Published online May 14, 2021. doi: 10.3748/wjg.v27.i18.2177
Peer-review started: January 26, 2021
First decision: February 27, 2021
Revised: March 13, 2021
Accepted: April 20, 2021
Article in press: April 20, 2021
Published online: May 14, 2021
The introduction of direct-acting antiviral drugs into clinical practice has revolutionized the treatment of chronic hepatitis C, making it highly effective and safe for patients. However, few researchers have analyzed the factors causing therapy failure in some patients.
To analyze factors influencing the failure of direct antiviral drugs in the large, multicenter EpiTer-2 cohort in a real-world setting.
The study cohort consisted of patients with chronic hepatitis C treated at 22 Polish centers from 2016-2020. Data collected from the online EpiTer-2 database included the following: hepatitis C virus (HCV) genotype, stage of fibrosis, hematology and liver function parameters, Child-Turcotte-Pugh and Model for End-stage Liver Disease scores, prior antiviral therapy, concomitant diseases, and drugs used in relation to hepatitis B virus (HBV) and/or human immunodeficiency virus (HIV) coinfections. Adverse events observed during the treatment and follow-up period were reported. Both standard and machine learning methods were used for statistical analysis.
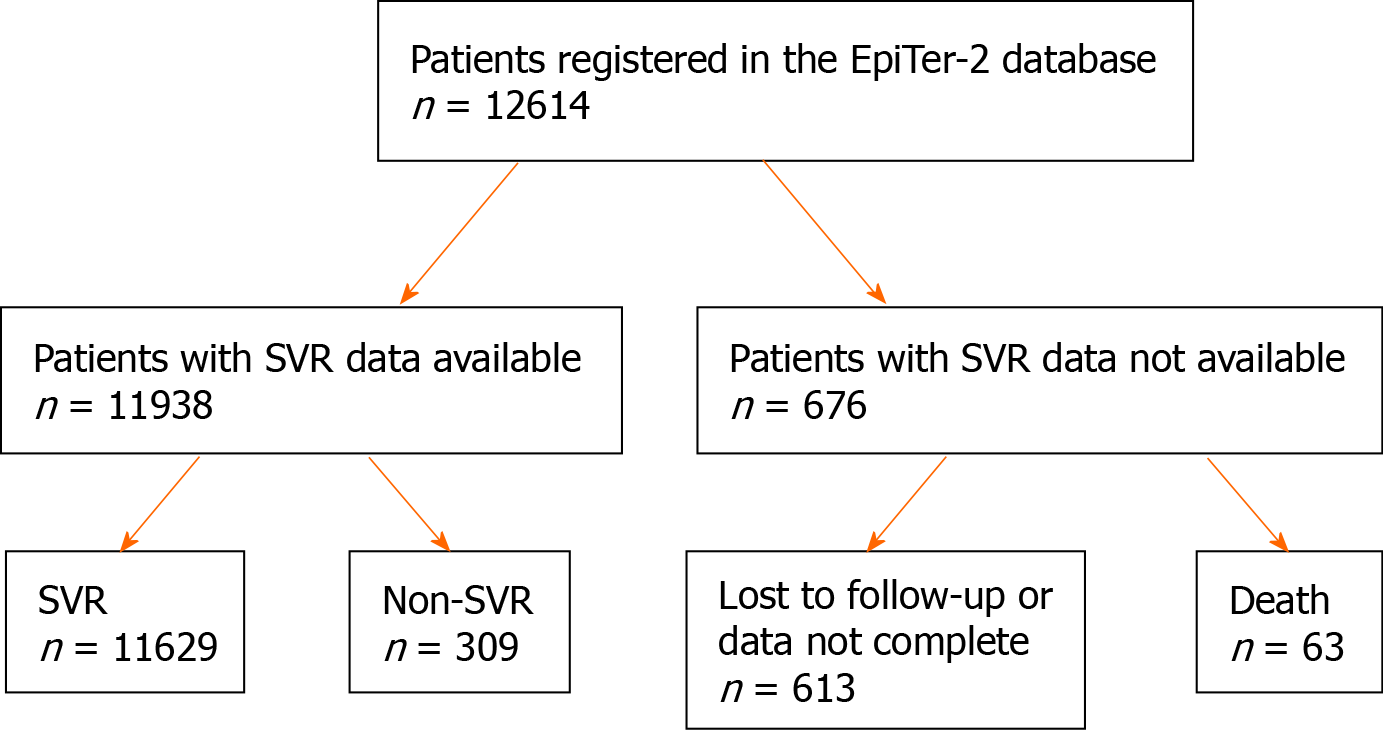
During analysis, 12614 patients with chronic hepatitis C were registered, of which 11938 (mean age: 52 years) had available sustained virologic response (SVR) data [11629 (97%) achieved SVR and 309 (3%) did not]. Most patients (78.1%) were infected with HCV genotype 1b. Liver cirrhosis was diagnosed in 2974 patients, while advanced fibrosis (F3) was diagnosed in 1717 patients. We included patients with features of hepatic failure at baseline [ascites in 142 (1.2%) and encephalopathy in 68 (0.6%) patients]. The most important host factors negatively influencing treatment efficacy were liver cirrhosis, clinical and laboratory features of liver failure, history of hepatocellular carcinoma, and higher body mass index. Among viral factors, genotype 3 and viral load also exerted an influence on treatment efficacy. Classical statistical analysis revealed that treatment ineffectiveness seemed to be influenced by the male sex, which was not confirmed by the multivariate analysis using the machine learning algorithm (random forest). Coinfection with HBV (including patients with on-treatment reactivation of HBV infection) or HIV, extrahepatic manifestations, and renal failure did not significantly affect the treatment efficacy.
In patients with advanced liver disease, individualized therapy (testing for resistance-associated variants and response-guided treatment) should be considered to maximize the chance of achieving SVR.
Core Tip: We analyzed factors influencing the failure of direct-acting antiviral drugs (DAAs) in a large, multicenter EpiTer-2 cohort of patients treated across 22 centers. Our findings demonstrate that failure of DAA treatment occurred mainly in patients with liver cirrhosis and deterioration of liver function. Our machine learning analysis further revealed that older age and creatinine and hemoglobin levels also influenced treatment failure, as did viral factors such as genotype 3 and viral load. Thus, in patients with advanced liver disease, individualized therapy (testing for resistance-associated variants, response-guided treatment) should be considered to maximize the chance of achieving a sustained virologic response.
- Citation: Janczewska E, Kołek MF, Lorenc B, Klapaczyński J, Tudrujek-Zdunek M, Sitko M, Mazur W, Zarębska-Michaluk D, Buczyńska I, Dybowska D, Czauż-Andrzejuk A, Berak H, Krygier R, Jaroszewicz J, Citko J, Piekarska A, Dobracka B, Socha Ł, Deroń Z, Laurans Ł, Białkowska-Warzecha J, Tronina O, Adamek B, Tomasiewicz K, Simon K, Pawłowska M, Halota W, Flisiak R. Factors influencing the failure of interferon-free therapy for chronic hepatitis C: Data from the Polish EpiTer-2 cohort study. World J Gastroenterol 2021; 27(18): 2177-2192
- URL: https://www.wjgnet.com/1007-9327/full/v27/i18/2177.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i18.2177
The introduction of direct-acting antiviral drugs (DAAs) into clinical practice has revolutionized the treatment of chronic hepatitis C, making it highly effective and safe for patients. Interferon-free therapies with a short duration and the absence of significant adverse events (AEs) allow for a sustained virologic response (SVR) in over 90% of patients, both in randomized clinical trials[1-3] and in real-world settings[4,5]. Previous research has demonstrated that pangenotypic therapies significantly increase the effectiveness of treatment in patients infected with hepatitis C virus (HCV) genotype 3, which is considered more difficult to treat[6-9].
Numerous studies have confirmed the high efficacy of treatment with DAAs in almost all groups of patients, including those with liver cirrhosis, renal failure, and organ transplants, regardless of age or concomitant diseases, including human immunodeficiency virus (HIV) or hepatitis B virus (HBV) coinfections[10,11].
Most of the publications evaluating interferon-free therapies are devoted to assessing their efficacy and safety. Few researchers, however, have analyzed the factors that cause the therapy to fail in some patients. Therefore, we aimed to analyze this issue in the large, multicenter EpiTer-2 cohort and to present the characteristics of patients in whom DAA treatment has failed in a real-world setting. Knowledge of these factors may aid in determining the qualifying criteria for antiviral therapy and the way it is conducted, which would further minimize the failure rate.
Achieving SVR does not completely exclude the development adverse consequences of chronic hepatitis C, but there are no better methods to prevent the progression of liver fibrosis, development of cirrhosis, liver failure, and hepatocellular carcinoma (HCC) than to eliminate the infection. Thus, all measures should be taken to maximize treatment efficacy.
In 2016, on the initiative of investigators, a group of 22 Polish centers began collecting data on the efficacy and safety of drugs used in the treatment of patients with chronic hepatitis C. This study, called EpiTer-2, was supported by the Polish Association of Epidemiologists and Infectologists. The data were collected using a web-based questionnaire, in accordance with the National General Data Protection Regulation.
The therapies were financed by the National Health Fund under general health insurance. The parameters collected in the database were as follows: HCV genotype, stage of fibrosis, hematology and liver function parameters, Child-Turcotte-Pugh and Model for End-stage Liver Disease (MELD) scores, prior antiviral therapy, concomitant diseases and drugs used in relation to them, HBV and/or HIV coinfections.
Hepatic fibrosis was evaluated via a liver biopsy based on the METAVIR or Scheuer scoring system, transient elastography using the FibroScan (Echosens, Paris) device, or real-time shear wave elastography) using the Aixplorer (Supersonic, Aix-en-Provence) device.
HCV RNA was monitored prior to and after the treatment (end of treatment virologic response), and then after at least a 12-wk follow-up period (SVR). Two assays were used to measure HCV RNA, depending on local practices at the testing site: Roche COBAS TaqMan with a lower limit of quantification (LLOQ) of 15 IU/mL or Abbott RealTime with an LLOQ of 12 IU/mL.
Peripheral blood counts, liver function, and kidney function were evaluated to assess the safety of the therapy. The basic scope of tests and schedule of patients’ visits were defined in the National Health Fund therapeutic program. For the safety of the patient, additional examinations were performed if necessary.
The drug used, the dosage and length of the treatment regimen, and the decision to add ribavirin were determined by treating physicians based on the applicable product characteristics and recommendations of the Polish Group of Experts for HCV[12].
AEs observed during the treatment and follow-up periods were reported as well, with particular attention to events related to liver disease.
This observational study was conducted in a real-world setting with approved drugs. Patients were not exposed to any experimental interventions, nor did the study intervene with the clinical management of the patient. The study only collected information from patient records. The analysis included routine examinations and tests performed in patients treated within the therapeutic program of the National Health Fund. The data were originally collected to assess treatment efficacy and safety in individual patients, not for scientific purposes. Hence, the treating physicians did not obtain approval from the ethics committee. According to local law (Pharmaceutical Law of 6th September 2001, art. 37al), non-interventional studies do not require ethics committee approval. Patients provided informed consent for treatment and the processing of personal data. Patient data were collected through an online system, and only physicians caring for patients had access to the patients’ personal information. Planning, conduct, and reporting of the study were in line with the tenets outlined in the Declaration of Helsinki, as revised in 2013.
To identify the best predictors of HCV detectability by at least the 12th week of follow-up, machine learning (ML) techniques were used to develop a statistical model. First, variables with more than 10% of missing observations were removed from the dataset.
Because the dataset was unbalanced for the dependent variable, an oversampling technique was used. The data were split into learning (75%) and testing (25%) sets. Four ML models were built using the following algorithm types: k-nearest neighbor, support vector machine, classification and regression tree, and random forest. The algorithm with the best accuracy was used for further analysis. Then, the selected parameters were optimized to boost the algorithm performance. Twelve variables with the highest predictive value were plotted.
Standard statistical methods were used to compare data between patients positive and negative for HCV at least 12 wk after the end of treatment.
Data are presented as the mean [95% confidence interval (CI)] for continuous variables and as counts (%) for categorical variables. Groups were compared with nonparametric Mann-Whitney U-tests and Pearson chi-square tests.
The parametric tests were not used because of the unequal sample size (SVR n = 11629; non-SVR n = 309).
Analysis was performed using the R programming language in RStudio (R Core Team, 2020) and IBM SPSS Statistics 25 (IBM Corp., 2017). The level of statistical significance was set at P < 0.05.
Patients’ disposition and treatment outcomes are presented in Figure 1. At the time of analysis, a total of 12614 patients with chronic hepatitis C were registered in the database, of which 11938 had available SVR data. Among them, 11629 (97%) achieved SVR, while 309 (3%) did not.
The baseline characteristics of patients with SVR and their relationships to treatment efficacy are presented in Tables 1 and 2. As there were missing data for some parameters, the number of patients for a given parameter is not always equal to the total number of patients.
| Variable | Total, n = 11938 | SVR, n = 11629 | Non-SVR, n = 309 | P value |
| Sex, n (%) | ||||
| Male | 5762 (48.3) | 5553 (47.8) | 209 (67.6) | < 0.001 |
| Female | 6176 (51.7) | 6076 (52.2) | 100 (32.4) | |
| Age | 52.24 (51.77-52.70) | 52.24 (51.77-52.71) | 52.16 (50.74-53.58) | 0.869 |
| BMI (kg/m2) | 26.47 (26.29-26.64) | 26.45 (26.26-26.62) | 27.20 (26.68-27.72) | < 0.001 |
| Fibrosis | ||||
| F0 | 277 (1.9) | 225 (99.1) | 2 (0.9) | |
| F1 | 4539 (38.8) | 4469 (98.5) | 70 (1.5) | |
| F2 | 2249 (19.2) | 2204 (98.0) | 45 (2.0) | < 0.001 |
| F3 | 1717 (14.7) | 1684 (98.1) | 33 (1.9) | |
| F4 | 2974 (25.4) | 2828 (95.1) | 146 (4.9) | |
| Liver stiffness (kPa) | 12.72 (12.45-12.99) | 12.53 (12.29-12.76) | 20.27 (14.78-25.76) | < 0.001 |
| Child Pugh, n (%) | ||||
| A | 11320 (96.9) | 11050 (97.1) | 270 (89.7) | |
| B | 346 (3.0) | 315 (2.8) | 31 (10.3) | < 0.001 |
| C | 13 (0.1) | 13 (0.1) | 0 (0.0) | |
| MELD score | 7.81 (7.76-7.85) | 7.79 (7.74-7.84) | 8.39 (8.11-8.67) | < 0.001 |
| Esophageal varices, n (%) | ||||
| Yes | 989 (10.5) | 922 (10.1) | 67 (27.1) | < 0.001 |
| No | 8413 (89.5) | 8233 (89.9) | 180 (72.9) | |
| Ascites at the start of the treatment, n (%) | ||||
| No | 11742 (98.8) | 11444 (98.9) | 298 (96.4) | |
| Moderate | 136 (1.2) | 125 (1.1) | 11 (3.6) | < 0.001 |
| Tense | 6 (0.0) | 6 (0.0) | 0 (0.0) | |
| Encephalopathy at the start of the treatment, n (%) | ||||
| No | 11808 (99.4) | 11507 (99.5) | 301 (97.8) | |
| Grade 1-2 | 67 (0.6) | 60 (0.5) | 7 (2.2) | 0.001 |
| Grade 3-4 | 1 (0.0) | 1 (0.0) | 0 (0.0) | |
| History of hepatocellular carcinoma, n (%) | ||||
| Yes | 179 (1.5) | 167 (1.5) | 12 (4.1) | < 0.001 |
| No | 11515 (98.5) | 11231 (98.5) | 284 (95.9) | |
| Extrahepatic manifestations, n (%) | ||||
| Yes | 948 (8.3) | 916 (8.2) | 32 (10.8) | 0.282 |
| No | 10513 (91.7) | 10248 (91.8) | 265 (89.2) | |
| HIV coinfection, n (%) | ||||
| Yes | 587 (5.0) | 564 (4.9) | 23 (7.6) | 0.098 |
| No | 11161 (95.0) | 10881 (95.1) | 280 (92.4) | |
| HBV coinfection, n (%) | ||||
| Yes | 1570 (13.5) | 1526 (13.4) | 44 (14.6) | 0.851 |
| No | 10098 (86.5) | 9840 (86.6) | 258 (85.4) | |
| HBsAg(+), n (%)) | 124 (8.0) | 122 (8.1) | 2 (4.5) | 0.543 |
| Reactivation of HBV infection, n (%) | ||||
| Yes | 11 (0.1) | 11 (0.1) | 0 (0.0) | 0.510 |
| No | 10707 (99.9) | 10435 (99.9) | 272 (100.0) | |
| History of liver transplantation, n (%) | ||||
| Yes | 146 (1.2) | 144 (1.3) | 2 (0.7) | 0.576 |
| No | 11555 (98.8) | 11253 (98.7) | 302 (99.3) | |
| Course of treatment, n (%) | ||||
| As planned | 11516 (97.1) | 11238 (97.3) | 278 (90.2) | |
| Terminated early | 86 (0.7) | 71 (0.6) | 15 (4.9) | < 0.001 |
| Modified | 257 (2.2) | 242 (2.1) | 15 (4.9) | |
| Ascites appearing while on treatment, n (%) | ||||
| Yes | 64 (0.5) | 57 (0.5) | 7 (2.3) | < 0.001 |
| No | 11773 (99.5) | 11473 (99.5) | 300 (97.7) | |
| Encephalopathy appearing while on treatment, n (%) | ||||
| Yes | 43 (0.4) | 35 (0.3) | 8 (2.6) | < 0.001 |
| No | 11773 (99.6) | 11474 (99.7) | 299 (97.4) | |
| Gastrointestinal bleeding while on treatment, n (%) | ||||
| Yes | 16 (0.1) | 14 (0.1) | 2 (0.7) | 0.044 |
| No | 11798 (99.9) | 11494 (99.9) | 304 (99.3) |
| Variable | Total, n = 11938 | SVR, n = 11629 | Non-SVR, n = 309 | P value |
| Albumin (g/dL) | 4.37 (4.2-4.45) | 4.38 (4.29-4.46) | 4.02 (3.76-4.28) | < 0.001 |
| Bilirubin (mg/dL) | 0.80 (0.79-0.81) | 0.79 (0.78-0.80) | 1.06 (0.98-1.15) | < 0.001 |
| INR | 1.10 (1.07-1.12) | 1.10 (1.07-1.12) | 1.11 (1.08-1.13) | < 0.001 |
| PLT (K/μL) | 191.47 (190.07-192.85) | 192.62 (191.22-194.02) | 147.63 (139.07-156.19) | < 0.001 |
| ALT (U/L) | 78.39 (77.19-79.59) | 77.92 (76.71-79.13) | 96.07 (87.16-104.98) | < 0.001 |
| Creatinine (mg/dL) | 0.92 (0.89-0.96) | 0.93 (0.89-0.96) | 0.81 (0.79-0.83) | 0.749 |
| eGFR, n (%) | ||||
| < 30 mL/min | 137 (29.2) | 137 (29.7) | 0 (0.0) | |
| > 60 mL/min | 160 (34.0) | 157 (34.0) | 3 (37.5) | 0.252 |
| 30-60 mL/min | 173 (36.8) | 168 (36.3) | 5 (62.5) | |
| Hemoglobin (g/dL) | 14.40 (14.37-14.43) | 14.40 (14.37-14.44) | 14.35 (14.15-14.55) | 0.812 |
| HCV genotype, n (%) | ||||
| 1A | 434 (3.6) | 426 (3.7) | 8 (2.6) | |
| 1B | 9327 (78.1) | 9147 (78.7) | 180 (58.3) | |
| 2 | 20 (0.2) | 20 (0.2) | 0 (0.0) | < 0.001 |
| 3 | 1328 (11.1) | 1221 (10.5) | 107 (34.6) | |
| 4 | 575 (4.8) | 565 (4.9) | 10 (3.2) | |
| 5 | 1 (0.0) | 1 (0.0) | 0 (0.0) | |
| 6 | 2 (0.0) | 2 (0.0) | 0 (0.0) | |
| HCV RNA (log10) | 6.37 (6.34-6.39) | 6.36 (6.34-6.39) | 6.39 (6.31-6.46) | 0.004 |
The studied population consisted of 5762 men (48.3%) and 6176 women (51.7%), with a mean age of 52 years. Most patients (78.1%) were infected with HCV genotype 1b, which is typical for the population of Polish patients with chronic hepatitis C. Liver cirrhosis was diagnosed in 2974 patients, while advanced fibrosis (F3) was diagnosed in 1717 patients. The study group also included patients with features of hepatic failure at baseline: ascites in 142 (1.2%) patients and encephalopathy in 68 (0.6%) patients. Esophageal varices were diagnosed in 989 (10.5%) patients.
A history of HCC was documented in 179 patients (1.5%), and 146 (1.2%) patients underwent liver transplantation prior to the antiviral treatment. HIV coinfection was diagnosed in 587 (5%) patients, while HBV coinfection was diagnosed in 1570 (13.5%) patients, among whom 124 patients were HBsAg-positive.
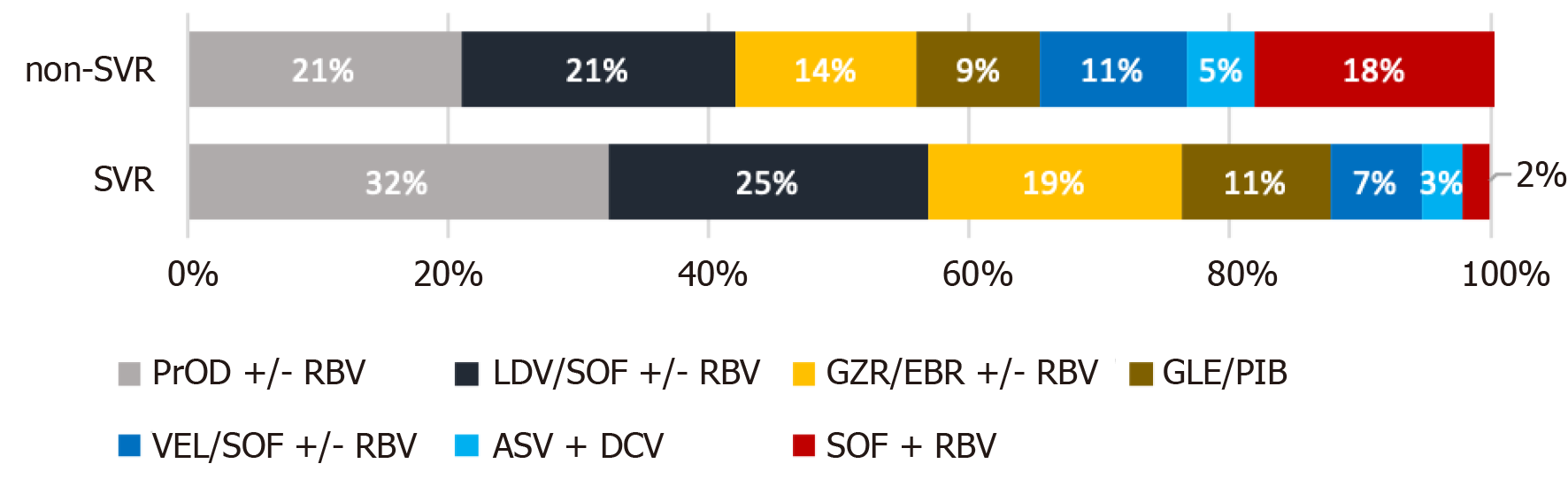
Treatment regimens and the percentages of patients taking particular medications among patients who achieved or did not achieve SVR are presented in Figure 2. The most commonly used drugs during the study (2016-2020) were paritaprevir/ ritonavir/ombitasvir +/- dasabuvir +/- ribavirin (RBV); ledipasvir/sofosbuvir (LDV/SOF) +/- RBV; grazoprevir/elbasvir +/- RBV, glecaprevir/pibrentasvir, and velpatasvir/SOF +/- RBV.
The remaining drugs, asunaprevir plus daclatasvir (ASV+DCV) or SOF + RBV, were used during the initial period of the EpiTer-2 study, and fewer patients were treated with these drugs.
The percentages of patients treated with VEL/SOF +/- RBV, ASV + DCV, and SOF + RBV were higher among patients without SVR than among those who achieved SVR.
The vast majority of patients received complete therapy as planned, although treatment was terminated prematurely in 86 (0.7%) and modified in 275 (2.2%). The modifications were mainly related to changes in the dose of RBV. The proportion of patients not completing full scheduled therapy was higher in the non-SVR group than in the SVR group; however, overall, it was not high (4.9% for both terminated and modified treatments).
Host factors: In the group of patients who did not achieve SVR, men predominated (67.7%). No age-related differences between the SVR and non-SVR groups were observed in the standard statistical analysis. However, body mass index (BMI) and mean liver stiffness (20.27 vs 12.35 kPa) were higher in the non-SVR group than in the SVR group.
DAA therapy was more often ineffective in patients with liver cirrhosis (F4 4.9% vs F3 1.9%; F2 2.0%; F1 1.5%; F0 0.9%), and with symptoms of liver failure (ascites, encephalopathy, esophageal varices, or higher Child-Pugh or MELD scores) at baseline. In addition, the occurrence of symptoms of liver failure during therapy decreased the probability of achieving SVR. Significant differences in laboratory markers of liver injury and function [alanine aminotransferase, albumin, bilirubin, international normalized ratio (INR), and platelet count] were also observed between the SVR and non-SVR groups (P < 0.001). However, it is worth noting that all 13 patients classified into Child-Pugh class C at baseline achieved SVR.
The percentage of patients with a history of HCC was significantly higher in the non-SVR group than in the SVR group (4.2% vs 1.5%, P < 0.001). Coinfection with HBV (including patients with on-treatment reactivation of HBV infection) or HIV, extrahepatic manifestations, and renal failure did not significantly affect the efficacy of therapy.
Viral factors: Infection with the HCV genotype 3 was more common in the non-SVR group than in the SVR group (34.6% vs 10.5%). Viral load was also higher among those without SVR (6.36 log10 vs 6.39 log10).
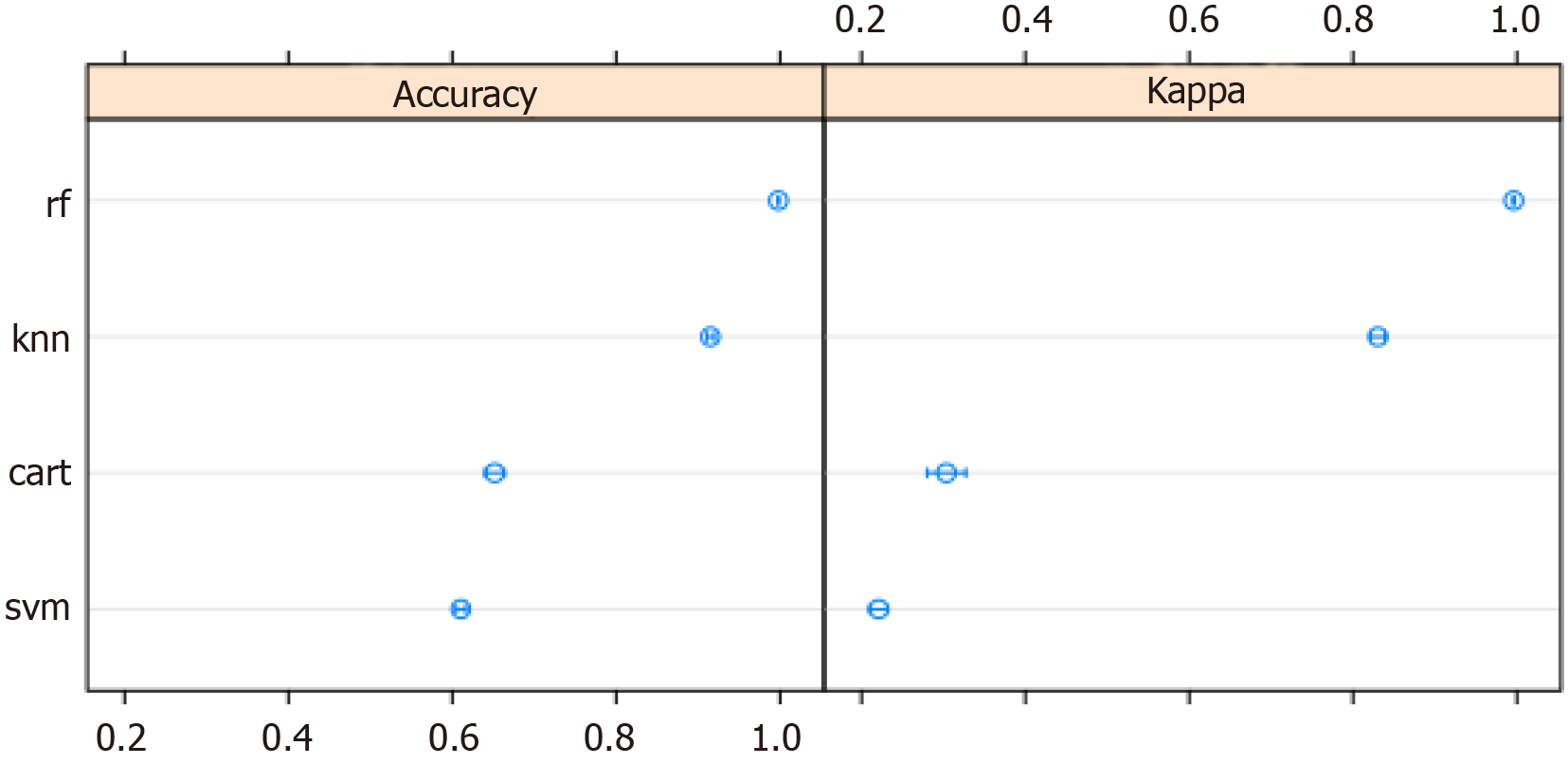
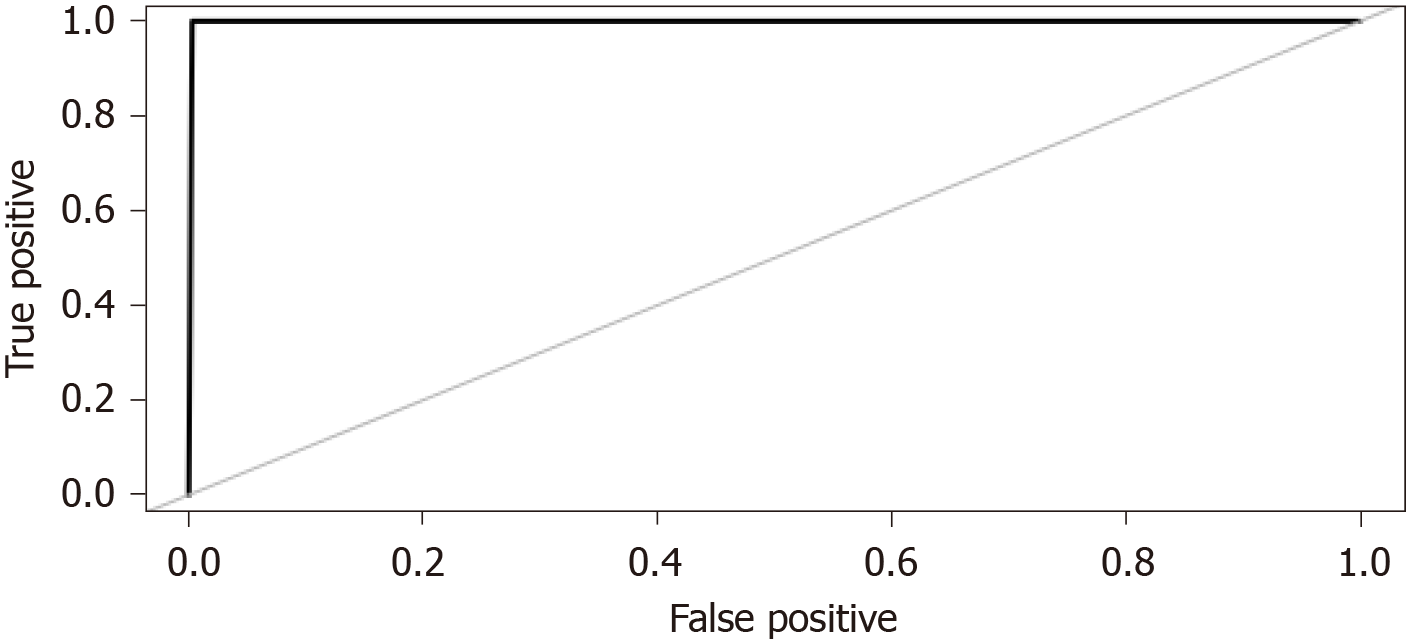
ML techniques were used to develop a predictive model. The variables in which the missing data accounted for over 10% of all observations were extracted from the database and removed from the analysis. Although four models were constructed using ML algorithms, the random forest model was selected because it yielded the best prediction accuracy. The accuracy of the models is presented in Figure 3. The area under the receiver operating characteristic curve for the random forest model was 0.999 (Figure 4).
The accuracy of the final model was checked using 10-fold cross-validation. The model was "taught" on the training data and then validated using the test data. The final model was built based on 14012 observations and 36 variables. Its accuracy was 0.9993 on the training set, and Cohen's κ statistic was 0.9985. When validating the model using the test set (n = 4670), an accuracy of 0.9985 (95%CI: 0.9969-0.9994) and a Cohen κ statistic of 0.9970 were obtained.
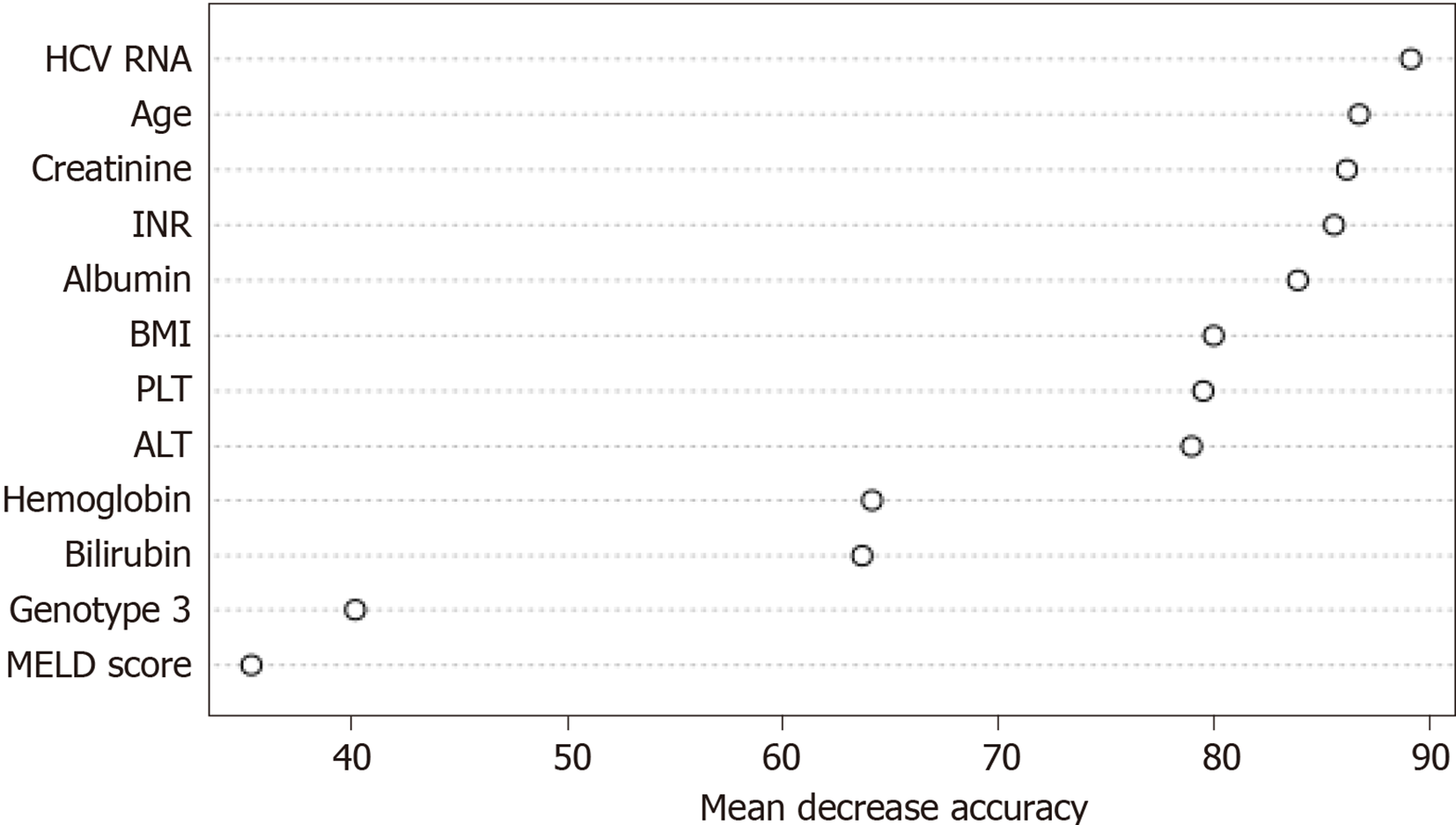
Based on the constructed model, the weights of variables influencing HCV RNA detectability at least 12 wk after the end of treatment (non-SVR) were determined. Two measures were used for this purpose: the average loss of accuracy, which is determined by how much the accuracy of the model has decreased after the removal of a specific variable (Figure 5), and the average loss of the Gini coefficient (Figure 6), on which the random forest algorithm is based. The greater the value of the loss of the Gini coefficient, the more important the variable is, because it leads to a reduction in the entropy of the output variable.
Figures 5 and 6 show the top 12 factors contributing to treatment failure.
In the case of the remaining variables, the degree of accuracy decrease indicated their minor importance in predicting therapy ineffectiveness.
Based on the statistical analysis of the factors influencing the failure of antiviral therapy performed with the use of the random forest algorithm, the following factors seem to be the most important: advancement of liver disease (platelets, albumin, INR, bilirubin, fibrosis), HCV characteristics (viral load, genotype 3), patient characteristics (age, BMI), hemoglobin levels, and creatinine levels. Conversely, the following factors seem to be of minor importance: HBV and HIV coinfections, extrahepatic manifestations of HCV infection, and coexistence of HCC.
Male sex was statistically significant in the conventional statistics. However, in the ML analysis, this parameter did not significantly affect treatment outcome (average accuracy decrease: 31st position, loss of the Gini coefficient: 14th position).
Treatment of chronic hepatitis C with DAAs rarely does not eliminate HCV infection. In our group, 97% of patients achieved SVR, which is consistent with the results of clinical trials[1-3,8,9] and other cohort studies[13-17]. In the analysis of our large, multicenter cohort of over 11000 patients, we assessed the factors that may have contributed to the ineffectiveness of DAA therapy in the remaining 3%.
Due to the specifics of the collected data (a very large study group, a large number of variables, a large disproportion between the number of patients who achieved and did not achieve SVR), we utilized ML techniques in addition to the traditional statistical analysis. Both types of statistical analysis revealed that the factors that have the greatest negative impact on the efficacy of DAA treatment are those related to the advancement of liver disease and impairment of its function. These observations are in line with the results of other cohort studies[18-22]. Because of the very low treatment failure rate, the numbers of patients failing to achieve SVR in these studies were significantly smaller than that in the present study. Gathering a group of 309 patients who did not achieve SVR makes our analysis more reliable than those conducted for groups with only several dozen non-SVR patients and can only be comparable to a few large study cohorts, such as the Veterans cohort[18].
In the classical statistical analysis, male sex seemed to be of importance, which is consistent with the findings of previous studies[17,18]. However, in the ML analysis, this factor was not among those with the greatest impact on treatment efficacy. Another difference between conventional statistics and the ML algorithm is the significance of age, creatinine, and hemoglobin in relation to treatment failure. These factors appeared to be irrelevant in the conventional analysis; however, they were among the important determinants of treatment failure in the random forest algorithm. Higher BMI was also an unfavorable prognostic factor in both types of statistical analysis. Among the virological factors examined, genotype 3 and viral load appeared to influence the efficacy of DAA treatment as well. Different results were obtained by Ioannou et al[18] in a large cohort of Veterans, in which, using conventional statistical methods, viral load and age had no effect on the treatment efficacy.
Neither HBV nor HIV coinfection influenced the results of therapy in our group.
Rial-Crestelo et al[23] in their publication, describes a cohort of 316 patients with HCV/HIV coinfection treated with DAAs between 2014 and 2018 (including 43.9% cirrhotics), in which the SVR rate was 90.9%. The factors with the greatest impact on the therapy ineffectiveness in this group were alcohol abuse and higher bilirubin levels. In our cohort, patients co-infected with HIV accounted for 5%, and only patients without active addictions were eligible for treatment. Higher bilirubin levels in our study were also associated with less effective therapy, as demonstrated by both traditional statistics and ML.
Part of the analyzed parameters showed a significant impact on the treatment effect in both “traditional” statistics and ML. For other parameters, we observed differences in statistical significance between these methods.
Direct comparison of these methods is difficult, because they involve different aspects of data collected. "Traditional" statistics cannot assess the interaction between many continuous variables and many factors simultaneously. We used this method for univariate analysis only.
ML algorithms are a multivariate way to analyze the data. It takes into account interaction between all variables, and this is a reason for inconsistency between "traditional" and ML sections. In our opinion, ML algorithms perform better because of the following reasons—the large data frame with more than 11000 observations and a significant disproportion between patients who achieved and did not achieve SVR (97% vs 3%); ML counts interactions between all variables; the oversampling technique allows us to have equal groups of HCV RNA detectability (in standard statistics, oversampling does not work because multiplication of data may result in biased outcomes).
The percentages of patients treated with VEL/SOF +/- RBV, ASV + DCV, and SOF + RBV were higher among patients without SVR than among those who achieved SVR.
The lower efficacy of treatment with SOF + RBV or ASV + DCV has been observed in previous studies and in clinical practice[24-27].
The relatively high percentage of patients treated with VEL/SOF +/- RBV among therapy failures is somewhat surprising. However, patients with hepatic failure were treated with this drug because treatment with a regimen containing protease inhibitors is contraindicated in this group. Thus, higher treatment failure rates may be associated with more severe liver disease. On the other hand, no such effect was observed in patients treated with LDV/SOF +/- RBV in the earlier period of the EpiTer-2 study, when relatively more patients with advanced liver disease were included[28].
Resistance-associated variants (RASs) were not analyzed in this study because they are not routinely used in clinical practice and are determined only in a few cases at select centers. Pre-treatment RAS data were not available for all patients, although some who qualified had them assessed as part of a separate study[29]. A study carried out on a population partially overlapping our study group reported an increased frequency of baseline NS5A RASs (particularly Y93H) in patients with advanced fibrosis and cirrhosis. Data from the study by Parczewski et al[29], which included 265 patients, some of whom were subsequently treated in the EpiTer-2 study, suggest that the incidence of NS5A RASs increases in patients with advanced fibrosis and cirrhosis in comparison to levels observed in those with mild fibrosis, even in those with no history of antiviral therapy.
The frequent occurrence of RASs was also observed in the Italian cohort of 87 patients after failing DAAs therapy, 79.5% of whom were patients with cirrhosis[30].
Considering the lower efficacy of treatment in patients with advanced liver disease and a greater tendency to develop RASs in these groups[29-31], sequencing tests should be considered in patients with cirrhosis, especially those with the features of deteriorating liver function (both with signs of overt failure and those meeting the Child-Pugh A criteria, but with decreased platelet count and albumin and/or elevated bilirubin). This would allow for the selection of personalized therapy with the maximum chance of eliminating HCV infection, which is important in this particular group of patients at risk of complications of liver cirrhosis and/or the development of HCC.
Recently, there has been a tendency to shorten therapy in patients with cirrhosis, making its duration equal to that utilized in patients with less advanced liver disease[32,33]. Our data suggest that longer treatment durations should be considered in patients with cirrhosis, especially those with borderline or overt liver failure and high HCV viral load, with monitoring of early responses during the treatment period (response-guided therapy). Although the percentage of patients not achieving SVR was statistically low, this applies mainly to patients with cirrhosis, in whom the elimination of HCV sometimes determines their further health and even life. Therefore, a special approach to the treatment of these patients should be considered[33,34]. Due to the serious prognosis in these patients, it is important to maximize the effectiveness of the therapy in order to eliminate HCV infection as soon as possible, to prevent the development of additional RASs that can limit the efficacy of possible re-therapy, and to start the process of liver regeneration.
It would be advisable to conduct a further study to verify our findings and, if necessary, to develop guidelines for personalized therapy of such patients.
The strength of our study lies primarily in the inclusion of a large group of non-SVR patients treated in the setting of everyday clinical practice, which allowed us to assess the effects of treatment in patients with more advanced liver injury and concomitant diseases, in contrast to most clinical trials. There are, however, several limitations in our study. This was a cohort study in which various drugs were used depending on their availability over the 4-year data collection period. At that time, manufacturers also made changes to the characteristics of medicinal products used (e.g., shortening the treatment period, recommended treatment with or without RBV). However, it is known that these changes were introduced after evidence from clinical trials showed that they did not significantly reduce the efficacy of treatment and may improve the patient’s safety[32].
In addition, some data were not entered for all patients, which is the case in such large, multicenter, real-world projects. However, despite the omitted data, the size of the group was large enough to draw reliable conclusions.
In conclusion, our findings demonstrate that failure of treatment with DAAs occurs mainly in patients with liver cirrhosis and deterioration of liver function. Our ML analysis further revealed that older age, creatinine, and hemoglobin levels also influenced treatment failure, as did viral factors such as genotype 3 and viral load. In patients with advanced liver disease, individualization of therapy (RAS testing, response-guided treatment) should be considered to maximize the chance of achieving SVR.
Treatment with direct-acting antiviral drugs (DAAs) is highly effective and safe. Interferon-free therapies allow for a sustained virologic response (SVR) in over 90% of patients, both in randomized clinical trials and in real-world settings.
Treatment of chronic hepatitis C with DAAs rarely does not eliminate hepatitis C virus (HCV) infection. Numerous studies have confirmed the high efficacy of treatment with direct-acting antivirals. Most of the publications evaluating interferon-free therapies are devoted to assessing their efficacy and safety. Few researchers, however, have analyzed the factors that cause the therapy to fail in some patients.
To analyze factors influencing the failure of direct antiviral drugs in the large, Polish multicenter EpiTer-2 cohort of 12614 patients in a real-world setting.
The study cohort consisted of patients treated at 22 centers from 2016-2020. Both standard and machine learning methods were used for statistical analysis.
Among 11938 patients with SVR data available, 11629 (97%) achieved SVR and 309 (3%) did not. Most patients (78.1%) were infected with HCV genotype 1b. Liver cirrhosis was diagnosed in 2974 patients, advanced fibrosis (F3) in 1717 patients. The most important host factors negatively influencing treatment efficacy were liver cirrhosis, clinical and laboratory features of liver failure, history of hepatocellular carcinoma, and higher body mass index. Among viral factors, genotype 3 and viral load also exerted an influence on treatment efficacy.
In patients with advanced liver disease, individualized therapy (testing for resistance-associated variants and response-guided treatment) should be considered to maximize the chance of achieving SVR.
The EpiTer-2 is still an active study, and data on patients treated for HCV infection are still being collected. The obtained data will allow us to confirm the results of our research on a larger group of patients and to verify the validity of the hypothesis that individualization of therapy in patients with liver cirrhosis may improve the treatment efficacy.
We would like to thank Polish Association of Epidemiologists and Infectiologists for the creation and maintenance of the database.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver, No. 2039.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anastasiou I, Derviş Hakim G, Feng QS, Grawish ME, Irato P S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A, Box TD, Younes Z, Enayati P, Green S, Baruch Y, Bhandari BR, Caruntu FA, Sepe T, Chulanov V, Janczewska E, Rizzardini G, Gervain J, Planas R, Moreno C, Hassanein T, Xie W, King M, Podsadecki T, Reddy KR; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-1992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 584] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 2. | Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, Brown DD, Wan S, DiNubile MJ, Nguyen BY, Robertson MN, Wahl J, Barr E, Butterton JR. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015;163:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 415] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 3. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, Foster GR, Bräu N, Buti M, Jacobson IM, Subramanian GM, Ding X, Mo H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Mangia A, Marcellin P; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1329] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 4. | Mangia A, Milligan S, Khalili M, Fagiuoli S, Shafran SD, Carrat F, Ouzan D, Papatheodoridis G, Ramji A, Borgia SM, Wedemeyer H, Losappio R, Pérez-Hernandez F, Wick N, Brown RS Jr, Lampertico P, Doucette K, Ntalla I, Ramroth H, Mertens M, Vanstraelen K, Turnes J. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts. Liver Int. 2020;40:1841-1852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Puenpatom A, Cao Y, Yu X, Kanwal F, El-Serag HB, Kramer JR. Effectiveness of Elbasvir/Grazoprevir in US Veterans with Chronic Hepatitis C Virus Genotype 1b Infection. Infect Dis Ther. 2020;9:355-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Zuckerman E, Gutierrez JA, Dylla DE, de Ledinghen V, Muir AJ, Gschwantler M, Puoti M, Caruntu F, Slim J, Nevens F, Sigal S, Cohen S, Fredrick LM, Pires Dos Santos AG, Rodrigues L Jr, Dillon JF. Eight Weeks of Treatment With Glecaprevir/Pibrentasvir Is Safe and Efficacious in an Integrated Analysis of Treatment-Naïve Patients With Hepatitis C Virus Infection. Clin Gastroenterol Hepatol 2020; 18: 2544-2553. e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Boyle A, Marra F, Peters E, Datta S, Ritchie T, Priest M, Heydtmann M, Barclay ST. Eight weeks of sofosbuvir/velpatasvir for genotype 3 hepatitis C in previously untreated patients with significant (F2/3) fibrosis. J Viral Hepat. 2020;27:371-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Zoratti MJ, Siddiqua A, Morassut RE, Zeraatkar D, Chou R, van Holten J, Xie F, Druyts E. Pangenotypic direct acting antivirals for the treatment of chronic hepatitis C virus infection: A systematic literature review and meta-analysis. EClinicalMedicine. 2020;18:100237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166:637-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 487] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 10. | Li T, Qu Y, Guo Y, Wang Y, Wang L. Efficacy and safety of direct-acting antivirals-based antiviral therapies for hepatitis C virus patients with stage 4-5 chronic kidney disease: a meta-analysis. Liver Int. 2017;37:974-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Peters MG, Kottilil S, Terrault N, Amara D, Husson J, Huprikar S, Florman S, Sulkowski MS, Durand CM, Luetkemeyer AF, Rogers R, Grab J, Haydel B, Blumberg E, Dove L, Emond J, Olthoff K, Smith C, Fishbein T, Masur H, Stock PG. Retrospective-prospective study of safety and efficacy of sofosbuvir-based direct-acting antivirals in HIV/HCV-coinfected participants with decompensated liver disease pre- or post-liver transplant. Am J Transplant. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Polish Group of Experts for HCV; Halota W, Flisiak R, Juszczyk J, Małkowski P, Pawłowska M, Simon K, Tomasiewicz K. Recommendations for the treatment of hepatitis C in 2017. Clin Exp Hepatol. 2017;3: 47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, Gea F, Lens S, García-Samaniego J, Sacristán B, García-Eliz M, Llerena S, Pascasio JM, Turnes J, Torras X, Morillas RM, Llaneras J, Serra MA, Diago M, Rodriguez CF, Ampuero J, Jorquera F, Simon MA, Arenas J, Navascues CA, Bañares R, Muñoz R, Albillos A, Mariño Z; Spanish Group for the Study of the Use of Direct-acting Drugs Hepatitis C Collaborating Group. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. J Hepatol. 2017;66:1138-1148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Bischoff J, Mauss S, Cordes C, Lutz T, Scholten S, Moll A, Jäger H, Cornberg M, Manns MP, Baumgarten A, Rockstroh JK. Rates of sustained virological response 12 weeks after the scheduled end of direct-acting antiviral (DAA)-based hepatitis C virus (HCV) therapy from the National German HCV registry: does HIV coinfection impair the response to DAA combination therapy? HIV Med. 2018;19:299-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Huang CF, Iio E, Jun DW, Ogawa E, Toyoda H, Hsu YC, Haga H, Iwane S, Enomoto M, Lee DH, Wong G, Liu CH, Tada T, Chuang WL, Cheung R, Hayashi J, Tseng CH, Yasuda S, Tran S, Kam L, Henry L, Jeong JY, Nomura H, Park SH, Nakamuta M, Huang JF, Tai CM, Lo GH, Lee MH, Yang HI, Kao JH, Tamori A, Eguchi Y, Ueno Y, Furusyo N, Tanaka Y, Yu ML, Nguyen MH; REAL-C Investigators. Direct-acting antivirals in East Asian hepatitis C patients: real-world experience from the REAL-C Consortium. Hepatol Int. 2019;13:587-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Salmon D, Trimoulet P, Gilbert C, Solas C, Lafourcade E, Chas J, Piroth L, Lacombe K, Katlama C, Peytavin G, Aumaitre H, Alric L, Boué F, Morlat P, Poizot-Martin I, Billaud E, Rosenthal E, Naqvi A, Miailhes P, Bani-Sadr F, Esterle L, Carrieri P, Dabis F, Sogni P, Wittkop L; ANRS CO13 Hepavih study Group. Factors associated with DAA virological treatment failure and resistance-associated substitutions description in HIV/HCV coinfected patients. World J Hepatol. 2018;10:856-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Dalgard O, Weiland O, Noraberg G, Karlsen L, Heggelund L, Färkkilâ M, Balslev U, Belard E, Øvrehus A, Skalshøi Kjær M, Krarup H, Thorup Røge B, Hallager S, Madsen LG, Lund Laursen A, Lagging M, Weis N. Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections-A Scandinavian real-life study. PLoS One. 2017;12:e0179764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, Su F, Berry K. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients with Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology 2016; 151: 457-471. e5 [PMID: 2726705. [Cited in This Article: ] |
| 19. | Chang CY, Nguyen P, Le A, Zhao C, Ahmed A, Daugherty T, Garcia G, Lutchman G, Kumari R, Nguyen MH. Real-world experience with interferon-free, direct acting antiviral therapies in Asian Americans with chronic hepatitis C and advanced liver disease. Medicine (Baltimore). 2017;96:e6128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Ippolito AM, Milella M, Messina V, Conti F, Cozzolongo R, Morisco F, Brancaccio G, Barone M, Santantonio T, Masetti C, Tundo P, Smedile A, Carretta V, Gatti P, Termite AP, Valvano MR, Bruno G, Fabrizio C, Andreone P, Zappimbulso M, Gaeta GB, Napoli N, Fontanella L, Lauletta G, Cuccorese G, Metrangolo A, Francavilla R, Ciracì E, Rizzo S, Andriulli A. HCV clearance after direct-acting antivirals in patients with cirrhosis by stages of liver impairment: The ITAL-C network study. Dig Liver Dis. 2017;49:1022-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Jiménez-Macías FM, Cabanillas-Casafranca M, Maraver-Zamora M, Romero-Herrera G, García-García F, Correia-Varela-Almeida A, Cabello-Fernández A, Ramos-Lora M. Experience in real clinical practice with new direct acting antivirals in chronic hepatitis C. Med Clin (Barc). 2017;149:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Terrault NA, Zeuzem S, Di Bisceglie AM, Lim JK, Pockros PJ, Frazier LM, Kuo A, Lok AS, Shiffman ML, Ben Ari Z, Akushevich L, Vainorius M, Sulkowski MS, Fried MW, Nelson DR; HCV-TARGET Study Group. Effectiveness of Ledipasvir-Sofosbuvir Combination in Patients With Hepatitis C Virus Infection and Factors Associated With Sustained Virologic Response. Gastroenterology 2016; 151: 1131-1140. e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 23. | Rial-Crestelo D, Sepúlveda MA, González-Gasca FJ, Geijo-Martínez P, Martínez-Alfaro E, Barberá JR, Yzusqui M, Casallo S, García M, Muñoz Hornero C, Espinosa-Gimeno A, Torralba M; Grupo de Estudio de Castilla la Manche de enfermedades Infecciosas (GECMEI). Impact of interferon-free therapies in HIV/HCV co-infected patients on real clinical practice: results from a multicenter region-wide cohort study (2014-2018). Eur J Gastroenterol Hepatol. 2021;32:279-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Suzuki F, Hatanaka N, Bando E, Nakamura K, Komoto A. Safety and effectiveness of daclatasvir and asunaprevir dual therapy in patients with genotype 1 chronic hepatitis C: results from postmarketing surveillance in Japan. Hepatol Int. 2018;12:244-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, Izumi N, Koike K, Takehara T, Kawada N, Sata M, Miyagoshi H, Eley T, McPhee F, Damokosh A, Ishikawa H, Hughes E. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 488] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 26. | Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 603] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 27. | Herbst DA Jr, Reddy KR. Sofosbuvir, a nucleotide polymerase inhibitor, for the treatment of chronic hepatitis C virus infection. Expert Opin Investig Drugs. 2013;22:527-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Flisiak R, Zarębska-Michaluk D, Janczewska E, Staniaszek A, Gietka A, Mazur W, Tudrujek M, Tomasiewicz K, Belica-Wdowik T, Baka-Ćwierz B, Dybowska D, Halota W, Lorenc B, Sitko M, Garlicki A, Berak H, Horban A, Orłowska I, Simon K, Socha Ł, Wawrzynowicz-Syczewska M, Jaroszewicz J, Deroń Z, Czauż-Andrzejuk A, Citko J, Krygier R, Piekarska A, Laurans Ł, Dobracki W, Białkowska J, Tronina O, Pawłowska M. Treatment of HCV infection in Poland at the beginning of the interferon-free era-the EpiTer-2 study. J Viral Hepat. 2018;25:661-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Parczewski M, Kordek J, Janczewska E, Pisula A, Łojewski W, Socha Ł, Wawrzynowicz-Syczewska M, Bociąga-Jasik M, Szymczak A, Cielniak I, Siwak E, Mularska E, Aksak-Wąs B, Urbańska A, Lübke N. Hepatitis C virus (HCV) genotype 1 NS5A resistance-associated variants are associated with advanced liver fibrosis independently of HCV-transmission clusters. Clin Microbiol Infect 2019; 25: 513.e1-513. e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Starace M, Minichini C, De Pascalis S, Macera M, Occhiello L, Messina V, Sangiovanni V, Adinolfi LE, Claar E, Precone D, Stornaiuolo G, Stanzione M, Ascione T, Caroprese M, Zampino R, Parrilli G, Gentile I, Brancaccio G, Iovinella V, Martini S, Masarone M, Fontanella L, Masiello A, Sagnelli E, Punzi R, Salomone Megna A, Santoro R, Gaeta GB, Coppola N. Virological patterns of HCV patients with failure to interferon-free regimens. J Med Virol. 2018;90:942-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Di Stefano M, Faleo G, Farhan Mohamed AM, Morella S, Bruno SR, Tundo P, Fiore JR, Santantonio TA. Resistance Associated Mutations in HCV Patients Failing DAA Treatment. New Microbiol. 2021;44:12-18. [PubMed] [Cited in This Article: ] |
| 32. | Brown RS Jr, Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, Horváth G, Zuckerman E, Carrion BR, Rodriguez-Perez F, Urbánek P, Abergel A, Cohen E, Lovell SS, Schnell G, Lin CW, Zha J, Wang S, Trinh R, Mensa FJ, Burroughs M, Felizarta F. Glecaprevir/pibrentasvir for 8 wk in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J Hepatol. 2020;72:441-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 33. |
Lampertico P, Mauss S, Persico M, Barclay ST, Marx S, Lohmann K, Bondin M, Zhang Z, Marra F, Belperio PS, Wedemeyer H, Flamm S Real-World Clinical Practice Use of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naive Patients with Compensated Cirrhosis.
Lampertico P, Mauss S, Persico M, Barclay ST, Marx S, Lohmann K, Bondin M, Zhang Z, Marra F, Belperio PS, Wedemeyer H, Flamm S Real-World Clinical Practice Use of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naive Patients with |
| 34. | Peiffer KH, Vermehren J, Kuhnhenn L, Susser S, Dietz J, Finkelmeier F, Weiler N, Welzel T, Grammatikos G, Zeuzem S, Sarrazin C. Interferon-free treatment choice according to baseline RASs leads to high SVR rates in HCV genotype 1 infected patients. J Infect Chemother. 2018;24:524-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |