Published online Jun 14, 2021. doi: 10.3748/wjg.v27.i22.2963
Peer-review started: February 2, 2021
First decision: February 27, 2021
Revised: March 10, 2021
Accepted: April 21, 2021
Article in press: April 21, 2021
Published online: June 14, 2021
Ulcerative colitis (UC) is a chronic, nonspecific, relapsing inflammatory bowel disease. The colorectum is considered the chief target organ of UC, whereas upper gastrointestinal (UGI) tract manifestations are infrequent. Recently, emerging evidence has suggested that UC presents complications in esophageal, stomachic, and duodenal mucosal injuries. However, UC-related UGI tract manifestations are varied and frequently silenced or concealed. Moreover, the endoscopic and microscopic characteristics of UGI tract complicated with UC are nonspecific. Therefore, UGI involvement may be ignored by many clinicians. In addition, no standard criteria have been established for patients with UC who should undergo fibrogastroduodenoscopy. Furthermore, specific treatment recommendations may be needed for patients with UC-associated UGI lesions. Herein, we review the esophageal, gastric, and duodenal mucosal lesions of the UC-associated UGI tract, as well as the potential pathogenesis and therapy.
Core Tip: This is a minireview to summarize the esophageal, gastric, and duodenal mucosal lesions of the ulcerative colitis (UC)-associated upper gastrointestinal (UGI) tract, together with the potential pathogenesis and treatment recommendations. UC-related UGI manifestations are diverse and normally quite or covered by the manifestations of the lower gastrointestinal tract. In addition, the endoscopic and microscopic characteristics of UGI tract complicated with UC are typically unspecified. However, it is very important to identify UC-associated UGI tract diseases. Our research may assist in the diagnosis and monitoring of UC-associated UGI tract diseases.
- Citation: Sun Y, Zhang Z, Zheng CQ, Sang LX. Mucosal lesions of the upper gastrointestinal tract in patients with ulcerative colitis: A review. World J Gastroenterol 2021; 27(22): 2963-2978
- URL: https://www.wjgnet.com/1007-9327/full/v27/i22/2963.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i22.2963
Ulcerative colitis (UC) is an idiopathic, chronic, relapsing inflammatory bowel disease (IBD) occurring in the colon and rectum. It has long been agreed that UC starts in the rectum and commonly spreads to a part of or the entire colon[1,2]. The main typical symptoms of UC include frequent bowel movement, mucus pus, bloody diarrhea, abdominal pain and discomfort, urgency, weight loss, and tenesmus[3,4]. Nevertheless, tremendous advances and progress have been made in our understanding of this disease over the last 2 decades. Apart from the above typical symptoms, emerging evidence supports that there are a variety of accompanying symptoms involving the upper gastrointestinal (UGI) tract in patients with UC upon macroscopic and microscopic analyses[5-9], such as eosinophilic esophagitis[10], gastroduodenitis[11], ulcers, or UGI inflammation[12,13]. In addition, the positive rate of UGI endoscopy in asymptomatic individuals was lower than that in symptomatic patients. The reported clinically significant UGI lesions include multiple erosions, granular changes, bamboo joint-like appearance, white spots, friable mucosa, ulcer, and purulent deposits during esophagogastroduodenoscopy[11,14-16], in which multiple erosions are extremely rare (0%-3%) in UC patients[17]. The UC-related UGI endoscopic and microscopic presentations are summarized in Figure 1 and Table 1.
| Classification | Presentations of UGI in UC | |
| Endoscopy | Erythema | |
| Edema | ||
| Inconspicuous vascular morphology | ||
| Granularity | ||
| Friability | ||
| Oral ulcers, superficial ulcers | ||
| Bamboo joint-like appearance | ||
| White spots | ||
| Purulent deposits | ||
| Microscopy | Esophagus | Esophageal ulcers |
| Esophagitis | ||
| Unspecified/nonspecific | ||
| Stomach | Unspecified chronic gastritis | |
| Lymphocytic gastritis | ||
| FEG | ||
| Ulcers | ||
| Duodenum | Diffuse chronic duodenitis in the presence of mucosal dilatation | |
| Structural deformation | ||
| Intraepithelial lymphocytic disease with normal mucosal structure | ||
Millions of people worldwide are affected by UC, which has been considered a global disorder[18]. The incidence of UC has been rising since the mid-20th century[19]. Nevertheless, pediatric IBD patients have been generally thought to present more common UGI involvement than adult patients. This discrepancy may be because UGI endoscopy was less routinely performed on asymptomatic adult patients with IBD than on their pediatric counterparts[20]. UC patients receive targeted treatment at various organizations ranging from community hospitals and public hospitals to clinics. However, UGI involvement may be unnoticed by the attending physician due to the lack of the knowledge of gastroduodenal lesions, which should raise concerns during UC treatment[15]. Furthermore, there is no established standard or criteria available for patients with UC who should undergo fibrogastroduodenoscopy (FGDS), because the clinical backgrounds of these patients with UC-associated UGI lesions have not been fully and exactly demonstrated. Specific management may be required for patients with UC-associated UGI lesions.
Henceforth, we conducted this review with the aim of describing various UGI tract presentations in UC and their differential diagnosis and management. Our study may help physicians and other clinicians make a correct and timely diagnosis of UC while avoiding needless examinations.
Traditionally, UC is considered a purely colon disease other than limited terminal ileal inflammation. In recent years, however, the prevalence at initial presentation of UC-associated esophageal disease has been 12%-50%[10], while it is as high as 70% to 90% in Crohn’s disease (CD) individuals[21,22]. These numbers may underestimate the prevalence of the disease due to the difficulty of precise diagnosis and the lack of standard upper endoscopy in asymptomatic individuals with UC. It is very challenging to diagnose esophageal complications of UC because these complications can present as diverse esophageal symptoms, such as erosive-ulcerative esophagitis or esophageal stricture, which share many characteristics with other common esophageal diseases. In addition, the microscopic manifestations and characteristics of esophageal complications with UC are typically unspecified. Although the prevalence of esophageal disease complicated with UC is lower than esophageal disease complicated with CD, it is still important to identify UC-associated esophageal disease.
The first case of esophagitis was reported by Margoles et al[23] in 1961. The occurrence of esophagitis has been described more frequently in pediatric patients than in adult individuals[24]. However, the endoscopic and histologic characteristics are unspecified or nonspecific. In addition, it is difficult to evaluate other irrelevant disorders, such as reflux disease, medications, or Candida[13,25,26]. Lymphocytic esophagitis (LE) is defined as large numbers of peripapillary lymphocytoses (PLs) or intraepithelial lymphocytes (IELs) without significant granulocytes (neutrophils and eosinophils)[27], and LE was originally demonstrated as a UGI tract manifestation in children with IBD[28]. LE has been reported in 7% of pediatric patients with UC and mucosal injury (edema or dyskeratosis) is commonly seen in patients with UC[28,29]; however, a significant difference was not found between IBD and non-IBD control individuals[28]. In contrast, a previous study suggested that PL found in LE might be a marker for disease activity in IBD[27]. The diagnosis of LE in UC patients should be distinguished from candidiasis, lichen planus esophagitis, and lichenoid esophagitis[30]. Granulomatous esophagitis has been reported in patients with CD[31-33] but not in patients with UC. The histologic diagnostic criteria for granulomatous esophagitis include chronic granulomatous disease, common variable immunodeficiency, and infection. Moreover, a previous analysis also revealed that there were statistically significant inverse associations between eosinophilic esophagitis and CD or microscopic colitis, but not UC[34].
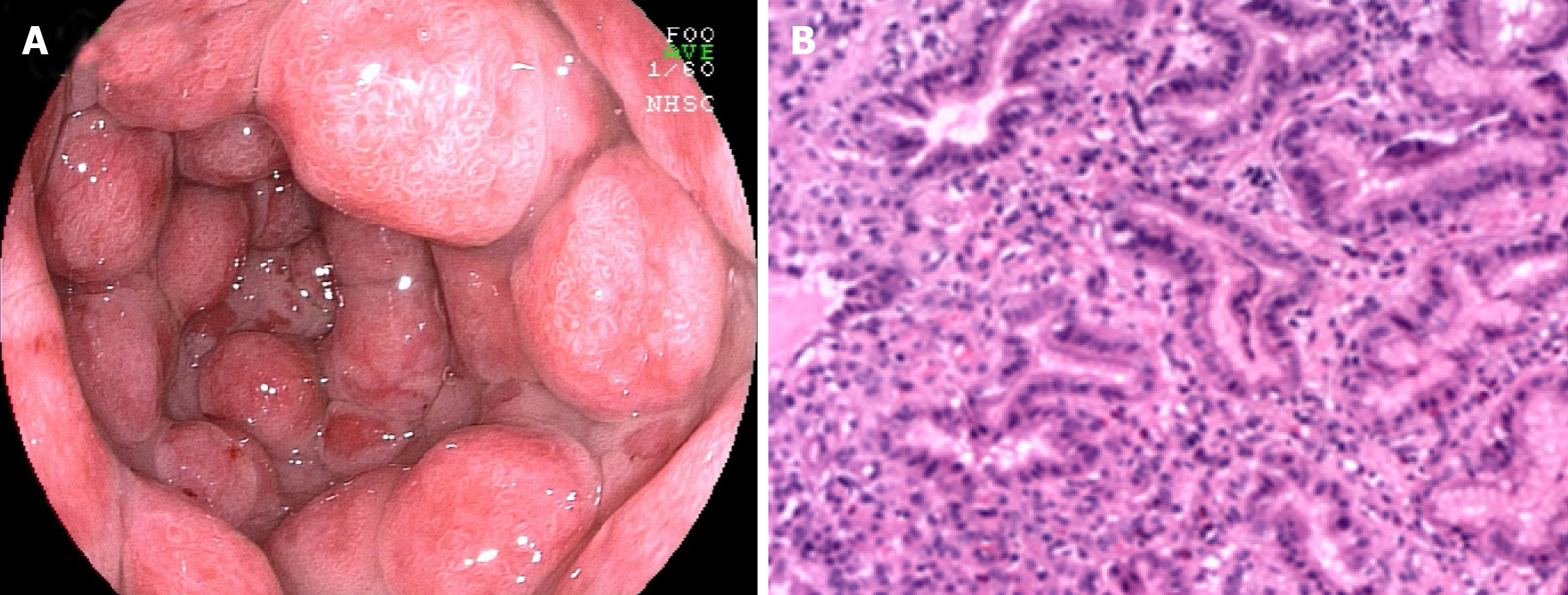
There are relatively few reports of esophageal ulcers associated with UC. Until now, there have been only 20 cases of esophageal ulcers (including ours) accompanied by UC[23,24,35-45]. The clinical characteristics of these patients are listed in Table 2. It is a punched-out ulcer and frequently seen in the middle to the lower esophagus[24]. According to the previous findings, esophageal ulcers were more commonly seen in the young individuals than in elderly individuals. Additionally, patients often have a complicated esophageal ulcer at the onset of colitis, or when it recurs. However, the endoscopic manifestations of UC patients with esophageal ulcers were unlike gastroduodenitis associated with UC (GDUC). GDUC is characterized as friable and granular mucosa[11,46], but in contrast, the esophageal ulcers complicated by UC are punched-out ulcers and frequently occur in the middle to lower esophagus (Figure 2A). Pathologically, only nonspecific inflammatory cell infiltration was demonstrated in all reported cases, without any UC-specific characteristics (Figure 2B). Nevertheless, regarding histology, the association between esophageal ulcers and UC was not established; esophageal lesions would still be effective in the treatment of UC. In addition, most UC patients with esophageal ulcers present other extraintestinal manifestations, including ocular injuries, skin disorders, and peripheral arthritis[47]. Nonetheless, the underlying pathological mechanisms are currently unidentified. Therefore, UC-complicated esophageal ulcers are particularly uncommon. They may occur as an extraintestinal manifestation in most cases of UC. The pathological discoveries of esophageal lesions are unspecified for UC. Consequently, a comprehensive diagnosis is needed to exclude diseases related to infection and drugs.
| Case No. | Ref. | Age | Gender | Duration of UC | Esophageal ulcer | Location | Symptoms | Treatment |
| 1 | Margoles et al[23], 1961 | 28 | M | 17 yr | Web formation | Middle esophagus | No | NA |
| 2 | Knudsen et al[36], 1967 | 15 | M | 2 yr | Severe esophagitis | Lower esophagus | Parasternal chest pain and dysphagia | PSL |
| 3 | Rosendorff et al[35], 1967 | 23 | M | 5 yr | Shallow ulceration with gross diffuse irregularity | Middle and lower esophagus | Dysphagia | PSL |
| 4 | Christopher et al[37], 1969 | 21 | F | 0 | Active ulcerative esophagitis | NA | NA | NA |
| 5 | Christopher et al[37], 1969 | 19 | M | 0 | Marked ulcerative esophagitis | NA | Substenal pain and dysphagia | NA |
| 6 | Christopher et al[37], 1969 | 26 | M | 2 mo | Esophageal ulcerations with perforaton into theanterior mediastinum | NA | Dysphagia, fever, hypotension | NA |
| 7 | Christopher et al[37], 1969 | 14 | F | 5 yr | Necrotizing fibrinopurulent ulceration | NA | NA | NA |
| 8 | Christopher et al[37], 1969 | 24 | M | 9 yr | Ulcerative and membranous esophagitis | NA | Nausea, vomiting and hematemesis | NA |
| 9 | Zimmerman et al[38], 1984 | 21 | M | 3 yr | Multiple friable ulcerations | Whole esophagus | Severe odynophagia and dysphagia | PSL, tetracycline |
| 10 | Konishi et al[40], 1998 | 47 | F | 0 | Multiple irregular esophageal ulcers | Whole esophagus | Anterior chest pain and dysphagia | 5ASA, H2blocker |
| 11 | Asakawa et al[44], 2000 | 18 | F | 7 yr | Longitudinal esophageal ulcer with hemorrhage | Middle and lower esophagus | Sore throat and pain on swallowing | PSL |
| 12 | Ikeda et al[42], 2000 | 18 | M | 1 yr | Punched-out esophageal ulcer | Middle esophagus | Sore throat and anterior chest pain | PSL |
| 13 | Higashi et al[41], 2004 | 19 | M | 0 | Punched-out esophageal ulcer | Middle esophagus | General fatigue | PSL, SASP |
| 14 | Sato et al[45], 2005 | 33 | M | 0 | Punched-out esophageal ulcer | Middle and lower esophagus | Anterior chest pain and dysphagia | PPI |
| 15 | Izawa et al[39], 2015 | 52 | F | 3 yr | Punched-out esophageal ulcer | Middle esophagus | Anterior chest pain on swallowing | PSL |
| 16 | Kuroki et al[43], 2019 | 47 | M | 3 yr | Necrosis | Lower esophagus | Epigastric pain | PPI |
| 17 | Kuroki et al[43], 2019 | 53 | M | NA | Necrosis | Middle and lower esophagus | Hematemesis | PPI |
| 18 | Tominaga et al[24], 2020 | 16 | F | 0 | An esophageal ulcer with aphthae | Middle esophagus | Chest pain | PSL |
| 19 | Tominaga et al[24], 2020 | 19 | F | 0 | Longitudinal esophageal ulcer | Low esophagus | Chest pain on swallowing | PSL |
| 20 | Ours | 24 | M | 1 yr | Multiple ulcerative lesions | Multiple lesions | Burning sensation | Remicade |
UC complicated by gastroduodenal lesions was first reported by Sasaki et al[14]. Multiple erosions and granular changes in the greater curvature of the gastric antrum and descending part of the duodenum (Figure 3A) have been demonstrated. Histologically, they confirmed obvious inflammatory cell infiltration and crypt abscesses in the gastric and duodenum. Subsequently, an increasing body of evidence suggests gastroduodenal mucosal lesions in UC patients[13,46,48-52]. More recently, the concept of ulcerative gastroduodenal lesion (UGDL) or GDUC was proposed[46,53]. The standard of diagnosis of UGDL includes: The lesions could be improved after UC treatment and the pathological examination is similar to the UC. Nevertheless, the diagnosis criteria are not rigorous enough because of the rare reports. It has been demonstrated that several factors are the major risk factors for GDUC, including more extensive colitis, administration of a lower dose of prednisolone, occurrence of pouchitis, post-colectomy status, and a longer postoperative period[54-56].
The occurrence of gastritis or gastroduodenitis has been estimated in 5%-19% of patients with UC[11,46,48,57-61]. Most UC patients present with mild local symptoms or no symptoms at all. Severe gastritis or gastroduodenitis is usually seen in subjects with long postoperative intervals after colectomy[60] or in individuals with extensive colitis, ileoanal pouchitis, or pancolitis[46,48,58]. Focal enhanced gastritis (FEG) has been considered as the most frequent UGI inflammatory form in patients with UC, followed by gastric basal mixed inflammation and superficial plasmacytosis[58,62-64]. FEG is characterized by localized accumulation of lymphocytes, neutrophils, and macrophages in at least one pit, neck, or gland of the adjacent lamina propria[58]. This focal inflammation pattern can be observed anywhere in the mucosa (Figure 3B), from the basal paramucosa of the muscularis to the superficial subepithelial layers, with the occurrence of a single focal point or as multiple focal points. Previously, FEG was described as a specific diagnostic marker for CD patients[65-67]. However, different opinions have also been proposed[68,69]. Scholars have also found that FEG could be seen in up to 20.8% of children with UC[65]. Although it is still uncertain whether the utility of FEG could distinguish CD from UC in adults, it is unreliable in children[65,70-72]. Basal and patchy inflammation is the second pattern of gastric inflammation, which includes a loose mixture of lymphocytes, eosinophils, mast cells, and plasma cells. These cells were found in the lamina propria between the deepest glands and the muscularis mucosae[58]. Moreover, superficial plasmacytosis is the third most common pattern of gastric inflammation. It is regarded as a diffuse band of plasma cells in the superficial lamina propria adjacent to the pits and necks. Furthermore, chronic, diffuse gastritis also seems to be a frequent feature in the gastric biopsy specimens from UC individuals[73]. Moreover, a recent study suggested that the active inflammation of the stomach and/or duodenum might be related to drug-refractory UC[61]. Notably, erosions or ulcers complicated with UC are infrequent and granulomas are always absent. Regarding to the gross and endoscopic features, UC-related gastritis is characterized by diffuse granular or brittle mucosa, as well as aphthous lesions[11,48]. For the differential diagnosis, patients with UC present with mild chronic sinusitis in the presence or absence of neutrophils and have a questionable specificity, which may be correlated with dietary and environmental factors. The infiltration of inflammatory cells observed in Helicobacter pylori-relevant gastritis and gastric CD-relevant chronic superficial gastritis is denser and more diffuse than that in UC.
The duodenum is not usually regarded as a target organ of UC. However, as early as the 1960s, chronic duodenitis associated with UC was first demonstrated[74]. Subsequently, emerging evidence implies the occurrence of chronic duodenitis associated with UC[11,17,46,48,51,53,75-83]. Nevertheless, it remains infrequent. Dyspepsia is the most common symptom of chronic duodenitis associated with UC, but it is often covered by the symptoms of UC[59]. Notably, most symptomatic duodenitis individuals present with severe colitis, which in some cases requires a colectomy. More interestingly, some individuals with a continental ileoanal pouch also have pouchitis[48]. A unique type of UGI inflammation in UC individuals is diffuse chronic duodenitis, which occurs in 10% of duodenal biopsy patients and combined resection occurs in 40% of UC patients who have had pouchitis. The reported endoscopic findings in symptomatic patients are diverse and include diffuse edema, granular, fragile, and ulcers, etc. Furthermore, the microscopic characteristics of duodenitis associated with UC include a dilated mucosa with diffuse inflammatory infiltration of monocytes and neutrophilic inflammation, glandular deformation, and erosion or ulceration[78]. Moreover, duodenal CD3 (+) and CD8 (+) IELs or lamina propria mononuclear cells may be present in a series of UC patients[84,85]. In summary, duodenitis complicated with UC is infrequently identified due to its aspecific capillary and microscopic features as well as its overlap with celiac disease, peptic duodenitis, and drug-related duodenitis. Nevertheless, clinical consciousness should be considered in diagnosing UC, especially if the patients have accomplished previous colectomy for widespread and severe colitis or if the patients also experience concomitant pouchitis or enterocolitis.
It is believed that UC may be mainly determined by a complex combination of environmental and host factors, genetic variations, immune response, and gut microbiota. The onset of this disease is activated by disturbance of the mucosal barrier, gut microbiota, and abnormal immune response. Many scholars support that the abnormal immune response (innate and adaptive) is a key direct pathogenesis, in which gut microbiota is an important stimulus for this immune damage process and the environmental and host factors may be the causative factors of the disease.
It has been well acknowledged that environmental and host factors play critical roles in increasing the susceptibility of developing UC. The increasing incidence of UC worldwide implies the significance of environmental factors in the progression of this disease[86,87]. This is similar to the pattern detected in the Western world in the early 20th century[88]. UC has specifically occurred in urban zones, and its incidence is faster and then slower. Westernization and its accompanying urbanization, sedentary lifestyle, exposure to environmental pollution, dietary changes, antibiotics usage, refrigeration, better sanitation, and fewer infections are regarded as contributing factors[89]. For example, former cigarette smoking has been reported as one of the UC strongest risk factors, whereas compared to the former and non-smokers, active smokers are less probably to suffer from UC and they mainly present with a milder clinical course[90-92]. Furthermore, appendectomy is considered to have a protective impact on future development of UC[93].
Genetic studies have been predominantly effective in recognizing both common and infrequent genetic variants associated with susceptibility to UC[94-96]. Human leukocyte antigen[97] and adenylate cyclase type 7[98] are the two important UC-specific genes. Moreover, many UC-specific genes are confirmed to be responsible for mediation of epithelial barrier function. However, it has been established that UC and CD share most genetic factors. These shared genetic factors could encode cytokine, innate and adaptive immune signal pathways, and immune sensing, such as interleukin (IL)-10, -12, -23R, and caspase recruitment domain containing protein 9. In addition, it has been demonstrated that about 70% of genetic variants are also commonly seen in some other autoimmune diseases, such as ankylosing spondylitis and psoriasis[86]. Overall, genetic factors deliberate a small but certain increase in susceptibility to UC. Nevertheless, many individuals did not present with genetic susceptibility when all susceptibility loci were evaluated by polygenic risk scores[99]. These findings imply that an abnormal adaptive immune response and epithelial barrier dysfunction may play critical roles in the pathogenesis of UC.
A series of animal experiments and clinical trials have confirmed the presence of significant intestinal flora dysbiosis in patients with UC[100,101]. The dysbiosis of intestinal flora is featured by decreased biodiversity, irregular composition of gut microbiota, and changes of spatial distribution, together with interactions between microbiota and the host[101]. There is a significant change in the number of intestinal bacteria in patients with UC, which is reflected in a decrease in probiotic bacteria (e.g., Bifidobacterium and lactobacillus, etc.) and an increase in conditionally pathogenic bacteria (such as Enterococci and Enterobacteria, etc.). Therefore, increasing studies have paid attention to the therapeutic effects of faecal microbial transplantation from healthy donors on patients with UC[100,102-105].
UC is a pathogenic inflammatory disease mediated by the immune system including innate immunity and adaptive immunity[106]. The innate immunity is the first line of defense against pathogens. Unlike adaptive immunity, innate immunity is non-specific and persistent. Immune cells in innate immunity, such as dendritic cells (DCs), macrophages, natural killer cells, intestinal epithelial cells, and myofibroblasts, can sense the intestinal microbiota and respond to conserved structural motifs of microorganisms, which can trigger a rapid and effective inflammatory response and prevent bacterial invasion[107]. Among them, DCs are specialized antigen-presenting cells responsible for T-cell activation and induction of adaptive immune responses and they are key players in the interplay between innate and adaptive immunity[107]. For the adaptive immune system, the components of this system cooperate with each other, with the molecules, and with the cells in the innate immune system to mount an effective immune response that eliminates invading pathogens under normal conditions. Unlike innate immunity, adaptive immunity has highly specific long-term immunity and is adaptive because antigen specificity is the result of complex maturation and development of immune cells. T helper (Th) cells are key elements to the adaptive immune response. UC is mainly activated by Th2 cells-induced immune response[108]. Th2 cells are mainly induced by IL-13 and then subsequently secrete IL-4, IL-5, and IL-13, in which IL-13 is considered essential to the pathogenesis of UC[109].
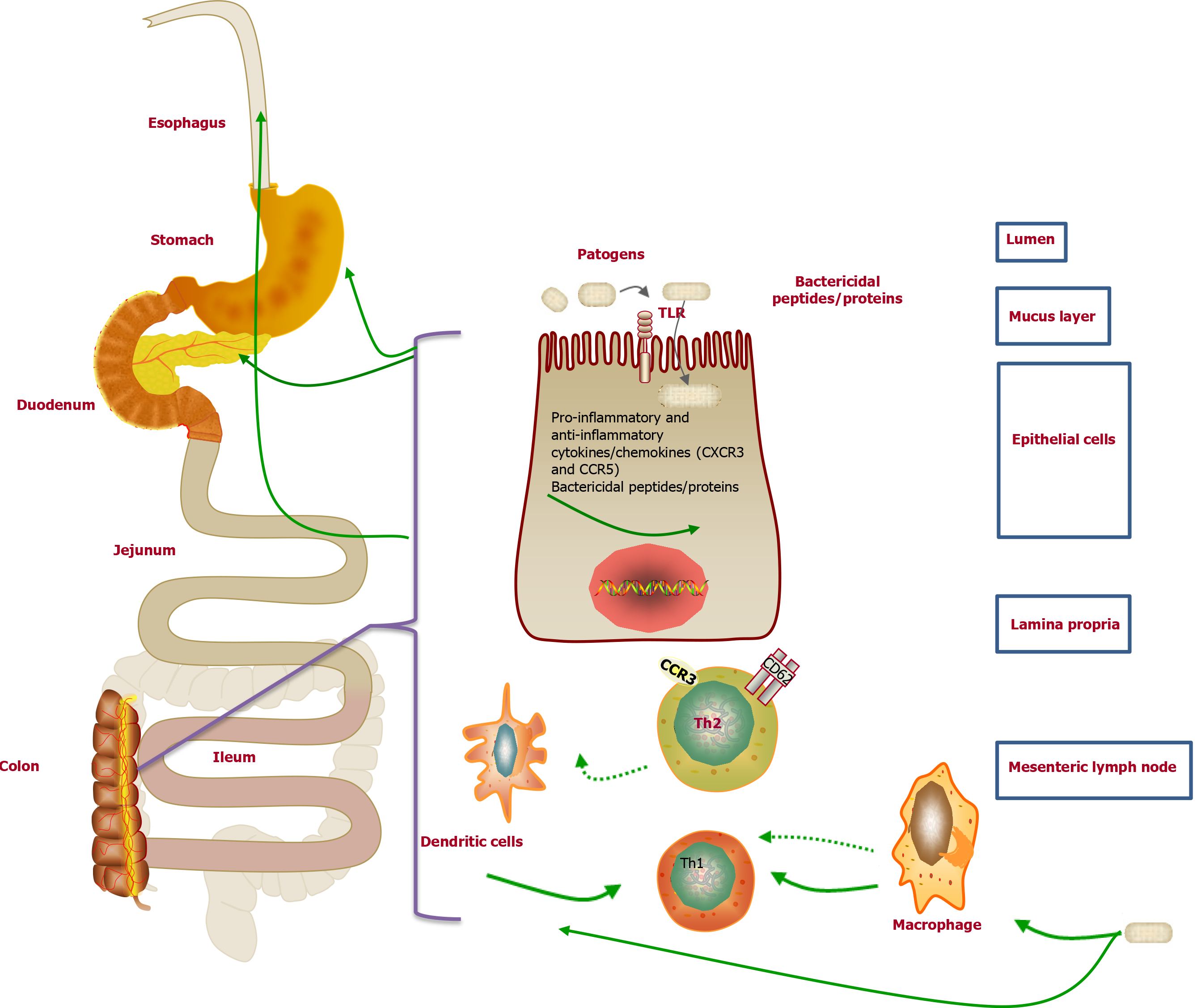
The pathogenesis of UGI mucosal lesions has not been clearly clarified. Nevertheless, many researchers supported that it was similar to that of the colonic lesions of UC. The cellular immune response to certain bacteria may contribute to the potential pathogenesis of UC[110]. Interestingly, similar immune molecules were also demonstrated in both the UGI tissue and the colon tissue (Figure 4). For example, a previous study suggested that the Th2 cells activated by CCR3+ and CD62L in the UGI tract might originate from mucosal lesions of the colon[111]. Moreover, Berrebi et al[112] reported that the chemokine receptor CXCR3 was unanimously expressed in the epithelium and lamina propria of perivascular areas of gastric biopsy specimens from the UC patients, while the chemokine receptor CCR5 was found in the lymphocytes surrounding the lamina propria. Therefore, the increased lymphocyte population upon stimulation in the colon of UC patients may result in lymphocyte infiltration in the stomach[73]. Furthermore, the excessive autoimmune response to bacterial antigens caused by genetic susceptibility to abnormal immune responses may result in the enrollment of memory T cells in the colorectal mucosa in the UGI tract[112]. However, there is no related evidence of the correlation between H. pylori and UGDL[46,113].
Molecular pathological epidemiology (MPE) has been reported as an integrative transdisciplinary science, which integrates the academic disciplines of molecular pathology and epidemiology[114-117]. Unlike conventional epidemiologic research, such as genome-wide association studies, MPE mainly figures out the underlying heterogeneity of disease processes, whereas traditional molecular epidemiology typically treats a disease as an individual entity[118,119]. Similar to the biology systems and WebMed[120,121], MPE combines the analysis of overall populations and macroenvironments, which is involved in the molecular analysis and microenvironments. In addition, the goal of MPE is to explore the mutual relation between exogenous and endogenous elements, molecular markers, and progression of malignancy[117,122,123]. More importantly, MPE research could cover all human diseases. Therefore, MPE studies can offer insightful aspects on the pathogenesis of disease by validating specific mechanisms in disease development and progression[122].
It has been well demonstrated that traditional epidemiology investigation reveals many factors, such as lifestyle, dietary, and environmental exposures, could be positively or negatively related with risk of illness[117,124]. Nevertheless, it is still unclear how these exposures impact the disease pathogenesis. Previous studies have confirmed that these above factors probably affect the pathogenic process by changing the local tissue microenvironment, and epigenetics play an important role in cellular response to alterations in the microenvironment[114]. An increasing body of evidence demonstrates application of MPE in colorectal cancer research to find out the possible etiologic factors[125-129]. Nonetheless, little information is available with respect to the research of MPE on UC. UC has been considered a heterogeneous disease, in which smoking, alcohol, diet, obesity, microbiome, inflammation, immunity, germline genetic variations, and gene-by-environment interactions are responsible for the progression of UC[130,131]. Therefore, MPE may be helpful to investigate those factors in relation to molecular pathologies, immunity, and clinical outcomes.
Although the MPE research has many advantages, the challenges should also be taken into consideration. Primarily, the reported challenges in the MPE study comprise the selected sample size, requirement for severe corroboration of molecular examines and study discoveries, and lack of multidisciplinary experts, international forums, as well as the standardized strategies[119]. Furthermore, MPE studies have to confront the problem of various hypothesis challenging and therefore require the formation of a priori hypotheses on the basis of early exploratory discoveries or possible biological mechanisms[117]. Moreover, MPE may also generate more opportunities for false discoveries[119]. In consideration of the pathogenesis of UC, MPE paradigm may be a promising direction and improve prediction of response to pharmacological, dietary, and lifestyle interventions for UC, together with the pathomechanism and treatment of mucosal lesions of the UGI tract in patients with UC.
5-aminosalicylate, infliximab, corticosteroid, antitumor necrosis factor (TNF), or calcium antagonist has been reported to be effective for managing UC[81-83,132]. Nevertheless, currently, standard treatment for UGI lesions in UC has not yet reached agreement. Steroids, leukocytapheresis, and/or peroral mesalazine can be used to treat UGI lesions in UC[46]. Moreover, immunosuppression such as cyclosporine, tacrolimus, or infliximab may be the treatment option for UGI inflammation[77,82,133,134] (Figure 5). For example, for individuals with UC-associated esophageal ulcers, intravenous prednisolone (50 mg/d or 60 mg/d) was administered, and some symptoms including chest pain, bloody stool, pain on swallowing, and diarrhea were improved within a couple of days[24]. For individuals with UGDL, particularly those presenting with mild symptoms, some drugs, such as sulfasalazine or mesalazine, could effectively relieve the symptoms[54], while for some individuals with severe UGDL, TNF antagonists, infliximab, or calcium antagonists might be more effective than sulfasalazine or steroid hormones[82,132]. Therefore, administration of sulfasalazine or mesalazine is a quick and efficient therapy to relieve the symptoms of UGDL, steroids can be utilized as second-line treatment, and TNF antagonists, infliximab, or calcium antagonists could be applied for patients who are insensitive to hormone therapy or subjects whose symptoms progress rapidly.
UC-related UGI manifestations are infrequent and normally nonspecific compared with intestinal manifestations, and hence, an ongoing diagnostic challenge should be extensively considered. Although the occurrence of UGI involvement in UC is rare, FGDS is specifically recommended for patients with UC who show extraintestinal manifestations and have undergone a colectomy or present pancolitis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ogino S S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1151] [Cited by in F6Publishing: 1295] [Article Influence: 107.9] [Reference Citation Analysis (3)] |
| 2. | Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc. 2019;94:1357-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 3. | Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014;89:1553-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 185] [Article Influence: 18.5] [Reference Citation Analysis (3)] |
| 4. | Gajendran M, Loganathan P, Jimenez G, Catinella AP, Ng N, Umapathy C, Ziade N, Hashash JG. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019;65:100851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 5. | Kovacs M, Muller KE, Arato A, Lakatos PL, Kovacs JB, Varkonyi A, Solyom E, Polgar M, Nemes E, Guthy I, Tokodi I, Toth G, Horvath A, Tarnok A, Tomsits E, Csoszánszky N, Balogh M, Vass N, Bodi P, Dezsofi A, Gardos L, Micskey E, Papp M, Szucs D, Cseh A, Molnar K, Szabo D, Veres G; Hungarian IBD Registry Group (HUPIR). Diagnostic yield of upper endoscopy in paediatric patients with Crohn's disease and ulcerative colitis. Subanalysis of the HUPIR registry. J Crohns Colitis. 2012;6:86-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Lemberg DA, Clarkson CM, Bohane TD, Day AS. Role of esophagogastroduodenoscopy in the initial assessment of children with inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1696-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Castellaneta SP, Afzal NA, Greenberg M, Deere H, Davies S, Murch SH, Walker-Smith JA, Thomson M, Srivistrava A. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004;39:257-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Abdullah BA, Gupta SK, Croffie JM, Pfefferkorn MD, Molleston JP, Corkins MR, Fitzgerald JF. The role of esophagogastroduodenoscopy in the initial evaluation of childhood inflammatory bowel disease: a 7-year study. J Pediatr Gastroenterol Nutr. 2002;35:636-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88:995-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 291] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Goldstein NS, Amin M. Upper Gastrointestinal Tract in Inflammatory Bowel Disease. Surg Pathol Clin. 2010;3:349-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Hori K, Ikeuchi H, Nakano H, Uchino M, Tomita T, Ohda Y, Hida N, Matsumoto T, Fukuda Y, Miwa H. Gastroduodenitis associated with ulcerative colitis. J Gastroenterol. 2008;43:193-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Harpaz N, Polydorides AD. Upper Gastrointestinal Manifestations of Inflammatory Bowel Disease. Surg Pathol Clin. 2020;13:413-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Tobin JM, Sinha B, Ramani P, Saleh AR, Murphy MS. Upper gastrointestinal mucosal disease in pediatric Crohn disease and ulcerative colitis: a blinded, controlled study. J Pediatr Gastroenterol Nutr. 2001;32:443-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Sasaki M, Okada K, Koyama S, Yoshioka U, Inoue H, Fujiyama Y, Bamba T. Ulcerative colitis complicated by gastroduodenal lesions. J Gastroenterol. 1996;31:585-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Kato R, Iwamuro M, Hiraoka S, Takashima S, Inokuchi T, Takahara M, Kondo Y, Tanaka T, Okada H. Evaluation of the Upper Gastrointestinal Tract in Ulcerative Colitis Patients. Acta Med Okayama. 2018;72:105-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 16. | Song J, Chang J, Kim C, Kim J, Han S, Kim T. The Endoscopic Findings of the Upper Gastrointestinal Tract in Patients with Ulcerative Colitis and Its Correlation with Disease Extent and Upper Gastrointestinal Symptoms. Gut Liver. 2019;13. [Cited in This Article: ] |
| 17. | Rubenstein J, Sherif A, Appelman H, Chey WD. Ulcerative colitis associated enteritis: is ulcerative colitis always confined to the colon? J Clin Gastroenterol. 2004;38:46-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Pravda J. Can ulcerative colitis be cured? Discov Med. 2019;27:197-200. [PubMed] [Cited in This Article: ] |
| 19. | Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol. 2020;35:380-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 282] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 20. | Siow VS, Bhatt R, Mollen KP. Management of acute severe ulcerative colitis in children. Semin Pediatr Surg. 2017;26:367-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Dąbkowski K, Graca-Pakulska K, Zawada I, Ostrowski J, Starzyńska T. Clinical significance of endoscopic findings in the upper gastrointestinal tract in Crohn's disease. Scand J Gastroenterol. 2019;54:1075-1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Turner D, Griffiths AM. Esophageal, gastric, and duodenal manifestations of IBD and the role of upper endoscopy in IBD diagnosis. Curr Gastroenterol Rep. 2007;9:475-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Margoles JS, Wenger J. Stomal ulceration associated with pyoderma gangrenosum and chronic ulcerative colitis. Report of two cases. Gastroenterology. 1961;41:594-598. [PubMed] [Cited in This Article: ] |
| 24. | Tominaga K, Tsuchiya A, Sato H, Mizusawa T, Morita S, Ishii Y, Takeda N, Natsui K, Kawata Y, Kimura N, Arao Y, Takahashi K, Hayashi K, Yokoyama J, Terai S. Esophageal Ulcers Associated with Ulcerative Colitis: A Case Series and Literature Review. Intern Med. 2020;59:1983-1989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Ruuska T, Vaajalahti P, Arajärvi P, Mäki M. Prospective evaluation of upper gastrointestinal mucosal lesions in children with ulcerative colitis and Crohn's disease. J Pediatr Gastroenterol Nutr. 1994;19:181-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 159] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Ashton JJ, Bonduelle Q, Mossotto E, Coelho T, Batra A, Afzal NA, Vadgama B, Ennis S, Beattie RM. Endoscopic and Histological Assessment of Paediatric Inflammatory Bowel Disease Over a 3-Year Follow-up Period. J Pediatr Gastroenterol Nutr. 2018;66:402-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Basseri B, Vasiliauskas EA, Chan O, Wang HL, Basseri RJ, Pimentel M, Soffer E, Conklin JL. Evaluation of peripapillary lymphocytosis and lymphocytic esophagitis in adult inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:505-511. [PubMed] [Cited in This Article: ] |
| 28. | Ebach DR, Vanderheyden AD, Ellison JM, Jensen CS. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn's disease. Inflamm Bowel Dis. 2011;17:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 29. | Sutton LM, Heintz DD, Patel AS, Weinberg AG. Lymphocytic esophagitis in children. Inflamm Bowel Dis. 2014;20:1324-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 30. | Abuquteish D, Putra J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: A pathological review. World J Gastroenterol. 2019;25:1928-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | LiVolsi VA, Jaretzki A 3rd. Granulomatous esophagitis. A case of Crohn's disease limited to the esophagus. Gastroenterology. 1973;64:313-319. [PubMed] [Cited in This Article: ] |
| 32. | Mahadeva U, Martin JP, Patel NK, Price AB. Granulomatous ulcerative colitis: a re-appraisal of the mucosal granuloma in the distinction of Crohn's disease from ulcerative colitis. Histopathology. 2002;41:50-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | De Matos V, Russo PA, Cohen AB, Mamula P, Baldassano RN, Piccoli DA. Frequency and clinical correlations of granulomas in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2008;46:392-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Sonnenberg A, Turner KO, Genta RM. Comorbid Occurrence of Eosinophilic Esophagitis and Inflammatory Bowel Disease. Clin Gastroenterol Hepatol 2021; 19: 613-615. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Rosendorff C, Grieve NW. Ulcerative oesophagitis in association with ulcerative colitis. Gut. 1967;8:344-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Knudsen KB, Sparberg M. Ulcerative esophagitis and ulcerative colitis. JAMA. 1967;201:140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Christopher NL, Watson DW, Farber ER. Relationship of chronic ulcerative esophagitis to ulcerative colitis. Ann Intern Med. 1969;70:971-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Zimmerman HM, Rosenblum G, Bank S. Apthous ulcers of the esophagus in a patient with ulcerative colitis. Gastrointest Endosc. 1984;30:298-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Izawa N, Sugaya T, Kanamori A, Muraoka R, Hiraishi H. A case of ulcerative colitis with esophageal ulcer. Prog Digest Endosc. 2015;87:94-95. [Cited in This Article: ] |
| 40. | Konishi H, Fujino N. A case of right-sided ulcerative colitis associated with unusual esophageal ulcer. Chiba Med Assoc Mag. 1998;50:143-144. [Cited in This Article: ] |
| 41. | Higashi Y, Sunayama K. A case of ulcerative colitis combined with esophageal ulcer. J Japan Surg Assoc. 2004;65:143-146. [Cited in This Article: ] |
| 42. | Ikeda Y, Suzuki K. A case of ulcerative colitis associated with a hemorrhagic esophageal ulcer. J Kushiro City Gen. 2000;12:129-133. [Cited in This Article: ] |
| 43. | Kuroki H, Sugita A, Koganei K, Tatsumi K, Futatsuki R, Arai K. Two cases of esophageal ulcer after surgical treatment for ulcerative colitis. Clin J Gastroenterol. 2020;13:495-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Asakawa A, Kojima Y, Fujii E, Ohtaka M, Shimazaki R, Sato T, Nakamura T, Morozumi A, Akahane Y, Fujino MA. Case of ulcerative colitis associated with oesophageal ulcer. J Int Med Res. 2000;28:191-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Sato N, Kurakata H, Hirano N, Sakai K, Hamatani S. A case of ulcerative colitis combined with esophageal ulcer. Prog Digest Endosc. 2004;65:90-91. [Cited in This Article: ] |
| 46. | Hisabe T, Matsui T, Miyaoka M, Ninomiya K, Ishihara H, Nagahama T, Takaki Y, Hirai F, Ikeda K, Iwashita A, Higashi D, Futami K. Diagnosis and clinical course of ulcerative gastroduodenal lesion associated with ulcerative colitis: possible relationship with pouchitis. Dig Endosc. 2010;22:268-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore). 1976;55:401-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 805] [Cited by in F6Publishing: 673] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 48. | Ikeuchi H, Hori K, Nishigami T, Nakano H, Uchino M, Nakamura M, Kaibe N, Noda M, Yanagi H, Yamamura T. Diffuse gastroduodenitis and pouchitis associated with ulcerative colitis. World J Gastroenterol. 2006;12:5913-5915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Maeda K, Okada M, Seo M, Aoyagi K, Sakisaka S. Evaluation of gastroduodenal mucosal lesions in patients with Crohn's disease and ulcerative colitis. Digest Endosc. 2010;16:199-203. [Cited in This Article: ] |
| 50. | Uchino M, Ikeuchi H, Bando T, Matsuoka H, Hirata A, Takahashi Y, Takesue Y, Inoue S, Tomita N. Diffuse gastroduodenitis and enteritis associated with ulcerative colitis and concomitant cytomegalovirus reactivation after total colectomy: report of a case. Surg Today. 2013;43:321-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Choi YS, Kim JK, Kim WJ, Kim MJ. Remission of diffuse ulcerative duodenitis in a patient with ulcerative colitis after infliximab therapy: a case study and review of the literature. Intest Res. 2019;17:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Yang Y, Li CQ, Chen WJ, Ma ZH, Liu G. Gastroduodenitis associated with ulcerative colitis: A case report. World J Clin Cases. 2020;8:3847-3852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Li M, Liu Y, Cui J, Qin H, Shi Y, Zhang S, Zhao Y. Ulcerative colitis with mucosal lesions in duodenum: Two case reports. Medicine (Baltimore). 2019;98:e15035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | García Gavilán MDC, López Vega MC, Sánchez IM. Ulcerative colitis with gastric and duodenal involvement. Rev Esp Enferm Dig. 2017;109:535-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Kaufman SS, Vanderhoof JA, Young R, Perry D, Raynor SC, Mack DR. Gastroenteric inflammation in children with ulcerative colitis. Am J Gastroenterol. 1997;92:1209-1212. [PubMed] [Cited in This Article: ] |
| 56. | Annese V, Caruso N, Bisceglia M, Lombardi G, Clemente R, Modola G, Tardio B, Villani MR, Andriulli A. Fatal ulcerative panenteritis following colectomy in a patient with ulcerative colitis. Dig Dis Sci. 1999;44:1189-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:39-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Lin J, McKenna BJ, Appelman HD. Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: a controlled study. Am J Surg Pathol. 2010;34:1672-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Shen B, Wu H, Remzi F, Lopez R, Shen L, Fazio V. Diagnostic value of esophagogastroduodenoscopy in patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2009;15:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Hoentjen F, Hanauer SB, Hart J, Rubin DT. Long-term treatment of patients with a history of ulcerative colitis who develop gastritis and pan-enteritis after colectomy. J Clin Gastroenterol. 2013;47:52-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Sullivan KJ, Wei M, Chernetsova E, Hallani S, de Nanassy J, Benchimol EI, Mack DR, Nasr A, El Demellawy D. Value of upper endoscopic biopsies in predicting medical refractoriness in pediatric patients with ulcerative colitis. Hum Pathol. 2017;66:167-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Horjus Talabur Horje CS, Meijer J, Rovers L, van Lochem EG, Groenen MJ, Wahab PJ. Prevalence of Upper Gastrointestinal Lesions at Primary Diagnosis in Adults with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:1896-1901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Putra J, Ornvold K. Focally enhanced gastritis in children with inflammatory bowel disease: a clinicopathological correlation. Pathology. 2017;49:808-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Parente F, Cucino C, Bollani S, Imbesi V, Maconi G, Bonetto S, Vago L, Bianchi Porro G. Focal gastric inflammatory infiltrates in inflammatory bowel diseases: prevalence, immunohistochemical characteristics, and diagnostic role. Am J Gastroenterol. 2000;95: 705-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Sharif F, McDermott M, Dillon M, Drumm B, Rowland M, Imrie C, Kelleher S, Harty S, Bourke B. Focally enhanced gastritis in children with Crohn's disease and ulcerative colitis. Am J Gastroenterol. 2002;97:1415-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Hong CH, Park DI, Choi WH, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Kim DH, Kim MK, Chae SW, Lee KB, Sohn JH, Oh SJ. [The clinical usefulness of focally enhanced gastritis in Korean patients with Crohn's disease]. Korean J Gastroenterol. 2009;53:23-28. [PubMed] [Cited in This Article: ] |
| 67. | Roka K, Roma E, Stefanaki K, Panayotou I, Kopsidas G, Chouliaras G. The value of focally enhanced gastritis in the diagnosis of pediatric inflammatory bowel diseases. J Crohns Colitis. 2013;7:797-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Hummel TZ, Kate FT, Kindermann A, Benninga MA. 547 The Role of Endoscopy of the Upper Gastrointestinal Tract in the Diagnostic Assessment of Childhood Inflammatory Bowel Disease. Gastroenterology. 2010;138. [DOI] [Cited in This Article: ] |
| 69. | Choi WT, Lauwers GY. Patterns of Gastric Injury: Beyond Helicobacter Pylori. Surg Pathol Clin. 2017;10:801-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | McHugh JB, Gopal P, Greenson JK. The clinical significance of focally enhanced gastritis in children. Am J Surg Pathol. 2013;37:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Petrolla AA, Katz JA, Xin W. The clinical significance of focal enhanced gastritis in adults with isolated ileitis of the terminal ileum. J Gastroenterol. 2008;43:524-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Xin W, Greenson JK. The clinical significance of focally enhanced gastritis. Am J Surg Pathol. 2004;28:1347-1351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Hendrickson BA. Gastric inflammation as a feature of ulcerative colitis. J Pediatr Gastroenterol Nutr. 2003;37:228-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 74. | Thompson JW 3rd, Bargen JA. Ulcerative duodenitis and chronic ulcerative colitis: report of two cases. Gastroenterology. 1960;38:452-455. [PubMed] [Cited in This Article: ] |
| 75. | Endo K, Kuroha M, Shiga H, Kakuta Y, Takahashi S, Kinouchi Y, Shimosegawa T. Two cases of diffuse duodenitis associated with ulcerative colitis. Case Rep Gastrointest Med. 2012;2012:396521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Mitomi H, Atari E, Uesugi H, Nishiyama Y, Igarashi M, Arai N, Ihara A, Okayasu I. Distinctive diffuse duodenitis associated with ulcerative colitis. Dig Dis Sci. 1997;42:684-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Saito K, Katsuno T, Nakagawa T, Minemura S, Oyamada A, Kanogawa N, Saito M, Yoshihama S, Maruoka D, Matsumura T, Arai M, Tohma T, Miyauchi H, Matsubara H, Yokosuka O. Severe diffuse duodenitis successfully treated with intravenous tacrolimus after colectomy for ulcerative colitis. Intern Med. 2014;53:2477-2481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Valdez R, Appelman HD, Bronner MP, Greenson JK. Diffuse duodenitis associated with ulcerative colitis. Am J Surg Pathol. 2000;24:1407-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Terashima S, Hoshino Y, Kanzaki N, Kogure M, Gotoh M. Ulcerative duodenitis accompanying ulcerative colitis. J Clin Gastroenterol. 2001;32:172-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 80. | Kawai K, Watanabe T, Nakayama H, Roberts-Thomson I, Nagawa H. Images of interest. Gastrointestinal: small bowel inflammation and ulcerative colitis. J Gastroenterol Hepatol. 2005;20:1791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Akitake R, Nakase H, Tamaoki M, Ueno S, Mikami S, Chiba T. Modulation of Th1/Th2 balance by infliximab rescues postoperative occurrence of small-intestinal inflammation associated with ulcerative colitis. Dig Dis Sci. 2010;55:1781-1784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Chiba M, Ono I, Wakamatsu H, Wada I, Suzuki K. Diffuse gastroduodenitis associated with ulcerative colitis: treatment by infliximab. Dig Endosc. 2013;25:622-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Willington AJ, Taylor G, White J, Gearry RB. Gastrointestinal: Ulcerative colitis-associated duodenitis. J Gastroenterol Hepatol. 2018;33:973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Patterson ER, Shmidt E, Oxentenko AS, Enders FT, Smyrk TC. Normal villous architecture with increased intraepithelial lymphocytes: a duodenal manifestation of Crohn disease. Am J Clin Pathol. 2015;143:445-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Vidali F, Di Sabatino A, Broglia F, Cazzola P, Biancheri P, Viera FT, Vanoli A, Alvisi C, Perego M, Corazza GR. Increased CD8+ intraepithelial lymphocyte infiltration and reduced surface area to volume ratio in the duodenum of patients with ulcerative colitis. Scand J Gastroenterol. 2010;45:684-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Porter RJ, Kalla R, Ho GT. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F1000Res. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 87. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1542] [Cited by in F6Publishing: 1873] [Article Influence: 267.6] [Reference Citation Analysis (1)] |
| 88. | Kirsner JB. Historical aspects of inflammatory bowel disease. J Clin Gastroenterol. 1988;10:286-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 89. | Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017; 152: 313-321. e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 566] [Cited by in F6Publishing: 634] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 90. | Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 930] [Cited by in F6Publishing: 1022] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 91. | Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 469] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 92. | Odes HS, Fich A, Reif S, Halak A, Lavy A, Keter D, Eliakim R, Paz J, Broide E, Niv Y, Ron Y, Villa Y, Arber N, Gilat T. Effects of current cigarette smoking on clinical course of Crohn's disease and ulcerative colitis. Dig Dis Sci. 2001;46:1717-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Sahami S, Kooij IA, Meijer SL, Van den Brink GR, Buskens CJ, Te Velde AA. The Link between the Appendix and Ulcerative Colitis: Clinical Relevance and Potential Immunological Mechanisms. Am J Gastroenterol. 2016;111:163-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 94. | Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium; Barrett JC, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Kubo M, Anderson CA, Weersma RK. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1401] [Cited by in F6Publishing: 1537] [Article Influence: 170.8] [Reference Citation Analysis (0)] |
| 95. | Festen EA, Goyette P, Scott R, Annese V, Zhernakova A, Lian J, Lefèbvre C, Brant SR, Cho JH, Silverberg MS, Taylor KD, de Jong DJ, Stokkers PC, Mcgovern D, Palmieri O, Achkar JP, Xavier RJ, Daly MJ, Duerr RH, Wijmenga C, Weersma RK, Rioux JD. Genetic variants in the region harbouring IL2/IL21 associated with ulcerative colitis. Gut. 2009;58:799-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Cleynen I, Boucher G, Jostins L, Schumm LP, Zeissig S, Ahmad T, Andersen V, Andrews JM, Annese V, Brand S, Brant SR, Cho JH, Daly MJ, Dubinsky M, Duerr RH, Ferguson LR, Franke A, Gearry RB, Goyette P, Hakonarson H, Halfvarson J, Hov JR, Huang H, Kennedy NA, Kupcinskas L, Lawrance IC, Lee JC, Satsangi J, Schreiber S, Théâtre E, van der Meulen-de Jong AE, Weersma RK, Wilson DC; International Inflammatory Bowel Disease Genetics Consortium; Parkes M, Vermeire S, Rioux JD, Mansfield J, Silverberg MS, Radford-Smith G, McGovern DP, Barrett JC, Lees CW. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387:156-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 493] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 97. | Sugimura K, Asakura H, Mizuki N, Inoue M, Hibi T, Yagita A, Tsuji K, Inoko H. Analysis of genes within the HLA region affecting susceptibility to ulcerative colitis. Hum Immunol. 1993;36:112-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Luo Y, de Lange KM, Jostins L, Moutsianas L, Randall J, Kennedy NA, Lamb CA, McCarthy S, Ahmad T, Edwards C, Serra EG, Hart A, Hawkey C, Mansfield JC, Mowat C, Newman WG, Nichols S, Pollard M, Satsangi J, Simmons A, Tremelling M, Uhlig H, Wilson DC, Lee JC, Prescott NJ, Lees CW, Mathew CG, Parkes M, Barrett JC, Anderson CA. Exploring the genetic architecture of inflammatory bowel disease by whole-genome sequencing identifies association at ADCY7. Nat Genet. 2017;49:186-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 99. | Lee HS, Cleynen I. Molecular Profiling of Inflammatory Bowel Disease: Is It Ready for Use in Clinical Decision-Making? Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 100. | Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Paramsothy R, Xuan W, Lin E, Mitchell HM, Borody TJ. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 710] [Cited by in F6Publishing: 752] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 101. | Guo XY, Liu XJ, Hao JY. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis. 2020;21:147-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 102. | Sha S, Liang J, Chen M, Xu B, Liang C, Wei N, Wu K. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39:1003-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 103. | Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015; 149: 102-109. e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 930] [Cited by in F6Publishing: 942] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 104. | Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, Löwenberg M, van den Brink GR, Mathus-Vliegen EM, de Vos WM, Zoetendal EG, D'Haens GR, Ponsioen CY. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015; 149: 110-118. e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 619] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 105. | Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C, Rosewarne CP, Bickley C, Peters C, Schoeman MN, Conlon MA, Roberts-Thomson IC, Andrews JM. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2019;321:156-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 481] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 106. | Choy MC, Visvanathan K, De Cruz P. An Overview of the Innate and Adaptive Immune System in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:2-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 107. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 595] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 108. | Buzza MS, Johnson TA, Conway GD, Martin EW, Mukhopadhyay S, Shea-Donohue T, Antalis TM. Inflammatory cytokines down-regulate the barrier-protective prostasin-matriptase proteolytic cascade early in experimental colitis. J Biol Chem. 2017;292:10801-10812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 109. | Di Sabatino A, Biancheri P, Rovedatti L, MacDonald TT, Corazza GR. New pathogenic paradigms in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:368-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42:16-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 111. | Honma J, Mitomi H, Murakami K, Igarashi M, Saigenji K, Toyama K. Nodular duodenitis involving CD8+ cell infiltration in patients with ulcerative colitis. Hepatogastroenterology. 2001;48:1604-1610. [PubMed] [Cited in This Article: ] |
| 112. | Berrebi D, Languepin J, Ferkdadji L, Foussat A, De Lagausie P, Paris R, Emilie D, Mougenot JF, Cezard JP, Navarro J, Peuchmaur M. Cytokines, chemokine receptors, and homing molecule distribution in the rectum and stomach of pediatric patients with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2003;37:300-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Matsumura M, Matsui T, Hatakeyama S, Matake H, Uno H, Sakurai T, Yao T, Oishi T, Iwashita A, Fujioka T. Prevalence of Helicobacter pylori infection and correlation between severity of upper gastrointestinal lesions and H. pylori infection in Japanese patients with Crohn's disease. J Gastroenterol. 2001;36:740-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Ogino S, Lochhead P, Chan AT, Nishihara R, Cho E, Wolpin BM, Meyerhardt JA, Meissner A, Schernhammer ES, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol. 2013;26:465-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 115. | Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 116. | Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 117. | Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 436] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 118. | Ogino S, King EE, Beck AH, Sherman ME, Milner DA, Giovannucci E. Interdisciplinary education to integrate pathology and epidemiology: towards molecular and population-level health science. Am J Epidemiol. 2012;176:659-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 119. | Ogino S, Nishihara R, VanderWeele TJ, Wang M, Nishi A, Lochhead P, Qian ZR, Zhang X, Wu K, Nan H, Yoshida K, Milner DA Jr, Chan AT, Field AE, Camargo CA Jr, Williams MA, Giovannucci EL. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology. 2016;27:602-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 120. | Ghosh S, Matsuoka Y, Asai Y, Hsin KY, Kitano H. Software for systems biology: from tools to integrated platforms. Nat Rev Genet. 2011;12:821-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 121. | Papp B, Notebaart RA, Pál C. Systems-biology approaches for predicting genomic evolution. Nat Rev Genet. 2011;12:591-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 122. | Hamada T, Keum N, Nishihara R, Ogino S. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J Gastroenterol. 2017;52:265-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 123. | Ogino S, Nowak JA, Hamada T, Phipps AI, Peters U, Milner DA Jr, Giovannucci EL, Nishihara R, Giannakis M, Garrett WS, Song M. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018;67:1168-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 124. | Stein RA. Epigenetics--the link between infectious diseases and cancer. JAMA. 2011;305:1484-1485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 125. | Curtin K, Slattery ML, Samowitz WS. CpG island methylation in colorectal cancer: past, present and future. Patholog Res Int. 2011;2011:902674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 126. | Kosumi K, Mima K, Baba H, Ogino S. Dysbiosis of the gut microbiota and colorectal cancer: the key target of molecular pathological epidemiology. J Lab Precis Med. 2018;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 127. | Hughes LAE, Simons CCJM, van den Brandt PA, van Engeland M, Weijenberg MP. Lifestyle, Diet, and Colorectal Cancer Risk According to (Epi)genetic Instability: Current Evidence and Future Directions of Molecular Pathological Epidemiology. Curr Colorectal Cancer Rep. 2017;13:455-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 128. | Lochhead P, Chan AT, Giovannucci E, Fuchs CS, Wu K, Nishihara R, O'Brien M, Ogino S. Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am J Gastroenterol. 2014;109:1205-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 129. | Li W, Qiu T, Ling Y, Guo L, Li L, Ying J. Molecular pathological epidemiology of colorectal cancer in Chinese patients with KRAS and BRAF mutations. Oncotarget. 2015;6:39607-39613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 130. | Yang H, Rotter JI, Toyoda H, Landers C, Tyran D, McElree CK, Targan SR. Ulcerative colitis: a genetically heterogeneous disorder defined by genetic (HLA class II) and subclinical (antineutrophil cytoplasmic antibodies) markers. J Clin Invest. 1993;92:1080-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 104] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 131. | Targan SR, Karp LC. Defects in mucosal immunity leading to ulcerative colitis. Immunol Rev. 2005;206:296-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 132. | Corporaal S, Karrenbeld A, van der Linde K, Voskuil JH, Kleibeuker JH, Dijkstra G. Diffuse enteritis after colectomy for ulcerative colitis: two case reports and review of the literature. Eur J Gastroenterol Hepatol. 2009;21:710-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 133. | Queliza K, Ihekweazu FD, Schady D, Jensen C, Kellermayer R. Granulomatous Upper Gastrointestinal Inflammation in Pediatric Ulcerative Colitis. J Pediatr Gastroenterol Nutr. 2018;66:620-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Rush B, Berger L, Rosenfeld G, Bressler B. Tacrolimus Therapy for Ulcerative Colitis-Associated Post-Colectomy Enteritis. ACG Case Rep J. 2014;2:33-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |