles were used alternatively to puncture the same lesion with a total of four punctures per session. Similar to Kim et al[47], needle size was dependent on tumor size on EUS, and the ProCore needle was used for all EUS-FNB. The primary outcome was diagnostic yield. The authors found that the rate of correct diagnosis on immunohistochemical staining tended to be higher for EUS-FNB (91.3%) than for EUS-FNA (73.9%, P = 0.120), although this failed to reach statistical significance. When sub-characterized by tumor size, they found that EUS-FNB had significantly higher rates of positive histology among tumors 21-30 mm. The study was limited by sample size and was underpowered, as several of their findings trended towards significance.
A larger 2018 RCT performed by Hedenstrom et al[48] similarly found superiority of EUS-FNB to EUS-FNA for SELs, utilizing the reverse bevel ProCore EUS-FNB needle. The study randomized 70 patients with GI SELs to dual sampling with EUS-FNA and EUS-FNB in an alternating fashion until the yield was regarded as satisfactory by the cytotechnician, with a maximum of six passes. Similar to Iwai et al[43], in the absence of ROSE, gross examination was performed by the endoscopist. The cohort consisted of mostly gastric SELs (66/70). The study found significantly higher overall diagnostic accuracy for EUS-FNB than EUS-FNA (83% vs 49%, P < 0.001). A trend of lower sensitivity of EUS-FNA for extramural lesions compared to intramural lesions was also observed, a trend that did not exist for EUS-FNB. The authors hypothesized that this may be related to increased mobility of extramural lesions, preferentially affecting EUS-FNA diagnostic accuracy. The characterization of intramural and extramural was based on appearance at EUS. The authors reported few adverse events.
These findings are in contrast to Nagula et al[49] who found in the SELs cohort (n = 18) that there was no significant difference in diagnostic yield between EUS-FNB ProCore and EUS-FNB (EUS-FNB 75% vs EUS-FNA 83.3%, P = 0.754).
Lymphadenopathy may arise from many different etiologies, ranging from benign inflammatory or infectious, to malignant etiologies. Evaluation of lymphadenopathy must include tissue sampling, as lymph node size has demonstrated poor specificity for differentiating malignant from benign lymphadenopathy[50,51]. Clarifying the malignant potential of lymphadenopathy is essential for clinical management[52].
The modality for sampling lymph nodes depends on anatomic location. For mediastinal lymph node sampling, EUS-TA is safer and less invasive compared to alternative techniques[10]. Additionally, for abdominal lymph nodes, EUS-sampling is successful in 92% of patients[53].
EUS-TA for lymph nodes is typically performed with EUS-FNA. However, the sensitivity of EUS-FNA for providing material for cytological evaluation is suboptimal, with reported rates of 88%-96%[54]. The suboptimal results are often attributed to damaged lymph node architecture[51,54]. This limitation of EUS-FNA is important in the evaluation of lymphadenopathy of unknown etiology, where the differential diagnosis includes lymphoma, metastasis, mycobacterial infection, and sarcoidosis, and core biopsy with preservation of lymph node architecture is particularly important for diagnostic purposes[53,55,56].
We found no published prospective RCTs of EUS-FNA vs EUS-FNB for only lymph node biopsy. In the large RCT by Nagula et al[49] mentioned above, the subgroup of lymph node biopsies (n = 46) found no difference between EUS-FNA and EUS-FNB Procore in diagnostic yield (92.9% vs 94.4%) or number of passes needed to make a diagnosis (median 2, P = 0.43) (Table 3).
De Moura et al[52] performed a prospective study comparing EUS-FNA vs EUS-FNB exclusively for lymph node diagnosis. The authors performed an analysis on a prospectively collected database of 209 patients undergoing either EUS-FNA (n = 108) or EUS-FNB (n = 101) to evaluate lymph nodes. No predefined protocol was used in the study, and as such several different EUS-FNB needles were used including Acquire, SharkCore, and ProCore. The cohort consisted mostly of peri-hepatic lymph nodes (60%) followed by peri-pancreatic (10.4%) and mediastinal (10.4%), and were mostly accessed via a transgastric approach (45%). The pathology of most specimens was benign (61%). Their primary outcome was diagnostic yield from cytological and histological analysis with and without immunohistochemical staining.
Overall, the authors found similar diagnostic accuracy between EUS-FNA and EUS-FNB (78.8% vs 83.2%, P = 0.423). However, the specificity for EUS-FNB demonstrated significant superiority (100% vs 93.62%, P = 0.01). In the subgroup analysis, EUS-FNB showed significantly higher sensitivity and specificity for abdominal lymph nodes. The diagnostic accuracy tended to be greater in the EUS-FNB cohort, but this failed to reach statistical significance. Following further analysis of lymph node location, EUS-FNB was associated with significantly higher sensitivity, specificity, and overall diagnostic accuracy for peri-hepatic lesions (88.9% vs 70.5%, P = 0.038).
Taken together, the study forms an important backdrop for further research, and argues for consideration of EUS-FNB over EUS-FNA for lymph node biopsy, specifically for peri-hepatic lesions.
Additional studies have assessed the additive benefit of sampling lesions with both EUS-FNA and EUS-FNB. One such study by Hedenstrom et al[33] found that EUS-FNA/FNB compared to EUS-FNA alone had a higher diagnostic sensitivity for pancreatic tumors (89% vs 69%, P = 0.02), but not for pancreatic adenocarcinoma. However, compared to the diagnostic accuracy of EUS-FNB in isolation, Keswani et al[57] found no additional diagnostic accuracy by including EUS-FNA for pancreatic adenocarcinoma.
Endoscopic ultrasound tissue acquisition is routinely utilized in the evaluation of pancreatic mass lesions, subepithelial lesions, and lymph node biopsies. Ongoing confusion surrounds the ideal modality for EUS-TA, whether by EUS-FNA or EUS-FNB. While more robust comparative clinical trials exist for pancreatic lesions compared to subepithelial lesions and lymph nodes, the data continue to be mixed. Randomized controlled trials with homogenous populations and homogenous sampling protocols are needed in order to truly understand which needle is superior.
Based on the literature reviewed in this article, the authors conclude the following: EUS-FNA and EUS-FNB both provide high diagnostic accuracy, with low technical failure and adverse events, and thus either needle can be utilized for EUS-TA of pancreatic lesions, subepithelial lesions, and lymph nodes. In our experience we prefer FNB with a new generation needle as it allows us fewer passes of the needle, allows us to forgo ROSE which adds significant time and resources to a procedure, and gives a sample suitable for molecular testing. When increased quantity of DNA is desired for next generation sequencing, the utilization of EUS-FNB should be considered. For extramural subepithelial lesions, the utilization of EUS-FNB should be considered. Despite the dearth of prospective literature, we would recommend EUS-FNB for lymph node biopsy, specifically for peri-hepatic nodes.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Havre RF, Tomizawa M, Zhang Y S-Editor: Zhang H L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Conti CB, Cereatti F, Grassia R. Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come? World J Gastrointest Endosc. 2019;11:454-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Brand B, Pfaff T, Binmoeller KF, Sriram PV, Fritscher-Ravens A, Knöfel WT, Jäckle S, Soehendra N. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand J Gastroenterol. 2000;35:1221-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Delbeke D, Pinson CW. Pancreatic tumors: role of imaging in the diagnosis, staging, and treatment. J Hepatobiliary Pancreat Surg. 2004;11:4-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Pannala R, Hallberg-Wallace KM, Smith AL, Nassar A, Zhang J, Zarka M, Reynolds JP, Chen L. Endoscopic ultrasound-guided fine needle aspiration cytology of metastatic renal cell carcinoma to the pancreas: A multi-center experience. Cytojournal. 2016;13:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Rickes S, Unkrodt K, Neye H, Ocran KW, Wermke W. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol. 2002;37:1313-1320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Wani S, Muthusamy VR, McGrath CM, Sepulveda AR, Das A, Messersmith W, Kochman ML, Shah J. AGA White Paper: Optimizing Endoscopic Ultrasound-Guided Tissue Acquisition and Future Directions. Clin Gastroenterol Hepatol. 2018;16:318-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, Eltoum IA. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Hikichi T, Irisawa A, Bhutani MS, Takagi T, Shibukawa G, Yamamoto G, Wakatsuki T, Imamura H, Takahashi Y, Sato A, Sato M, Ikeda T, Hashimoto Y, Tasaki K, Watanabe K, Ohira H, Obara K. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009;44:322-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Dumonceau JM, Deprez PH, Jenssen C, Iglesias-Garcia J, Larghi A, Vanbiervliet G, Aithal GP, Arcidiacono PG, Bastos P, Carrara S, Czakó L, Fernández-Esparrach G, Fockens P, Ginès À, Havre RF, Hassan C, Vilmann P, van Hooft JE, Polkowski M. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy. 2017;49:695-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | ASGE Standards of Practice Committee, Eloubeidi MA, Decker GA, Chandrasekhara V, Chathadi KV, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Saltzman JR, Sharaf R, Shergill AK, Cash BD, DeWitt JM. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc. 2016;83:17-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Bhutani MS, Gress FG, Giovannini M, Erickson RA, Catalano MF, Chak A, Deprez PH, Faigel DO, Nguyen CC; No Endosonographic Detection of Tumor (NEST) Study. The No Endosonographic Detection of Tumor (NEST) Study: a case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy. 2004;36:385-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Best Pract Res Clin Gastroenterol. 2009;23:743-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: prospects and problems. Pancreatology. 2007;7:163-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Levy MJ, Wiersema MJ. EUS-guided Trucut biopsy. Gastrointest Endosc. 2005;62:417-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-36; quiz 751, 753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Tempero MM, M. Al-Hawary, M. Behrman, S. Benson III, Al. Cardin, D. CHa, C. Chiorean, E. Chung, V. Czito, B. Del Chiaro, M. Dillhoff, M. Donahue, T. Dotan, E. Ferrone, C. Fountzilas, C. Hardacre, J. Hawkins, W. Klute, K. Ko, A. LoConte, N. Lowy, A. Moravek, C. Nakakura, E. Narang, A. Obando, J. Polanco, P. Reddy, S. Reyngold, M. Scaife, C. Shen, J. Vollmer C. Wolff, R. Wolpin, B. NCCN Clinical Practice Guidelines in Oncology Pancreatic Adenocarcinoma Version 1.2021 2020. [cited 2021 January 1]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. [Cited in This Article: ] |
| 19. | Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc. 2004;59:185-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Facciorusso A, Wani S, Triantafyllou K, Tziatzios G, Cannizzaro R, Muscatiello N, Singh S. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: a network meta-analysis. Gastrointest Endosc 2019; 90: 893-903. e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 21. | Adler DG, Muthusamy VR, Ehrlich DS, Parasher G, Thosani NC, Chen A, Buscaglia JM, Appannagari A, Quintero E, Aslanian H, Taylor LJ, Siddiqui A. A multicenter evaluation of a new EUS core biopsy needle: Experience in 200 patients. Endosc Ultrasound. 2019;8:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 22. | DiMaio CJ, Kolb JM, Benias PC, Shah H, Shah S, Haluszka O, Maranki J, Sharzehi K, Lam E, Gordon SR, Hyder SM, Kaimakliotis PZ, Allaparthi SB, Gress FG, Sethi A, Shah AR, Nieto J, Kaul V, Kothari S, Kothari TH, Ho S, Izzy MJ, Sharma NR, Watson RR, Muthusamy VR, Pleskow DK, Berzin TM, Sawhney M, Aljahdi E, Ryou M, Wong CK, Gupta P, Yang D, Gonzalez S, Adler DG. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): results of a large North American multicenter study. Endosc Int Open. 2016;4:E974-E979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 67] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 23. | Fabbri C, Luigiano C, Maimone A, Tarantino I, Baccarini P, Fornelli A, Liotta R, Polifemo A, Barresi L, Traina M, Virgilio C, Cennamo V. Endoscopic ultrasound-guided fine-needle biopsy of small solid pancreatic lesions using a 22-gauge needle with side fenestration. Surg Endosc. 2015;29:1586-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Iwashita T, Nakai Y, Samarasena JB, Park DH, Zhang Z, Gu M, Lee JG, Chang KJ. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Larghi A, Iglesias-Garcia J, Poley JW, Monges G, Petrone MC, Rindi G, Abdulkader I, Arcidiacono PG, Costamagna G, Biermann K, Bories E, Doglioni C, Dominguez-Muñoz JE, Hassan C, Bruno M, Giovannini M. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc. 2013;27:3733-3738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Wang J, Zhao S, Chen Y, Jia R, Zhang X. Endoscopic ultrasound guided fine needle aspiration versus endoscopic ultrasound guided fine needle biopsy in sampling pancreatic masses: A meta-analysis. Medicine (Baltimore). 2017;96:e7452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Li H, Li W, Zhou QY, Fan B. Fine needle biopsy is superior to fine needle aspiration in endoscopic ultrasound guided sampling of pancreatic masses: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e0207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Guedes HG, Moura DTH, Duarte RB, Cordero MAC, Santos MELD, Cheng S, Matuguma SE, Chaves DM, Bernardo WM, Moura EGH. A comparison of the efficiency of 22G versus 25G needles in EUS-FNA for solid pancreatic mass assessment: A systematic review and meta-analysis. Clinics (Sao Paulo). 2018;73:e261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Bang JY, Ramesh J, Trevino J, Eloubeidi MA, Varadarajulu S. Objective assessment of an algorithmic approach to EUS-guided FNA and interventions. Gastrointest Endosc. 2013;77:739-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Aadam AA, Wani S, Amick A, Shah JN, Bhat YM, Hamerski CM, Klapman JB, Muthusamy VR, Watson RR, Rademaker AW, Keswani RN, Keefer L, Das A, Komanduri S. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016;4:E497-E505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Tian L, Tang AL, Zhang L, Liu XW, Li JB, Wang F, Shen SR, Wang XY. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: a prospective comparison study. Surg Endosc. 2018;32:3533-3539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Chen YI, Chatterjee A, Berger R, Kanber Y, Wyse J, Lam E, Gan I, Auger M, Kenshil S, Telford J, Donnellan F, Quinlan J, Lutzak G, Alshamsi F, Parent J, Waschke K, Alghamdi A, Barkun J, Metrakos P, Chaudhury P, Martel M, Dorreen A, Candido K, Miller C, Adam V, Barkun A, Zogopoulos G, Wong C. Endoscopic ultrasound (EUS)-guided fine needle biopsy alone vs. EUS-guided fine needle aspiration with rapid onsite evaluation in pancreatic lesions: a multicenter randomized trial. Endoscopy. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 33. | Hedenström P, Demir A, Khodakaram K, Nilsson O, Sadik R. EUS-guided reverse bevel fine-needle biopsy sampling and open tip fine-needle aspiration in solid pancreatic lesions - a prospective, comparative study. Scand J Gastroenterol. 2018;53:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Oppong KW, Bekkali NLH, Leeds JS, Johnson SJ, Nayar MK, Darné A, Egan M, Bassett P, Haugk B. Fork-tip needle biopsy versus fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized crossover study. Endoscopy. 2020;52:454-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Kandel P, Nassar A, Gomez V, Raimondo M, Woodward TA, Crook JE, Fares NS, Wallace MB. Comparison of endoscopic ultrasound-guided fine-needle biopsy versus fine-needle aspiration for genomic profiling and DNA yield in pancreatic cancer: a randomized crossover trial. Endoscopy. 2021;53:376-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 36. | Standards of Practice Committee, Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Khashab MA, Lightdale JR, Muthusamy VR, Shaukat A, Qumseya BJ, Wang A, Wani SB, Yang J, DeWitt JM. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85:1117-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 37. | Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Landi B, Palazzo L. The role of endosonography in submucosal tumours. Best Pract Res Clin Gastroenterol. 2009;23:679-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Polkowski M, Gerke W, Jarosz D, Nasierowska-Guttmejer A, Rutkowski P, Nowecki ZI, Ruka W, Regula J, Butruk E. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy. 2009;41:329-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal DV, Kim MK. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Ji JS, Lee BI, Choi KY, Kim BW, Choi H, Huh M, Chung WC, Chae HS, Chung IS. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med. 2009;24:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Wani S, Muthusamy VR, Komanduri S. EUS-guided tissue acquisition: an evidence-based approach (with videos). Gastrointest Endosc 2014; 80: 939-59. e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 43. | Iwai T, Kida M, Imaizumi H, Miyazawa S, Okuwaki K, Yamauchi H, Kaneko T, Hasegawa R, Miyata E, Koizumi W. Randomized crossover trial comparing EUS-guided fine-needle aspiration with EUS-guided fine-needle biopsy for gastric subepithelial tumors. Diagn Cytopathol. 2018;46:228-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Asokkumar R, Yung Ka C, Loh T, Kah Ling L, Gek San T, Ying H, Tan D, Khor C, Lim T, Soetikno R. Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): a randomized study. Endosc Int Open. 2019;7:E955-E963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 45. | van Riet PA, Erler NS, Bruno MJ, Cahen DL. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: a systemic review and meta-analysis. Endoscopy. 2021;53:411-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 46. | van Riet PA, Larghi A, Attili F, Rindi G, Nguyen NQ, Ruszkiewicz A, Kitano M, Chikugo T, Aslanian H, Farrell J, Robert M, Adeniran A, Van Der Merwe S, Roskams T, Chang K, Lin F, Lee JG, Arcidiacono PG, Petrone M, Doglioni C, Iglesias-Garcia J, Abdulkader I, Giovannini M, Bories E, Poizat F, Santo E, Scapa E, Marmor S, Bucobo JC, Buscaglia JM, Heimann A, Wu M, Baldaque-Silva F, Moro CF, Erler NS, Biermann K, Poley JW, Cahen DL, Bruno MJ. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc. 2019;89:329-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 47. | Kim GH, Cho YK, Kim EY, Kim HK, Cho JW, Lee TH, Moon JS; Korean EUS Study Group. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014;49:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 48. | Hedenström P, Marschall HU, Nilsson B, Demir A, Lindkvist B, Nilsson O, Sadik R. High clinical impact and diagnostic accuracy of EUS-guided biopsy sampling of subepithelial lesions: a prospective, comparative study. Surg Endosc. 2018;32:1304-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Nagula S, Pourmand K, Aslanian H, Bucobo JC, Gonda TA, Gonzalez S, Goodman A, Gross SA, Ho S, DiMaio CJ, Kim MK, Pais S, Poneros JM, Robbins DH, Schnoll-Sussman F, Sethi A, Buscaglia JM; New York Endoscopic Research Outcomes Group (NYERO). Comparison of Endoscopic Ultrasound-Fine-Needle Aspiration and Endoscopic Ultrasound-Fine-Needle Biopsy for Solid Lesions in a Multicenter, Randomized Trial. Clin Gastroenterol Hepatol 2018; 16: 1307-1313. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Dietrich CF, Jenssen C, Arcidiacono PG, Cui XW, Giovannini M, Hocke M, Iglesias-Garcia J, Saftoiu A, Sun S, Chiorean L. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound. 2015;4:176-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Lisotti A, Ricci C, Serrani M, Calvanese C, Sferrazza S, Brighi N, Casadei R, Fusaroli P. Contrast-enhanced endoscopic ultrasound for the differential diagnosis between benign and malignant lymph nodes: a meta-analysis. Endosc Int Open. 2019;7:E504-E513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | de Moura DTH, McCarty TR, Jirapinyo P, Ribeiro IB, Farias GFA, Ryou M, Lee LS, Thompson CC. Endoscopic Ultrasound Fine-Needle Aspiration versus Fine-Needle Biopsy for Lymph Node Diagnosis: A Large Multicenter Comparative Analysis. Clin Endosc. 2020;53:600-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Puri R, Mangla R, Eloubeidi M, Vilmann P, Thandassery R, Sud R. Diagnostic yield of EUS-guided FNA and cytology in suspected tubercular intra-abdominal lymphadenopathy. Gastrointest Endosc. 2012;75:1005-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Puli SR, Batapati Krishna Reddy J, Bechtold ML, Ibdah JA, Antillon D, Singh S, Olyaee M, Antillon MR. Endoscopic ultrasound: it's accuracy in evaluating mediastinal lymphadenopathy? World J Gastroenterol. 2008;14:3028-3037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | García Sabater JF, Amador Yscla A, Perales Obenich JA. [Classification and identification of the genus Mycobacterium]. Rev Latinoam Microbiol. 1977;19:7-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Nieuwoudt M, Lameris R, Corcoran C, Rossouw TM, Slavik T, Du Plessis J, Omoshoro-Jones JA, Stivaktas P, Potgieter F, Van der Merwe SW. Polymerase chain reaction amplifying mycobacterial DNA from aspirates obtained by endoscopic ultrasound allows accurate diagnosis of mycobacterial disease in HIV-positive patients with abdominal lymphadenopathy. Ultrasound Med Biol. 2014;40:2031-2038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Keswani RN, Krishnan K, Wani S, Keefer L, Komanduri S. Addition of Endoscopic Ultrasound (EUS)-Guided Fine Needle Aspiration and On-Site Cytology to EUS-Guided Fine Needle Biopsy Increases Procedure Time but Not Diagnostic Accuracy. Clin Endosc. 2014;47:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |