Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.3064
Peer-review started: February 6, 2021
First decision: March 14, 2021
Revised: March 27, 2021
Accepted: May 7, 2021
Article in press: May 7, 2021
Published online: June 14, 2021
The rapid global spread of coronavirus disease 2019 (COVID-19) infection has become a major health issue with higher morbidity and mortality rates. Besides respiratory symptoms, a growing body of evidence indicates a variety of gastrointestinal manifestations including liver involvement. In this regard, several data supported an association between COVID-19 infection and liver injury in adults, while in children there is compelling but currently limited evidence. In particular, patients with COVID-19 have shown a higher risk of liver injury (mainly expressed as increased transaminase levels or hepatic steatosis). Conversely, a greater risk of more severe forms of COVID-19 infection has been observed in subjects with pre-existing chronic liver diseases. The dramatic interplay between COVID-19 and liver damage has been related to the inflammatory pathways chronically active in patients with nonalcoholic fatty liver disease and acutely in those affected by COVID-19, but other different pathogenic mechanisms have also been supposed. Of note, patients with previous metabolic comorbidities also had a higher risk of severe COVID-19 infection. This emphasizes the pathogenic interrelation of the inflammatory pathways with a dysregulated metabolic milieu in COVID-19 patients. Taking into account the prognostic role of fatty liver in COVID-19 patients and its intrinsic relationship with metabolic abnormalities even in childhood, a strict monitoring of this condition is recommended. We aimed to summarize the most recent evidence regarding the potential interplay between pediatric fatty liver and COVID-19.
Core Tip: Both adult and pediatric data recently reported a liver involvement in coronavirus disease 2019 (COVID-19). Although several pathogenic mechanisms have been proposed, inflammatory pathways seem to play a pivotal role in the pathophysiology of liver damage in this viral infection. In particular, a complex and bidirectional relationship has been highlighted between fatty liver and COVID-19. Several data suggested this intriguing interplay by underscoring the need for an early close monitoring of this liver condition with an intrinsic greater cardiometabolic burden.
- Citation: Di Sessa A, Lanzaro F, Zarrilli S, Picone V, Guarino S, Miraglia del Giudice E, Marzuillo P. COVID-19 and pediatric fatty liver disease: Is there interplay? World J Gastroenterol 2021; 27(22): 3064-3072
- URL: https://www.wjgnet.com/1007-9327/full/v27/i22/3064.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i22.3064
Since late 2019, the whole world has been suddenly disrupted by the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). At the beginning of February 2021, this global acute pandemic produced more than 102 million worldwide confirmed cases and a total number of deaths to 2.2 million, representing an unprecedented healthcare challenge[1].
The pathogenic role of COVID-19 depends on binding of spike viral proteins to angiotensin I converting enzyme 2 (ACE-2) receptors facilitating the entry into the target cells[2-4]. Although mainly expressed in the respiratory tract, in particular in the nasopharynx epithelium, these receptors are also located in multiple sites such as the gastrointestinal tract and vascular endothelium[5-8]. Along with clinical respiratory symptoms, growing evidence supported a COVID-19 related gastrointestinal involvement[8-13]. COVID-19 positive adult patients might experience gastrointestinal symptoms due to the presence of ACE-2 receptors in the glandular cells of gastric, duodenal, and distal enterocytes that favor malabsorption, impaired intestinal secretion, and enteric nervous system activation[8,9,12,14]. The virus might also affect the liver through a direct viral translocation from gut or by an indirect mechanism involving systemic inflammation, pre-existing liver diseases, or drug-related liver damage[11,13-16].
Pediatric COVID-19 reports have shown both a milder course and a better prognosis of the disease in this population, probably due to the special immune response system pertaining to children. Gastrointestinal manifestations of the disease at this age are various and not uncommon, in particular in the early phase of the infection.
Nonalcoholic fatty liver disease (NAFLD) has become the most common worldwide chronic liver disease with an epidemic rate both in adults and children[17,18]. It has been largely recognized for its close relationship with insulin resistance and other metabolic features leading to an increased cardiometabolic risk from childhood to adulthood[19-23]. Nevertheless, NAFLD pathophysiology is still far from being fully clarified. To date, the “multiple hits” represents the most favored pathogenic hypothesis, including a role for several different factors (e.g., inflammation, oxidative stress, gut axis, mitochondrial dysfunction, diet, hormones, and genetics)[24].
The mechanism underlying the increase of liver enzymes seemed to be different between adult and pediatric patients, but further studies are needed for a better elucidation[25] .
In adults, two mechanisms for liver injury have been postulated such as a possible role of ACE-2 receptor binding of the virus to the epithelial cells of the bile ducts or a dysregulated hepatic innate immune response, which represents the most probable hypothesis. No evidence supported the involvement of ACE-2 receptor in pediatric liver injury. Moreover, both interleukin (IL)-6 and IL-10 levels did not significantly differ between infected children with and without elevated liver enzymes[25]. Of interest, the presence of a protective mechanism against liver involvement in children accounting for the mild increase of aspartate aminotransferase and/or alanine aminotransferase (ALT) compared to adults has been suggested. Zhou et al[25] reported a higher prevalence of liver involvement (expressed as abnormal liver enzymes) in children aged 0-3 years than in those aged > 3 years, by speculating that this finding might be related to a more pronounced liver immaturity.
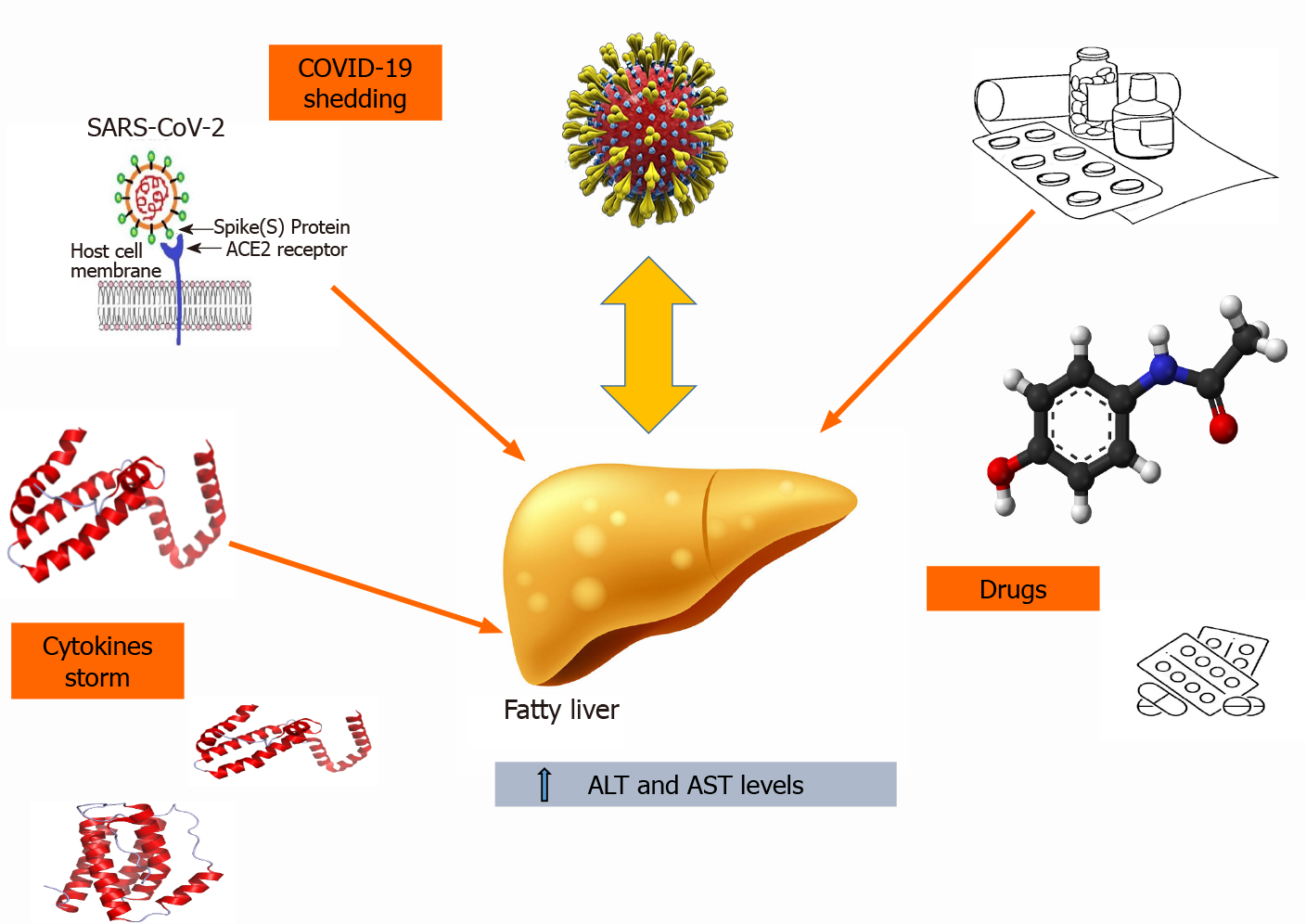
The exact mechanism linking COVID-19 to pediatric fatty liver remains poorly known[8,11,25] (Figure 1). In fact, the low incidence of COVID-19 infection in children and its relatively asymptomatic course at this age make it difficult to better understand the pathophysiology of this concern[8,11]. To complicate matters, one of the most likely hypotheses supporting the abnormal liver enzymes in adults such as the inflammatory cytokine storm (including IL-6-and IL-10) seemed to not play a central role in pediatric COVID-19 related liver damage[25]. In fact, studies in infected children have demonstrated that levels of these cytokines are linked to greater severity of the disease, but no statistical differences of both IL-6 and IL-10 serum concentrations have been detected between patients with and without increased transaminases[25]. Moreover, the pediatric clinical practice to first give paracetamol for fever manage
However, all these studies suffered from some limitations. The first was relatively small sample sizes. Lack of a more accurate laboratory assessment and of longitudinal data represented another limitation. Finally, lack of previous data about comorbidities or drug administration before the hospitalization needed to be mentioned.
Nevertheless, given the growing amount of data regarding liver involvement during this pandemic, the American Association for the Study of Liver Diseases recommends to regularly monitor the mild increase of transaminase in patients with COVID-19 and (mainly in children) to further evaluate for potential underlying liver diseases because of the uncommon presentation and the current paucity of data at this age[26].
A large body of evidence has shown an association between COVID-19 and NAFLD in adults (Table 1). Findings from adult studies reported that the prevalence of steatosis (mainly detected by ultrasound abdominal scan or computed tomography) in the confirmed COVID-19 cases was significantly higher than in healthy subjects, even after adjustments for gender and age[27].
| Ref. | Study design | Population | NAFLD | Main findings |
| Mahamid et al[34] | Retrospective case-control study, SZMC, Jerusalem | 71 hospitalized patients with COVID-19 infection, both genders, age ≥ 18.0 yr (mean age 51.0 ± 21.7), 22 NAFLD, 49 non-NAFLD | CT within hospitalization or recently made | Significant association between NAFLD and severity of COVID-19 even after adjustments for obesity, hypertension, metabolic syndrome, diabetes, and smoking. This association was independent of metabolic syndrome and/or its components. NAFLD patients have an increased risk of severe COVID-19 in both genders, in particular in males (Male: P = 0.001 Female: P = 0.002) |
| Ji et al[28] | Retrospective case-control study. Patients of two COVID Hospital in China | 202 patients with COVID-19 (hospitalized and follow-up within 12 mo of the diagnosis). Median age 44.5 (34.8-54.1), 163 patients with stable disease (37.6% of patients with NAFLD), 39 patients with progressive disease (25.8% of patients with NAFLD) | NAFLD defined as hepatic steatosis index: 8 × (ALT/AST) + BMI (+ 2 if type 2 diabetes, + 2 if female) > 36 and/or US | Male patients aged > 60 yr, with higher BMI, underlying comorbidities, and NAFLD were associated with COVID-19 progression. Patients with NAFLD had higher risk of disease progression, longer viral shedding times, and higher likelihood of abnormal liver function from admission to discharge than patients without NAFLD |
| Zhou et al[31] | Cohort study, Asian ethnicity | 55 MAFLD patients with COVID-19 were 1:1 matched by age (± 5 yr), sex, and BMI (± 1 unit) to COVID-19 patients without MAFLD. Age < 60 yr | CT | The presence of MAFLD was associated with severity of COVID-19 even after full adjustment (age, sex, smoking status, obesity, diabetes, hypertension) and a trend to increased duration of hospitalization. MAFLD patients had higher levels of CRP, ALT, AST, GGT, fasting blood glucose, and triglycerides |
| Medeiros et al[27] | Retrospective case-control study. Radiology Departments of Hospital Beneficiencia Portuguesa, San Paolo- Brasil | 316 patients clinically suspected of having COVID-19 infection: -n.204: RT-PCR positive; -n.112: RT-PCR and chest CT negative pattern. Age > 18 yr | CT: Attenuation value of ≤ 40 HU, measured in the region of interest (commonly in the right hepatic lobe) in non-enhanced phase | Higher prevalence of steatosis in affected patients, even after adjustments for sex and age |
| Forlano et al[29] | Retrospective cohort study. Imperial College Healthcare NHS Trust (London, United Kingdom) | 193 hospitalized, adult patients with COVID-19 infection and CT imaging, NAFLD: 61 (31%); Non-NAFLD: 132 (66%), excluded: 5 (3%) | US or CT dated within 1 yr from the admission for COVID-19 or a known diagnosis of NAFLD. FIB-4 index for fibrosis | No difference in terms of admission to ICU and in mortality between NAFLD and non-NAFLD patients. NAFLD patients were significantly younger at presentation |
| Gao et al[30] | Cohort study. Four hospitals in China | 130 nondiabetic patients with COVID-19: 65 MAFLD and 65 controls were 1:1 matched by age (± 5 yr) and sex | CT | MAFLD presence in nondiabetic patients was associated with a 4-fold increased risk of severe COVID-19, even after adjusting for age, sex, and coexisting comorbidities. The risk of severe COVID-19 increased with increasing numbers of metabolic risk factors |
| Targher et al[33] | Retrospective cohort study. Four hospitals in China | 310 hospitalized, adult patients with COVID-19 infection | CT: FIB-4 index and NFS used to categorize liver fibrosis in low, intermediate, or high | In patients with MAFLD the presence of intermediate or high fibrosis (FIB-4 or NFS) was associated with a higher risk of severe COVID-19, even after adjusting for sex, obesity, and diabetes |
| Sharma et al[32] | Review | Adult patients | CT/FIB-4 index and NFS | Patients with MAFLD had higher risk of disease progression, longer viral shedding times, higher likelihood of abnormal liver function, and 4-6-fold increased risk of severe disease than patients with no MAFLD. Younger patients (age < 60 yr) were also at greater risk for increased severity of COVID-19 |
Additional data suggested that male patients aged > 60 years, with higher body mass index, underlying comorbidities, and NAFLD were at higher risk of COVID-19 progression[28]. Another study reported no difference in terms of admission to intensive care unit and mortality between NAFLD and non-NAFLD patients. Mortality was associated to male gender (similarly to non-NAFLD patients) and a pronounced inflammatory response (including high ferritin and early weaning score as main predictors). The latter might account for the presence of a dysregulated hepatic innate immune response, one of the most acclaimed mechanisms postulated for liver injury. In addition, NAFLD patients were significantly younger at presen
Interestingly, taking into account the new definition of metabolic-associated fatty liver disease (MAFLD), authors investigated the potential relationship between COVID-19 infection and MAFLD patients[30-32]. MAFLD patients had a greater risk of disease progression, longer viral shedding times, and a higher probability of liver dysfunction from admission to discharge than patients without MAFLD[30-32]. Sharma et al[32] found that younger patients (aged < 60 years) with MAFLD showed an increased severity of COVID-19 disease. Overall, a significant association between MAFLD and younger presentation of COVID-19 was found[31,32].
Notably, as observed in NAFLD subjects, the direct relationship of MAFLD with COVID-19 infection emphasizes the relevance of the metabolic milieu in this viral condition, by pointing out the role of dysregulated hepatic innate immunity to trigger liver damage[29-32]. Moreover, overwhelming evidence supported the association of COVID-19 infection severity (defined as the occurrence of any of the following parameters: arterial PaO2/FiO2 ≤ 300 mmHg, SpO2 ≤ 93%, ≥ 30 breath per minute) with both NAFLD and/or MAFLD, even after full adjustment for common cardiometabolic risk factors such as obesity, hypertension, metabolic syndrome, diabetes, and smoking[30,33]. Hence, this association was found to be independent of metabolic syndrome and/or its components[33]. In particular, MAFLD presence in nondiabetic patients was associated with a 4-fold increased risk of severe COVID-19, even after adjusting for age, gender, and coexisting comorbidities, and the risk was even higher with increasing numbers of metabolic risk factors[30]. Moreover, the presence of intermediate or high fibrosis was also linked to a higher risk of severe COVID-19, even after adjusting for gender, obesity, and diabetes[34].
Compared to adult findings, evidence in children is currently limited but in line with the aforementioned association (Table 2). In fact, data from 6 pediatric case-series studies and a review reported that liver involvement, defined as an increase of ALT and/or aspartate aminotransferase, was rare and mild (< 2 × ULN)[35-41]. In contrast to adults, Zhou et al[25] found no gender difference in children with COVID-19 and elevated liver enzymes.
| Ref. | Study design | Population | NAFLD | Main findings |
| Qiu et al[36] | Cohort study. Three hospitals in Zhejiang Province, China | 36 pediatric patients (aged 0-16 yr) with laboratory confirmed COVID-19 infection; mild cases (n = 17); moderate cases (n = 19) compared with adults with COVID-19 (n = 175); Children with SARS (n = 44); Children with H1N1 influenza (n = 167) | Increased liver enzymes | Elevated ALT in two patients (mild cases) and AST in three patients (two mild and one moderate case), 6% of cases with elevated liver enzymes, 18% of cases with elevated liver enzymes, 48% of cases with elevated liver enzymes, 17% of cases with elevated liver enzymes |
| Sun et al[37] | Retrospective analysis. Intensive Care Unit, Wuhan Children’s Hospital | 8 severe or critically ill COVID-19 patients admitted to the ICU. Age: 2 mo-15 yr | Increased liver enzymes | Elevated ALT levels in 4 out of 8 patients. Total bilirubin level in all patients was normal |
| Wang et al[39] | Retrospective study. Children from six provinces (autonomous region) in northern China. | 31 cases of COVID-19. Age: 6 mo-17 yr | Increased liver enzymes | Elevation of liver enzyme (22%) |
| Xia et al[38] | Retrospective study. Wuhan Children’s Hospital | 20 COVID-19 pediatric patients. Age: 1 d-14 yr, 7 mo (median age: 2 yr and 1.5 mo) | No NAFLD screening. CT was performed for pneumonia | Elevated ALT (> 40 U/L) in 25% of cases |
| Jiehao et al[40] | Case series. Children’s Hospital in Shanghai, Hainan, Hefei in Anhui province, and Qingdao in Shandong province | 10 patients aged 3-131 mo (mean: 74 mo). Male: female 1:1.5 | No NAFLD screening. CT was performed for pneumonia | Median ALT: 18.5 U/L, AST: 27.7 U/L. One patient had ALT: 100 U/L and AST: 142 U/L |
| Tan et al[41] | Retrospective study. North Hospital of Changsha First Hospital | 10 children with confirmed COVID-19 infection. Mean age of 7 yr (1-12 yr) | Increased liver enzymes | Elevated AST in two patients |
Pediatric studies examining different variables such as demographic data, clinical and laboratory features, chest imaging results, treatments, and clinical outcomes of the patients with COVID-19 showed a prevalence of possible liver involvement from 6% to 50%[25,35-41].
A retrospective analysis including eight severe or critically ill patients with COVID-19 (aged 2 mo-15 years) admitted to the intensive care unit of the Wuhan Children’s Hospital reported a mild increase of ALT levels in half of the population, while total bilirubin levels in all patients were normal[36]. Similarly, a retrospective study of 20 pediatric patients with COVID-19 infection showed increased ALT levels (> 40 U/L) in 25% of cases[37]. A slight prevalence (6%) of elevated liver enzymes in confirmed-COVID-19 Chinese patients (aged 0-16 years) was also described in a cohort study[35]. In addition, it provided evidence for a minor liver involvement compared to adults with COVID-19 (18%), children with severe acute respiratory syndrome (48%), and children with influenza A (7%)[35].
It has been observed in children that both critically ill patients admitted to the intensive care unit and mild patients (defined as the presence of upper respiratory symptoms or asymptomatic infection, positive real-time reverse transcription polymerase chain reaction test for SARS-CoV-2, and no abnormal radiographic and septic presentation) rarely showed abnormal liver enzymes[35,36]. In these studies, all the patients underwent an antiviral treatment, therefore the possibility that transaminase abnormalities were drug-induced cannot be ruled out[35,36]. Moreover, it should be noted that the most common symptoms at admission were cough and fever, usually treated with paracetamol, of which an incorrect administration might also influence transaminase levels[41].
Gastrointestinal symptoms have been largely described in COVID-19 infected patients. Indeed, the wide presence of ACE-2 receptors through the entire gastrointestinal tract has been considered to be responsible for the spectrum of gastrointestinal manifestations including abdominal pain, nausea, vomiting, or less commonly fatty liver and acute hepatitis. In particular, liver involvement has been reported both in adult and pediatric affected patients with a higher prevalence in the former population[7,42,43]. Several pathogenic mechanisms have been hypothesized to explain liver damage, but its exact pathophysiology is still under debate[42,44]. First, it could be considered as a consequence of the “cytokine storm” resulting in an aberrant immune system reaction with an excessive inflammatory response, as observed in many affected subjects. Moreover, liver injury might represent a side effect of the administered drugs or a secondary effect of the hypoxia consequent to failure of other organs such as heart and lung. On the contrary, a direct viral effect is unlikely, as supported by the scarce evidence of an increase of the classical markers of biliary tract involvement such as gamma-glutamyl transferase and alkaline phosphatase in affected patients in the face of a large hepatic expression of ACE-2 on cholangiocyte surfaces[6,45].
Of concern, liver damage in patients affected by COVID-19 seems to be enhanced by the intrinsic chronic activation of inflammatory pathways in NAFLD, potentially worsening outcomes in subjects with previous coexisting metabolic diseases[7,8]. Moreover, patients with pre-existing chronic liver diseases have been found to be at higher risk to develop more severe forms of COVID-19 infection.
NAFLD expressed as hepatic steatosis and/or elevated liver enzymes represents one of the most common liver abnormalities reported during this pandemic, in particular in pediatric patients[7]. Studies reported an overall prevalence of mild liver enzyme increases of almost 25% in COVID-19 infected children. Although different pathogenic mechanisms than adults have been supposed, further studies are required to better clarify the pathophysiology of abnormal liver enzymes in children with COVID-19.
The potential prognostic role for NAFLD as well as its intrinsic close relationship with metabolic traits require a close monitoring of fatty liver in these patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xi D S-Editor: Zhang H L-Editor: Filipodia P-Editor: Ma YJ
| 1. | World Health Organization. Coronavirus Disease (COVID-2019) Situation Reports. Geneva: WHO; 2020. [cited 5 February 2021] Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation. [Cited in This Article: ] |
| 2. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4113] [Cited by in F6Publishing: 4360] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 3. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11946] [Cited by in F6Publishing: 13074] [Article Influence: 3268.5] [Reference Citation Analysis (0)] |
| 4. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1286] [Cited by in F6Publishing: 1444] [Article Influence: 361.0] [Reference Citation Analysis (0)] |
| 5. | Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614-14621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 619] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 6. | Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 566] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 7. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3643] [Cited by in F6Publishing: 3934] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 8. | Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology 2020; 159: 373-375. e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 252] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 10. | Liang W, Feng Z, Rao S, Xiao C, Xue X, Lin Z, Zhang Q, Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 11. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 338] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 12. | Fu Y, Zhu R, Bai T, Han P, He Q, Jing M, Xiong X, Zhao X, Quan R, Chen C, Zhang Y, Tao M, Yi J, Tian D, Yan W. Clinical Features of Patients Infected With Coronavirus Disease 2019 With Elevated Liver Biochemistries: A Multicenter, Retrospective Study. Hepatology. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 13. | Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286-2293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 101] [Cited by in F6Publishing: 85] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 14. | Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J Gastroenterol. 2020;26:2323-2332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 114] [Cited by in F6Publishing: 90] [Article Influence: 22.5] [Reference Citation Analysis (2)] |
| 15. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 189] [Article Influence: 47.3] [Reference Citation Analysis (2)] |
| 16. | Méndez-Sánchez N, Valencia-Rodríguez A, Qi X, Yoshida EM, Romero-Gómez M, George J, Eslam M, Abenavoli L, Xie W, Teschke R, Carrion AF, Keaveny AP. What Has the COVID-19 Pandemic Taught Us so Far? J Clin Transl Hepatol. 2020;8:0024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Sahota AK, Shapiro WL, Newton KP, Kim ST, Chung J, Schwimmer JB. Incidence of Nonalcoholic Fatty Liver Disease in Children: 2009-2018. Pediatrics. 2020;146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Paul S, Davis AM. Diagnosis and Management of Nonalcoholic Fatty Liver Disease. JAMA. 2018;320:2474-2475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Morandi A, Di Sessa A, Zusi C, Umano GR, El Mazloum D, Fornari E, Miraglia Del Giudice E, Targher G, Maffeis C. Nonalcoholic Fatty Liver Disease and Estimated Insulin Resistance in Obese Youth: A Mendelian Randomization Analysis. J Clin Endocrinol Metab. 2020;105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Di Bonito P, Valerio G, Licenziati MR, Miraglia Del Giudice E, Baroni MG, Morandi A, Maffeis C, Campana G, Spreghini MR, Di Sessa A, Morino G, Crinò A, Chiesa C, Pacifico L, Manco M. High uric acid, reduced glomerular filtration rate and non-alcoholic fatty liver in young people with obesity. J Endocrinol Invest. 2020;43:461-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1326] [Cited by in F6Publishing: 1361] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 22. | Targher G. What's Past Is Prologue: History of Nonalcoholic Fatty Liver Disease. Metabolites. 2020;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 205] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 24. | Di Sessa A, Umano GR, Cirillo G, Passaro AP, Verde V, Cozzolino D, Guarino S, Marzuillo P, Miraglia Del Giudice E. Pediatric non-alcoholic fatty liver disease and kidney function: Effect of HSD17B13 variant. World J Gastroenterol. 2020;26:5474-5483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Zhou YH, Zheng KI, Targher G, Byrne CD, Zheng MH. Abnormal liver enzymes in children and infants with COVID-19: A narrative review of case-series studies. Pediatr Obes. 2020;15:e12723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 394] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 27. | Medeiros AK, Barbisan CC, Cruz IR, de Araújo EM, Libânio BB, Albuquerque KS, Torres US. Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom Radiol (NY). 2020;45:2748-2754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 377] [Article Influence: 94.3] [Reference Citation Analysis (2)] |
| 29. | Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, Judge R, Soubieres A, Middleton P, Daunt A, Perez-Guzman P, Selvapatt N, Lemoine M, Dhar A, Thursz MR, Nayagam S, Manousou P. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. 2020;15:e0240400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, George J, Zheng MH. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2021;36:204-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 31. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 32. | Sharma P, Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab Syndr. 2020;14:825-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 34. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 35. | Zhong ZF, Huang J, Yang X, Peng JL, Zhang XY, Hu Y, Fu N, Lin HL, Jiang B, Tian YY, Yao HY, Deng LP, Tang XQ, Zhou JC, Tang J, Xie X, Liu Q, Liu J, Dou CY, Dai RJ, Yan B, Yang XF. Epidemiological and clinical characteristics of COVID-19 patients in Hengyang, Hunan Province, China. World J Clin Cases. 2020;8:2554-2565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 759] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 37. | Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 371] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 38. | Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55:1169-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 617] [Cited by in F6Publishing: 611] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 39. | Wang D, Ju XL, Xie F, Lu Y, Li FY, Huang HH, Fang XL, Li YJ, Wang JY, Yi B, Yue JX, Wang J, Wang LX, Li B, Wang Y, Qiu BP, Zhou ZY, Li KL, Sun JH, Liu XG, Li GD, Wang YJ, Cao AH, Chen YN. [Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China]. Zhonghua Er Ke Za Zhi. 2020;58:269-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 96] [Reference Citation Analysis (0)] |
| 40. | Jiehao C, Jin X, Daojiong L, Zhi Y, Lei X, Zhenghai Q, Yuehua Z, Hua Z, Ran J, Pengcheng L, Xiangshi W, Yanling G, Aimei X, He T, Hailing C, Chuning W, Jingjing L, Jianshe W, Mei Z. A Case Series of Children With 2019 Novel Coronavirus Infection: Clinical and Epidemiological Features. Clin Infect Dis. 2020;71:1547-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 533] [Article Influence: 133.3] [Reference Citation Analysis (0)] |
| 41. | Tan YP, Tan BY, Pan J, Wu J, Zeng SZ, Wei HY. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2020;127:104353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 42. | Mameli S, Marcialis MA, Bassareo PP, Fanos V. COVID-19 and hepatic damage: what we know? Panminerva Med. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 44. | Rohani P, Ahmadi Badi S, Moshiri A, Siadat SD. Coronavirus disease 2019 (COVID-19) and pediatric gastroenterology. Gastroenterol Hepatol Bed Bench. 2020;13:351-354. [PubMed] [Cited in This Article: ] |
| 45. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |