Published online Dec 7, 2020. doi: 10.3748/wjg.v26.i45.7131
Peer-review started: July 25, 2020
First decision: September 14, 2020
Revised: September 28, 2020
Accepted: November 12, 2020
Article in press: November 12, 2020
Published online: December 7, 2020
Altered tight junction (TJ) proteins are correlated with carcinogenesis and tumor development. Nimbolide is a tetranotriterpenoid that has been shown to have antioxidant and anti-proliferative properties; however, its anticancer effects and molecular mechanism in hepatocellular carcinoma (HCC) remains obscure.
To investigate the effect of nimbolide on TJ proteins, cell cycle progression, and hepatic inflammation in a mouse model of HCC.
HCC was induced in male Swiss albino mice (CD-1 strain) by a single intraperitoneal injection of 100 mg/kg diethylnitrosamine (DEN) followed by 80 ppm N-nitrosomorpholine (NMOR) in drinking water for 28 wk. After 28 wk, nimbolide (6 mg/kg) was given orally for four consecutive weeks in DEN/NMOR induced HCC mice. At the end of the 32nd week, all the mice were sacrificed and blood and liver samples were collected for various analyses. Macroscopic examinations of hepatic nodules were assessed. Liver histology and HCC tumor markers such as alpha-fetoprotein (AFP) and glypican-3 were measured. Expression of TJ proteins, cell proliferation, and cell cycle markers, inflammatory markers, and oxidative stress markers were analyzed. In silico analysis was performed to confirm the binding and modulatory effect of nimbolide on zonula occludens 1 (ZO-1), nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-κB), and tumor necrosis factor alpha (TNF-α).
We found nimbolide treatment at a concentration of 6 mg/kg to HCC mice reduced hepatic tumor size by 52.08% and tumor volume (P < 0.01), and delayed tumor growth in HCC mice with a concomitant reduction in tumor markers such as AFP levels (P < 0.01) and glypican-3 expression (P < 0.05). Furthermore, nimbolide treatment increased tight junction proteins such as ZO-1 and occludin expression (P < 0.05, respectively) and reduced ZO-1 associated nucleic acid binding protein expression (P < 0.001) in HCC mice liver. Nimbolide treatment to HCC mice also inhibited cell proliferation and suppressed cell cycle progression by attenuating proliferating cell nuclear antigen (P < 0.01), cyclin dependent kinase (P < 0.05), and CyclinD1 (P < 0.05) expression. In addition, nimbolide treatment to HCC mice ameliorated hepatic inflammation by reducing NF-κB, interleukin 1 beta and TNF-α expression (P < 0.05, respectively) and abrogated oxidative stress by attenuating 4-hydroxynonenal expression (P < 0.01). Molecular docking studies further confirmed that nimbolide interacts with ZO-1, NF-κB, and TNF-α.
Our current study showed for the first time that nimbolide exhibits anticancer effect by reducing tumor size, tumor burden and by suppressing cell cycle progression in HCC mice. Furthermore, nimbolide treatment to HCC mice ameliorated inflammation and oxidative stress, and improved TJ proteins expression. Consequently, nimbolide could be potentially used as a natural therapeutic agent for HCC treatment, however further human studies are warranted.
Core Tip: The effects of nimbolide on tight junction (TJ) proteins, cell cycle progression, and hepatic inflammation in diethylnitrosamine (DEN) and N-nitrosomorpholine (NMOR) induced hepatocellular carcinoma (HCC) mouse model is unknown. This study revealed for the first time that nimbolide suppresses tumor growth by restoring hepatic TJ proteins, inhibiting cell proliferation and cell cycle progression, and attenuating inflammation and oxidative stress in HCC mice. Moreover, nimbolide showed a modulatory effect on zonula occludens 1, nuclear factor of kappa light polypeptide gene enhancer in B-cells and tumor necrosis factor alpha proteins confirmed by in silico analysis. All the above results clarified that nimbolide abolished DEN and NMOR induced hepatocarcinogenesis and suggested that nimbolide could be a future potential drug candidate for the treatment of human HCC.
- Citation: Ram AK, Vairappan B, Srinivas BH. Nimbolide inhibits tumor growth by restoring hepatic tight junction protein expression and reduced inflammation in an experimental hepatocarcinogenesis. World J Gastroenterol 2020; 26(45): 7131-7152
- URL: https://www.wjgnet.com/1007-9327/full/v26/i45/7131.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i45.7131
Hepatocellular carcinoma (HCC) is one of the most lethal malignancies and ranks third in the annual cancer mortality rate with overall 6-20 months of median survival after diagnosis[1]. The etiological factors such as hepatitis viral infection and alcohol abuse instigate chronic inflammation in the liver and lead to cirrhosis and ultimately HCC at the terminal stage[2]. The treatment modalities for HCC are limited with surgical resection and liver transplantation. The frequency of recurrence of HCC is high[1]. Since most of the HCC patients are diagnosed in an advanced stage, surgical options are unsuitable and only systemic chemotherapy (doxorubicin or sorafenib) and supportive care are the best available treatment options, however, only minimally increase the lifespan. Moreover, chemotherapy has low efficiency and side effects, and do not improve the 5-year survival rates[3]. Besides, due to the development of drug resistance and the existence of genetic heterogeneity within the individuals, the clinical outcome of targeted therapies is less effective[4]. Therefore, the discovery and development of successful new molecular chemotherapeutic agents targeting multiple pathways simultaneously are an immediate need to prevent the disease progression and recurrence in HCC patients.
Tight junction (TJ) proteins are intercellular adhesion molecules located at the apical and basolateral membrane and play a fundamental role in maintaining tissue barrier and cellular integrity[5]. It regulates paracellular movement in endothelial cells, whereas in epithelial cells it tightly seals the gap between adjacent cells in an adhesive manner and prevents cell dissociation[6]. Of note, HCC is a type of epithelial cell carcinoma[7]. In the liver, TJ proteins are present in the hepatocytes and biliary epithelial cells, which form a blood-biliary barrier[8]. Zonula occludens (ZO)-1, a membrane-associated scaffolding protein that belongs to membrane associated guanylate kinase family, contains three PDZ domains, a single SH3 domain and a GK domain[9]. ZO-1 binds to occludin (transmembrane protein) via its GK domain and interacts with homologous family members ZO-2 and ZO-3 via the second PDZ domain[10,11]. Apart from barrier function, TJs proteins have been implicated in cellular signal transduction to modulate cell proliferation and differentiation[12]. It has been found that SH3 domain of ZO-1 shares homology with SH3 domain of lethal disc large-1 tumor suppressor gene of drosophila, and its deletion or mutation leads to cell overgrowth and neoplastic changes, suggesting ZO-1 acts as a tumor suppressor[13]. ZO-1 binds to Y-box transcription factor ZO-1 associated nucleic acid binding protein (ZONAB), which interacts with the cell cycle kinase cyclin dependent kinase (CDK4), and regulates transcription of cell cycle related genes such as proliferating cell nuclear antigen (PCNA) and CyclinD1, providing a molecular link between ZO-1 and ZONAB, thereby regulating cellular proliferation[14,15]. Alteration in TJ proteins leads to a decrease in cell-cell adhesion, which is a crucial step to the cellular phenotypic transformation for tumor initiation and progression. Indeed, loss of TJ proteins such as ZO-1, occludin, and claudin have been shown to correlate with increased invasiveness, metastasis, and poor prognosis in breast cancer and colorectal cancer[16-18]. Similarly, although occludin-/- mice exhibited no gross anatomical defects, the gastric epithelial cells became hyperplastic after 40 wk[19]. Of note, our human study in HCC patients indicated a significant correlation between hepatic ZO-1 and high-sensitive C-reactive protein, however, the causal relationship remains unclear[20].
Nimbolide is a tetranotriterpenoid, and an active compound isolated from leaves and flowers of the Neem tree (Azadirachta indica), which is a traditional medicinal plant of Meliaceae family which is widely distributed in Asia, Africa, and other tropical parts of the world[21]. Several published studies have shown evidence that nimbolide induces apoptosis, inhibits cell proliferation, and metastasis in a variety of cancer cells including prostate, breast cancer, and choriocarcinoma cell lines[22-24]. Furthermore, the antitumor activities of nimbolide have been shown to have beneficial effects in pre-clinical models of various cancers such as oral squamous cell carcinoma[25], prostate cancer, and pancreatic cancer[26,27]. Nimbolide also induces cell cycle arrest and apoptosis in renal cell carcinoma[28] and its antioxidant and anti-inflammatory activities were widely studied using in vivo and in vitro models[29,30]. In the current study, we aimed to investigate the effect of nimbolide on TJ protein expression, cell cycle progression, and inflammation in diethylnitrosamine (DEN) and N-nitrosomorpholine (NMOR) induced experimental HCC in mice.
DEN (N0756) and NMOR (N7382) were purchased from Sigma-Aldrich, St. Louis, Missouri, United States. Nimbolide of 97% purity (AHRF/NP/001) was procured from Asthagiri Herbal Research Foundation, Chennai, Tamilnadu, India. Doxorubicin hydrochloride (10 mg vial, Biochem pharmaceutical industries Ltd., Mumbai, India) was obtained from the Department of Medical Oncology, JIPMER.
Male Swiss mice (CD-1 strain) weighing 10-15 g were obtained from the central animal facility, JIPMER, and maintained in a specific pathogen-free environment. All the animals were housed in polypropylene cages and maintained at 21-25 °C with 12 h light/dark cycle and free (ad libitum) access to water and standard rodent pellets. All experimental study was approved by JIPMER Institute Animal Ethics Committee and was carried out in accordance with the Committee for the Purpose of Control and Supervision of Experiments in Animals (CPCSEA) guidelines.
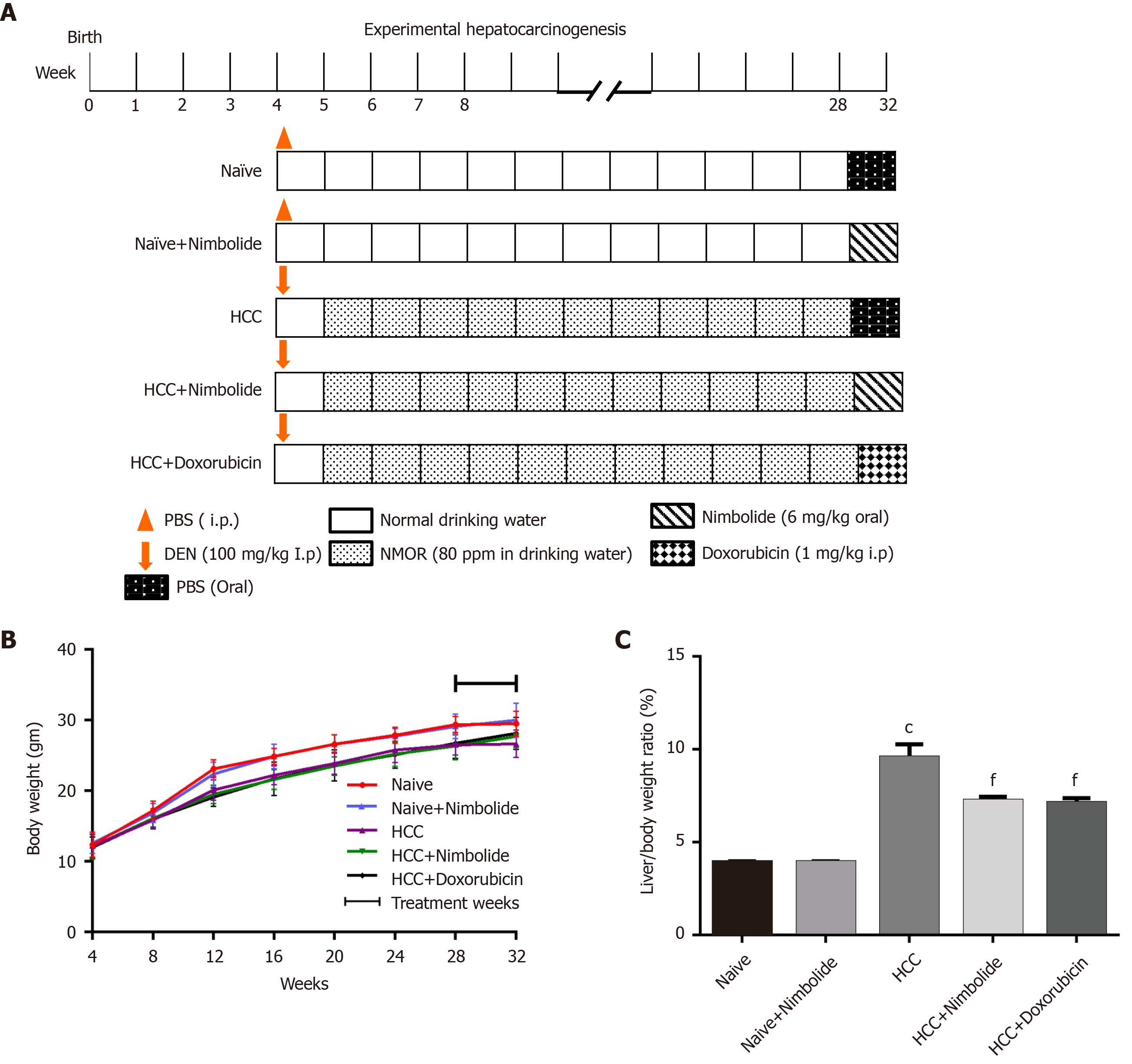
Induction of HCC: Hepatocellular carcinoma was induced in mice by administering a single intraperitoneal (i.p.) injection of DEN [100 mg/kg body weight in phosphate buffer saline (PBS)] and subsequent administration of NMOR in the drinking water (80 ppm) for 28 wk. After tumor formation at 28th week, the mice were randomized into five groups. Group I Naive mice continued to receive standard pellet diet and water ad libitum for four weeks. Group II naïve + treatment mice continued to receive standard pellet diet and water ad libitum, and were administered with nimbolide (6 mg/kg body weight in PBS) daily for four weeks through oral gavage. Group III HCC mice continued to receive standard pellet diet and water ad libitum, and were administered PBS (oral) for four weeks. Group IV HCC mice were administered with nimbolide (6 mg/kg body weight in PBS) daily for four weeks through oral gavage. Group V HCC mice were treated with the standard anticancer drug doxorubicin hydrochloride (1 mg/kg body weight in saline; i.p.) weekly thrice for the next four weeks to compare the chemotherapeutic efficacy of nimbolide. The final study groups were: (1) Naïve (n = 8); (2) Naïve + nimbolide (n = 8); (3) HCC (n = 10); (4) HCC + nimbolide (n = 10); and (5) HCC + doxorubicin (n = 10). The experimental protocol and treatment schedule is depicted in Figure 1A.
At termination (end of 32nd week) mice in different study groups were sacrificed by exsanguination under anaesthesia (ketamine hydrochloride 100 mg/kg). Blood was collected from the cardiac puncture in a heparinized tube, centrifuged at 3500 rpm at
The entire visible and macroscopic nodules on the surface of the liver were counted for each mouse in all experimental groups. The length (L) and width (W) of superficial tumor nodules were measured by using a digital vernier caliper. The largest length (L) of the nodule was considered as the maximum tumor diameter. The volume of the tumor was calculated using the formula; volume (V) = L × (W)2/2. Tumor burden was calculated by multiplying mean tumor volume with the no. of nodules for each mouse in the experimental groups. Percentage tumor growth inhibition (% TGI) was defined as the difference between mean tumor volume (MTV) of DEN + NMOR treated (MTVD) and chemotherapeutic group (MTVT) at 32nd week, using the formula: % TGI = [(MTVD - MTVT)/MTVD] × 100.
For histopathological analysis, 10% formalin-fixed liver tissues were dehydrated with a series of graded alcohol, cleared in xylene, and embedded in paraffin wax. Thereafter, 5 μm tissue sections were cut and stained with hematoxylin and eosin (HE) with standard protocol. HE stained sections were assessed for histological features of HCC by an independent pathologist in a blind fashion. Numbers of mitotic cells in 10 high-power fields (HPF, 400 ×) were counted on HE stained slides and the highest number of mitotic cells among them was defined as the mitotic index.
Plasma liver function parameters such as aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were measured by autoanalyzer using Beckman coulter kits (United States), according to manufacturer instruction. HCC tumor marker alpha-fetoprotein (AFP) was measured in plasma by ELISA kit (CKbio-15638; Shanghai, China).
Liver tissues were snap-frozen in liquid nitrogen and stored at -80 °C for western blot analysis. About 150 mg of tissue was weighed and homogenized in 500 μL ice-cold RIPA buffer (G-Biosciences, St. Louis, MO, United States) with protease inhibitor cocktail (P8340, Sigma-Aldrich, St. Louis, MO, United States) and 10% protein methyl sulfonyl fluoride (in ethanol). Tissue homogenate was centrifuged at 14000 g for 10 min at 4 °C and supernatant was collected. The protein content of the individual sample was estimated by Bradford assay using the Pierce coomassie protein assay kit (23200, Thermo Fisher Scientific, Rockford, IL, United States). About 30 μg of total protein of individual sample was diluted in SDS sample buffer and were resolved using SDS-polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane (S045A330R, Advantec, Tokyo, Japan). Then the membranes were blocked for non-specific binding with 5% non-fat dry milk (GRM1254; Himedia, Mumbai, India) in Tris-buffered saline containing 0.01% Tween 20 (TBST) for 1 h at room temperature, followed by overnight incubation with primary antibody at 4 °C. The primary antibodies used were rabbit polyclonal anti-ZO1 (1:1000, 61-7300; Thermo Fisher, Rockford, IL, United States), rabbit polyclonal anti-ZONAB (1:500, 40-2800; Thermo Fisher, Rockford, IL, United States), mouse monoclonal anti-occludin (1:1000, sc-133256; Santa Cruz, Dallas, TX, United States), rabbit polyclonal anti-CDK4 (1:1000, A0366; Abclonal, Woburn, Massachusetts, United States), rabbit polyclonal anti-CyclinD1 (1:1000, A2708; Abclonal, Woburn, MA, United States), rabbit monoclonal anti-nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-κB)p65 (1:1000, 8242S; CST, Danvers, MA, United States), rabbit polyclonal anti-tumor necrosis factor alpha (TNF-α) (1:1000, 2081R; BIOSS, Woburn, Massachusetts, United States), rabbit polyclonal anti-Interleukin 1 beta (IL-1β) (1:1000, A11370; Abclonal, Woburn, MA, United States) and mouse monoclonal anti-β-actin (1:1000, AC004; Abclonal, Woburn, MA, United States). After overnight incubation, membranes were washed with TBST three times and incubated with peroxidase-conjugated goat anti-rabbit (1:10000, 406401; BioLegend, San Diego, CA, United States) or goat anti-mouse (1:10000, 405306; BioLegend, San Diego, CA, United States) for 1 h at room temperature and the bands were visualized using an enhanced chemiluminescent substrate (34087; West pico detection kit, Thermo Scientific, Rockford, IL, United States) in Chemidoc system (Bio-rad, United States). Protein bands were quantified using Image Lab software (Bio-rad, United States).
3-5 μm thick sections were cut using an automated microtome (Leica Biosystems) and mounted on a silane coated slide. After deparaffinization, slides were immersed in xylene and rehydrated in different graded ethanol followed by 3% H2O2 in methanol for 15 min to quench the endogenous peroxidase activity. Then the sections were rinsed with PBS, and antigen retrieval was performed using citrate buffer (PH = 6.0) in the decloaking system at 110°C for 10 min. After blocking, the sections were incubated with primary antibody overnight at 4°C. Primary antibodies used were rabbit polyclonal anti-glypican-3 (1:100, A13988; Abclonal, Woburn, MA, United States), rabbit polyclonal anti-PCNA (1:100, A0264; Abclonal, Woburn, MA, United States), and rabbit polyclonal anti-4-Hydroxynonenal (4HNE) (1:100, bs-6313R; Bioss, Woburn, Massachusetts, United States). After washing with PBS, sections were incubated with universal anti-mouse/rabbit Ig (MP-7500; Vector lab, Burlingame, CA, United States) for 30 min at room temperature and immunostaining was performed with ImmPACT DAB kit (SK4105; Vector lab, Burlingame, CA, United States) and counterstained with hematoxylin. After mounting with Histamount solution, sectioned were examined under EVOS FLc imaging system (Life Techonologies, United States).
Molecular docking using Schodinger AutoDock 4.0 software (United States) was performed to predict the binding affinity of nimbolide with ZO-1, NF-κB, and TNF-α. The X-ray crystal structure of ZO-1 [Protein Data Bank archive (PDB) ID: 2RRM], NF-κB (PDB ID: 1VKX), and TNF-α (PDB ID: 2TNF) were retrieved from RCSB protein data bank (http://www.rcsb.org). The proteins were prepared before molecular docking using PyMol software (Schrodinger LLC, Cambridge, United States). Nimbolide (CID: 100017) 2D structure was retrieved from the Pubchem database (https://pubchem.ncbi.nlm.nih.gov/compound) in SDF file format and then converted to .mol2 format using Open Babel software version 2.4.1. After the simulation, different docked conformations were obtained and conformation with the strongest binding affinity towards ligand binding cavity of ZO-1, NF-κB, and TNF-α were selected as possible binding conformations and taken for further analysis. The final assessment of protein-ligand interaction was done and interpreted as Glide score (kcal/moL).
All the data are expressed as mean ± standard error of mean (SEM). Results were analyzed using GraphPad Prism 6.0 software. The comparison between different groups was analyzed by one-way ANOVA followed by Tukey’s multiple comparison post-hoc test or by Kruskal-Wallis followed by Dunn’s multiple comparison post-hoc test. A P < 0.05 was considered as statistical significance.
Experimental mice were treated with nimbolide or doxorubicin (Figure 1A) and the average weekly bodyweight of the naive and experimental mice was represented in Figure 1B. HCC induction in mice showed a decreased bodyweight throughout the experimental period compared to naive mice. Nimbolide or doxorubicin treatment to HCC mice showed a trend in increased body weight from 28th to 32nd week (P = 0.6781) (Figure 1B). Furthermore, the liver/body weight ratio measured was higher (P < 0.0001) in HCC mice compared to naïve mice and this may be due to the induction of tumors by DEN and NMOR. On the contrary, treatment with nimbolide to HCC mice showed reduced liver/body weight ratio (P < 0.001) when compared to DEN/NMOR induced HCC mice, suggesting nimbolide possibly alleviated tumor burden (Figure 1C). Similar effects were seen in mice treated with doxorubicin in DEN/NMOR induced HCC mice (Figure 1C). Naïve mice treated with nimbolide shows similar body weight and liver/body weight ratio when compared to the naïve mice alone group.
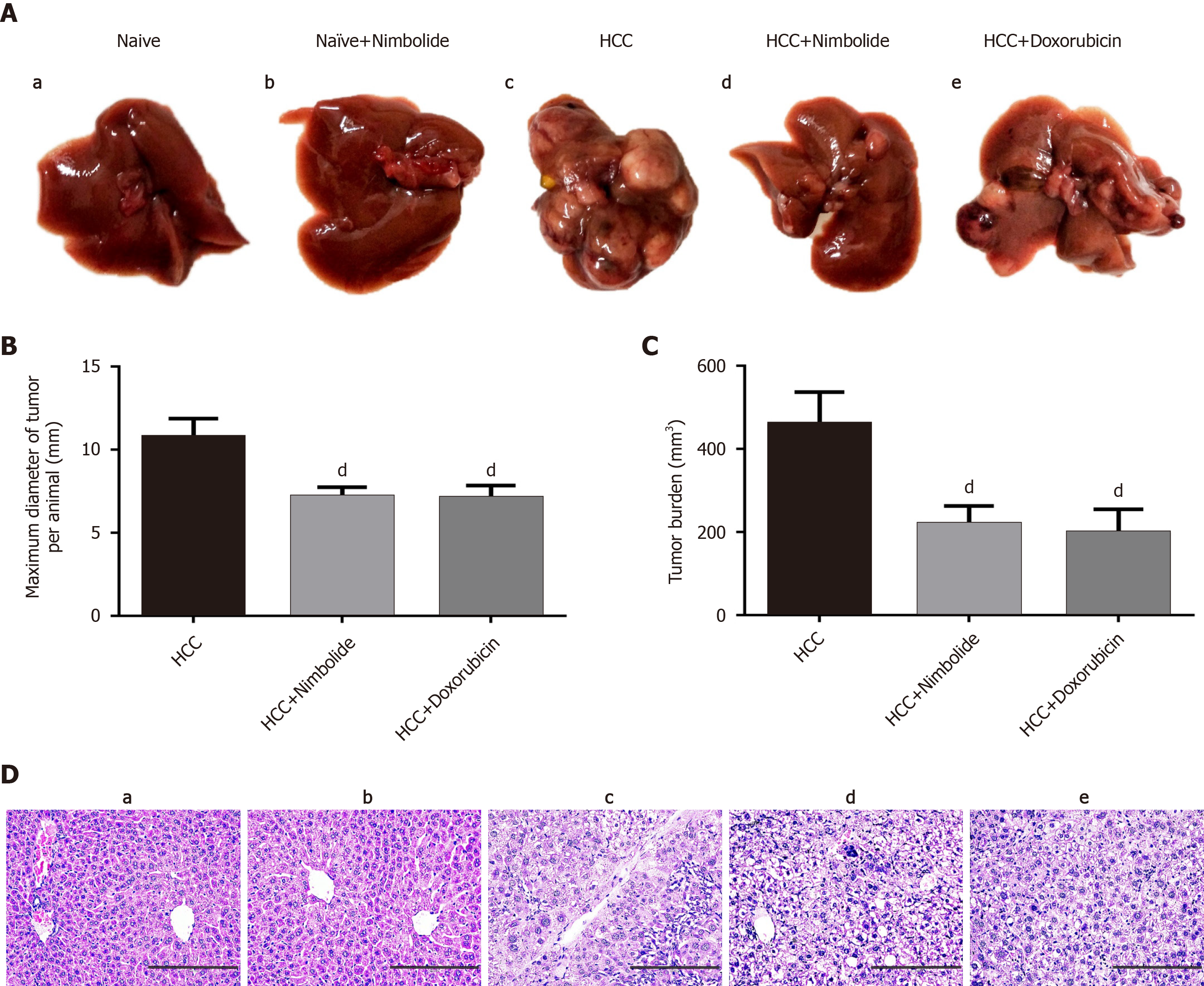
Macroscopically visible hepatic nodules were evident on the liver surface of HCC mice, whereas, treatment with nimbolide or doxorubicin showed much lesser hepatic nodules (Figure 2A). Moreover, the maximum tumor diameter measured was higher in HCC mice and was reduced following treatment with nimbolide (P < 0.05) or doxorubicin (P < 0.05) to HCC mice (Figure 2B). The calculated tumor burden was also higher in HCC mice. Treatment with nimbolide (P < 0.05) or doxorubicin (P < 0.05) to HCC mice showed reduced tumor burden compared to HCC mice alone group (Figure 2C). Also, nimbolide or doxorubicin treatment to HCC mice inhibited tumor growth by 52.08% and 54.45%, respectively, with the reduction in mean tumor volume (P < 0.01) compared to HCC mice alone group (Table 1).
To identify the effective dose of nimbolide, we performed a dose-response study with three different doses of nimbolide (1.5 mg/kg, 3 mg/kg, and 6 mg/kg body weight) in DEN/NMOR induced HCC mice. We found treatment with 6 mg/kg nimbolide reduced maximum tumor diameter (P < 0.05) (Supplementary Figure 1) and tumor volume (P < 0.05) (Supplementary Figure 2) in HCC mice, while 1.5 mg/kg and 3 mg/kg nimbolide treatment were not significant compared to HCC mice alone group. Liver function parameters and AFP levels were also significantly decreased to HCC mice following 6 mg/kg b.w. nimbolide treatment (Supplementary Table 1). Based on the above preliminary data, the effective dose of 6 mg/kg b.w. nimbolide was chosen for subsequent experiments.
To confirm the malignancy potential of DEN/NMOR induced hepatocarci-nogenesis, representative hematoxylin and eosin stained sections of the liver was studied and the results were shown in Figure 2D. Naive (Figure 2D, panel a) and naïve + nimbolide (Figure 2D, panel b) mice liver showed normal hepatic lobular architecture whereas, HCC mice liver showed neoplastic changes (Figure 2D, panel c). The neoplastic changes in the liver showed classical features of HCC characterized by hepatocyte arranged in cords (3 cell layer), cellular atypia, eosinophilic to clear cytoplasm, enlarged nucleus, and hyperchromasia. Treatment with nimbolide or doxorubicin showed minimal cellular atypia and decreased nuclear: Cytoplasmic ratio (Figure 2D, panel d and e). Furthermore, the mitotic index is a simple method for the evaluation of cellular proliferation rate and is a hallmark feature of cancer. Indeed, the mitotic index is a predictor of prognosis in patients with HCC[31]. Hematoxylin and eosin stained liver section of HCC mice showed a higher mitotic index (3-4/10 HPF) whereas, a lower mitotic index (1-2/10 HPF) was observed in nimbolide or doxorubicin treated HCC mice liver (Table 2).
| Experimental groups | Mitotic index/10 HPF |
| Naïve | 0/10 HPF |
| Naïve + nimbolide | 0/10 HPF |
| HCC | 3-4/10 HPF |
| HCC + nimbolide | 1-2/10 HPF |
| HCC + doxorubicin | 1-2/10 HPF |
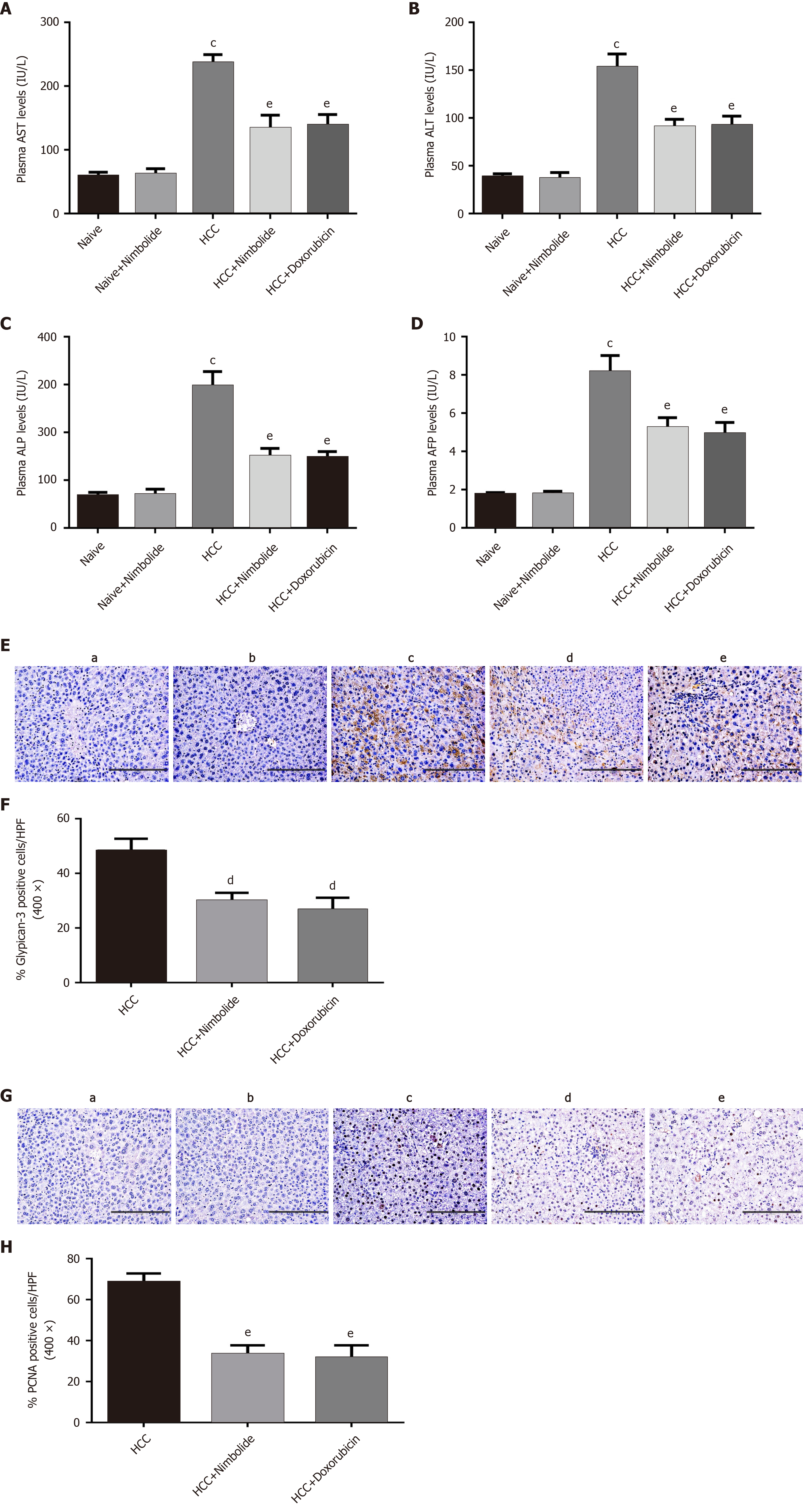
Elevated levels of hepatic dysfunction marker enzymes such as AST, ALT, and ALP are widely used to assess hepatic dysfunction, and are associated with HCC progression[32]. DEN/NMOR induced HCC mice showed elevated levels of AST, ALT, and ALP (P < 0.0001) when compared to naive mice. Treatment with nimbolide or doxorubicin to HCC mice lowered the plasma levels of AST, ALT, and ALP (P < 0.01) compared to HCC mice alone group (Figure 3A-C). Plasma alpha-fetoprotein (AFP) levels and glypican-3 are clinically used as tumor markers for the diagnosis and prognosis of HCC[33,34]. Reduced AFP levels was reported to have prognostic significance after hepatic tumor resection and transarterial chemoembolization in HCC patients[35,36]. We found a higher plasma AFP levels in HCC mice (P < 0.0001) compared to naive mice. Nimbolide or doxorubicin treatment to HCC mice showed decreased plasma AFP levels (P < 0.01) compared to HCC mice alone group (Figure 3D). Increased hepatic glypican-3 expression was associated with a significantly lower 5-year survival rate and poor prognosis in HCC patients[34]. Figure 3E shows the immunostaining of glypican-3 on the liver sections of naive and experimental mice. Naive mice (Figure 3E, panel a) and naïve + nimbolide mice (Figure 3E, panel b) liver showed negative reactivity to glypican-3 whereas, strong glypican-3 cytoplasmic positivity was seen in HCC mice liver (Figure 3E, panel c), while nimbolide or doxorubicin treatment to HCC mice showed reduced glypican-3 cytoplasmic positivity (Figure 3E, panel d and e). Quantification of glypican-3 positive cells in the experimental group was represented in Figure 3F. PCNA is a putative marker for cell proliferation and hepatocyte damage and its high expression was associated with poor survival in HCC patients[37]. Figure 3G shows the immunostaining of PCNA on the liver sections of naive and experimental mice. Negative staining was observed in naïve (Figure 3G, panel a) and naïve + nimbolide (Figure 3G, panel b) mice liver whereas, HCC mice liver (Figure 3G, panel c) displayed much higher PCNA-positive nuclei, while treatment with nimbolide (Figure 3G, panel d) or doxorubicin (Figure 3G, panel e) to HCC mice showed reduced PCNA-positive nuclei. Quantification of PCNA positive nuclei in the experimental group was represented in Figure 3H.
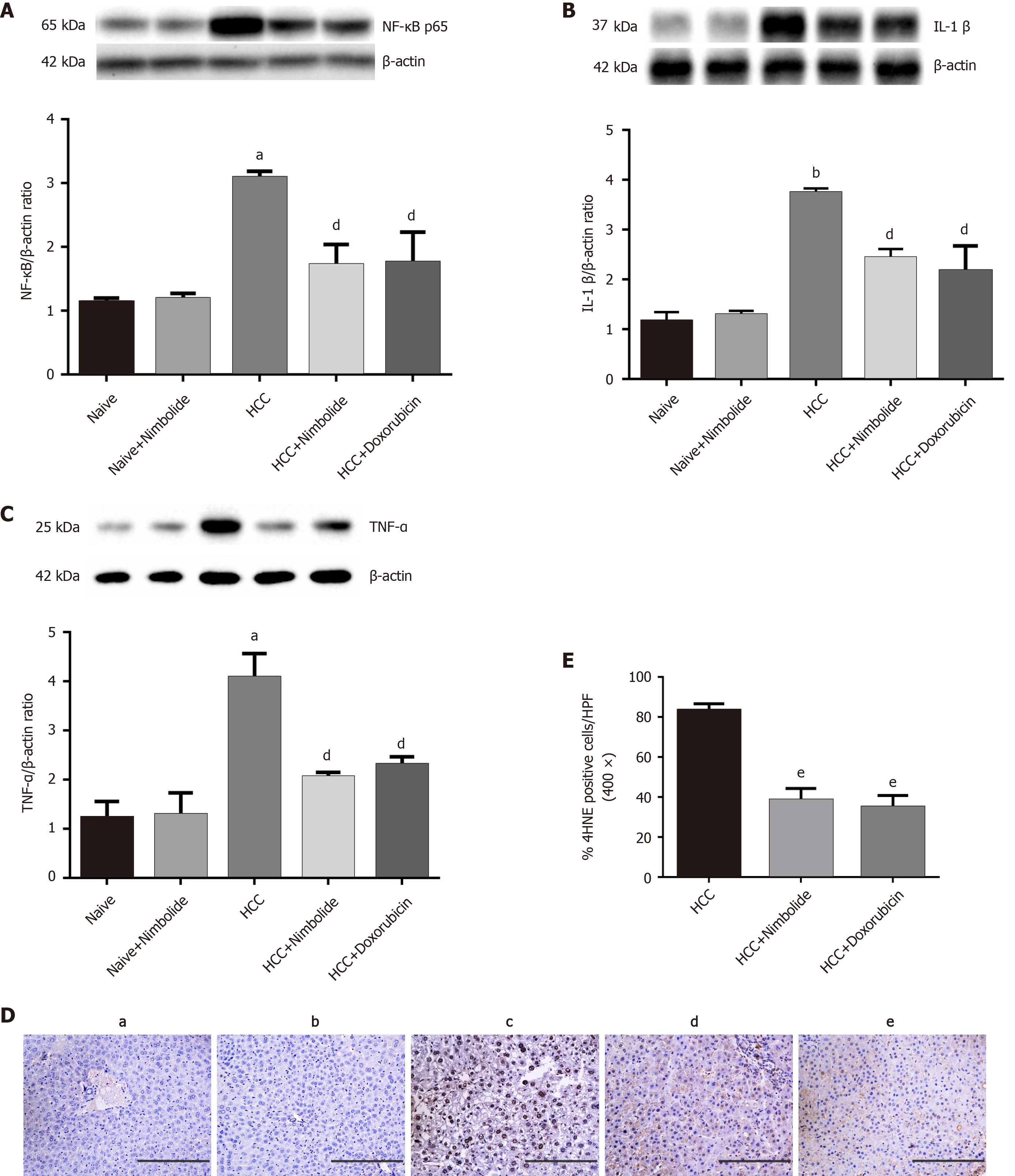
Figure 4A-C shows the hepatic NF-κB, TNF-α, and IL-1β protein expressions by western blot in the naive and experimental mice. The hepatic protein expression of NF-κB (P < 0.01), IL-1β (P < 0.001), and TNF-α (P < 0.01) were increased in HCC mice compared to naive mice. Nimbolide or doxorubicin treatment to HCC mice showed decreased NF-κB (P < 0.05), IL-1β (P < 0.05), and TNF-α (P < 0.05) protein expressions compared to HCC mice alone. Figure 4D shows immunostaining of 4HNE on the liver sections of naive and experimental mice. Naive (Figure 4D, panel a) and naïve + nimbolide (Figure 4D, panel b) mice liver showed negative reactivity to 4HNE while 4HNE expression has increased in HCC mice liver (Figure 4D, panel c). Nimbolide (Figure 4D, panel d) or doxorubicin (Figure 4D, panel e) treatment to HCC mice showed reduced hepatic 4HNE nuclear positivity compared to HCC mice alone. Moreover, 4HNE positive nuclei quantification was done and represented in Figure 4E.
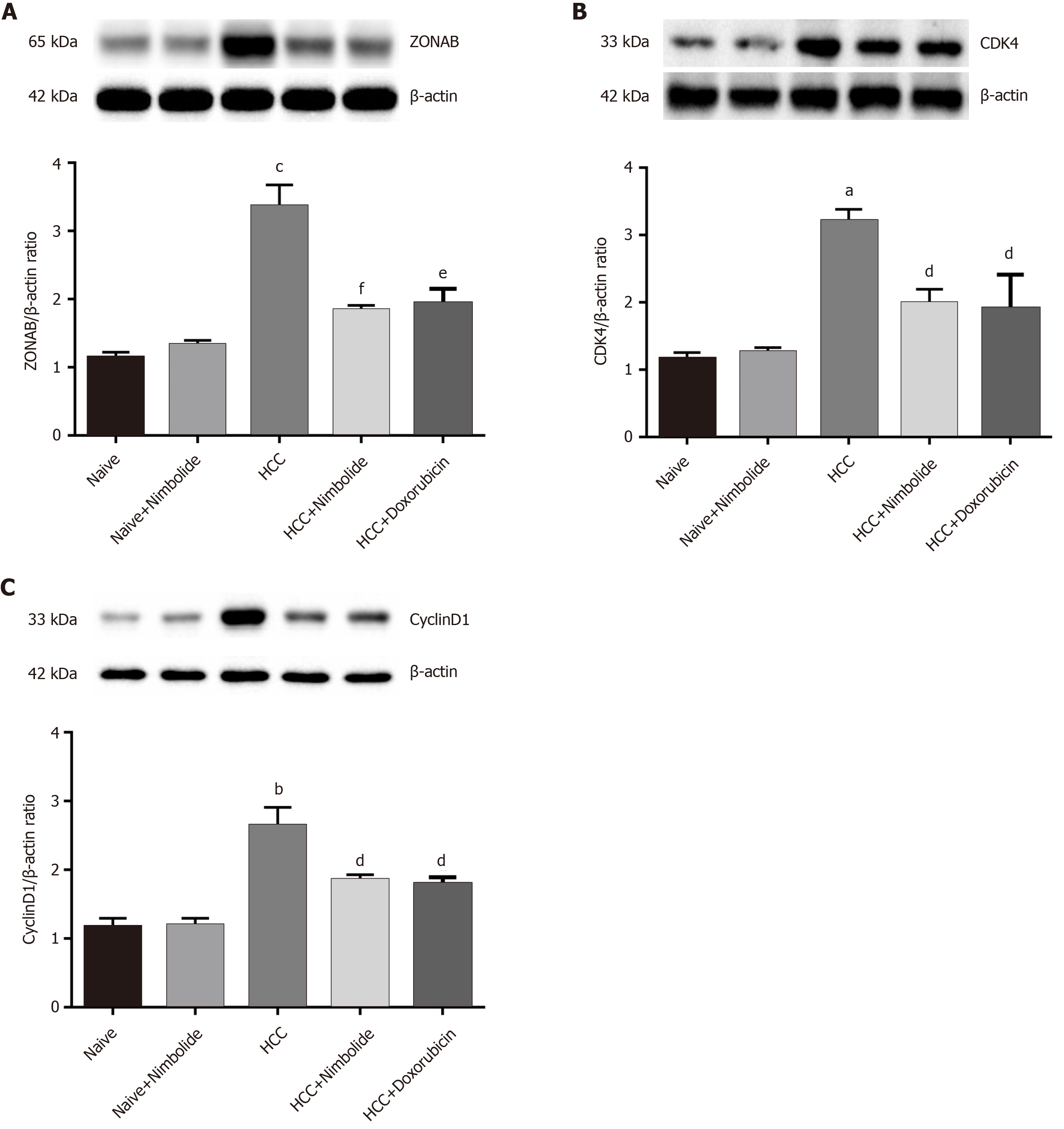
Figure 5A shows the hepatic ZONAB protein expression by western blot in naive and experimental mice. The hepatic ZONAB protein expression was higher (P < 0.0001) in HCC mice compared to naive mice while treatment with nimbolide (P < 0.001) or doxorubicin (P < 0.01) to HCC mice showed reduced ZONAB protein expression compared to HCC mice alone. Figure 5B and C shows the cell cycle markers CDK4 and CyclinD1 protein expressions by western blot in naive and experimental mice. The hepatic CDK4 (P < 0.01) and CyclinD1 (P < 0.001) protein expressions were increased in HCC mice compared to naive mice. Treatment with nimbolide (P < 0.05) or doxorubicin (P < 0.05) to HCC mice showed decreased CDK4 and cyclinD1 protein expression compared to HCC mice alone.
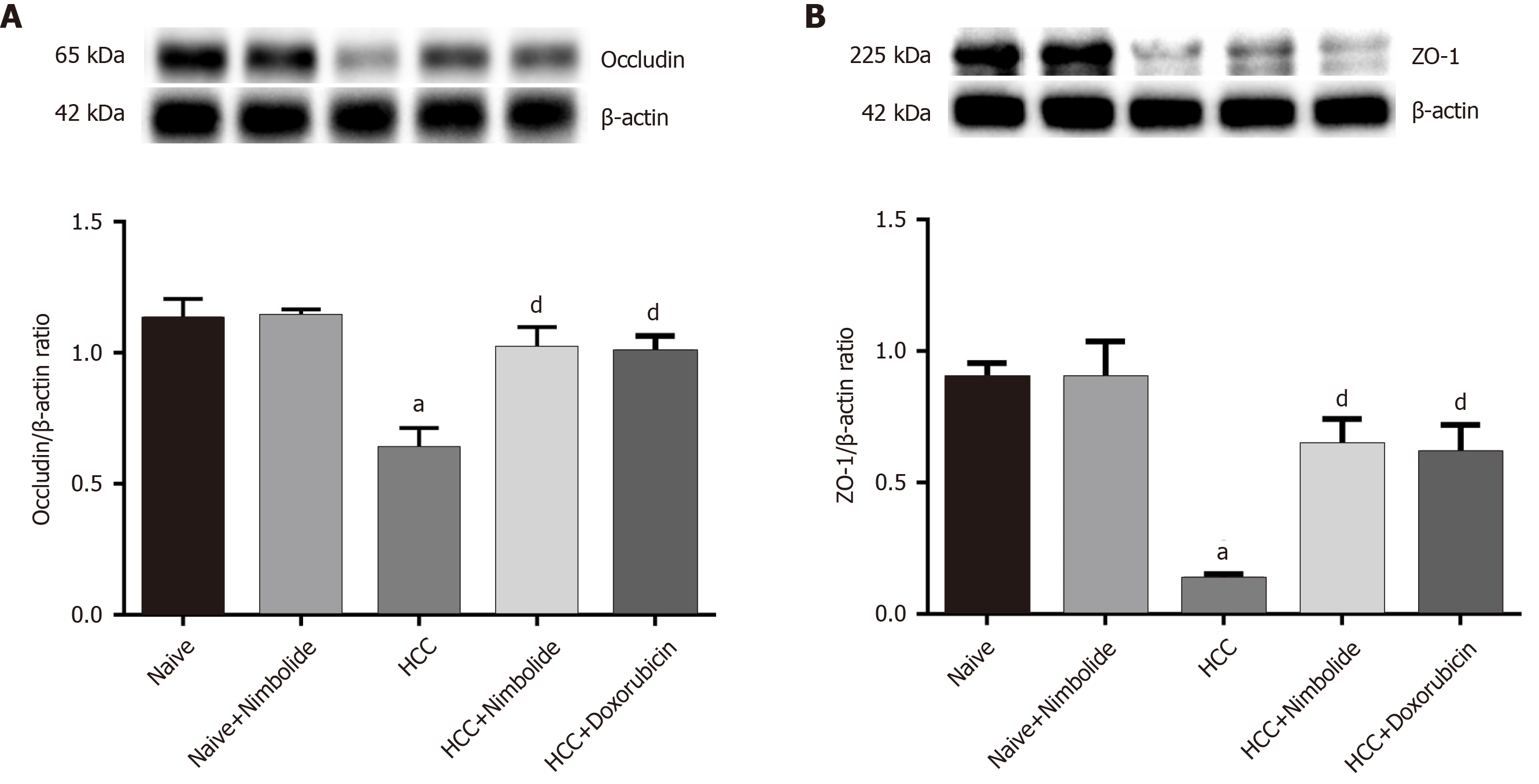
Figure 6A shows the hepatic occludin protein expression by western blot in naive and experimental mice. The hepatic occludin protein expression was down-regulated (P < 0.01) in HCC mice compared to naive mice while treatment with nimbolide (P < 0.05) or doxorubicin (P < 0.05) to HCC mice, occludin protein expression was up-regulated compared to HCC mice alone. Figure 6B shows the hepatic ZO-1 protein expression by western blot in naive and experimental mice. Moreover, hepatic ZO-1 protein expression was reduced (P < 0.01) in HCC mice compared to naive mice. Nimbolide (P < 0.05) or doxorubicin (P < 0.05) treatment to HCC mice showed significantly increased ZO-1 protein expression when compared to lone HCC.
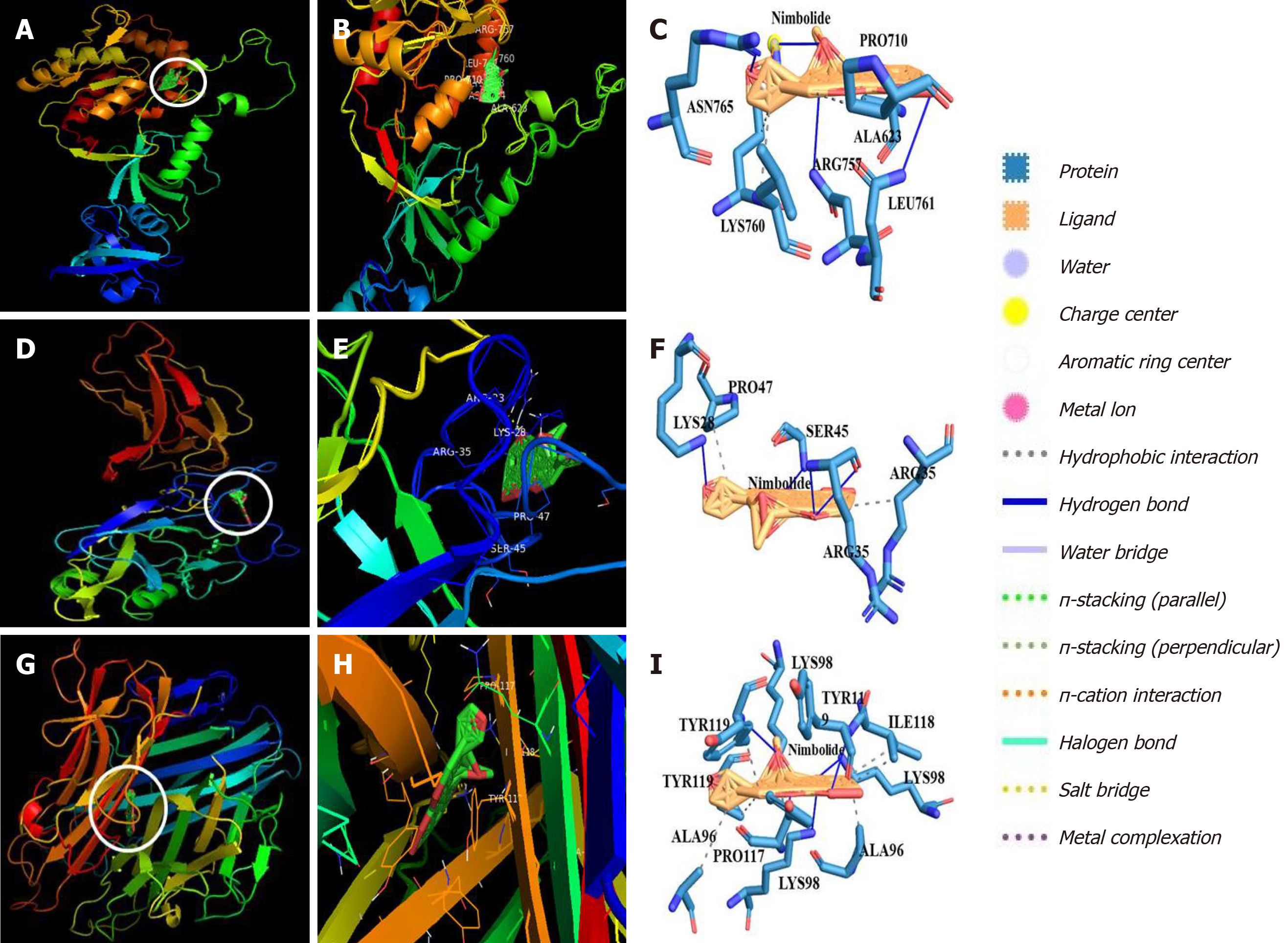
The 2D structure of nimbolide is represented in Supplementary Figure 3. The 3D structure and ligand binding cavity of ZO-1, NF-κB, and TNF-α was shown in Supplementary Figure 4A-F. Docked complexes of nimbolide and ZO-1 protein showed nimbolide formed hydrogen bonds with amino acid residues Arg 757, Lys 760, Asn 764, Asn 765 and hydrophobic interaction with amino acid residues Ala 623, Pro 710, Lys 760, Leu 761 towards predicted ligand-binding cavity of ZO-1 with a glide score of -9.28 kcal/moL which indicates nimbolide has a strong binding affinity and modulatory effect on ZO-1 protein (Figure 7A-C and Table 3). Molecular docking also revealed nimbolide formed hydrogen bonds with amino acid residues Lys 28, Arg 33, Ser 45 and hydrophobic interaction with amino acid residues Arg 33, Arg 35, Pro 47 towards predicted ligand-binding cavity of NF-κB protein with a glide score of -7.62 kcal/moL suggesting nimbolide has a modulatory effect on NF-κB protein (Figure 7D-F and Table 3). Besides, nimbolide also formed hydrogen bonds with amino acid residues Lys 98, Tyr 119 and hydrophobic interaction with amino acid residues Ala 96, Pro 117, Ile 118, Tyr 119 towards predicted ligand-binding cavity of TNF-α protein with a glide score of -8.84 kcal/moL indicating nimbolide has a strong binding and modulatory effect on TNF-α protein (Figure 7G-I and Table 3).
| S. No. | Proteins | Nimbolide (CID: 100017) | ||
| Glide score (kcal/moL) | Hydrogen bonds | Hydrophobic interaction | ||
| 1 | ZO-1 (PDB ID: 2RRM) | -9.28 | Arg 757, Lys 760, Asn 764, Asn 765 | Ala 623, Pro 710, Lys 760, Leu 761 |
| 2 | NF-κB (PDB ID: 1VKX) | -7.62 | Lys 28, Arg 33, Ser 45 | Arg 33, Arg 35, Pro 47 |
| 3 | TNF-α (PDB ID: 2TNF) | -8.84 | Lys 98, Tyr 119 | Ala 96, Pro 117, Ile 118, Tyr 119 |
Our study showed for the first time that nimbolide treatment to DEN and NMOR induced HCC mice exhibits anticancer effect as evidenced by decreased plasma AFP levels and hepatic glypican-3 expression, inhibition of cell cycle progression (ZONAB, CDK4, and cyclinD1), and amelioration of inflammation and oxidative stress. Nimbolide treatment also reduced tumor size, tumor burden, and pathological grade in HCC mice. The other major finding of the current study is increased TJ proteins such as ZO-1 and occludin following treatment with nimbolide to HCC mice. Further, the molecular docking study revealed that nimbolide formed several hydrogen bonds and hydrophobic interactions with amino acid residues of ligand binding cavity of ZO-1, NF-κB, and TNF-α, showing higher binding affinity as observed by high glide score between the docked complexes.
Doxorubicin is a standard anticancer drug that was also administered to HCC mice to compare the effectiveness of nimbolide. Our results revealed that almost 52.08% of tumor growth was inhibited by nimbolide, whereas doxorubicin inhibited 54.45% tumor growth in HCC mice. Additionally, reduction in liver coefficient, tumor diameter, tumor burden, and tumor volume in HCC mice by nimbolide treatment was also comparable with the standard anticancer drug doxorubicin.
A key finding of this study was that nimbolide treatment improved hepatic TJ protein (ZO-1 and occludin) expression in HCC mice. Our previous human study showed evidence that decreased hepatic ZO-1 expression was positively correlated with disease severity in HCC patients[20]. Moreover, occludin was also found to be decreased in HCC patients[38]. Of note, our previous study also showed evidence that cirrhotic mice brain exhibited significantly decreased ZO-1, occludin, and claudin-5 protein expression, which were associated with increased inflammation observed in cirrhotic mice[39,40]. The TJ protein ZO-1 has been reported to be involved in cell proliferation[12]. ZO-1 knock-down in mice showing embryonically lethal and was associated with defective angiogenesis of the yolk sac and impaired apoptosis of the embryonic cells[41]. Besides, the deletion of ZO-1 showed increased cell proliferation and neoplastic changes which suggests ZO-1 may have a tumor suppressor role[13]. In this context, lycopene (carotenoid) administration inhibited human cutaneous squamous cell carcinoma (cSCC) cell proliferation by up-regulating ZO-1 expression[42]. Moreover, earlier studies have suggested that ZO-1 regulates cell proliferation by binding to ZONAB, a Y-box transcription factor that has DNA binding element inverted CCAAT box, whose nuclear accumulation leads to transcription of cell cycle related genes such as CDK4, Cyclin D1, and PCNA[14,15]. This was supported by the fact that, in a sparse culture where the proliferation rate is high, the cellular ZONAB expression is high and the ZO-1 expression is low. By contrast, the ZONAB expression is down-regulated and ZO-1 expression is up-regulated in the confluent quiescent monolayer cells[43]. Knock-down of ZO-1 or overexpression of ZONAB resulted in increased proliferation of retinal pigment epithelial cells and induced epithelial to mesenchymal transition phenotype in mice[44]. In the present study, we found increased hepatic protein expression of ZONAB and its transcriptionally regulated cell cycle mediators such as CDK4, cyclinD1, and PCNA expressions in HCC mice, which were decreased following nimbolide treatment. Furthermore, we noted a reciprocal relationship between the protein expression pattern of ZO-1 and ZONAB in HCC mice which was reversed by nimbolide treatment. Thus, our study demonstrated for the first time that ZO-1/ZONAB pathway plays a crucial role in the pathogenesis of HCC by regulating cell cycle progression. Moreover, we also identified that nimbolide interacts with the ligand-binding cavity of ZO-1, forming hydrogen bonds and hydrophobic interaction with amino acids which may have a modulatory effect.
Another finding in our study is that nimbolide could regulate cell cycle progression in DEN and NMOR induced HCC in mice. Cell cycle is a major event for cell proliferation and an uncontrolled tumor cell proliferation is responsible for HCC progression[45,46]. CDK4 and cyclinD1 are important mediators of cell cycle progression which are found to be overexpressed and associated with poor prognosis in HCC patients[45,46]. In the presence of extracellular stimuli such as cytokines (TNF-α), the active complex is formed by CDK4/CyclinD1 which phosphorylates retinoblastoma protein (pRb), and promotes the transition of the cell cycle from G1 to S phase through the restriction (R) checkpoint. The pRb induces gene expression necessary for DNA replication such as PCNA during S phase[47]. A previous study has shown that nimbolide regulates cell cycle progression in both cell lines and animal models[48]. In glioblastoma, nimbolide inhibited CDK4/CDK6 kinase activity and arrested cell cycle at the G1 phase[49]. Priyadarsini et al[50] reported that in HeLa cells nimbolide treatment inhibited major cell cycle regulatory proteins such as cyclin D1, cyclin B, and PCNA and restricted cell cycle at G0/G1 phase[50]. Concurrent with the above studies, we also observed that nimbolide treatment reduces CDK4 and Cyclin D1 protein expression in HCC mice. Therefore, we speculate that nimbolide could inhibit cell proliferation by preventing cell cycle progression from G1 to S phase and prevents HCC pathogenesis.
In the current study, we found nimbolide might dampen the inflammation through NF-κB pathway in HCC mice. Inflammation has a wide impact on the pathogenesis of cancer development starting from initiation to progression and metastasis. 70% of HCC develops in cirrhotic patients on the background of inflammation[1]. Activation of the NF-κB pathway plays a critical role in inflammation-induced cancer by affecting cell proliferation, apoptosis, angiogenesis, and metastasis[2]. Stimulation of hepatic kupffer cells during chronic inflammation releases proinflammatory cytokines such as TNF-α and IL-1β via the NF-κB pathway which is known to regulate cell proliferation, survival, and inhibition of apoptosis[2]. In this study, we found increased hepatic protein expression of NF-κB and its downstream mediator proinflammatory cytokines TNF-α and IL-1β in HCC mice, indicating the hyperinflammatory state of hepatic tissue. A previous study indicated that nimbolide administration inhibits NF-κB signaling and proinflammatory cytokines in both cell lines and animal models[48]. In line with this evidence, Kavitha et al[51] reported that nimbolide exerts anticancer effects in HepG2 cells by inhibiting NF-κB signaling and its downstream mediator Wnt/β-catenin pathway[51]. Similarly, Gupta et al[52] reported that nimbolide treatment inhibited xenograft progression in colorectal cancer by targeting the NF-κB pathway and by suppressing pro-inflammatory microenvironment[52]. Concomitant with the above results we also proved that nimbolide treatment attenuated the protein expression of NF-κB, TNF-α, and IL-1β in HCC mice. Moreover, molecular docking study revealed that nimbolide formed hydrogen bonds and hydrophobic interaction with amino acid residues of the ligand binding cavity of NF-κB and TNF-α. These results suggest that nimbolide ameliorated inflammation and suppressed NF-κB signaling in DEN and NMOR induced HCC mice. Therefore it is postulated that nimbolide might regulate cell proliferation by targeting the NF-κB signaling pathway which is considered as a central pathway linking inflammation and cancer[2]. The proposed mechanism of action by which nimbolide exerts its therapeutic effects in HCC mice is depicted in Figure 8.
DEN is known to induce HCC by generating reactive oxygen species (ROS) which causes oxidative stress in the cell leading to oxidative DNA damage, membrane lipid peroxidation, and mitochondrial damage favoring cell survival and proliferation[53]. 4HNE, a potent and stable by-product of lipid peroxidation, is considered as a marker of oxidative stress, and can independently form DNA adducts, and causes mutation and genetic instability, thus favoring hepatocarcinogenesis[54]. In the current study, we found increased nuclear 4HNE protein expression in HCC mice liver while nimbolide treatment to HCC mice significantly reduced hepatic 4HNE protein expression. This suggests that nimbolide could protect ROS induced oxidative damage and lipid peroxidation in HCC. In this context, Alshammari et al[29] reported that nimbolide treatment abrogates oxidative stress and promotes antioxidants capacity in the primary hepatocytes[29]. Similarly, another study in DMBA induced oral cancer showed that nimbolide inhibited oxidative stress-induced DNA damage by improving antioxidant enzymes[30]. Thus, collectively our results indicate that nimbolide has a potent antioxidant activity and abrogates oxidative stress by quenching free radicals and might restore cellular redox balance thereby attenuating the progression of carcinogenesis. Our study has some limitations that we did not study the nimbolide effects in ZO-1 or ZONAB conditional knock-out mice to prove the exact mechanism of HCC regulation. Indeed, our future experimental approach would delineate this either in vivo or in vitro. Moreover, with data obtained in this work, it is not possible to establish the mechanisms involved in the anticancer effect of nimbolide. The study show different mechanisms that occur at the same time than the anticancer effect but a direct relationship between them has not been showed. The efficacy of doxorubicin is very limited in the treatment of human HCC so a limited effect of nimbolide could be also expected.
In conclusion, the results of our study showed evidence in HCC mice that nimbolide treatment suppresses tumor growth and cell cycle progression, and ameliorates inflammation and oxidative stress. Importantly, nimbolide treatment restored hepatic TJ proteins expression and regulates ZO-1/ZONAB pathway in HCC. Our study also demonstrated that the anticancer efficiency of nimbolide was comparable with standard anticancer drug doxorubicin in a preclinical model of HCC. Treatment with natural bioactive compounds, therefore, represents promising chemoprevention agents with minimal side effects, and thus nimbolide could be used as a novel potential candidate drug against HCC progression. Indeed, further molecular and population-based studies are warranted.
Worldwide, hepatocellular carcinoma (HCC) is a prevalent lethal disease exhibiting the highest cancer mortality rate with limited chemotherapeutic options. The discovery of a new chemotherapeutic agent is an urgent demand for the treatment of HCC patients.
Tight Junction (TJ) proteins have been implicated to regulate various signal transductions in carcinogenesis. Recent evidence has shown that nimbolide possesses anticancer activity in various cancers; however, its specific mechanism in HCC remains elusive.
We aimed to study the effect of nimbolide on TJ proteins, cell cycle progression, and hepatic inflammation in an experimental hepatocarcinogenesis mouse model.
Diethylnitrosamine (DEN) and N-nitrosomorpholine (NMOR) induced mouse model of HCC was performed in the present study. Nimbolide was given orally for four consecutive weeks to DEN/NMOR induced HCC mice from 28th to 32nd week. At the end of the study period (32nd week), all the mice were sacrificed, blood and liver samples were collected for various analysis. HCC tumor markers such as alpha-fetoprotein (AFP) levels and glypican-3 protein expression were analysed. Hepatic TJ proteins, cell cycle, and inflammatory marker protein expressions were evaluated by western blot analysis. Cell proliferation and oxidative stress markers were analyzed by immunohistochemistry. Molecular docking study was performed to confirm the interaction of nimbolide with zonula occludens 1 (ZO-1), nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-κB) and tumor necrosis factor alpha (TNF-α).
Hepatic tumor size and HCC tumor markers AFP and glypican-3 were reduced following nimbolide treatment. TJ protein (ZO-1 and occludin) expression levels were restored after nimbolide treatment, while ZO-1 associated nucleic acid binding protein expression was attenuated. Additionally, nimbolide treatment also reduced cell proliferation and cell cycle markers. Moreover, nimbolide treatment ameliorated hepatic inflammation and oxidative stress in HCC mice. The binding affinity and modulatory effects of nimbolide with ZO-1, NF-κB, and TNF-α were confirmed by molecular docking analysis.
Nimbolide treatment showed anticancer effects by improving TJ proteins expression, suppressing cell cycle and cell proliferation, and ameliorating inflammation and oxidative stress in DEN and NMOR induced HCC mice.
Nimbolide may be used as a potential chemotherapeutic agent for HCC treatment. Further molecular research and human studies may delineate nimbolide as a candidate drug for HCC treatment.
We thank Dr. Athithan V, PhD, for analyzing molecular docking studies.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver, No. 11313.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aureliano M, Heneberg P, Wang X, Zapater P S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 526] [Cited by in F6Publishing: 570] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 2. | Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin Liver Dis. 2019;39:26-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 3. | Vogel A, Saborowski A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat Rev. 2020;82:101946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Block KI, Gyllenhaal C, Lowe L, Amedei A, Amin ARMR, Amin A, Aquilano K, Arbiser J, Arreola A, Arzumanyan A, Ashraf SS, Azmi AS, Benencia F, Bhakta D, Bilsland A, Bishayee A, Blain SW, Block PB, Boosani CS, Carey TE, Carnero A, Carotenuto M, Casey SC, Chakrabarti M, Chaturvedi R, Chen GZ, Chen H, Chen S, Chen YC, Choi BK, Ciriolo MR, Coley HM, Collins AR, Connell M, Crawford S, Curran CS, Dabrosin C, Damia G, Dasgupta S, DeBerardinis RJ, Decker WK, Dhawan P, Diehl AME, Dong JT, Dou QP, Drew JE, Elkord E, El-Rayes B, Feitelson MA, Felsher DW, Ferguson LR, Fimognari C, Firestone GL, Frezza C, Fujii H, Fuster MM, Generali D, Georgakilas AG, Gieseler F, Gilbertson M, Green MF, Grue B, Guha G, Halicka D, Helferich WG, Heneberg P, Hentosh P, Hirschey MD, Hofseth LJ, Holcombe RF, Honoki K, Hsu HY, Huang GS, Jensen LD, Jiang WG, Jones LW, Karpowicz PA, Keith WN, Kerkar SP, Khan GN, Khatami M, Ko YH, Kucuk O, Kulathinal RJ, Kumar NB, Kwon BS, Le A, Lea MA, Lee HY, Lichtor T, Lin LT, Locasale JW, Lokeshwar BL, Longo VD, Lyssiotis CA, MacKenzie KL, Malhotra M, Marino M, Martinez-Chantar ML, Matheu A, Maxwell C, McDonnell E, Meeker AK, Mehrmohamadi M, Mehta K, Michelotti GA, Mohammad RM, Mohammed SI, Morre DJ, Muralidhar V, Muqbil I, Murphy MP, Nagaraju GP, Nahta R, Niccolai E, Nowsheen S, Panis C, Pantano F, Parslow VR, Pawelec G, Pedersen PL, Poore B, Poudyal D, Prakash S, Prince M, Raffaghello L, Rathmell JC, Rathmell WK, Ray SK, Reichrath J, Rezazadeh S, Ribatti D, Ricciardiello L, Robey RB, Rodier F, Rupasinghe HPV, Russo GL, Ryan EP, Samadi AK, Sanchez-Garcia I, Sanders AJ, Santini D, Sarkar M, Sasada T, Saxena NK, Shackelford RE, Shantha Kumara HMC, Sharma D, Shin DM, Sidransky D, Siegelin MD, Signori E, Singh N, Sivanand S, Sliva D, Smythe C, Spagnuolo C, Stafforini DM, Stagg J, Subbarayan PR, Sundin T, Talib WH, Thompson SK, Tran PT, Ungefroren H, Vander Heiden MG, Venkateswaran V, Vinay DS, Vlachostergios PJ, Wang Z, Wellen KE, Whelan RL, Yang ES, Yang H, Yang X, Yaswen P, Yedjou C, Yin X, Zhu J, Zollo M. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin Cancer Biol. 2015;35 Suppl:S276-S304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 5. | Piontek J, Krug SM, Protze J, Krause G, Fromm M. Molecular architecture and assembly of the tight junction backbone. Biochim Biophys Acta Biomembr. 2020;1862:183279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in F6Publishing: 700] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 7. | Berman JJ. Tumor classification: molecular analysis meets Aristotle. BMC Cancer. 2004;4:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol. 2015;63:1023-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 9. | Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1148] [Cited by in F6Publishing: 1192] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745-29753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1010] [Cited by in F6Publishing: 1005] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 11. | de Mendoza A, Suga H, Ruiz-Trillo I. Evolution of the MAGUK protein gene family in premetazoan lineages. BMC Evol Biol. 2010;10:93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | González-Mariscal L, Lechuga S, Garay E. Role of tight junctions in cell proliferation and cancer. Prog Histochem Cytochem. 2007;42:1-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834-7838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 366] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Spadaro D, Tapia R, Jond L, Sudol M, Fanning AS, Citi S. ZO proteins redundantly regulate the transcription factor DbpA/ZONAB. J Biol Chem. 2014;289:22500-22511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004;40:2717-2725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Kaihara T, Kusaka T, Nishi M, Kawamata H, Imura J, Kitajima K, Itoh-Minami R, Aoyama N, Kasuga M, Oda Y, Hattori M, Fujimori T. Dedifferentiation and decreased expression of adhesion molecules, E-cadherin and ZO-1, in colorectal cancer are closely related to liver metastasis. J Exp Clin Cancer Res. 2003;22:117-123. [PubMed] [Cited in This Article: ] |
| 18. | Kleeff J, Shi X, Bode HP, Hoover K, Shrikhande S, Bryant PJ, Korc M, Büchler MW, Friess H. Altered expression and localization of the tight junction protein ZO-1 in primary and metastatic pancreatic cancer. Pancreas. 2001;23:259-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131-4142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 875] [Cited by in F6Publishing: 852] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 20. | Ram AK, Pottakat B, Vairappan B. Increased systemic zonula occludens 1 associated with inflammation and independent biomarker in patients with hepatocellular carcinoma. BMC Cancer. 2018;18:572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Ekong DEU. Chemistry of the meliacins (limonoids). The structure of nimbolide, a new meliacin from Azadirachta indica. Chem Commun (London). 1967;16:808a–808a. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Raja Singh P, Sugantha Priya E, Balakrishnan S, Arunkumar R, Sharmila G, Rajalakshmi M, Arunakaran J. Inhibition of cell survival and proliferation by nimbolide in human androgen-independent prostate cancer (PC-3) cells: involvement of the PI3K/Akt pathway. Mol Cell Biochem. 2017;427:69-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Elumalai P, Gunadharini DN, Senthilkumar K, Banudevi S, Arunkumar R, Benson CS, Sharmila G, Arunakaran J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012;215:131-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Harish Kumar G, Chandra Mohan KV, Jagannadha Rao A, Nagini S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest New Drugs. 2009;27:246-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Sophia J, Kiran Kishore T K, Kowshik J, Mishra R, Nagini S. Nimbolide, a neem limonoid inhibits Phosphatidyl Inositol-3 Kinase to activate Glycogen Synthase Kinase-3β in a hamster model of oral oncogenesis. Sci Rep. 2016;6:22192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Ahn KS, Kim C, Shanmugam MK, Siveen KS, Arfuso F, Samym RP, Deivasigamanim A, Lim LH, Wang L, Goh BC, Kumar AP, Hui KM, Sethi G. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid Redox Signal. 2016;24:575-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Subramani R, Gonzalez E, Arumugam A, Nandy S, Gonzalez V, Medel J, Camacho F, Ortega A, Bonkoungou S, Narayan M, Dwivedi Ak, Lakshmanaswamy R. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition. Sci Rep. 2016;6:19819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 28. | Hsieh YH, Lee CH, Chen HY, Hsieh SC, Lin CL, Tsai JP. Induction of cell cycle arrest, DNA damage, and apoptosis by nimbolide in human renal cell carcinoma cells. Tumour Biol. 2015;36:7539-7547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Alshammari GM, Balakrishnan A, Chinnasamy T. Nimbolide attenuate the lipid accumulation, oxidative stress and antioxidant in primary hepatocytes. Mol Biol Rep. 2017;44:463-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Priyadarsini RV, Manikandan P, Kumar GH, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Radic Res. 2009;43:492-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Ha SY, Choi M, Lee T, Park CK. The Prognostic Role of Mitotic Index in Hepatocellular Carcinoma Patients after Curative Hepatectomy. Cancer Res Treat. 2016;48:180-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | von Felden J, Wege H, Schulze K. Elevated Aspartate Aminotransferase to Alanine Aminotransferase Ratio Predicts Poor Outcome in Hepatocellular Carcinoma. Hepatol Commun. 2020;4:1382-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan D, Cao X, Han M, Du H, Ye Y. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One. 2020;15:e0228857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 34. | Guo M, Zhang H, Zheng J, Liu Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J Cancer. 2020;11:2008-2021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 35. | Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11:212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 37. | Stroescu C, Dragnea A, Ivanov B, Pechianu C, Herlea V, Sgarbura O, Popescu A, Popescu I. Expression of p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in hepatocellular carcinoma. J Gastrointestin Liver Dis. 2008;17:411-417. [PubMed] [Cited in This Article: ] |
| 38. | Orbán E, Szabó E, Lotz G, Kupcsulik P, Páska C, Schaff Z, Kiss A. Different expression of occludin and ZO-1 in primary and metastatic liver tumors. Pathol Oncol Res. 2008;14:299-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Mohandas S, Vairappan B. Pregnane X receptor activation by its natural ligand Ginkgolide-A improves tight junction proteins expression and attenuates bacterial translocation in cirrhosis. Chem Biol Interact. 2020;315:108891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Vairappan B, Sundhar M, Srinivas BH. Resveratrol Restores Neuronal Tight Junction Proteins Through Correction of Ammonia and Inflammation in CCl4-Induced Cirrhotic Mice. Mol Neurobiol. 2019;56:4718-4729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S, Tsukita S. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465-2475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 42. | Bi S, Li L, Gu H, Li M, Xu S, Bu W, Zhang M, Zhou Z, Chen X. Lycopene upregulates ZO-1 and downregulates claudin-1 through autophagy inhibition in the human cutaneous squamous cell carcinoma cell line COLO-16. J Cancer. 2019;10:510-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024-2033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 44. | Georgiadis A, Tschernutter M, Bainbridge JW, Balaggan KS, Mowat F, West EL, Munro PM, Thrasher AJ, Matter K, Balda MS, Ali RR. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS One. 2010;5:e15730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Lu JW, Lin YM, Chang JG, Yeh KT, Chen RM, Tsai JJ, Su WW, Hu RM. Clinical implications of deregulated CDK4 and Cyclin D1 expression in patients with human hepatocellular carcinoma. Med Oncol. 2013;30:379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Joo M, Kang YK, Kim MR, Lee HK, Jang JJ. Cyclin D1 overexpression in hepatocellular carcinoma. Liver. 2001;21:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway and the restriction point. Curr Opin Cell Biol. 1996;8:805-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 303] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Elumalai P, Arunakaran J. Review on molecular and chemopreventive potential of nimbolide in cancer. Genomics Inform. 2014;12:156-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Karkare S, Chhipa RR, Anderson J, Liu X, Henry H, Gasilina A, Nassar N, Ghosh J, Clark JP, Kumar A, Pauletti GM, Ghosh PK, Dasgupta B. Direct inhibition of retinoblastoma phosphorylation by nimbolide causes cell-cycle arrest and suppresses glioblastoma growth. Clin Cancer Res. 2014;20:199-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Priyadarsini RV, Murugan RS, Sripriya P, Karunagaran D, Nagini S. The neem limonoids azadirachtin and nimbolide induce cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells. Free Radic Res. 2010;44:624-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Kavitha K, Vidya Priyadarsini R, Anitha P, Ramalingam K, Sakthivel R, Purushothaman G, Singh AK, Karunagaran D, Nagini S. Nimbolide, a neem limonoid abrogates canonical NF-κB and Wnt signaling to induce caspase-dependent apoptosis in human hepatocarcinoma (HepG2) cells. Eur J Pharmacol. 2012;681:6-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Gupta SC, Prasad S, Sethumadhavan DR, Nair MS, Mo YY, Aggarwal BB. Nimbolide, a limonoid triterpene, inhibits growth of human colorectal cancer xenografts by suppressing the proinflammatory microenvironment. Clin Cancer Res. 2013;19:4465-4476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Moreira AJ, Rodrigues G, Bona S, Cerski CT, Marroni CA, Mauriz JL, González-Gallego J, Marroni NP. Oxidative stress and cell damage in a model of precancerous lesions and advanced hepatocellular carcinoma in rats. Toxicol Rep. 2015;2:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |