Published online May 21, 2021. doi: 10.3748/wjg.v27.i19.2312
Peer-review started: January 24, 2021
First decision: February 23, 2021
Revised: February 27, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: May 21, 2021
Hepatitis B virus reactivation (HBVr) can occur in patients treated with immunosuppressive medications. Risk stratification for HBVr based on hepatitis B virus (HBV) serology and viral load is an important strategy to determine appropriate HBV monitoring and antiviral prophylaxis use. Recent advances in the understanding of pathophysiology of autoimmune diseases have led the development of cytokine-targeted therapies. Tumor necrosis factor (TNF)-α inhibitors have been widely used for patients with inflammatory bowel disease, psoriasis, and rheumatic diseases. Further, the clinical benefits of interleukin (IL)-12/23, IL-17, or Janus kinases inhibitors have been demonstrated in these patients. It is well known that TNF-α inhibitor use can lead to HBVr, however, the risk of HBVr in patients undergoing non-TNF-targeted biologics have not been fully understood. In this review, we discuss the risk of HBVr in patients treated with non-TNF-targeted biologics, and immunological mechanisms of these medications causing HBVr.
Core Tip: Although the risk of hepatitis B virus reactivation (HBVr) in patients undergoing non-tumor necrosis factor (TNF)-targeted biologics have not been fully understood, some previous studies showed that the risk of HBVr in patients with non-TNF-targeted biologics might be higher than that in patients with TNF-α inhibitors. While patients with chronic hepatitis B virus (HBV) should receive antiviral prophylaxis when they start non-TNF-targeted biologics, antiviral prophylaxis may be a favorable strategy rather than the pre-emptive strategy in patients with resolved HBV. Large-scale studies are needed to ascertain the differential risk of HBVr between patients with TNF-α inhibitors and non-TNF-targeted biologics.
- Citation: Akiyama S, Cotter TG, Sakuraba A. Risk of hepatitis B virus reactivation in patients with autoimmune diseases undergoing non-tumor necrosis factor-targeted biologics. World J Gastroenterol 2021; 27(19): 2312-2324
- URL: https://www.wjgnet.com/1007-9327/full/v27/i19/2312.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i19.2312
Hepatitis B virus reactivation (HBVr) can occur in patients treated with immuno-suppressive therapy and chemotherapy. In the current era of biologics, physicians need to understand the risk of HBVr in patients with autoimmune diseases undergoing anti-cytokine therapies.
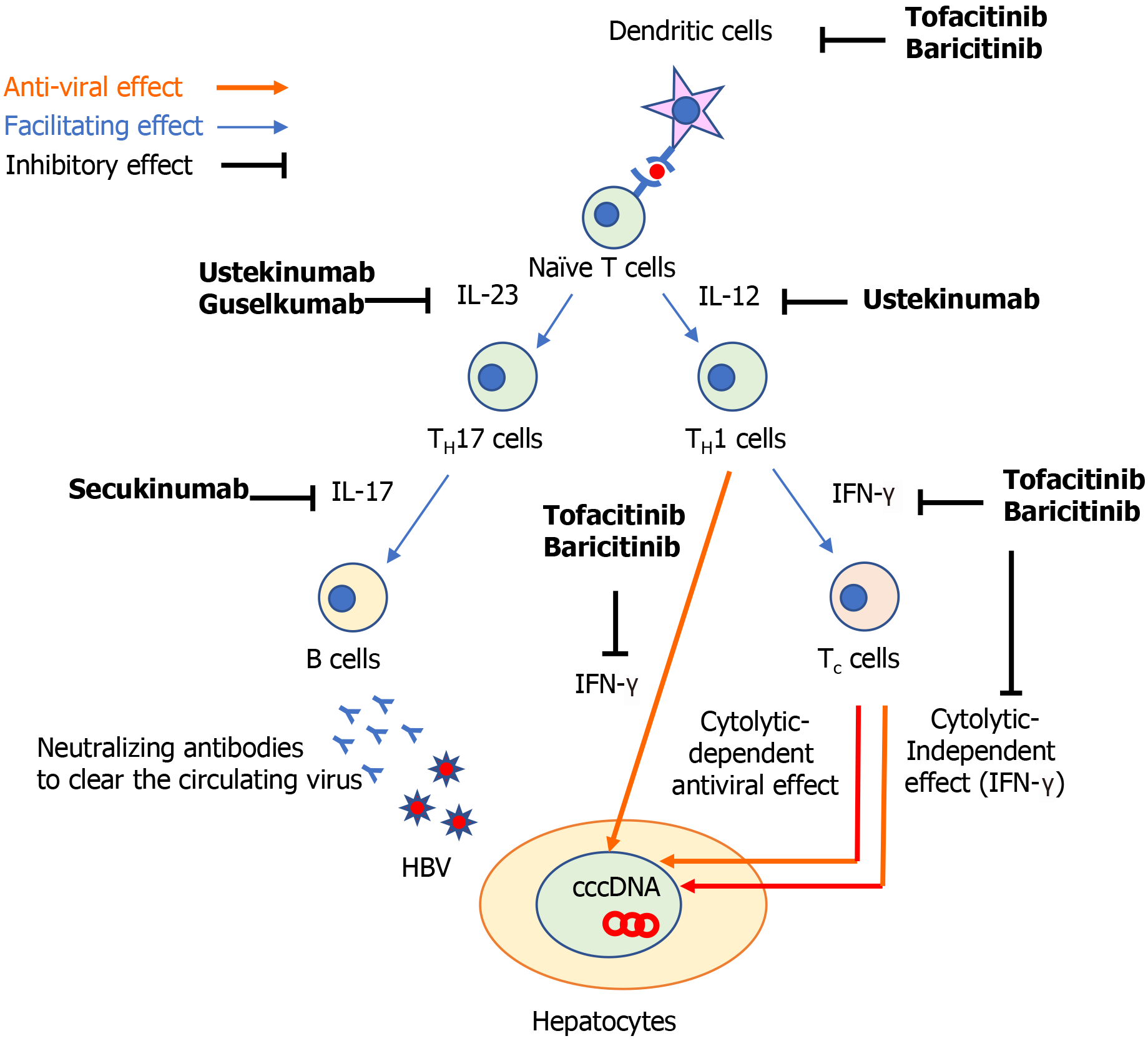
The following three components are important for the development of HBVr: (1) The host immune response; (2) The covalently closed circular DNA of the viral genome of HBV (cccDNA); and (3) The use of immunosuppressive drugs[1]. HBV infection induces a series of innate[2] and adaptive[3] immune responses[1]. The host immune responses against hepatitis B virus (HBV) infection recruit adaptive cytotoxic T (Tc) cells to induce both cytolytic-dependent and -independent antiviral effects. In the cytolytic-independent effect, interferons (IFN) play an important role to suppress the HBV replication. To produce neutralizing antibodies to clear circulating HBV, B cells are also recruited to limit the viral spread of HBV (Figure 1)[4]. However, even when clinical resolution of HBV infection is achieved, it does not mean complete elimination of HBV-DNA because cccDNA can persist in the nucleus of hepatocytes and it can be a source of HBVr when immunosuppressive medications are used.
Tumor necrosis factor (TNF)-α is a key cytokine not only in the pathogenesis of autoimmune diseases but also in the host immune reactions against HBV infection. TNF-α is synthesized by macrophages and T cells and induce the production of a variety of inflammatory cytokines, suppressing viral replication[5]. TNF-α is also necessary for the proliferation of HBV-specific Tc cells that are essential for suppression of HBV replication[6]. Hence, TNF-α inhibitors (e.g., infliximab, adalimumab, and etanercept) can inhibit the anti-HBV immune response, leading to HBV replication[7]. Indeed, the pooled prevalence of HBVr in patients with autoimmune diseases undergoing TNF-α inhibitors was reported to be 4.2% (95%CI: 1.4%-8.2%)[8].
The signaling pathways involving interleukin (IL)-12/23, IL-17, and Janus kinases (JAKs) have been highlighted as novel specific therapeutic targets for autoimmune diseases. A recent multicenter observational study for patients with psoriasis showed that HBVr was significantly more common among patients receiving anti-TNF-α therapies than IL-17 inhibitors[9]. However, there is still limited data in understanding the risk of HBVr in patients who are treated with biologics which inhibit such specific inflammatory pathways. In the present article, we aimed to review previous literatures which assessed the risk of HBVr in patients treated with non-TNF-targeted biologics and discuss how each medication can influence the development of HBVr.
The professional societies in the United States [American Association for the Study of Liver Diseases (AASLD); American Gastroenterological Association (AGA)], Europe [European Association for the Study of the Liver (EASL)] and Asia [Asian Pacific Association for the Study of the Liver (APASL)] have published guidelines to assist providers with HBVr management[10-13]. In this review article, we divide patients into 2 risk groups which is consistent with the professional society guidelines[10-13] when assessing practical management of HBVr.
Chronic HBV (CHB) [i.e., Hepatitis B surface antigen (HBsAg)-positive and antibody to hepatitis B core antigen (anti-HBc)-positive] which includes patients with chronic active [serum HBV DNA ≥ 2000 IU/mL and normal or elevated serum alanine transaminase (ALT)] or inactive (serum HBV DNA < 2000 IU/mL and normal ALT) HBV infection.
Resolved HBV (i.e., HBsAg-negative and anti-HBc-positive). Of note, there is insufficient evidence to support the use of anti-HBs titers as a decision aid when making a recommendation regarding prophylaxis[11].
There are subtle differences in the definition of HBVr among the professional society guidelines, however, the general concept is the same[10-13]. In patients with CHB, HBVr is defined by a rise in HBV DNA above baseline. In patients with resolved HBV, HBVr is defined by either the appearance of HBV DNA in the blood or conversion to the HBsAg+ state (i.e., seroreversion). The heterogeneity observed in HBVr definition is also reflected in the existing studies on HBVr. The majority of studies included the following parameters: (1) An acute rise in HBV-DNA levels compared with baseline; (2) Elevated levels of serum aminotransferases; and (3) Seroreversion[1]. In this review article, we followed the criteria of HBVr described in each article.
Patients with CHB have an increased risk of HBVr when undergoing immuno-suppressive therapy compared to patients with resolved HBV. For example, among patients treated with TNF-α inhibitors there is an estimated 5-fold increased risk of HBVr in patients with CHB compared to patients with resolved HBV (15.4% vs 3.0% risk of HBVr)[8]. Further, another study showed that the pooled rate of HBVr without antiviral prophylaxis was 15.6% (95%CI: 2.3-35.7) in patients with CHB who were treated with TNF-α inhibitors. In patients with resolved HBV, the pooled rates of HBVr without antiviral prophylaxis in patients who were treated with TNF-α inhibitors and non-TNF-targeted biologics were 1.4% (95%CI: 0.5%-2.6%) and 6.1% (95%CI: 0.0%-16.6%), respectively[14]. Each of the 4 professional societal guidelines recommend testing HBV serology on all candidates for immunosuppressive therapy or chemotherapy to enable appropriate risk stratification (i.e. CHB vs resolved HBV)[10-13].
Next, the degree of expected iatrogenic immunosuppression should be assessed. Hematopoietic stem cell transplant (HSCT) recipients and B cell–depleting therapies (e.g., rituximab) are high-potency regimens and confer the highest risk of HBVr[10-13]. The AGA guidelines ascertain that anthracyclines (e.g., doxorubicin) and moderate- to high-dose corticosteroids (CS) (i.e., ≥ 10 mg of daily prednisone or equivalent for ≥ 4 wk) confer higher risk than other immunosuppressants[11].
CHB: In general, the professional societal guidelines recommend HBV prophylaxis, typically entecavir or tenofovir, for all candidates for immunosuppression who have CHB, apart from patients treated with traditional immunosuppressive agents (e.g., thiopurines, methotrexate), intra-articular CSs, or oral CSs ≤ 1 wk[10-13]. The AGA risk stratify this cohort of patients into moderate (1%-10%) and high risk (> 10%) groups for HBVr[11]. Antiviral prophylaxis should be started before and continued after cessation of immunosuppression, generally 12 to 18 mo if high-potency therapies are used and 6 to 12 mo for other therapies[10-13].
Resolved HBV: Guidelines largely agree that resolved HBV patients on high-potency immunosuppression (HSCT recipients and B cell–depleting therapies) should receive HBVr prophylaxis, with the AGA placing this group of patients in the high risk HBVr group (> 10%)[10-13].
For resolved HBV patients not on a high-potency regimen, the guidelines are more dissimilar. AGA recommend prophylaxis for resolved HBV patients at moderate risk (1%-10%) of HBVr, which include patients treated with TNF-α inhibitors, other cytokine or integrin inhibitors, tyrosine kinase inhibitors, moderate- or high-dose CSs for ≥ 4 wk and anthracycline derivatives. In contrast, AASLD, EASL and APASL recommend a pre-emptive therapy for this patient cohort, not prophylaxis, whereby serial lab monitoring (HBV DNA, HBsAg) is performed at 1- to 3-mo intervals on therapy and up to 12 mo after cessation of immunosuppression with on-demand antiviral therapy if needed[10,12,13]. Given that HBsAg seroreversion can lead to fatal acute hepatitis, antiviral therapy should be started immediately, independently of ALT level[11]. Of note, both EASL and APASL recommend treating resolved HBV patients similarly to HBsAg-positive patients if baseline serum HBV-DNA is positive[12,13].
AGA classify resolved HBV patients who are treated with traditional immunosuppressive agents (e.g., thiopurines, methotrexate), low-dose CSs ≥ 4 wk, intra-articular CSs, or any dose of oral CSs for ≤ 1 wk, as low-risk (< 1%) for HBVr and do not recommend prophylaxis, similar to the other society guidelines[10-13].
Given the paucity of data on the HBVr risk among patients treated with non-TNF-targeted biologics, we reviewed the existing literature on the risk of HBVr in patients with autoimmune diseases who received non-TNF-targeted biologics and summarized the findings in Tables 1-3. A majority of articles focused on patients with CHB or resolved infection. According to the AGA guideline, CHB and resolved HBV patients treated with non-TNF-targeted therapies are categorized into the moderate-risk HBVr group and therapeutic prophylaxis is recommended. However, AASLD, EASL and APASL recommend serial monitoring among resolved HBV (if HBV DNA is negative) with pre-emptive prophylaxis if HBVr is observed. Therefore, it is important to determine the precise HBVr with non-TNF-targeted biologics to ascertain if a strategy of monitoring/pre-emptive may be too lax, and perhaps a uniform strategy of prophylaxis may be more optimal as recommended by the AGA. Given that patients with resolved infection should be treated similarly to those with CHB patients if their serum HBV-DNA tests are positive at baseline[12,13], we present their baseline HBV-DNA in Tables 1-3.
| Ref. | Number of HBV patients | HBV status | Disease | Drugs | Prophylaxis | Follow-up | HBV reactivation |
| Ting et al[27], 2018 | 54 | (1) 10 CHB; and (2) 44 resolved HBV. HBV-DNA at baseline (-) | Psoriasis | Ustekinumab | Yes: 2 patients with CHB | 24 mo | (1) 2 patients with CHB without prophylaxis. (no hepatitis); and (2) 1 patient with resolved HBV (mild hepatitis) |
| Solay et al[5], 2018 | 29 | 29 resolved HBV. HBV-DNA at baseline (-) | Psoriasis/HS/AS/RA/CD | Ustekinumab (n = 7) | NA | 22 wk | 1 patient with psoriasis without prophylaxis (no data regarding hepatitis) |
| Sanz-Bueno et al[68], 2015 | 20 | 20 resolved HBV. HBV-DNA at baseline (-) but viral load was assessed in 7 of 20 patients | Psoriasis | Ustekinumab (n = 6) | No | 40 mo | 0 |
| Chiu et al[28], 2013 | 14 | (1) 11 CHB; and (2) 3 resolved HBV. HBV-DNA at baseline was not available | Psoriasis | Ustekinumab | Yes: 4 patients with CHB | 10 mo | (1) 2 patients with CHB without prophylaxis (No hepatitis); and (2) 0 |
| Navarro et al[69], 2013 | 5 | 5 CHB | Psoriasis | Ustekinumab (n = 1) | Yes | 25 mo | 0 |
| Hayashi et al[70], 2014 | 5 | 5 resolved HBV. HBV-DNA at baseline was not available | Psoriasis | Ustekinumab | No | 52 wk | 0 |
| Koskinas et al[41], 2013 | 1 | Resolved HBV. HBV-DNA at baseline was not available | Psoriasis | Ustekinumab | No | 16 mo | 1 with hepatitis (ALT 65 IU/mL) |
| Steglich et al[71], 2014 | 1 | Resolved HBV. HBV-DNA at baseline (-) | Psoriasis | Ustekinumab | Yes | 36 mo | 0 |
| Duncan et al[43], 2019 | 1 | Resolved HBV. HBV-DNA at baseline was not available | Palmoplantar Psoriasis | Guselkumab | No | 12 mo | 0 |
| Ref. | Number of HBV patients | HBV status | Disease | Drugs | Prophylaxis | Follow-up | HBV reactivation |
| Chiu et al[52], 2018 | 49 | (1) 25 CHB; and (2) 24 resolved HBV. HBV-DNA at baseline (-) in 11 patients with resolved HBV | Psoriasis | Secukinumab | Yes: 3 patients with CHB | 3 mo | (1) 6 patients with CHB without prophylaxis. (no hepatitis); and (2) 1 patient with resolved HBV with positive viral load at baseline (no hepatitis) |
| Moneva-Leniz et al[72], 2020 | 4 | (1) 2 CHB; and (2) 2 resolved HBV. HBV-DNA at baseline (-) | Psoriasis/palmoplantar psoriasis | Secukinumab | Yes: 1 patient with CHB and 1 patient with resolved HBV | 20 mo | (1) 0; and (2) 0 |
| Feaster et al[73], 2018 | 1 | A carrier of congenital HBV infection1 | Psoriasis and PsA | Secukinumab | No | 24 mo | 0 |
| Bevans et al[74], 2018 | 1 | Seropositive hepatitis1 | Palmoplantar psoriasis and AS | Secukinumab | No | 14 mo | 0 |
| Yanagihara et al[75], 2017 | 1 | CHB | Psoriasis vulgaris | Secukinumab | Yes | 9 mo | 0 |
| Peccerillo et al[76], 2018 | 1 | Resolved HBV. HBV-DNA at baseline (-) | Psoriasis | Secukinumab | Yes | 14 mo | 0 |
| Koike et al[53], 2019 | 1 | CHB | Psoriasis and PsA | Ixekizumab | Yes | 18 mo | 0 |
| Lora et al[54], 2019 | 1 | Resolved HBV. HBV-DNA at baseline (-) | Psoriasis | Ixekizumab | Yes | 12 mo | 0 |
| Ref. | Number of HBV patients | Serology for HBV infectious | Disease | Drugs | Prophylaxis | Follow-up | HBV reactivation |
| Chen et al[66], 2018 | 81 | (1) 6 CHB; and (2) 75 resolved HBV. HBV-DNA at baseline (-) but viral load was assessed in 53 patients with resolved HBV | RA | Tofacitinib | Yes: 2 patients with CHB | 3-6 mo | (1) 2 patients with CHB without prophylaxis (1 patient developed hepatitis); and (2) 0 |
| Serling-Boyd et al[67], 2021 | 8 | 8 resolved HBV. HBV-DNA was assessed in 6 patients, but viral loads were not available | 7 RA, 1 PsA | Tofacitinib | Yes: 2 patients | 3.1 yr | 0 |
| Harigai et al[62], 2020 | 215 | 215 resolved HBV. HBV-DNA (-) at baseline in 30 patients with resolved HBV who had detectable post-baseline HBV-DNA | RA | Baricitinib | NA | 2.7 yr | 8 patients with resolved HBV had HBV-DNA ≥ 29 IU/mL (4 patients med the criteria of HBVr in this study, no hepatitis) |
The cytokine IL-12 contributes to the differentiation of naïve T cells to T helper 1 (TH1) cells and IL-23 maintains and expand IL-17 producing T helper (TH17) cells (Figure 1)[15]. These two cytokines play a central role to regulate T cell-mediated immune responses, which are dysregulated in various autoimmune diseases including psoriasis and Crohn’s disease (CD)[15,16]. The clinical benefit of IL-12 and IL-23 inhibition has been demonstrated in psoriasis, CD, and ulcerative colitis by ustekinumab[17-19], which is an antibody against p40, the common subunit of IL-12 and IL-23. IL-12 plays an important role in achieving sustained control of HBV replication. IL-12 can promote cell-mediated immunity by facilitating the production of IFN-γ production by TH1 cells, resulting in the inhibition of HBV replication[20,21] and the induction of antiviral effects of HBV-specific Tc cells[22,23]. Indeed, patients with CHB who were treated with recombinant human IL-12 exhibited a high proportion of HBV clearance in a dose-dependent manner[24] and the addition of IL-12 to lamivudine enhanced T cell reactivity to HBV and IFN-γ production[25]. Furthermore, patients with CHB responding to IFN-α treatment were shown to have higher IL-12 and IFN-γ expression levels during the treatment[26]. These findings suggest that ustekinumab might theoretically increase the risk of HBVr.
Several studies have assessed the risk of HBVr in patients treated with ustekinumab (Table 1). A retrospective study showed that no HBVr occurred among 2 patients with CHB taking antiviral prophylaxis after starting ustekinumab, whereas, 2 patients (25%) developed HBVr without hepatitis among 8 patients with CHB without antiviral prophylaxis[27]. Another study demonstrated a 29% rate of HBVr after ustekinumab initiation without antiviral prophylaxis in patients with CHB[28]. Given that patients with CHB have a high risk of HBVr without antiviral prophylaxis, these patients require antiviral prophylaxis and appropriate monitoring for HBV-DNA and serology tests after initiating ustekinumab treatment.
A retrospective study on 44 patients with resolved HBV who initiated ustekinumab without antiviral prophylaxis found that 1 patient (2.3%) developed HBVr complicated with mild hepatitis[27]. This patient discontinued concurrent methotrexate when reactivation occurred and HBV-DNA became undetectable without antiviral therapy in 6 mo. In another study involving 7 patients with resolved HBV on ustekinuamb, 1 patient (14.3%) developed HBVr. This patient was not on antiviral prophylaxis and started entecavir for treatment of HBVr[5]. These data suggest that there is a certain risk of HBVr in patients with resolved HBV even without detectable HBV-DNA at baseline after starting ustekinumab, suggesting that these patients might need antiviral prophylaxis as is the AGA guidelines preferred option. While the guidelines of AASLD, EASL, APASL recommend pre-emptive therapy if HBV DNA is negative, further studies are warranted in order to understand if these patients require antiviral prophylaxis.
IL-23-specific antagonists, such as tildrakizumab[29,30], risankizumab[31,32], guselku-mab[33,34], and brazikumab[35], have been shown to be effective for psoriasis and CD. These medications bind to the p19 subunit on IL-23 and inhibit its interaction with the IL-23 receptors[36]. The potential mechanism of HBVr in patients treated with IL-23 inhibitors is still unclear. Previous studies found that TH17 cells, which are expanded by IL-23 (Figure 1), increase with the severity of liver damage in patients with CHB[37-39]. An observational, clinical-controlled study also demonstrated that the expression levels of IL-23 and IL-17 were associated with increased possibilities of hepatitis B e-antigen (HBeAg) clearance and HBsAg decline in patients with HBeAg-positive CHB during pegylated IFN therapy[40]. This study also found that high serum IL-23 Levels can predict the response to IFN therapy in patients with HBeAg-positive CHB[40]. Given that TH17 cells promote the differentiation and function of B cells[41,42], IL-23 might activate the humoral immune response against circulating HBV and play a role to facilitate HBV clearance by IFN therapy (Figure 1). Although this hypothesis suggest that IL-23 inhibitors may abrogate the HBV clearance, it still remains to be elucidated whether these medications contribute to the development of HBVr.
The data regarding the safety of IL-23 inhibitors in patients with HBV infection is limited. A case report showed that a patient with resolved HBV infection did not develop HBVr 1 year after starting guselkumab (Table 1)[43]. There have been no reported studies focusing on the risk of HBVr in patients treated with other IL-23 inhibitors.
IL-17 is a major effector cytokine of TH17 cells and mediate host defense mechanisms[44]. Inhibition of IL-17 with secukinumab, ixekizumab, and brodalumab have demonstrated clinical benefits in patients with psoriasis[45-47], psoriatic arthritis[48], and ankylosing spondylitis[49]. TH17/IL-17 axis is involved in the process of fibrogenesis and increases the expression of proinflammatory cytokines, promoting the recruitment of inflammatory cells in patients with CHB[50]. A previous study showed that Th17 cells were significantly increased in patients with CHB, as well as the expression level of IL-17[51]. They also demonstrated that the suppression of viral replication induced by IFN-α resulted in a decrease in TH17 cells and IL-17 expression, suggesting that TH17 cells might play an important role during IFN-α treatment to eliminate HBV[51]. As we described above, TH17 cells also facilitate B cells[41,42] and would enhance the humoral response to clear circulating HBV (Figure 1). These findings implicate that TH17/IL-17 axis might be associated with HBV clearance and its inhibition may increase the risk of HBVr.
A prospective multicenter study on 22 patients with CHB with no antiviral prophylaxis after starting secukinumab showed that 6 patients (27.3%) developed HBVr (Table 2)[52]. Three patients with HBVr started antiviral treatments and their viral loads decreased rapidly within 3 mo. The remaining three patients with HBVr were followed without antiviral drugs and their viral loads remained low without acute hepatitis. Notably, none of the 3 patients with CHB who received antiviral prophylaxis developed HBVr[52]. Hence, this study reinforced the importance of antiviral prophylaxis in patients with CHB starting treatment with IL-17 inhibitors. This study also included 24 patients with resolved HBV who did not receive antiviral prophylaxis and identified one patient (4.2%) with a positive viral load at baseline who developed HBVr without acute hepatitis[52]. This study re-affirmed the EASL and APASL guidelines which recommend antiviral prophylaxis in patients with resolved HBV if their baseline viral loads are positive.
A case report on a patient with CHB treated with ixekizumab and entecavir simultaneously did not develop HBVr after 18 mo of treatment[53]. Another report showed that a patient with resolved HBV did not experience HBVr during follow-up (Table 2)[54]. Given that data regarding the risk of HBVr in patients treated with ixekizumab or brodalumab are still limited, further studies with larger sample sizes are warranted.
JAKs bind to type I and II cytokine receptors and transmit extracellular cytokine signals to activate various signal transducers and activators of transcription, which drive the proinflammatory machinery of the cellular immune response[55]. The clinical benefit of JAK inhibitors has been demonstrated in patients with rheumatoid arthritis[56,57], psoriatic arthritis[58,59], and ulcerative colitis[60,61]. Important signaling pathways in host-defense include innate antiviral responses via IFN-α/β mediated by JAK1-tyrosine kinase 2 complexes, and IFN-γ mediated by JAK1-JAK2 complexes[55]. Hence, JAK inhibitors might counteract the suppressive effects of IFN on viral replication[62,63]. Further, dendritic cells and effective T cell lineages including TH cells and Tc cells play important roles to defense against HBV-infection (Figure 1)[64]. A previous study demonstrated that a JAK inhibitor can block the differentiation and function of dendritic cells, leading to impaired T cell activation (Figure 1)[65]. Thus, it was suggested that JAK inhibitors might negatively interact with the defense mechanism against HBV infection. Further studies investigating how JAK inhibitors influence the development of HBVr are warranted.
A retrospective cohort study including 6 patients with CHB showed that 2 out of 4 patients (50%) without antiviral prophylaxis developed HBVr after starting tofacitinib. One patient had an elevated ALT level and started entecavir, resulting in declines in HBV-DNA and ALT levels. Another patient started entecavir and did not develop acute hepatitis. Both patients continued tofacitinib after the development of HBVr. Meanwhile, 2 patients with CHB who received antiviral prophylaxis did not develop HBVr after initiating tofacitinib. Further, in this study, none of 75 patients with resolved HBV received antiviral prophylaxis and no HBVr was observed in this group[66]. Another study also demonstrated that patients with resolved HBV did not develop HBVr after starting tofacitinib (Table 3)[67].
A study assessing data which was integrated from four phase 3 trials of baricitinib in patients showed that, among 215 patients with resolved HBV, 8 patients (3.7%) had a single quantifiable result of HBV-DNA viral load (HBV-DNA level ≥ 29 IU/mL) after initiating baricitinib. Among these 8 patients, 4 patients met the definition of HBVr (HBV-DNA ≥ 100 IU/mL), but no patients developed hepatitis. HBV-DNA at baseline was assessed in 6 patients and all examined patients did not have detectable HBV-DNA level. Antiviral therapy was not used in 5 of 8 patients[62].
All these findings suggest that patients with CHB should receive antiviral prophylaxis when they start JAK inhibitors. As for patients with resolved HBV infection, given that HBVr was occasionally reported even if their HBV-DNA levels were not detected at baseline, an appropriate consultation with hepatologists is necessary. There has been limited data regarding the risk of HBVr in patients with autoimmune diseases who are treated with other JAK inhibitors (e.g., upadacitinib, filgotinib, peficitinib).
In summary, considering antiviral prophylaxis with an appropriate risk stratification is necessary when we start non-TNF-targeted biologics for patients with autoimmune diseases. The frequencies of HBVr without antiviral prophylaxis in patients with CHB on IL-12/23, IL-17, and JAK inhibitors are up to 29%, 27%, and 50%, respectively. A meta-analysis demonstrated that the pooled rate of HBVr without antiviral prophylaxis was 15.6% (95%CI: 2.3-35.7) in patients with CHB who were treated with TNF-α inhibitors, suggesting that non-TNF-targeted biologics, particularly JAK inhibitors, may have a higher risk of HBVr compared with TNF-α inhibitors. Given that no patients who received antiviral prophylaxis developed HBVr, HBVr is preventable with antiviral therapy in patients with CHB on non-TNF-targeted biologics. As all of professional societies recommended in their guidelines, patients with CHB should receive antiviral prophylaxis when they start non-TNF-targeted biologics. In patients with resolved HBV, the rates of HBVr without antiviral prophylaxis in patients on IL-12/23, IL-17, and JAK inhibitors are up to 2.3%, 4.2%, and 0%, respectively. The meta-analysis showed that the pooled rates of HBVr without antiviral prophylaxis in patients who were treated with TNF-α inhibitors and non-TNF-targeted biologics were 1.4% (95%CI: 0.5%-2.6%) and 6.1% (95%CI: 0.0%-16.6%), respectively[14]. These data supported that the risk of HBVr in patients treated with non-TNF-targeted biologics might be higher than that in patients with TNF-α inhibitors even if their HBV status is resolved HBV. According to the AGA guideline, patients with resolved HBV who are treated with non-TNF-targeted biologics are categorized into the moderate risk group and antiviral prophylaxis are recommended for this patient cohort[11]. However, as stated previously, AASLD, EASL and APASL recommend the pre-emptive therapeutic strategy for this cohort, although APASL and EASL do include the caveat of potentially using HBV DNA assessment to aid decision-making[10,12,13]. Given the higher risk of HBVr with non-TNF-targeted biologics compared with TNF-α inhibitors, antiviral prophylaxis may be a favorable strategy rather than the pre-emptive strategy to prevent HBVr in patients with resolved HBV. Large-scale studies are needed to ascertain the differential risk of HBVr between patients with TNF-α inhibitors and non-TNF-targeted biologics and to stratify the risk of HBVr by the type of non-TNF-targeted biologics. While HBsAg seroreversion can lead to fatal acute hepatitis, a consultation with hepatologists or infectious disease specialists is recommended.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Georgiev T, Karagiannakis D, Mohsenzadegan M S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Gentile G, Antonelli G. HBV Reactivation in Patients Undergoing Hematopoietic Stem Cell Transplantation: A Narrative Review. Viruses. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 2. | Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol. 2016;64:S60-S70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64:S71-S83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 300] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 4. | Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 557] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 5. | Solay AH, Acar A, Eser F, Kuşcu F, Tütüncü EE, Kul G, Şentürk GÇ, Gürbüz Y. Reactivation rates in patients using biological agents, with resolved HBV infection or isolated anti-HBc IgG positivity. Turk J Gastroenterol. 2018;29:561-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Kasahara S, Ando K, Saito K, Sekikawa K, Ito H, Ishikawa T, Ohnishi H, Seishima M, Kakumu S, Moriwaki H. Lack of tumor necrosis factor alpha induces impaired proliferation of hepatitis B virus-specific cytotoxic T lymphocytes. J Virol. 2003;77:2469-2476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Manzano-Alonso ML, Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011;17:1531-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Cantini F, Boccia S, Goletti D, Iannone F, Leoncini E, Panic N, Prignano F, Gaeta GB. HBV Reactivation in Patients Treated with Antitumor Necrosis Factor-Alpha (TNF-α) Agents for Rheumatic and Dermatologic Conditions: A Systematic Review and Meta-Analysis. Int J Rheumatol. 2014;2014:926836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Chiu HY, Chiu YM, Chang Liao NF, Chi CC, Tsai TF, Hsieh CY, Hsieh TY, Lai KL, Chiu TM, Wu NL, Hui RC, Lee CN, Wang TS, Chen PH, Yang CC, Huang YH. Predictors of hepatitis B and C virus reactivation in patients with psoriasis treated with biological agent: A nine-year multicenter cohort study. J Am Acad Dermatol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2290] [Cited by in F6Publishing: 2247] [Article Influence: 374.5] [Reference Citation Analysis (0)] |
| 11. | Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215-9; quiz e16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2771] [Cited by in F6Publishing: 3132] [Article Influence: 447.4] [Reference Citation Analysis (0)] |
| 13. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1652] [Cited by in F6Publishing: 1661] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 14. | Lin TC, Yoshida K, Tedeschi SK, de Abreu MM, Hashemi N, Solomon DH. Risk of Hepatitis B Virus Reactivation in Patients With Inflammatory Arthritis Receiving Disease-Modifying Antirheumatic Drugs: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken). 2018;70:724-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1153] [Cited by in F6Publishing: 1179] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 16. | Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 673] [Cited by in F6Publishing: 683] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 17. | Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S, Dooley LT, Reich K; PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1092] [Cited by in F6Publishing: 1038] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 18. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519-1528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 779] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 19. | Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, Adedokun OJ, Li K, Peyrin-Biroulet L, Van Assche G, Danese S, Targan S, Abreu MT, Hisamatsu T, Szapary P, Marano C; UNIFI Study Group. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2019;381:1201-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 600] [Article Influence: 120.0] [Reference Citation Analysis (1)] |
| 20. | Zeuzem S, Carreño V. Interleukin-12 in the treatment of chronic hepatitis B and C. Antiviral Res. 2001;52:181-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236-3243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 196] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Guidotti LG. The role of cytotoxic T cells and cytokines in the control of hepatitis B virus infection. Vaccine. 2002;20 Suppl 4:A80-A82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74:2255-2264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Carreño V, Zeuzem S, Hopf U, Marcellin P, Cooksley WG, Fevery J, Diago M, Reddy R, Peters M, Rittweger K, Rakhit A, Pardo M. A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis B. J Hepatol. 2000;32:317-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Rigopoulou EI, Suri D, Chokshi S, Mullerova I, Rice S, Tedder RS, Williams R, Naoumov NV. Lamivudine plus interleukin-12 combination therapy in chronic hepatitis B: antiviral and immunological activity. Hepatology. 2005;42:1028-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Rossol S, Marinos G, Carucci P, Singer MV, Williams R, Naoumov NV. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J Clin Invest. 1997;99:3025-3033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 174] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Ting SW, Chen YC, Huang YH. Risk of Hepatitis B Reactivation in Patients with Psoriasis on Ustekinumab. Clin Drug Investig. 2018;38:873-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Chiu HY, Chen CH, Wu MS, Cheng YP, Tsai TF. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295-1303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, Nograles K, Mehta A, Cichanowitz N, Li Q, Liu K, La Rosa C, Green S, Kimball AB. Tildrakizumab vs placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 354] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 30. | Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, Gottlieb AB, Nakagawa H, Bowman EP, Mehta A, Li Q, Zhou Y, Shames R. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 31. | Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, Papp KA, Sofen H, Puig L, Foley P, Ohtsuki M, Flack M, Geng Z, Gu Y, Valdes JM, Thompson EHZ, Bachelez H. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 386] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 32. | Feagan BG, Sandborn WJ, D'Haens G, Panés J, Kaser A, Ferrante M, Louis E, Franchimont D, Dewit O, Seidler U, Kim KJ, Neurath MF, Schreiber S, Scholl P, Pamulapati C, Lalovic B, Visvanathan S, Padula SJ, Herichova I, Soaita A, Hall DB, Böcher WO. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn's disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 317] [Article Influence: 45.3] [Reference Citation Analysis (1)] |
| 33. | Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, Shen YK, Szapary P, Randazzo B, Reich K. A Phase 2 Trial of Guselkumab vs Adalimumab for Plaque Psoriasis. N Engl J Med. 2015;373:136-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 34. | Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, Li S, Shen YK, Gordon KB. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 433] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 35. | Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, Higgins PDR, Newbold P, Faggioni R, Patra K, Li J, Klekotka P, Morehouse C, Pulkstenis E, Drappa J, van der Merwe R, Gasser RA Jr. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn's Disease: A Phase 2a Study. Gastroenterology 2017; 153: 77-86. e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 36. | Frieder J, Kivelevitch D, Haugh I, Watson I, Menter A. Anti-IL-23 and Anti-IL-17 Biologic Agents for the Treatment of Immune-Mediated Inflammatory Conditions. Clin Pharmacol Ther. 2018;103:88-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, Fu JL, Shi F, Shi M, Wang HF, Wang FS. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 315] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 38. | Xia L, Tian D, Huang W, Zhu H, Wang J, Zhang Y, Hu H, Nie Y, Fan D, Wu K. Upregulation of IL-23 expression in patients with chronic hepatitis B is mediated by the HBx/ERK/NF-κB pathway. J Immunol. 2012;188:753-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Ge J, Wang K, Meng QH, Qi ZX, Meng FL, Fan YC. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol. 2010;30:60-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Yu C, Gong X, Yang Q, Lian J, Xu K, Ruan B, Li L. The serum IL-23 Level predicts the response to pegylated interferon therapy in patients with chronic hepatitis B. Liver Int. 2015;35:1549-1556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Koskinas J, Tampaki M, Doumba PP, Rallis E. Hepatitis B virus reactivation during therapy with ustekinumab for psoriasis in a hepatitis B surface-antigen-negative anti-HBs-positive patient. Br J Dermatol. 2013;168:679-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci USA. 2010;107:14292-14297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 389] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 43. | Duncan JR, Orlowski TJ, Elewski BE. Safety of guselkumab in hepatitis B virus infection. Dermatol Online J. 2019;25. [PubMed] [Cited in This Article: ] |
| 44. | Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 45. | Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C; ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1392] [Cited by in F6Publishing: 1400] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 46. | Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, Reich K, Amato D, Ball SG, Braun DK, Cameron GS, Erickson J, Konrad RJ, Muram TM, Nickoloff BJ, Osuntokun OO, Secrest RJ, Zhao F, Mallbris L, Leonardi CL; UNCOVER-1 Study Group; UNCOVER-2 Study Group; UNCOVER-3 Study Group. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N Engl J Med. 2016;375:345-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 561] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 47. | Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, Papp K, Spelman L, Toth D, Kerdel F, Armstrong AW, Stingl G, Kimball AB, Bachelez H, Wu JJ, Crowley J, Langley RG, Blicharski T, Paul C, Lacour JP, Tyring S, Kircik L, Chimenti S, Callis Duffin K, Bagel J, Koo J, Aras G, Li J, Song W, Milmont CE, Shi Y, Erondu N, Klekotka P, Kotzin B, Nirula A. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015;373:1318-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 568] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 48. | Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, Adams DH, Kerr L, Lee C, Shuler CL, Genovese M; SPIRIT-P2 Study Group. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389:2317-2327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 49. | Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M, Mpofu S, Richards HB; MEASURE 1 Study Group; MEASURE 2 Study Group. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med. 2015;373:2534-2548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 676] [Cited by in F6Publishing: 685] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 50. | Paquissi FC. Immunity and Fibrogenesis: The Role of Th17/IL-17 Axis in HBV and HCV-induced Chronic Hepatitis and Progression to Cirrhosis. Front Immunol. 2017;8:1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 51. | Feng H, Yin J, Han YP, Zhou XY, Chen S, Yang L, Yan JR, Zhang GX. Sustained Changes of Treg and Th17 Cells During Interferon-α Therapy in Patients with Chronic Hepatitis B. Viral Immunol. 2015;28:412-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Chiu HY, Hui RC, Huang YH, Huang RY, Chen KL, Tsai YC, Lai PJ, Wang TS, Tsai TF. Safety Profile of Secukinumab in Treatment of Patients with Psoriasis and Concurrent Hepatitis B or C: A Multicentric Prospective Cohort Study. Acta Derm Venereol. 2018;98:829-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Koike Y, Fujiki Y, Higuchi M, Fukuchi R, Kuwatsuka S, Murota H. An interleukin-17 inhibitor successfully treated a complicated psoriasis and psoriatic arthritis patient with hepatitis B virus infection and end-stage kidney disease on hemodialysis. JAAD Case Rep. 2019;5:150-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Lora V, Graceffa D, De Felice C, Morrone A, Bonifati C. Treatment of severe psoriasis with ixekizumab in a liver transplant recipient with concomitant hepatitis B virus infection. Dermatol Ther. 2019;32:e12909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13:234-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 340] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 56. | Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Soma K, Bradley J, Mebus C; ORAL Step investigators. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 515] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 57. | Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, Isaacs JD, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Riese R, Bradley J. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 337] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 58. | Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, Kudlacz E, Wang C, Menon S, Hendrikx T, Kanik KS. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N Engl J Med. 2017;377:1525-1536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 334] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 59. | Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, Cieślak D, Graham D, Wang C, Menon S, Hendrikx T, Kanik KS. Tofacitinib or Adalimumab vs Placebo for Psoriatic Arthritis. N Engl J Med. 2017;377:1537-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 360] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 60. | Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1; OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723-1736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 896] [Cited by in F6Publishing: 981] [Article Influence: 140.1] [Reference Citation Analysis (0)] |
| 61. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 585] [Cited by in F6Publishing: 583] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 62. | Harigai M, Winthrop K, Takeuchi T, Hsieh TY, Chen YM, Smolen JS, Burmester G, Walls C, Wu WS, Dickson C, Liao R, Genovese MC. Evaluation of hepatitis B virus in clinical trials of baricitinib in rheumatoid arthritis. RMD Open. 2020;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 63. | Kirito K, Sakamoto M, Enomoto N. Elevation of the Hepatitis B Virus DNA during the Treatment of Polycythemia Vera with the JAK Kinase Inhibitor Ruxolitinib. Intern Med. 2016;55:1341-1344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1202] [Cited by in F6Publishing: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 65. | Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, Wolf D, Brossart P. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122:1192-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 66. | Chen YM, Huang WN, Wu YD, Lin CT, Chen YH, Chen DY, Hsieh TY. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real-world study. Ann Rheum Dis. 2018;77:780-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 67. | Serling-Boyd N, Mohareb AM, Kim AY, Hyle EP, Wallace ZS. The use of tocilizumab and tofacitinib in patients with resolved hepatitis B infection: a case series. Ann Rheum Dis. 2021;80:274-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Sanz-Bueno J, Vanaclocha F, García-Doval I, Torrado R, Carretero G, Daudén E, Patricia Ruiz-Genao D, Alsina-Gibert MM, Pérez-Zafrilla B, Pérez-Rial G, Rivera R; members of the BIOBADADERM group. Risk of Reactivation of Hepatitis B Virus Infection in Psoriasis Patients Treated With Biologics: A Retrospective Analysis of 20 Cases From the BIOBADADERM Database. Actas Dermosifiliogr. 2015;106:477-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Navarro R, Vilarrasa E, Herranz P, Puig L, Bordas X, Carrascosa JM, Taberner R, Ferrán M, García-Bustinduy M, Romero-Maté A, Pedragosa R, García-Diez A, Daudén E. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol. 2013;168:609-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 70. | Hayashi M, Umezawa Y, Fukuchi O, Ito T, Saeki H, Nakagawa H. Efficacy and safety of ustekinumab treatment in elderly patients with psoriasis. J Dermatol. 2014;41:974-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 71. | Steglich RB, Meneghello LP, Carvalho AV, Cheinquer H, Muller FM, Reginatto FP. The use of ustekinumab in a patient with severe psoriasis and positive HBV serology. An Bras Dermatol. 2014;89:652-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Moneva-Leniz LM, Sahuquillo-Torralba A, Vila-Payeras A, Mateu-Puchades A. Risk of Hepatitis B Virus Reactivation in Patients on Secukinumab for Psoriasis: A Series of 4 Cases. Actas Dermosifiliogr. 2020;111:613-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Feaster B, Cline A, Feldman SR. Secukinumab for psoriasis in a patient with hepatitis B. Dermatol Online J. 2018;24. [PubMed] [Cited in This Article: ] |
| 74. | Bevans SL, Mayo TT, Elewski BE. Safety of secukinumab in hepatitis B virus. J Eur Acad Dermatol Venereol. 2018;32:e120-e121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Yanagihara S, Sugita K, Yoshida Y, Tsuruta D, Yamamoto O. Psoriasis vulgaris in a hepatitis B virus carrier successfully treated with secukinumab and entecavir combination therapy. Eur J Dermatol. 2017;27:185-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 76. | Peccerillo F, Odorici G, Pellacani G, Conti A. Secukinumab: A positive outcome in a patient with severe psoriasis and HBV-HCV co-infection. Dermatol Ther. 2018;31:e12601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |