Published online May 14, 2021. doi: 10.3748/wjg.v27.i18.2073
Peer-review started: January 16, 2021
First decision: February 28, 2021
Revised: February 28, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: May 14, 2021
Infection with hepatotropic viruses is not limited to the liver and can lead to the development of various immunological disorders (the formation of cryoglobulins, rheumatoid factor, antinuclear antibodies, autoantibodies specific for autoimmune hepatitis and primary biliary cholangitis, and others), which can manifest as glomerulonephritis, arthritis, uveitis, vasculitis (cryoglobulinemic vasculitis, polyarteritis nodosa, Henoch-Schonlein purpura, isolated cutaneous necrotizing vasculitis), and other rheumatologic disorders, and be a trigger for the subsequent development of autoimmune hepatitis and primary biliary cholangitis. A further study of the association between autoimmune liver diseases and hepatotropic virus infection would be useful to assess the results of treatment of these associated diseases with antiviral drugs. The relationship of these immune disorders and their manifestations with hepatotropic viruses is best studied for chronic hepatitis B and C. Only isolated cases of these associations are described for hepatitis A. These links are least studied, and are often controversial for hepatitis E, possibly due to their relatively rare diagnoses. Patients with uveitis, glomerulonephritis, arthritis, vasculitis, autoimmune liver diseases should be tested for biomarkers of viral hepatitis, and if present, these patients should be treated with antiviral drugs.
Core Tip: Infection with hepatotropic viruses is not limited to the liver and can lead to the development of various immunological disorders, which can manifest itself as glomerulonephritis, arthritis, uveitis, vasculitis, and other rheumatologic disorders, and be a trigger for the subsequent development of autoimmune hepatitis and primary biliary cholangitis. These associations are best studied for chronic hepatitis B and C. Only isolated cases of these are described for hepatitis A. These links are least studied, and are often controversial for hepatitis E. Patients with uveitis, glomerulonephritis, arthritis, vasculitis, autoimmune liver diseases should be tested for biomarkers of viral hepatitis.
- Citation: Maslennikov R, Ivashkin V, Efremova I, Shirokova E. Immune disorders and rheumatologic manifestations of viral hepatitis. World J Gastroenterol 2021; 27(18): 2073-2089
- URL: https://www.wjgnet.com/1007-9327/full/v27/i18/2073.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i18.2073
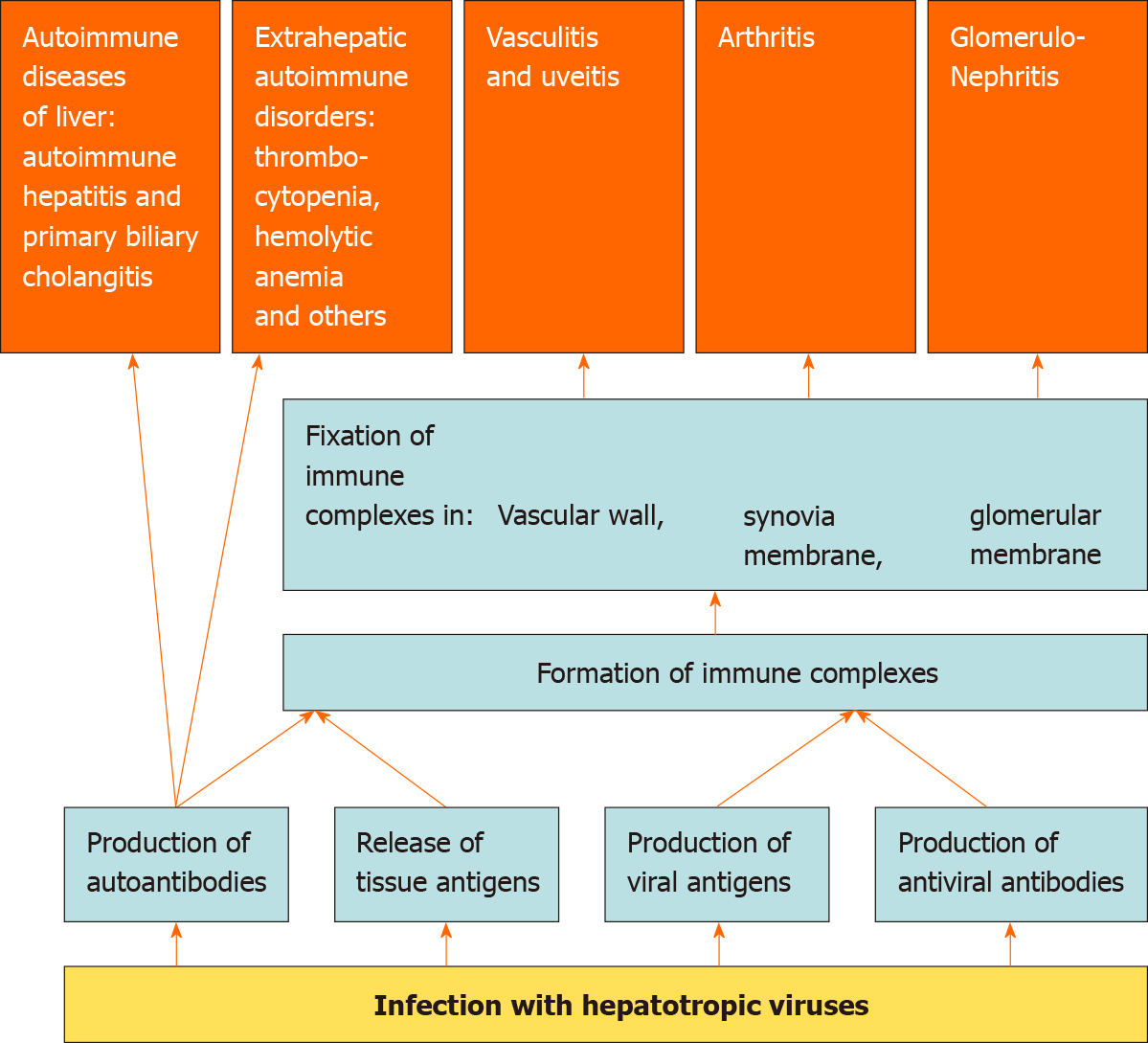
Viruses, unlike bacteria and fungi, cannot reproduce on their own and need the resources of a host cell as they are obligate intracellular parasites[1]. The viral proteins produced by the cell undergo partial proteolysis in the proteosomes, after which their fragments in conjunction with the molecules of the major histocompatibility complex type 1, are exposed on the cell surface to stimulate the immune system[2]. The partial proteolytic products of the cell’s own proteins are exposed in a similar way. If these peptides have sufficient similarity to the partial proteolytic peptides of viral proteins, it is possible to develop an autoimmune cross-reaction in response to a viral infection[3]. Antiviral immunity is directed not only and not so much against the virus itself, but against the host cell infected with it, which serves as another predictor for the development of autoimmune aggression[4,5]. Among the autoantibodies produced in response to a viral infection, rheumatoid factor [(RF) an autoantibody against IgG], and cryoglobulins (antibodies that precipitate in the cold), are of utmost importance. The release of viral antigens into the bloodstream also plays a major role. Damage to the tissues, both directly by viruses and as a result of immune aggression against infected cells, also results in the release of a large number of tissue antigens. Tissue and viral antigens interact with antibodies to form immune complexes, which are fixed in the synovial membrane of the joints, glomerular membrane, and vascular wall, including the choroid. This leads to the development of arthritis, glomerulonephritis, vasculitis, and uveitis. Thus, viral infections can be triggers for autoimmune reactions and other immune disorders, which predispose to the development of rheumatologic and immunological complications of viral infections (Figure 1). However, the exact mechanisms of the development of autoimmune reactions in viral infections are yet to be established.
Many viruses can infect the liver, but only for five of them it is the main target organ: hepatitis A viruses (HAV), hepatitis B viruses (HBV), hepatitis C viruses (HCV), hepatitis D viruses (HDV) and hepatitis E viruses (HEV)[6]. HDV is a defective virus and can only replicate when co-infected with HBV[7].
Our review is devoted to describe the development of the aforementioned immunological disorders and caused by them rheumatologic and other extrahepatic manifestations in viral hepatitis. Knowing these complications is very important as infection with hepatotropic viruses can manifest only in the form of these disorders, leading to misdiagnosis and inadequate treatment.
Hepatitis A occurs only in an acute form[8]. Perhaps due to the short-term contact of the macroorganism with the virus, immune and rheumatologic disorders rarely develop.
Joint pain without arthritis occurs on average in 10%-20% of patients with hepatitis A[9-11]. True arthritis in hepatitis A develops within vasculitis (see below).
RF was found in only two patients with hepatitis A who had cryoglobulinemic vasculitis[12].
Cryoglobulinemia is observed in 95% of patients with hepatitis A in one study. Cryoprecipitate was represented by IgM, including antibodies against HAV. IgA and/or IgG were also represented in the cryoprecipitate of 15% of patients. After recovery, the cryoglobulin content decreased to normal. Interestingly, the cryoglubulin level in hepatitis A was higher than in acute hepatitis B[13].
Mesangioproliferative glomerulonephritis with deposits of IgM and complement components was found in a 7-year-old boy with hepatitis A. This was accompanied by the development of severe nephrotic syndrome, neutrophilic leukocytosis, and a decrease in the blood complement (components C3 and C4) levels. The disease ended in complete recovery[14].
In another case, mesangioproliferative glomerulonephritis in a young woman led to deposits of all three types of immunoglobulins and complement component C1q, and was accompanied by the development of acute renal failure, but not nephrotic syndrome; blood complement levels were normal. Complete recovery was observed here also[15].
The development of IgA nephropathy in hepatitis A has also been reported[16,17].
Polymyositis with myoglobulinuria, increased creatine kinase activity and electromyographic changes was verified by muscle biopsy in a patient with hepatitis A[18].
A 23-year-old man with hepatitis A developed adult-onset Still's syndrome: Fever, maculopapular rash on the trunk and legs, generalized arthralgia, severe neutrophilic leukocytosis, and hyperferritinemia. The disease was successfully controlled by glucocorticoids[19].
A case of unilateral autoimmune parotitis with left-sided pain and swelling in the face, generalized arthralgia, and rash was reported. Biopsy revealed mononuclear infiltration of the affected parotid gland. The disease was resistant to antibiotics, but was quickly treated with prednisolone[20].
We found 13 published cases of hepatitis A-associated vasculitis (Tables 1 and 2)[12,21-30]. These were Henoch-Schonlein purpura (HSP) (53.8%), cryoglobulinemic vasculitis (CGV) (30.8%), and isolated cutaneous necrotizing vasculitis (15.4%). Most of them (76.9%) occurred in children. The pathological process involved the skin (92.3%), joints (69.2%), intestines (46.2%) and kidneys (7.7%). Arthralgia without synovitis was observed in 15.4% of cases and arthritis in 54.8% of cases (mainly in the knees and ankles). Vasculitis developed about 5wk after the onset of the disease, and was accompanied by a second wave of hepatitis in 30.8% of cases and a protracted course (more than one month) of hepatitis in 23.1% of cases. All patients experienced complete recovery.
| Case | Dan et al[21] | Press et al[22] | Nassih et al[23] | Chemli et al[24] | Mohan et al[25] | Altınkaynak et al[26] |
| Age, yr | 30 | 2 | 8 | 10 | < 1 | 10 |
| Sex | Female | Female | Female | Male | Female | Male |
| Diagnosis | CNV | CNV | CGV | HSP | HSP | HSP |
| Body temperature | N | N/A | N | N/A | Mild fever | N |
| Time of the onset after the onset of hepatitis | 2 wk | N/A | 8 wk | 3 d after admission to hospital | During the first month of illness | 2 wk |
| Second wave of hepatitis | - | N/A | - | - | - | - |
| Pruritus | + | N/A | + | + | - | - |
| Jaundice | + | N/A | + | N/A | + | + |
| Rash | EPR over the hips, but also involving the buttocks and arms, and rare petechiae | Ecchymotic lesions | PP on the legs, forearms, and the back | PP on the declivous regions | Bluish PP on both lower limbs, swelling over dorsum of hands and feet | PP on the legs and on the gluteal regions. |
| Joints | N | N/A | Arthritis in the knees | Arthralgia | Arthritis in the right knee | Arthralgia |
| GN | No | N/A | Dipstick test was positive to proteins (2+) and blood (3+) | No | No | No |
| Gut | N | N/A | N | Abdominal pain | Small amount of blood in stool | Abdominal pain |
| RF | N/A | N/A | Negative | N/A | Negative | Negative |
| ANA | N/A | N/A | Negative | N/A | Negative | Negative |
| WBC, 109/L | 6.3 | N/A | 7.2 | N/A | ↑ | 9.9 |
| CRP, mg/L | N/A | N/A | 38 | N/A | N/A | N |
| Platelets, 109/L | 408 | N/A | N/A | N/A | N | 416 |
| ESR, mm/h | 80 | N/A | 80 | N/A | N/A | N/A |
| Cryoglobulins | N/A | N/A | IgM, IgA, and IgG | N/A | N/A | N/A |
| Transaminases | ↑ | N/A | ↑ | ↑ | ↑ | ↑ |
| Complement | N | N/A | N/A | N/A | N/A | N |
| Skin biopsy | LCV with deposition of IgM and C3 | Necrotizing vasculitis with fibrin thrombi | LCV | N/A | LCV | N/A |
| Treatment | N/A | GC | GC | N/A | Analgetics | N/A |
| Case | Garty et al[27] | Sasan et al[28] | Islek et al[29] | Islek et al[29] | Bozaykut et al[30] | Inman et al[12] | Inman et al[12] |
| Age, yr | 8 | 8 | 13 | 11 | < 1 | 26 | 26 |
| Sex | Male | Male | Male | Female | Female | Female | Female |
| Diagnosis | HSP | HSP | HSP | HSP | CGV | CGV | CGV |
| Body temperature | N/A | 37.2 | N | N | N | N | N |
| Time of the onset after the onset of hepatitis | 5 wk | 5 wk | 13 wk | 5 wk | N/A | 20 wk | 19 wk |
| Second wave of hepatitis | - | - | + | + | N/A | + | + |
| Pruritus | - | - | - | + | - | - | - |
| Jaundice | - | - | - | + | - | + | - |
| Rash | PP on on the buttocks, penis and legs, which began with darkening of the right half of the scrotum | Non blanching red-brown papules over both thighs and legs | PP on the legs | PP on the legs | Oedema and ecchymosis on the dorsum of the hands and from feet to the knees | PP on on the legs, buttocks, arms, which began with ankles | No rash |
| Joint | N | Arthritis in the knees and ankles | Arthritis in the right knee and ankle | Arthritis in unspecified joints | N | Arthritis in the knees and ankles | Arthritis in the ankles, right fourth and fifth right metatarso-phalangeal joints |
| GN | No | No | No | No | No | No | No |
| Gut | N | Abdominal pain | Abdominal pain | Abdominal pain | N | N | N |
| RF | N/A | N/A | Negative | Negative | N/A | 1:160 | 1:320 |
| ANA | N/A | N/A | Negative | Negative | N/A | N/A | N/A |
| WBC, 109/L | N/A | 10.1 | 4.6 | 7.2 | 12 | N/A | N/A |
| CRP, mg/L | N/A | N/A | N | N/A | N/A | N/A | N/A |
| Platelets, 109/L | N/A | 516 | 250 | 407 | 300 | N/A | N/A |
| ESR, mm/h | N/A | 34 | 22 | 42 | N/A | N/A | N/A |
| Cryoglobulins | N/A | N/A | N/A | N/A | Positive | Anti-HAV IgG | Anti-HAV IgG |
| Transaminases | ↑ | N | ↑ | ↑ | N | ↑ | ↑ |
| Complement | N/A | N/A | N | C3↑, C4 - N | N | N/A | N/A |
| Skin biopsy | N/A | N/A | LCV with deposition of IgM in the dermo-epidermal junction | LCV | LCV | LCV | N/A |
| Treatment | N/A | GC | Sympto-matically | N/A | No | No | NSAID |
Experimental infection of monkeys marmosets with HAV led to the development of proliferative glomerulonephritis with deposits of IgM, component C3 (less often other immunoglobulins), and vasculitis[31].
Isolated cases of uveitis associated with hepatitis A have also been described[32,33]. Moreover, uveitis appeared before the symptoms of hepatitis[32].
One patient with hepatitis A developed lupus-like syndrome: symmetrical arthralgia in the wrists, metacarpophalangeal and proximal interphalangeal joints, left-sided pleurisy, Lupus cells, and antinuclear antibodies (ANA), antibodies against double-stranded deoxyribonucleic acid (DNA) and cardiolipin, which disappeared within a few months[34].
Autoimmune hepatitis (AIH) was triggered by hepatitis A in 11 published cases[35-44]. In some of these patients, this was accompanied by the appearance of ANA. A case of a 24-year-old woman with an autoimmune hepatitis/primary biliary cirrhosis overlap syndrome triggered by an acute hepatitis A infection was reported[45]. No case of isolated primary biliary cholangitis (PBC) associated with HAV was found.
Thus, immunological disorders and rheumatologic manifestations in hepatitis A are rare, have a favorable prognosis, and run their course without treatment or after treatment with glucocorticoids or non-steroidal anti-inflammatory drugs.
Hepatitis B is characterized by lifelong infection, which predisposes to more frequent immunological disorders and caused by them rheumatologic and other extrahepatic manifestations.
Among patients with chronic hepatitis B, Raynaud's phenomenon occurs in 2%, arthralgia or arthritis in 3%, myalgia in 3%, Sjogren’s syndrome in 3%, glomerulonephritis in 3%, uveitis in 2%, cryoglobulins in 2%[46].
RF is detected more often in asymptomatic carriers of HBV surface antigen (HBsAg) than in healthy individuals (11.8% vs 3.4%). RF positive rate was not significantly associated with the level of alanine aminotransferase or C-reactive protein in individuals with HBsAg[47]. HBV DNA levels significantly correlated with the titers of RF[48]. Antibodies against cyclic citrullated peptide (anti-CCP), a more specific marker of rheumatoid arthritis, were detected in individuals with HBsAg less often than was RF (4.6% vs 11.8%). Among patients with HBsAg, RF is found in 46% of patients with arthritis, 5% of patients with arthralgia, and in 8% of patients without rheumatologic complaints. Anti-CCP is found in 36%, 0% and less than 1% of these patients, respectively. Joint disorders and biochemical changes classified as rheumatoid arthritis were observed in 4.1% of patients with HBsAg. These patients accounted for 32.1% of HBV infected individuals with RF, 81.8% of those with anti-CCP and 90% of them have both RF and anti-CCP[49].
Patients with HBV infection accounted for 6.3% of patients with complaints of pain in joints and/or muscles. Moreover, only 26.3% of them had true arthritis[50].
HBV-associate non-rheumatoid arthritis can develop simultaneously in all affected joints, be migratory, or have an additive pattern. Synovitis develops abruptly and is severe. Arthritis develops within 12 wk after the onset of the disease, but in some cases, it is the first manifestation of hepatitis B. The age of patients with arthritis is 14-35 years in 82.8% of cases. Monoarthritis of a large joint is observed in about 40% of cases, polyarthritis of the small joints of the fingers in 10%, and a combined lesion of large and small joints in 50%. Among the large joints, the knee joints are most often affected, followed by the wrist, ankle, elbow, shoulder and hip joints. The metacarpophalangeal and proximal interphalangeal joints of the hands are affected as often as the knee joints, whereas the small joints of the feet are less frequently affected. Synovitis is usually symmetrical. The cervical and lumbar intervertebral joints are involved in about 10% of cases, usually together with other joints. ANA is determined in approximately 10% of such patients, and RF in approximately 25%, anti-CCP in approximately 5%. Complement is reduced in almost half of these patients. In all cases, arthritis resolved spontaneously or after treatment with non-steroidal anti-inflammatory drugs within 3-7 d. No development of chronic arthritis or recurrence of arthritis has been observed[49,51].
On average, 3% of glomerulonephritis is associated with the presence of HBsAg. It is membranous glomerulonephritis in 40% of cases, membranoproliferative glomerulonephritis in 20%, focal segmental glomerulosclerosis in 20%, IgA nephropathy in 10%; the remaining 10% account for the other forms. There are nephrotic syndrome in 60% of cases of HBV-associated glomerulonephritis, and nephritic syndrome or isolated changes in urine analysis in other cases[52].
Fibromyalgia is more often detected in patients with hepatitis B than in those without infection (32% vs 5%). Moreover, there was no difference in the incidence of this disease between patients with untreated active chronic hepatitis B, an inactive hepatitis B virus infection, and patients receiving treatment for this infection[53].
Autoantibodies are detected on average in 60% of patients with chronic hepatitis B. Most often, these are ANA (approximately 25%), anti-Ro52 (approximately 30%), anti-gp210 and anti-PML (approximately 10%), AMA-M2 (approximately 7%), anti-Sp100, anti-SMA, anti-LC-1 and anti-SLA/LP (all constituting approximately 3%), and anti-LKM-1 (< 1%). The frequency of detection of ANA was higher in the pre-cirrhotic stage than in cirrhosis (30% vs 20%). The frequency of detection of other autoantibodies did not differ significantly between these stages of the disease[54].
The high percentage of autoantibodies that are specific for PBC (anti-gp210, anti-PML, and AMA-M2) in HBV infection may indicate that HBV could be trigger for the development of this disease. Thus, in one study, signs of silent HBV infection (anti-HBc without HBsAg) were found in 40% of patients with PBC. Bilirubin level is higher and the degree of fibrosis is greater in these patients than in those with idiopathic PBC[55]. PBC will be diagnosed in 2%-3% of patients with hepatitis B over the next 15 years[56].
Specific biomarkers of AIH (anti-SMA, anti-LC-1, anti-SLA/LP, and anti-LKM-1) have been identified in 1%-3% of patients with hepatitis B[54]. Among patients with AIH, HBV DNA was detected in almost 25%, and serological biomarkers of HBV infection without signs of viral replication were found in another 30%[57].
About 35% of polyarteritis nodosa (PAN) cases are associated with hepatitis B. For this variant of the disease, neuropathies (approximately 85% vs 65%), arterial hypertension (49% vs 27%), abdominal pain (50% vs 28%), testicular involvement (24% vs 13%), cardiomyopathy (13% vs 4%) were more characteristic than for idiopathic PAN unlike livedo (10% vs 20%). The prognosis in patients with HBV-associated PAN was worse than in idiopathic PAN: 60% of patients from the first group and 74% from the second were alive after 10 years[58]. Hepatitis B was diagnosed before the development of PAN in approximately 30% of these patients. Vasculitis developed before the end of the clinical manifestations of hepatitis or in the next few days after this in half of these cases, and within the first 6 mo after this in the others. Transaminases are normal in 33%-50% of patients at the time of the onset of PAN[59,60].
Biomarkers of hepatitis B are detected in 2%-6% of cases with mixed cryoglobulinemia[61-63] and in almost 10% of cases with non-HCV CGV[64]. Manifestations of HBV-associated CGV are: Purpura (100%), arthralgias (71%), peripheral neuropathy (29%), glomerulonephritis (18%), Raynaud phenomenon (18%), and leg ulcer (6%)[65]. Cryoglobulins disappear in the serum leading to the regression of vasculitis in the majority of patients treated with entecavir, adefovir, and lamivudine. Corticosteroid therapy is effective for clinical symptoms of vasculitis, but ineffective for suppression of HBV and immunological features. Immunosuppressive agents are not recommended because of possible flare-up of viral replication. The use of interferons in these cases does not always lead to a positive effect[66].
There are only a few published cases of the association of HSP with hepatitis B[67-71].
Interestingly, according to a meta-analysis, biomarkers of hepatitis B are less common in systemic lupus erythematosus than in the general population: Odds ratio of HBsAg was 0.24 [95% confidence interval (CI): 0.17-0.33] and odds ratio of anti-HBc was 0.4 (95%CI: 0.31 - 0.50)[72].
HCV (like HBV) persists for a long time in the body; therefore, the frequency of immunological disorders and rheumatologic manifestations is also quite high[73].
Patients with HCV infection accounted for 5.3% of patients with complaints of pain in the joints and/or muscles. Moreover, 40% of them had true arthritis, 50% had arthralgia, and 10% had no joint disorders[50].
Pain in the joints and/or muscles are noted in 70% of patients with chronic hepatitis C if the medical history was collected carefully. Backache is the most common complaint (54%), followed by early morning stiffness (45%), arthralgia (42%), myalgia (38%), neck pain (33%), generalized pain (21%), and subjective joint swelling (20%). Diffuse pain was present in 23% of patients, non-diffuse regional plus axial pain in 18%, axial pain in 17%, and regional pain in 12%[74].
Among patients with HCV having no rheumatologic complaints, RF is detected more than in the general population (15% vs 5%), unlike anti-CCP with the same incidence (about 5%)[75]. Patients with HCV and joint involvement have RF in 60% of cases, cryoglobulins in almost 50%, and both in 40%. Anti-CCP was detected only in patients without RF and cryoglobulins[76].
Cryoglobulins are found in almost 65% of a general population of HCV patients, but they clinically manifest only in 5% of them. The achievement of a stable viral response leads to a decrease in the detection rate of cryoglobulins from 57% to 33%, whereas the detection rate remains unchanged among those in whom it was not achieved. In approximately 20% of patients with cryoglobulinemia, it persists for 8 years after achieving a stable viral response. Moreover, 80% of these patients with persisted cryoglobulinemia have its clinical manifestations during this time[77]. The high incidence of cryoglobulinemia in chronic hepatitis C can be explained by the fact that HCV can replicate in lymphocytes, protecting them from apoptosis and resulting in polyclonal proliferation, including clones that produce cryoglobulins[78].
Anti-HCV antibodies are detected in 92%-95% of patients with mixed cryoglobulinemia, and HCV RNA in 90%[61,63].
Arthritis in HCV infection develops in 5%-10% of cases[79]: Symmetric polyarthritis in 2/3 of cases, and oligo- or monoarthritis in the rest 1/3 of cases. Morning stiffness for more than 1 h occurs in 70% of HCV infected patients with arthritis, whereas erosion in the joints and subcutaneous nodules are not observed. Cryoglobulins, RF and ANA are detected in 43%, 60% and 20% of these cases, respectively. The level of complement C3 is reduced in 15% of these patients, and C4 in 30% of these patients. In addition, 86% of them had elevated transaminases. The use of anti-inflammatory or disease-modifying drugs is not effective[80], but the use of direct-acting antivirals (DAA) drugs is the most promising[81]. This arthritis is very similar to rheumatoid arthritis, and anti-CCP (unlike RF) should be used to differentiate between these diseases[82,83].
Autoantibodies are detected in 66% of patients with chronic hepatitis C. These are ANA (20%-32%), anti-LKM-1 (1%-22%), anti-Ro52 (about 15%), anti-SMA (3%-8%), AMA-M2 (approximately 3%), and anti-LC-1 (approximately 1%)[54,84].
Furthermore, 8%-12% of patients with PBC had biomarkers of HCV-infection (anti-HCV antibodies or RNA HCV)[85-87]. Despite the frequent detection of AIH-specific antibodies, it is very rarely diagnosed in HCV-infection[88]. HCV biomarkers are also rarely detected in AIH[89]. However, AIH was successfully treated with DAA in a patient with concomitant HCV infection[90].
Antibodies against HCV are detected in 40% of patients with glomerulonephritis[91]. Occult HCV infection (presence of HCV RNA by an ultrasensitive method in the absence of antibodies against HCV) is detected in 40% of the rest part of these patients: in 40% with membranous glomerulonephritis, 30% with membranoproliferative glomerulonephritis, 50% with IgA nephropathy, 30% with idiopathic nephrotic syndrome (including minimal change disease, focal segmental glomerulosclerosis, and IgM nephropathy), 50% with lupus nephropathy, and 40% with anti-neutrophil cytoplasmic antibody (ANCA) positive glomerulonephritis and 4% in the control group (hereditary glomerular nephropathy)[92]. The most common form of HCV-nephropathy is membranoproliferative glomerulonephritis, followed by focal segmental glomerulosclerosis, mesangioproliferative, and membranous glomerulonephritis[91]. Anti-HCV is detected in 98% of cases of glomerulonephritis with mixed cryoglobulinemia and in 2% glomerulonephritis without cryoglobulinemia[93]. Autopsies of patients with hepatitis C revealed glomerulopathies: Mesangioproliferative glomerulonephritis (18%), membranoproliferative glomerulonephritis (11%), membranous glomerulonephritis (3%), and mesangial expansion without hypercellularity (23%). No glomerular pathology was observed in 45% of autopsies in hepatitis C[94].
The HCV antigen is detected in the glomeruli in almost 30% glomerulonephritis with antibodies against HCV in the blood and in almost 60% in glomerulonephritis with HCV RNA in the blood[95].
Extrarenal manifestations of HCV-glomerulopathy were absent in 80% of patients even though 54% had cryoglobulinemia. Electron microscopy revealed virus-like particles in 50% of renal biopsies[91].
There are publications showing the effectiveness of DAA in the treatment of HCV-nephropathy[96-98].
Among rheumatologic diseases in patients with HCV infection, Sjogren's syndrome is most often detected (almost 50% of cases) followed by rheumatoid arthritis (15%), systemic lupus erythematosus (11%), PAN (8%), antiphospholipid syndrome (6%), inflammatory myopathies (4%), systemic sclerosis (1%). The rest of rheumatic diseases, including HSP, accounted for less than 1%[99]. A meta-analysis showed that in patients with HCV infection, cryoglobulinemia [30% vs 2%, odd ratio (OR) is 11.5], CGV (5%), Sjogren's syndrome (12% vs 0.7%, odd ratio is 2.3) and arthritis (1% vs 0.1%, OR is 2.4) are significantly more often detected than in the general population[100]. Raynaud phenomenon was found in 8% of patients with HCV[101].
Vasculitis associated with HCV-infection are CGV in approximately 80% of cases and PAN in approximately 20% of cases[102]. Other vasculitis, including HSP, are rare[99].
Among patients with PAN, antibodies against HCV were detected in 20%, and HCV RNA in 5%[103]. PAN was diagnosed on average 2 years after the diagnosis of HCV infection. These patients had purpura (68%), livedo reticularis (20%-60%), arthralgia (61%), weight loss (60%), multiplex mononeuritis (70%), myalgias or weakness (58%), altered arteriography (49%), hypertension (37%-55%), abdominal pain (30%), raised creatinine (26%), fever (20%), polyneuropathy (16%), proteinuria (16%), hematuria (16%), intestinal bleeding (16%), diarrhea (13%), orchitis (0%-7%)[99,102].
CGV was also diagnosed on average 2 years after the diagnosis of HCV infection. The most common manifestations of HCV-associated CGV are purpura (67%), polyneuropathy (65%), arthralgia (50%), proteinuria (30%), hematuria (22%), and arterial hypertension (22%). Myalgia (9%), multiplex mononeuritis (9%), livedo reticularis (3%), weight loss (4%), and abdominal pain (1.5%) are less common. Fever, intestinal bleeding, diarrhea, and orchitis are usually absent[102].
The differential diagnoses of vasculitis in HCV infection are presented in Table 3.
| Cryoglobulinemic vasculitis | Polyarteritis nodosa | |
| Weight loss | +/- | +++ |
| Fever | - | ++ |
| Myalgia | + | ++ |
| Polyneuropathy | +++ | ++ |
| Mononeuritis multiplex | + | +++ |
| Livedo | +/- | ++ |
| Arterial hypertension | ++ | +++ |
| Orchitis | - | + |
| Abdominal pain | +/- | ++ |
| Diarrhea | - | + |
| Intestinal bleeding | - | + |
| Microaneurysms or stenosis | - | +++ |
| C-reactive protein level | Normal | ↑ |
Rituximab allows to achieve remission in HCV-associated CGV in 87% of cases[104]. The effectiveness of DAA in the treatment of CGV in HCV-infection is under study. Experts currently recommend DAAs as first line treatment for mild to moderate CGV and rituximab with or without aphaeresis for severe cases[105].
Patients with HCV-associated Sjogren's syndrome present with xerophthalmia (97%), xerostomia (97%), positive Schirmer test (98%), altered salivary flow (81%), ANA (68%), RF (53%), and anti-Ro/La (25%)[99].
Very few cases of uveitis in HCV-infection were noted[106].
Hepatitis E, as a rule, is an acute infection but it can become chronic in persons with immunodeficiency[107].
Joint or/and muscle pain without arthritis and myositis is present in approximately 60% of patients with acute hepatitis E[108]. Arthralgias were observed in 5% of patients with chronic hepatitis E[109].
Two cases of arthritis development in acute hepatitis E have been described (Table 4)[110,111].
| Case | Al-Shukri et al[110] | Serratrice et al[111] |
| Age | 52 | 51 |
| Sex | Female | Female |
| Joints | Shoulders, elbows, hips, knees, ankles, left second and third metacarpophalangeal | Ankles and knees followed by the wrists and fingers |
| Duration of arthritis | No data | 3 mo |
| Rash | Maculopapular, non-itchy rash all over her body | No |
| Fever | No | No |
| Jaundice | No | No |
| Pruritus | No | No |
| Other symptoms and signs | Retroorbital pain, eye discharge, headache, and loss of appetite | No |
| Rheumatoid factor | No | No |
| Antinuclear antibodies | No | No |
| White blood cells, 109/L | Normal | 2.8 |
| Erythrocyte sedimentation rate, mm/h | 35 | 24 |
| C-reactive protein, mg/L | 25 | 3 |
| Transaminases | ↑ | ↑ |
| Complement | C3 - ↑; C4 -Normal | No data |
| Treatment | No data | No specific treatment |
ANA were found in 9% of patients with acute hepatitis E and in 24% of patients with chronic hepatitis E. Cryoglobulins were found in 7% and 27%, respectively. Cryoglobulinemia persisted for a median of 4 mo (range 3–15 mo). Patients with cryoglobulins had higher levels of creatinine, IgM, and HEV RNA in the blood. There were no clinical manifestations of cryoglobulinemia in all patients in this study except for a patient with neuralgic amyotrophy[112]. In another study, ANA was detected in 37% of patients with acute hepatitis E, and anti-SMA test was positive in 23% of them. These antibodies remained in the blood for more than 1 year in 37% of these cases. Moreover, no patients developed AIH during this follow-up period[113]. Similar results were published in the third study[114]. Seroprevalence of HEV in patients with AIH did not differ from that of the general population[115,116]. Another study showed that antibodies against HEV were detected slightly more often in AIH than the average for the population, but the detection rate was significantly lower than in the previous study[117]. In general, the relationship between HEV and AIH remains unclear.
Antibodies against HEV were not found in any of the 25 patients with PBC; thus, the relationship between these diseases is extremely unlikely[118].
ANCA was positive in 15% of patients with acute hepatitis E[114].
Most cases of glomerulonephritis and manifested cryoglobulinemia in hepatitis E have been described after transplantation[119,120]. Therefore, the exact cause of their development is unclear: HEV infection, the consequences of transplantation, or their combination. In any case, testing for HEV biomarkers should be performed in these patients. A cure for cryoglobulinemic membranoproliferative glomerulonephritis has been described in such a patient after treatment with ribavirin[121].
Thus far, only one case of HEV-associated cryoglobulinemic membranoproliferative glomerulonephritis in a non-transplanted person has been published. The condition appeared a month after the onset of acute hepatitis E. The activity of the inflammatory process in the liver remained elevated. This patient had RF in the blood and was successfully treated with plasmaphoresis and pulse glucocorticoids[122].
The only case of HSP triggered by acute hepatitis E virus infection has been described[123].
There are no published studies on the frequency of detection of HEV biomarkers in patients with uveitis or PAN.
There is a wide array of extrahepatic manifestations potentially associated with hepatitis viruses. These viruses can lead to the development of various immunological disorders (the formation of autoantibodies and cryoglobulins), which can manifest as glomerulonephritis, arthritis, uveitis, vasculitis, and other rheumatologic disorders. In addition, it is quite possible that this infection could be a trigger for the subsequent development of AIH and PBC.
The relationship between autoimmune liver diseases and hepatotropic virus infection is very interesting, especially when treated with antiviral drugs. A further study of this field would be useful to test for biomarkers of hepatotropic viruses in these diseases and to analyze the results of their treatment with antiviral drugs.
In addition to the manifestations described above, immunological disorders due to infection with hepatotropic viruses can lead to the development of many other autoimmune diseases (autoimmune thyroiditis, thrombocytopenia, hemolytic anemia, diabetes mellitus, pulmonary fibrosis, and others), the consideration of which is beyond the scope of this review.
These associations are best shown for chronic viral hepatitis B and C. Only isolated cases of these are described for hepatitis A. These links are least studied, and often controversial for hepatitis E, possibly due to its relatively rare diagnoses.
We have summarized the data presented in the review in Table 5.
| Hepatitis A | Hepatitis B | Hepatitis C | Hepatitis E | |
| Rheumatoid factor | Only 2 cases | Approximately 12% of patients | 15% of patients | 1 case in a patient without transplant |
| Antinuclear antibodies | In a case of lupus-like reaction and some cases of AIH triggered by HAV infection | Approximately 25% of patients | 20%-30% of patients | 9%-37% (acute) and 24% (chronic) of patients |
| Cryoglobulins | 95% of patients in 1 study | 2% of patients | 65% of patients | 7% (acute) and 27% (chronic) of patients |
| Arthralgia | 10%-20% of patients | 3% of patients | 42% of patients | Approximately 60% of patients |
| Arthritis | Only in patients with vasculitis (7 cases) | About a quarter of patients with pain in joints | 5%-10% of patients | 2 cases |
| GN | 4 cases of mesangio-proliferative GN | 3% of GN: membranous GN (40%); membrano-proliferative GN (20%); focal segmental glomerulo-sclerosis (20%); mesangio-proliferative GN (10%), others (10%) | Approximately 50% of GN: membranous GN (5%); membrano-proliferative GN (55%); focal segmental glomerulo-sclerosis (25%); mesangio-proliferative GN (20%), others (5%) | 1 case of membrano-proliferative GN; some cases of GN after transplantation |
| Henoch-Schonlein purpura | 7 cases | Some cases | Some cases | 1 case |
| CGV | 4 cases | 10% of non-HCV CGV cases | 90%-95% of CGV cases | Some cases |
| PAN | No cases | 35% of PAN cases | 20% of PAN cases | No cases |
| Uveitis | Some cases | 2% of patients | Some cases | No cases |
| AIH | 11 cases | 55% of AIH cases | Very rare | No cases |
| PBC | 1 case of overlap syndrome | 40% of PBC cases | Approximately 10% of PBC cases | No cases |
To date, few studies have been published on the effectiveness of modern DAA in the treatment of patients with rheumatologic and autoimmune manifestations of hepatotropic virus infection, which represents a large field for future research.
Nevertheless, patients with uveitis, glomerulonephritis, arthritis, vasculitis, autoimmune liver diseases should be tested for biomarkers of viral hepatitis, and if these infections are present, they should be treated.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fteiha B, Mohamed GA S-Editor: Zhang L L-Editor: A P-Editor: Liu JH
| 1. | Yin J, Redovich J. Kinetic Modeling of Virus Growth in Cells. Microbiol Mol Biol Rev. 2018;82:e00066-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110:163-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 263] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Getts DR, Chastain EM, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. 2013;255:197-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Panoutsakopoulou V, Cantor H. On the relationship between viral infection and autoimmunity. J Autoimmun. 2001;16:341-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Jara LJ, Medina G, Saavedra MA. Autoimmune manifestations of infections. Curr Opin Rheumatol. 2018;30:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Gallegos-Orozco JF, Rakela-Brödner J. Hepatitis viruses: not always what it seems to be. Rev Med Chil. 2010;138:1302-1311. [PubMed] [Cited in This Article: ] |
| 7. | Abbas Z, Afzal R. Life cycle and pathogenesis of hepatitis D virus: A review. World J Hepatol. 2013;5:666-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Jeong SH, Lee HS. Hepatitis A: clinical manifestations and management. Intervirology. 2010;53:15-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Routenberg JA, Dienstag JL, Harrison WO, Kilpatrick ME, Hooper RR, Chisari FV, Purcell RH, Fornes MF. Foodborne outbreak of hepatitis A: clinical and laboratory features of acute and protracted illness. Am J Med Sci. 1979;278:123-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171 Suppl 1:S15-S18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Koff RS. Clinical manifestations and diagnosis of hepatitis A virus infection. Vaccine. 1992;10 Suppl 1:S15-S17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Inman RD, Hodge M, Johnston ME, Wright J, Heathcote J. Arthritis, vasculitis, and cryoglobulinemia associated with relapsing hepatitis A virus infection. Ann Intern Med. 1986;105:700-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 78] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Shalit M, Wollner S, Levo Y. Cryoglobulinemia in acute type-A hepatitis. Clin Exp Immunol. 1982;47:613-616. [PubMed] [Cited in This Article: ] |
| 14. | Mathur RC, Mathur NC. Mesangial proliferative glomerulonephritis and nephrotic syndrome with hepatitis A virus infection. Indian Pediatr. 1996;33:1051-1053. [PubMed] [Cited in This Article: ] |
| 15. | McCann UG 2nd, Rabito F, Shah M, Nolan CR 3rd, Lee M. Acute renal failure complicating nonfulminant hepatitis A. West J Med. 1996;165:308-310. [PubMed] [Cited in This Article: ] |
| 16. | al-Homrany M. Immunoglobulin A nephropathy associated with hepatitis A virus infection. J Nephrol. 2001;14:115-119. [PubMed] [Cited in This Article: ] |
| 17. | Cheema SR, Arif F, Charney D, Meisels IS. IgA-dominant glomerulonephritis associated with hepatitis A. Clin Nephrol. 2004;62:138-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Aggarwal SP, Khurana SB, Sabharwal BD. Hepatitis A associated with myoglobinuria. Indian J Gastroenterol. 1996;15:107. [PubMed] [Cited in This Article: ] |
| 19. | Seo SR, Kim SS, Lee SJ, Kim TJ, Park YW, Lee SS. Adult-onset Still disease in a patient with acute hepatitis A. J Clin Rheumatol. 2011;17:444-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Bhatt G, Sandhu VS, Mitchell CK. A rare presentation of hepatitis a infection with extrahepatic manifestations. Case Rep Gastrointest Med. 2014;2014:286914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Dan M, Yaniv R. Cholestatic hepatitis, cutaneous vasculitis, and vascular deposits of immunoglobulin M and complement associated with hepatitis A virus infection. Am J Med. 1990;89:103-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Press J, Maslovitz S, Avinoach I. Cutaneous necrotizing vasculitis associated with hepatitis A virus infection. J Rheumatol. 1997;24:965-967. [PubMed] [Cited in This Article: ] |
| 23. | Nassih H, Bourrahouat A, Sab IA. Hepatitis A Virus Infection Associated with Cryoglobulinemic Vasculitis. Indian Pediatr. 2020;57:71-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Chemli J, Zouari N, Belkadhi A, Abroug S, Harbi A. [Hepatitis A infection and Henoch-Schonlein purpura: a rare association]. Arch Pediatr. 2004;11:1202-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Mohan N, Karkra S. Henoch schonlein purpura as an extra hepatic manifestation of hepatitis A. Indian Pediatr. 2010;47:448. [PubMed] [Cited in This Article: ] |
| 26. | Altinkaynak S, Ertekin V, Selimoglu MA. Association of Henoch-Schonlein purpura and hepatitis A. J Emerg Med. 2006;30:219-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Garty BZ, Danon YL, Nitzan M. Schoenlein-Henoch purpura associated with hepatitis A infection. Am J Dis Child. 1985;139:547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Sasan MS, Doghaee MA. Association of henoch-schoenlein purpura with hepatitis a. Iran J Pediatr. 2012;22:571-572. [PubMed] [Cited in This Article: ] |
| 29. | Islek I, Kalayci AG, Gok F, Muslu A. Henoch-Schönlein purpura associated with hepatitis A infection. Pediatr Int. 2003;45:114-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Bozaykut A, Atay E, Atay Z, Ipek IO, Akin M, Dursun E. Acute infantile haemorrhagic oedema associated with hepatitis A. Ann Trop Paediatr. 2002;22:59-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Morita M, Kitajima K, Yoshizawa H, Itoh Y, Iwakiri S, Shibata C, Mayumi M. Glomerulonephritis associated with arteritis in marmosets infected with hepatitis A virus. Br J Exp Pathol. 1981;62:103-113. [PubMed] [Cited in This Article: ] |
| 32. | Azimi A, Shirvani M, Hosseini S, Bazojoo V, Masihpoor N, Mohaghegh S, Sadeghi SM. Acute bilateral granulomatous anterior uveitis as an extra-hepatic manifestation of hepatitis A virus (HAV) infection: a case report. J Ophthalmic Inflamm Infect. 2020;10:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Tien PT, Lin CJ, Tsai YY, Chen HS, Hwang DK, Muo CH, Lin JM, Chen WL. Relationship Between Uveitis, Different Types Of Viral Hepatitis, And Liver Cirrhosis: A 12-Year Nationwide Population-Based Cohort Study. Retina. 2016;36:2391-2398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Segev A, Hadari R, Zehavi T, Schneider M, Hershkoviz R, Mekori YA. Lupus-like syndrome with submassive hepatic necrosis associated with hepatitis A. J Gastroenterol Hepatol. 2001;16:112-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | S-Are V, Yoder L, Samala N, Nephew L, Lammert C, Vuppalanchi R. An Outbreak Presents An Opportunity to Learn About A Rare Phenotype: Autoimmune Hepatitis After Acute Hepatitis A. Ann Hepatol. 2020;19:694-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Subramanian SK, Patel JM, Younes M, Nevah Rubin MI. Postinfectious Autoimmune Hepatitis-Induced Liver Failure: A Consequence of Hepatitis A Virus Infection. ACG Case Rep J. 2020;7:e00441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Grave A, Juel J, Vyberg M, Olesen SS, Hansen JB. [Autoimmune hepatitis preceded by hepatitis A]. Ugeskr Laeger. 2015;177:V12140669. [PubMed] [Cited in This Article: ] |
| 38. | Tanaka H, Tujioka H, Ueda H, Hamagami H, Kida Y, Ichinose M. Autoimmune hepatitis triggered by acute hepatitis A. World J Gastroenterol. 2005;11:6069-6071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Kim YD, Kim KA, Rou WS, Lee JS, Song TJ, Bae WK, Kim NH. [A case of autoimmune hepatitis following acute hepatitis A]. Korean J Gastroenterol. 2011;57:315-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Mikata R, Yokosuka O, Imazeki F, Fukai K, Kanda T, Saisho H. Prolonged acute hepatitis A mimicking autoimmune hepatitis. World J Gastroenterol. 2005;11:3791-3793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Huppertz HI, Treichel U, Gassel AM, Jeschke R, Meyer zum Büschenfelde KH. Autoimmune hepatitis following hepatitis A virus infection. J Hepatol. 1995;23:204-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Singh G, Palaniappan S, Rotimi O, Hamlin PJ. Autoimmune hepatitis triggered by hepatitis A. Gut. 2007;56:304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Bouyahia O, Naija O, Matoussi N, Khemiri M, Ben Mansour F, Khaldi F. [Autoimmune hepatitis following acute hepatitis A: a care report]. Tunis Med. 2008;86:87-88. [PubMed] [Cited in This Article: ] |
| 44. | Skoog SM, Rivard RE, Batts KP, Smith CI. Autoimmune hepatitis preceded by acute hepatitis A infection. Am J Gastroenterol. 2002;97:1568-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Heurgué A, Bernard-Chabert B, Picot R, Cadiot G, Thiéfin G. Overlap syndrome triggered by acute viral hepatitis A. Eur J Gastroenterol Hepatol. 2009;21:708-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Cacoub P, Saadoun D, Bourlière M, Khiri H, Martineau A, Benhamou Y, Varastet M, Pol S, Thibault V, Rotily M, Halfon P. Hepatitis B virus genotypes and extrahepatic manifestations. J Hepatol. 2005;43:764-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Shim CN, Hwang JW, Lee J, Koh EM, Cha HS, Ahn JK. Prevalence of rheumatoid factor and parameters associated with rheumatoid factor positivity in Korean health screening subjects and subjects with hepatitis B surface antigen. Mod Rheumatol. 2012;22:885-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Choi ST, Lee HW, Song JS, Lee SK, Park YB. Analysis of rheumatoid factor according to various hepatitis B virus infectious statuses. Clin Exp Rheumatol. 2014;32:168-173. [PubMed] [Cited in This Article: ] |
| 49. | Lim MK, Sheen DH, Lee YJ, Mun YR, Park M, Shim SC. Anti-cyclic citrullinated peptide antibodies distinguish hepatitis B virus (HBV)-associated arthropathy from concomitant rheumatoid arthritis in patients with chronic HBV infection. J Rheumatol. 2009;36:712-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Oliveira ÍMX, Silva RDSUD. Rheumatological Manifestations Associated with Viral Hepatitis B or C. Rev Soc Bras Med Trop. 2019;52:e20180407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Duffy J, Lidsky MD, Sharp JT, Davis JS, Person DA, Hollinger FB, Min KW. Polyarthritis, polyarteritis and hepatitis B. Medicine (Baltimore). 1976;55:19-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 156] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Raveendran N, Beniwal P, D'Souza AV, Tanwar RS, Kimmatkar P, Agarwal D, Malhotra V. Profile of glomerular diseases associated with hepatitis B and C: A single-center experience from India. Saudi J Kidney Dis Transpl. 2017;28:355-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Yazmalar L, Deveci Ö, Batmaz İ, İpek D, Çelepkolu T, Alpaycı M, Hattapoğlu E, Akdeniz D, Sarıyıldız MA. Fibromyalgia incidence among patients with hepatitis B infection. Int J Rheum Dis. 2016;19:637-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Li BA, Liu J, Hou J, Tang J, Zhang J, Xu J, Song YJ, Liu AX, Zhao J, Guo JX, Chen L, Wang H, Yang LH, Lu J, Mao YL. Autoantibodies in Chinese patients with chronic hepatitis B: prevalence and clinical associations. World J Gastroenterol. 2015;21:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Zhang Y, Shi Y, Wu R, Wang X, Gao X, Niu J. Primary biliary cholangitis is more severe in previous hepatitis B virus infection patients. Eur J Gastroenterol Hepatol. 2018;30:682-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Rigopoulou EI, Zachou K, Gatselis NK, Papadamou G, Koukoulis GK, Dalekos GN. Primary biliary cirrhosis in HBV and HCV patients: Clinical characteristics and outcome. World J Hepatol. 2013;5:577-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Chen XX, Xiang KH, Zhang HP, Kong XS, Huang CY, Liu YM, Lou JL, Gao ZH, Yan HP. Occult HBV infection in patients with autoimmune hepatitis: A virological and clinical study. J Microbiol Immunol Infect. 2020;53:946-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Le Guern V, Bienvenu B, Mouthon L, Guillevin L; French Vasculitis Study Group. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum. 2010;62:616-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 306] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 59. | Guillevin L, Mahr A, Callard P, Godmer P, Pagnoux C, Leray E, Cohen P; French Vasculitis Study Group. Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine (Baltimore). 2005;84:313-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 325] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 60. | Guillevin L, Lhote F, Jarrousse B, Bironne P, Barrier J, Deny P, Trepo C, Kahn MF, Godeau P. Polyarteritis nodosa related to hepatitis B virus. A retrospective study of 66 patients. Ann Med Interne (Paris). 1992;143 Suppl 1:63-74. [PubMed] [Cited in This Article: ] |
| 61. | Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C, Zignego AL. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 293] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 62. | Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, Pietrogrande M, Renoldi P, Bombardieri S, Bordin G. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM. 1995;88:115-126. [PubMed] [Cited in This Article: ] |
| 63. | Mazzaro C, Maso LD, Mauro E, Gattei V, Ghersetti M, Bulian P, Moratelli G, Grassi G, Zorat F, Pozzato G. Survival and Prognostic Factors in Mixed Cryoglobulinemia: Data from 246 Cases. Diseases. 2018;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Galli M, Oreni L, Saccardo F, Castelnovo L, Filippini D, Marson P, Mascia MT, Mazzaro C, Origgi L, Ossi E, Pietrogrande M, Pioltelli P, Quartuccio L, Scarpato S, Sollima S, Riva A, Fraticelli P, Zani R, Giuggioli D, Sebastiani M, Sarzi Puttini P, Gabrielli A, Zignego AL, Scaini P, Ferri C, De Vita S, Monti G. HCV-unrelated cryoglobulinaemic vasculitis: the results of a prospective observational study by the Italian Group for the Study of Cryoglobulinaemias (GISC). Clin Exp Rheumatol. 2017;35 Suppl 103:67-76. [PubMed] [Cited in This Article: ] |
| 65. | Mazzaro C, Dal Maso L, Urraro T, Mauro E, Castelnovo L, Casarin P, Monti G, Gattei V, Zignego AL, Pozzato G. Hepatitis B virus related cryoglobulinemic vasculitis: A multicentre open label study from the Gruppo Italiano di Studio delle Crioglobulinemie - GISC. Dig Liver Dis. 2016;48:780-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Mazzaro C, Dal Maso L, Visentini M, Gitto S, Andreone P, Toffolutti F, Gattei V. Hepatitis B virus-related cryogobulinemic vasculitis. The role of antiviral nucleot(s)ide analogues: a review. J Intern Med. 2019;286:290-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Kurokawa M, Hisano S, Ueda K. Hepatitis B virus and Schönlein-Henoch purpura. Am J Dis Child. 1985;139:861-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 68. | Maggiore G, Martini A, Grifeo S, De Giacomo C, Scotta MS. Hepatitis B virus infection and Schönlein-Henoch purpura. Am J Dis Child. 1984;138:681-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | Ergin S, Sanli Erdoğan B, Turgut H, Evliyaoğlu D, Yalçin AN. Relapsing Henoch-Schönlein purpura in an adult patient associated with hepatitis B virus infection. J Dermatol. 2005;32:839-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Stemerowicz R, Möller B, Lobeck H, Oertel J, Hopf U. [Schoenlein-Henoch purpura in chronic HBsAG-positive hepatitis]. Immun Infekt. 1988;16:12-15. [PubMed] [Cited in This Article: ] |
| 71. | Shin JI, Lee JS. Hepatitis B virus infection and Henoch-Schönlein purpura. J Dermatol. 2007;34:156; author reply 157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Wang S, Chen Y, Xu X, Hu W, Shen H, Chen J. Prevalence of hepatitis B virus and hepatitis C virus infection in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Oncotarget. 2017;8:102437-102445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Kuna L, Jakab J, Smolic R, Wu GY, Smolic M. HCV Extrahepatic Manifestations. J Clin Transl Hepatol. 2019;7:172-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 74. | Barkhuizen A, Rosen HR, Wolf S, Flora K, Benner K, Bennett RM. Musculoskeletal pain and fatigue are associated with chronic hepatitis C: a report of 239 hepatology clinic patients. Am J Gastroenterol. 1999;94:1355-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Orge E, Cefle A, Yazici A, Gürel-Polat N, Hulagu S. The positivity of rheumatoid factor and anti-cyclic citrullinated peptide antibody in nonarthritic patients with chronic hepatitis C infection. Rheumatol Int. 2010;30:485-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Liu FC, Chao YC, Hou TY, Chen HC, Shyu RY, Hsieh TY, Chen CH, Chang DM, Lai JH. Usefulness of anti-CCP antibodies in patients with hepatitis C virus infection with or without arthritis, rheumatoid factor, or cryoglobulinemia. Clin Rheumatol. 2008;27:463-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Cheng YT, Cheng JS, Lin CH, Chen TH, Lee KC, Chang ML. Rheumatoid factor and immunoglobulin M mark hepatitis C-associated mixed cryoglobulinaemia: an 8-year prospective study. Clin Microbiol Infect. 2020;26:366-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Fuentes A, Mardones C, Burgos PI. Understanding the Cryoglobulinemias. Curr Rheumatol Rep. 2019;21:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Ferucci ED, Choromanski TL, Varney DT, Ryan HS, Townshend-Bulson LJ, McMahon BJ, Wener MH. Prevalence and correlates of hepatitis C virus-associated inflammatory arthritis in a population-based cohort. Semin Arthritis Rheum. 2017;47:445-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Zuckerman E, Keren D, Rozenbaum M, Toubi E, Slobodin G, Tamir A, Naschitz JE, Yeshurun D, Rosner I. Hepatitis C virus-related arthritis: characteristics and response to therapy with interferon alpha. Clin Exp Rheumatol. 2000;18:579-584. [PubMed] [Cited in This Article: ] |
| 81. | Koga T, Kawashiri SY, Nakao K, Kawakami A. Successful ledipasvir + sofosbuvir treatment of active synovitis in a rheumatoid arthritis patient with hepatitis C virus-related mixed cryoglobulinemia. Mod Rheumatol. 2017;27:917-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Siloşi I, Boldeanu L, Biciuşcă V, Bogdan M, Avramescu C, Taisescu C, Padureanu V, Boldeanu MV, Dricu A, Siloşi CA. Serum Biomarkers for Discrimination between Hepatitis C-Related Arthropathy and Early Rheumatoid Arthritis. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Ezzat WM, Raslan HM, Aly AA, Emara NA, El Menyawi MM, Edrees A. Anti-cyclic citrullinated peptide antibodies as a discriminating marker between rheumatoid arthritis and chronic hepatitis C-related polyarthropathy. Rheumatol Int. 2011;31:65-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Amin K, Rasool AH, Hattem A, Al-Karboly TA, Taher TE, Bystrom J. Autoantibody profiles in autoimmune hepatitis and chronic hepatitis C identifies similarities in patients with severe disease. World J Gastroenterol. 2017;23:1345-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Chen HW, Huang HH, Lai CH, Chang WE, Shih YL, Chang WK, Hsieh TY, Chu HC. Hepatitis C virus infection in patients with primary biliary cirrhosis. Ann Hepatol. 2013;12:78-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | Floreani A, Baragiotta A, Leone MG, Baldo V, Naccarato R. Primary biliary cirrhosis and hepatitis C virus infection. Am J Gastroenterol. 2003;98:2757-2762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Bertolini E, Battezzati PM, Zermiani P, Bruno S, Moroni GA, Marelli F, Villa E, Manenti F, Zuin M, Crosignani A. Hepatitis C virus testing in primary biliary cirrhosis. J Hepatol. 1992;15:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 88. | Badiani RG, Becker V, Perez RM, Matos CA, Lemos LB, Lanzoni VP, Andrade LE, Dellavance A, Silva AE, Ferraz ML. Is autoimmune hepatitis a frequent finding among HCV patients with intense interface hepatitis? World J Gastroenterol. 2010;16:3704-3708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 89. | Dvir R, Sautto GA, Mancini N, Racca S, Diotti RA, Clementi M, Memoli M. Autoimmune hepatitis and occult HCV infection: A prospective single-centre clinical study. Autoimmun Rev. 2017;16:323-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | López Couceiro L, Gómez Domínguez E, Muñoz Gómez R, Castellano Tortajada G, Ibarrola de Andrés C, Fernández Vázquez I. Healing of autoimmune hepatitis associated with hepatitis C virus infection treated with direct-acting antivirals. Rev Esp Enferm Dig. 2019;111:159-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Sabry AA, Sobh MA, Irving WL, Grabowska A, Wagner BE, Fox S, Kudesia G, El Nahas AM. A comprehensive study of the association between hepatitis C virus and glomerulopathy. Nephrol Dial Transplant. 2002;17:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 92. | Castillo I, Martinez-Ara J, Olea T, Bartolomé J, Madero R, Hernández E, Bernis C, Aguilar A, Quiroga JA, Carreño V, Selgas R. High prevalence of occult hepatitis C virus infection in patients with primary and secondary glomerular nephropathies. Kidney Int. 2014;86:619-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Misiani R, Bellavita P, Fenili D, Borelli G, Marchesi D, Massazza M, Vendramin G, Comotti B, Tanzi E, Scudeller G. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117:573-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 374] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 94. | Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med. 1998;37:836-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 95. | Cao Y, Zhang Y, Wang S, Zou W. Detection of the hepatitis C virus antigen in kidney tissue from infected patients with various glomerulonephritis. Nephrol Dial Transplant. 2009;24:2745-2751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Nayak S, Kataria A, Sharma MK, Rastogi A, Gupta E, Singh A, Tiwari SC. Hepatitis C Virus-associated Membranoproliferative Glomerulonephritis Treated with Directly Acting Antiviral Therapy. Indian J Nephrol. 2018;28:462-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Shimada M, Nakamura N, Endo T, Yamabe H, Nakamura M, Murakami R, Narita I, Tomita H. Daclatasvir/asunaprevir based direct-acting antiviral therapy ameliorate hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis: a case report. BMC Nephrol. 2017;18:109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Obata F, Murakami T, Miyagi J, Ueda S, Inagaki T, Minato M, Ono H, Nishimura K, Shibata E, Tamaki M, Yoshimoto S, Kishi F, Kishi S, Matsuura M, Nagai K, Abe H, Doi T. A case of rapid amelioration of hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis treated by interferon-free directly acting antivirals for HCV in the absence of immunosuppressant. CEN Case Rep. 2017;6:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Ramos-Casals M, Muñoz S, Medina F, Jara LJ, Rosas J, Calvo-Alen J, Brito-Zerón P, Forns X, Sánchez-Tapias JM; HISPAMEC Study Group. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J Rheumatol. 2009;36:1442-1448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 100. | Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 101. | Lee YH, Ji JD, Yeon JE, Byun KS, Lee CH, Song GG. Cryoglobulinaemia and rheumatic manifestations in patients with hepatitis C virus infection. Ann Rheum Dis. 1998;57:728-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Saadoun D, Terrier B, Semoun O, Sene D, Maisonobe T, Musset L, Amoura Z, Rigon MR, Cacoub P. Hepatitis C virus-associated polyarteritis nodosa. Arthritis Care Res (Hoboken). 2011;63:427-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Carson CW, Conn DL, Czaja AJ, Wright TL, Brecher ME. Frequency and significance of antibodies to hepatitis C virus in polyarteritis nodosa. J Rheumatol. 1993;20:304-309. [PubMed] [Cited in This Article: ] |
| 104. | Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:835-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 105. | Mazzaro C, Mauro E, Ermacora A, Doretto P, Fumagalli S, Tonizzo M, Toffolutti F, Gattei V. Hepatitis C virus- related cryoglobulinemic vasculitis. Minerva Med. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 106. | Disdier P, Bolla G, Veit V, Ridings B, Gambarelli-Mouillac N, Harle JR, Weiller PJ. [Association of uveitis and hepatitis C. 5 cases]. Presse Med. 1994;23:541. [PubMed] [Cited in This Article: ] |
| 107. | European Association for the Study of the Liver. ; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 343] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 108. | Shinde N, Patil T, Deshpande A, Gulhane R, Patil M, Bansod Y. Clinical profile, maternal and fetal outcomes of acute hepatitis e in pregnancy. Ann Med Health Sci Res. 2014;4:S133-S139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 454] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 110. | Al-Shukri I, Davidson E, Tan A, Smith DB, Wellington L, Johannessen I, Ramalingam S. Rash and arthralgia caused by hepatitis E. Lancet. 2013;382:1856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 111. | Serratrice J, Disdier P, Colson P, Ene N, de Roux CS, Weiller PJ. Acute polyarthritis revealing hepatitis E. Clin Rheumatol. 2007;26:1973-1975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Horvatits T, Schulze Zur Wiesch J, Polywka S, Buescher G, Lütgehetmann M, Hussey E, Horvatits K, Peine S, Haag F, Addo MM, Lohse AW, Weiler-Normann C, Pischke S. Significance of Anti-Nuclear Antibodies and Cryoglobulins in Patients with Acute and Chronic HEV Infection. Pathogens. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Wu J, Guo N, Zhu L, Zhang X, Xiong C, Liu J, Xu Y, Fan J, Yu J, Pan Q, Yang J, Liang H, Jin X, Ye S, Wang W, Liu C, Zhang J, Li G, Jiang B, Cao H, Li L. Seroprevalence of AIH-related autoantibodies in patients with acute hepatitis E viral infection: a prospective case-control study in China. Emerg Microbes Infect. 2020;9:332-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Terziroli Beretta-Piccoli B, Ripellino P, Gobbi C, Cerny A, Baserga A, Di Bartolomeo C, Bihl F, Deleonardi G, Melidona L, Grondona AG, Mieli-Vergani G, Vergani D, Muratori L; Swiss Autoimmune Hepatitis Cohort Study Group. Autoimmune liver disease serology in acute hepatitis E virus infection. J Autoimmun. 2018;94:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 115. | Llovet LP, Gratacós-Ginés J, Ortiz O, Rodriguez-Tajes S, Lens S, Reverter E, Ruiz-Ortiz E, Costa J, Viñas O, Forns X, Parés A, Londoño MC. Higher seroprevalence of hepatitis E virus in autoimmune hepatitis: Role of false-positive antibodies. Liver Int. 2020;40:558-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | van Gerven NM, van der Eijk AA, Pas SD, Zaaijer HL, de Boer YS, Witte BI, van Nieuwkerk CM, Mulder CJ, Bouma G, de Man RA; Dutch Autoimmune Hepatitis Study Group. Seroprevalence of Hepatitis E Virus in Autoimmune Hepatitis Patients in the Netherlands. J Gastrointestin Liver Dis. 2016;25:9-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | Pischke S, Gisa A, Suneetha PV, Wiegand SB, Taubert R, Schlue J, Wursthorn K, Bantel H, Raupach R, Bremer B, Zacher BJ, Schmidt RE, Manns MP, Rifai K, Witte T, Wedemeyer H. Increased HEV seroprevalence in patients with autoimmune hepatitis. PLoS One. 2014;9:e85330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 118. | Le Cann P, Tong MJ, Werneke J, Coursaget P. Detection of antibodies to hepatitis E virus in patients with autoimmune chronic active hepatitis and primary biliary cirrhosis. Scand J Gastroenterol. 1997;32:387-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Bazerbachi F, Leise MD, Watt KD, Murad MH, Prokop LJ, Haffar S. Systematic review of mixed cryoglobulinemia associated with hepatitis E virus infection: association or causation? Gastroenterol Rep (Oxf). 2017;5:178-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 120. | Fousekis FS, Mitselos IV, Christodoulou DK. Extrahepatic manifestations of hepatitis E virus: An overview. Clin Mol Hepatol. 2020;26:16-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 121. | Del Bello A, Guilbeau-Frugier C, Josse AG, Rostaing L, Izopet J, Kamar N. Successful treatment of hepatitis E virus-associated cryoglobulinemic membranoproliferative glomerulonephritis with ribavirin. Transpl Infect Dis. 2015;17:279-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 122. | Guinault D, Ribes D, Delas A, Milongo D, Abravanel F, Puissant-Lubrano B, Izopet J, Kamar N. Hepatitis E Virus-Induced Cryoglobulinemic Glomerulonephritis in a Nonimmunocompromised Person. Am J Kidney Dis. 2016;67:660-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (2)] |
| 123. | Thapa R, Biswas B, Mallick D. Henoch-Schönlein purpura triggered by acute hepatitis E virus infection. J Emerg Med. 2010;39:218-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |