Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3372
Peer-review started: January 28, 2021
First decision: April 5, 2021
Revised: April 14, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: June 21, 2021
Acute-on-chronic liver failure (ACLF) patients have a high short-term mortality rate, and the severity evaluation of ACLF is necessary for prognostication. Therefore, it was meaningful to evaluate the association between type 2 diabetic mellitus (DM) and ACLF and further explore the feasibility of using DM as a prognostic indicator in ACLF patients. The association between type 2 DM and the prognosis of patients with severe liver disease remains unclear.
To examine the effect of type 2 DM on the prognosis of patients with ACLF.
Clinical data from 222 ACLF patients were collected and analyzed. The patients were categorized into two groups depending on whether they had DM or not, and the clinical data of ACLF patients were measured within 48 h after admission. Complications of ACLF were documented during treatment, such as hepatic encephalopathy, hepatorenal syndrome, acute upper gastrointestinal hemorrhage, and spontaneous peritonitis (SBP). Values of laboratory parameters, complication rates, and hospital mortality rates were compared between two groups.
Among 222 ACLF patients, 38 cases were categorized into DM groups, the mean age was 56.32 years and 73.68% were male. The prognosis of ACLF patients was significantly correlated with DM in univariate [hazard ratio (HR) = 2.4, 95% confidence interval (CI) =1.5-3.7, P < 0.001] and multivariable analysis (HR = 3.17, 95%CI =1.82-5.523, P < 0.001). The incident of SBP (34.21% vs 13.59%, P = 0.038) and other infections like lung, urinary, blood, and cholecyst (44.74% vs 28.26%, P = 0.046) were higher in DM patients than non-DM counterparts. In addition, the ACLF patients with DM tended to have a high mortality rate (P < 0.001). Cumulative survival time was also significantly shorter in the ACLF patients with DM than non-DM.
A significant association between DM and the prognosis of ACLF patients was found in China. The ACLF patients with DM had higher incidence of hospital mortality and infection than those without DM.
Core Tip: This study evaluated the association between type 2 diabetic mellitus (DM) and the prognosis of acute-on-chronic liver failure (ACLF) patients. The 222 ACLF patients were categorized into two groups depending on whether they had DM or not. Values of laboratory parameters, complication rates, and hospital mortality rates were compared between two groups. We observed a significant association between DM and the prognosis of ACLF patients in the study. The ACLF patients with DM had higher incidence of hospital mortality and infection than those without DM.
- Citation: Lai RM, Chen TB, Hu YH, Wu G, Zheng Q. Effect of type 2 diabetic mellitus in the prognosis of acute-on-chronic liver failure patients in China. World J Gastroenterol 2021; 27(23): 3372-3385
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3372.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3372
Liver failure is usually manifested as coagulation dysfunction, jaundice, ascites, and hepatic encephalopathy (HE). Acute-on-chronic liver failure (ACLF) is a clinical type of liver failure, and the term was firstly raised in order to describe a condition with two insults operating on the liver simultaneously[1]. ACLF has been featured as a clinical syndrome in which an acute injury factor in a patient with chronic liver disease caused rapid deterioration of liver function, resulting in one or more organ failure. Causes of ACLF could be attributed to hepatitis B virus (HBV), autoimmune hepatitis, severe nonalcoholic fatty liver disease (NAFLD), and other sources. According to Asian Pacific Association for the Study of the Liver (APASL) ACLF Research Consortium consensus, which was published in 2014 and subsequently updated in 2019, ACLF was defined as acute hepatic insult manifested as jaundice and coagulopathy, complicated within 4 wk by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease/cirrhosis, and associated with a high 28-d mortality[1,2]. ACLF diagnostic criteria is uniform in the world, and the Asia-Pacific consensus emphasizes the emergence of liver failure based on chronic liver disease, mainly focusing on the performance of liver failure in order to get early diagnosis and intervention of disease[3]. It was found that ACLF patients had a high short-term mortality rate (> 15% at 28 d)[4] and the severity evaluation of ACLF was necessary for prognostication. Hence well-established prognostic indicators are extremely important, which are helpful for early intervention and reversibility of ACLF as well as improving survival rate[5,6].
Diabetic mellitus (DM) is one of the leading causes of morbidity and mortality across the globe[7]. Previous studies showed that DM was associated with higher incidence of encephalopathy, spontaneous bacterial peritonitis, and hepatocellular carcinoma in chronic liver disease[8-10]. With the improvement of people's living standards, the prevalence of fatty liver disease and DM has increased significantly in China. NAFLD is the most common chronic liver disease, and the risk of progressed liver fibrosis in NAFLD patients is significantly associated with DM[11,12]. The prevalence of DM has increased significantly in recent decades, results in the coexistence of DM and chronic liver disease being common[13]. DM, NAFLD, and obesity are manifestations of symptoms known as metabolic syndrome, and these symptoms are all related to each other. A recent study showed that morbid obesity is an important risk factor for the development of ACLF in liver cirrhosis patients[14]. However, there are very few studies that have evaluated the association between DM and ACLF. Therefore, it may be meaningful to investigate the association between DM and ACLF and further explore the feasibility of using DM as a prognostic indicator in ACLF patients.
In this retrospective single-center study, all patients diagnosed with liver failure who were hospitalized at the Department of Hepatology Research Institute of the First Affiliated Hospital, Fujian Medical University, China from July 2013 to July 2020 were recruited in this study. ACLF was defined by APASL definition in 2019, an acute hepatic insult manifesting as jaundice [serum bilirubin ≥ 5 mg/dL (85 mmol/L) and coagulopathy (international normalized ratio (INR) ≥ 1.5 or prothrombin activity < 40%)] complicated within 4 wk by clinical ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease/cirrhosis and was associated with a high 28-d mortality[1]. The diagnosed complications of ACLF patients also were in accord with this definition. Type 2 DM was defined using any of the following criteria: (1) The World Health Organization 2013 criteria; and (2) documented history of diabetes. All ACLF patients were included as follow criteria: (1) patients aged 18 years and 85 years; (2) ACLF diagnostic criteria accord with APASL definition in 2019; and (3) sufficient data for the study. Patients were excluded if they had the following conditions: (1) de novo tumors; (2) liver transplantation or liver operation; (3) current use of hormone medication; and (4) other fatal disease, or gestation. After exclusion, a total of 222 patients with basis for chronic liver disease were recruited, including 190 HBV infection patients, 17 alcoholic liver disease patients, 15 other disease patients, among which 94 deaths were observed. This prospective study was approved by the ethics committee at the First Affiliated Hospital of Fujian Medical University, China.
All parameters were collected from the electronic medical record system. The demographic parameters included age, gender, and body mass index (BMI). The clinical laboratory parameters collected within 48 h after admission, included prothrombin time (PT), INR, total bilirubin, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, blood urea nitrogen (BUN), serum creatinine, albumin (ALB), fasting blood-glucose (FBG), total cholesterol (TCHO), triglyceride, K+, Na+, leukocyte count (white blood cell), erythrocyte count [red blood cell (RBC)], hemoglobin, platelet count (PLT), alpha fetoprotein, and ammonia. The BMI was calculated as weight (kg)/height2 (m2). This study used the West Haven criteria for grading HE. This grading system differentiated four grades of clinically manifest HE[15]. Ascites can be divided into 3 grades based on the volume of fluid by ultrasound examination[16]. The Child-Turcotte-Pugh (CTP) score, Model for End-Stage Liver Disease (MELD) score, and MELD with serum sodium (MELD-Na) score were calculated using the latest published criteria[5,17,18]. All prognostic scores were calculated according to all parameters at 48 h of admission.
Statistical analyses were performed using SPSS 24.0 (Armonk, NY, United States). The normally distributed continuous variables are presented as mean ± SD, which were further evaluated by Student’s t-test between those with and without DM, whereas variables showing skewed distributions were evaluated by the Mann-Whitney U test, which are presented as median (interquartile range). Categorical variables were described using frequencies and proportions, and Pearson’s chi-squared test was used to compare categorical variables.
The Cox proportional hazard regression analysis was used to estimate the hazard ratio (HR) for the association between DM and all-cause mortality. Participants were followed up from date of hospital admission to date of death or date of discharge. Proportional hazard assumption was tested using Kaplan-Meier survival curves method. A multivariable Cox model was chosen by considering all variables with P < 0.1 in univariate analysis, meanwhile DM was considered as the main variable in statistical analysis model. Further, the nomogram and forest plot were built in terms of results of Cox regression analyses. A two sided P value less than 0.05 were considered significant.
The baseline demographics characteristics and clinical laboratory parameters of the patients are shown in Table 1, and HBV was the most common etiology of ACLF in both groups (68.42% and 75.54%, respectively). The study included 38 DM patients, the mean age was 56.32 years, and 73.68% were male. As expected, BMI and fasting glucose were higher in DMs than their non-DM counterparts. In contrast, the level of ALB was higher in non-DM (P = 0.045). The scoring systems included CTP, MELD, and MELD-Na, and there were no statistically significant differences between the two groups. The mortality rate in DM patients was significantly higher than non-DM patients (65.79% vs 37.5%, P = 0.001).
| DM (yes) (n = 38) | DM (no) (n = 184) | P value | |
| Age (yr) | 56.32 ± 14.23 | 49.16 ± 12.84 | 0.002 |
| Gender, n (%) | 0.309 | ||
| Male | 28 (73.68) | 149 (80.98) | |
| Female | 10 (26.32) | 35 (19.02) | |
| Cause of disease, n (%) | 0.201 | ||
| Hepatitis B virus | 26 (68.42) | 139 (75.54) | |
| Hepatitis B virus + other | 5 (13.16) | 20 (10.87) | |
| Alcohol | 2 (5.26) | 15 (8.15) | |
| Others | 5 (13.16) | 10 (5.44) | |
| WBC (109/L) | 6.17 ± 4.03 | 7.35 ± 3.58 | 0.07 |
| RBC (1012/L) | 3.68 ± 0.87 | 3.94 ± 0.84 | 0.084 |
| Hb (g/L) | 117.21 ± 24.71 | 121.95 ± 23.13 | 0.257 |
| PLT (109/L) | 100.34 ± 42.20 | 118.79 ± 59.09 | 0.069 |
| PT (s) | 23.01 ± 5.38 | 24.45 ± 6.95 | 0.229 |
| INR | 1.97 ± 0.45 | 2.10 ± 0.59 | 0.229 |
| ALT (U/L) | 396.08 ± 448.56 | 560.36 ± 693.06 | 0.163 |
| AST (U/L) | 365.95 ± 391.18 | 419.99 ± 513.42 | 0.541 |
| γ-GGT (U/L) | 174.16 ± 305.61 | 137.57 ± 127.33 | 0.231 |
| TBIL (μmol/L) | 320.71 ± 141.31 | 309.56 ± 134.00 | 0.644 |
| ALB (g/L) | 29.25 ± 4.51 | 30.73 ± 4.03 | 0.045 |
| Scr (μmol/L) | 56.37 ± 22.00 | 63.45 ± 27.28 | 0.134 |
| BUN (mmol/L) | 3.94 ± 2.65 | 4.25 ± 2.98 | 0.56 |
| TCHO (mmol/L) | 2.67 ± 0.81 | 2.65 ± 1.05 | 0.919 |
| TG (mmol/L) | 1.45 ± 0.67 | 1.26 ± 0.70 | 0.124 |
| Na+ (mmol/L) | 136.98 ± 3.97 | 136.86 ± 4.43 | 0.878 |
| K+ (mmol/L) | 3.90 ± 0.46 | 4.08 ± 0.58 | 0.072 |
| AMON (μmol/L) | 64.40 ± 40.39 | 71.60 ± 41.85 | 0.332 |
| AFP (ng/mL) | 118.63 ± 202.80 | 128.19 ± 192.02 | 0.784 |
| BMI (kg/m2) | 24.99 ± 3.32 | 22.78 ± 3.03 | < 0.001 |
| FBG (mmol/L) | 5.34 ± 1.87 | 3.83 ± 1.07 | < 0.001 |
| Scoring systems | |||
| CTP | 10.79 ± 1.49 | 10.40 ± 1.35 | 0.115 |
| MELD | 19.38 ± 4.52 | 20.74 ± 5.06 | 0.128 |
| MELD-Na | 20.89 ± 5.00 | 22.27 ± 6.84 | 0.239 |
| Death, n (%) | 25 (65.79) | 69 (37.5) | 0.001 |
The incidence of common complications in ACLF patients with or without DM is shown in Table 2, and ascites was the most frequent complication in both groups (89.47% and 75.54%, respectively), most of whom was mild. Compared to non-DM participants, DMs had a higher incidence of HE complication (P = 0.026). There was no statistical difference in other clinical complications including ascites, hepatorenal syndrome (HRS), and acute upper gastrointestinal bleeding (AUGIB) between the two groups. Spontaneous peritonitis (SBP) was the common infection complication of ACLF patients, and the incidence of SBP was higher in DMs than their non-DM counterparts (P = 0.038). Other infections, including lung infections, urinary infections, blood infections, and cholecyst infections, were statistically different (P = 0.046), though the total incidence rates were low. Furthermore, lung infections were the main infection (34.21% and 23.37%, respectively).
| DM (yes) (n = 38) | DM (no) (n = 184) | P value | |
| Ascites, n (%) | 34 (89.47) | 139 (75.54) | 0.271 |
| Mild | 21 (55.26) | 80 (43.48) | |
| Moderate | 5 (13.16) | 26 (14.13) | |
| Severe | 8 (21.05) | 33 (17.93) | |
| HE, n (%) | 4 (10.53) | 5 (2.72) | 0.026 |
| Grade (1-2) | 4 (10.53) | 3 (1.63) | |
| Grade (3-4) | 0 (0) | 2 (1.09) | |
| HRS, n (%) | 4 (10.53) | 12 (6.52) | 0.385 |
| AUGIB, n (%) | 3 (7.89) | 4 (2.17) | 0.066 |
| SBP, n (%) | 13 (34.21) | 25 (13.59) | 0.038 |
| Other infections, n (%) | 17 (44.74) | 52 (28.26) | 0.046 |
| Lung | 13 (34.21) | 43 (23.37) | |
| Urinary | 1 (2.63) | 5 (2.72) | |
| Blood | 2 (5.26) | 2 (1.09) | |
| Cholecyst | 1 (2.63) | 2 (1.09) |
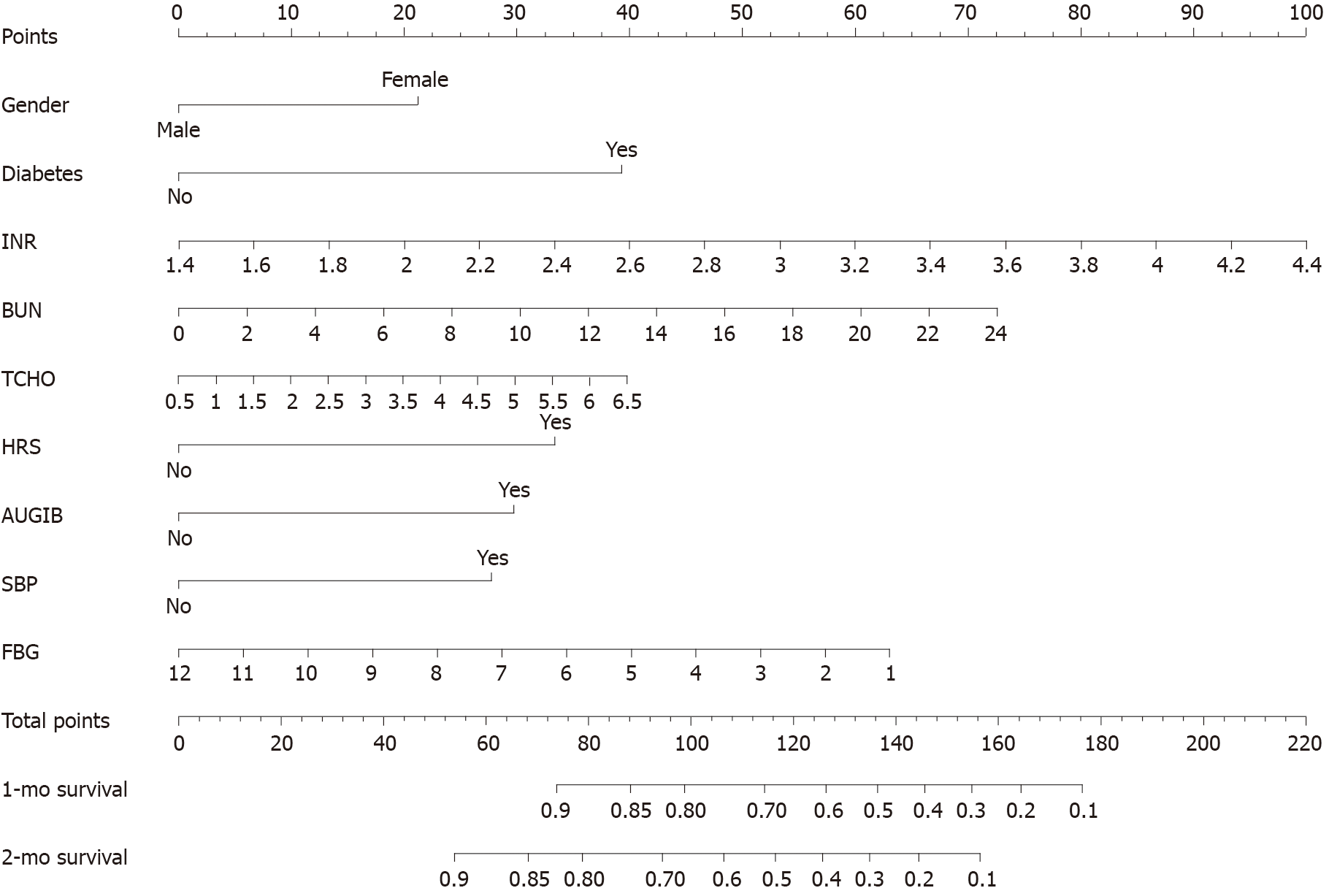
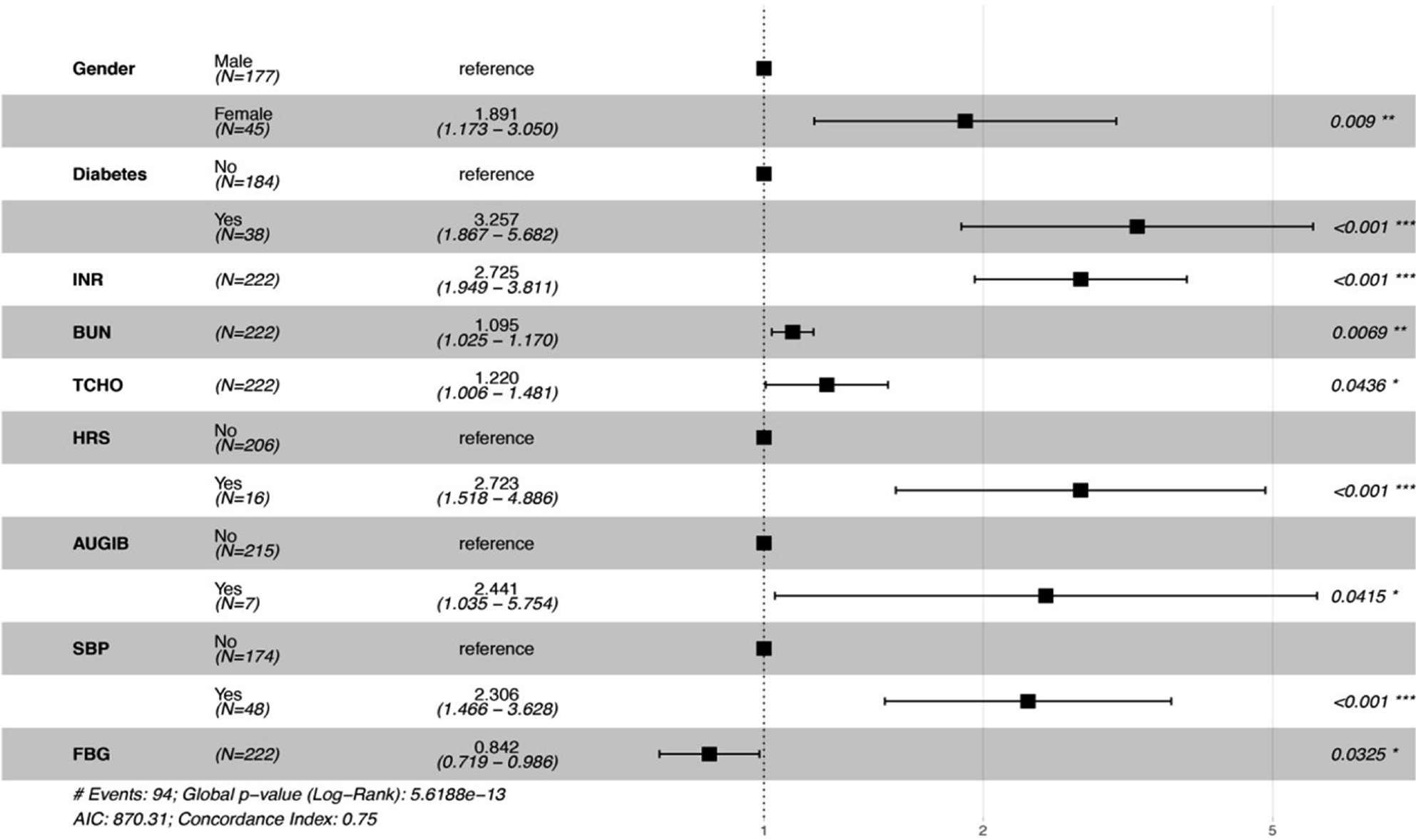
As shown in Table 3, univariate and multivariable analysis were used to evaluate the predictors of prognosis for ACLF patients. Among demographic risk factors, gender and BMI were associated with the prognosis of ACLF patients in univariate analysis. With regard to clinical laboratory parameters, PT, INR, Na+, FBG, BUN, PLT, and RBC were significantly associated with the prognosis of ACLF patients in univariate analysis, moreover, INR, FBG, and BUN also had significant association in multivariable analysis. However, TCHO was not significantly associated with the prognosis of ACLF patients in univariate analysis, while it was significantly in multivariable analysis. Among scoring systems reflecting severity of liver dysfunction, CTP, MELD, and MELD-Na were associated with the prognosis of ACLF patients in univariate analysis, while they had no significant association in multivariable analysis. All four risk factors identified in the univariate analysis (DM, HRS, AUGIB, and SBP) remained significant in the multivariable analysis. Further, nomogram and forest plots that incorporate parameters previously shown to be associated with the prognosis of ACLF patients in the multivariable analysis, including gender, DM, INR, BUN, TCHO, HRS, AUGIB, and SBP, were then constructed and are presented in Figures 1 and 2. As shown by nomogram plot in Figure 1, the total points accumulated by the various variables correspond to the predicted probability of survival for individual patient, and the predicted probability of 1- or 2-mo survival ranged from 0.1 to 0.9. The forest plot in Figure 2 illustrated the intuitive correlation between each risk variable, and the prognosis of ACLF and DM was the most important risk factor (HR = 3.257) influencing the prognosis of ACLF patients.
| Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender | 0.58 (0.37-0.91) | 0.019 | 0.535 (0.332-0.861) | 0.01 |
| Age (yr) | 1 (0.99-1) | 0.23 | ||
| WBC (109/L) | 1 (0.99-1.1) | 0.12 | ||
| RBC (1012/L) | 0.73 (0.57-0.92) | 0.009 | ||
| Hb (g/L) | 0.99 (0.98-1) | 0.01 | ||
| PLT (109/L) | 0.99 (0.99-1) | 0.021 | ||
| PT (s) | 1.1 (1-1.1) | < 0.001 | ||
| INR | 1.9 (1.4-2.5) | < 0.001 | 2.725 (1.949-3.811) | < 0.001 |
| ALT (U/L) | 1 (1-1) | 0.53 | ||
| AST (U/L) | 1 (1-1) | 0.8 | ||
| γ-GGT (U/L) | 1 (1-1) | 0.93 | ||
| TBIL (μmol/L) | 1 (1-1) | 0.77 | ||
| ALB (g/L) | 0.95 (0.9-1) | 0.079 | ||
| Scr (μmol/L) | 1 (1-1) | 0.15 | ||
| BUN (mmol/L) | 1.1 (1-1.2) | 0.021 | 1.095 (1.025-1.170) | 0.0069 |
| TCHO (mmol/L) | 1 (0.85-1.3) | 0.76 | 1.220 (1.006-1.170) | 0.0436 |
| TG (mmol/L) | 0.78 (0.55-1.1) | 0.18 | ||
| Na+ (mmol/L) | 0.94 (0.9-0.98) | 0.005 | ||
| K+ (mmol/L) | 0.83 (0.58-1.2) | 0.34 | ||
| AMON (μmol/L) | 1 (0.99-1) | 0.64 | ||
| AFP (ng/ml) | 1 (1-1) | 0.075 | ||
| BMI (kg/m2) | 0.99 (0.93-1.1) | 0.7 | ||
| FBG (mmol/L) | 0.58 (0.39-0.87) | 0.009 | 0.842 (0.719-0.986) | 0.0325 |
| CTP | 1.7 (1-2.9) | 0.042 | ||
| MELD | 1.1 (1-1.1) | 0.02 | ||
| MELD-Na | 1 (1-1.1) | 0.002 | ||
| DM | 2.4 (1.5-3.7) | < 0.001 | 3.17 (1.82-5.523) | < 0.001 |
| Ascites | 1.2 (1-1.5) | 0.036 | ||
| HE | 1.3 (0.65-2.5) | 0.48 | ||
| HRS | 3.3 (1.9-5.8) | < 0.001 | 2.71 (1.513-4.857) | 0.001 |
| AUGIB | 2.6 (1.1-5.9) | 0.027 | 2.444 (1.037-5.76) | 0.041 |
| SBP | 2.5 (1.6-3.9) | < 0.001 | 2.262 (1.438-3.558) | < 0.001 |
| Other infections | 1.2 (0.82-1.9) | 0.32 | ||
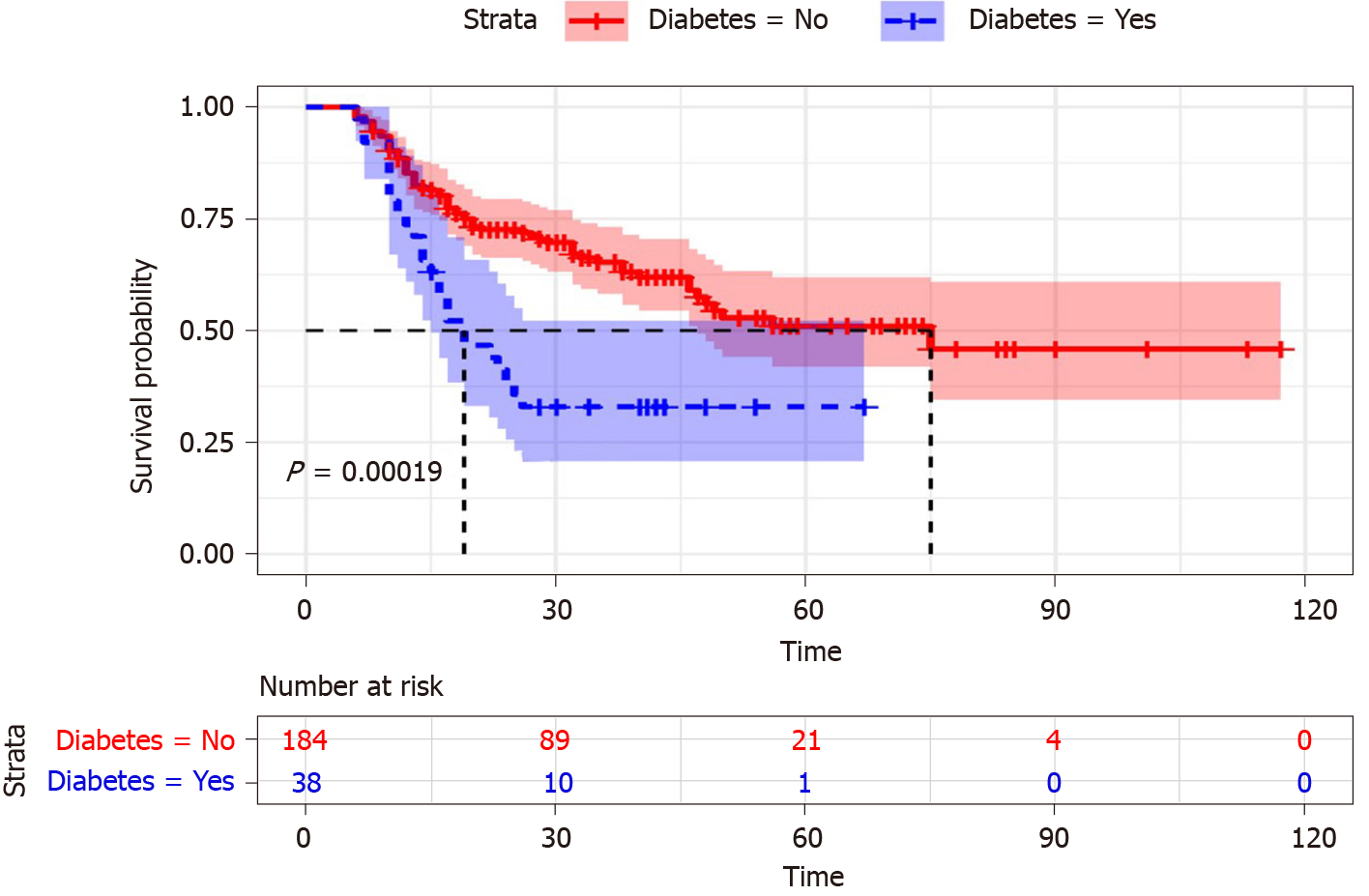
Survival curves were used to describe the survival status of DM and non-DM patients with ACLF. Figure 3 showed that the cumulative survival rate of ACLF patients was significantly distinguished between DM and non-DM (P = 0.00019). The survival time of ACLF patients with non-DM was longer than that in the patients with DM. With the follow-up time increased, the survival time of patients with DM decreased significantly. Therefore, DM was associated with a higher death risk in ACLF patients.
ACLF was characterized by acute decompensation of chronic liver disease, and the course of ACLF was complex and associated with high short-term mortality, complicated with multiple organ failure[19]. The effective prognostic predictors of ACLF would help in identifying assessment of patients at specific time points either requiring early organ support treatment or urgent liver transplantation for ACLF patients, thereafter, doctors may provide a rational therapy for the patients in time[20,21]. Previous study demonstrated that patients who survived for the first 3 mo usually had a good prognosis[22]. Therefore, the exploration of ACLF related prognostic indicators was helpful to reduce the mortality by predicting the outcome of patients and providing early clinical intervention.
It has been reported that there is a strong association between DM and liver disease. During a 12-year follow-up cohort study, compared to non-DM patients with chronic hepatitis B (CHB), DM patients with CHB developed more frequently to cirrhosis and cirrhosis decompensation[23]. Similarity, it has been shown that the presence of DM was an independent predictor of mortality in CHB patients[24]. Therefore, it is necessary to pay attention to the impact of DM on the clinical outcomes with ACLF. In this study, it was found that DM was as an independent prognostic factor for ACLF patients, and the incidence of infection and mortality were higher in DMs than that in non-DM patients. In addition to DM, gender, INR, BUN, TCHO, HRS, AUGIB, SBP, and FBG were independent risk factors for the prognosis of ACLF patients in the multivariate analysis.
Previous studies had shown that nomogram enabled more-accurate individualized prediction of survival than MELD, MELD-Na, CTP, sequential organ failure assessment, or CLIF-C scores for HBV patients and demonstrated superior net benefits over other score models[25,26]. Therefore, an effective prognostic nomogram for ACLF patients was established, as shown in Figure 1; the nomogram of our study could accurately predict 1- and 2-mo survival in patients with ACLF.
Liver plays an important role in glucose homeostasis, and it is the major storage site of glycogen. Massive damage and necrosis of liver cells can cause serious deficiency of liver glycogen synthesis and storage, which are the main features of ACLF disease. Meanwhile, gastrointestinal dysfunction and gastrointestinal mucous edema could lead to impaired nutrient absorption in ACLF patients. Therefore, the mechanism of blood glucose regulation may be abnormal when liver function was severely damaged due to ACLF. In addition, the influence of DM on chronic liver related clinical outcomes had become a focus of research in recent years. It was proved that DM emerged as a risk factor of experiencing liver related death in the chronic liver patients[27]. Furthermore, a meta-analysis showed that DM was independently associated with poor survival in hepatocellular carcinoma patients[28]. Thus, the presence of DM looked as an “at risk” sub-population of liver-related patients. In a conclusion, DM had a tremendous impact on liver disease, which should be fully considered by clinicians. Our study was consistent with previous studies, as shown in Figures 2 and 3, which implied that the level of fast blood glucose and DM were important prognostic indicators for ACLF patients. The cumulative survival time of ACLF patients with non-DM was significantly longer than the patients with DM (P = 0.00019). DM was identified as a risk factor in the univariate analysis (P = 0.009) and remained significant in the multivariable analysis (P = 0.038) in ACLF patients.
DM is a disorder of glucose metabolism; in order to maintain body composition and nitrogen balance, metabolic control and sufficient protein and energy intake are required[29]. ACLF is in a hyper-metabolism state, and upregulated inflammatory response may lead to accelerated proteolysis and increased body energy expenditure, which contributes to negative nitrogen balance[30]. Our study showed that increased level of BUN, which may due to negative nitrogen balance, was associated with the prognosis of ACLF patients in Cox regression analysis. In ACLF patients, the function of protein synthesis was significantly reduced, and ALB had become an effective indicator for ACLF nutritional surveillance[31,32]. A retrospective study had shown that ALB was associated with prognosis in ACLF patients; the patients with hypoproteinemia tended to have a higher mortality rate[33]. However, in this study, ALB was not associated with the prognosis of liver failure, the result may be interfered by exogenous ALB infusion during treatment. Therefore, ACLF patients with DM should be paid more attention to ensure sufficient protein and energy intake.
Malnutrition, protein consumption, and chronic inflammation could lead to sarcopenia in chronic liver disease, which is related with adverse clinical outcomes and the increased risk of mortality[34,35]. Further research found that sarcopenia was strongly associated with ACLF development and impacted the poor prognosis of ACLF[36]. Previous study revealed that DM was significantly associated with sarcopenia[37]. DM increased the risk of sarcopenia, which may be a risk factor to induce the poor prognosis in ACLF patients. Therefore, it was important to give adequate nutritional support for the patients with liver failure in order to reduce the incidence of sarcopenia.
DM, BMI, and NALFD as metabolic risk factors were strongly related with the pathogenesis of metabolic syndrome. This research showed that BMI in DM patients were higher than that in non-DM counterparts. Our previous study showed BMI was associated with hospital mortality in ACLF patients[33], however, this study showed that BMI was not related with ACLF, which may do with the lack of subgroup analysis of BMI. At the same time, our study found that ACLF patients with DM had a higher BMI than the patients with non-DM, which may be a risk factor for the high mortality in ACLF patients with DM.
DM was a significant risk factor for progression of the chronic liver disease[38]. In fact, several previous surveys indicated that survival was significantly lower in DM than in non-DM cirrhotic[10,39]. DM patients were accompanied by high occurrence of NAFLD, and the prevalence of NAFLD in DM varied from 40% to 70%[40,41]. A recent study implied that NAFLD emerged as the rapidly growing etiology of chronic liver disease associated with ACLF[42]. DM was a significant risk factor for progression of the chronic liver disease. In fact, several previous surveys indicated that survival was significantly lower in DM than in non-DM cirrhotic. Our research further confirmed this finding that DM could increase mortality rate in ACLF patients. DM is an important metabolic risk factor that leads to increased mortality in ACLF patients (HR = 3.257, P = 0.001). For all of the above reasons, DM may increase the incidence of NAFLD and affect the prognosis of ACLF patients.
Presence of DM was also shown to be related with the high incidence of HE in patients with liver cirrhosis[43,44]. DM increased the risk of HE in cirrhosis patients, the previous studies showed that insulin resistance, muscle breakdown, and glutaminase activity may be mechanistic[44,45]. In terms of complications of liver failure, DM patients had a higher incidence of HE complication than non-DM participants (P = 0.026); our study produced exactly the same result as above studies. Compared with the patients without DM, it was known that the patients with DM had higher rates for all infections[46]. Previous studies had shown that DM was associated with high incidence of infection in cirrhotic individuals[10]. Our study showed the incidence of infection was higher in ACLF patients with DM than in counterparts without DM (P = 0.038). SBP and pneumonia were common infectious complication of ACLF patients; especially SBP was significantly related with the prognosis of ACLF patients in Cox regression analysis. Therefore, it was extremely important to prevent and control infection in patients with ACLF, especially in the patients with concurrent DM. Among all possible infections, SBP and pulmonary infection should be considered in priority.
There are several limitations in our research. Firstly, this was not a multicenter study and the number of DM patients with ACLF was relatively small. Therefore, the magnitude of association between DM and the prognosis of ACLF could have been less precise. Secondly, because of the retrospective design of the study, not all relevant variables were obtained continuously; glycosylated hemoglobin (HBA1c) parameter and blood sugar control level were available sporadically in ACLF patients with DM. Therefore, these variables were not taken into account in the statistical analysis. Finally, Because of the drawback of this retrospective research, to assess further the impact of DM on the prognosis in ACLF patients, a larger cohort study should be conducted.
DM and chronic liver disease in terms of prevalence and mortality are on the rise worldwide. Because of the liver's important role in glucose metabolism, the correlation between liver disease and DM was close. Through this study, we found that DM could predict the prognosis of ACLF patients, and the ACLF patients with DM had higher incidence of mortality and infection, especially in abdominal and pulmonary infections. Consequently, the clinician should pay attention to the result that DM was a major predictor of short-term mortality in ACLF.
The acute-on-chronic liver failure (ACLF) patients have a high short-term mortality rate, and the severity evaluation of ACLF is necessary for prognostication. The prevalence of diabetic mellitus (DM) has increased significantly in recent decades, resulting in coexistence of DM and chronic liver disease be common. There were very few studies that have evaluated the association between DM and ACLF. Therefore, the association between type 2 DM and the prognosis of patients with ACLF remained unclear.
Previous studies suggested that DM increased higher incidences of complications in chronic liver disease, included encephalopathy, spontaneous bacterial peritonitis, and hepatocellular carcinoma, but it was not clear whether DM would similarly affect ACLF patients. We needed further to evaluate the association between DM and the prognosis of ACLF patients in order to explore the feasibility of using DM as a prognostic indicator in ACLF patients.
We aimed to examine the effect of DM on the prognosis of patients with ACLF and established a predictive model to predict the risk of mortality for ACLF patients. A well-established prognostic predictive model was extremely important, which was helpful for early intervention of ACLF patients as well as improved survival rate.
Clinical data from 222 ACLF patients were retrospectively collected and analyzed between July 2013 and July 2020 from the Department of Hepatology Research Institute of the First Affiliated Hospital, Fujian Medical University. The patients were categorized into two groups depending on whether they had DM or not, and complications of ACLF were documented during treatment. Values of laboratory parameters, complication rates, and hospital mortality rates were compared between two groups. The Cox proportional hazard regression analysis was used to estimate hazard ratio (HR) for the association between DM and all-cause mortality. DM was independent risk factor to predict mortality in ACLF patients. Further, the nomogram and forest plot were built in terms of results of Cox regression analyses. A survival nomogram model was constructed to predict the probability of 1- and 2-mo survival for ACLF patients.
The prognosis of ACLF patients was significantly correlated with DM in univariate (HR = 2.4, P < 0.001) and multivariable analysis (HR = 3.17, P < 0.001). The ACLF patients with DM tended to have a high mortality rate (P < 0.001). Survival curves showed that the cumulative survival rate of ACLF patients was significantly distinguished between DM and non-DM (P = 0.00019). The survival time of ACLF patients with non-DM was longer than the patients with DM. As a good prognostic indicator, DM was helpful to predict the outcome of ACLF patients. The incidence of infection was higher in DM patients than non-DM counterparts (P = 0.038). Among all possible infections, spontaneous peritonitis and pulmonary infection should be considered a priority for ACLF patients. ACLF patients’ survival nomogram could predict the probability of 1- and 2-mo survival, which would be helpful to provide early clinical intervention. Because of the retrospective design of the study, we needed to assess further the impact of DM on the prognosis in ACLF patients by a prospective cohort study.
Our study found a significant association between DM and the prognosis of ACLF patients in China. The survival time of ACLF patients with non-DM was longer than that of patients with DM. The ACLF patients with DM had higher incidence of hospital mortality and infection than those without DM. DM was an independent risk factor affecting the prognosis of ACLF patients.
This was not a multicenter study and the number of DM patients with ACLF was relatively small. Therefore, a multi-center prospective cohort study would evaluate further the impact of DM on the prognosis in ACLF patients. Meanwhile, a noninvasive model should be established based on an extensive clinical database to predict effectively the survival time of ACLF patients.
The author thanks the medical and nursing staff of Department of Hepatology of The First Affiliated Hospital of Fujian Medical University for their dedication. All patients participated in this study are appreciated.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lashen SA, Zhang X S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 409] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 2. | Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, Hamid S, Kim DJ, Komolmit P, Lata S, Lee GH, Lesmana LA, Mahtab M, Maiwall R, Moreau R, Ning Q, Pamecha V, Payawal DA, Rastogi A, Rahman S, Rela M, Saraya A, Samuel D, Saraswat V, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Butt AS, Tan SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O; APASL ACLF Working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 457] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 3. | Olson JC, Wendon JA, Kramer DJ, Arroyo V, Jalan R, Garcia-Tsao G, Kamath PS. Intensive care of the patient with cirrhosis. Hepatology. 2011;54:1864-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 175] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 4. | Dhiman RK, Agrawal S, Gupta T, Duseja A, Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol. 2014;20:14934-14941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 59] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 5. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 624] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 6. | Xue R, Duan Z, Liu H, Chen L, Yu H, Ren M, Zhu Y, Jin C, Han T, Gao Z, Meng Q. A novel dynamic model for predicting outcome in patients with hepatitis B virus related acute-on-chronic liver failure. Oncotarget. 2017;8:108970-108980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Carracher AM, Marathe PH, Close KL. International Diabetes Federation 2017. J Diabetes. 2018;10:353-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Wlazlo N, van Greevenbroek MM, Curvers J, Schoon EJ, Friederich P, Twisk JW, Bravenboer B, Stehouwer CD. Diabetes mellitus at the time of diagnosis of cirrhosis is associated with higher incidence of spontaneous bacterial peritonitis, but not with increased mortality. Clin Sci (Lond). 2013;125:341-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, Roberts LR. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:907-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 10. | Elkrief L, Chouinard P, Bendersky N, Hajage D, Larroque B, Babany G, Kutala B, Francoz C, Boyer N, Moreau R, Durand F, Marcellin P, Rautou PE, Valla D. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60:823-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Khan R, Foster GR, Chowdhury TA. Managing diabetes in patients with chronic liver disease. Postgrad Med. 2012;124:130-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 12. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2383] [Cited by in F6Publishing: 3032] [Article Influence: 505.3] [Reference Citation Analysis (2)] |
| 13. | Silva TE, Ronsoni MF, Schiavon LL. Challenges in diagnosing and monitoring diabetes in patients with chronic liver diseases. Diabetes Metab Syndr. 2018;12:431-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Sundaram V, Jalan R, Ahn JC, Charlton MR, Goldberg DS, Karvellas CJ, Noureddin M, Wong RJ. Class III obesity is a risk factor for the development of acute-on-chronic liver failure in patients with decompensated cirrhosis. J Hepatol. 2018;69:617-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1158] [Cited by in F6Publishing: 1221] [Article Influence: 122.1] [Reference Citation Analysis (1)] |
| 16. | Chinese Society of Hepatology; Chinese Medical Association; Xu X, Duan Z, Ding H, Li W, Jia J, Wei L, Linghu E, Zhuang H. Chinese guidelines on the management of ascites and its related complications in cirrhosis. Hepatol Int. 2019;13:1-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Perdigoto DN, Figueiredo P, Tomé L. The Role of the CLIF-C OF and the 2016 MELD in Prognosis of Cirrhosis with and without Acute-on-Chronic Liver Failure. Ann Hepatol. 2019;18:48-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Song DS, Kim TY, Kim DJ, Kim HY, Sinn DH, Yoon EL, Kim CW, Jung YK, Suk KT, Lee SS, Lee CH, Kim TH, Choe WH, Yim HJ, Kim SE, Baik SK, Jang JY, Kim HS, Kim SG, Yang JM, Sohn JH, Choi EH, Cho HC, Jeong SW, Kim MY; Korean Acute-on-Chronic Liver Failure (KACLiF) Study Group. Validation of prognostic scores to predict short-term mortality in patients with acute-on-chronic liver failure. J Gastroenterol Hepatol. 2018;33:900-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 20. | Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, Laleman W, Trebicka J, Elkrief L, Hopf C, Solís-Munoz P, Saliba F, Zeuzem S, Albillos A, Benten D, Montero-Alvarez JL, Chivas MT, Concepción M, Córdoba J, McCormick A, Stauber R, Vogel W, de Gottardi A, Welzel TM, Domenicali M, Risso A, Wendon J, Deulofeu C, Angeli P, Durand F, Pavesi M, Gerbes A, Jalan R, Moreau R, Ginés P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 407] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 21. | Wu SJ, Yan HD, Zheng ZX, Shi KQ, Wu FL, Xie YY, Fan YC, Ye BZ, Huang WJ, Chen YP, Zheng MH. Establishment and validation of ALPH-Q score to predict mortality risk in patients with acute-on-chronic hepatitis B liver failure: a prospective cohort study. Medicine (Baltimore). 2015;94:e403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Lin S, Zhang K, Zhang J, Wang M, Velani B, Zhu Y. Long-term outcomes of patients with hepatitis B virus-related acute on chronic liver failure: An observational cohort study. Liver Int. 2019;39:854-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Huang YW, Wang TC, Lin SC, Chang HY, Chen DS, Hu JT, Yang SS, Kao JH. Increased risk of cirrhosis and its decompensation in chronic hepatitis B patients with newly diagnosed diabetes: a nationwide cohort study. Clin Infect Dis. 2013;57:1695-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Lin S, Chen J, Wang M, Han L, Zhang H, Dong J, Zeng D, Jiang J, Zhu Y. Prognostic nomogram for acute-on-chronic hepatitis B liver failure. Oncotarget. 2017;8:109772-109782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Gao F, Li X, Wan G, Li Y, Zhang Q, Liu Y, Liu H, Li H, Wang X. Development and external validation of a prognostic nomogram for acute decompensation of chronic hepatitis B cirrhosis. BMC Gastroenterol. 2018;18:179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Bertot LC, Jeffrey GP, de Boer B, MacQuillan G, Garas G, Chin J, Huang Y, Adams LA. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int. 2018;38:1793-1802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 28. | Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ, Yan SL. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2014;9:e95485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Franz MJ. Protein and diabetes: much advice, little research. Curr Diab Rep. 2002;2:457-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Patel JJ, McClain CJ, Sarav M, Hamilton-Reeves J, Hurt RT. Protein Requirements for Critically Ill Patients With Renal and Liver Failure. Nutr Clin Pract. 2017;32:101S-111S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Bémeur C, Desjardins P, Butterworth RF. Role of nutrition in the management of hepatic encephalopathy in end-stage liver failure. J Nutr Metab. 2010;2010:489823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol. 2008;23:527-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Lai R, Chen T, Wu Z, Lin S, Zhu Y. Associations between body mass index and mortality in acute-on-chronic liver failure patients. Ann Hepatol. 2019;18:893-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Ooi PH, Hager A, Mazurak VC, Dajani K, Bhargava R, Gilmour SM, Mager DR. Sarcopenia in Chronic Liver Disease: Impact on Outcomes. Liver Transpl. 2019;25:1422-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 35. | Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, Dunn MA; Fitness; Life Enhancement; and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23:625-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 36. | Mauro E, Crespo G, Martinez-Garmendia A, Gutierrez-Acevedo MN, Diaz JM, Saidman J, Bermudez C, Ortiz-Patron J, Garcia-Olveira L, Zalazar F, Narvaez A, Spina JC, Orta R, Savluk L, Piano S, Marciano S, Gadano A. Cystatin C and Sarcopenia Predict Acute on Chronic Liver Failure Development and Mortality in Patients on the Liver Transplant Waiting List. Transplantation. 2020;104:e188-e198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Hayashida I, Kusabiraki T, Tamaki J. Sarcopenia and falls in community-dwelling elderly subjects in Japan: Defining sarcopenia according to criteria of the European Working Group on Sarcopenia in Older People. Arch Gerontol Geriatr. 2014;59:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 38. | Hamed AE, Elsahar M, Elwan NM, El-Nakeep S, Naguib M, Soliman HH, Ahmed Aboubakr A, AbdelMaqsod A, Sedrak H, Assaad SN, Elwakil R, Esmat G, Salh S, Mostafa T, Mogawer S, Sadek SE, Saber MM, Ezelarab H, Mahmoud AA, Sultan S, El Kassas M, Kamal E, ElSayed NM, Moussa S. Managing diabetes and liver disease association. Arab J Gastroenterol. 2018;19:166-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Elkrief L, Rautou PE, Sarin S, Valla D, Paradis V, Moreau R. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36:936-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 40. | Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Non-alcoholic fatty liver disease in diabetics--prevalence and predictive factors in a multiracial hospital clinic population in Malaysia. J Gastroenterol Hepatol. 2013;28:1375-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Kabir MA, Uddin MZ, Siddiqui NI, Robi IH, Malek MS, Islam MS, Rahman S, Hossain MS, Mahapatra SK, Alam MJ, Ahmad F, Alam MS, Islam MA. Prevalence of Non-Alcoholic Fatty Liver Disease and Its Biochemical Predictors in Patients with Type-2 Diabetes Mellitus. Mymensingh Med J. 2018;27:237-244. [PubMed] [Cited in This Article: ] |
| 42. | Axley P, Ahmed Z, Arora S, Haas A, Kuo YF, Kamath PS, Singal AK. NASH Is the Most Rapidly Growing Etiology for Acute-on-Chronic Liver Failure-Related Hospitalization and Disease Burden in the United States: A Population-Based Study. Liver Transpl. 2019;25:695-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 43. | Jepsen P, Watson H, Andersen PK, Vilstrup H. Diabetes as a risk factor for hepatic encephalopathy in cirrhosis patients. J Hepatol. 2015;63:1133-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 44. | Kalaitzakis E, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Jalan R, Björnsson E. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007;27:1194-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Ampuero J, Ranchal I, del Mar Díaz-Herrero M, del Campo JA, Bautista JD, Romero-Gómez M. Role of diabetes mellitus on hepatic encephalopathy. Metab Brain Dis. 2013;28:277-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic Control and Risk of Infections Among People With Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care. 2018;41:2127-2135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |