Published online Jan 7, 2021. doi: 10.3748/wjg.v27.i1.107
- This article has been corrected.
- See: World J Gastroenterol. Oct 14, 2021; 27(38): 6511-6512
Peer-review started: November 9, 2020
First decision: November 23, 2020
Revised: December 7, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: January 7, 2021
Shifting on lifestyle, diet, and physical activity contributed on increasing number of obese people around the world. Multiple factors influence the development of obesity. Some research suggested that gut microbiota (GM) plays an important role in nutrient absorption and energy regulation of individuals, thus affecting their nutritional status. Report of Indonesia Basic Health Research showed that the prevalence of obesity in every province tended to increase. Although the root cause of obesity is excessive calorie intake compared with expenditure, the differences in gut microbial ecology between healthy and obese humans may affect energy homeostasis. GM affect body weight, especially obesity. Probiotics that are consumed while alive and able to colonize in the intestine are expected to increase the population of good bacteria, especially Bifidobacteria and Lactobacilli, and suppress pathogens such as Enterobacteriaceae and Staphylococcus. The strain of L. plantarum Dad-13 has been demonstrated to survive and colonize in the gastrointestinal tract of healthy Indonesian adults who consume fermented milk containing L. plantarum Dad-13. The consumption of probiotic L. plantarum Dad-13 powder decreased E. coli and non-E. coli coliform bacteria in school-aged children in Indonesia. L. plantarum is a dominant bacterium in the average Indonesian’s GM. For this reason, this bacterium is probably a more suitable probiotic for Indonesians.
To determine the effect of the consumption of indigenous probiotic Lactobacillus plantarum Dad-13 powder in overweight adults in Yogyakarta (Indonesia).
Sixty overweight volunteers with a body mass index (BMI) equal to or greater than 25 consume indigenous probiotic powder L. plantarum Dad-13 (2 × 109 CFU/gram/sachet) for 90 d. The study was a randomized, double-blind, placebo-controlled study. The volunteers filled in a diary on a daily basis, which consisted of questions on study product intake (only during ingestion period), other food intake, number of bowel movements, fecal quality (consistency and color), any medications received, and any symptom of discomfort, such as diarrhea, constipation, vomiting, gassing, sensation of illness, etc. Fecal samples and the subjects’ diaries were collected on the morning of day 10 + 1, which was marked as the end of the baseline period and the start of the ingestion period. During the ingestion period (from day 11 to day 101), several parameters to measure and analyze the results included body weight and height (once a month), the lipid profile, GM analysis using MiSeq, short-chain fatty acid (SCFA) analysis using gas chromatography, and the measurement of fecal pH using a pH meter.
The consumption of indigenous probiotic powder L. plantarum Dad-13 caused the average body weight and BMI of the probiotic group to decrease from 84.54 ± 17.64 kg to 83.14 ± 14.71 kg and 33.10 ± 6.15 kg/m2 to 32.57 ± 5.01 kg/m2, respectively. No significant reduction of body weight and BMI in the placebo group was observed. An analysis of the microbiota showed that the number of Bacteroidetes, specifically Prevotella, increased significantly, while that of Firmicutes significantly decreased. No significant change in lipid profile in both groups was found. Also, no significant change in SCFAs (e.g., butyrate, propionate, acetic acid) and pH level was found after the consumption of the probiotic.
No significant differences in pH before and after ingestion were observed in both the probiotic and placebo groups as well as in the lipid profile of both cholesterol and triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and the LDL/HDL ratio. In addition, no significant changes in the concentration of SCFAs (e.g., acetic acid, propionate, and butyrate) were found after con-sumption. Interestingly, a significant decrease in body weight and BMI (P < 0.05) was determined in the treatment group. An analysis of GM shows that L. plantarum Dad-13 caused the Firmicutes population to decrease and the Bacteroidetes population (especially Prevotella) to increase.
Core Tip: Obesity and overweight are corelated with unhealthy lifestyle that affect the health of intestine and affect the ecosystem of gut microbiota (GM). Consumption of probiotics help to maintain the ecosystem of GM to stay balance and healthy. L. plantarum Dad-13 is potential probiotics for Indonesians to maintain health of the gastrointestinal ecosystem. This research was conducted to investigate and determine the effect of consumption of indigenous probiotic L. plantarum Dad-13 powder in overweight adults in Yogyakarta (Indonesia). The results show decreasing body mass index and weight on overweight subject and increasing of Bacteroidetes specifically Prevotella.
- Citation: Rahayu ES, Mariyatun M, Putri Manurung NE, Hasan PN, Therdtatha P, Mishima R, Komalasari H, Mahfuzah NA, Pamungkaningtyas FH, Yoga WK, Nurfiana DA, Liwan SY, Juffrie M, Nugroho AE, Utami T. Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J Gastroenterol 2021; 27(1): 107-128
- URL: https://www.wjgnet.com/1007-9327/full/v27/i1/107.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i1.107
Changes in lifestyle, diet, and physical activity have resulted in an exponential increase in the number of obese people around the world. Multiple factors influence the development of this disease, and gut microbiota (GM) have been suggested to play an important role in the nutrient absorption and energy regulation of individuals, thus affecting their nutritional status. Different levels of GM have been observed between individuals with normal nutritional status and those who are obese.
The World Health Organization defines obesity as an accumulation of abnormal or excessive fat that can interfere with health[1]. Body mass index (BMI) is the easiest way to identify whether someone is obese or not, namely, by calculating body weight (kg) divided by height squared (m²). A person is categorized as overweight if his/her BMI is greater than or equal to 25.0, while an obese person is someone with a BMI greater than or equal to 30.0[2]. A report from Indonesia Basic Health Research showed that the prevalence of obesity in every province tended to increase from 2007 to 2013 to 2018. In addition, it reported that adult women had higher obesity prevalence compared with adult men[3].
Although the root cause of obesity is excessive calorie intake compared with expenditure, the differences in gut microbial ecology between healthy and obese humans may affect energy homeostasis. In other words, individuals predisposed to obesity may have gut microbial communities that promote the more efficient extraction and/or storage of energy from a given diet compared with the communities in lean individuals[4].
GM affect body weight, especially obesity. A study showed that after the GM of fat mice were moved to thin mice, the latter gradually increased in weight and became fat[5]. A link is presumed to exist between GM and body weight. The biomarker of the GM of obesity is high-phylum Firmicutes bacteria[6]. Delzenne et al[7] found that the number of Bifidobacteria in obese individuals is lower than that in normal individuals. Other bacteria that were reported to consistently increase in obese individuals include Enterobacteriaceae, Escherichia coli, and Staphylococcus aureus[7].
Bifidobacteria are known as good bacteria as they produce short-chain fatty acids (SCFAs), such as acetate, propionate, butyrate, and lactate. This metabolite result has important effects on host metabolism. SCFAs can regulate (suppress or activate) the expression of specific genes associated with adiposity and inflammation that somewhat benefits the host. Given this description, we know that the population of Bifidobacteria is decreasing and that some pathogen bacteria, such as Enterobacteriaceae and Staphylococcus, are inclined to increase in obese individuals. Probiotics that are consumed while alive and able to colonize in the intestine are expected to increase the population of good bacteria, especially Bifidobacteria and Lactobacilli, and suppress pathogens such as Enterobacteriaceae and Staphylococcus. Kobyliak et al[8] proved that the consumption of probiotics, especially Lactobacilli, for nine weeks could suppress body weight gain and reduce adipose tissue in obese mice.
The probiotic research team of Universitas Gadjah Mada (UGM) came up with an indigenous probiotic from Indonesia that was obtained from various sources, but it has not been thoroughly studied. A study by Rahayu et al[9] revealed that the Indonesian indigenous probiotic strains have been molecularly confirmed and could inhibit the growth of pathogenic bacteria. In addition, the indigenous probiotic strains were shown to be resistant to pH 2 and bile salt of 3% concentration. Some probiotic cultures owned by the UGM research team are Lactobacillus plantarum Dad-13 (obtained from dadih, fermented buffalo milk), L. plantarum Mut-7 and Mut-13 (taken from gatot, fermented cassava), L. plantarum T3 (obtained from growol, fermented raw cassava), and Lactobacillus paracasei SNP-2 (taken from healthy baby feces).
The strain of L. plantarum Dad-13 has been demonstrated to survive and colonize in the gastrointestinal tract of healthy Indonesian adults who consume fermented milk containing L. plantarum Dad-13[10]. A safety assessment of L. plantarum Dad-13 on Sprague Dawley rats reported no adverse effects in general health, organ weight, leukocyte profiles, GOT activity, MDA concentration, and intestinal morphology after the consumption of the probiotic[11]. The indigenous probiotic L. plantarum Dad-13 also did not cause translocation in the organs and blood of the rats[11].
Apart from significantly increasing the population of L. plantarum and Lactobacillus, the consumption of probiotic L. plantarum Dad-13 powder decreased E. coli and non–E. coli coliform bacteria in school-aged children in Indonesia[12]. L. plantarum is a dominant bacterium in the average Indonesian’s GM[13]. For this reason, this bacterium is probably a more suitable probiotic for Indonesians. Thus, this study aimed to determine the effect of the consumption of indigenous probiotic L. plantarum Dad-13 powder in overweight adults in Yogyakarta (Indonesia).
This study involved 60 overweight volunteers, consisting of 24 males and 36 females. The age of the subjects ranged between 35 and 56 years old. The inclusion criteria of the subjects covered having a BMI equal to or greater than 25, no history of gastrointestinal disorder (such as constipation, diarrhea, abdominal pain, and irritable bowel syndrome), and no allergies to certain foods. The subjects had not taken antibiotics/antimycotics or any specific drugs and did not consume antidiarrheal or laxative medicine for 100 d during the study.
This study was conducted in accordance with Good Clinical Practice (GCP) as defined by the International Conference of Harmonization (ICH) and in accordance with the Indonesian National Agency for Drug and Food Control Guidance. Approval by the Ethics Committee of the Faculty of Medicine, Public Health, and Nursing of UGM, Yogyakarta, was received on January 2, 2018, as stated in the committee’s letter, with reference number KE/FK/0002/2018.
The product of this study was 1 g of skimmed milk powder containing the probiotic L. plantarum Dad-13 of 2 × 109 CFU in sachet packing. The product was prepared using a halal medium by the Center for Food and Nutrition Studies, UGM. One gram of skimmed milk obtained from a local supermarket was used in the placebo group. The study products were stored in a refrigerator (< 4 °C) before being consumed. L. plantarum Dad-13, the indigenous probiotic strain, was deposited in ampoules at the Food and Nutrition Culture Collection (FNCC), Center for Food and Nutrition Studies, UGM. Labelling and product blinding were prepared by the Unit Production of Probiotics and Starter Cultures, Center for Food and Nutrition Studies, UGM.
DNA fecal extraction was performed using phenol–chloroform extraction. The SsoFast™ Evagreen® Supermix Kit from PT Sciencewerke (Indonesia) was used as a mixture of DNA extracts in super-mix real-time PCR. The primers for qPCR analysis consisted of Bifidobacteria[14], the L. plantarum subgroup[15], Clostridium coccoides[15], and Enterobacteriaceae[14]. The main instrument used for GM analysis was real-time PCR. Stool sampling equipment included stool tubes, sterile tissue paper, gloves, masks, ice gel, and cool boxes. DNA extraction equipment included a centrifuge, a vortex, analytical scales, and other kinds of glassware. The equipment for the probiotic powder included freeze dryers and vacuum sealing.
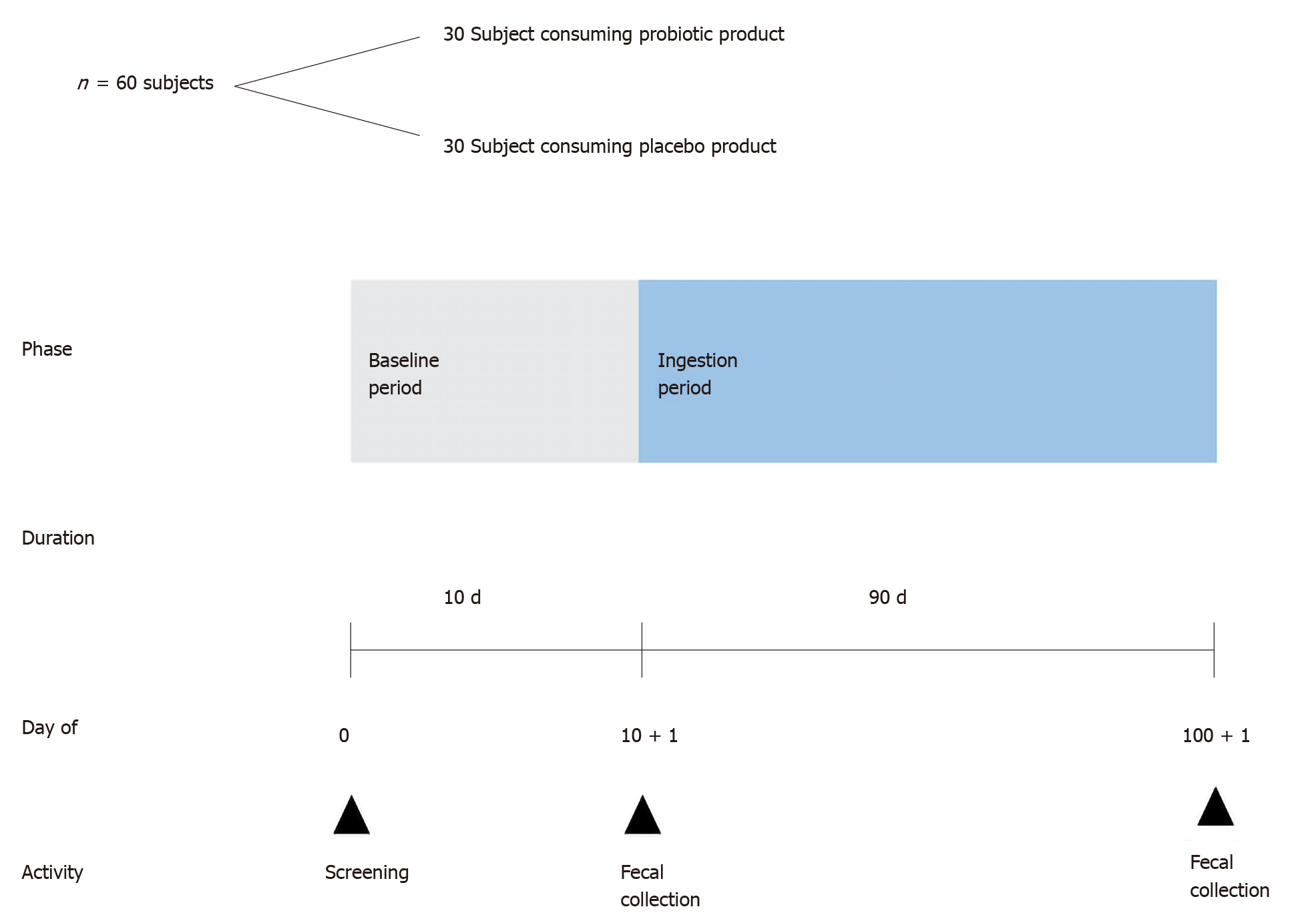
The study was a randomized, placebo-controlled study; 60 volunteers were divided into an intervention group (probiotic) and a control group (placebo). All the subjects and the researcher were blinded to the treatment administrated (double-blind study). This study used simple randomization, performed in such a way that leaves no significant difference between the study groups (BMI, age, or sex). The placebo product used was skimmed milk without probiotics. The study consisted of 10 d without the consumption of the study product (baseline period) followed by 90 d of ingestion, as shown in Figure 1. During the baseline period, the volunteers consumed their normal dietaries with the exception of probiotic products. The baseline period was a “washout” period to eliminate the effect of previously used probiotics. The volunteers filled in a diary on a daily basis, which consisted of questions on study product intake (only during the ingestion period), other food intake, number of bowel movements, fecal quality (consistency and color), any medications received, and any symptom of discomfort, such as diarrhea, constipation, vomiting, gassing, sensation of illness, etc. Fecal samples and the subjects’ diaries were collected on the morning of day 10 ± 1, which was marked as the end of the baseline period and the start of the ingestion period. During the ingestion period (from day 11 to day 101), the volunteers consumed one sachet of the study product per day after having lunch for 90 consecutive days. The volunteers were not allowed to consume any other probiotic products. They were requested to fill in a new diary on a daily basis. Upon the completion of the ingestion period, on the morning of day 100 ± 1, fecal samples and the subjects’ diaries were collected.
A fecal sample was collected into a sterile tube with a kind of scoop built into the inside of the lid by the subjects at home, and the sample was immediately transported to the laboratory in a cold storage container (< 10 °C). Two tubes were used to collect the samples, containing buffer/stabilizer RNA later and glass beads, one tube for GM analysis and the other for SCFA analysis. The materials and instructions for fecal sample collection were provided to the subjects prior to the fecal collection schedule. The subjects were instructed to defecate on the trail paper (smooth side up) and were prevented from wetting the fecal paper with urine or water. Then they were required to immediately take a sample by scraping the feces with the scooper and capping the tube tightly.
Several parameters to measure and analyze the results included: (1) The measurement of body weight and height once a month; (2) The lipid profile; (3) GM analysis; (4) SCFA analysis using gas chromatography; and (5) The measurement of fecal pH using a pH meter.
A high-throughput analysis of 16 rRNA gene sequences was carried out according to the previous method. Areas V3-V4 of the sequences from the bacteria were amplified with the fecal DNA genome (approximately 1 ng) using TaKaRa Ex TaqTM HS (Takara Bio, Japan) and universal primer Bakt_341F (5’-CGCTCTTCCGATCTCTG CCTACGGGGGGGCWGCAG-355)GGCTATICCCACCATTCCCCATTCCACCA CCACCACCACCACCACCACCACCA UTAA. The amplification results were used as a template for the second PCR using barcode-tag primers. The second PCR results were purified using the FastGene Gel/PCR Extraction Kit (NIPPON Genetics, Japan) according to company protocol. The purified products were quantified using the PicoGreen® dsDNA Assay Kit (Life Technologies, United States) based on company protocol. All the PCR samples were of the same total amount (approximately 200 ng total), and they were purified using electrophoresis in 2% (w/v) agarose gel (classic-type Agarose-LE: Nacalai Tesque, Japan), followed by extraction from the gel by the FastGene Gel/PCR Extraction Kit. The purified mixture was applied to the final-pair sequence of Illumina MiSeq v3 (Illumina, United States).
The data was displayed as mean ± standard deviation unless stated otherwise. IBM Statistic SPSS 20.0 with a 95% confidence interval (α = 5%) was used to perform statistical analysis. A chi-square test or independent t-test or Wilcoxon test was carried out to evaluate the significant differences of the observed parameters between the probiotic-treated group and the placebo group depending on the normality and equality of variance of the data. In addition, a paired t-test was used to analyze the observed parameters before and after the consumption of the indigenous probiotic powder or placebo powder.
Sixty overweight subjects who participated in the research signed informed consent forms. The subjects were divided into two groups, namely, the probiotic-treated group and the placebo group. Neither the researcher nor the participants knew which subject entered the probiotic group or the placebo group. The research began on January 5, 2019. Fifteen days were allotted for the prescreening period, and the baseline period started on January 21-30, 2019, the intervention period started on January 31 and ended on April 30, 2019. The research ended when all the subjects finished giving their fecal samples to the researcher. The demographic data of the subjects showed no significant differences in age, height, weight, and BMI, and the number of female participants was higher than that of male participants (Table 1).
The body weight, height, and BMI of the subjects were measured every 10 d. Table 2 presents a significant decrease (P < 0.05) in the body weight and BMI of the subjects after 90 d of probiotic ingestion. Table 3 further shows the different effects of consuming probiotics between the female and male subjects.
| Group | Baseline period | Ingestion period | P value | |
| Weight | Probiotic-treated | 84.54 ± 17.62 | 83.14 ± 14.71 | 0.042a |
| Placebo | 79.37 ± 11.76 | 78.80 ± 11.77 | 0.121 | |
| Height | Probiotic-treated | 159.66 ± 8.27 | 159.66 ± 8.27 | 1.002 |
| Placebo | 157.92 ± 9.58 | 157.92 ± 9.58 | 1.002 | |
| BMI | Probiotic-treated | 33.10 ± 6.15 | 32.57 ± 5.01 | 0.042a |
| Placebo | 31.80 ± 3.71 | 31.56 ± 3.67 | 0.181 |
| Gender | Group | Baseline period | Ingestion period | P value | |
| Women | Weight | Probiotic-treated | 77.91 ± 14.16 | 77.08 ± 13.68 | 0.011a |
| Placebo | 73.20 ± 9.93 | 72.69 ± 9.93 | 0.331 | ||
| Height | Probiotic-treated | 153.82 ± 4.05 | 153.82 ± 4.05 | 1.002 | |
| Placebo | 151.42 ± 5.92 | 151.42 ± 5.92 | 1.002 | ||
| BMI | Probiotic-treated | 32.90 ± 5.73 | 32.58 ± 5.58 | 0.021a | |
| Placebo | 31.96 ± 4.25 | 31.72 ± 4.14 | 0.311 | ||
| Men | Weight | Probiotic-treated | 94.48 ± 18.11 | 92.22 ± 11.45 | 0.381 |
| Placebo | 88.63 ± 7.51 | 87.97 ± 7.77 | 0.161 | ||
| Height | Probiotic-treated | 168.42 ± 3.92 | 168.42 ± 3.92 | 1.002 | |
| Placebo | 167.67 ± 3.87 | 167.67 ± 3.87 | 1.002 | ||
| BMI | Probiotic-treated | 33.39 ± 6.98 | 32.54 ± 4.25 | 0.371 | |
| Placebo | 31.56 ± 2.87 | 31.32 ± 2.96 | 0.151 |
Some studies also reported that probiotics could reduce body weight. Kadooka et al[16] found that the probiotic Lactobacillus gasseri SBT2055 (LG2055) caused abdominal adiposity, body weight, and other measures to decrease, suggesting its beneficial influence on metabolic disorders. According to Higashikawa et al[17], the heat-killed Pediococcus pentosaceus LP28 displayed an anti-obesity effect that reduced BMI, body fat, and waist circumference. Another study revealed that the mean of weight loss in female subjects consuming Lactobacillus rhamnosus CGMCC1.3724 (LPR) supplementation was significantly higher than that in women who belonged to the placebo group after the first 12 wk. The body weight and fat mass of the male subjects were not affected by the treatment[18].
The lipid profile showed that in both groups, there was no significant difference in each parameter measured after consuming the study product. The results of the lipid profile are shown in Table 4.
| Lipid profile | Group | Baseline period | Ingestion period | P value |
| Cholesterol (mg/dL) | Probiotic-treated | 194.93 ± 37.64 | 192.20 ± 36.55 | 0.462 |
| Placebo | 193.70 ± 29.47 | 192.37 ± 29.75 | 0.412 | |
| Triglyceride (mg/dL) | Probiotic-treated | 151.50 ± 63.92 | 166.83 ± 75.02 | 0.162 |
| Placebo | 191.40 ± 133.60 | 187.73 ± 111.58 | 0.542 | |
| HDL (mg/dL) | Probiotic-treated | 40.33 ± 9.77 | 40.00 ± 9.28 | 0.691 |
| Placebo | 39.93 ± 7.29 | 40.60 ± 8.18 | 0.391 | |
| LDL (mg/dL) | Probiotic-treated | 141.43 ± 32.17 | 136.97 ± 33.12 | 0.182 |
| Placebo | 134.50 ± 24.84 | 133.50 ± 27.06 | 0.711 | |
| Ratio of LDL/HDL | Probiotic-treated | 3.63 ± 0.95 | 3.55 ± 0.87 | 0.381 |
| Placebo | 3.44 ± 0.70 | 3.39 ± 0.84 | 0.611 |
Fecal characteristics indicate intestinal conditions in humans. These characteristics include volume, type, color, odor, and pH. The fecal volume of 1 is equal to the volume of a chicken egg. The color is indicated in four scales (1: yellow; 2: brownish yellow; 3: brown; 4: green). The Bristol stool chart was used to identify the type of feces. The aroma of the feces was expressed using a three-point scale (1: normal; 2: strong; 3: very strong). Table 5 shows the fecal characteristics and defecation frequency.
| Group | Baseline period | Ingestion period | P value | |
| Volume | Probiotic-treated | 2.20 ± 0.79 | 2.23 ± 0.96 | 0.861 |
| Placebo | 2.37 ± 0.95 | 2.29 ± 0.90 | 0.232 | |
| Type | Probiotic-treated | 3.59 ± 0.93 | 3.22 ± 1.17 | 0.081 |
| Placebo | 4.02 ± 1.14 | 3.89 ± 0.93 | 0.342 | |
| Color | Probiotic-treated | 1.86 ± 0.57 | 1.73 ± 0.55 | 0.201 |
| Placebo | 2.00 ± 0.59 | 1.95 ± 0.54 | 0.701 | |
| Odor | Probiotic-treated | 1.11 ± 0.37 | 1.10 ± 0.28 | 0.932 |
| Placebo | 1.26 ± 0.35 | 1.32 ± 0.41 | 0.572 | |
| pH | Probiotic-treated | 5.72 ± 0.31 | 5.76 ± 0.28 | 0.513 |
| Placebo | 5.58 ± 0.40 | 5.75 ± 0.34 | 0.073 | |
| Defecation frequency4 | Probiotic-treated | 12.93 ± 3.61 | 13.40 ± 4.52 | 0.582 |
| Placebo | 14.67 ± 4.93 | 15.70 ± 7.57 | 0.512 |
Table 5 indicates that in both the probiotic-treated group and the placebo group, the volume, type, color, odor, and pH of the feces during the baseline and ingestion periods were not significantly changed. The defecation frequency was expressed as the total number or frequency of defecation in 10 d. Overall, the fecal samples from both groups had the following characteristics: Banana-like shape, brownish yellow color, normal odor, and pH of 5.58-5.76.
An analysis of SCFAs was performed using gas chromatography. The SCFAs analyzed in this study included acetic acid, propionate, and butyrate. Table 6 shows the SCFA concentration of the probiotic-treated and placebo groups. It also shows that the SCFAs did not significantly change (P > 0.05) in both groups after the ingestion period. The SCFAs function via diverse host molecular mechanisms to regulate host energy intake, energy expenditure, and storage[19]. The production of SCFAs by bacteria that ferment carbohydrates contribute 10% of the total energy to be absorbed in the colon, and the rest would be lost through the feces[20]. One study proved that the administration of L. salivarius Ls-33 to obese adolescent subjects did not have a significant effect[21]. Likewise, the administration of L. plantarum Dad-13 in this study did not affect the SCFA concentration of the overweight subjects. The pH value in the treatment group was 5.72 ± 0.31 before ingestion and 5.76 ± 0.28 after ingestion. The pH value in the placebo group was 5.58 ± 0.40 before ingestion and 5.75 ± 0.34 after ingestion. This insignificant change of fecal pH was attributed to insignificant SCFA concentration, so the intestines’ condition did not change.
| Group | Baseline period | Ingestion period | P valuea | |
| Acetic acid (mmol/kg) | Probiotic-treated | 63.19 ± 34.97 | 64.76 ± 17.61 | 0.89 |
| Placebo | 67.09 ± 19.56 | 63.76 ± 13.05 | 0.65 | |
| Propionic acid (mmol/kg) | Probiotic-treated | 22.02 ± 14.17 | 20.65 ± 9.76 | 0.67 |
| Placebo | 21.98 ± 10.44 | 17.16 ± 2.04 | 0.17 | |
| Butyrate acid (mmol/kg) | Probiotic-treated | 14.78 ± 6.68 | 14.97 ± 7.24 | 0.95 |
| Placebo | 19.45 ± 8.60 | 15.59 ± 9.40a | 0.33 |
Diet or food intake is associated with obesity. This study also recorded the daily diet of the subjects. The dietary records were analyzed using the NutriSurvey 2007 software. The household size based on the standard issued by the Republic of Indonesia Ministry of Health in 2014 was used to measure the amount of food intake. Table 7 below summarizes the diet profile of the subjects.
| Group | Baseline period | Ingestion day 11-20 | Ingestion day 21-30 | Ingestion day 31-40 | Ingestion day 41-60 | Ingestion day 61-80 | Ingestion day 81-100 | |
| Energy (kcal) | Probiotic-treated | 1518.17 ± 484.46 | 1322.04 ± 431.90 | 1338.23 ± 376.17 | 1274.13 ± 390.37 | 1124.89 ± 378.82a | 1060.65 ± 286.86a | 1103.54 ± 311.74a |
| Placebo | 1642.88 ± 599.33 | 1660.02 ± 611.62 | 1562.79 ± 1144.53 | 1396.65 ± 476.01a | 1085.16 ± 346.08a | 1047.90 ± 313.37a | 1105.73 ± 313.58a | |
| Water (g) | Probiotic-treated | 744.06 ± 526.92 | 633.17 ± 397.27 | 593.60 ± 377.72 | 691.70 ± 470.44 | 720.66 ± 551.62 | 656.24 ± 419.94 | 712.81 ± 438.11 |
| Placebo | 1122.19 ± 615.82 | 1016.66 ± 665.44 | 1041.80 ± 568.23 | 1100.16 ± 601.35 | 688.97 ± 533.39a | 813.86 ± 565.46 | 841.27 ± 595.64 | |
| Protein (g) | Probiotic-treated | 52.72 ± 19.49 | 44.69 ± 16.73 | 46.23 ± 14.61 | 46.81 ± 12.61 | 38.49 ± 14.10a | 38.30 ± 10.23a | 39.62 ± 11.30a |
| Placebo | 53.92 ± 24.02 | 55.03 ± 25.52 | 47.12 ± 33.52 | 47.02 ± 18.70 | 38.38 ± 12.95a | 35.67 ± 10.98a | 39.09 ± 9.72a | |
| Lipid (g) | Probiotic-treated | 61.41 ± 30.24 | 47.15 ± 26.32 | 47.60 ± 21.41 | 49.86 ± 19.25 | 40.78±15.24a | 39.63 ± 12.02a | 40.78 ± 12.99a |
| Placebo | 64.15 ± 34.36 | 63.17 ± 38.78 | 54.22 ± 36.98 | 52.15 ± 25.09a | 36.99 ± 13.85a | 36.88 ± 12.74a | 37.68 ± 9.70a | |
| Carbo-hydrate (g) | Probiotic-treated | 190.85 ± 68.00 | 179.93 ± 57.44 | 180.95 ± 65.23 | 160.50 ± 59.23 | 150.80 ± 56.88 | 137.97 ± 43.53a | 144.63 ± 46.33a |
| Placebo | 216.39 ± 88.21 | 221.71 ± 83.07 | 224.10 ± 190.74 | 186.27 ± 71.98 | 149.82 ± 48.91a | 144.70 ± 49.45a | 153.74 ± 60.37a | |
| Fiber (g) | Probiotic-treated | 10.96 ± 5.88 | 8.66 ± 3.04 | 8.71 ± 2.91 | 8.49 ± 2.63 | 7.08 ± 2.16a | 6.24 ± 2.01a | 6.61 ± 2.48a |
| Placebo | 12.17 ± 7.15 | 13.34 ± 9.00 | 11.90 ± 8.61 | 10.20 ± 4.67 | 6.96 ± 2.99a | 7.17 ± 2.30a | 7.45 ± 2.30a | |
| PUFA (g) | Probiotic-treated | 16.87 ± 13.02 | 12.80 ± 7.73 | 12.17 ± 5.87 | 13.03 ± 7.64 | 12.17 ± 7.36 | 10.91 ± 4.50 | 10.86 ± 4.31 |
| Placebo | 18.20 ± 13.73 | 18.49 ± 15.71 | 15.70 ± 13.47 | 16.29 ± 13.52 | 10.50 ± 5.94a | 10.03 ± 4.70a | 9.73 ± 3.1a | |
| Choles-terol (mg) | Probiotic-treated | 191.39 ± 163.58 | 163.28 ± 83.55 | 168.44 ± 92.31 | 182.29 ± 68.95 | 160.32 ± 84.56 | 172.70 ± 77.78 | 179.23 ± 57.13 |
| Placebo | 170.42 ± 154.32 | 168.91 ± 169.50 | 142.45 ± 159.96 | 164.19 ± 124.86 | 173.22 ± 86.80 | 165.31 ± 85.03 | 182.64 ± 81.91 |
Based on the analysis of the dietary patterns of the subjects, the standard deviation was high, which indicates that the nutrient intake of the subjects was very diverse. Compared to the intake during the baseline period, both the probiotic-treated group and the placebo group consumed less energy, protein, lipid, carbohydrate, and PUFA sources in the last month of the ingestion period. In addition, the average daily energy intake of the subjects was around 1518.17-1642.88 kcal/d, less than that of a normal adult (around 2000 kcal/d)[22]. The consumption of dietary fiber sources experienced a gradual drop from day 41 to the end of the study period. Meanwhile, no significant differences were observed in the intake of cholesterol between the baseline period and the end of the ingestion period in both the probiotic-treated group and the placebo group.
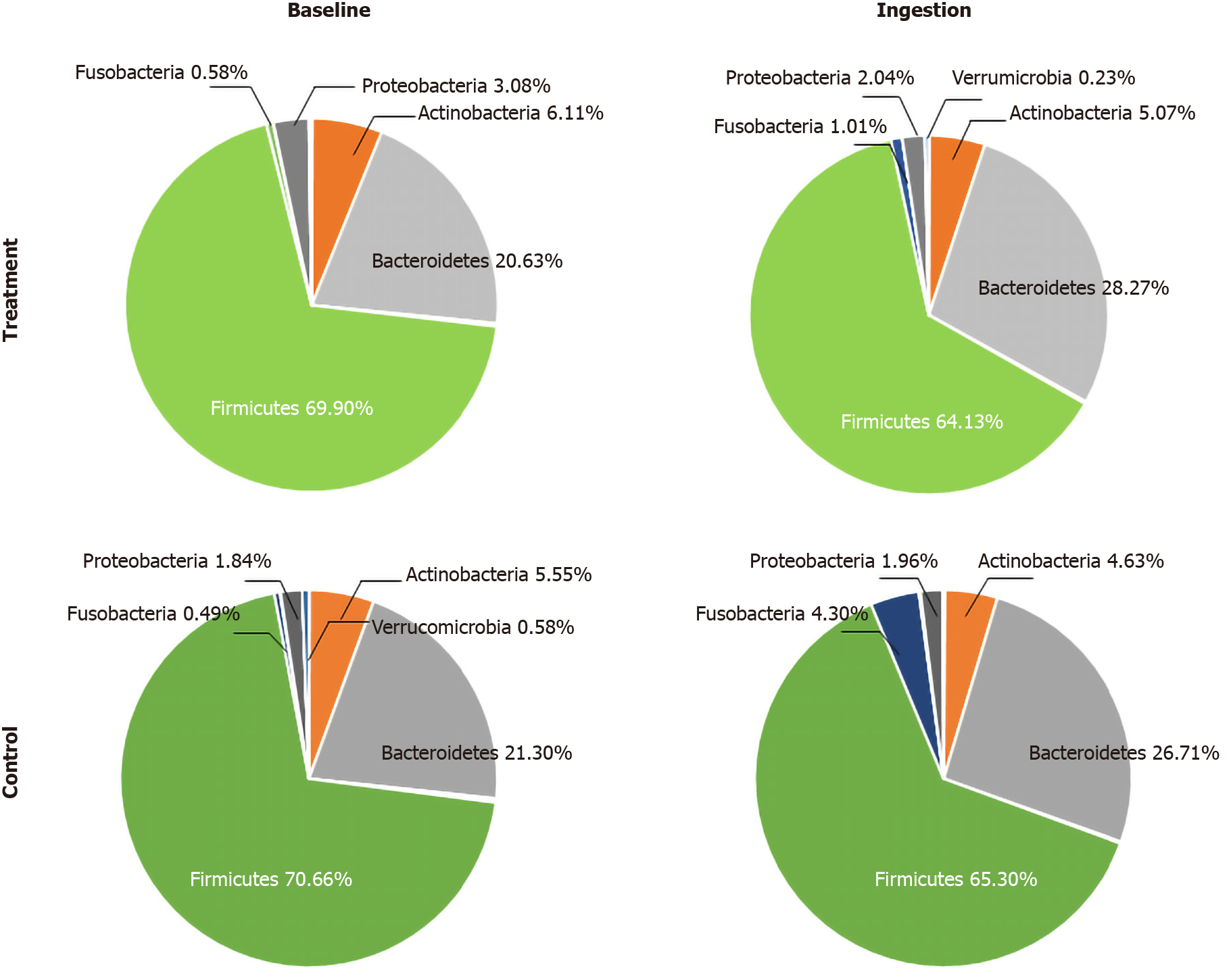
Based on the results of the 16 RNA sequences using MiSeq performed in both the probiotic-treated and placebo groups, the bacterial group was dominated by the phyla Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia (Figure 2). A small portion of the phyla Cyanobacteria, Lentisphaerae, Elusimicrobia, and Synergistetes appeared in both the treatment and control groups (Table 8).
| Phylum | Genus | Probiotic-treated | Placebo | ||||
| Baseline, mean (%) ± SD | Ingestion, mean (%) ± SD | P value | Baseline, mean (%) ± SD | Ingestion, mean (%) ± SD | P value | ||
| Firmicutes | Faecalibacterium | 11.43 ± 5.03 | 10.94 ± 4.21 | 0.614 | 15.30 ± 7.07 | 11.82 ± 5.50 | 0.01a |
| Coprococcus | 7.53 ± 3.55 | 6.23 ± 1.85 | 0.037a | 7.34 ± 3.71 | 5.82 ± 2.67 | 0.116 | |
| Other | 7.63 ± 5.05 | 8.22 ± 4.15 | 0.491 | 6.03 ± 4.13 | 8.40 ± 4.56 | 0.012a | |
| Ruminococcus | 4.49 ± 4.06 | 3.69 ± 2.90 | 0.271 | 3.80 ± 3.84 | 3.70 ± 4.79 | 0.572 | |
| Roseburia | 1.36 ± 1.31 | 1.54 ± 1.10 | 0.072b | 1.49 ± 1.55 | 2.04 ± 1.28 | 0.037a | |
| Clostridium | 0.16 ± 0.22 | 0.16 ± 0.27 | 0.829 | 0.25 ± 0.53 | 0.35 ± 0.49 | 0.202 | |
| Paenibacillus | 0.01 ± 0.01 | 0.00 ± 0.01 | 0.1 | 0.03 ± 0.06 | 0.00 ± 0.01 | 0.002a | |
| Bacteroidetes | Prevotella | 14.56 ± 11.57 | 19.25 ± 13.03 | 0.066b | 14.15 ± 13.69 | 14.28 ± 14.13 | 0.75 |
| Bacteroides | 3.78 ± 5.43 | 5.57 ± 8.21 | 0.019a | 5.59 ± 9.97 | 10.30 ± 13.83 | 0.04a | |
| Actino-bacteria | Bifidobacterium | 3.38 ± 4.96 | 2.74 ± 4.01 | 0.6 | 3.07 ± 3.95 | 2.71 ± 2.91 | 0.957 |
| Collinsella | 2.02 ± 1.40 | 1.52 ± 0.94 | 0.069b | 2.00 ± 1.80 | 1.51 ± 0.98 | 0.271 | |
| Brevibacterium | 0.01 ± 0.01 | 0.00 ± 0.01 | 0.307 | 0.03 ± 0.07 | 0.01 ± 0.02 | 0.082b | |
| Proteo-bacteria | Succinivibrio | 2.19 ± 4.51 | 1.44 ± 3.37 | 0.548 | 2.06 ± 5.29 | 2.51 ± 6.89 | 0.534 |
| Phyllobacterium | 0.02 ± 0.04 | 0.02 ± 0.02 | 0.037a | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.167 | |
| Sphingomonas | 0.02 ± 0.03 | 0.02 ± 0.02 | 0.75 | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.833 | |
| Verrucomicrobia | Akkermansia | 0.13 ± 0.56 | 0.04 ± 0.18 | 0.028a | 0.35 ± 1.64 | 0.02 ± 0.07 | 0.024a |
The phylum distribution composition of each subject from both the treatment and control groups can be seen in Figure 3. Three genera - Firmicutes, Bacteroidetes, and Actinobacteria - are the most dominant genera appearing on almost all the subjects, while some phyla, such as Proteobacteria and Fusobacteria, appear dominantly in only a few subjects.
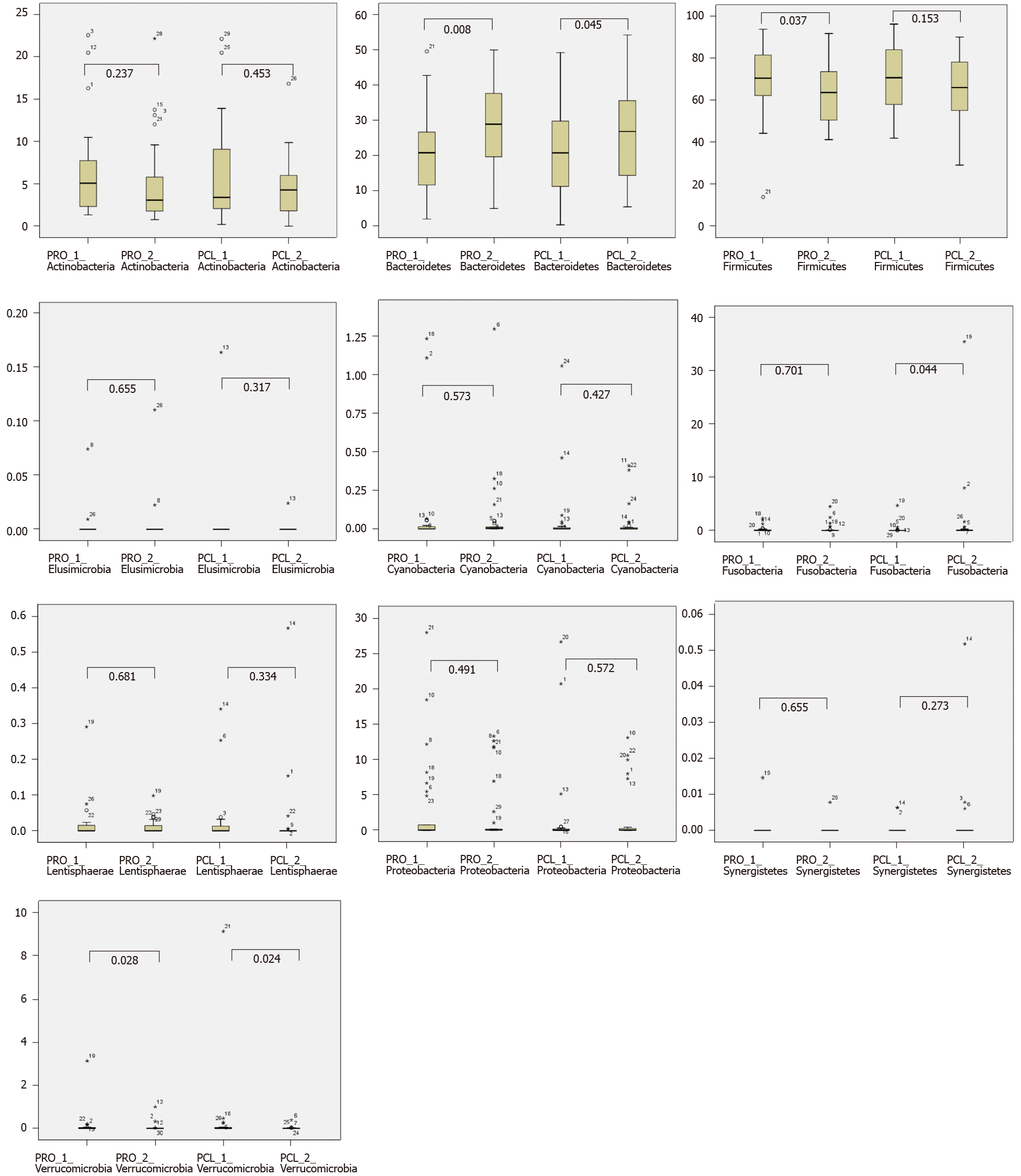
Concerning the two dominant phyla, the number of Bacteroidetes significantly increased (P < 0.05) in both the treatment and placebo groups after the ingestion period (Table 9), and the number of Firmicutes significantly decreased (P < 0.05) in the treatment group (Figure 4). Meanwhile, the Fusobacteria population was only found in a few subjects. The Verrucomicrobia population significantly decreased in both the treatment and placebo groups after the ingestion period. Verrucomicrobia was often associated with gastrointestinal health and glucose homeostasis. No significant changes (P > 0.05) were found in the phyla of Cyanobacteria, Elusimicrobia, Lentisphaerae, and Synergistetes in the treatment and placebo groups before and after the ingestion period. The changes in phylum of bacterial composition in both the probiotic-treated and placebo groups before and after the ingestion period are presented in Figure 4.
| No. | Phylum | Group | Baseline period | Ingestion period | P valuea |
| 1 | Firmicutes | Probiotic-treated | 69.90 ± 15.95 | 64.13 ± 15.22 | 0.037a |
| Placebo | 70.66 ± 14.41 | 65.30 ± 14.52 | 0.153 | ||
| 2 | Bacteroidetes | Probiotic-treated | 20.63 ± 11.49 | 28.27 ± 14.26 | 0.008a |
| Placebo | 21.30 ± 12.72 | 26.71 ± 13.93 | 0.045a | ||
| 3 | Actinobacteria | Probiotic-treated | 6.11 ± 5.37 | 5.07 ± 5.48 | 0.237 |
| Placebo | 5.55 ± 4.90 | 4.63 ± 3.61 | 0.453 | ||
| 4 | Proteobacteria | Probiotic-treated | 2.87 ± 6.39 | 2.04 ± 6.05 | 0.491 |
| Placebo | 1.84 ± 4.32 | 1.69 ± 3.77 | 0.572 | ||
| 5 | Fusobacteria | Probiotic-treated | 0.23 ± 0.57 | 0.34 ± 0.86 | 0.701 |
| Placebo | 0.20 ± 0.94 | 1.58 ± 6.57 | 0.044a | ||
| 6 | Verrucomicrobia | Probiotic-treated | 0.13 ± 0.57 | 0.04 ± 1.66 | 0.028a |
| Placebo | 0.35 ± 0.19 | 0.02 ± 0.07 | 0.024a | ||
| 7 | Cyanobacteria | Probiotic-treated | 0.09 ± 0.29 | 0.07 ± 0.21 | 0.537 |
| Placebo | 0.06 ± 0.24 | 0.04 ± 0.10 | 0.427 | ||
| 8 | Lentisphaerae | Probiotic-treated | 0.02 ± 0.05 | 0.01 ± 0.08 | 0.681 |
| Placebo | 0.03 ± 0.02 | 0.03 ± 0.11 | 0.334 | ||
| 9 | Elusimicrobia | Probiotic-treated | 0.00 ± 0.01 | 0.00 ± 0.03 | 0.655 |
| Placebo | 0.01 ± 0.02 | 0.00 ± 0.00 | 0.317 | ||
| 10 | Synergistetes | Probiotic-treated | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.655 |
| Placebo | 0.00 ± 0.00 | 0.00 ± 0.01 | 0.273 |
At the genus level (as shown in Table 8), some microbiota showed some changes in composition. However, not all genera of the whole phylum experienced some changes. In the phylum Firmicutes, Faecalibacterium was quite significant in the placebo group compared with the treatment group (P < 0.05). One species of Faecalibacterium that is quite abundant in the human digestive tract is Faecalibacterium prausnitzii. Faecalibacterium is a fairly dominant digestive microbiota, as indicated by the fact that 5%-15% of total bacteria are F. prausnitzii species[23]. F. prausnitzii is also considered one of the health indicators of gastrointestinal health. Healthy subjects normally showed an abundance of F. prausnitzii compared with subjects with Crohn’s disease[24]. The genus Coprococcus showed a significant decrease in the treatment group (P < 0.05). Coprococcus is characterized as comprising anaerobic microbes able to produce butyrate acid. Countless studies have associated Coprococcus with the health conditions of the human digestive tract. One study showed that its healthy subjects had a high abundance of Coprococcus compared with subjects with colorectal cancer[25]. Other studies showed that the number of Bacteroides and Coprococcus in subjects with colorectal pre-cancerous conditions was much lower than that in healthy subjects[26]. Body conditions in humans, such as obesity or being overweight, can also affect the conditions of microbiota. The populations of Blautia, Coprococcus, and Enterobacteriaceae were quite high in overweight children in Mexico compared with those with normal conditions[27].
Table 8 shows that Roseburia significantly increased in both the treatment (P < -0.01) and placebo groups (P < 0.05). Roseburia is a microbiota of the genus Firmicutes that has the characteristics of gram-positive, obligate anaerobes and can produce the SCFA butyrate. In the human digestive tract, one of the species of Roseburia - namely, Roseburia hominis - can regulate immunity[28]. An increase in the population of Roseburia can also be attributed to the type of food consumed. The consumption of resistant starch is said to increase Eubacterium rectale and Roseburia[29]. The Roseburia population in humans is quite varied. A study of groups of obese and overweight children showed a fairly high Roseburia population compared with the normal group[27].
The Paenibacillus genus, as shown in Table 8, decreased in both groups, but only the placebo control group experienced a significant decrease. The genus Clostridium did not significantly change in both groups, but the placebo group experienced a slight increase in abundance. The genus Ruminococcus decreased significantly in the probiotic-treated group.
The Coprococcus genus experienced a significant decrease in the treatment group after consumption, inversely proportional to the genus Roseburia, which experienced a significant increase in the treatment group after consumption. Roseburia also experienced a significant increase in the control group after the ingestion period. The genera Faecalibacterium and Paenibacillus experienced a decrease in the placebo group after the ingestion period. No significant differences were observed in the genera Clostridium and Ruminococcus in both the treatment and placebo groups after the ingestion period.
The second dominant phylum (Table 9) is Bacteroidetes, which increased in the treatment group. The genus Bacteroides significantly increased in both the probiotic-treated and placebo groups (P < 0.05). However, the genus Prevotella significantly increased (P < 0.1) in the probiotic-treated group. Previous papers mentioned that Prevotella is the dominant genus in the phylum Bacteroidetes for healthy school-aged children (Murugesan et al[27] 2015) and adult Indonesians (Rahayu et al[13] 2019). In this study, the population of Prevotella is much higher than that of Bacteroides. This finding supports previous reports stating that Indonesians have the Prevotella enterotype.
The relative abundance of the phylum Actinobacteria - namely, the genera Brevibacterium, Bifidobacteria, and Collinsella - is shown in Table 8. Brevibacterium in the treatment group significantly decreased after the ingestion period and showed a significant decrease in the genus Collinsella in the treatment group (P < 0.1) compared with the placebo group. Collinsella is a microbiota of the phylum Actinobacteria. These microbiota were often said to be pathobionts, which have the potential to influence the nature of the pathogen to its host[30]. Subjects with obesity and having type 2 diabetes are said to have a high abundance of Collinsella compared with healthy people[31].
Table 8 shows that the genus Phyllobacterium of the phylum Proteobacteria increased significantly in the treatment group, while no significant change in the genera Succinivibrio and Sphingomonas was found in both the treatment and placebo groups. Meanwhile, the abundance of the genus Akkermansia in the phylum Verrucomicrobia decreased significantly.
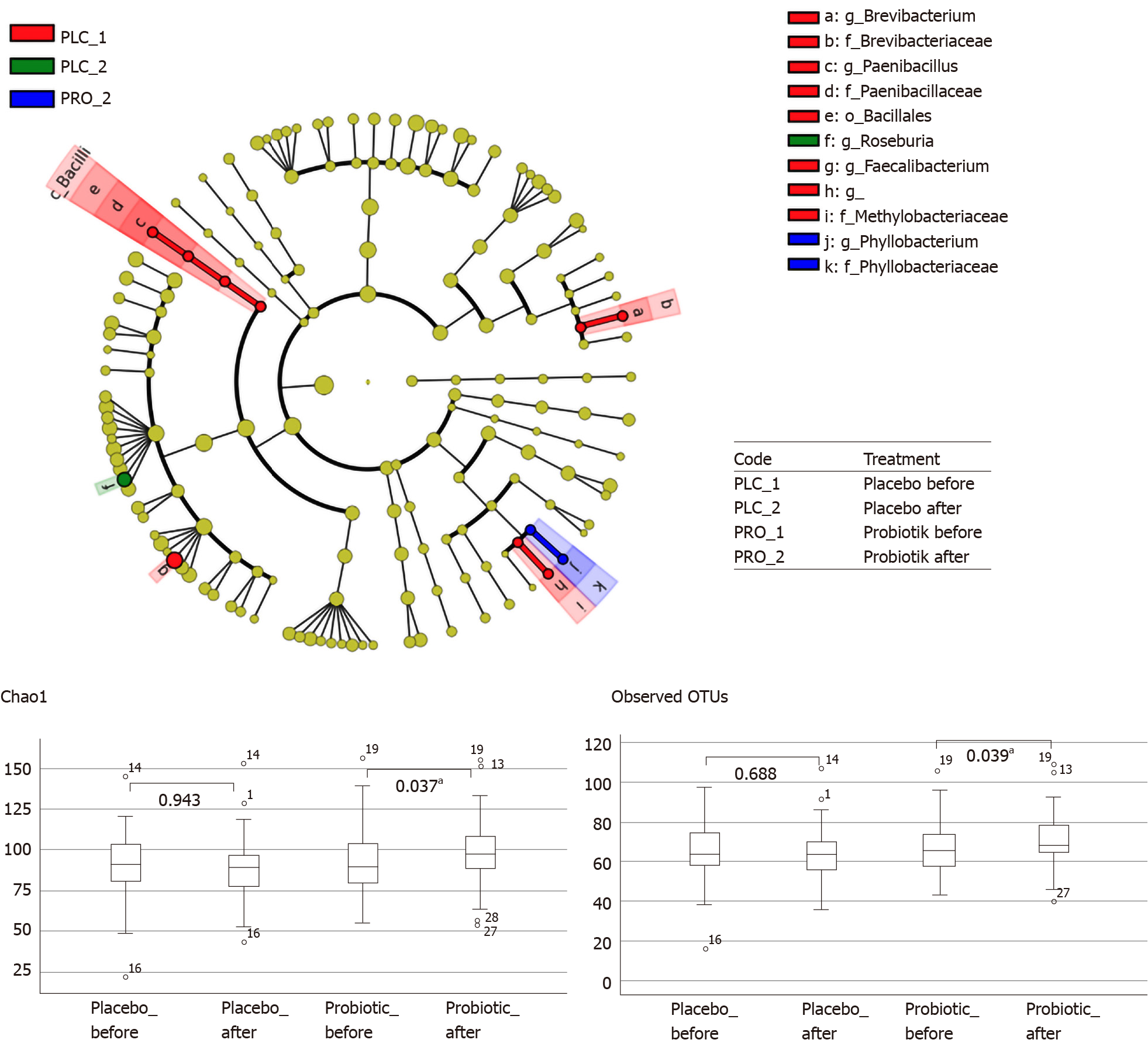
From the analyses using LeSfe with a P value of < 0.05 (LDA > 2.0), one against all showed a significant difference in bacterial abundance in the probiotic-treated and placebo groups before and after the ingestion period. Alpha diversity analysis (Figure 5) showed that the abundance of bacteria in the probiotic-treated group significantly increased after they consumed the probiotic powder. This indicates that the consumption of probiotics could increase the abundance of bacteria in obese people, who have a diversity and wealth of microbiota gut components compared with eutrophic subjects[32]. At the genus level, a significant increase was observed in the abundance of the genus Phyllobacterium in the probiotic-treated group after consumption, whereas in the control group, Roseburia abundance increased significantly after consumption. The Brevibacterium, Paenibacillus, Bacillales, and Faecalibacterium groups were abundant in the placebo group before the consumption of the placebo product.
Ley et al[33] authored one of the first studies linking GM to obesity in humans[4]. The results from the 16 rRNA gene sequences in mouse models indicated that the two most abundant bacterial phyla were Firmicutes (60%-80%) and Bacteroidetes (20%-40%). In particular, the ob/ob mice had a 50% decrease in the population of Bacteroidetes and a proportional increase in Firmicutes. These changes indicate that obesity affects the diversity of GM and suggest that the intentional manipulation of the community structure may be useful to regulate the energy balance in the obese individual[4,33]. Meanwhile, Turnbaugh et al[34] and Furet et al[35] found a lower representation of Bacteroidetes (Bacteroides/Prevotella) in obese individuals, with no differences in the Firmicutes phylum.
In addition, an ongoing review of GM and obesity found evidence of the association between gut bacteria and obesity[36,37]. Normally, the subclass distributions of GM are composed of the following: Bacteroidetes (23%), comprising the genus Bacteroides; Firmicutes (64%), including Bacilli, Clostridia, and Mollicutes; Proteobacteria (8%), gram-negative bacteria, such as E. coli and Helicobacter pylori; Fusobacteria, Verrucomicrobia, and Actinobacteria (3%), which include species such as Bifidobacteria; and only about 2% of other phyla. Our findings also indicate that an obese person has a different microbial proportion of the dominant phyla, which consists of the higher Firmicutes of about 70% and the lower Bacteroidetes of about 21%, compared with a normal person, as mentioned in another study by Abenavoli et al[37]. The other phyla comprise Actinobacteria at about 6%, Proteobacteria at 3%, and less than 1% of other bacteria, such as Fusobacteria, Verrucomicrobia, Cyanobacteria, and Lentisphaerae.
Jumpertz et al[38] investigated the dynamic changes of GM during diets that varied in caloric content in the feces of lean and obese individuals by measuring ingested and stool calories using bomb calorimetry. The alteration of the nutrient load induced rapid changes in the GM. These changes were directly correlated with stool energy loss in lean individuals, such as a 20% increase in Firmicutes and a corresponding decrease in Bacteroidetes, which were associated with an increased energy harvest. A high degree of overfeeding in lean individuals was accompanied by a greater fractional decrease in stool energy loss. These results show that the nutrient load is a key variable that can influence the gut (fecal) bacterial community structure over short periods. Furthermore, the observed associations between gut microbes and nutrient absorption indicate a possible role of the human GM in the regulation of the nutrient harvest. Recent studies have shown that the increase of bile acids in the intestine when comparing sterile rats with normal rats would show that the GM are related to not only obesity but also a diverse range of metabolic diseases[39].
Several mechanisms have been proposed for GM causative action in obesity physiopathology. In fact, gut commensal bacteria interact with our metabolism at several points. They help convert ingested complex nutrients to SCFAs, transform mucins and dietary fibers into simple sugars ready for absorption, stimulate intestinal epithelial proliferation, and favor nutrient absorption and metabolism. They are the main actor in shaping the gut crucial defense barrier constituted by the systemic and mucosal immune system and activate bio-inactive compounds[40]. Nevertheless, GM play an important role in human adipose tissue formation and deposition. Indeed, our intestinal bacteria can maintain the human body’s energy balance mainly because of their ability to share the otherwise indigestible components of a mammalian’s diet[41]. In this study, the average daily energy intake of the subjects was around 1518.17-1642.88 kcal/d, less than that of a normal adult (around 2000 kcal/d). No significant differences were observed in the diet profile of the subjects in both the probiotic-treated and placebo groups.
Abenavoli et al[37] mentioned in their review that evidence of the association between gut bacteria and obesity exists in both infants and adults. Several genetic, metabolic, and inflammatory pathophysiological mechanisms are involved in the interplay between gut microbes and obesity. Microbial changes in the human gut can be considered a factor in obesity development in humans. The modulation of the bacterial strains in the digestive tract can help reshape the metabolic profile in the human obese host, as suggested by data from several animal and human studies. Several reports have also been conducted on the probiotic treatment of obese individuals. In adults, different strains of Lactobacillus and Bifidobacterium, alone or in combination, as well as P. pentosaceus led to a significant reduction of body weight, BMI, waist circumference, and fat mass[17,42-46].
As the administered dosage of probiotics affects the efficacy of the treatment, reduced visceral adiposity and waist circumference were observed after exposure to a high dose of L. gasseri BNR17[44]. These results were not so unambiguous given the different doses of Ecologic® (a mixture of multi-strains of Lactobacillus and Bifidobacterium), although this study was only conducted on obese women[47]. Interestingly, a report by Sanchez et al[48] showed the gender-specific effects of probiotics in human obese subjects. Indeed, the administration of L. rhamnosus CGMCC1.3724 and a restricted caloric diet resulted in significantly higher weight loss in obese women than in men. This finding can be explained by a greater impact on satiety, eating habits, and mood in women vs men[48]. Finally, scant evidence exists on the potential preventive effect on obesity of some probiotics in non-obese subjects. Specifically, VSL#3 can reduce body weight and fat accumulation via L. gasseri SBT2055 administration[16,49].
No significant differences in pH were found before and after ingestion in both the probiotic and placebo groups as well as in the lipid profile of both cholesterol and triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and the LDL/HDL ratio. In addition, no significant changes in the concentration of SCFAs (acetic acid, propionate, and butyrate) were observed after the consumption of the probiotic powder L. plantarum Dad-13.
An interesting finding is a significant decrease in body weight and BMI (P < 0.05) in the treatment group. This weight loss was particularly observed in the female subjects. GM analysis shows that L. plantarum Dad-13 was able to decrease Firmicutes and increase Bacteroidetes (especially Prevotella).
Gut microbiota (GM) play an important role in the nutrient absorption and energy regulation of individuals, thus affecting their nutritional status. GM also affect body weight, especially obesity, a condition wherein the accumulation of abnormal or excessive fat can interfere with health. Obesity in Indonesia showed an increasing prevalence in every province from 2007 to 2018. One study found a link between GM and body weight. Probiotics, as healthy bacteria, can improve an individual’s health status by affecting GM composition. The consumption of probiotics may maintain this status and reduce the weight gain of adults with obesity in Indonesia.
This research aimed to investigate the effect of the consumption of an indigenous probiotic on overweight people. The results obtained may be used to determine the condition of GM in overweight people and the effect of indigenous probiotics on the GM of overweight adults. These results may also be used to determine the treatment of probiotic consumption that is most suitable and effective for overweight individuals in Indonesia to improve their health status.
The objective of this study was to determine the effect of the consumption of the indigenous probiotic powder L. plantarum Dad-13 on overweight adults in Indonesia.
Sixty overweight volunteers with body mass index (BMI) equal to or greater than 25 consumed indigenous probiotic powder L. plantarum Dad-13 (2 × 109 CFU/gram/sachet) for 90 d. The study was a randomized, double-blind, placebo-controlled study. The volunteers filled in a diary on a daily basis, which consisted of questions on study product intake (only during the ingestion period), other food intake, number of bowel movements, fecal quality (consistency and color), any medications received, and any symptom of discomfort, such as diarrhea, constipation, vomiting, gassing, sensation of illness, etc. Fecal samples and the subjects’ diaries were collected on the morning of day 10 + 1, marked as the end of the baseline period and the start of the ingestion period. During the ingestion period (from day 11 to day 101), several parameters to measure and analyze the results included body weight and height (once a month), the lipid profile, GM analysis using MiSeq, short-chain fatty acid (SCFA) analysis using gas chromatography, and the measurement of fecal pH using a pH meter.
The consumption of indigenous probiotic powder L. plantarum Dad-13 by overweight people caused the average body weight and BMI of the probiotic group to decrease from 84.54 ± 17.64 kg to 83.14 ± 14.71 kg and from 33.10 ± 6.15 kg/m2 to 32.57 ± 5.01 kg/m2, respectively. No significant reduction in the body weight and BMI of the placebo group was found. An analysis of the microbiota showed that the number of Bacteroidetes, specifically Prevotella, increased significantly, while Firmicutes significantly decreased. No significant change in lipid profile was observed in both groups. Also, no significant change in SCFAs (butyrate, propionate, acetic acid) and pH level were found after the consumption of the probiotic.
No significant differences in pH were found before and after ingestion in both the probiotic and placebo groups as well as in the lipid profile of both cholesterol and triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and the LDL/HDL ratio. In addition, no significant changes were observed in the concentration of SCFAs (acetic acid, propionate, and butyrate) after consumption. Interestingly, a significant decrease in body weight and BMI (P < 0.05) was found in the treatment group. An analysis of the GM shows that L. plantarum Dad-13 was able to decrease Firmicutes and increase Bacteroidetes (especially Prevotella).
These results proved that the consumption of probiotics among overweight adults helps significantly reduce body weight, especially in women, and affects the composition of GM.
The researchers would like to sincerely thank the Indonesian Ministry of Research and Higher Education and Kalbe PT for providing support, as well as the study subjects for their participation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin X S-Editor: Huang P L-Editor: A P-Editor: Ma YJ
| 1. | World Health Organization. What is malnutrition? [Internet]. 2020. Available from: https://www.who.int/features/qa/malnutrition/en/. [Cited in This Article: ] |
| 2. | World Health Organization. Obesity and overweight [Internet]. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Cited in This Article: ] |
| 3. | Ministry of Health of the Republic of Indonesia. Report of Indonesia Basic Health Research in 2018. 2018. Available from: https://www.litbang.kemkes.go.id/Laporan-riset-kesehatan-dasar-riskesdas/. [Cited in This Article: ] |
| 4. | Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070-11075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4639] [Cited by in F6Publishing: 4164] [Article Influence: 219.2] [Reference Citation Analysis (1)] |
| 5. | Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2001] [Cited by in F6Publishing: 2044] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
| 6. | De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691-14696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3584] [Cited by in F6Publishing: 3703] [Article Influence: 264.5] [Reference Citation Analysis (0)] |
| 7. | Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr. 2011;31:15-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 280] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 8. | Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L, Fusek J, Rodrigo L, Kruzliak P. Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab (Lond). 2016;13:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 9. | Rahayu ES, Yogeswara A, Mariyatun, Windiarti L, Utami T, Watanabe K. Molecular characteristics of indigenous probiotic strains from Indonesia. Int J Probiotics Prebiotics. 2016;11:109-116. [Cited in This Article: ] |
| 10. | Rahayu ES, Cahyanto M, Windiarti L, Sutriyanto J, Kandarina T, Utami T. Effects of Consumption of Fermented Milk Containing Indigenous Probiotic Lactobacillus Plantarum Dad-13 on the Fecal Microbiota of Healthy Indonesian Volunteers. Int J Probiotics Prebiotics. 2016;11:91-98. [Cited in This Article: ] |
| 11. | Rahayu ES, Rusdan IH, Athennia A, Kamil RZ, Pramesi PC, Marsono Y, Utami T, Widada J. Safety Assessment of Indigenous Probiotic Strain Lactobacillus plantarum Dad-13 Isolated from Dadih Using Sprague Dawley Rats as a Model. Am J Pharmacol Toxicol. 2019;1:38-47. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Maghfirotin Marta B, Tyas U, Muhammad Nur C, Jaka W, Endang Sutriswati R. Effects of Consumption of Probiotic Powder Containing Lactobacillus Plantarum Dad-13 on Fecal Bacterial Population in School-Age Children in Indonesia. Int J Probiotics Prebiotics. 2019;14:1-8. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Rahayu ES, Utami T, Mariyatun M, Hasan PN, Kamil RZ, Setyawan RH, Pamungkaningtyas FH, Harahap IA, Wiryohanjoyo DV, Pramesi PC, Cahyanto MN, Sujaya IN, Juffrie M. Gut microbiota profile in healthy Indonesians. World J Gastroenterol. 2019;25:1478-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, Tanaka R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl Environ Microbiol. 2004;70:167-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 360] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Matsuda K, Tsuji H, Asahara T, Matsumoto K, Takada T, Nomoto K. Establishment of an analytical system for the human fecal microbiota, based on reverse transcription-quantitative PCR targeting of multicopy rRNA molecules. Appl Environ Microbiol. 2009;75:1961-1969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 432] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 17. | Higashikawa F, Noda M, Awaya T, Danshiitsoodol N, Matoba Y, Kumagai T, Sugiyama M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: a randomized, double-blind, placebo-controlled clinical trial. Eur J Clin Nutr. 2016;70:582-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, Ngom-Bru C, Berger B, Philippe L, Ammon-Zuffrey C, Leone P, Chevrier G, St-Amand E, Marette A, Doré J, Tremblay A. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. 2014;111:1507-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 20. | Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1231] [Cited by in F6Publishing: 1234] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 21. | Larsen N, Vogensen FK, Gøbel RJ, Michaelsen KF, Forssten SD, Lahtinen SJ, Jakobsen M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin Nutr. 2013;32:935-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Institute of Medicine, Food and Nutrition Board. A Report of the Panel on Macronutrients, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference intakes for energy, carbohydrates, fiber, fat, fatty acids, cholesterol, protein and amino acids. The National Academies Press. 2005 . [DOI] [Cited in This Article: ] |
| 23. | Duncan SH, Hold GL, Harmsen HJM, Stewart CS, Flint HJ. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol. 2002;52:2141-2146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 608] [Cited by in F6Publishing: 633] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 25. | Ai D, Pan H, Li X, Gao Y, Liu G, Xia LC. Identifying Gut Microbiota Associated With Colorectal Cancer Using a Zero-Inflated Lognormal Model. Front Microbiol. 2019;10:826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 26. | Brugère JF, Borrel G, Gaci N, Tottey W, O'Toole PW, Malpuech-Brugère C. Archaebiotics: proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes. 2014;5:5-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 27. | Murugesan S, Ulloa-Martínez M, Martínez-Rojano H, Galván-Rodríguez FM, Miranda-Brito C, Romano MC, Piña-Escobedo A, Pizano-Zárate ML, Hoyo-Vadillo C, García-Mena J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis. 2015;34:1337-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Patterson AM, Mulder IE, Travis AJ, Lan A, Cerf-Bensussan N, Gaboriau-Routhiau V, Garden K, Logan E, Delday MI, Coutts AGP, Monnais E, Ferraria VC, Inoue R, Grant G, Aminov RI. Human Gut Symbiont Roseburia hominis Promotes and Regulates Innate Immunity. Front Immunol. 2017;8:1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 29. | Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1101] [Cited by in F6Publishing: 1106] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 30. | Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol. 2011;23:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 289] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 31. | Bunney PE, Zink AN, Holm AA, Billington CJ, Kotz CM. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol Behav. 2017;176:139-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 951] [Cited by in F6Publishing: 762] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 32. | Al-Assal K, Martinez AC, Torrinhas RS, Cardinelli C, Waitzberg D. Gut microbiota and obesity. Clin Nutr Exp. 2018;20:60-64. [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5789] [Cited by in F6Publishing: 5918] [Article Influence: 348.1] [Reference Citation Analysis (0)] |
| 34. | Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153-4158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 670] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 35. | Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, Rizkalla S, Clément K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049-3057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 898] [Cited by in F6Publishing: 831] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 36. | Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192:373-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 820] [Cited by in F6Publishing: 780] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 37. | Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, Aiello V, Romano B, De Lorenzo A, Izzo AA, Capasso R. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients. 2019;11:2690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 286] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 38. | Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 801] [Cited by in F6Publishing: 782] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 39. | Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59:617–628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 40. | Sanmiguel C, Gupta A, Mayer EA. Gut Microbiome and Obesity: A Plausible Explanation for Obesity. Curr Obes Rep. 2015;4:250-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 41. | Stephens RW, Arhire L, Covasa M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity. Obesity (Silver Spring). 2018;26:801-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 42. | Jung S, Lee YJ, Kim M, Kim M, Kwak JH, Lee JW, Ahn YT, Sim JH, Lee JH. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduced body adiposity and Lp-PLA2 activity in overweight subjects. J Funct Foods. 2015;19:744-752. [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Gomes AC, de Sousa RG, Botelho PB, Gomes TL, Prada PO, Mota JF. The additional effects of a probiotic mix on abdominal adiposity and antioxidant Status: A double-blind, randomized trial. Obesity (Silver Spring). 2017;25:30-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Kim J, Yun JM, Kim MK, Kwon O, Cho B. Lactobacillus gasseri BNR17 Supplementation Reduces the Visceral Fat Accumulation and Waist Circumference in Obese Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J Med Food. 2018;21:454-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 45. | Minami J, Iwabuchi N, Tanaka M, Yamauchi K, Xiao JZ, Abe F, Sakane N. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: a randomized, double-blind, placebo-controlled trial. Biosci Microbiota Food Health. 2018;37:67-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Pedret A, Valls RM, Calderón-Pérez L, Llauradó E, Companys J, Pla-Pagà L, Moragas A, Martín-Luján F, Ortega Y, Giralt M, Caimari A, Chenoll E, Genovés S, Martorell P, Codoñer FM, Ramón D, Arola L, Solà R. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes (Lond). 2019;43:1863-1868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 47. | Szulińska M, Łoniewski I, van Hemert S, Sobieska M, Bogdański P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients. 2018;10:773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 48. | Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, Doré J, Tremblay A. Effects of a Diet-Based Weight-Reducing Program with Probiotic Supplementation on Satiety Efficiency, Eating Behaviour Traits, and Psychosocial Behaviours in Obese Individuals. Nutrients. 2017;9:284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 49. | Osterberg KL, Boutagy NE, McMillan RP, Stevens JR, Frisard MI, Kavanaugh JW, Davy BM, Davy KP, Hulver MW. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity (Silver Spring). 2015;23:2364-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |