Published online May 7, 2021. doi: 10.3748/wjg.v27.i17.2039
Peer-review started: January 28, 2021
First decision: March 6, 2021
Revised: March 19, 2021
Accepted: April 21, 2021
Article in press: April 21, 2021
Published online: May 7, 2021
High-dose intravenous iron is an effective treatment option for iron deficiency (ID) or ID anaemia (IDA) in inflammatory bowel disease (IBD). However, treatment with ferric carboxymaltose (FCM) has been associated with the development of hypophosphatemia.
To investigate mechanisms behind the development of hypophosphatemia after intravenous iron treatment, and disclose symptoms and clinical manifestations related to hypophosphatemia short-term.
A prospective observational study of adult IBD patients with ID or IDA was conducted between February 1, 2017 and July 1, 2018 at two separate university hospitals in the southeast region of Norway. Patients received one dose of 1000 mg of either FCM or ferric derisomaltose (FDI) and were followed for an observation period of at least 7 wk. Blood and urine samples were collected for relevant analyses at baseline, week 2 and at week 6. Clinical symptoms were assessed at the same timepoints using a respiratory function test, a visual analogue scale, and a health-related quality of life questionnaire.
A total of 106 patients was available for analysis in this study. The FCM treatment group consisted of 52 patients and hypophosphatemia was present in 72.5% of the patients at week 2, and in 21.6% at week 6. In comparison, the FDI treatment group consisted of 54 patients and 11.3% of the patients had hypophosphatemia at week 2, and 3.7% at week 6. The difference in incidence was highly significant at both week 2 and 6 (P < 0.001 and P < 0.013, respectively). We observed a significantly higher mean concentration of intact fibroblast growth factor 23 (P < 0.001), a significant rise in mean urine fractional excretion of phosphate (P = 0.004), a significant decrease of 1,25-dihydroxyvitamin D (P < 0.001) and of ionised calcium levels (P < 0.012) in the FCM-treated patients compared with patients who received FDI. No clinical symptoms could with certainty be related to hypophosphatemia, since neither the respiratory function test, SF-36 (36-item short form health survey) or the visual analogue scale scores resulted in significant differences between patients who developed hypophosphatemia or not.
Fibroblast growth factor 23 has a key role in FCM induced hypophosphatemia, probably by inducing loss of phosphate in the urine. Short-term clinical impact of hypophosphatemia was not demonstrated.
Core Tip: High-dose intravenous iron is an effective treatment for iron deficiency anaemia (IDA) in inflammatory bowel disease (IBD). However, ferric carboxymaltose (FCM) is associated with development of hypophosphatemia. This study of adult IBD patients with IDA investigated the mechanisms and clinical manifestations related to hypophosphatemia after treatment of either FCM or ferric derisomaltose (FDI). The incidence of hypophosphatemia was significantly higher after FCM than FDI, and fibroblast growth factor 23 had a key role, inducing loss of phosphate in the urine along with a significant lowering of 1,25-dihydroxyvitamin D and ionised calcium levels. Short-term clinical impact was not demonstrated.
- Citation: Detlie TE, Lindstrøm JC, Jahnsen ME, Finnes E, Zoller H, Moum B, Jahnsen J. Hypophosphatemia after high-dose intravenous iron treatment in patients with inflammatory bowel disease: Mechanisms and possible clinical impact. World J Gastroenterol 2021; 27(17): 2039-2053
- URL: https://www.wjgnet.com/1007-9327/full/v27/i17/2039.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i17.2039
Iron replacement therapy is often needed in patients with inflammatory bowel disease (IBD) because iron deficiency (ID) and ID anaemia (IDA) occur frequently in this patient group[1-3]. A large proportion of IBD patients experience intolerance to oral iron[4]. Additionally, it is asserted that oral iron can lead to an exacerbation of inflammation in the bowel mucosa due to a local effect on the enterocytes[5-7]. Therefore, administration of high-dose iron as an intravenous infusion is an effective, suitable and convenient treatment option in IBD. Ferric carboxymaltose (FCM; Ferinject®; Vifor Pharma) and ferric derisomaltose (FDI), previously known as iron isomaltoside (Monofer®; Pharmacosmos A/S), are the most widely used preparations in Europe when high-dose intravenous iron is indicated.
In a recent publication, we described a high incidence of hypophosphatemia in IBD patients who had received treatment with FCM[8]. The mechanism behind the development of hypophosphatemia has been described by Wolf et al[9], but probably is not yet fully understood, and has not been investigated in patients with IBD. Fibroblast growth factor 23 (FGF23) is a small peptide hormone, synthesized by osteocytes, which regulates phosphate and vitamin D homeostasis[9]. FGF23 consists of a biologically-active component (full-length, intact FGF23) and inactive C-terminal fragments (C-terminal FGF23). FCM causes an increase in intact FGF23, which triggers the pathophysiological cascade of renal phosphate wasting, suppressed levels of 1,25-dihydroxyvitamin D, and secondary hyperparathyroidism[9]. In contrast, FDI does not appear to induce increased intact FGF23 levels, and is associated with a low incidence of hypophosphatemia[9].
Moderate to severe hypophosphatemia over time, as well as acute severe hypophosphatemia, can lead to serious complications, e.g., respiratory failure, haemolysis, left ventricular failure, and rhabdomyolysis[10-13]. Development of osteomalacia with pseudo-fractures has been found in patients with sustained hypophosphatemia [14-17]. However, there are uncertainties with regard to both the frequency of symptoms and the clinical impact of hypophosphatemia.
Reduced quality of life (QoL) is common and well-documented in IBD patients due to chronic inflammation in the gut and the occurrence of extra-intestinal manifesta
In this short-term study, we aimed to investigate the mechanisms causing the development of hypophosphatemia in IBD patients, with ID or IDA, who received one high-dose (1000 mg) infusion of iron. Moreover, we aimed to document symptoms and clinical manifestations related to hypophosphatemia.
This prospective observational study was conducted between February 1, 2017 and July 1, 2018. The study design and patient recruitment have previously been described in detail (Detlie et al[8]). In brief, adult IBD patients (> 18 years) diagnosed with ID or IDA (according to European Crohn’s and Colitis Organisation guidelines)[2] were recruited at two separate study sites in the southeast region of Norway and treated with either FCM or FDI.
Eligible patients were prescribed 1000 mg of high-dose intravenous iron, FCM (50 mg/mL) or iron derisomaltose (100 mg/mL), administered as a single dose. Patients who had received high-dose intravenous iron treatment or a packed red blood cell transfusion within 3 mo of study entry, or for whom high-dose intravenous iron treatment was contraindicated, were not included in the study.
Enrolment continued until at least 50 consecutive patients with complete adherence to the study protocol were recruited at each site (a total of more than 100 patients) (Supplementary Figure 1). The enrolment period was followed by a prospective observation period, which lasted ≤ 7 wk for each patient and included three study visits.
Study inclusion was performed at baseline, at which time intravenous iron treatment was administered. Patients attended the clinic at week 2 (10-15 d) and at week 6 (5-7 wk) following intravenous iron treatment. Each patient could receive only one infusion within an approximate 2-mo period after consenting to study participation.
Blood analysis at each study visit included ionised calcium, creatinine, phosphate, parathyroid hormone (PTH) and vitamin D (25-hydroxyvitamin D).
Blood samples were also frozen and sent to Medizinische Universität Innsbruck, Universitätsklinik für Innere Medizin I, for analysis of 1,25-dihydroxyvitamin D, intact and C-terminal FGF23. The Kainos FGF-23 ELISA Kit was used for the FGF23 analysis. The assay for intact FGF23 measures only full-length peptide, whereas the assay for C-terminal FGF23 measures full-length peptide and the C-terminal fragments thereby representing total FGF23.
Spot urine samples were collected at each study visit and analysed for urine phosphate and urine creatinine. A calculation of the fractional excretion of phosphate rate (FEPO4) was then performed using the formula, FEPO4 = (urine phosphate × plasma creatinine × 100)/(plasma phosphate × urine creatinine). Oslo University Hospital Ullevål used the Roche analysis method (Roche/Hitachi Cobas® C systems PHOS2 and CREP2) while Akershus University Hospital used the Vitros analysis (VITROS® MicroSlide Assay 5.1 FS Diluent Pack 3). The slight sensitivity difference between the two analytical methods was minimized by recalculating FEPO4 using the above-mentioned formula.
Symptoms that might be related to hypophosphatemia were assessed at each of the three study visits using the MicroRPMTM (CareFusion) test to determine respiratory muscle function by measuring maximum inspiratory and maximum expiratory pressure, a health-related QoL questionnaire (36-item short form health survey, SF-36), and a visual analogue scale (VAS).
For the MicroRPMTM respiratory function test, patients were asked to inhale and exhale as hard as possible. The test was repeated three times at every visit, and the best result of the three attempts was registered.
The SF-36 is a generic, self-administered questionnaire containing 36 items[20]. The items are divided into eight multi-item scales that reflect general health, physical functioning, role limitations due to physical problems, bodily pain, vitality, mental health, social functioning, and role limitations due to emotional problems. Each scale is transformed into a 1-100 scale, where a lower score represents more disability. The processing of raw SF-36 data into results was executed according to the SF-36 scoring algorithms[21].
The VAS is a 10 cm line on which the patient is asked to place a vertical mark to indicate the level of intensity of a symptom that best fits his or her experience. Scores range from 0-100 (mm) where a higher score represents greater symptom intensity. The VAS was used to assess general weakness, fatigue, joint pain, joint stiffness, muscle pain, bone and skeletal pain, and difficulties performing daily activities.
All demographic information was collected from patients’ medical records and was entered into an electronic case report form.
The study was completed when all enrolled patients had received intravenous iron administration, had attended all three study visits, and had fulfilled the requirements of the study protocol.
Serum phosphate, PTH, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, ionised calcium, creatinine, intact and C-terminal FGF23, and FEPO4 (urine phosphate and urine creatinine) were measured in order to assess possible mechanisms behind the development of hypophosphatemia after intravenous iron treatment. Results from the FCM treatment group were compared with results from the FDI treatment group.
Hypophosphatemia was defined as a serum phosphate level < 0.8 mmol/L (< 2.5 mg/dL). The clinical impact of hypophosphatemia was evaluated at week 2 and week 6 using the respiratory muscle function test, SF-36, and the VAS score. In relation to the assessment of clinical impact, the hypophosphatemia group was defined as patients experiencing hypophosphatemia at both week 2 and week 6. Results for patients with hypophosphatemia were compared with results for patients without hypophosphatemia, independent of treatment group.
This study was designed to achieve 80% power to detect a difference in the primary outcome, which was the incidence of hypophosphatemia (previously described by Detlie et al[8]). Hence, the MicroRPMTM respiratory test, SF-36, and VAS scores were not used to justify sample size.
Data are presented descriptively, as mean with SD or 95% confidence intervals for continuous variables, and as the number of exposed patients (with proportions) for categorical variables. Hypothesis tests for differences in change between treatment groups, change from baseline, and groups with or without hypophosphatemia, were conducted using paired t-tests. All analyses were performed in R. A P value of < 0.05 was considered significant.
The study protocol was approved by the relevant local regulatory and ethical committees and adhered to the applicable laws on data protection. A study registration application was sent to the EudraCT system with the application No. 2016-003476-41, but the application was deemed unnecessary since there were no indications of a medical intervention study.
All patients gave informed consent before inclusion into the study, and the study was performed in accordance with the principles for post-authorisation safety studies, according to Good Clinical Practice guidelines.
All biological material obtained from patients was destroyed after analysis, as were the frozen blood samples sent to the Medical University of Innsbruck.
Study nurses were blinded to the results of laboratory findings but, for safety reasons, the primary investigator at each study centre was not blinded.
Of the 130 patients screened for this study, 106 patients (52 patients at Oslo University Hospital Ullevål and 54 patients at Akershus University Hospital) were included in the analyses. Demographic and clinical characteristics of the patients have previously been described[8].
Data for serum phosphate, FEPO4, intact and C-terminal FGF23, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, ionised calcium, and PTH at baseline and at each study visit are shown in Table 1. A sub-analysis of the same data, stratified according to hypophosphatemia status (with/without) at week 2 and at week 6, is shown in Table 2.
| Analysis | High-dose intravenous iron | Baseline, mean ± SD | Week 2, mean ± SD | Difference from baseline at week 2 | Difference between FCM and FDI at week 2 (95%CI) | P value | Week 6, mean ± SD | Difference from baseline at week 6 | Difference between FCM and FDI at week 6 (95%CI) | P value |
| Serum phosphate, mmol/L (ref. value 0.8-1.65) | FCM | 1.07 ± 0.2 | 0.65 ± 0.2 | -0.417 | -0.344 (-0.427 to -0.260) | < 0.001 | 1.00 ± 0.3 | -0.072 | -0.070 (-0.144 to 0.004) | 0.064 |
| FDI | 1.15 ± 0.2 | 1.07 ± 0.2 | -0.073 | 1.14 ± 0.2 | -0.002 | |||||
| Intact FGF23, pg/mL (ref. value 11.50-48.90) | FCM | 43.42 ± 14.2 | 91.61 ± 63.8 | 49.205 | 45.312 (25.982 to 64.697) | < 0.001 | 44.79 ± 23.1 | 1.718 | 1.559 (-6.407 to 9.525) | 0.698 |
| FDI | 43.88 ± 14.5 | 47.77 ± 22.1 | 3.892 | 44.04 ± 16.6 | 0.159 | |||||
| C-terminal FGF23, pmol/L (ref. value 0.30-3.00) | FCM | 2.46 ± 3.2 | 1.68 ± 1.3 | -0.756 | 6.783 (-1.319 to 14.885) | 0.099 | 0.94 ± 1.2 | -1.507 | 5.124 (-1.310 to 11.558) | 0.116 |
| FDI | 8.87 ± 30.6 | 1.33 ± 1.4 | -7.539 | 2.24 ± 7.6 | -6.632 | |||||
| FEPO4, % (ref. value N/A) | FCM | 9.95 ± 5.8 | 18.70 ± 10.8 | 9.946 | 5.375 (1.801 to 8.95) | 0.004 | 13.68 ± 11.3 | 4.210 | 3.326 (-0.309 to 6.96) | 0.072 |
| FDI | 12.55 ± 5.9 | 17.03 ± 8.6 | 4.570 | 13.37 ± 6.0 | 0.884 | |||||
| PTH, pmol/L (ref. value 1.5-7.0) | FCM | 5.46 ± 2.6 | 7.02 ± 3.4 | 1.608 | 0.442 (-0.561 to 1.445) | 0.384 | 5.97 ± 3.3 | 0.767 | 0.590 (-0.358 to 1.539) | 0.220 |
| FDI | 5.51 ± 2.6 | 6.72 ± 3.4 | 1.166 | 5.69 ± 2.3 | 0.176 | |||||
| Ionised calcium, mmol/L (ref. value 1.16-1.32) | FCM | 1.21 ± 0.0 | 1.20 ± 0.0 | -0.015 | -0.020 (-0.035 to -0.004) | 0.012 | 1.21 ± 0.0 | 0.000 | -0.018 (-0.036 to -0.001) | 0.044 |
| FDI | 1.23 ± 0.0 | 1.23 ± 0.1 | 0.005 | 1.25 ± 0.0 | 0.018 | |||||
| 25-hydroxyvitamin D, nmol/L (ref. value 50-125) | FCM | 58.35 ± 24.4 | 57.13 ± 23.1 | -1.212 | -2.133 (-6.238 to 1.972) | 0.305 | 57.48 ± 20.8 | -0.865 | -0.160 (-7.078 to 6.759) | 0.964 |
| FDI | 63.51 ± 21.9 | 64.63 ± 20.0 | 0.922 | 62.75 ± 21.1 | -0.706 | |||||
| 1,25-dihydroxyvitamin D, ng/L (ref. value 20-79) | FCM | 51.10 ± 19.2 | 28.77 ± 17.9 | -21.074 | -16.463 (-24.487 to -8.438) | < 0.001 | 53.78 ± 20.2 | 3.218 | 5.357 (-3.164 to 13.878) | 0.215 |
| FDI | 52.85 ± 20.4 | 48.24 ± 17.7 | -4.611 | 50.71 ± 19.8 | -2.139 |
| Analysis | High-dose intravenous iron | Week 2 serum phosphate ≥ 0.8 mmol/L, mean ± SD | Week 2 serum phosphate < 0.8 mmol/L, mean ± SD | Difference | P value | Week 6 serum phosphate ≥ 0.8 mmol/L, mean ± SD | Week 6 serum phosphate < 0.8 mmol/L, mean ± SD | Difference | P value |
| Intact FGF23, pg/mL (ref. value 11.50-48.90) | FCM | 33.71 ± 10.56 (n = 14/51) | 114.33 ± 62.22 (n = 37/51) | 80.62 | < 0.001 | 39.30 ± 16.79) (n = 40/51) | 66.86 ± 30.05 (n = 11/51) | 27.56 | 0.013 |
| FDI | 45.82 ± 16.90 (n = 47/53) | 57.16 ± 46.35 (n = 6/53) | 11.43 | 0.577 | 43.85 ± 16.83 (n = 52/54) | 48.93 ± 10.13 (n = 2/54) | 5.08 | 0.604 | |
| C-terminal FGF23, pmol/L (ref. value 0.30-3.00) | FCM | 1.03 ± 0.99 (n = 14/51) | 1.93 ± 1.33 (n = 37/51) | 0.9 | 0.014 | 0.76 ± 0.63 (n = 40/51) | 1.64 ± 2.14 (n = 11/51) | 0.88 | 0.206 |
| FDI | 1.36 ± 1.47 (n = 47/53) | 1.08 ± 0.72 (n = 6/53) | -0.28 | 0.465 | 2.27 ± 7.79 (n = 52/54) | 1.31 ± 0.33 (n = 2/54) | -0.97 | 0.384 | |
| FEPO4, % (ref. value N/A) | FCM | 13.35 ± 5.94 (n = 12/46) | 20.59 ± 11.60 (n = 34/46) | 7.24 | 0.009 | 9.62 ± 6.56 (n = 36/47) | 26.99 ± 13.38 (n = 11/47) | 17.37 | 0.001 |
| FDI | 15.97 ± 7.85 (n = 46/52) | 25.11 ± 10.22 (n = 6/52) | 9.14 | 0.080 | 13.00 ± 5.79 (n = 52/54) | 22.85 ± 4.41 (n = 2/54) | 9.85 | 0.176 | |
| PTH, pmol/L (ref. value 1.5-7.0) | FCM | 5.46 ± 2.83 (n = 14/48) | 7.46 ± 3.23 (n = 34/48) | 2 | 0.042 | 5.22 ± 2.39 (n = 39/47) | 9.93 ± 4.43) (n = 8/47) | 4.71 | 0.020 |
| FDI | 6.28 ± 2.97 (n = 47/53) | 10.13 ± 4.70 (n = 6/53) | 3.85 | 0.102 | 5.66 ± 2.32 (n = 52/54) | 6.40 ± 1.70 (n = 2/54) | 0.74 | 0.649 | |
| Ionised calcium, mmol/L (ref. value 1.16-1.32) | FCM | 1.21 ± 0.32 (n = 13/50) | 1.19 ± 0.05 (n = 37/50) | -0.02 | 0.336 | 1.21 ± 0.05 (n = 40/51) | 1.22 ± 0.04 (n = 11/51) | 0.01 | 0.496 |
| FDI | 1.24 ± 0.45 (n = 46/52) | 1.21 ± 0.09 (n = 6/52) | -0.03 | 0.422 | 1.25 ± 0.05 (n = 51/53) | 1.23 ± 0.02 (n = 2/53) | -0.02 | 0.314 | |
| 25-hydroxyvitamin D, nmol/L (ref. value 50-125) | FCM | 63.00 ± 30.94 (n = 14/51) | 54.54 ± 19.69 (n = 37/51) | -8.46 | 0.354 | 60.05 ± 21.68 (n = 40/51) | 45.45 ± 10.00 (n = 11/51) | -14.6 | 0.002 |
| FDI | 64.16 ± 20.30 (n = 45/51) | 64.83 ± 18.93 (n = 6/51) | 0.67 | 0.937 | 63.68 ± 20.97 (n = 50/52) | 39.50 ± 2.12 (n = 2/52) | -24.18 | < 0.001 | |
| 1,25-dihydroxyvitamin D, ng/L (ref. value 20-79) | FCM | 46.34 ± 19.10 (n = 14/50) | 21.46 ± 11.63 (n = 36/50) | -24.88 | < 0.001 | 56.76 ± 20.27 (n = 40/50) | 40.89 ± 15.95 (n = 10/50) | -15.87 | 0.016 |
| FDI | 49.05 ± 18.52 (n = 47/53) | 45.72 ± 7.15 (n = 6/53) | -3.33 | 0.414 | 50.88 ± 19.92 (n = 52/54) | 46.45 ± 21.85 (n = 2/54) | -4.43 | 0.822 |
As previously described, following treatment with FCM, hypophosphatemia was present in 72.5% (37/51) of patients at week 2, and in 21.6% (11/51) of patients at week 6. In comparison, in the FDI treatment group, 11.3% (6/53) of patients had hypophosphatemia at week 2, and 3.7% (2/54) at week 6. There were no new incidences of hypophosphatemia at week 6. The difference in incidence was highly significant at both week 2 and 6 (P < 0.001 and P < 0.013, respectively)[8]. These findings are consistent with the mean urine FEPO4 that was significantly (P = 0.004) higher at week 2 in the FCM treatment group compared with the FDI treatment group, and still elevated (though declining) at week 6 in the FCM group (Table 1 and Figure 1A). In the sub-analysis, the FDI-treated patients with hypophosphatemia (n = 6) had numerically increased FEPO4 (Table 2).
Patients in both treatment groups without hypophosphatemia at week 2 also experienced an increase in FEPO4 at week 2 compared to baseline values, but urinary phosphate excretion declined again at week 6 in these patients.
There was a significant (P < 0.001) increase in intact FGF23 from baseline to week 2 after infusion of FCM, compared with the FDI treatment group (Table 1 and Figure 1B). At week 6, intact FGF23 values in the FCM treatment group had returned close to baseline. In comparison, after FDI treatment no such increases were found (Figure 1B). At baseline, the serum concentration of C-terminal FGF23 was higher in the FDI treatment group than in the FCM treatment group (Table 1), and declined after FDI infusion (Figure 1C). This high value at baseline was not seen in the FCM treatment group (Table 1), which is probably compatible with the less severe ID/IDA seen in the FCM group[8].
In the sub-analysis, for the FCM-treated patients with hypophosphatemia, intact FGF23 was significantly increased compared with FCM-treated patients without hypophosphatemia at week 2 and at week 6 (Table 2). In the FCM-treated patients who had normal phosphate, intact FGF23 was not increased at week 2 or week 6.
For FDI-treated patients, the sub-analysis showed that there was no significant difference in mean intact FGF23 Levels between patients with/without hypophospha
There were no significant differences between the treatment groups in the concentration of 25-hydroxyvitamin D throughout the study period (Figure 1D). However, the sub-analysis showed that 25-hydroxyvitamin D concentrations were lower at week 6 in the two FDI-treated patients with hypophosphatemia when compared with baseline concentrations within the same group (Tables 1 and 2). At week 2, 1,25-dihydroxyvitamin D concentrations were significantly lower in patients who received FCM compared with patients who received FDI (Table 1). In the FCM-treatment group, the mean concentration of 1,25-dihydroxyvitamin D returned to baseline at week 6 (Table 1 and Figure 1E). However, the sub-analysis revealed that, for the FCM-treated patients with hypophosphatemia, low 1,25-dihydroxyvitamin D levels persisted at week 6 (Table 2). In the subgroups of patients without hypophosphatemia, 1,25-dihydroxyvitamin D levels were relatively unchanged.
In our cohort, we identified 36 patients (34.0%) with vitamin D deficiency (25-hydroxyvitamin D < 50 nmol/L) at baseline; 10 of these patients had severe vitamin D deficiency (25-hydroxyvitamin D < 30 nmol/L). The distribution of these patients was equal in the two treatment groups, as well as equally distributed across disease states – ulcerative colitis and Crohn’s disease. Moreover, we found no association between low levels of vitamin D and development of hypophosphatemia.
Ionised calcium values dropped significantly from baseline to week 2 in the FCM treatment group compared with the FDI treatment group (P < 0.012) but stayed within normal range. The mean values in the FCM group had increased by week 6, but the between-group difference was still significant (P < 0.044). Calcium values remained stable throughout the study in the FDI treatment group (Figure 1F), and in the subgroup of FCM-treated patients who did not develop hypophosphatemia. The sub-analysis showed that there was a numerically lower level of ionised calcium in the FDI-treated patients with hypophosphatemia than in the FDI-treated patients without hypophosphatemia (Table 2).
PTH values were elevated (> 7 pmol/L) in 28 patients (26.4%) at baseline; the distribution was similar between treatment groups. PTH concentrations were similar between treatment groups at baseline, and no significant between-group differences were observed in mean PTH concentrations at week 2, and at week 6 (Table 1). PTH values increased in both treatment groups at week 2 and decreased again at week 6 (Figure 1G). The sub-analysis indicated that the increase in PTH in both treatment groups was mainly driven by the patients who developed hypophosphatemia, with significant differences at week 2 and week 6 for the FCM-treated patients with hypophosphatemia compared to FCM-treated patients without hypophosphatemia (Table 2).
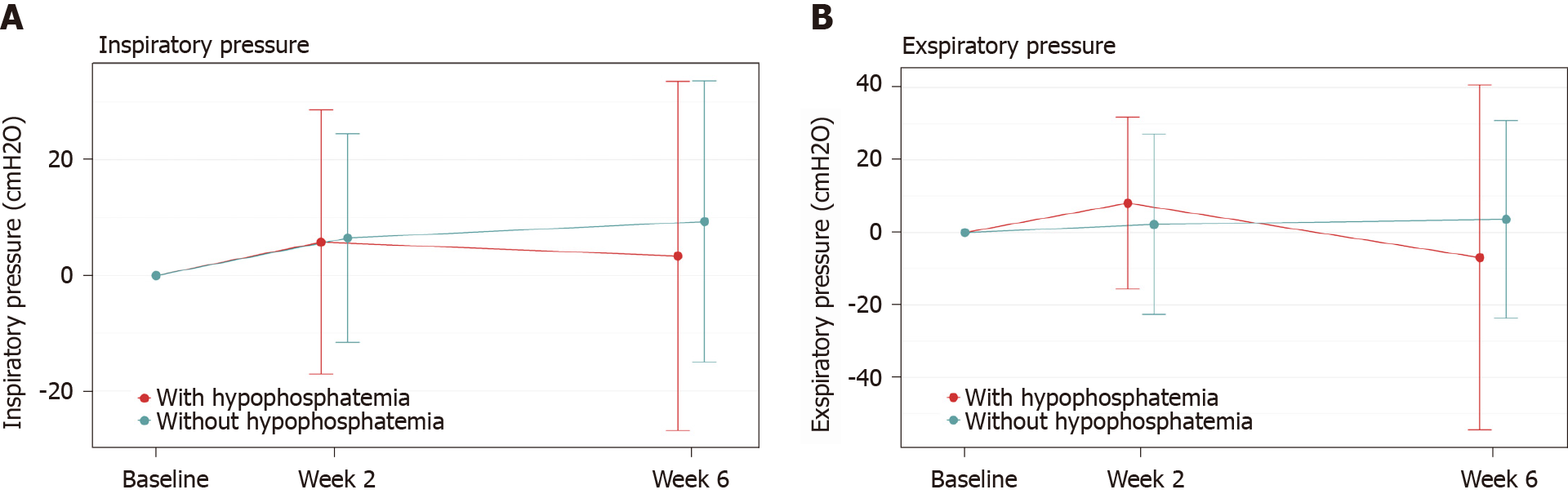
In the comparison of patients who developed hypophosphatemia vs those who did not develop hypophosphatemia, independent of treatment group, no significant differences were observed in the respiratory muscle function test results. The differences between patients with hypophosphatemia and those with normal phosphate values were minimal and the standard deviation was wide in both groups (Figure 2).
The results of the SF-36 QoL assessment are presented in Table 3. Overall, there were no significant differences between patient groups with or without hypophosphatemia at baseline and at any time point during the study. The mean scores at baseline in both treatment groups were generally low.
| SF-36 scale item | Normal phosphate population, baseline, mean ± SD | Hypophosphatemia population, baseline, mean ± SD | Difference1 at baseline, mean (95%CI) | Normal phosphate population, change at week 2, mean ± SD | Hypophosphatemia population, change at week 2, mean ± SD | Difference1 at week 2, mean (95%CI) | Normal phosphate population, change at week 6, mean ± SD | Hypophosphatemia population, change at week 6, mean ± SD | Difference1 at week 6, mean (95%CI) |
| General health | 50.91 ± 20.1 | 48.50 ± 26.1 | 2.4 (-14.5 to 19.4) | -0.45 ± 12.9 | 0.75 ± 12.2 | -1.2 (-9.2 to 6.8) | 3.06 ± 16.3 | 3.25 ± 15.1 | -0.2 (-10.2 to 9.8) |
| Physical functioning | 78.72 ± 22.4 | 86.11 ± 21.1 | -7.4 (-21.3 to 6.6) | 2.63 ± 10.9 | -0.28 ± 6.3 | 2.9 (-1.6 to 7.4) | 3.97 ± 14.5 | -0.28 ± 4.7 | 4.2 (0.2 to 8.3) |
| Role limitations due to physical problems | 47.61 ± 42.8 | 66.67 ± 40.4 | -19.1 (-45.7 to 7.6) | -4.08 ± 28.1 | -6.25 ± 18.8 | 2.2 (-10.8 to 15.1) | 11.32 ± 39.2 | 2.08 ± 16.7 | 9.2 (-3.7 to 22.1) |
| Bodily pain | 65.65 ± 25.1 | 67.17 ± 27.6 | -1.5 (-19.6 to 16.5) | 0.68 ± 18.9 | 4.42 ± 12.2 | -3.7 (-12.2 to 4.7) | 3.40 ± 19.8 | 10.83 ± 19.1 | -7.4 (-20.0 to 5.2) |
| Vitality | 37.98 ± 22.0 | 40.00 ± 22.4 | -2.0 (-16.7 to 12.7) | 4.05 ± 13.1 | 7.50 ± 15.9 | -3.4 (-13.8 to 6.9) | 10.89 ± 18.0 | 12.92 ± 16.0 | -2.0 (-12.7 to 8.6) |
| Mental health | 71.72 ± 18.9 | 70.33 ± 16.9 | 1.4 (-9.9 to 12.6) | 3.37 ± 9.0 | 4.33 ± 5.0 | -1.0 (-4.5 to 2.6) | 4.89 ± 13.2 | 1.67 ± 11.1 | 3.2 (-4.2 to 10.7) |
| Social functioning | 68.35 ± 26.3 | 68.75 ± 30.4 | -0.4 (-20.2 to 19.4) | 3.86 ± 16.7 | 7.29 ± 13.5 | -3.4 (-12.5 to 5.6) | 8.70 ± 20.7 | 7.29 ± 15.5 | 1.4 (-9.1 to 11.9) |
| Role limitations due to emotional problems | 67.91 ± 41.5 | 72.22 ± 42.2 | -4.3 (-32.1 to 23.4) | -2.66 ± 34.8 | -8.33 ± 37.9 | 5.7 (-19.1 to 30.5) | 6.52 ± 38.0 | -2.78 ± 26.4 | 9.3 (-8.8 to 27.4) |
There were no significant differences in VAS scores between the groups of patients with/without hypophosphatemia at week 2 and at week 6 (Table 4). Overall, VAS scores were elevated at baseline. However, the group of patients who developed hypophosphatemia had lower VAS scores at baseline for the items joint pain, muscle pain, and bone and skeletal pain, compared to the group of patients who did not develop hypophosphatemia; between-group differences were not significant for these items. There was, however, a significant between-group difference (P < 0.001) at baseline for the VAS joint stiffness item score, with lower values in the group of patients who developed hypophosphatemia.
| VAS item | Normal phosphate group, baseline, mean ± SD | Hypophosphatemia group, baseline, mean ± SD | Difference1 at baseline, mean (95%CI) | P value | Normal phosphate group, change at week 2, mean ± SD | Hypophosphatemia group, change at week 2, mean ± SD | Difference1 at week 2, mean (95%CI) | P value | Normal phosphate group, change at week 6, mean ± SD | Hypophosphatemia group, change at week 6, mean ± SD | Difference1 at week 6, mean (95%CI) | P value |
| General weakness | 43.54 ± 28.7 | 31.17 ± 30.5 | 12.4 (-7.6 to 32.4) | 0.205 | -4.98 ± 14.9 | -1.50 ± 17.2 | -3.5 (-14.7 to 7.7) | 0.515 | -10.64 ± 21.9) | -6.33 ± 25.7 | -4.3 (-21.1 to 12.5) | 0.589 |
| Fatigue | 37.53 ± 30.0 | 29.58 ± 30.1 | 7.9 (-11.8 to 27.7) | 0.403 | -3.31 ± 19.4 | -1.33 ± 17.9 | -2.0 (-13.9 to 9.9) | 0.728 | -8.93 ± 22.0 | -5.17 ± 26.7 | -3.8 (-21.2 to 13.6) | 0.648 |
| Joint pain | 17.30 ± 23.3 | 8.25 ± 14.8 | 9.1 (-1.2 to 19.3) | 0.081 | 0.32 ± 14.3 | -3.09 ± 6.9 | 3.4 (-1.9 to 8.7) | 0.197 | 2.17 ± 18.0 | 0.17 ± 7.4 | 2.0 (-3.8 to 7.8) | 0..489 |
| Joint stiffness | 13.31 ± 22.3 | 1.42 ± 3.4 | 11.9 (6.9 to 16.8) | < 0.001 | -0.57 ± 18.1 | -0.64 (2.1) | 0.1 (-3.9 to 4.0) | 0.972 | 0.99 ± 17.7 | 1.75 ± 8.5 | -0.8 (-7.1 to 5.6) | 0.807 |
| Muscle pain | 15.35 ± 23.1 | 5.83 ± 14.5 | 9.5 (-0.5 to 19.6) | 0.062 | -1.71 ± 14.6 | 2.45 (8.9) | -4.2 (-10.7 to 2.3) | 0.194 | -0.50 ± 20.6 | -0.17 ± 11.0 | -0.3 (-8.3 to 7.6) | 0.932 |
| Bone and skeletal pain | 7.24 ± 19.4 | 1.92 ± 6.6 | 5.3 (-0.3 to 10.9) | 0.061 | 1.85 ± 20.2 | 2.64 (8.7) | -0.8 (-7.7 to 6.1) | 0.817 | 3.42 ± 18.7 | -1.67 ± 6.8 | 5.1 (-0.5 to 10.7) | 0.076 |
| Difficulties performing daily activities | 34.38 ± 28.5 | 29.67 ± 29.7 | 4.7 (-14.8 to 24.2) | 0.611 | -3.80 ± 14.9 | -2.17 (23.5) | -1.6 (-16.8 to 13.5) | 0.819 | -9.45 ± 20.0 | -11.00 ± 24.8 | 1.6 (-14.6 to 17.7) | 0.839 |
Our study indicates that FGF23 plays an important role in the development of hypophosphatemia in IBD patients treated with FCM. In these patients, a high level of intact FGF23, an increased excretion of phosphate in the urine, a decrease of 1,25-dihydroxyvitamin D and of serum calcium levels, and a slight elevation of PTH, was demonstrated.
Previous clinical trials of FCM have shown similar results[9,22]. However, for the most part, these studies have been conducted in healthy and, predominantly, female populations. The role of FGF23 has also been described in earlier publications[23-26]. Regulation of phosphate concentrations in the body seems to be strongly influenced by intact FGF23, which reduces phosphate reabsorption in the proximal tubules in the kidneys and inhibits production of 1,25-dihydroxyvitamin D, probably by inhibiting the activity of the enzyme 25-hydroxyvitamin D-1a-hydroxylase and increased expression of 24-hydroxylase[24,26]. Our findings suggest that FCM could have a direct impact on cleavage of FGF23, resulting in a high level of intact FGF23 and consequent phosphate wasting. This might also explain why baseline phosphate level does not predict the development of mild or severe hypophosphatemia, due to the inappropriate excretion of available phosphate in the urine, following FCM treatment[8]. We also observed a decrease in 1,25-dihydroxyvitamin D (the active vitamin D metabolite), a decrease in ionised calcium, and development of secondary hyperparathyroidism. This might explain why some patients treated with FCM still had hypophosphatemia six weeks after treatment, when the intact FGF23 values had normalized (Table 2) since elevated PTH promotes excretion of phosphate in the urine[9,27,28].
The majority of patients in the FCM treatment group developed hypophosphatemia at week 2. The remaining patients did not develop hypophosphatemia and had unchanged levels of intact FGF23. So, there is a clear association between the development of high levels of intact FGF23 and hypophosphatemia. Therefore, it can only be speculated that there might be some individual factors related to the handling of FCM that cause the majority of patients treated with FCM to develop hypophosphatemia, whereas others do not. Neither is it known if any individual patient would develop hypophosphatemia on subsequent administrations of FCM, or if the effect of FCM treatment on phosphate wasting is indiscriminate. Perhaps some patients are protected against the influence of FCM on the enzyme responsible for FGF23 protein cleavage. From our results, we postulate that the mechanism of FCM-induced hypophosphatemia is not related to IBD; instead, it appears to be independently connected to the drug itself.
A few patients who received treatment with FDI also developed hypophosphatemia but, unlike those receiving FCM, these patients did not on average have significantly elevated intact FGF23 Levels when assessed at week 2, which would suggest a different underlying mechanism. A transient increase in intact FGF23 during the first 2 wk in patients experiencing hypophosphatemia cannot be ruled out, as data were not collected during this time period. A numerical increase in PTH was observed at week 2 along with decreased ionised calcium, and decreased 25-hydroxyvitamin D at week 6. It is not clear whether these observations are the result of a transient increase of intact FGF23 during the first 2 wk, or solely a physiological response to a rapid correction of ID, or simply an artefact due to the low numbers of FDI patients who developed hypophosphatemia. The general physiological response of mineral metabolism markers to rapid ID correction is not fully elucidated and is an area of further research.
An important observation is that 34% of the study population was vitamin D deficient at baseline with 25-hydroxyvitamin D values < 50 nmol/L and, perhaps more interestingly, 24% of the patients had PTH values compatible with secondary hyperparathyroidism. These findings were equally distributed between the two treatment groups. This disturbance in vitamin D metabolism is unlikely to be a consequence of previous iron infusions since no patients received high-dose intravenous iron treatment during the 6 mo prior to inclusion in this study. The high prevalence of vitamin D deficiency at baseline is in agreement with previous studies of patients with IBD[29]. However, it is important to note that, in our study, many of the samples were taken during the winter months when sun exposure is reduced in Norway, and individuals could therefore be expected to be somewhat vitamin D deficient during this time. Nevertheless, this finding is important since both hypophosphatemia and vitamin D deficiency can contribute to the development of metabolic bone disease, including osteomalacia.
Guidelines regarding hypophosphatemia diagnosis, treatment, and follow-up are available, but the possible risk or incidence rate of developing hypophosphatemia with symptoms or complications are rarely mentioned[30]. A risk of developing respiratory failure, rhabdomyolysis, and left ventricular failure due to severe hypophosphatemia has been reported in case series[10]. More recent data also predict an increased risk of developing osteomalacia, especially in long-standing hypophosphatemia[14,15]. What is less well known is the number of patients developing more subtle, but identifiable, symptoms related to hypophosphatemia that are experienced as troublesome and might influence QoL.
With respect to the clinical impact of hypophosphatemia, measuring forced inspiratory and expiratory respiratory pressure can be used as a proxy to assess the physical effect of hypophosphatemia on skeletal and proximal muscles. There are no specific questionnaires available to evaluate the clinical impact of hypophosphatemia. The SF-36 is, however, one of the most commonly applied QoL questionnaires used world-wide in health surveys. Additionally, the VAS score can be used as a general assessment of impact of symptoms, such as fatigue, general weakness, bone and skeletal pain, and joint and muscle conditions. In our study, these three methods were applied to assess clinical impact in patients who developed hypophosphatemia compared to those who did not develop hypophosphatemia. All three methods failed to demonstrate significant differences in clinical impact following one administration of high-dose intravenous iron in this short-term study.
We hypothesize several reasons that might explain these results. In addition to the fact that a type II error cannot be excluded, it can be speculated that the positive effect of the correction of ID or IDA plays a more important role than any short-term negative clinical impact of hypophosphatemia and, hence, the effects of hypophospha
The already affected baseline recordings in SF-36 and the VAS score should not go unnoticed. These findings mirror previous studies of IBD populations[31], and reflect the reduced QoL and the intensity of symptoms that these patients experience in general. Finally, the fact that we did not detect clinical consequences in patients who developed hypophosphatemia suggests that, in order to detect overt symptoms and complications, the population size needs to be larger than our sample, as one might expect such complications to be relatively rare. Hence, this needs to be taken into account when considering the expectation of finding significant changes in the clinical outcomes in this study.
In summary, our study has implicated the small peptide hormone FGF23 in the development of hypophosphatemia in IBD patients treated with FCM. An increase in intact FGF23 occurs, which probably results in phosphate wasting in the urine. Assessment of symptoms did not exclude, nor did they demonstrate, any short-term clinical impact of hypophosphatemia in IBD patients treated for ID or IDA with high-dose intravenous iron.
High-dose intravenous iron is an effective and frequently used treatment option for iron deficiency (ID) or ID anaemia (IDA) in inflammatory bowel disease (IBD). However, treatment with ferric carboxymaltose (FCM) has been associated with the development of hypophosphatemia.
We aimed to investigate the occurrence of hypophosphatemia after treatment with either FCM and ferric derisomaltose (FDI) for ID or IDA in patients with IBD.
In this part of the study, we aimed to disclose underlying mechanism behind the development of hypophosphatemia after treatment with high dose intravenous iron and whether hypophosphatemia had a clinical impact on these patients.
A prospective observational study of adult IBD patients with ID or IDA was conducted between February 1, 2017 and July 1, 2018 at two separate university hospitals in the southeast region of Norway. Patients were recruited consecutively and received one dose of 1000 mg of either FCM or FDI, and were followed for an observation period of at least 7 wk at three timepoints; baseline, week 2 and week 6. Blood and urine samples were collected for relevant analyses at all three visits in addition to assessment of clinical symptoms using a respiratory function test, a visual analogue scale, and a health-related quality of life questionnaire.
Our study results demonstrate an association between FCM treatment and the development of hypophosphatemia by increasing the level of intact Fibroblast Growth Factor 23 (iFGF23) and phosphate wasting in the urine. Moreover, we observed a significant decline in active Vitamin D and ionised calcium. No clinical impact was detected by applying Short Form-36 questionnaire, visual analogue scale score and real-time position management breathing test in an observation period of 6 wk.
FCM treatment is associated with the development of hypophosphatemia in patients with IBD. This is due to increased formation of iFGF23 which in turn probably results in an increase of urinary phosphate output. No clinical impact was detected nor excluded. Assumably our study is underpowered together with a too short observation period to provide solid information with regard to clinical impact of hypophosphatemia.
Based on our results we encourage clinicians to be aware of the risk of developing hypophosphatemia after treatment with FCM. Larger studies with a longer observation period to detect possible clinical impact of hypophosphatemia is desirable.
We would like to acknowledge Wondrak P at the Medical University of Innsbruck, Austria, for her great assistance and work with the analysis of 1,25-dihydroxyvitamin D and fibroblast growth factor 23 in this study.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Norwegian Gastroenterological Association; United European Gastroenterology; and European Crohn’s and Colitis Organization.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Norway
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Henderson P, Howarth GS, Lukin DJ S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther. 2006;24:1507-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, Gomollon F, Iqbal T, Katsanos K, Koutroubakis I, Magro F, Savoye G, Stein J, Vavricka S; European Crohn’s and Colitis Organisation [ECCO]. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 346] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 3. | Eriksson C, Henriksson I, Brus O, Zhulina Y, Nyhlin N, Tysk C, Montgomery S, Halfvarson J. Incidence, prevalence and clinical outcome of anaemia in inflammatory bowel disease: a population-based cohort study. Aliment Pharmacol Ther. 2018;48:638-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Rampton DS, Goodhand JR, Joshi NM, Karim AB, Koodun Y, Barakat FM, Macken L, Ward DG, Iqbal TH, Epstein J, Fell JM, Sanderson IR. Oral Iron Treatment Response and Predictors in Anaemic Adolescents and Adults with IBD: A Prospective Controlled Open-Label Trial. J Crohns Colitis. 2017;11:706-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3006] [Cited by in F6Publishing: 2266] [Article Influence: 161.9] [Reference Citation Analysis (0)] |
| 6. | Mahalhal A, Williams JM, Johnson S, Ellaby N, Duckworth CA, Burkitt MD, Liu X, Hold GL, Campbell BJ, Pritchard DM, Probert CS. Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS One. 2018;13:e0202460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Carrier J, Aghdassi E, Platt I, Cullen J, Allard JP. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment Pharmacol Ther. 2001;15:1989-1999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Detlie TE, Lindstrøm JC, Jahnsen ME, Finnes E, Zoller H, Moum B, Jahnsen J. Incidence of hypophosphatemia in patients with inflammatory bowel disease treated with ferric carboxymaltose or iron isomaltoside. Aliment Pharmacol Ther. 2019;50:397-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Wolf M, Rubin J, Achebe M, Econs MJ, Peacock M, Imel EA, Thomsen LL, Carpenter TO, Weber T, Brandenburg V, Zoller H. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA. 2020;323:432-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 10. | Subramanian R, Khardori R. Severe hypophosphatemia. Pathophysiologic implications, clinical presentations, and treatment. Medicine (Baltimore). 2000;79:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Amanzadeh J, Reilly RF Jr. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol. 2006;2:136-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Padelli M, Leven C, Sakka M, Plée-Gautier E, Carré JL. [Causes, consequences and treatment of hypophosphatemia: A systematic review]. Presse Med. 2017;46:987-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ. Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care. 2010;14:R147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Schaefer B, Glodny B, Zoller H. Blood and Bone Loser. Gastroenterology. 2017;152:e5-e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018;2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Urbina T, Belkhir R, Rossi G, Carbonnel F, Pavy S, Collins M, Mariette X, Seror R. Iron Supplementation-Induced Phosphaturic Osteomalacia: FGF23 is the Culprit. J Bone Miner Res. 2018;33:540-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Reyes M, Diamond T. Hypophosphataemic Rickets Due to Parenteral Ferrous Carboxymaltose in a Young Man with Crohn Disease and Iron Deficiency: A Case Report and Review of Literature. J Clin Case Rep. 2017;7:931. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982-1992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 399] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 20. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] [Cited in This Article: ] |
| 21. | Ware JE, Snow KK, Kosinski M, Gandek B. Health survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center, 1993. [Cited in This Article: ] |
| 22. | Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Fukumoto S. Phosphate metabolism and vitamin D. Bonekey Rep. 2014;3:497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1334] [Cited by in F6Publishing: 1251] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 26. | Prié D, Friedlander G. Reciprocal control of 1,25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clin J Am Soc Nephrol. 2010;5:1717-1722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820-3828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 361] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 28. | Potts JT. Parathyroid hormone: past and present. J Endocrinol. 2005;187:311-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Fletcher J, Cooper SC, Ghosh S, Hewison M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 30. | Nottingham University Hospitals. Guideline for the Treatment of Hypophosphatemia in Adults. [cited 15 September 2020]. In: Nottingham University Hospitals [Internet]. Available from: https://studyres.com/doc/14104630/hypophosphataemia-in-adults---nottingham-university-hospi. [Cited in This Article: ] |
| 31. | Jelsness-Jørgensen LP, Bernklev T, Henriksen M, Torp R, Moum BA. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:106-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |